Abstract
In this study, the physio pathological effects of Aspergillus alliaceus (Aa, fungi, biocontrol agent) on Orobanche (parasitic plant) were investigated by hormone and phenolic substance tests. In experimental group, Orobanches were treated with the fungi, considering control group was fungus-free. Based on the hormonal tests, in the experimental group, salicylic acid (SA), jasmonic acid (JA), abscisic acid (ABA) and gibberellic acid (GA) levels significantly decreased, and only indole acetic acid (IAA) hormone levels were fairly higher than the control group. According to phenolic substance tests, it was found that only gallic acid, syringic acid and caffeic acid values significantly increased compared with control, and catechin and p-coumaric acid values were significantly lower. Consequently, it was determined that Aa pathogenesis (1) considerably reduces the effects of all defence hormones (JA, ABA, SA), (2) operates an inadequate defence based solely on the IAA hormone and several phenolic substances (gallic acid, syringic acid and caffeic acid), (3) and inevitably the fungi lead the Orobanche to a slow and continuous death. The results were evaluated in detail in the light of similar recent article and current literature in terms of biocontrol and pathology.
Keywords: Phytoparasite, Herbicide, Asteraceae, Phelipanche, Mycoherbicide, Bioherbicide
Introduction
Orobanche (Broomrape) is a parasitic plant that is rooted in various plants such as tomatoes, sunflowers, cotton, tobacco and eggplants and it is widely found in the fields of Asia, Mediterranean and Europe [1, 2]. As the plant is holoparasite, it provides all water and nutrients from its host [3]. And naturally, Orobanche is one of the most problematic and hard to fight parasites in the world agriculture [4] and it causes to increasing rates of yield losses every year.
Although many methods have been applied around the world, including cultural, agricultural, prevention, biological, mechanical and chemicals, no effective control has not been found yet. Chemical control, in particular, has prevented more Orobanche parasitism than others. For instance, better results were obtained from especially herbicides with chloritfuron and triasulfuron, in the fight against Orobanche aegyptiaca in tomato production [5]. Moreover, imidazolinone herbicides from synthetic compounds are useful at this point. However, these chemicals have negative effects to the environment and human health. Therefore, alternative new practices are needed in the fight against parasitic Orobanche [6].
The healthiest remedy is the use of biological agents effective only to Orobanche, that is, the biocontrol method [7]. Until now, some effective fungal pathogens have been identified in biocontrol studies [8–10]. For example, Fusarium oxysporum Schlecht. f. sp. ortoceras (FOO) inhibited the Orobanche parasitism up to 90% in tomatoes [11]. Indeed, Fusarium caused hormonal disorders and intense accumulation of phenolic substances in Orobanche and killed quickly the parasite [12, 13].
Recently, we discovered a new fungal biological control agent of Orobanche, Aspergillus alliaceus Thom & Church [14]. A. alliaceus is very different from other fungal biocontrol agents with its sclerotial structure, which are composed of dense fungal mycelia and food reserves. Thanks to the sclerot, the fungus has been dormant for years in unfavourable environments and can return to normal mycelial phase under favourable conditions. This ability increases their survival capability under adverse conditions.
In the Mycobank website [15], there is no record of any pathogenicity of the fungus on humans, other than the Orobanche plant species. However, the fungus caused a pulmonary infection in an acute myeloid leukaemia patient by another Aspergillus taxon, Aspergillus flavus [16].
Ochratoxin A is a mycotoxin produced by A. alliaceus [17]; however, no mycotoxin studies of the origin of this fungus have been found. Though, in general, this mycotoxin has several harmful effects such as hepatotoxicity, teratogenicity and immunosuppression, while it was also carcinogenic in the kidney and the liver [18]. In studies carried out, the fungus was effective only on Orobanche due to its very dense starch storage and did not cause any toxic effect on sunflower [14, 19, 20]. Nevertheless, Ochratoxin A tests should be performed on both Orobanche and sunflower in the future.
In our recent works, the irreversible lethal pathological effects of Aspergillus alliaceus on Orobanche have been confirmed by histological and transcriptomic studies [14, 21]. In this study, it is aimed to clarify the physiological effects of fungi on Orobanche by some hormonal and secondary metabolite tests in order to fill this gap. Thus, the pathology of fungus on Orobanche will be better understood.
Materials and methods
As material, 3 objects were used in the study: Orobanche, Sunflower, Fungus (Orobanche biocontrol agent).
Orobanche seeds were collected from sunflower (cv. HA-89B) fields Edirne, Thrace region of Turkey, and were identified as Orobanche cernua L. The sunflower plant is the host for Orobanche, and the sunflower (cv. HA-89B) used is susceptible to Orobanche; thus, Orobanche parasitism is provided.
The third object is a fungus used as a Orobanche biocontrol agent, Aspergillus alliaceus Thom & Church (Fig. 1c). This fungus was collected from dead Orobanche samples from the same fields. Fungal inoculation and pure culture trials were partially modified by Aybeke [12, 13] and Herron et al. [22]. Infected Orobanche tissue with the fungus was surface-disinfected for 1 min in a solution containing 1.5% (v/v) sodium hypochlorite, and after rinsing with sterile distilled water, it was immersed in 70% (v/v) ethanol for 1 min and air-dried. Small pieces of tissue cut from the leading edges of lesions were plated directly onto half-strength potato dextrose agar medium (1/2 PDA) [12, 13]. After the incubation at 27.5 °C, the material was transferred to fresh 20% Czapek yeast–sucrose–agar media (CYA20S), and grown for 7 days at 23 °C, which was followed by preparation of pure cultures. This is because CYA20S was found to be the most efficient formulation for fungi in trials with different agars [14, 19, 20].
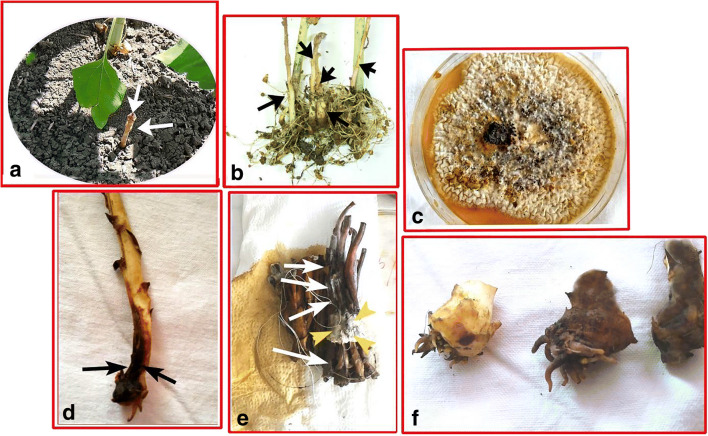
Fig. 1.
a One healthy Orobanche belonging to the control group (fungus-free) in pots (arrows). b Control group Orobanches on the host (sunflower), removed from the soil (arrows). c The fungus used, A. alliaceus, its view on 20% Czapek yeast–sucrose–agar media (CYA20S). d Due to fungal infection, an Orobanche stem with darkened bottom (arrows) (the upper sides are still healthy). e Orobanche stems (white arrows) completely dead by fungal infection, and fungal mycelium residues (yellow arrowheads) on it. f Small Orobanches, healthy (on the left), completely dark and dead (in the middle and right ones) with the accumulation of phenols after the fungal infection
The isolated micro fungi were identified according to Klich [23] and Raper and Fennell [24] and also the fungal culture was stocked into CBS (Fungal Biodiversity Centre, Utrecht, The Netherlands) collection with the code ‘CBS 563.65’.
Plants (sunflower and Orabanche) were grown in pots and the method of Muller-Stover et al. [25] was applied partially modified by Aybeke et al. [19]. The pots (140 × 130 mm, no. 3) were filled up to two-thirds with a 1/1 mixture of clay and sandy soil. Orobanche seeds (fifty milligrammes per kg of soil; [25]) are poured into the soil and mixed well with a hand shovel, and then, three sunflower seeds were sown in the pot. After 14 days, the seedlings were reduced to one so that one sunflower seedling was left in each pot [19]. The fungus was kept in stock for future experiments on a special nutrient-poor agar (SNA) consisting of 1 g KH2PO4, 1 g KNO3, 0.5 g MgSO4 x7H2O, 0.5 g KCl, 0.2 g glucose, 0.2 g sucrose and 20 g agar/l at 5 °C [26].
Fungal inoculation experiments were performed by applying fungus directly onto the post-emergence Orobanche with a sterile forceps. Two to three square millimetres of CYA20S agar fungal cultures (Fig. 1c) at the tip of the forceps were used for Orobanche infection tests [12, 13, 21].
Fungus-free Orobanche (uninfected with the fungus) constituted the control group.
All experiments were performed in greenhouse conditions at temperatures between 15 and 25 °C (within the same day) or in temperature-calibrated solarium rooms by using HQLR-lamps (1000 W) under a 16-h light period and 35–40% humidity [27], by adapting the weather conditions to the specific sunflower–Orobanche life cycle.
Approximately 4–5 days after fungus inoculation, necrotic damaged Orobanches were (Fig. 1e–f) removed from the pots and immediately stored in a deep freezer at − 86 °C, for below specific physiological experiments [12, 13, 21].
Hormonal analysis
Healthy (control) and infected Orobanche stems (experimental/test group) (200 mg frozen Orobanche samples) were extracted with the TissueLyser LT (Qiagen, TissueLyser LT) [28] with minor modifications [29]. After lysis in a 2-ml extraction tube for 2 min, a 100-mg pellet was mixed with 1 ml extraction solvent (methanol/isopropanol, 50:50 (v/v) with 0.5% of ammonium formate) in an Eppendorf tube. This mixture was vortexed rapidly under freezing conditions and then centrifuged at 10,000 rpm for 10 min at 4 °C (Bioer Mixing Block MB-102). The supernatant was filtered through a 0.22-μm PTFE filter. Extracted samples (200 μl) were analysed by UPLC-ESI–MS/MS (API4000 QTrap; Applied Biosystems).
For authentic hormonal activity analysis, standards of each hormone (indole acetic acid, IAA; gibberellic acid, GA; abscisic acid, ABA; salicylic acid, SA; jasmonic acid, JA) were loaded onto the MS/MS system to determine fragments and voltage conditions. Then, mixtures of the five hormones were prepared in concentrations ranging from 0.05 to 50 μg/kg to establish five points for calibration. Calibration curves for each hormone were generated using Analyst software (Applied Biosystems). Samples (50 μl) were then analysed by UPLC-ESI/MS–MS and Spark UPLC system integrated with an Applied Biosystems QTRAP 4000 (Applied Biosystems).
Chromatographic separation was performed on a Phenomenex Luna 3 μm C18(2) 100 9 2.0 mm column at 40 °C. The solvent gradient used was 100% A (99.5% H2O:0.5% ammonium formate) to 100% B (99.5% MeOH:0.5% ammonium formate) over 5 min. The gradient profile for hormones was constructed as follows: ((time in min)/A %): (0/98), (1/2), (3/2), (4/98), (5/98). Hormone analyses were performed with a Turbo ion spray source in negative ion mode with MRM options in the Analyst software. The curtain gas was set at 10 a.u., the source temperature was 400 °C, and ion source gases 1 and 2 were both 20 a.u. The declustering potential was set at 100 V. The source voltage was 3500 V [29].
Secondary metabolite analysis
Chemicals, standards and reagents
Formic acid (98–100%), methanol (Hypergrade LC MS), isopropyl alcohol (2-propanol) and DMSO were purchased from Merck, Darmstadt, Germany. Ammonium formate (HPLC grade) was from Sigma–Aldrich, Germany. Reference standards, gallic acid, catechin, 2–5 dihydroxybenzoic acid, trans-caffeic acid, syringic acid, trans-sinapic acid, trans-p-coumaric acid, trans-ferulic acid, resveratrol and salicylic acid were from Fluka and protocatechuic acid was purchased from HWI Analytik Gmbh, Germany. MTT (3(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazo-lium-bromide) was purchased from Biomatik Cambridge, Ontario. Phosphate-buffered saline (PBS) and molecular biology grade water were from Gibco, Invitrogen, Carlsbad, CA, USA. The resistance of ultra-distilled water used for instrumental analysis was 18.2 MΩ.
Liquid chromatography and mass spectrometry conditions
Phenol analyses were performed on an Agilent 1200 infinity LC in combination with the Agilent 6460 Triple Quadrupole MS/MS System, equipped with a Jet Stream Electrospray ionization source (Agilent Technologies, Palo Alto, CA, USA). The analytical column was Agilent Poroshell 120 EC-C18 (4.6 9 50 mm, 2.7 lm particle size) and set at 25 °C. Mobile phase A consisted of UPW, 0.2% ammonium formate (v/v) and 0.2% formic acid (v/v). Mobile phase B consisted of methanol, 0.2% ammonium formate (v/v) and 0.2% formic acid (v/v). The flow rate was 0.3 ml/min at ambient temperature. The injection volume was 1 μl and the LC gradient conditions were as follows: 0–1 min, 70% A, 30% B; 3–7 min 30% A, 70% B; 9–10 min 50% A, 50% B; 11–12 min; 70% A, 30% B. The run time was 12 min.
The optimized MS analysis parameters were as follows: gas temperature was set at 325 °C, the nebuliser gas pressure was set at 45 psi, the nozzle voltage was set at 500 V, the capillary at 3000 V, sheath gas temperature at 400 °C and sheath gas flow at 12 L/min. Multiple reaction monitoring (MRM) was performed on the positive and negative ion mode. Data acquisition was performed with Mass Hunter (version B.06.01) software. Nitrogen (N2) was used as the collision gas at 1.12 mTorr. Calibration standard mixes were prepared in 50% UPW, 25% methanol and 25% isopropanol at calibration concentrations of 1–200 ng/ml.
Statistical analysis
All tests were repeated independently three times and differences in data of, hormonal, and metabolic tests of control and experimental groups were compared by ANOVA, which means separation by Duncan’s test using SPSS 18 software at a significance level of p ≤ 0.05, as described our previous studies [12, 13, 21].
Results
Hormone analysis results
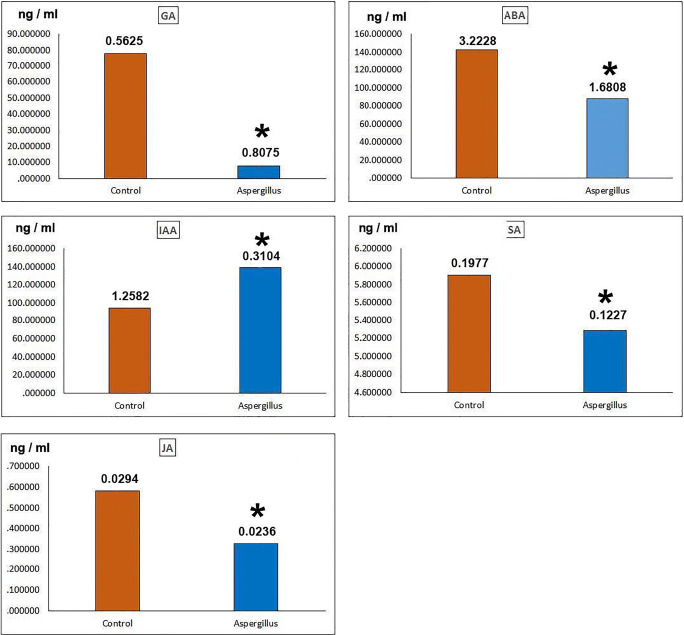
GA, the amount of hormone in the experimental group (A. alliaceus-infected Orobanche group) was almost 10 times lower than the control. Amount of GA hormone in experimental group 7.58 ng/ml (± 80), control hormone value 77.74 ng/ml (± 56) (Fig. 2).
Fig. 2.
Hormone levels in control and experimental groups. IAA, indoleacetic acid, F = 5819,094, df = 3, P = 0.0000*; GA, gibberellic acid, F = 1710,269, df = 3, P = 0.0000*; ABA, abscisic acid, F = 993,159, df = 3, P = 0.0000*; SA, salicylic acid, F = 55,878, df = 3, P = 0.0000*, JA, jasmonic acid, F = 72,827, df = 3, P = 0.0000*. The numbers in the columns define standard error data. * indicates significant differences between control and Aspergillus infected experimental groups according to one-way ANOVA, Duncan test (p ≤ 0.05)
ABA, the amount of hormone in the experimental group is quite low compared with the control. Experimental group, 88.11 ng/ml (± 1.68); control, 142.49 ng/ml (± 3.22) (Fig. 2).
IAA, the hormone of the test group (139.43 ng/ml; ± 31) was significantly higher than the control group (93.62 ng/ml; ± 1.25) (Fig. 2).
SA, the values of the control and test groups (5.90 ng/ml; ± 19) and (5.28 ng/ml; ± 12), respectively, were close to each other.
JA, values of the test group (32 ng/ml; ± 02) were significantly lower than the control (58 ng/ml; ± 029) (Fig. 2).
The values of all hormones except IAA were considerably lower than those of the control.
The highest hormone levels were observed in the ABA control group and then in the IAA experimental group (Fig. 2). The hormone values of the SA group are similar.
The lowest hormone values were observed in JA control and experimental groups (Fig. 2). According to statistical analysis, hormone values of all experimental groups were significantly different from those of control (Fig. 2).
Secondary metabolite (phenols) analysis results
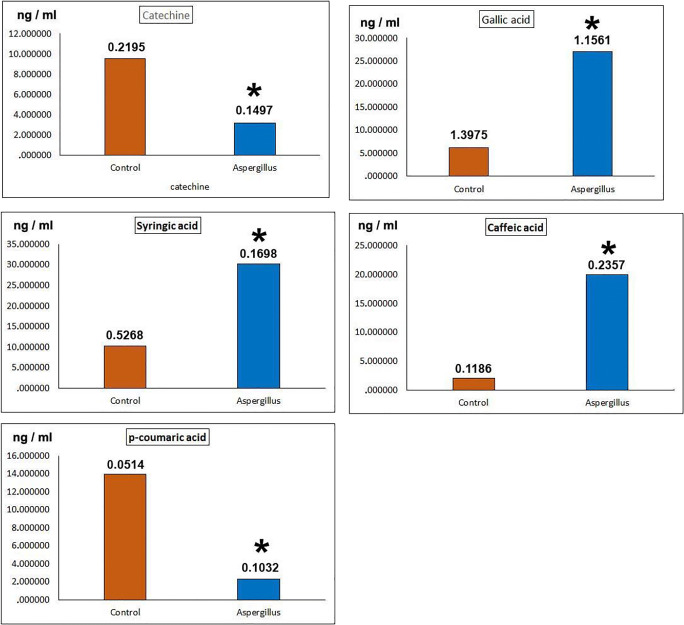
Catechine, the amount of this phenol in the control group was 9.55 ng/ml (± 21), while in the test group it was 3.16 ng/ml (± 14). The value in the test group is considerably lower than the control (Fig. 3).
Fig. 3.
Phenol levels in control and experimental groups. Catechine, F = 292,923, df = 3, P = 0.0000*; Gallic acid, F = 70,490, df = 3, P = 0.0000*; Syringic acid, F = 503,436, df = 3, P = 0.0000*; Caffeic acid, F = 403,271, df = 3, P = 0.0000*; P-coumaric acid, F = 154,457,138, df = 3, P = 0.0000*. The numbers in the columns define standard error data. * indicates significant differences between control and Aspergillus infected experimental groups according to one-way ANOVA, Duncan test (p ≤ 0.05)
Gallic acid, in the test group, the value of this phenol increased almost 4-fold compared with the control. The control value was 6.22 ng/ml (± 1.39) and the test group value was 27.02 ng/ml (± 1.15) (Fig. 3).
Syringic acid, the experimental group increased 3 times more than the control group. Control, 10.24 ng/ml (± 52); the experimental group, 30.15 ng/ml (± 16) (Fig. 3).
Caffeic acid, in the test group, the value of this phenol increased almost 10-fold compared with the control.
P-coumaric acid, the value in the test group is well below the control (Fig. 3).
Phenol amounts in catechine and p-coumaric acids are considerably lower than those of control; on the contrary, in gallic acid, syringic acid and caffeic acid, phenol amounts are quite higher than control values. The highest increase is caffeic acid. The value of this phenol is 10 times higher than in the control. The value increases/decreases in other phenols changed almost 3–6 times compared with the control (Fig. 3). According to statistical analysis, all experimental group phenol values were significantly different from those in the control (Fig. 3).
Discussion
According to hormonal tests
GA, ABA and JA hormones decreased significantly compared with control. Although the SA values were close to each other in the control and experimental groups, the difference was significant. The IAA hormone in the experimental group was significantly higher than the control group. In other words, during the pathogenesis of A. alliaceus, only the IAA hormone was significantly elevated in the Orobanche, while the other hormones decreased considerably.
Several functions of plant hormones
As a hormone, gibberellins (GA) have different effects such as seed germination, elongation, flowering, stimulation of photosynthetic activity, the formation of sex features flowering and fruit formation [30].
GA rapidly decreases when the plant encounters biotic and abiotic stress [31]. GA is a dense crosstalk with other hormones, and its levels fall rapidly if a stress is met during development. Therefore, GA has a negative role in defence, but GA signalling mediates stress tolerance through the control of cellular redox homeostasis [32].
ABA is a hormone that makes seed dormancy and suppresses floral induction and is particularly important in response to abiotic stress [33, 34]. Additionally, ABA can increase or decrease plant resistance compared with pathogenic agents (bacteria, fungi), whereas it always increases resistance to viral pathogens [35–37]. Therefore, ABA is a multifaceted effective hormone; therefore, its response varies according to bacteria and fungi and to their function in the infection process. As a general information, ABA response develops in necrotrophic interactions [38]. For example, ABA activated disease-resistant and callose formation against fungal pathogen Leptosphaeria maculans in Brassica napus and Arabidopsis [39].
Various phytopathogenic fungal agents synthesise ABA as a virulence factor ABA is important in mutualistic interactions between host plant and pathogen [40, 41], and its deficiency reduced the pathogen effect as described in the examples below (tomato, Bortyis cinerea; tobacco, Ralstonia solanacearum; Arabidopsis, Plectosphaerella cucumerina; and rice, Magnaporthe oryzae) [42–45].
SA is involved in plant defence activation against fungal pathogens, bacteria, sap-absorbing herbivore biotrophs and hemibiotrophs [46, 47]. SA-dependent responses are triggered by chewing herbivor insects and other biotrophic pathogen attacks [48]. Following local pathogen attacks, systemic acquired resistance (SAR) is initiated by activation of the SA and activation of the pathogen-related genes [49]. For example, SA-inducing genes are activated in Strawberry during Colletotrichum infection.
JA is an important hormone especially in defence against mechanical injury, insect herbivor, abiotic and biotic stress conditions, defence against nematodes, heavy metal, cold stress, drought stress, salt stress and UV stress, and changes caused by seasonal and circadian rhythms [50, 51].
Auxin (IAA) is known as multifunctional growth and development regulators in plants [52]. Auxin is the main regulator of almost all aspects of plant growth, morphogenesis and including adaptive responses to environmental stimuli. It also plays a key role in integrating environmental stimuli into growth adaptations [53].
When plants are exposed to environmental stresses, auxin signalling decreases under ROS accumulation, and thus cause a decrease in plant growth and development [54]. Thus, it is more resistant to stress. For instance, auxin accumulation decreased in rice and tomatoes have under salt stress [55]. Indeed, in tomato, IAA concentration decreased by 75% with salt stress. Nevertheless, the accumulation of IAA in in wheat ears was 300 times higher than control (fungus-free) during Fusarium graminearum (fungus) infection, and IAA application significantly suppressed Fusarium culmorum infection in barley [56]. Against Al toxicity in wheat, auxin signal transduction provides resistance by increasing malic acid concentration [57]. In addition, under some viral attacks, the plant could partially run into the auxin signalling pathway [58]. In potato, auxin caused resistance or sensitivity to infection depending on the type of pathogen (necrotrophic or biotroph) [59]. Therefore, upon these examples above, it is understood that resistance is provided by auxin or other auxin antagonist hormones, depending on the host plant and the type of pathogen [60].
Cross relations between hormones and comparison with the present results
In addition, there is an antagonistic relationship between JA and other hormones (IAA, ABA and SA) in the process of fighting stress [61], and depending on the type of pathogen, defence response generated by fine tuning crosstalk relations between these hormones [62, 63]. At this point, the JA and SA signal form the backbone of the defence response [64]. However, according to present results, all 3 hormones (ABA, SA and JA), which are very important in defence, are significantly lower than the control group (Fig. 2). In addition, according to Maggio et al. [65], GA decline and ABA increase are important in adaptation and struggle against stress. However, GA values have decreased considerably like these 3 defence hormones (Fig. 2).
According to the analysis of phenolic substances (secondary metabolite)
Gallic acid, syringic acid and caffeic acid values were significantly increased in experimental groups. On the contrary, catechine and p-coumaric acid values were significantly lower than the control. All these differences are significant (Fig. 3).
Various effects of phenolic substances
Syringic acid has anti-oxidant, anti-endotoxin, and anti-bacterial, anti-cancer and also has cardio protective effect [66, 67].
Gallic acid has strong anti-fungal, anti-oxidant, anti-carcinogenic, anti-oxidative, anti-mutagenic and anti-inflammatory activities [68]. Gallic acid also protects tissues and organs from the damage of toxic compounds [69].
Caffeic acid derivatives are anti-inflammatory, anti-apoptotic, anti-oxidant, anti-viral, anti-proliferative, neuroprotective, immunomodulatory, anti-inflammatory, anti-carcinogenic and neuroprotective effects [70]. It prevents cancer metastasis, reduces cancer proliferation and viability [71].
Catechin and p-coumaric acid have also anti-oxidant, anti-cancer, anti-bacterial effects are available [72, 73] and their values were significantly lower than other phenols and control group (Fig. 3). At this juncture, no information on the synergistic/antagonistic interaction between phenols was found; however, it is clear that the pathogenicity of A. alliaceus accelerates the metabolism of gallic acid, syringic acid and caffeic acid in the Orobanche while reducing the biosynthesis of other phenols. As phenolic compounds are used against the attack of microorganisms, insects, herbivores, and they have anti-microbial effects [74]. These changes in phenol levels are thought to be due to A. alliaceus pathogenicity and its different effects on phenol biosynthesis pathways. Indeed, after fungal infection, all Orobanches have darkened and died, as described in Fig. 1e–f.
Relationship between present results and previous A. alliaceus study
Based on the our recent transcriptomic study, during Orobanche pathogenesis of A. alliaceus, it was determined that A. alliaceus (1) activates only süperoxide dismutase (SOD) from anti-oxidant enzymes, (2) induces free radical damage, (3) damages protein synthesis metabolism, (4) inhibits other anti-oxidant (except SOD) and apoptosis-based defence reactions and ultimately lead to a slow and continuous death [21].
According to present hormonal results, A. alliaceus decreases considerably the effects of all defence hormones (JA, ABA, SA), and activates IAA hormone at highest level. In addition, it is evident that mainly secondary metabolite-based defence is carried out and which could not reduce the damage of the fungus. This is because Orobanche is dying completely (Fig. 1e, f) and none of the abovementioned damages have been repaired by the IAA. In short, our previous study [21] and present results showed parallelism.
Different pathogenic effects of Fusarium and A. alliaceus on Orobanche (for comparison purposes)
In a previous study, Fusarium oxysporum (fungus) was found to cause serious hormonal disorders in Orobanche, inducing an only SA-based defence, and rapidly increasing the values of some phenols and finally killing quickly Orobanche [13]. In other words, the effect mechanisms of the Fusarium and Aspergillus fungi on Orobanche were different from each other, and this was probably due to their unique mycotoxins. Consequently, A. alliaceus disrupted the hormonal balances in the Orobanche, weakened its defence and eventually killed Orobanche slowly.
Acknowledgements
I thank Prof. Dr. Oguzhan Doganlar for his valuable comments, TUBAP (Trakya University, Scientific Researches Foundations) with the project number TUBAP 2016-15, and Assoc. Prof. Dr. Burhan Sen for cultivation and identification efforts of fungal material.
Compliance with ethical standards
Conflict of interest
The author declares that he has no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Qasem JR. Parasitic weeds of the Orobanchaceae family and their natural hosts in Jordan. Weed Biol Manag. 2009;9(2):112–122. [Google Scholar]
- 2.Parker C. The parasitic weeds of the Orobanchaceae. In: Joel DM, Gressel J, Musselman LJ, editors. Parasitic Orobanchaceae: parasitic mechanisms and control strategies. Berlin: Springer; 2013. pp. 313–344. [Google Scholar]
- 3.Delavault P. Knowing the parasite: biology and genetics of Orobanche. Helia. 2015;38(62):15–29. [Google Scholar]
- 4.Fernandez-Aparicio M, Reboud X, Gibot-Leclerc S. Broomrape weeds, underground mechanisms of parasitism and associated strategies for their control: a review. Front Plant Sci. 2016;7:1–23. doi: 10.3389/fpls.2016.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghannam I, Al-Masri M, Barakat R. The effect of herbicides on the Egyptian broomrape (Orobanche aegyptiaca) in tomato fields. Am J Plant Sci. 2012;3:346–352. [Google Scholar]
- 6.Qasem JR. Branched broomrape (Orobanche ramosa L.) control in tomato (Lycopersicon esculentum Mill.) by trap crops and other plant species in rotation. Crop Prot. 2019;120:75–83. [Google Scholar]
- 7.Mamdouh M, Yasser N, Shabana A, Mamdouh M, et al. Granular formulation of Fusarium oxysporum for biological control of faba bean and tomato Orobanche. Pest Manag Sci. 2008;64:1237–1249. doi: 10.1002/ps.1625. [DOI] [PubMed] [Google Scholar]
- 8.Shabana YM, Müller-Stöver D, Sauerborn J. Granular Pesta formulation of Fusarium oxysporum f.sp. orthoceras for biological control of sunflower broomrape: efficacy and shelf-life. Biol Control. 2003;26:189–201. [Google Scholar]
- 9.Boari A, Vurro M. Evaluation of Fusarium spp. and other fungi as biological control agents of broomrape (Orobanche ramosa) Biol Control. 2004;30:212–219. [Google Scholar]
- 10.Kohlschmid E, Sauerborn J, Müller-Stöver D. Impact of Fusarium oxysporum on the holoparasitic weed Phelipanche ramosa: biocontrol efficacy under field-grown conditions. Weed Res. 2009;49(Suppl. 1):56–65. [Google Scholar]
- 11.Hodosy AS, Hornok L. Occurrence of hyperparasite Fusarium species and their use for control of broomrape on tomato. Proc Internat Conf Integr Plant Prot. 1983;4:48–52. [Google Scholar]
- 12.Aybeke M. Fusarium infection causes genotoxic disorders and antioxidant-based damages in Orobanche spp. Microbiol Res. 2017;201:46–51. doi: 10.1016/j.micres.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Aybeke M. Fusarium infection causes phenolic accumulations and hormonal disorders in Orobanche spp. Indian J Microbiol. 2017;57(4):416–421. doi: 10.1007/s12088-017-0669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aybeke M, Sen B, Okten S. Aspergillus alliaceus, a new potential biological control of the root parasitic weed Orobanche. J Basic Microbiol. 2014;54:93–101. doi: 10.1002/jobm.201300080. [DOI] [PubMed] [Google Scholar]
- 15.Mycobank, 2020, http://www.mycobank.org/Biolomics.aspx?Table=Mycobank&MycoBankNr_=256402, acc.date: 9.3.2020
- 16.Ozhak-Baysan B, Alastruey-Izquıerdo A, Saba R, Ogunc D, Ongut G, Tımuragaoglu A, Arslan G, Cuenca-Estrella M, Rodrıguez-Tudela JL. Aspergillus alliaceus and Aspergillus flavus co-infection in an acute myeloid leukemia patient. Med Mycol. 2010;48:995–999. doi: 10.3109/13693781003749418. [DOI] [PubMed] [Google Scholar]
- 17.Bayman P, Baker JL, Doster MA, Michailides TJ, Mahoney NE. Ochratoxin production by the Aspergillus ochraceus group and Aspergillus alliaceus. Appl Environ Microbiol. 2002;68:2326–2329. doi: 10.1128/AEM.68.5.2326-2329.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Wang L, Liu F, Wang Q, Selvaraj JN, Xing F, Zhao Y, Yang L. Ochratoxin a producing fungi, biosynthetic pathway and regulatory mechanisms. Toxins (Basel) 2016;8(3):83. doi: 10.3390/toxins8030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aybeke M, Şen B, Ökten S. Pesta granule trials with Aspergillus alliaceus for the biocontrol of Orobanche spp. J Bioc Sci Technol. 2015;25(7):803–813. [Google Scholar]
- 20.Aybeke M. Several pesta tablet trials with Aspergillus alliaceus Thom & Church for effective underground and aboveground Orobanche L. Biocontrol. Trakya Uni J Nat Sci. 2016;17(1):65–70. [Google Scholar]
- 21.Aybeke M. Transcriptomic effects of Aspergillus alliaceus on Orobanche during its pathogenesis. J Plant Dis Prot. 2018;125:33–39. [Google Scholar]
- 22.Herron DA, Wingfield MJ, Wingfield BD, Rodas CA, Marincowitz S, Steenkamp E. Novel taxa in the Fusarium fujikuroi species complex from Pinus spp. Stud Mycol. 2015;80:131–150. doi: 10.1016/j.simyco.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klich MA. Identification of common Aspergillus species. 1. Utrecht: Centralbureau voor Schimmelcultures; 2002. p. 122. [Google Scholar]
- 24.Raper KB, Fennell DI. The genus Aspergillus. Baltimore: Williams & Wilkins; 1965. [Google Scholar]
- 25.Muller-Stover D, Kroschel J, Thomas H, Sauerborn J. Chlamydospores of Fusarium oxysporum Schlecht f.sp. orthoceras (Appel & Wollenw.) Bilai as inoculum for wheat-flourkaolin granules to be used for biological control of Orobanche cumana Wallr. Eur J Plant Pathol. 2002;108:221–228. [Google Scholar]
- 26.Nirenberg HI. Untersuchungen u ber die morphologische und biologische Differenzierung in der Fusarien Sektion Liseola. Mitt Biol Bundesanstalt Land- und Forstwirtsch Berlin-Dahlem, Germany. 1976;169:1–117. [Google Scholar]
- 27.Louarn J, Boniface M-C, Pouilly N, Velasco L, Pérez-Vich B, Vincourt P, Muños S. Sunflower resistance to broomrape (Orobanche cumana) is controlled by specific QTLs for different parasitism stages. Front Plant Sci. 2016;7:590. doi: 10.3389/fpls.2016.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller M, Munne-Bosch S. Rapid and sensitive hormonal profiling of complex plant samples by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Plant Methods. 2011;7:37. doi: 10.1186/1746-4811-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doganlar ZB. Physiological and genetic responses to pesticide mixture treatment of Veronica beccabunga. Water Air Soil Pollut doi. 2012;223:6201–6212. doi: 10.1007/s11270-012-1350-y. [DOI] [Google Scholar]
- 30.Li JR, Yu K, Wei JR, Ma Q, Wang BQ, Yu D. Gibberellin retards chlorophyll degradation during senescence of Paris polyphylla. Biol Plant. 2010;54(2):395–399. [Google Scholar]
- 31.Colebrook EH, Thomas SG, Phillips AL, Hedden P. The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol. 2014;217:67–75. doi: 10.1242/jeb.089938. [DOI] [PubMed] [Google Scholar]
- 32.Xia X-J, Zhou Y-H, Shi K, Zhou J, Foyer CH, Yu J-Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J Exp Bot. 2015;66(10):2839–2856. doi: 10.1093/jxb/erv089. [DOI] [PubMed] [Google Scholar]
- 33.Sung CL, Luan S. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 2012;35:53–60. doi: 10.1111/j.1365-3040.2011.02426.x. [DOI] [PubMed] [Google Scholar]
- 34.Humplik JF, Bergougnoux V, Van Volkenburgh E. To stimulate or inhibit? That is the question for the function of abscisic acid. Trends Plant Sci. 2017;22:830–841. doi: 10.1016/j.tplants.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Zhang L, Li D, Wang F, Yu D. WRKY8 transcription factor functions in the TMV-cg defense response by mediating both abscisic acid and ethylene signaling in Arabidopsis. Proc Natl Acad Sci U S A. 2013;110:E1963–E1971. doi: 10.1073/pnas.1221347110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alazem M, Lin KY, Lin NS. The abscisic acid pathway has multifaceted effects on the accumulation of Bamboo mosaic virus. Mol Plant-Microbe Interact. 2014;27:177–189. doi: 10.1094/MPMI-08-13-0216-R. [DOI] [PubMed] [Google Scholar]
- 37.Alazem M, Lin NS. Roles of plant hormones in the regulation of host-virus interactions. Mol Plant Pathol. 2015;16:529–540. doi: 10.1111/mpp.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curvers KHS, Mouille G, de Rycke R, Asselbergh B, Van Hecke A, Vanderschaeghe D, Höfte H, Callewaert N, Van Breusegem F, Höfte M. Abscisic acid deficiency causes changes in cuticle permeability and pectin composition that influence tomato resistance to Botrytis cinerea. Plant Physiol. 2010;154:847–860. doi: 10.1104/pp.110.158972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaliff M, Staal J, Myrenås M, Dixelius C. ABA is required for Leptosphaeria maculans resistance via ABI1- and ABI4-dependent signaling. Mol Plant-Microbe Interact. 2007;20:335–345. doi: 10.1094/MPMI-20-4-0335. [DOI] [PubMed] [Google Scholar]
- 40.Mbengue M, Navaud O, Peyraud R, Barascud M, Badet T, Vincent R, et al. Emerging trends in molecular interactions between plants and the broad host range fungal pathogens Botrytis cinerea and Sclerotinia sclerotiorum. Front Plant Sci. 2016;7:422. doi: 10.3389/fpls.2016.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stec N, Banasiak J, Jasinski M. Abscisic acid–an overlooked player in plant-microbe symbioses formation? Acta Biochim Pol. 2016;63:53–58. doi: 10.18388/abp.2015_1210. [DOI] [PubMed] [Google Scholar]
- 42.Sivakumaran A, Akinyemi A, Mandon J, Cristescu SM, Hall MA, Harren FJ, et al. ABA suppresses Botrytis cinerea elicited NO production in tomato to influence H2O2 generation and increase host susceptibility. Front Plant Sci. 2016;7:709. doi: 10.3389/fpls.2016.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou J, Zhang H, Yang Y, Zhang Z, Zhang H, Hu X, Chen J, Wang XC, Huang R. Abscisic acid regulates TSRF1-mediated resistance to Ralstonia solanacearum by modifying the expression of GCC box-containing genes in tobacco. J Exp Bot. 2008;59:645–652. doi: 10.1093/jxb/erm353. [DOI] [PubMed] [Google Scholar]
- 44.Sánchez-Vallet A, López G, Ramos B, Delgado-Cerezo M, Riviere M-P, Llorente F, Fernández PV, Miedes E, Estevez JM, Grant M, Molina A. Disruption of abscisic acid signaling constitutively activates Arabidopsis resistance to the necrotrophic fungus Plectosphaerella cucumerina. Plant Physiol. 2012;160:2109–2124. doi: 10.1104/pp.112.200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ulferts S, Delventhal R, Splivallo R, Karlovsky P, Schaffrath U. Abscisic acid negatively interferes with basal defence of barley against Magnaporthe oryzae. BMC Plant Biol. 2015;15:7. doi: 10.1186/s12870-014-0409-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joshi JR, Burdman S, Lipsky A, Yariv S, Yedidia I. Plant phenolic acids affect the virulence of Pectobacterium aroidearum and P. carotovorum ssp. brasiliense via quorum sensing regulation. Mol Plant Pathol. 2016;17:487–500. doi: 10.1111/mpp.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi G, Chen J, Chang M, Chen H, Hall K, Korin J, Liu F, Wang D, Fu ZQ. Pandemonium breaks out: disruption of salicylic acid-mediated defense by plant pathogens. Mol Plant. 2018;11:1427–1439. doi: 10.1016/j.molp.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Schweiger R, Heise A, Persicke M, Muller C. Interactions between the jasmonic and salicylic acid pathway modulate the plant metabolome and affect herbivores of different feeding types. Plant Cell Environ. 2014;37:1574–1585. doi: 10.1111/pce.12257. [DOI] [PubMed] [Google Scholar]
- 49.Shoresh M, Harman GE, Mastouri F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol. 2010;48:21–43. doi: 10.1146/annurev-phyto-073009-114450. [DOI] [PubMed] [Google Scholar]
- 50.Wasternack C. Action of jasmonates in plant stress responses and development—applied aspects. Biotechnol Adv. 2014;32:31–39. doi: 10.1016/j.biotechadv.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 51.Zhao S, Ma Q, Xu X, Li G, Hao L. Tomato jasmonic acid-deficient mutant spr2 seedling response to cadmium stress. J Plant Growth Regul. 2016;35(3):603–610. [Google Scholar]
- 52.Taylor-Teeples M, Lanctot A, Nemhauser JL. As above, so below: auxin’s role in lateral organ development. Dev Biol. 2016;419:156–164. doi: 10.1016/j.ydbio.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Y. Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu Rev Plant Biol. 2018;69:417–435. doi: 10.1146/annurev-arplant-042817-040226. [DOI] [PubMed] [Google Scholar]
- 54.Potters G, Pasternak TP, Guise Y, Palme KJ, Jansen MAK. Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci. 2007;12:98–105. doi: 10.1016/j.tplants.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Kazan K. Auxin and the integration of environmental signals into plant root development. Ann Bot. 2013;112:1655–1665. doi: 10.1093/aob/mct229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petti C, Reiber K, Ali SS, Berney M, Doohan FM. Auxin as a player in the biocontrol of Fusarium head blight disease of barley and its potential as a disease control agent. BMC Plant Biol. 2012;12:224. doi: 10.1186/1471-2229-12-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu X, Lin Y, Liu D, Wang C, Zhao Z, Cui X, et al. MAPKmediated auxin signal transduction pathways regulate the malic acid secretion under aluminum stress in wheat (Triticum aestivum L.) Sci Rep. 2017;7:1620. doi: 10.1038/s41598-017-01803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benjamins R, Scheres B. Auxin: the looping star in plant development. Annu Rev Plant Biol. 2008;59:443–465. doi: 10.1146/annurev.arplant.58.032806.103805. [DOI] [PubMed] [Google Scholar]
- 59.Kolachevskaya OO, Lomin SN, Arkhipov DV, Romanov GA. Auxins in potato: molecular aspects and emerging roles in tuber formation and stress resistance. Plant Cell Rep. 2019;38:681–698. doi: 10.1007/s00299-019-02395-0. [DOI] [PubMed] [Google Scholar]
- 60.Bieleszová K, Pařízková B, Kubeš M et al (2018) New fluorescently labeled auxins exhibit promising anti-auxin activity. New Biotechnol. 10.1016/j.nbt.2018.06.003 [DOI] [PubMed]
- 61.Pieterse CM, Leon-Reyes A, Van der Ent S, Van Wees SC. Networking by small molecule hormones in plant immunity. Nat Chem Biol. 2009;5(5):308–316. doi: 10.1038/nchembio.164. [DOI] [PubMed] [Google Scholar]
- 62.Kazan K, Manners JM. MYC2: the master in action. Mol Plant. 2013;6:686–703. doi: 10.1093/mp/sss128. [DOI] [PubMed] [Google Scholar]
- 63.Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Ann of Botany. Ann Bot. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pieterse CMJ, Van Der D, Zamioudis DC, Leon-Reyes A, Van Wees SCM. Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol. 2012;28:489–521. doi: 10.1146/annurev-cellbio-092910-154055. [DOI] [PubMed] [Google Scholar]
- 65.Maggio A, Barbieri G, Raimondi G, De Pascale S. Contrasting effects of GA3 treatments on tomato plants exposed to increasing salinity. J Plant Growth Regul. 2010;29:63–72. [Google Scholar]
- 66.Shahzad S, Mateen S, Naeem SS, Akhtar K, Rizvi W, Moin S. Syringic acid protects from isoproterenol induced cardiotoxicity in rats. Europ J of Pharmac. 2019;849:135–145. doi: 10.1016/j.ejphar.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 67.Fang W, Zhu S, Niu Z, Yin Y (2019) The protective effect of syringic acid on dextran sulfate sodium induced experimental colitis in BALB/c mice. Drug Dev Res. 2019 Sep;80(6):731-740. 10.1002/ddr.21524 [DOI] [PubMed]
- 68.Babak G, Houshmand G, Hosseinzadeh A, Kalantar M, Mehrzadi S, Goudarzi M (2019) Gallic acid ameliorates sodium arsenite-induced renal and hepatic toxicity in rats. Drug Chem Toxicol:1–12. 10.1080/01480545.2019.1591434 [DOI] [PubMed]
- 69.Safaei F, Mehrzadi S, Khadem Haghighian H, Hosseinzadeh A, Nesari A, Dolatshahi M, Esmaeilizadeh M, Goudarzi M. Protective effects of gallic acid against methotrexate-induced toxicity in rats. Acta Chir Belg. 2018;118(3):152–160. doi: 10.1080/00015458.2017.1394672. [DOI] [PubMed] [Google Scholar]
- 70.Prudêncio ER, Cardoso CM, Castro RN, Riger CJ. Antioxidant effect of caffeic acid derivatives on sod and glutathione defective yeasts. Appl Biochem Microbiol. 2019;55(3):264–269. [Google Scholar]
- 71.Pelinson LP, Assmann CE, Palma TV, da Cruz IBM, et al. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol Biol Rep. 2019;46:2085–2092. doi: 10.1007/s11033-019-04658-1. [DOI] [PubMed] [Google Scholar]
- 72.Jing X, Zhang J, Huang Z, Sheng Y, Ji L. The involvement of Nrf2 antioxidant signalling pathway in the protection of monocrotaline-induced hepatic sinusoidal obstruction syndrome in rats by (+)-catechin hydrate. Free Radic Res. 2018;52:402–414. doi: 10.1080/10715762.2018.1437914. [DOI] [PubMed] [Google Scholar]
- 73.Yue Y, Shen P, Xu Y, Park Y. p-Coumaric acid improves oxidative and osmosis stress responses in Caenorhabditis elegans. J Sci Food Agric. 2019;99:1190–1197. doi: 10.1002/jsfa.9288. [DOI] [PubMed] [Google Scholar]
- 74.Rossetti A, Mazzaglia A, Muganu M, Paolocci M, Sguizzato M, Esposito E, Cortesi R, Balestr GM. Microparticles containing gallic and ellagic acids for the biological control of bacterial diseases of kiwifruit plants. J Plant Dis Prot. 2017;124:563–575. [Google Scholar]