Abstract
Candida parapsilosis produces biofilm, which colonizes catheters and other invasive medical devices that are manipulated by health care workers. In previous studies, C. parapsilosis in vitro biofilms have exhibited high resistance rates against conventional antifungals, but susceptibility to both echinocandins and lipid formulations of amphotericin B (lipid complex and liposomal). However, a recent study showed good activity of amphotericin B deoxycholate on the biomass of C. parapsilosis biofilms. Although moderate activity of echinocandins has been demonstrated against low metabolic activity biofilms of C. parapsilosis, few studies have analyzed the action of these drugs on high metabolic activity biofilms. Moreover, high biofilm-forming isolates have been associated with central venous catheter-related fungemia outbreaks and higher mortality rates. Therefore, it is relevant to verify the activity of the main antifungal drugs against high metabolic activity biofilms of C. parapsilosis. Our study aimed to evaluate the in vitro activity of amphotericin B deoxycholate, anidulafungin, caspofungin, and micafungin against high biofilm-forming and high metabolic activity clinical isolates of C. parapsilosis. Our results showed good activity of amphotericin B against C. parapsilosis biofilms, but none of the echinocandin drugs was effective. This suggests that amphotericin B deoxycholate may be a better choice than echinocandins for the treatment of biofilm-associated infections by C. parapsilosis, mainly in countries with insufficient health care resources to purchase lipid formulations of amphotericin B. These results warn of the possibility of persistent catheter-related candidemia caused by high biofilm-forming C. parapsilosis strains when treated with echinocandin drugs.
Keywords: Candida parapsilosis, Biofilm, XTT, Amphotericin B, Echinocandins, Antifungal resistance
Introduction
Candida spp. are among the main agents of bloodstream infections worldwide, not only due to implementation of high immunosuppressive therapies (e.g., chemotherapy, transplants) but also due to the increasing use of invasive devices such as central venous catheters (CVCs) [1], leading to mortality rates of 25–40% [2]. Candida spp., the third most common pathogen after coagulase-negative staphylococci and Staphylococcus aureus, are responsible for approximately 8% of catheter-associated infections [3].
Although C. albicans remains the most frequently isolated species worldwide, its incidence is decreasing, whereas C. parapsilosis infections are emerging related to the increased use of intravascular devices [4, 5]. C. parapsilosis is the major non-C. albicans species causing vascular catheter candidemia, primarily in pediatric patients [6], with the largest increase in incidence since 1990 [7]. Among the Candida spp., C. parapsilosis is one of the major biofilm-forming species, colonizing catheters and other invasive medical devices that are manipulated by health care workers [7–9].
Biofilms are microbial communities embedded in an extracellular matrix, irreversibly attached to the surface of inert materials or living tissues, and exhibit lower growth rates and higher resistance to antibiotics. They can develop on the surface of many hospital devices, such as cardioverter defibrillators, prostheses, and catheters, hindering the eradication of Candida from the hospital environment [10].
The phenotype of Candida biofilms has a high resistance rate against conventional antifungals (azoles and polyenes) due to a complex and multifactorial process that includes extracellular matrix production, overexpression of sterols and efflux pumps, the presence of persistent cells, among others [11]. However, biofilm resistance to amphotericin B (AMB) is less reported than that for azole drugs [12].
Echinocandins have fungicidal activity against Candida spp. and are the recommended first-line treatment for candidemia in the main clinical guidelines [13, 14]. This antifungal class decreases extracellular matrix production by inhibiting 1,3-β-d-glucan synthesis [9] and it has been considered the first choice for treatment of biofilm-associated invasive candidiasis [15]. The development of biofilm resistance to echinocandins is relatively slow [16]. Furthermore, previous reports demonstrated the effectiveness of these drugs against catheter infections in vivo and in vitro, indicating their use as a potential anti-Candida biofilm therapy [17, 18].
On the other hand, high biofilm-forming (HBF) isolates of Candida parapsilosis have been associated with outbreaks [19] and higher mortality rates [8]. HBF strains of this pathogen have been associated with CVC-related fungemia and death within 30 days from the onset of the episode [20].
Recently, an investigation involving cultures of clinical isolates obtained from blood and non-sterile sites showed that C. parapsilosis (66.7%) had the highest biofilm production rate followed by C. tropicalis (44.7%) and C. albicans (20.8%). Furthermore, C. parapsilosis strains showed the highest metabolic activity and biofilm biomass [21].
Therefore, to better address the issue of C. parapsilosis nosocomial infections, it is important to identify the most suitable antifungal drugs for treatment of C. parapsilosis biofilm-associated infections caused by HBF isolates and in those presenting high metabolic activity (HMA). The purpose of this study was to evaluate the in vitro activity of AMB deoxycholate (d-AMB), anidulafungin (ANF), caspofungin (CAF), and micafungin (MIF) against sessile cells of HBF and HMA C. parapsilosis clinical isolates.
Materials and methods
First, we determined the biofilm formation profile of 38 C. parapsilosis clinical isolates obtained from cases of invasive candidiasis. Biofilm biomass and metabolic activity were measured by crystal violet staining [22] and 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay [23], respectively. The results were interpreted according to a previous published protocol [24]. Each biofilm experiment was performed at least three times on different days and C. albicans SC5314 was used as a quality control strain.
Isolates classified as HBF and HMA were selected for antifungal susceptibility testing. The sessile minimum inhibitory concentrations (SMICs) for each isolate were calculated from the measurement of biofilm metabolic activity by the XTT reduction assay after antifungal treatment with AMB (Sigma-Aldrich, St. Louis, MO, USA), ANF (Pfizer, New York, NY, USA), CAF (Sigma-Aldrich, St. Louis, MO, USA), or MIF (Astellas Pharma, Tokyo, Japan). SMIC50 and SMIC80 were defined as the antifungal concentration at which a 50% or 80% decrease in absorption was detected in comparison with the untreated biofilm, respectively [23]. Each experiment was performed at least three times on different days and C. albicans SC5314 was used as a quality control strain. The minimal inhibitory concentrations (MICs) of d-AMB, ANF, CAF, and MIF for planktonic cells of the isolates were determined by the European Committee for Antimicrobial Susceptibility Testing (EUCAST) microdilution assay [25] and correlated with the SMICs of the respective biofilms. Each MIC experiment was performed at least three times on different days and C. parapsilosis ATCC22019 and C. krusei ATCC6258 were used as quality control strains.
Results and discussion
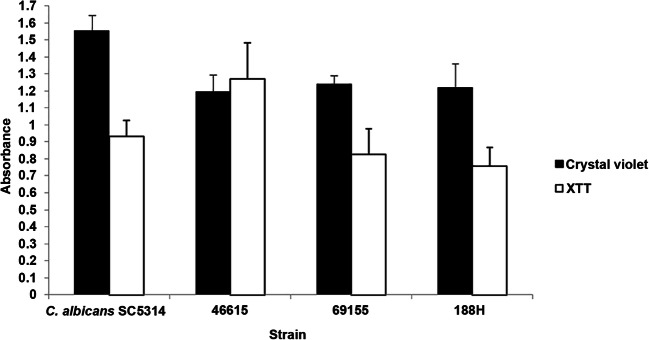
All 38 isolates were positive for biofilm formation capability, although the level of biofilm production was highly variable; most of the isolates showed a low level of biofilm formation (71.1%). Most biofilms presented low metabolic activity (71.1%), and biofilm metabolic activity did not correlate with the amount of biofilm biomass (data not shown). The three isolates classified as HBF and HMA (numbers 46615, 69155, and 188H, Fig. 1) were submitted to antifungal susceptibility testing.
Fig. 1.
Biofilm quantification of the Candida parapsilosis clinical isolates and Candida albicans SC5314 reference strain by crystal violet staining and XTT reduction assay. Error bars represent the standard deviation
The planktonic cells of all isolates were susceptible to d-AMB (0.125–0.5 mg/L) and intermediate to ANF (0.25–1 mg/L), CAF (1 mg/L), and MIF (1 mg/L), according to the EUCAST Antifungal Clinical Breakpoints Table v.9.0 [26]. The echinocandins did not show activity against sessile cells of the isolates at the highest concentration tested (16 mg/L) (Table 1).
Table 1.
Activity of amphotericin B and echinocandins against biofilms with high metabolic activity from Candida parapsilosis clinical isolates and Candida albicans SC5314
| Isolate | Amphotericin B (mg/L) | Anidulafungin (mg/L) | Caspofungin (mg/L) | Micafungin (mg/L) | ||||
|---|---|---|---|---|---|---|---|---|
| SMIC50 | SMIC80 | SMIC50 | SMIC80 | SMIC50 | SMIC80 | SMIC50 | SMIC80 | |
| 46615 | 0.25 | 0.5 | > 16 | > 16 | > 16 | > 16 | > 16 | > 16 |
| 69155 | 0.25 | 0.5 | > 16 | > 16 | > 16 | > 16 | > 16 | > 16 |
| 188H | 0.25 | 0.5 | > 16 | > 16 | > 16 | > 16 | > 16 | > 16 |
| SC5314 | 0.5 | 1 | 0.03 | 0.25 | 0.03 | 0.06 | 0.03 | 0.25 |
SMIC50, SMIC80, 50%, and 80% reduction, respectively, in the metabolic activity of the biofilm treated with the antifungal compared with the control
Limited therapeutic options make biofilm formation a significant clinical problem for critically ill patients [16], and it is important to investigate whether HBF and HMA isolates are a complicating factor in C. parapsilosis infections. In this study, we found that d-AMB presented activity against both planktonic and sessile cells of C. parapsilosis clinical isolates. Moreover, we demonstrated the lack of efficacy of the three echinocandin agents against sessile cells of HBF and HMA C. parapsilosis clinical isolates.
The main antifungal drugs available have been found to have minimal activity against Candida spp. biofilms [12]. C. parapsilosis planktonic cells demonstrate innately high MICs for echinocandins [27], and some studies have demonstrated activity of these antifungal agents against biofilms of this species [28–32]. However, only moderate susceptibility to echinocandins was reported in low metabolic activity biofilms of C. parapsilosis [31, 32].
Taking into account the variable susceptibility of C. albicans biofilms to MIF, depending on biomass production or metabolic activity, and the fact that isolates with HBF or HMA were more susceptible to this antifungal agent [33], we evaluated echinocandins activity against C. parapsilosis biofilms. In contrast with C. albicans, our results did not show any activity of MIF against sessile cells of HBF and HMA C. parapsilosis isolates, in accordance with previous observations [34]. Interestingly, recent in vivo findings suggest that lock therapy with MIF may promote C. parapsilosis biofilm dispersal rather than biofilm-cidal activity [35].
Biofilm formation by C. parapsilosis has shown a high degree of variability among isolates [5, 36] and the anti-biofilm activity of d-AMB has been reported to be species and strain dependent [37, 38]. An earlier study showed that C. parapsilosis biofilms were resistant to d-AMB and susceptible to lipid formulations of AMB (both lipid complex and liposomal) [28]. Conversely, our results are in agreement with recently published data demonstrating good activity of d-AMB on the biomass reduction of C. parapsilosis biofilms [38, 39]. Moreover, a previous study showed higher activity of d-AMB than ANF against C. parapsilosis biofilms [39], in agreement with our results, which extend this observation to CAF and MIF.
In conclusion, C. parapsilosis sessile cells showing HBF and HMA were susceptible only to d-AMB, indicating that this agent as a better choice than echinocandins for the treatment of biofilm-related infections by this species, mainly in countries with insufficient health care resources to purchase lipid formulations of AMB. A larger cohort of isolates from multiple centers and in vivo models should confirm our results. However, these data may alert physicians who empirically prescribe echinocandins as therapy for catheter-related candidemia regarding the possible persistence of infections caused by C. parapsilosis.
Author contributions
All authors contributed to the conception and design of the study. Material preparation, data collection, and analysis were performed by DYT and GDN. The first draft of the manuscript was written by DYT, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding information
This work was supported by grants from Programa de Fomento às Atividades de Pesquisa (PROFAP- LIM) and CNPq – the National Science and Technology Development Council, in Brazil (455905/2014-2). DYT conducted this work supported by a postdoctoral fellowship (2018/15491-3), São Paulo Research Foundation (FAPESP).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Enoch DA, Yang H, Aliyu SH, Micallef C. The changing epidemiology of invasive fungal infections. Methods Mol Biol. 2017;1508:17–65. doi: 10.1007/978-1-4939-6515-1_2. [DOI] [PubMed] [Google Scholar]
- 2.Clancy CJ, Shields RK, Nguyen MH (2016) Invasive candidiasis in various patient populations: incorporating non-culture diagnostic tests into rational management strategies. J Fungi (Basel) 2. 10.3390/jof2010010 [DOI] [PMC free article] [PubMed]
- 3.Bouza E, Guinea J, Guembe M. The role of antifungals against Candida biofilm in catheter-related candidemia. Antibiotics (Basel) 2014;4:1–17. doi: 10.3390/antibiotics4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamoth F, Lockhart SR, Berkow EL, Calandra T. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother. 2018;73:i4–i13. doi: 10.1093/jac/dkx444. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Cerdeira C, Gregorio MC, Molares-Vila A, López-Barcenas A, Fabbrocini G, Bardhi B, Sinani A, Sánchez-Blanco E, Arenas-Guzmán R, Hernandez-Castro R. Biofilms and vulvovaginal candidiasis. Colloids Surf B Biointerfaces. 2019;174:110–125. doi: 10.1016/j.colsurfb.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Puig-Asensio M, Padilla B, Garnacho-Montero J, Zaragoza O, Aguado JM, Zaragoza R, Montejo M, Muñoz P, Ruiz-Camps I, Cuenca-Estrella M, Almirante B; CANDIPOP Project; GEIH-GEMICOMED (SEIMC); REIPI Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a population-based surveillance in Spain. Clin Microbiol Infect. 2014;20:O245–O254. doi: 10.1111/1469-0691.12380. [DOI] [PubMed] [Google Scholar]
- 7.Singaravelu K, Gácser A, Nosanchuk JD. Genetic determinants of virulence - Candida parapsilosis. Rev Iberoam Micol. 2014;31:16–21. doi: 10.1016/j.riam.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Trofa D, Gácser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008;21:606–625. doi: 10.1128/CMR.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larkin EL, Dharmaiah S, Ghannoum MA. Biofilms and beyond: expanding echinocandin utility. J Antimicrob Chemother. 2018;73:i73–i81. doi: 10.1093/jac/dkx451. [DOI] [PubMed] [Google Scholar]
- 10.Cavalheiro M, Teixeira MC. Biofilms: threats, challenges, and promising strategies. Front Med (Lausanne) 2018;5:28. doi: 10.3389/fmed.2018.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva S, Rodrigues CF, Araújo D, Rodrigues ME, Henriques M (2017) Candida species biofilms’ antifungal resistance. J Fungi (Basel) 3. 10.3390/jof3010008 [DOI] [PMC free article] [PubMed]
- 12.Taff HT, Mitchell KF, Edward JA, Andes DR. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013;8:1325–1337. doi: 10.2217/fmb.13.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Flörl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18(Suppl 7):19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 15.Almirante B, Garnacho-Montero J, Maseda E, Candel FJ, Grau S, Guinea J, Moreno I, Muñoz P, Ruiz-Santana S. Candidemia and invasive candidiasis approach in critically ill patients: role of the echinocandins. Rev Esp Quimioter. 2017;30:355–367. [PubMed] [Google Scholar]
- 16.Swaminathan S, Kamat S, Pinto NA. Echinocandins: their role in the management of Candida biofilms. Indian J Med Microbiol. 2018;36:87–92. doi: 10.4103/ijmm.IJMM_17_400. [DOI] [PubMed] [Google Scholar]
- 17.Katragkou A, Roilides E, Walsh TJ. Role of echinocandins in fungal biofilm-related disease: vascular catheter-related infections, immunomodulation, and mucosal surfaces. Clin Infect Dis. 2015;61(Suppl 6):S622–S629. doi: 10.1093/cid/civ746. [DOI] [PubMed] [Google Scholar]
- 18.Lazzell AL, Chaturvedi AK, Pierce CG, Prasad D, Uppuluri P, Lopez-Ribot JL. Treatment and prevention of Candida albicans biofilms with caspofungin in a novel central venous catheter murine model of candidiasis. J Antimicrob Chemother. 2009;64:567–570. doi: 10.1093/jac/dkp242. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn DM, Mikherjee PK, Clark TA, Pujol C, Chandra J, Hajjeh RA, Warnock DW, Soil DR, Ghannoum MA. Candida parapsilosis characterization in an outbreak setting. Emerg Infect Dis. 2004;10:1074–1081. doi: 10.3201/eid1006.030873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soldini S, Posteraro B, Vella A, De Carolis E, Borghi E, Falleni M, Losito AR, Maiuro G, Trecarichi EM, Sanguinetti M, Tumbarello M. Microbiologic and clinical characteristics of biofilm-forming Candida parapsilosis isolates associated with fungaemia and their impact on mortality. Clin Microbiol Infect. 2018;24:771–777. doi: 10.1016/j.cmi.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Munusamy K, Vadivelu J, Tay ST. A study on Candida biofilm growth characteristics and its susceptibility to aureobasidinA. Rev Iberoam Micol. 2018;35:68–72. doi: 10.1016/j.riam.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Melo AS, Bizerra FC, Freymüller E, Arthington-Skaggs BA, Colombo AL. Biofilm production and evaluation of antifungal susceptibility amongst clinical Candida spp. isolates, including strains of the Candida parapsilosis complex. Med Mycol. 2011;49:253–262. doi: 10.3109/13693786.2010.530032. [DOI] [PubMed] [Google Scholar]
- 23.Pierce CG, Uppuluri P, Tristan AR, Wormley FL, Jr, Mowat E, Ramage G, Lopez-Ribot JL. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3:1494–1500. doi: 10.1038/nport.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcos-Zambrano LJ, Escribano P, Bouza E, Guinea J. Production of biofilm by Candida and non-Candida spp. isolates causing fungemia: comparison of biomass production and metabolic activity and development of cut-off points. Int J Med Microbiol. 2014;304:1192–1198. doi: 10.1016/j.ijmm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Arendrup MC, Meletiadis J, Mouton JW, Lagrou K, Hamal Petr, Guinea J, the Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST) (2017) EUCAST DEFINITIVE DOCUMENT E.DEF 7.3.1: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7_3_1_Yeast_testing__definitive.pdf. Accessed 22 May 2018
- 26.EUCAST (2018) Antifungal agents. Breakpoint tables for interpretation of MICs (version 90 valid from 2018-02-12) European Committee on Antimicrobial Susceptibility Testing http://wwweucastorg/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/Antifungal_breakpoints_v_90_180212pdf. Accessed 22 May 2018
- 27.Garcia-Effron G, Katiyar SK, Park S, Edlind TD, Perlin DS. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob Agents Chemother. 2008;52:2305–2312. doi: 10.1128/AAC.00262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother. 2002;46:1773–1780. doi: 10.1128/AAC.46.6.1773-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katragkou A, Chatzimoschou A, Simitsopoulou M, Dalakiouridou M, Diza-Mataftsi E, Tsantali C, Roilides E. Differential activities of newer antifungal agents against Candida albicans and Candida parapsilosis biofilms. Antimicrob Agents Chemother. 2008;52:357–360. doi: 10.1128/AAC.00856-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cocuaud C, Rodier MH, Daniault G, Imbert C. Anti-metabolic activity of caspofungin against Candida albicans and Candida parapsilosis biofilms. J Antimicrob Chemother. 2005;56:507–512. doi: 10.1093/jac/dki269. [DOI] [PubMed] [Google Scholar]
- 31.Prażyńska M, Bogiel T, Gospodarek-Komkowska E. In vitro activity of micafungin against biofilms of Candida albicans, Candida glabrata, and Candida parapsilosis at different stages of maturation. Folia Microbiol (Praha) 2018;63:209–216. doi: 10.1007/s12223-017-0555-2. [DOI] [PubMed] [Google Scholar]
- 32.Simitsopoulou M, Peshkova P, Tasina E, Katragkou A, Kyrpitzi D, Velegraki A, Walsh TJ, Roilides E. Species-specific and drug-specific differences in susceptibility of Candida biofilms to echinocandins: characterization of less common bloodstream isolates. Antimicrob Agents Chemother. 2013;57:2562–2570. doi: 10.1128/AAC.02541-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcos-Zambrano LJ, Escribano P, Bouza E, Guinea J. Susceptibility of Candida albicans biofilms to caspofungin and anidulafungin is not affected by metabolic activity or biomass production. Med Mycol. 2016;54:155–161. doi: 10.1093/mmy/myv094. [DOI] [PubMed] [Google Scholar]
- 34.Kovács R, Tóth Z, Nagy F, Daróczi L, Bozó A, Majoros L. Activity of exogenous tyrosol in combination with caspofungin and micafungin against Candida parapsilosis sessile cells. J Appl Microbiol. 2017;122:1529–1536. doi: 10.1111/jam.13452. [DOI] [PubMed] [Google Scholar]
- 35.Fujimoto K, Takemoto K. Efficacy of liposomal amphotericin B against four species of Candida biofilms in an experimental mouse model of intravascular catheter infection. J Infect Chemother. 2018;24:958–964. doi: 10.1016/j.jiac.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Adherence and biofilm formation of non-Candida albicans Candida species. Trends Microbiol. 2011;19:241–247. doi: 10.1016/j.tim.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Prażyńska M, Gospodarek E. In vitro effect of amphotericin B on Candida albicans, Candida glabrata and Candida parapsilosis biofilm formation. Mycopathologia. 2014;177:19–27. doi: 10.1007/s11046-014-9727-7. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues CF, Henriques M (2017) Liposomal and deoxycholate amphotericin B formulations: effectiveness against biofilm infections of Candida spp. Pathogens 6. 10.3390/pathogens6040062 [DOI] [PMC free article] [PubMed]
- 39.Valentín A, Cantón E, Pemán J, Fernandez-Rivero ME, Tormo-Mas MA, Martínez JP. In vitro activity of anidulafungin in combination with amphotericin B or voriconazole against biofilms of five Candida species. J Antimicrob Chemother. 2016;71:3449–3452. doi: 10.1093/jac/dkw316. [DOI] [PubMed] [Google Scholar]