Abstract
Papain-like cysteine proteases (PLCPs) in plants are essential to prevent phytopathogen invasion. In order to search for cysteine protease inhibitors and to investigate compounds that could be associated to pineapple Fusarium disease, a chemistry investigation was performed on Fusarium proliferatum isolated from Ananas comosus (pineapple) and cultivated in Czapek medium. From F. proliferatum extracts, nine secondary metabolites were isolated and characterized by nuclear magnetic resonance spectroscopy and mass spectrometry experiments: beauvericin (1), fusaric acid (2), N-ethyl-3-phenylacetamide (3), N-acetyltryptamine (4), cyclo(L-Val-L-Pro) cyclodipeptide (5), cyclo(L-Leu-L-Pro) cyclodipeptide (6), cyclo(L-Leu-L-Pro) diketopiperazine (7), 2,4-dihydroxypyrimidine (8), and 1H-indole-3-carbaldehyde (9). Compounds 1, 3, and 6 showed significant inhibition of papain, with IC50 values of 25.3 ± 1.9, 39.4 ± 2.5, and 7.4 ± 0.5 μM, respectively. Compound 1 also showed significant inhibition against human cathepsins V and B with IC50 of 46.0 ± 3.0 and 6.8 ± 0.7 μM, respectively. The inhibition of papain by mycotoxins (fusaric acid and beauvericin) may indicate a mechanism of Fusarium in the roles of infection process.
Electronic supplementary material
The online version of this article (10.1007/s42770-020-00256-7) contains supplementary material, which is available to authorized users.
Keywords: Cathepsin; Cysteine peptidases; Fusaric acid; Fusarium proliferatum, papain
Introduction
Protease inhibitors in plants are key factors for defense and promotion of balance of the ecological system interaction between plants, microorganisms, and insects [1, 2]. Most proteolytic enzymes are papain-like cysteine proteases (PLCPs), and they play an important role in plant immunity preventing pathogen invaders [3]. In humans, PLCPs are known as cathepsins, and the upregulation of these enzymes in the organism is related to pathologic disorders (osteoporosis, arthritis, cancer, metastasis, etc.) [4, 5]. Plants’ PLCPs belong to protease family C1A of clan CA, which catalytic triad contains the amino acids Cys, His, and Asn [3]. The most studied PLCP of plants is papain in latex from Carica papaya (Caricaceae) and bromelain from pineapple, Ananas comosus (Bromeliaceae) [3, 6]. Bromelain was found as the major protein of pineapple crown leaf extract [7].
Experiments of knockout for protease depletion have shown that mutants lose resistance against fungus, such as tomato rcr3 null mutants [3]. In addition, studies of papain from papaya tree and wild fig (Ficus virgata, Moracease) corroborate with the previous study demonstrating that cysteine protease expression is crucial to resistance to herbivore attack [8, 9]. Recently, bromelain from pineapple stem showed to be essential to prevent phytopathogen invasion [10]. Pineapple (Ananas comosus, Bromeliaceae) is a perennial plant of considerable economic importance in tropical and subtropical areas. The fruits are considered phytoceutical, and different parts of the plant are pointed with medicinal usage [7]. Fungal diseases caused by Fusarium species carry out damage of fruits and stem apices [11]. Among the Fusarium species, Fusarium proliferatum has been described as phytopathogenic on pineapple [11], banana [12], and citrus [13].
Previously, diverse compounds were identified from Fusarium proliferatum, such as the beauvericin [14], fusaproliferin [15], and naphytoquinones, and some of the characterized compounds have shown significant pharmacological activities [16]. In this view, this work searched for compounds from F. proliferatum, which could be related to the process of fungal invasion on pineapple (A. comosus) by protease inhibition. Additionally, compounds isolated from F. proliferatum were screened in human cathepsins V and B (CatV and CatB).
Material and methods
Fusarium strains DNA extraction and sequencing of PCR products
Pineapple (Ananas comosus) infected by fungi was commercially acquired in São Carlos, SP, Brazil. Afterward, using sterile procedure, the fruit tissue with fungi was cut out and transferred to potato dextrose agar (PDA, Neogen Corporation, Lansing, MI, USA) medium plates. In order to purify the fungi, the hyphae of fungi were transferred into new plates, and the strains were successively replicated in new plates with PDA.
The genomic DNA was isolated, and the microorganism was identified by the BPI biotechnology company (Biotecnologia Pesquisa e Inovação, Botucatu, SP, Brazil) using Sange method [17]. The DNAs of fungi isolated were extracted using kit ZR fungal/bacterial DNA MicroPrep™ (D6007) (Zymo Research, Irvine, CA, USA) [18]. The DNA was evaluated by electrophoresis agarose gel and quantified by fluorescence (Qubit, Thermo Fisher, Waltham, MA, USA). The DNA was stored at − 20 °C. The PCR assays were performed using 10 μL of GoTaq® Colorless Master Mix 2× (Promega, Madison, WI, USA), 0.6 μM of oligonucleotide forward and 0.6 μM of reverse oligonucleotide, 40 ng of genomic DNA, and ultrapure sterile water to complete the final volume of 20 μL. The primers ITS-5 forward (5′GGAAGTAAAAGTCGTAACAAGG) and ITS-4 reverse (5′TCCTCCGCTTATTGATATGC3′) were used to amplify the region of ITS [18]. The PCR was conducted in a Veriti™ Thermal Cycler (Applied Biosystems, Foster, CA, USA) using the following conditions: at 95 °C for 5 min, followed by 30 cycles at 95 °C for 30 s; 56 °C for 40 s (annealing temperature); 72 °C for 1 min; and 72 °C for 5 min. PCR samples were evaluated by electrophorese in 2% agarose gels with UniSafe Dye (Uniscience, Miami Lakes, FL, USA) and visualized by UV light. After purification it was used in Agencourt AMPure XP (Beckman Coulter Inc., Barueri, SP, USA) and quantified by fluorescence in Qubit (Thermo Fisher, Waltham, MA, USA).
The primers ITS-5 and ITS-4 were used for sequencing reaction labeled using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) and purified by precipitating procedure with ethanol/EDTA/sodium acetate. Automated sequencing was obtained by capillary electrophoresis at ABI 3500 Genetic Analyzer (Applied Biosystems). The software used to evaluate electropherograms was EMBOSS (European Molecular Biology Open Software)/Merger, Chromas Lite, Geneious 4.8.3. The BLAST tool was used to compare the genomic sequences of nucleotides obtained with the references in the NCBI (Bethesda, MD, USA)/GenBank, which is a public database of nucleotide sequences available for identification purposes.
Culture media for Fusarium
In order to evaluate the best environmental conditions for a large-scale culture of fungi, the chemical profile was determined by ultra-performance liquid chromatography coupled with mass spectrometry (UPLC-MS/MS) (Waters, Milford, MA, USA) for the fungus isolated, which was inoculated in four culture media: nutrient agar, potato dextrose agar (PDA) (Neogen Corporation, Lansing, MI, USA), Czapek (HiMedia Laboratories, Vadhani, India) yeast extract agar (Neogen), and malt agar (HiMedia). The different culture media agar plates were inoculated with three 9-mm mycelial plugs of the fungus, each plate with 3 plugs (9 mm diameter). After 14 days of incubation at room temperature, the plugs were removed from the plates and placed in the solvent mixture, methanol/dichloromethane/ethyl acetate (1:2:3), in ultrasound equipment for 1 h, in order to conduct a solid-phase microextraction (SPME) [19]. Afterward, the extraction mixture was filtered and dried for analyses on UPLC-MS/MS, an equipment system with electrospray and triple quadrupole mass spectrometer (Xevo® G2-XS, Waters). The fungal extracts were analyzed using a C18 column (1.7 μm; 2.1 × 100 mm) as chromatographic stationary phase, at a flow rate of 0.5 mL/min and the mobile phase in gradient mode starting from 97% water (A), 1.5% acetonitrile (B), and 1.5% methanol (C) going to 0% (A), 50% (B), and 50% (C) after 15 min and then maintaining this conditions for 4 min. Both eluents contained formic acid (0.02%) (v/v).
The Czapek (HiMedia) yeast extract (Neogen) liquid medium was selected for large-scale cultivation, preparing 12 L of sterile medium (40 × 300 mL in 1000 mL Erlenmeyer flasks) for fungi inoculation. The 100 μL spore suspension (1 × 106 spores/mL) was added to the Czapek medium in each flask and cultured at room temperature for 14 days. Czapek medium (3 × 300 mL in 1000 mL Erlenmeyer flasks) without microorganism inoculation was used as control. The resulting fungal cultures obtained after 14 days were filtrated using 0.45 μm membrane to separate mycelium. The aqueous filtrate was extracted with ethyl acetate (EtOAc) three times, and the organic phase was distilled using rotary evaporator to dryness obtaining EtOAc extract (FA). Into a mycelium was added ethanol, and after 3 days in a sealed Erlenmeyer, the solvent was evaporated at 40 °C on a rotary evaporator obtaining the ethanolic extract (ME). Solvents used were from Vetec (Duque de Caxias, RJ, Brazil).
Extraction and isolation
The ME (698 mg) was purified by reversed-phase preparative high-performance liquid chromatography (HPLC, Shimadzu, Columbia, MD, USA) (C18 Luna Phenyl-Hexyl, φ = 300 mm × h = 4.6 mm, 5 μ) in gradient mode and a flow of 4.7 mL/min. The mobile phase started from 5% acetonitrile (ACN) (A) and 95% of water (B) for 45 min, following 10 min with 100% (A) and 0% (B), affording 13 fractions (ME1 to ME13). Further purification of the fractions yielded compound 1 (10 mg, fraction ME13), compound 2 (7.8 mg, fraction ME5), compound 3 (3.8 mg, fraction ME7), compound 6 (1 mg, fraction ME4), and compound 8 (5.6 mg, fraction (ME2).
The EtOAc extract (FA) (306 mg) was also purified by preparative HPLC (C18 Luna Phenyl-Hexyl, φ = 300 mm × h = 4.6 mm, 5 μ) in gradient mode and a flow of 4.7 mL/min, starting from 5% ACN (A) and 95% of water (B) for 40 min, following 30% (A) and 70% (B) for 2 min, and 100% (A) in 8 min, affording 13 fractions (FA1 to FA13). Fractions FA5, FA6, FA7, FA10, FA11, and FA13 were chromatographed over Sephadex LH-20 (Amersham Pharmacia Biotech AB, Staffanstorp, Sweden) (φ = 2 cm × h = 32 cm) eluting with MeOH to obtain compounds 1 (7 mg, second fraction eluted), 4 (2.2 mg, second fraction eluted), 5 (1.2 mg, first fraction eluted), 6 (3.3 mg, second fraction eluted), 7 (0.8 mg, fourth fraction eluted), and 9 (1.1 mg, third fraction eluted). The compounds isolated from the fungus were characterized by nuclear magnetic resonance spectroscopy (NMR) in one-dimensional (1D) and two-dimensional (2D) experiments and mass spectrometry (MS) (Online Resource) and compared with the literature data [20–27]. A DRX-400 NMR spectrometer (Bruker, Billerica, MA, USA), 9.4 T (1H, 400 MHz; 13C, 100 MHz), was used for NMR (1D and 2D) acquisition. Deuterated methanol (CD3OD) and TMS (internal reference) were obtained from Merk (Darmstadt, Germany) and Sigma-Aldrich (St. Louis, MO, USA).
For isolated compounds analysis by gas chromatography mass spectrometry (GC-MS), a GC 2010 Plus coupled with mass spectrometer MSTQ8030 (Shimadzu), using electron impact ionization mode at 70 eV was used. The experimental condition was in the splitless mode, injecting 1 μL oof sample in the GC-MS, starting at 80 °C (1 min) and then increasing 10 °C/min until 280 °C; the flow rate was 1.2 mL/min; and the column was a Restek Rtx-5 ms (30 m × 250 μm and film 0.25 μm; Shimadzu). To evaluate the data, the software GC-MS Real-Time Analysis® (Shimadzu) and NIST library were used.
Enzymatic assays
Papain and human liver cathepsins B and reagents were purchased from Sigma-Aldrich. Recombinant human cathepsin V was produced using the Pichia pastoris expression system as previously described [28]. The molar concentration of the enzyme was determined by active site titration with E-64 following the conditions previously described [29]. The enzyme activities were measured by similar assay conditions except for the concentration of substrate, Z-Phe-Arg-MCA (benzyloxycarbonyl-phenylalanyl-arginine-4-methyl-7-coumarylamide), which were based on Km values for each enzyme. The concentrations of substrate were 90 μM, 10 μM, and 185 μM for papain, CatV, and CatB, respectively [28, 30, 31].
Compounds 1 to 9 were subjected to enzymatic assay screenings with papain and human liver cathepsins B and V. The compounds were prepared in dimethyl sulfoxide (DMSO) (concentration of 50 μM), and negative control (without inhibitor) and positive control (irreversible inhibitor, E-64) were used. Experiment was performed in triplicate in 96-well plate by incubating the compounds with enzyme solution previously prepared (2.0 nM of enzyme, 2.5 mM of DTE, 1,4-dithioerythritol, buffer solution pH 5.5, 2.5 mM EDTA, 5 min, at 27 °C), for 5 min, at 27 °C. Afterward, 45 μL of substrates were added starting the reaction (final volume of 200 μL), and measurements were carried out for 5 min. For enzymatic assays, a microplate spectrophotometer, SpectraMax M3 (SoftMax Pro®, Molecular Devices, LLC. San Jose, CA, USA), was used. In addition, IC50 was determined for compounds with inhibition greater than 60% by rate values using SigmaPlot 12.0 (Systat Software, San Jose, CA, USA) [32, 33].
Results and discussion
The fungus isolated showed 99% of homology with Fusarium proliferatum genomic sequences according to NCBI/GenBank database, allowing to confirm the fungus species (n. HF930594.1).
The fractionation of the EtOAc extract (FA) and mycelium ethanolic extract (ME) obtained from large-scale cultivation of F. proliferatum leads to nine compounds characterized by 1D and 2D NMR, GC-MS, and UPLC-MS/MS and by comparing them with the literature data (Table 1). Additional data are given in Supplementary Files (Online Resource). From ME extract were isolated compounds beauvericin (1), fusaric acid (2), N-ethyl-3-phenylacetamide (3), and cyclo(L-Leu-L-Pro) cyclodipeptide (6), and 2,4-dihydroxypyrimidine (8). From FA extract were isolated compounds beauvericin (1), N-acetyltryptamine (4), cyclo(L-Val-L-Pro) cyclodipeptide (5), cyclo(L-Leu-L-Pro) cyclodipeptide (6), cyclo(L-Leu-L-Pro) diketopiperazine (7), and 1H-indole-3-carbaldehyde (9) (Table 1). The compounds characterized from 1 to 9 mostly have been commonly identified in Fusarium species. Beauvericin (1) and fusaric acid (2) are mycotoxins, which have been identified in many species of Fusarium likewise in Fusarium proliferatum [34, 35].
Table 1.
Proton nuclear magnetic resonance (1H NMR) spectroscopic data for compounds 1 to 9 (400 MHz NMR spectrometer)
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | |
|---|---|---|---|---|---|---|---|---|---|
| Ha | δH (multiplicity,b J) | ||||||||
| 1 | 4.84 (d, 8.9) | 1.9 (s) | 9.90 (s) | ||||||
| 2 | 1.8 (m) | 3.38 (t) | 7.00 (m) | ||||||
| 3 | 0.85 (d, 6.6) | 8.10 (d, 8.0) | 2.80 (t) | 3.47–3.58 (m) | 3.49–3.54 (m) | 3.64 (m) | |||
| 4 | 0.25 (d, 6.9) | 7.90 (dd, 8.0, 2.1) | 1.94 (m); 2.03 (m) | 1.83–2.09 (m) | 8.15 (m) | ||||
| 5 | 7.28 (m) | 7.57 (d, 7.9) | 1.94 (m); 2.32 (m) |
2.31 (m) 1.83–2.09 (m) |
5.60 (d, 7.7) | 7.22–7.30 (m) | |||
| 6 | 8.51 (s) | 7.20 (m) | 7.08 (m) | 4.03 (m) | 4.13 (m) | 4.31 (td, 5.0, 1.7) | 7.40 (d, 7.7) | 7.22–7.30 (m) | |
| 7 | 3.14 (s) | 2.76 (t, 7.8) | 7.20 (m) | 7.08 (m) | 3.03 (dd, 13.8, 4.9); 3.24 (dd, 13.8, 5.1) | 7.48 (m) | |||
| 8 | 5.80 (dd, 12.7, 4.5) | 1.66 (quint, 7.6) | 7.20 (m) | 7.32 (d, 8.1) | |||||
| 9 |
3.04 (dd, 14.7, 12.7) 3.40 (dd, 14.7, 4.6) |
1.40 (sext, 7.4) | 7.28 (m) | 4.19 (m) | 4.26 (ddd, 9.5, 7.0, 1.7) | 7.28 (m) | |||
| 10 | 0.96 (t, 7.3) | 2.96 (t, 7.1) | 2.48 (dhept, 7.1, 2.5) |
1.53 (m) 1.83–2.09 (m) |
7.23 (m) | ||||
| 11 | 7.26 (m) | 3.48 (t, 7.3) | 0.93 (d, 6.9) | 1.83–2.09 (m) | 7.23 (m) | ||||
| 12 | 7.26 (m) | 1.09 (d, 6.9) | 0.95 (d, 2.3) | 7.23 (m) | |||||
| 13 | 7.18 (m) | 0.97 (d, 2.3) | 7.23 (m) | ||||||
| 14 | 1.93 (s) | 2.15 (m) | |||||||
| 15 | 0.79 (d, 6.9) | ||||||||
| 16 | 0.44 (d, 7.0) | ||||||||
aH number of hydrogen position. bThe following abbreviations were used to designate multiplicities (d, doublet; dd, doublet of doublets; ddd, double double-doublet; dhept, doublet of heptet; m, multiplet; quint, quintet; s, singlet; sext, sextet; t, triplet; td, triplet of doublets)
Deuterated methanol (CD3OD) was used as solvent. Chemical shifts are reported in δ (ppm) and coupling constants (J) are reported in Hz
Among the compounds evaluated on papain and cathepsins V and B, beauvericin (1), N-ethyl-3-phenylacetamide (3), and cyclo(L-Leu-L-Pro) cyclodipeptide (6) showed significant inhibition of papain catalytic activity (Fig. 1, 79%, 57%, and 94% of inhibition, respectively), with IC50 values of 25.3 ± 1.9, 39.4 ± 2.5, and 7.4 ± 0.5 μM, respectively. In addition, beauvericin (1) also inhibited CatV and CatB with IC50 of 46.0 ± 3.0 and 6.8 ± 0.7 μM, respectively. Beauvericin (1) (BEA) was a major compound isolated from both extracts (FA and ME). BEA is a depsipeptide known as bioactive compound with activity against Mycobacterium tuberculosis, Plasmodium falciparum, and pathogens of gastrointestinal tract [34]. Beauvericin (1) and fusaric acid (2) (200 μM) showed 99% and 58% of inhibition of papain activity, respectively. Fusaric acid (2) is a fungal toxin, and it has been mentioned as a virulence factor in Fusarium infecting banana [36], tomato [37], and cotton [38]. Mycotoxins from fungi are key factors on the process of establishment of infection in plants, and fusaric acid from F. oxysporum may interfere on metabolic pathways of host plant during invasion process [35]. Recently, a study with fusaric acid-deficient mutant was evaluated and showed to increase the resistance in watermelon by Fusarium proliferation [39].
Fig. 1.
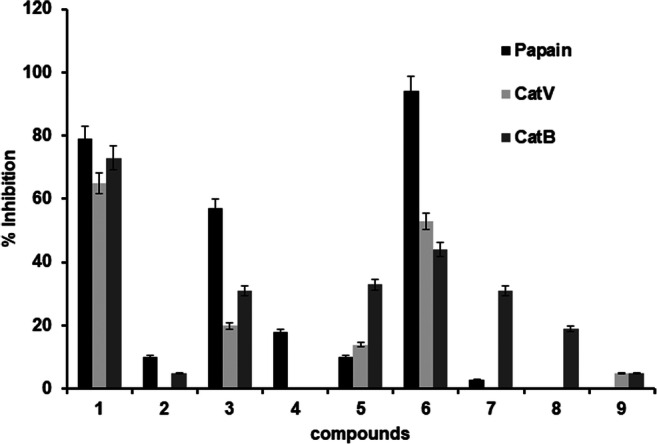
Results of screening of compounds 1 to 9 (50 μM) against papain, CatV, and CatB
Beauvericin (1) showed potential on inhibiting humans’ PLCPs CatV and CatB. Human cathepsins V and B are related to cancer diseases; specifically, CatB is involved in facilitating invasion process and metastasis of tumor cell lines [5, 33]. Previously, beauvericin revealed to be a promising compound with anticancer effect in vitro and in vivo reducing tumor expressively [40].
The compound N-ethyl-3-phenylacetamide (3), revealed as papain inhibitor in this work, was previously reported as a α-glucosidase inhibitor (from baker’s yeast) as well, an enzyme target for type 2 diabetes mellitus therapy [41].
The known indole derivatives N-acetyltryptamine (4) and 1H-indole-3-carbaldehyde (9) and the diketopiperazines (5), (6), and (7) were isolated previously from Fusarium genus, but it is the first time isolated from F. proliferatum species [42, 43]. Compound 2,4-dihydroxypyrimidine (8) (uracil) is commonly identified in endophytic fungus Fusarium sp. [44].
Diketopiperazines are natural cyclic dipeptides produced by microorganisms with a broad range of biological activities such as antimicrobial, anticancer, antiviral, antifungal, antibacterial, and antihyperglycemic [45, 46]. Likewise, cyclo(L-Leu-L-Pro) cyclodipeptide (6), a diketopiperazine known as gancidin W, has been reported as potent antimalarial and antimicrobial agent with MIC values between 0.25 and 12.5 μg/mL against Enterococcus faecalis, Escherichia coli, Staphylococcus aureus, Micrococcus luteus, Candida albicans, and Cryptococcus neoformans [46, 47].
The N-acetyltryptamine (4) has antimicrobial potential and has been isolated previously from different natural sources, Fusarium solani JK10, Fusarium incarnatum (HKI00504), Penicillium chrysogenum, P. expansum, P. vitale, Rhizoctonia solani, and Streptomyces sp. [48–51].
Besides 1H-indole-3-carbaldehyde (9) did not show significant inhibition on papain and PLCPs, this indole compound has showed previously to be potent inhibitor of other enzymes, xanthine oxidase (IC50 = 13.36 ± 0.39 μM), an enzyme related to gout and diabetes, and against kinases (COX-2, ROCK1/2, and JAK3) [52, 53].
The mycotoxins (beauvericin (1) and fusaric acid (2)) and the compounds N-ethyl-3-phenylacetamide (3) and cyclo(L-Leu-L-Pro) cyclodipeptide (6) are reported for the first time as papain inhibitors. Considering the fact that bromelain from pineapple prevents infection by phytopathogens [10], the mechanism of action discovered corroborates with previous findings. Although further studies are needed to confirm that cysteine protease inhibition by mycotoxins influences in the Fusarium invasion process, our findings may be a clue in the roles of the development of Fusarium in pineapple.
Electronic supplementary material
(DOCX 2216 kb)
Acknowledgments
The authors would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brasília, DF, Brazil) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brasília, DF, Brazil, Finance Code 001).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Valueva TA, Mosolov VV. Role of inhibitors of proteolytic enzymes in plant defense against phytopathogenic microorganisms. Biochem. 2004;69(11):1305–1309. doi: 10.1007/s10541-005-0015-5. [DOI] [PubMed] [Google Scholar]
- 2.Mosolov VV, Valueva TA. Proteinase inhibitors in plant biotechnology: a review. Appl Biochem Microbiol. 2008;44:233–240. doi: 10.1134/S0003683808030010. [DOI] [PubMed] [Google Scholar]
- 3.Misas-Villamil JC, van der Hoorn RAL, Doehlemann G. Papain-like cysteine proteases as hubs in plant immunity. New Phytol. 2016;212(4):799–801. doi: 10.1111/nph.14117. [DOI] [PubMed] [Google Scholar]
- 4.Stoka V, Turk V, Turk B. Lysosomal cathepsins and their regulation in aging and neurodegeneration. Ageing Res Rev. 2016;32:22–37. doi: 10.1016/j.arr.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Silva TL, Fernandes JB, Silva MF das GF da, de Sousa LRF, Vieira PC (2019) New cathepsin V inhibitor from stems of Bowdichia virgilioides. Brazilian J Pharmacogn 29(4):491–494. 10.1016/j.bjp.2019.04.004
- 6.Morihara K, Oda K. Microbial degradation of proteins. In: Guenther W, editor. Microbial degradation of natural products. Weinheim: VCH; 1993. pp. 293–364. [Google Scholar]
- 7.Dutta S, Bhattacharyya D. Enzymatic , antimicrobial and toxicity studies of the aqueous extract of Ananas comosus (pineapple) crown leaf. J Ethnopharmacol. 2013;150(2):451–457. doi: 10.1016/j.jep.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Domsalla A, Melzig MF. Occurrence and properties of proteases in plant latices. Planta Med. 2008;74(7):699–711. doi: 10.1055/s-2008-1074530. [DOI] [PubMed] [Google Scholar]
- 9.Konno K, Hirayama C, Nakamura M, Tateishi K, Tamura Y, Hattori M, Kohno K. Papain protects papaya trees from herbivorous insects: role of cysteine proteases in latex. Plant J. 2004;37:370–378. doi: 10.1046/j.1365-313X.2003.01968.x. [DOI] [PubMed] [Google Scholar]
- 10.López-Garcia B, Hernández M, Segundo BS. Bromelain, a cysteine protease from pineapple (Ananas comosus) stem, is an inhibitor of fungal plant pathogens. Lett Appl Microbiol. 2012;55:62–67. doi: 10.1111/j.1472-765X.2012.03258.x. [DOI] [PubMed] [Google Scholar]
- 11.Stępién Ł, Koczyk G, Waśkiewicz A. Diversity of Fusarium species and mycotoxins contaminating pineapple. J Appl Genet. 2013;54:367–380. doi: 10.1007/s13353-013-0146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiménez M, Logrieco A, Bottalico A. Ocurrence and pathogenicity of Fusarium species in banana fruits. J Phytopathol. 1993;137:214–220. doi: 10.1111/j.1439-0434.1993.tb01341.x. [DOI] [Google Scholar]
- 13.Denis-Sandoval M, Guarnaccia V, Polizzi G, Crous PW. Symptomatic citrus trees reveal a new pathogenic lineage in Fusarium and two new Neocosmospora species. Persoonia. 2018;40:1–25. doi: 10.3767/persoonia.2018.40.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moretti A, Logrieco A, Bottalico A, Ritteni A, Randazzo G. Production of beauvericin by Fusarium proliferatum from maize in Italy. Mycotoxin Res. 1994;10:73–78. doi: 10.1007/BF03192255. [DOI] [PubMed] [Google Scholar]
- 15.Santini A, Ritieni A, Fogliano V, Randazzo G, Mannina L, Logrieco A, Benedetti E. Structure and absolute stereochemistry of fusaproliferin, a toxic metabolite from Fusarium proliferatum. J Nat Prod. 1996;59:109–112. doi: 10.1021/np960023k. [DOI] [PubMed] [Google Scholar]
- 16.Dame ZT, Silima B, Gryzenhout M, Ree VT. Bioactive compounds from the endophytic fungus Fusarium proliferatum. Nat Prod Res. 2016;30(11):1301–1304. doi: 10.1080/14786419.2015.1053089. [DOI] [PubMed] [Google Scholar]
- 17.Petrini O, Sieber TN, Toti L, Viret O. Ecology , metabolite production and substrate utilization in endophytic fungi. Nat Toxins. 1992;1:185–196. doi: 10.1002/nt.2620010306. [DOI] [PubMed] [Google Scholar]
- 18.White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Academic press Inc. PCR protocols: a guide to methods and applications, pp 315–322. 10.1016/B978-0-12-372180-8.50042-1
- 19.Smedsgaard J. Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. J Chromatograpgy A. 1997;760(2):264–270. doi: 10.1016/S0021-9673(96)00803-5. [DOI] [PubMed] [Google Scholar]
- 20.Alshaibani M, Mohamadzin N, Jalil J, Sidik N, Ahmad S, Kamal N, Edrada-Ebel R. Isolation, purification, and characterization of five active diketopiperazine derivatives from endophytic Streptomyces SUK 25 with antimicrobial and cytotoxic activities. J Microbiol Biotechnol. 2017;27(7):1249–1256. doi: 10.4014/jmb.1608.08032. [DOI] [PubMed] [Google Scholar]
- 21.He R, Wang B, Wakimoto T, Wang M, Zhu L, Abe I. Cyclodipeptides from metagenomic library of a japanese marine sponge. J Braz Chem Soc. 2013;24(12):1926–1932. doi: 10.5935/0103-5053.20130240. [DOI] [Google Scholar]
- 22.Hu L, Rychilik M. Biosynthesis of 15N3-labeled enniatins and beauvericin and their application to stable isotope dilution assays. J Agric Food Chem. 2012;60:7129–7136. doi: 10.1021/jf3015602. [DOI] [PubMed] [Google Scholar]
- 23.Sakai N, Moriya T, Konakahara T. An efficient one-pot synthesis of unsymmetrical ethers: a directly reductive deoxygenation of esters using an InBr3/Et3SiH catalytic system. J Organomet Chem. 2007;72(15):5920–5922. doi: 10.1021/jo070814z. [DOI] [PubMed] [Google Scholar]
- 24.Pavlát P, Halama A, Weidlich T, Fiala B, Bekárek V. Medium effect on 1H- and 13C- NMR spectra of melatonin. Acta Univ Palacki Olomuc, Chemica. 1999;38:59–64. [Google Scholar]
- 25.Pessoa-Mahana H, M IC, Pessoa-Mahana CD, Araya-Maturana R, Fajardo IA, Barria CS. Synthesis of 1-benzyl-3-[4-(aryl-1-piperazinyl) carbonyl]-1H-indoles. Novel ligands with potential D4 dopaminergic activity. J Chil Chem Soc. 2011;56(4):866–869. doi: 10.4067/S0717-97072011000400009. [DOI] [Google Scholar]
- 26.Sahabuddin S, Ghosh R, Achari B, Mandal SB. Nucleoside synthesis from 3-alkylated sugars: role of 3β-oxy substituents in directing nucleoside formation. Org Biomol Chem. 2006;4:551–557. doi: 10.1039/b514028e. [DOI] [PubMed] [Google Scholar]
- 27.Stipanovic RD, Wheeler MH, Puckhaber LS, Liu J, Bell AA, Williams HJ. Nuclear magnetic resonance (NMR) studies on the biosynthesis of fusaric acid from Fusarium oxysporum f. sp. vasinfectum. J Agric Food Chem. 2011;59(10):5351–5356. doi: 10.1021/jf200628r. [DOI] [PubMed] [Google Scholar]
- 28.Brömme D, Li Z, Barnes M, Mehler E. Human cathepsin V functional expression, tissue distribution, electrostatic surface, enzymatic characterization, and chromosomal localization. Biochemistry. 1999;38:2377–2385. doi: 10.1021/bi982175f. [DOI] [PubMed] [Google Scholar]
- 29.Barret AJ, Kembhavi AA, Brown MA, Kirschke H, Knight CG, Tamait M, Hanadat K. L-trans-epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982;201:189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almeida PC, Nates IL, Chagas JR, Rizzi CCA, Faljoni-alario A, Carmona E, Juliano L, Nader HB, Tersariol ILS. Cathepsin B activity regulation. Heparin-like glycosaminogylcans protect human cathepsin B from alkaline pH-induced inactivation. J Biol Chem. 2001;276(2):944–951. doi: 10.1074/jbc.M003820200. [DOI] [PubMed] [Google Scholar]
- 31.Davy A, Svendsen I, Sørensen SO, Sørensen MB, Rouster J, Meldal M, Simpson DJ, Cameron-mills V. Substrate specificity of barley cysteine endoproteases EP-A and EP-B1. Plant Physiol. 1998;117(1):255–261. doi: 10.1104/pp.117.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Severino RP, Guido RVC, Marques EF, Brömme D, das GFda SMF, Fernandes JB, Andricopulo AD, Vieira PC. Acridone alkaloids as potent inhibitors of cathepsin V. Bioorg Med Chem. 2011;19:1477–1481. doi: 10.1016/j.bmc.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 33.Ramalho SD, Bernades A, Demetrius G, Noda-Perez C, Vieira PC, dos Santos CY, da Silva JA, de Moraes MO, Mousinho KC (2013) Synthetic chalcone derivatives as inhibitors of cathepsins K and B , and their cytotoxic evaluation. Chem Biodivers 10:1999–2006. 10.1002/cbdv.201200344 [DOI] [PubMed]
- 34.Meca G, Sospedra I, Soriano JM, Ritieni A, Moretti A, Mañes J. Antibacterial effect of the bioactive compound beauvericin produced by Fusarium proliferatum on solid medium of wheat. Toxicon. 2010;56:349–354. doi: 10.1016/j.toxicon.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, Cheng Y, Ma Y, Chen C, Xu F. Role of phenolic acids from the rhizosphere soils of Panax notoginseng as a double-edge sword in the occurrence of root-rot disease. Molecules. 2018;23:819. doi: 10.3390/molecules23040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong X, Ling N, Wang M, Shen Q, Guo S. Fusaric acid is a crucial factor in the disturbance of leaf water imbalance in Fusarium infected banana plants. Plant Physiol Biochem. 2012;60:171–179. doi: 10.1016/j.plaphy.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 37.López-Diaz C, Rahjoo V, Sulyok M, Ghionna V, Martin-Vicente A, Capilla J, Di Pietro A, Lopez-Berges MS. Fusaric acid contributes to virulence of Fusarium oxyporum on plant and mamalian hosts. Mol Plant Pathol. 2018;19:440–453. doi: 10.1111/mpp.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crutcher FK, Puckhaber LS, Stipanovic RD, Bell AA, Nichols RL, Lawrence KS, Liu J. Microbial resistance mechanisms to the antibiotic and phytotoxin fusaric acid. J Chem Ecol. 2017;43:996–1006. doi: 10.1007/s10886-017-0889-x. [DOI] [PubMed] [Google Scholar]
- 39.Xie X, Huang C, Cai Z, Chen Y, Dai C. Targeted acquisition of Fusarium oxysporum f. sp. niveum toxin-deficient mutant and its effects on watermelon Fusarium wilt. J Agric Food Chem. 2019;67(31):8536–8547. doi: 10.1021/acs.jafc.9b02172. [DOI] [PubMed] [Google Scholar]
- 40.Heilos D, Roríguez-Carrasco Y, Englinger B, Timelthaler G, Sushilla VS, Sulyok M, Boecker S, Süssmuth RD, Heffeter P, Lemmens-Gruber R, Dornetshuber-Fleiss R, Walter B. The natural fungal metabolite beauvericin exerts anticancer activity in vivo: a pre-clinical pilot study. Toxins. 2017;9:258–263. doi: 10.3390/toxins9090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen S, Yong T, Xiao C, Su J, Zhang Y, Jiao C, Xie Y. Pyrrole alkaloids and ergosterols from Grifola frondosa exert anti-α-glucosidase and antiproliferative activities. J Funct Foods. 2018;43:196–205. doi: 10.1016/j.jff.2018.02.007. [DOI] [Google Scholar]
- 42.Wang ZF, Zhang W, Xiao L, Zhou YM, Du FY (2018) Characterization and bioactive potentials of secondary metabolites from Fusarium chlamydosporum. Nat Prod Res. 10.1080/14786419.2018.1508142 [DOI] [PubMed]
- 43.Cirigliano AM, Rodriguez MA, Gagliano ML, Bertinetti BV, Godeas AM, Cabrera GM. Liquid chromatography coupled to diferente atmospheric pressure ionization sources-quadrupole-time-of-flight mass spectrometry and post-column addition of metal salt solutions as a powerful tool for the metabolic profiling of Fusarium oxysporum. J Chormatogr A. 2016;1439:97–111. doi: 10.1016/j.chroma.2015.11.073. [DOI] [PubMed] [Google Scholar]
- 44.Liang YM, Yu Y, Wang GK, Liu JS, Zhang PL, Ma ZH, Liu HT, Wang G. Study on secondary metabolites of endophytic fungus Fusarium lactis from Dendrobium huoshanense. Chin Tradit Herbal Drugs. 2017;48:4608–4614. doi: 10.7501/j.issn.0253-2670.2017.22.003. [DOI] [Google Scholar]
- 45.Mishra AK, Choi J, Choi SJ, Baek KH. Cyclodipeptides: an overview of their biosynthesis and biological activity. Molecules. 2017;22:1796–1809. doi: 10.3390/molecules22101796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Carvalho MP, Abraham WR. Antimicrobial and biofilm inhibiting diketopiperazines. Curr Med Chem. 2012;19:3564–3577. doi: 10.2174/092986712801323243. [DOI] [PubMed] [Google Scholar]
- 47.Zin NM, Baba MS, Zainal-Abidin AH, Latip J, Mazlan NW, Edrada-Ebel R. Gancidin W, a potential low-toxicity antimalarial agent isolated from an endophytic Streptomyces SUK 10 noraziah. Drug Des Dev Ther. 2017;11:351–363. doi: 10.2147/DDDT.S121283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehdi RBA, Shaaban KA, Rebai IK, Smaoui S, Bejar S, Mellouli L. Five naturally bioactive molecules including two rhamnopyranoside derivatives isolated from the Streptomyces sp. strain TN58. Nat Prod Res. 2009;23:1095–1107. doi: 10.1080/14786410802362352. [DOI] [PubMed] [Google Scholar]
- 49.Pedras MSC, Yu Y, Liu J, Tandron-Moya YA. Metabolites produced by the phytopathogenic fungus Rhizoctonia solani: isolation, chemical structure determination, syntheses and bioactivity. Z Naturforsch C J Biosci. 2005;60:717–722. doi: 10.1515/znc-2005-9-1010. [DOI] [PubMed] [Google Scholar]
- 50.Kyekyeku JO, Kusari S, Adosraku RK, Bullach A, Golz C, Strohmann C, Spiteller M. Antibacterial secondary metabolites from an endophytic fungus, Fusarium solani JK10. Fitoterapia. 2017;119:108–114. doi: 10.1016/j.fitote.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Li LY, Ding Y, Growth I, Menzel KD, Peschel G, Kerstin V, Deng ZW, Sattler I, Lin WH. Pyrrole and indole alkaloids from an endophytic Fusarium incarnatum (HKI00504) isolated from the mangrove plant Aegiceras corniculatum. J Asian Nat Prod Res. 2008;10:765–770. doi: 10.1080/10286020802031106. [DOI] [PubMed] [Google Scholar]
- 52.Gómez-Betancur I, Zhao J, Tan L, Chen C, Yu G, Rey-Suárez P, Preciado L. Bioactive compounds isolated from marine bacterium Vibrio neocaledonicus and their enzyme inhibitory activities. Mar Drugs. 2019;17:401–420. doi: 10.3390/md17070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng T, Ming YY, Tu ZC, Xu FR, Cheng YX. Two new compounds from medicinal insect Blaps japanensis and their biological evaluation. Nat Prod Commun. 2018;13:149–151. doi: 10.1177/1934578X1801300210. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 2216 kb)


