Abstract
Gene therapies are powerful tools to prevent, treat, and cure human diseases. The application of gene therapies for skin diseases received little attention so far, despite the easy accessibility of skin and the urgent medical need. A major obstacle is the unique barrier properties of human skin, which significantly limits the absorption of biomacromolecules, and thus hampers the efficient delivery of nucleic acid payloads. In this review, we discuss current approaches, successes, and failures of cutaneous gene therapy and provide guidance toward the development of next-generation concepts. We specifically allude to the delivery strategies as the major obstacle that prevents the full potential of gene therapies – not only for skin disorders but also for almost any other human disease.
Keywords: gene therapy, skin, gene editing, topical gene delivery, genodermatoses, CRISPR, RNA therapy
Do We Need Gene Therapy for Skin Diseases?
Gene therapies, including RNA-based approaches, gene augmentation, and gene editing (Table 1 ), are powerful tools to prevent, treat, and cure a multitude of human diseases [1]. The first gene therapy, Glybera (see Glossary), obtained regulatory approval in 2012 and at least eight more followed since then. Modern cutaneous gene therapy was pioneered by Paul Khavari and his group, who published first studies on the delivery of the transglutaminase 1 gene into congenital ichthyosis patient cells using retroviruses back in 1996 [2]. Since then, incredibly fast advancements, especially in the field of CRISPR-based gene editing, have propelled the potential applications of gene therapies. Hence, the drug development pipelines and clinical trials are full of gene therapy-based approaches that provide a new lever for the treatment of previously untreatable conditions [3]. Interestingly, the application of gene therapies for skin-related diseases has received comparably little attention so far, despite the easy accessibility of human skin and the urgent medical need [4].
Table 1.
Overview of the Current Gene Therapy Tools
| Tools | Features | Advantages | Challenges | |
|---|---|---|---|---|
RNA-based therapies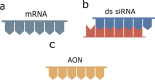
|
a) Messenger RNA (mRNA) | • Induce the production of desired protein to restore normal function |
 Promising for ‘undruggable’ targets Promising for ‘undruggable’ targets Easy and rapid chemical synthesis Easy and rapid chemical synthesis Cost effective and stable shelf life Cost effective and stable shelf life Allows personalized medicine Allows personalized medicine Low immunogenicity. Low immunogenicity. |
 Poor pharmacokinetic properties Poor pharmacokinetic properties Variable efficacy in suppression of target protein Variable efficacy in suppression of target protein Off-target effects Off-target effects |
| b) Silencing RNA | • Short double-stranded RNA fragments • Triggers mRNA degradation or blocks its transcriptions |
|||
| c) AON | • Short sequences of modified DNA or RNA • Inhibit mRNA translation into proteins |
|||
Gene augmentation
|
a) Plasmid DNA | • Wild-type copy of mutated gene • Transient gene knock-in or DNA-directed RNA interference (gene knockdown) |
 Regulated gene expression Regulated gene expression Easy to design and cost effective Easy to design and cost effective Flexible for personalized medicine Flexible for personalized medicine Low immunogenicity Low immunogenicity |
 Translocation into the cell nucleus is required Translocation into the cell nucleus is required Readministration is required for long-lasting effects Readministration is required for long-lasting effects |
| b) Minicircle DNA | ||||
| c) Mini-string DNA | ||||
|
Gene repair and editing Insertion, deletion, or replacement of genes 
|
a) ZFN and b) TALEN proteins detect target DNA sequences. | • Programmable, sequence-specific DNA-binding modules linked to a nonspecific DNA cleavage domain |
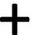 Broad range of gene editing Broad range of gene editing |
 Time-consuming and labor-intensive de novo protein engineering Time-consuming and labor-intensive de novo protein engineering |
| c) RNA-guided nuclease technology (CRISPR/Cas9) | • A target-specific guide RNA (gRNA) complexes with the Cas9 nuclease protein • Induction of double-strand breaks (DSBs) at target site •DSBs are repaired either by nonhomologous end joining or homology-directed repair |
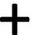 Cas nucleases can be delivered as DNA, mRNA, or RNP, which refers to preassembled Cas9 protein–gRNA complex Cas nucleases can be delivered as DNA, mRNA, or RNP, which refers to preassembled Cas9 protein–gRNA complex Fast, cheap, simple Fast, cheap, simple RNP: higher editing rates and less off-target effects than plasmid-based Cas expression RNP: higher editing rates and less off-target effects than plasmid-based Cas expression |
 Transfection is challenging Transfection is challenging Risk of off-target effects unclear Risk of off-target effects unclear |
|
| a) Base editing | •An impaired Cas9–sgRNA combined with deaminase (a catalytic enzyme which permanently alters the chemical sequence of single base). |
 Conversion of single bases or base pairs possible Conversion of single bases or base pairs possible No DSBs No DSBs Correction of single-nucleotide polymorphism Correction of single-nucleotide polymorphism |
 Transfection is challenging Transfection is challenging Risk of off-target effects remains unclear Risk of off-target effects remains unclear |
|
An impaired skin barrier may pose a serious threat to health, and skin diseases significantly affect physical and psychological well-being. In fact, they belong to the most frequent diseases of humans causing a significant economic burden worldwide [5]. In principle, all types of skin diseases are candidates for gene therapy, ranging from inflammatory diseases over skin cancer to genodermatoses. Genodermatoses are a diverse group of rare, often severe skin diseases that result from a variety of single mutations in 500 or more genes. One of the most common genodermatoses is epidermolysis bullosa (EB), a rare genetic condition that results in blistering of the skin and mucous membranes with severity ranging from mild to fatal [6]. While EB persists into adulthood, it is especially significant in neonates who suffer from higher mortality rates in some instances because of dehydration and infection [6,7]. Further examples for genodermatoses are congenital ichthyosis and the Netherton syndrome. So far, genodermatoses cannot be cured, and current treatment options purely rely on the relief of symptoms. A potentially curative strategy is to repair the disease-causing mutations within the host genome using gene editing [1,8]. This, together with the high medical need, makes genodermatoses the prime candidate for cutaneous gene therapy.
Overall, however, the absorption of biomacromolecules, such as gene therapeutics into the skin, is highly restricted due to its unique composition and structural organization (Figure 1 ; Box 1 ). In fact, human skin only allows efficient absorption of small [molecular weight (MW) ≤ 800 Da] and moderately lipophilic (logP 1–3) molecules [9,10], which makes it an exceptionally challenging target for gene delivery.
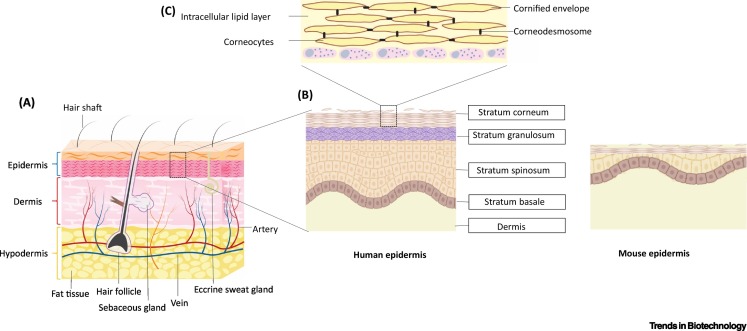
Figure 1.
Structure of Human Skin.
(A) Human skin is composed of three layers: the epidermis, which mainly consists of keratinocytes; the dermis, which contains connective tissue, sweat glands, and hair follicles; and the hypodermis, which is mainly composed of adipose tissue. (B) In the epidermal layer, keratinocytes undergo continuous maturation and differentiation resulting in four epidermal layers: stratum basale, stratum spinosum, stratum granulosum, and the stratum corneum (SC), which forms the outermost layer of the skin. A schematic depiction of mouse epidermis (created with BioRender.com) highlights the anatomical differences between human and mouse skin. (C) The SC consists of terminally differentiated keratinocytes (= corneocytes), which are connected by corneodesmosomes and surrounded by an insoluble cornified envelope. Corneocytes are embedded in highly lipophilic lipid layers mainly composed of ceramides, cholesterol, and fatty acids.
Box 1. Structure of Human Skin.
Human skin exerts several vital functions as it protects the human body from excessive transepidermal water loss and prevents the entry of xenobiotics and microbes. It is characterized by a highly sophisticated structural organization and is composed of three main layers: the epidermis, the dermis, and the hypodermis. Epidermis and dermis are connected by the basement membrane, epidermal–dermal junction, which also anchors the epidermis and dermis through proteins such as collagen and integrins, providing resistance against external shear stress.
The epidermis encompasses three main cell populations: keratinocytes, which make up 99% of the cells, melanocytes, and highly specialized immune cells, the Langerhans cells. Keratinocytes derive from the skin stem cells, which are located in the stratum basale, then undergo continuous maturation and differentiation, forming three different epidermal layers: the stratum spinosum, stratum granulosum, and SC (Figure 1) [9,10]. Except for the SC, all skin layers are metabolically active. The outermost layer of the human skin, the SC, consists of corneocytes – terminally differentiated keratinocytes which are interconnected by keratin filaments and are enclosed within an insoluble amalgam of crosslinked skin proteins. The corneocytes are embedded in a highly lipophilic skin lipid matrix. The lipid matrix is a mixture of ceramides, cholesterol, and fatty acids, organized in an orthorhombic lattice packing that determines the unique barrier properties of human skin.
The dermis is an elastic connective tissue which is highly vascularized and, thus, supports the epidermis as well as the skin appendages, such as hair follicles, sweat, and sebaceous glands. Fibroblasts, the primary cell type of the dermis, produce and secrete structural proteins such as collagen fibers, elastin, and proteoglycans that together form the extracellular matrix, in which further immune cells such as macrophages, mast cells, and dendritic cells are embedded.
The hypodermis is rich in adipose tissue, collagen, and elastic fibers and, thus, acts as a cushion and protects the body against temperature variations. This loose connective tissue further aids the support of nerves and blood vessels in the skin.
Alt-text: Box 1
Hence, the focus of this review is to provide insight into the strengths and limitations of skin-relevant gene delivery strategies. In fact, efficient, targeted, and safe gene delivery is the major obstacle that currently hampers the translation of gene therapies from bench-to-bedside – not only for skin disorders but also for almost any other human disease [11]. Further, we discuss the successes and failures of intradermal gene therapy and provide guidance toward the development of next-generation concepts.
Delivery of Gene Therapies to the Skin
Viral Gene Delivery
Viral vectors currently are the most effective carriers for gene delivery due to their innate capability to infect both dividing and quiescent cells. Currently, 70% of ongoing gene therapy clinical trials are viral vector based [12]. Nonintegrating viral vectors, such as adenoviral vectors and adeno-associated viral vectors (AAVs), offer the advantage of inherent infection ability without triggering potential problems due to insertional mutagenesis [13]. AAVs seem to be less immunogenic than other viruses, but the risk of pre-existing immunity limits their transfection efficacy and prevents re-dosing regimens [14]. By contrast, lentiviral vectors, which belong to the retroviruses, maintain stable long-term transgene expression but are capable of genome integration, which increases the risk for insertional mutagenesis [15].
Despite their efficacy, the use of viral vectors is accompanied by considerable challenges, the biggest of which is the limited cargo size. AAVs (20 nm), for example, can only encapsulate approximately 4.7 kb of genetic cargo. Gene-editing tools such as CRISPR require a much larger and/or additional carriers for efficient delivery. To overcome the size caveat, the use of dual vectors has been proposed: one for the Cas9 and the other for the single guide RNA (sgRNA) [16]. However, the success of this approach relies on the simultaneous intracellular delivery of sgRNA and Cas9. Further, curative approaches for recessive genodermatoses require the simultaneous delivery of donor templates, which cannot be achieved using viral vectors. Recent advancements of the CRISPR-Cas technology, such as prime editing, enable more precise cutting and higher editing efficacies, but viral vectors cannot serve as delivery tools as the reverse transcriptase (7 kb) cannot be encapsulated [17]. Although lentiviral vectors (80–100 nm) can encapsulate larger genomic materials (approximately 10 kb) [18,19], their safety remains controversially discussed [15].
In addition to packaging constraints, the broad tissue-targeting ability of viral vectors can be problematic as the long-term expression of gene-editing molecules may result in off-target effects and immunogenicity [20]. Finally, the design of viral vectors requires complex manufacturing processes at the highest standards, which raises cost-related problems [21].
Non-viral Gene Delivery Systems
Lipid-Based Nanoparticles
Today, lipid-based nanoparticles (LNPs) are the most advanced, non-viral gene delivery systems. In fact, the approval of ONPATTRO in 2018, the first-ever siRNA-based drug, has paved the way for a new class of gene therapies. LNPs have become a clinically validated platform technology to deliver genetic material of nearly any size, for example, ≤20-kbp DNA vectors have been successfully delivered in a preclinical setting [22], and about 11.5-kb self-amplifying RNAs encoding for SARS-CoV-2 (severe acute respiratory syndrome-coronavirus 2) proteins are currently being tested in the clinic as COVID-19 (coronavirus disease 2019) vaccines [23]. This revolutionary discovery offers the potential to treat human skin disorders by silencing pathogenic genes, expressing therapeutic proteins, or correcting genetic defects [24]. Rapid mixing and microfluidic procedures enable efficient large-scale manufacturing crucial for entering into routine clinical practice. Until recently, however, only a few studies explored the use of LNPs for treating skin disorders.
Historically, cationic lipids [such as 1,2-dioleoyl-3-trimethylammonium propane, (DOTAP)] in combination with helper lipids (e.g., phospholipids or cholesterol) have been used to complex, protect, and deliver nucleic acids [25]. These lipoplexes and related transfection reagents such as Lipofectamine are powerful agents for introducing gene constructs into cells in vitro. Although promising, these systems have little clinical utility due to carrier-related toxicity attributed to the permanent cationic charge and immune activation. In addition, certain cationic lipids, including DOTAP, have even shown inhibitory effects for efficient cutaneous gene transfer [26,27].
A key advancement of LNP technology was the identification of ionizable cationic lipids such as DLin-MC3-DMA [28,29]. This class of lipids possesses an acid dissociation constant (pK a) of approximately 6.5. This ensures that the lipid is neutral under physiological conditions [28], and positively charged at acidic pH to enable efficient entrapment of nucleic acids. This discovery significantly reduced carrier-related side effects and thus improved the therapeutic index by several orders of magnitude. Upon LNP internalization, the low endosomal pH allows for protonation of the ionizable lipid, resulting in destabilization of the endosome via interaction with negatively charged endosomal lipids, and finally cytoplasmic release of the genetic payload (Box 2 ) [30]. Conclusively, the ionizable lipid has a threefold function: efficient interaction and entrapment with nucleic acid drugs, reduction of particle toxicity, and facilitation of endosomal escape [28].
Box 2. Extra- and Intracellular Barriers for Non-viral Delivery Systems.
Non-viral gene delivery systems such as lipid nanoparticles (LNPs) or polymeric nanoparticles face several extracellular and intracellular barriers that must be overcome before generating any therapeutic effect. Consequently, non-viral delivery systems are designed to prevent the degradation of genetic material through endonucleases, escape immune detection, and reduce nonspecific interactions [25]. In addition, the delivery systems need to reach the target tissue, enter the cells, escape the endosomes, and unpack the genetic material. For RNA-based therapies, delivery into the cytosol is sufficient, whereas for DNA vectors and gene-editing tools, the nucleus needs to be reached. Cellular uptake mechanisms of non-viral delivery systems include adsorptive or receptor-mediated endocytosis. The latter is based on specific interactions of endogenous (i.e., serum proteins covering nanoparticles) or exogenous (i.e., chemically conjugated moieties) targeting ligands on the nanoparticle with cell surface receptors. Once internalized, the non-viral delivery systems become hostile within the endocytic vesicles that rapidly acidify to pH 5–6. In the case of polymer-based gene delivery systems, proton-accepting amines prevent the acidification, leading to osmotic vesicle swelling and finally endosomal membrane rupture (proton sponge hypothesis) [92]. By contrast, the protonation of lipids within the acidic endosomes triggers an association with anionic endosomal lipids. The formation of membrane disruptive non-lamellar structures facilitates the subsequent endosomal escape (shape hypothesis) [93].
Overall, the performance of non-viral delivery systems can be enhanced by both chemical and physical engineering strategies. Size, zeta potential, and geometric optimization are mandatory, and chemical engineering, such as the conjugation of targeting ligands and cell-penetrating peptides or the generation of stimuli-responsive nanoparticles, may facilitate the cellular entry and endosomal escape [30].
Alt-text: Box 2
Utilizing LNP technology to deliver gene-editing complexes holds great promise as a therapeutic approach for skin diseases. In fact, LNPs are capable of delivering CRISPR gene-editing components in DNA, RNA, or RNP (ribonucleoprotein) form, thereby overcoming typical delivery problems such as proteolytic degradation in the skin. Akin to nucleic acid drugs, Cas9 RNP complexes typically bear a net negative charge, and it has been shown that Cas9 RNP can also be delivered using LNP technology [31., 32., 33., 34.].
Future advances in LNP-based cutaneous gene therapy will rely on lipid library screens coupled with structure–activity–relationship analyses to tailor and optimize the LNP properties for skin applications. For example, a recent study demonstrated that the complexing lipid type significantly affects the delivery of self-amplifying messenger RNA to human skin explants [27]. The zwitterionic lipid cephalin enabled a sevenfold increase in luciferase expression as compared with the cationic lipid DOTAP. In addition, the effect of different administration routes needs to be assessed due to potential translational blockage caused by innate immune responses, as recently shown for LNP self-amplifying RNA systems injected intradermally [35].
Polymeric Nanoparticles
Polymer-based gene delivery systems are a diverse group of non-viral vectors. Cationic polymers easily form spherical complexes (= polyplexes; 50–200 nm diameter) with negatively charged genetic cargo due to electrostatic interactions. The most commonly used polymers for skin applications are polyethyleneimine (PEI) and its derivatives, as well as poly-(β-amino ester) (PBAE).
Numerous in vitro and in vivo studies have demonstrated the potential of PEI-based nanoparticles (NPs) for the treatment of skin diseases due to its high transfection efficacy and efficient endosomal escape (Box 2). At the same time, PEI is also known for its pronounced toxicity. Notably, both transfection efficacy and cytotoxicity of cationic polymers are directly proportional to their MW [36] – high MW PEI relates to high transfection efficacy and high toxicity. At the same MW, however, branched PEI complexes genetic cargo 15-fold more efficiently than linear PEI. Nevertheless, linear PEI is more suitable for in vivo applications due to its better biocompatibility [37,38]. For example, linear PEI was shown to complex viral RNA that activates innate immune receptors in melanoma (e.g., Toll-like receptors) highly efficiently. Intratumoral injection of these polyplexes caused prominent T-cell infiltration, resulting in potent antitumor activity without any major side effects [39]. These findings formed the basis for an ongoing Phase I/II clinical trial for melanoma immunotherapy (NCT02828098).
To increase the biocompatibility of PEI-based NPs, low MW PEI can be copolymerized with neutral and biocompatible moieties. For example, polymerization of cyclodextrins with LMW PEI showed improved biocompatibility compared with native PEI, and at the same time, a fourfold increased transfection efficacy [40]. Similarly, conjugation of PEI with PLGA [poly(lactic-co-glycolic acid)] yielded efficient siRNA encapsulation and transfection of dendritic cells, resulting in the restoration of cell maturation and functionality, yielding significant allogeneic T-cell proliferation in vitro [41].
Another avenue is the use of ‘smart’ polymers that respond to changes in the biological environment. For example, Chen and colleagues [42,43] evaluated the effect of fluorination and the bioreducibility of cationic hyperbranched poly(amido amines). Reducible fluorinated polymers complexed well with siRNA and induced superior gene knockdown compared with nonreducing polyplexes following intravenous and tumoral injection in mice. Liu and coworkers [44] engineered redox- and pH-sensitive NPs based on a galactose-functionalized n-butylamine-poly(l-lysine)-b-poly(l-cysteine) polypeptide core, coated with sheddable polyethylene glycol copolymers for targeted delivery of miR155 to tumor-associated macrophages. The polyethylene glycol shielded the cationic core at physiological pH but quickly switched to a positive charge in the acidic tumor environment and, thus, shed off to re-expose its core. These polyplexes effectively transfected tumor-associated macrophages and induced miR155 expression by 100–400 times both in vitro and after intratumoral injection in melanoma xenografted mouse models. Although this is a very elegant approach, complex NPs like that are often considered as too complicated for commercial applications.
PBAEs are another class of cationic polymers for gene delivery into the skin, yielding transfection efficacies comparable with viral vectors. So far, more than 2000 versatile linear PBAEs have been designed, and some linear PBAEs show superior effects compared with PEI [45] and viral vectors [46]. Again, branching of the cationic polymers dramatically increases the transfection efficacy [47]. In fact, highly branched PBAE-based NPs efficiently delivered minicircle DNA encoding for COL7A1 to EB patient-derived keratinocytes ex vivo [48]. The topically applied PBAE NPs efficiently restored the production of collagen VII in EB models and showed lower cytotoxicity than PEI [47,49]. These promising data led to the first topical gene therapy product candidate based on highly branched poly-(β-amino esters) (HPBAEs), which has been licensed by Amryt Pharma in 2018i.
Overall, polymeric NPs are increasingly used for gene delivery to the skin. However, it should be noted that most of the studies are still in an early preclinical phase, which leaves the actual translational value ambiguous [48,50,51]. Although polymeric NPs do not have the natural infective ability of viruses, by leveraging our understanding of cell biology and bioengineering of synthetic biomaterials, the development of stable, multifunctional, and highly effective gene delivery systems has advanced [52]. So far, however, the clinical translation of polymeric NPs is limited by efficient endosomal escape and lower transfection efficacies compared with viral vectors. In fact, PEI efficacy ranges between 1/10th and 1/1000th of an adenoviral vector depending on the MW [53].
Physical Methods
Harnessing physical and barrier disruptive methods holds great potential for gene delivery to the skin. Electroporation is the most extensively studied method and frequently used in experimental settings. High-intensity electrical pulses transiently create pores in the cell membrane, which facilitates the cellular entry of biomacromolecules yielding high transfection efficacies [54]. Nevertheless, pronounced cytotoxicity attributed to pH changes prevents widespread in vivo applications [55]. More suitable for clinical applications is iontophoresis, where low-intensity electrical currents are applied that enhance and guide the cell penetration of charged molecules [56]. For example, the application of noninvasive iontophoresis yielded comparable delivery efficiencies for STAT3 siRNA in melanoma mouse models as intratumoral injections [57].
The application of ultrasound (sonoporation) also yields successful gene delivery beyond tight biological barriers. Sonoporation temporarily permeabilizes the cell membrane and facilitates the penetration of genetic cargo into cells [58]. As such, sonoporation enabled the topical penetration of miR-197 in psoriatic mouse models, followed by a significant downregulation of interleukin-17 (IL-17) and IL-22 receptors [59]. For intradermal delivery, low and medium ultrasound frequencies are used, yielding high ‘cavitational effects’ and triggering the formation of vapor-filled bubbles, which ultimately collapse and form pores in the cell membrane. One limitation of this technique is the low transfection efficacy compared with electroporation since cavitation does not occur uniformly and cannot be controlled in skin tissue [60].
Of special interest for dermal applications are hollow and dissolvable microneedles (MNs; 50–1500 μm) that overcome the stratum corneum (SC) by the formation of microscopic pores through which small and biomacromolecules may freely penetrate the viable epidermis [61]. The pores close within 1 h and leave the skin fairly unaffected [62]. Up to a length of 500 μm, application of MNs is painless as they are too short to activate nerves in the dermis. MNs have been successfully used for intradermal delivery of DNA [63], siRNA [64], proteins [65,66], and peptides [67]. Especially hollow MNs seem promising for the topical delivery of nucleic acids [61]. For example, they enabled the intradermal delivery of STAT3 siRNA into melanoma mouse models, which reduced STAT3 messenger RNA expression by 60% and tumor weight and volume by 80% [68]. Among current limitations for clinical applications are the limited loading capacity and challenges when it comes to whole-body administrations.
State of the Art in the Gene Therapy for Skin Diseases
Currently, gene therapies for skin diseases are mainly explored in preclinical settings. In fact, of the 1052 clinical trials involving gene therapy, only 23 studies are focused on skin conditions [69], out of which, EB is the most frequently studied followed by melanoma and few trials that focus on the Netherton syndrome and congenital ichthyosis (Table 2 ). However, all current melanoma studies pursue a systemic approach by utilizing, for example, genetically modified T cells to increase their antitumor activity [70], which is beyond the scope of this review.
Table 2.
Non-Exhaustive List of Currently Ongoing Clinical Trials in Cutaneous Gene Therapy
| Clinical trial | Clinical phase | Estimated enrollment | Approach | Primary completion date | Sponsor |
|---|---|---|---|---|---|
|
NCT04186650: Clinical trial for RDEB using autologous skin equivalent grafts genetically corrected with a COL7A1-encoding SIN retroviral vector |
I/II | 3 | Ex vivo approach; regrafting of genetically corrected autologous skin equivalent grafts onto adult RDEB patients | July 2021 | Institut National de la Santé Et de la Recherche Médicale, France; part of the European Commission-funded GENEGRAFT project |
|
NCT04227106: Open-label clinical trial of EB-101 for the treatment of recessive dystrophic epidermolysis bullosa (RDEB) |
III | 15 | Ex vivo approach; one-time surgical application of approximately 30 autologous, gene-corrected keratinocyte sheets for the treatment of RDEB wound sites | September 2020 | Abeona Therapeutics, Inc. |
|
NCT02810951: A phase I/II study of FCX-007 (genetically-modified autologous human dermal fibroblasts) for recessive dystrophic epidermolysis bullosa (RDEB) |
I/II | 12 | Ex vivo approach; FCX-007 are genetically modified autologous skin fibroblasts. The cells are expanded and genetically modified to produce functional collagen VII followed by intradermal injection | July 2020 | Fibrocell Technologies, Inc. |
|
NCT01263379: A phase 1/2A single center trial of gene transfer for recessive dystrophic epidermolysis bullosa (RDEB) using the drug LZRSE-Col7A1 engineered autologous epidermal sheets (LEAES) |
I/IIa | 10 | Ex vivo approach; RDEB cells are transfected with a collagen 7 gene using a retrovirus followed by the growth of skin sheets using the gene-corrected cells and regrafting onto patients | December 2023 | Stanford University in coll. National Institute of Arthritis and Musculoskeletal and Skin Diseases & Abeona Therapeutics, Inc. |
|
NCT01545323: Phase I study of ex-vivo lentiviral gene therapy for the inherited skin disease Netherton syndrome |
I | 5 | Ex vivo approach; generation and regrafting of autologous skin sheet graft generated from SPINK5 transduced cells | February 2018 No published data yet available |
Great Ormond Street Hospital for Children NHS Foundation Trust |
|
NCT03536143: A phase II study of beremagene geperpavec (KB103), a non-integrating, replication-incompetent herpes simplex virus 1 (HSV-1) vector expressing the human collagen VII (COL7) protein, for the treatment of dystrophic epidermolysis bullosa (DEB) |
II | 4 | In situ; topical beremagene geperpavec (KB103, HSV1-COL7 vector) can safely and effectively promote healing of DEB patient wounds | February 2020 | Krystal Biotech, Inc. |
|
NCT04047732: A phase I/II clinical trial of topical KB105, a replication-incompetent, non-integrating HSV-1 vector expressing human transglutaminase 1 (TGM1) for the treatment of TGM1-deficient autosomal recessive congenital ichthyosis (ARCI) |
I/II | 6 | Topical application of KB105, a replication-incompetent, nonintegrating Herpes simplex virus type 1 (HSV-1) vector expressing human transglutaminase 1 (TGM1) formulated as a topical gel in TGM1-deficient ARCI patients | March 2020 | Krystal Biotech, Inc. |
|
NCT03605069: A first in human, double-blind, randomized, intra-subject placebo-controlled, multiple dose study of QR-313 evaluating safety, proof of mechanism, preliminary efficacy and systemic exposure in subjects with DDEB or RDEB due to mutation(s) in Exon 73 of the COL7A1 gene |
I/II | 14 | In situ; topical application of QR-313 [21-nucleotide AONs targeting COL7A1 pre-messenger RNA] in subjects with confirmed dominant dystrophic epidermolysis bullosa or recessive dystrophic epidermolysis bullosa mutations in the COL7A1 gene | September 2020 | Wings Therapeutics, Inc. |
Some clinical reports demonstrate the power gene therapies have for the treatment of severe skin diseases. For example, a 7-year-old boy who suffered from a life-threatening form of EB was treated with a gene augmentation approach [71,72]. A biopsy was taken from his skin; his keratinocytes were expanded ex vivo and transfected with a retrovirus expressing a full-length LAMB3 cDNA. Subsequently, large skin sheets were grown from the transfected keratinocytes, which were ultimately regrafted onto the boy. The transgenic skin grafts replaced 80% of the patient’s skin, and after 21 months, his skin had regained a normal appearance without any detachment from the dermis [8]. This long-term effect likely resulted from a co-incidental transfection of skin stem cells, which drive the skin regeneration.
The successful outcome of this study provided a blueprint for ex vivo gene therapies of genodermatoses. In fact, several clinical studies are currently investigating the efficacy and safety of viral vector-based gene therapy for EB and congenital ichthyosis, either by applying the vector topically or by regrafting approaches (Table 2). The first results are expected in 2020/2021.
With the emergence of CRISPR, gene editing is currently heavily investigated for the treatment of EB. CRISPR’s potential for the correction of both dominant and recessive disease-causing mutations has been repeatedly demonstrated so far only in vitro and in xenografted rodent models [72., 73., 74., 75., 76.]. Each of these studies yielded the restoration of the gene function in a significant number of cells (≤70%), which is considered sufficient for the development of a scarless phenotype after engraftment onto a human body. The application of CRISPR is also being expanded to in vivo approaches. Wu and colleagues [77] restored C7 gene function in EB mouse models by delivering sgRNA/Cas9 RNP complex via intradermal injections followed by electroporation to facilitate the transfection of skin stem cells. The skin adhesion area improved from 30% to 60%, but only 2% of stem cells were edited.
Despite the great interest in CRISPR, siRNA-based therapies are still the most widely studied approaches. Pachyonychia congenita, an autosomal dominant genodermatoses caused by mutations in keratin, was the first skin disease to undergo clinical studies involving siRNA treatments (NCT00716014). Here, siRNA that targets the disease-relevant mutations was intradermally injected, yielding promising regression of the disease [78]. Unfortunately, the pain associated with frequent intralesional administration precludes a clinical translation. Consequentially, considering the potential of siRNA therapeutics for skin disorders, the interest in efficient and less invasive delivery methods is increasing.
Another strategy for gene silencing is antisense oligonucleotide (AON)-based approaches, which have been widely explored for the treatment of EB. A study from 2006 pioneered the design of AONs for EB treatment. It achieved a restoration of C7 function in 6% of EB patient cells and, to a low extent, in a xenografted rat model. However, no anchoring fibrils were found, which would have provided the ultimate proof for the treatment success [79]. Since then, several studies have verified the therapeutic benefit of AONs in EB treatment [80,81]. Here, Lipofectamine has been used for the transfection of primary skin cells ex vivo, yielding the restoration of type VII collagen expression in 6%–50% of the cells. Notably, in vivo administration either intradermally or intravenously resulted in significantly lower restoration rates (10%–14%). Overall, preclinical CRISPR data indicate its superiority over AONs.
Nevertheless, AONs have also been explored for the treatment of inflammatory skin diseases [82] and melanoma [83]. For the latter, AONs mainly aim to inhibit the expression of antiapoptotic proteins such as survivin and B-cell lymphoma/leukemia 2, while cytokine production is targeted for the treatment of inflammatory diseases. Physical methods have been harnessed to increase AONs' penetration after topical application. For example, iontophoresis was used to enhance the topical absorption of AON-loaded polyplexes for skin cancer treatment. Synergetic effects between the polyplexes and iontophoresis increased AONs' skin delivery threefold compared with free AONs yielding reduced tumor volumes of 45% versus 20% in mice [83]. This is an excellent example of how to combine different delivery approaches for effective gene therapy. Recently, Liu and coworkers [84] reported the development of an LNP-based AON formulation targeting the IL-17A receptor expression as a topical treatment for psoriasis. The topical application to a psoriasis-like mouse model and 3D skin models and normal human explants resulted in a 72%–75% reduced protein expression of the IL-17A receptor compared with the untreated control.
Overall, there are five approved AON therapies to date, but none of these are for skin-related diseases [85]. Nevertheless, an AON gel formulation targeting disease-relevant mutations of type VII collagen in EB patients is currently in clinical phase 2 (NCT03605069) (Table 2).
Concluding Remarks and Future Directions
A variety of gene therapies have evolved over the past decades with great capacity to treat all types of skin diseases. Patients suffering from rare and currently untreatable genodermatoses like EB will be the first to benefit from cutaneous gene therapy due to the high medical need, simplified approval procedures due to orphan designations, and advanced clinical developments. Further, the restoration of only 10% of the normal gene function is considered sufficient to alleviate the skin conditions significantly [86,87]. This seems achievable even in the light of current gene delivery challenges [1].
In general, gene therapies are administered either in vivo or ex vivo. For systemic diseases or diseases of the inner organs, intravenous applications are favorable. With skin as a target, this approach will likely not yield the intended results. The lack of vasculature in the viable epidermis and the tight epidermal–dermal junction zone prevent the delivery or penetration of biomacromolecules to the viable epidermis, which is the target for most skin diseases. Consequentially, enabling gene therapy for skin diseases requires either a treatment of cells outside of the human body (ex vivo approach) followed by a re-transplantation or a topical, in situ application which seems favorable as its easily accessible (Figure 2 , Key Figure). Nevertheless, the latter is especially challenging due to the barrier properties of human skin and the unfavorable properties of genetic cargo, such as high MW, negative charge, and biological instabilities. Further, the target cells, such as keratinocytes and skin stem cells, are considered ‘hard to transfect’ among primary cells (see Outstanding Questions).
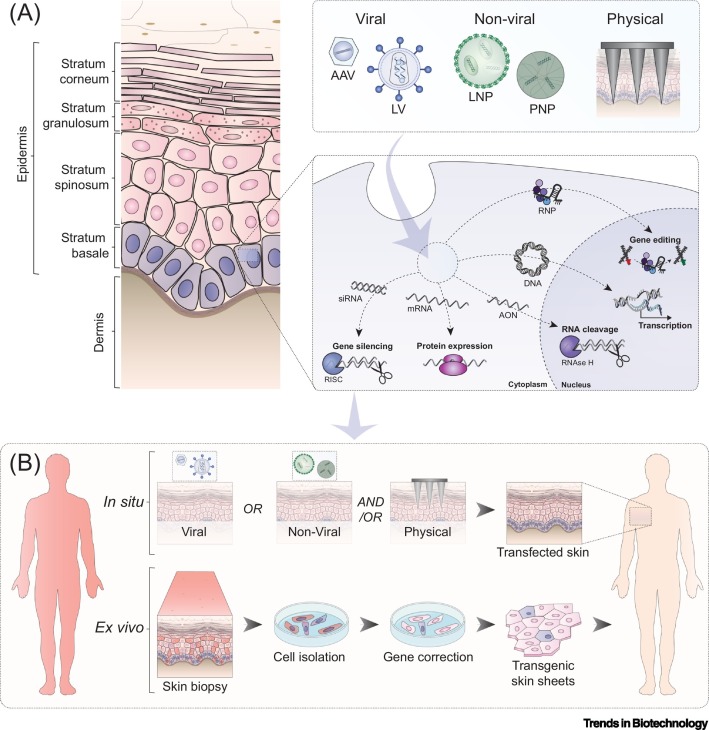
Figure 2.
Key Figure. Application of Gene Therapies for the Treatment of Skin Diseases
(A) Viral, non-viral, and physical methods can be employed to deliver genetic cargo efficiently into skin (stem) cells. (B) To enable gene therapy, the genetic cargo can be introduced ex vivo, after which transgenic skin sheets can be regrafted onto the human body, something which is currently already applied in the clinics. Another more elegant approach is in situ gene therapy which allows the topical application of gene therapies in vivo, but this still requires substantial research efforts. AAV, adeno-associated virus; AON, antisense oligonucleotide; LNP, lipid-based nanoparticle; LV, lentivirus; PNP, polymeric nanoparticle; RISC, RNA-induced silencing complex; RNP, ribonucleoprotein.
Outstanding Questions.
Which route of administration will yield the best results? It seems unlikely that a systemic application of gene therapeutics intended for the treatment of skin diseases may result in any therapeutic effect in the skin as reaching the target cells is hampered due to epidermal–dermal junction zone and the lack of blood vessels in the viable epidermis.
What about topical applications? Entering the viable layers of the human skin intact is the prerequisite for correcting or editing genes. However, it should be noted that the majority of topically applied delivery systems (viral and non-viral) are not able to overcome the stratum corneum, even in barrier-deficient skin.
Are physical methods such as iontophoresis or microneedles suitable to facilitate the topical delivery of delivery systems? A simultaneous application may prove beneficial. Alternatively, denudation of the upper skin layers may provide direct access to the viable epidermal layers and/or the skin stem cells.
What is the best strategy to enable the efficient and safe delivery of gene therapeutics to human skin? Viral delivery systems are still the gold standard but they have distinct limitations. Among non-viral delivery systems, lipid-based nanoparticles are the most advanced, but does this also hold true for gene delivery to the skin?
What transfection efficacy is required to achieve a cure for skin diseases? For genodermatoses, first studies indicate that the recovery of 10% of intrinsic gene function is sufficient to rescue the phenotype. Nevertheless, the effect will be limited to 28 days if keratinocytes are being targeted due to the continuous epidermal regeneration. For long-lasting effects, skin stem cells need to be targeted.
How can we target skin stem cells? Aiming for a curative approach, transfection of skin stem cells is inevitable. The knowledge about skin stem cells, however, is rather poor due to methodological and technical challenges.
Alt-text: Outstanding Questions
Today, viral delivery, especially AAV-mediated gene delivery, is the most commonly used approach. However, preliminary data now indicate that AAVs may pose a cancer risk as seen in a study in adult dogs with hemophilia A [88], which would have drastic consequences for this technology if these results are confirmed. The safety, capacity, and pre-existing immunity limitations of viral vectors propel the interest in non-viral delivery systems, which offer several advantages, such as tailorable sizes, a lack of genomic integration, low (or lower) immunogenicity, cost-effectiveness, and easier scale-up. Among them, LNPs are the most advanced systems that hold the biggest promise for future applications in skin. However, each delivery system faces the challenge of reaching the target cells intact, which is almost impossible considering the barrier properties of human skin. Many studies have demonstrated that the vast majority of topically applied nanoparticles do not overcome the SC, even in severely damaged skin [89,90]. Hence, when aiming for an in situ gene therapy for skin diseases, a combination with physical methods likely holds the greatest potential to enable an in vivo gene therapy (Figure 2).
Further, there is a general lack of suitable disease models, and most of the published preclinical work on gene delivery to the skin has been conducted in mice. Notably, mouse skin differs significantly from human skin. The difference in the sheer number of epidermal layers (1–3 in mice versus 15–20 in humans) [91] reflects how problematic the translation of findings in mice to the human situation is and how important it is to evaluate the efficiency of delivery systems in human-relevant settings.
Overall, to advance the field of cutaneous gene therapy, we first need a better understanding of how to deliver gene therapeutics safely and efficiently to the target cells. Head-to-head comparisons of clinically appropriate delivery strategies are required. Second, we need to increase the translational value of our preclinical work. This means incorporating the use of human-relevant models, such as pig skin, human skin explants, or tissue-engineered skin models, in early preclinical phases and, for in vivo studies, human-based tissue grafted onto mice. Overall, these are fundamental aspects that, once resolved, will ultimately increase the prospects for large-scale implementation.
To conclude, we have the tools for gene therapy; we now need to optimize their delivery to the target site in the skin.
Acknowledgments
Financial support of the Canadian Institute of Health Research (CIHR PJT-166035; for S.H., Q.U.A., and A.H.), the Faculty of Pharmaceutical Sciences of the University of British Columbia, and the NanoMedicines Innovation Network (NMIN; D.W. and S.H.) is greatly acknowledged. E.C. thanks the São Paulo Research Foundation (FAPESP #2017/24402-1 and #2019/05100-0). A.H. thanks the Lohn Foundation for her entry fellowship. Further, D.W. is supported by the Swiss National Science Foundation (#183923).
Glossary
- C7
type VII collagen which is frequently mutated in epidermolysis bullosa.
- Congenital ichthyosis
a group of rare genodermatoses; mild to fatal phenotypes possible; usually manifests as a severe keratinization disorder.
- CRISPR-Cas
clustered regularly interspaced short palindromic repeats and CRISPR-associated protein; efficient, fast, and simple gene-editing technology; second generation of gene-editing tools.
- Da
Dalton, unit of the molecular weight.
- Donor template
nucleic acid delivered into a cell to insert or change large sequences of DNA in the genomic target region. It provides a functional copy of a mutated gene.
- Epidermolysis bullosa (EB)
a heterogenic group of rare but severe genodermatoses.
- Glybera
the first-ever approved gene therapy to treat lipoprotein lipase deficiency, a rare inherited disorder which can cause severe pancreatitis.
- LAMB3 cDNA
a DNA copy of LAMB3 mRNA.
- Lipid-based nanoparticles
Colloidal drug carrier systems that consist of lipids.
- logP
a partition coefficient or distribution coefficient is the ratio of concentrations of a compound in a mixture of two immiscible solvents at equilibrium. Indicates the hydrophilicity or lipophilicity of a compound.
- Melanoma
highly malignant, often aggressive, metastatic, and drug-resistant type of skin cancer.
- miR
micro RNA; important role in post-transcriptional gene expression regulation.
- Monogenic
mutation in a single gene.
- Netherton syndrome
rare, monogenic skin disorder caused by damage in a gene called SPINK5, which leads to a significantly impaired skin barrier function.
- ONPATTRO
the first-ever siRNA therapeutic based on lipid nanoparticle technology for treating polyneuropathy in people with hereditary transthyretin-mediated amyloidosis.
- Orphan designations
special status to a drug or biological product (drug) to treat a rare disease or condition.
- PLGA
a biodegradable and non-toxic FDA-approved co-polymer of poly-lactic acid and poly-glycolic acid.
- pKa
indicates the strength of an acid. A lower pKa value indicates a stronger acid.
- Stratum corneum (SC)
outermost, nonviable layer of human skin; exerts the main barrier functions.
- TALEN
transcription activator-like effector nucleases; first generation of gene-editing tools.
- ZFNs
zinc-finger nucleases; first generation of gene-editing tools.
Resources
ihttps://www.amrytpharma.com/product-portfolio/ap103/References
- 1.Doudna J.A. The promise and challenge of therapeutic genome editing. Nature. 2020;578:229–236. doi: 10.1038/s41586-020-1978-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choate K.A., et al. Transglutaminase 1 delivery to lamellar ichthyosis keratinocytes. Hum. Gene Ther. 1996;7:2247–2253. doi: 10.1089/hum.1996.7.18-2247. [DOI] [PubMed] [Google Scholar]
- 3.Farboud B., et al. Enhanced genome editing with Cas9 ribonucleoprotein in diverse cells and organisms. J. Vis. Exp. 2018 doi: 10.3791/57350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilousova G. Gene therapy for skin fragility diseases: the new generation. J. Invest. Dermatol. 2019;139:1634–1637. doi: 10.1016/j.jid.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Laughter M.R., et al. The burden of skin and subcutaneous diseases in the United States from 1990 to 2017. JAMA Dermatol. 2020 doi: 10.1001/jamadermatol.2020.1573. Published online June 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pope E. Epidermolysis bullosa: a 2020 perspective. Br. J. Dermatol. 2020 doi: 10.1111/bjd.19125. Published online May 10, 2020. [DOI] [PubMed] [Google Scholar]
- 7.Oji V., et al. Revised nomenclature and classification of inherited ichthyoses: results of the First Ichthyosis Consensus Conference in Soreze 2009. J. Am. Acad. Dermatol. 2010;63:607–641. doi: 10.1016/j.jaad.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Arney K. Change the genes to fix the skin. Nature. 2018;564:S14–S15. doi: 10.1038/d41586-018-07640-2. [DOI] [PubMed] [Google Scholar]
- 9.Lundborg M., et al. Human skin barrier structure and function analyzed by cryo-EM and molecular dynamics simulation. J. Struct. Biol. 2018;203:149–161. doi: 10.1016/j.jsb.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Kabashima K., et al. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019;19:19–30. doi: 10.1038/s41577-018-0084-5. [DOI] [PubMed] [Google Scholar]
- 11.van Haasteren J., et al. The delivery challenge: fulfilling the promise of therapeutic genome editing. Nat. Biotechnol. 2020;38:845–855. doi: 10.1038/s41587-020-0565-5. [DOI] [PubMed] [Google Scholar]
- 12.Picanço-Castro V., et al. Emerging patent landscape for non-viral vectors used for gene therapy. Nat. Biotechnol. 2020;38:151–157. doi: 10.1038/s41587-019-0402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura T., et al. Production of adeno-associated virus vectors for in vitro and in vivo applications. Sci. Rep. 2019;9:13601. doi: 10.1038/s41598-019-49624-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valdmanis P.N., et al. rAAV-mediated tumorigenesis: still unresolved after an AAV assault. Mol. Ther. 2012;20:2014–2017. doi: 10.1038/mt.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palfi S., et al. Long-term follow-up of a phase I/II study of ProSavin, a lentiviral vector gene therapy for Parkinson’s disease. Hum. Gene Ther. Clin. Dev. 2018;29:148–155. doi: 10.1089/humc.2018.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y., et al. A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat. Biotechnol. 2016;34:334–338. doi: 10.1038/nbt.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anzalone A.V., et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576:149–157. doi: 10.1038/s41586-019-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koike-Yusa H., et al. Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat. Biotechnol. 2014;32:267–273. doi: 10.1038/nbt.2800. [DOI] [PubMed] [Google Scholar]
- 19.Kabadi A.M., et al. Multiplex CRISPR/Cas9-based genome engineering from a single lentiviral vector. Nucleic Acids Res. 2014;42:e147. doi: 10.1093/nar/gku749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlesworth C.T., et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat. Med. 2019;25:249–254. doi: 10.1038/s41591-018-0326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senior M. After Glybera’s withdrawal, what’s next for gene therapy? Nat. Biotechnol. 2017;35:491–492. doi: 10.1038/nbt0617-491. [DOI] [PubMed] [Google Scholar]
- 22.Fink T.L., et al. Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using compacted DNA nanoparticles. Gene Ther. 2006;13:1048–1051. doi: 10.1038/sj.gt.3302761. [DOI] [PubMed] [Google Scholar]
- 23.McKay P.F., et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-17409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akinc A., et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019;14:1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- 25.Buck J., et al. Lipid-based DNA therapeutics: hallmarks of non-viral gene delivery. ACS Nano. 2019;13:3754–3782. doi: 10.1021/acsnano.8b07858. [DOI] [PubMed] [Google Scholar]
- 26.Yu W.H., et al. Topical gene delivery to murine skin. J. Invest. Dermatol. 1999;112:370–375. doi: 10.1046/j.1523-1747.1999.00513.x. [DOI] [PubMed] [Google Scholar]
- 27.Blakney A.K., et al. The skin you are in: design-of-experiments optimization of lipid nanoparticle self-amplifying RNA formulations in human skin explants. ACS Nano. 2019;13:5920–5930. doi: 10.1021/acsnano.9b01774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayaraman M., et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Ed. Engl. 2012;51:8529–8533. doi: 10.1002/anie.201203263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semple S.C., et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni J.A., et al. Lipid nanoparticle technology for clinical translation of siRNA therapeutics. Acc. Chem. Res. 2019;52:2435–2444. doi: 10.1021/acs.accounts.9b00368. [DOI] [PubMed] [Google Scholar]
- 31.Zuris J.A., et al. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 2015;33:73–80. doi: 10.1038/nbt.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang M., et al. Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 2016;113:2868. doi: 10.1073/pnas.1520244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller J.B., et al. Non-viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co-delivery of Cas9 mRNA and sgRNA. Angew. Chem. Int. Ed. Engl. 2017;56:1059–1063. doi: 10.1002/anie.201610209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finn J.D., et al. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 2018;22:2227–2235. doi: 10.1016/j.celrep.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Huysmans H., et al. Expression kinetics and innate immune response after electroporation and LNP-mediated delivery of a self-amplifying mRNA in the skin. Mol. Ther. Nucleic Acids. 2019;17:867–878. doi: 10.1016/j.omtn.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blakney A.K., et al. Big is beautiful: enhanced saRNA delivery and immunogenicity by a higher molecular weight, bioreducible, cationic polymer. ACS Nano. 2020;14:5711–5727. doi: 10.1021/acsnano.0c00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malloggi C., et al. Comparative evaluation and optimization of off-the-shelf cationic polymers for gene delivery purposes. Polym. Chem. 2015;6:6325–6339. [Google Scholar]
- 38.Craciun B.F., et al. Synergistic effect of low molecular weight polyethylenimine and polyethylene glycol components in dynamic nonviral vector structure, toxicity, and transfection efficiency. Molecules. 2019;24 doi: 10.3390/molecules24081460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aznar M.A., et al. Immunotherapeutic effects of intratumoral nanoplexed poly I:C. J. Immunother. Cancer. 2019;7:116. doi: 10.1186/s40425-019-0568-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng L.H., et al. beta-Cyclodextrin-linked polyethylenimine nanoparticles facilitate gene transfer and enhance the angiogenic capacity of mesenchymal stem cells for wound repair and regeneration. J. Biomed. Nanotechnol. 2015;11:680–690. doi: 10.1166/jbn.2015.1970. [DOI] [PubMed] [Google Scholar]
- 41.Alshamsan A., et al. STAT3 silencing in dendritic cells by siRNA polyplexes encapsulated in PLGA nanoparticles for the modulation of anticancer immune response. Mol. Pharm. 2010;7:1643–1654. doi: 10.1021/mp100067u. [DOI] [PubMed] [Google Scholar]
- 42.Chen G., et al. Combining fluorination and bioreducibility for improved siRNA polyplex delivery. ACS Appl. Mater. Interfaces. 2017;9:4457–4466. doi: 10.1021/acsami.6b14184. [DOI] [PubMed] [Google Scholar]
- 43.Chen G., et al. Fluorination enhances serum stability of bioreducible poly(amido amine) polyplexes and enables efficient intravenous siRNA delivery. Adv. Healthc. Mater. 2018;7 doi: 10.1002/adhm.201700978. [DOI] [PubMed] [Google Scholar]
- 44.Liu L., et al. Tumor associated macrophage-targeted microRNA delivery with dual-responsive polypeptide nanovectors for anti-cancer therapy. Biomaterials. 2017;134:166–179. doi: 10.1016/j.biomaterials.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 45.Green J.J., et al. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc. Chem. Res. 2008;41:749–759. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green J.J., et al. Combinatorial modification of degradable polymers enables transfection of human cells comparable to adenovirus. Adv. Mater. 2007;19:2836–2842. [Google Scholar]
- 47.Zhou D., et al. The transition from linear to highly branched poly(beta-amino ester)s: branching matters for gene delivery. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng M., et al. Efficient and robust highly branched poly(beta-amino ester)/minicircle COL7A1 polymeric nanoparticles for gene delivery to recessive dystrophic epidermolysis bullosa keratinocytes. ACS Appl. Mater. Interfaces. 2019;11:30661–30672. doi: 10.1021/acsami.9b13135. [DOI] [PubMed] [Google Scholar]
- 49.Zhou D., et al. Highly branched poly(β-amino ester)s for skin gene therapy. J. Control. Release. 2016;244:336–346. doi: 10.1016/j.jconrel.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 50.Hainzl S., et al. COL7A1 editing via CRISPR/Cas9 in recessive dystrophic epidermolysis bullosa. Mol. Ther. 2017;25:2573–2584. doi: 10.1016/j.ymthe.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang M.Z., et al. Transdermal siRNA delivery by pH-switchable micelles with targeting effect suppress skin melanoma progression. J. Control. Release. 2020;322:95–107. doi: 10.1016/j.jconrel.2020.03.023. [DOI] [PubMed] [Google Scholar]
- 52.Lostalé-Seijo I., Montenegro J. Synthetic materials at the forefront of gene delivery. Nat. Rev. Chem. 2018;2:258–277. [Google Scholar]
- 53.Varga C.M., et al. Quantitative comparison of polyethylenimine formulations and adenoviral vectors in terms of intracellular gene delivery processes. Gene Ther. 2005;12:1023–1032. doi: 10.1038/sj.gt.3302495. [DOI] [PubMed] [Google Scholar]
- 54.Argus F., et al. Electroporation of tissue and cells: a three-equation model of drug delivery. Comput. Biol. Med. 2017;84:226–234. doi: 10.1016/j.compbiomed.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Lesueur L.L., et al. Overcoming the specific toxicity of large plasmids electrotransfer in primary cells in vitro. Mol. Ther. Nucleic Acids. 2016;5 doi: 10.1038/mtna.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pereira T.A., et al. Hydrogel increases localized transport regions and skin permeability during low frequency ultrasound treatment. Sci. Rep. 2017;7 doi: 10.1038/srep44236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Labala S., et al. Effective melanoma cancer suppression by iontophoretic co-delivery of STAT3 siRNA and imatinib using gold nanoparticles. Int. J. Pharm. 2017;525:407–417. doi: 10.1016/j.ijpharm.2017.03.087. [DOI] [PubMed] [Google Scholar]
- 58.Robertson J., et al. Circulation cooling in continuous skin sonoporation at constant coupling fluid temperatures. Ultrasound Med. Biol. 2020;46:137–148. doi: 10.1016/j.ultrasmedbio.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 59.Lifshiz Zimon R., et al. Ultrasound targeting of Q-starch/miR-197 complexes for topical treatment of psoriasis. J. Control. Release. 2018;284:103–111. doi: 10.1016/j.jconrel.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 60.Polat B.E., et al. A physical mechanism to explain the delivery of chemical penetration enhancers into skin during transdermal sonophoresis - insight into the observed synergism. J. Control. Release. 2012;158:250–260. doi: 10.1016/j.jconrel.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dul M., et al. Hydrodynamic gene delivery in human skin using a hollow microneedle device. J. Control. Release. 2017;265:120–131. doi: 10.1016/j.jconrel.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 62.Gualeni B., et al. Minimally invasive and targeted therapeutic cell delivery to the skin using microneedle devices. Br. J. Dermatol. 2018;178:731–739. doi: 10.1111/bjd.15923. [DOI] [PubMed] [Google Scholar]
- 63.Moreno E., et al. Skin vaccination using microneedles coated with a plasmid DNA cocktail encoding nucleosomal histones of Leishmania spp. Int. J. Pharm. 2017;533:236–244. doi: 10.1016/j.ijpharm.2017.09.055. [DOI] [PubMed] [Google Scholar]
- 64.Deng Y., et al. Transdermal delivery of siRNA through microneedle array. Sci. Rep. 2016;6:21422. doi: 10.1038/srep21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Witting M., et al. Feasibility study for intraepidermal delivery of proteins using a solid microneedle array. Int. J. Pharm. 2015;486:52–58. doi: 10.1016/j.ijpharm.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 66.Kim E., et al. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao X., et al. Formulation of hydrophobic peptides for skin delivery via coated microneedles. J. Control. Release. 2017;265:2–13. doi: 10.1016/j.jconrel.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 68.Pan J., et al. Intradermal delivery of STAT3 siRNA to treat melanoma via dissolving microneedles. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-19463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alliance for Regenerative Medicine . ARM Annual Report & Sector Year in Review – 2019. Alliance for Regenerative Medicine; 2019. Advancing gene, cell, and tissue-based therapies. [Google Scholar]
- 70.Marotte L., et al. Increased antitumor efficacy of PD-1-deficient melanoma-specific human lymphocytes. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2019-000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirsch T., et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551:327–332. doi: 10.1038/nature24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kocher T., et al. Predictable CRISPR/Cas9-mediated COL7A1 reframing for dystrophic epidermolysis bullosa. J. Invest. Dermatol. 2020 doi: 10.1016/j.jid.2020.02.012. Published online March 3, 2020. [DOI] [PubMed] [Google Scholar]
- 73.Bonafont J., et al. Clinically relevant correction of recessive dystrophic epidermolysis bullosa by dual sgRNA CRISPR/Cas9-mediated gene editing. Mol. Ther. 2019;27:986–998. doi: 10.1016/j.ymthe.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benati D., et al. CRISPR/Cas9-mediated in situ correction of LAMB3 gene in keratinocytes derived from a junctional epidermolysis bullosa patient. Mol. Ther. 2018;26:2592–2603. doi: 10.1016/j.ymthe.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Izmiryan A., et al. Ex vivo COL7A1 correction for recessive dystrophic epidermolysis bullosa using CRISPR/Cas9 and homology-directed repair. Mol. Ther. Nucleic Acids. 2018;12:554–567. doi: 10.1016/j.omtn.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jackow J., et al. CRISPR/Cas9-based targeted genome editing for correction of recessive dystrophic epidermolysis bullosa using iPS cells. Proc. Natl. Acad. Sci. U. S. A. 2019;116:26846–26852. doi: 10.1073/pnas.1907081116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu W., et al. Efficient in vivo gene editing using ribonucleoproteins in skin stem cells of recessive dystrophic epidermolysis bullosa mouse model. Proc. Natl. Acad. Sci. U. S. A. 2017;114:1660–1665. doi: 10.1073/pnas.1614775114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leachman S.A., et al. First-in-human mutation-targeted siRNA phase Ib trial of an inherited skin disorder. Mol. Ther. 2010;18:442–446. doi: 10.1038/mt.2009.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goto M., et al. Targeted skipping of a single exon harboring a premature termination codon mutation: implications and potential for gene correction therapy for selective dystrophic epidermolysis bullosa patients. J. Invest. Dermatol. 2006;126:2614–2620. doi: 10.1038/sj.jid.5700435. [DOI] [PubMed] [Google Scholar]
- 80.Bremer J., et al. Antisense oligonucleotide-mediated exon skipping as a systemic therapeutic approach for recessive dystrophic epidermolysis bullosa. Mol. Ther. Nucleic Acids. 2016;5 doi: 10.1038/mtna.2016.87. [DOI] [PubMed] [Google Scholar]
- 81.Turczynski S., et al. Targeted exon skipping restores type VII collagen expression and anchoring fibril formation in an in vivo RDEB model. J. Invest. Dermatol. 2016;136:2387–2395. doi: 10.1016/j.jid.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 82.Collard, J.D.B., FL, US), Khorkova Sherman, Olga (Tequesta, FL, US), Coito, Carlos (West Palm Beach, FL, US), Treatment of filaggrin (FLG) related diseases by modulation of flg expression and activity. 2019, CuRNA, Inc. (Miami, FL, US): United States.
- 83.Venuganti V.V., et al. Topical gene silencing by iontophoretic delivery of an antisense oligonucleotide-dendrimer nanocomplex: the proof of concept in a skin cancer mouse model. Nanoscale. 2015;7:3903–3914. doi: 10.1039/c4nr05241b. [DOI] [PubMed] [Google Scholar]
- 84.Liu H., et al. Targeting the IL-17 receptor using liposomal spherical nucleic acids as topical therapy for psoriasis. J. Invest. Dermatol. 2020;140:435–444. doi: 10.1016/j.jid.2019.06.146. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Levin A.A. Treating disease at the RNA level with oligonucleotides. N. Engl. J. Med. 2019;380:57–70. doi: 10.1056/NEJMra1705346. [DOI] [PubMed] [Google Scholar]
- 86.Aufenvenne K., et al. Transglutaminase-1 and bathing suit ichthyosis: molecular analysis of gene/environment interactions. J. Invest. Dermatol. 2009;129:2068–2071. doi: 10.1038/jid.2009.18. [DOI] [PubMed] [Google Scholar]
- 87.Fritsch A., et al. A hypomorphic mouse model of dystrophic epidermolysis bullosa reveals mechanisms of disease and response to fibroblast therapy. J. Clin. Invest. 2008;118:1669–1679. doi: 10.1172/JCI34292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaiser J. Virus used in gene therapies may pose cancer risk, dog study hints. Science. January 6, 2020 doi: 10.1126/science.aba7696. [DOI] [Google Scholar]
- 89.Hönzke S., et al. Tailored dendritic core-multishell nanocarriers for efficient dermal drug delivery: a systematic top-down approach from synthesis to preclinical testing. J. Control. Release. 2016;242:50–63. doi: 10.1016/j.jconrel.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 90.Giulbudagian M., et al. Breaking the barrier - potent anti-inflammatory activity following efficient topical delivery of etanercept using thermoresponsive nanogels. Theranostics. 2018;8:450–463. doi: 10.7150/thno.21668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gerber P.A., et al. The top skin-associated genes: a comparative analysis of human and mouse skin transcriptomes. Biol. Chem. 2014;395:577–591. doi: 10.1515/hsz-2013-0279. [DOI] [PubMed] [Google Scholar]
- 92.Lai W.F., Wong W.T. Design of polymeric gene carriers for effective intracellular delivery. Trends Biotechnol. 2018;36:713–728. doi: 10.1016/j.tibtech.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 93.Kulkarni J.A., et al. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale. 2019;11:21733–21739. doi: 10.1039/c9nr09347h. [DOI] [PubMed] [Google Scholar]