Abstract
Abstract
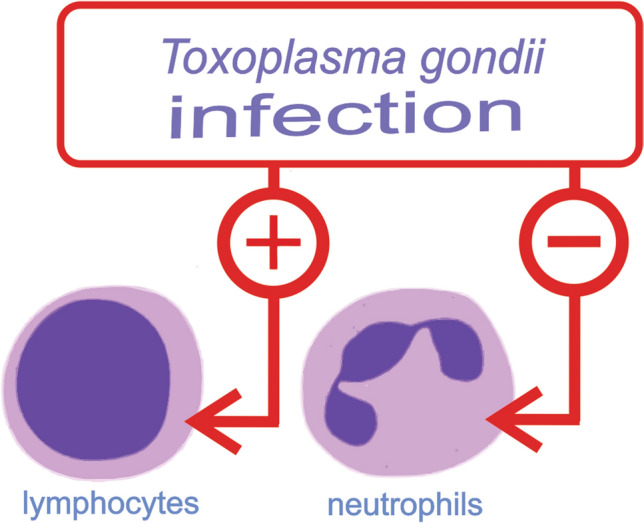
Toxoplasmosis is a zoonosis caused by Toxoplasma gondii, which can be acquired by oral contact and may cause severe health problems especially for pregnant (congenital toxoplasmosis) and immunocompromised patients. This study aimed to verify the diagnostic significance of hematological parameters and C-reactive protein (CRP) for toxoplasmosis acute detection. A case-control study was carried out between December 2017 and May 2018, in samples of convenience independent of age and sex. The case group was formed by 25 patients with positive anti-Toxoplasma gondii IgG/IgM antibody and the control group was formed by 21 patients with non-positive anti-Toxoplasma gondii IgG/IgM antibody. The results of the hematological parameters and CRP were analyzed in these patients. The patients with Toxoplasma gondii IgM antibody reagent showed higher lymphocytes counting and lower neutrophils counting than the control group. C-reactive protein levels were not different between the groups case and control. ROC curve analysis highlighted that the cut-off value of > 32.00% for lymphocytes and < 57.50% for neutrophils were able to produce specificity higher than 90% for IgM antibody detection. The Naïve Bayes classifier was considered suitable (AUC ≈ 0.700) to separate both groups according to their white cell counting. Changes in lymphocytes and neutrophils may be useful parameters for toxoplasmosis identification and may be used as a tool in the complementary diagnosis of toxoplasmosis.
Graphic abstract
Keywords: Toxoplasmosis, Complementary diagnosis, Hematological profile, IgG, IgM
Introduction
Toxoplasmosis is a zoonosis caused by Toxoplasma gondii, an intracellular parasite that is the unique specie able to produce the disease in all the hosts (Aguirre et al. 2019). Toxoplasmosis is acquired by oral contact with infecting forms eliminated in feline feces, who contaminate the soil and the water (Escotte-Binet et al. 2019). The ingestion of raw or undercook meat contaminated with parasite cysts is an important way of acquiring the disease (Paraboni et al. 2019; Sroka et al. 2020), while the congenital transmission is also extensively reported and represents a public health problem (de Melo et al. 2020). The prevalence of toxoplasmosis in the world varies from 10 to 80%, according to the geographical aspects and risk factors associated with social burdens. Moreover, it has been found a higher prevalence in Latin America and in tropical African countries (Robert-Gangneux and Dardé 2012). In many regions of Brazil toxoplasmosis outbakes have been reported in the last decade (Carmo et al. 2010; Nunes do Rego e Silva et al. 2019; Paraboni et al. 2019).
The T. gondii infection usually did not produce symptoms in healthy people; however, cervical lymphadenopathy or ocular disease can occur (Petersen and Liesenfeld 2007). In immunocompromised patients, T. gondii infection can produce severe clinical condition when cause pneumonia and disseminated disease, including death (Abbasi et al. 2020). In pregnant, the parasite can lead to spontaneous abortion, premature birth, fetal death or several sequels in the eyes and brain of the fetus, including hydrocephalus, chorioretinitis, intracerebral calcifications, mainly if the infection occurs in the first months of the gestation (Khan and Khan 2018).
Serological diagnosis plays a relevant role in the identification of T. gondii infections (Ybañez et al. 2020). The detection of IgM antibody anti-toxoplasmosis is the main analysis performed in the clinical routine for diagnostic of active recent infection, while the presence of IgG antibody anti-toxoplasmosis is related to a previously exposure and it is found in chronic cases (Pomares et al. 2017). Concomitantly with antibodies against T. gondii, other laboratory analyses may contribute to the accuracy of diagnostics. The presence of low avidity of IgG antibodies can be applied for the diagnosis of a recent toxoplasmosis infection (Rahbari et al. 2012). Also, molecular methods such as polymerase chain reaction may be useful for the detection of the infection by amplification of conserved sequences of parasite genes (Marín et al. 2018). Despite the availability of these laboratory analyses for toxoplasmosis diagnostic, the confirmation of ocular toxoplasmosis is usually clinical and when the clinical examination is not sufficient to confirm its presence, the laboratory diagnostic involves the analyze of ocular fluids (Greigert et al. 2019a; Rahimi-Esboei et al. 2018; Rahimi Esboei et al. 2019) using polymerase chain reaction and immunoblot techniques (Greigert et al. 2019b). Thus, there is an interest in finding new markers and predictors of disease useful for ocular toxoplasmosis diagnostics and monitoring, and some routine laboratory tests may be better explored for this purpose.
Simple techniques commonly available in most of the clinical laboratories may have critical importance in suggest the presence of infection by T. gondii, mainly when other methodologies were not available. In this context, the analysis of blood cells differential counting and C-reactive protein (CRP) are inserted. CRP is an essential component of the non-specific immune response, which is increased during infections and inflammation (Sproston and Ashworth 2018). Currently, new applications have been proposed for CRP measurements. Some recent investigations involving the use of CRP highlights the role of lymphocyte/CRP ratio for risk stratification in patients with intrahepatic cholangiocarcinoma (Lu et al. 2020); the use of CRP values for differentiation between severe malaria from uncomplicated malaria (Bhardwaj et al. 2019); and the use of CRP/albumin ratio in coronary artery disease detection (Tanriverdi et al. 2020). In the literature, there is a lack of investigations that explores the use of CRP and differential leucocytes counting for toxoplasmosis diagnostic. Considering these aspects, this work aimed to evaluate the significance of hematological parameters and CRP levels in the diagnostic of acute toxoplasmosis.
Materials and methods
A case-control study was performed in convenience samples obtained from patients attended at the clinical analysis laboratory of the Santa Terezinha Hospital Foundation of Erechim/RS (FHSTE), between December 2017 and May 2018. The samples were separated within two groups: the samples which showed serological tests positives to anti-T. gondii IgG/IgM antibody were enrolled in the case group. The control group was formed by samples with serological tests negatives (negative anti-T. gondii IgG/IgM antibody). All the measurements were performed by chemiluminescence methodology (Beckman Coulter®).
Hematological parameters were obtained in a XN 1000 Sysmex and the reference values were defined according to the age and sex of the patients. CRP levels were obtained from immunoturbidimetry technique in a Bioplus 2000 equipment (Biotecnica) with CRP turbilate diagnostic kit (Biotécnica®) and the analyses were performed according to the manufacturer’s protocol. The reference value was considered normal when below 6 mg/L. The study was approved by the URI-Erechim Ethical Committee under the number 38135014.8.0000.5351.
The data was analyzed using the t test or Mann-Whitney test considering as significant the difference when p < 0.05. The normality of the data was assessed by Shapiro–Wilk test. Receiver Operating Characteristic Curves (ROCs) were produced aiming to evaluate the efficacy of CRP or hematological parameters into differentiate the IgM reactive of non-reactive and Youden’s J statistic was used to define the optimized cut-off values. All analyses were performed in GraphPad Prism 6.0 software.
Furthermore, machine learning (ML) techniques were employed for the statistical classification of the data into two groups (IgM reactive and non-reactive) by using Orange (Demšar et al. 2013). Firstly, the absolute number of the hematological parameters were obtained and then normalized through mean centering and standard deviation scaling in order to avoid large differences among the individual values. The normalized data was checked for pairwise intercorrelation of the variables by using the Pearson’s correlation coefficient (r), which was considered suitable if it was lower than 0.80. Finally, the data was evaluated through the following supervised methods using default parameters: neural network, random forest, support vector machine (SVM), k-nearest neighbor (k-NN), naive Bayes and logistic regression (logreg). An internal validation method, leave-one-out (LOO) was employed to evaluate the robustness of the models. The data was evaluated according to the following metrics: Area Under the ROC-Curve (AUC), Recall (sensibility) and Precision.
Results
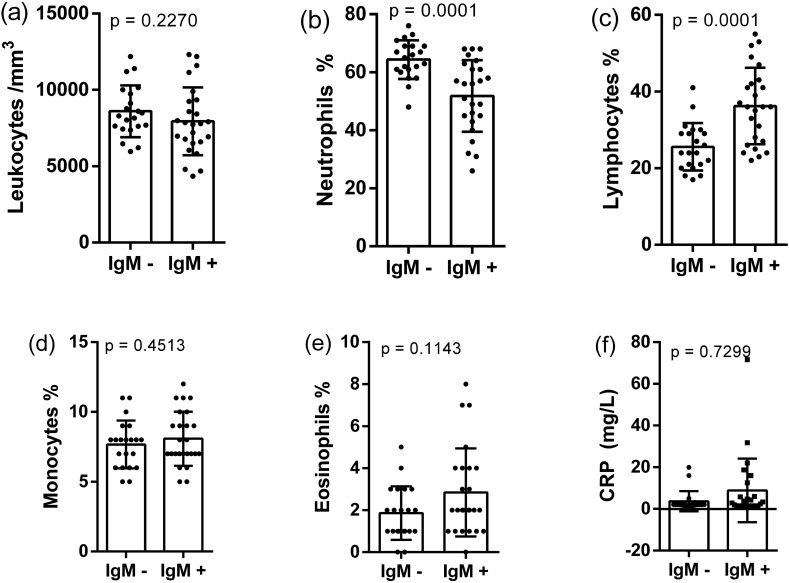
Twenty-five patients in the acute phase of toxoplasmosis were attended at the clinical laboratory of FHSTE between December 2017 and May 2018. The age of patients ranged from 13 to 49 years old (27 ± 9) and there were 6 men and 19 women. Twenty-one patients with 25 ± 6 years old formed the control group. The CRP concentration average in the case group was 8.84 ± 15.26 mg/L, and in the control group 3.66 ± 4.87 mg/L, thus was not found a difference between the two groups (p = 0.7299).
The hematological analysis showed a different profile in case and control groups. The total white cells counting was not different (p = 0.2270), otherwise the distribution of lymphocytes and neutrophils was intensively modified. The presence of acute infection by T. gondii promoted a significant increase in the lymphocytes percentage (36.20 ± 9.98%), when compared to the control group (25.57 ± 6.18%). On the other hand, the case group showed a lower neutrophil counting (51.84 ± 12.35%) than the control group (64.38 ± 6.65%). Considering other hematological parameters, the difference between the two groups was not found. Thus, the relative counting of monocytes, basophils and eosinophils were similar in the two groups (p > 0.05). These findings are represented in Fig. 1 and suggest strongly that the predominance of lymphocytes is associated with the presence of anti-T. gondii IgG/IgM antibodies.
Fig. 1.
Differences of white cells counting (a–e) and CRP (f) measurement in patients with IgM− and IgM+ . The values are represented as mean ± standard deviation
Another data collected from the hematological profile also did not allowed to differentiate the patients of two groups. A significant difference was not found between the groups for thrombocytes counting (p = 0.3087); erythrocytes counting (p = 0.0948), hemoglobin measurement (p = 0.3482) and hematocrit measurement (p = 0.3928). In the same way, the analyses of the hematimetric indices were not different among patients with IgG/IgM reagents.
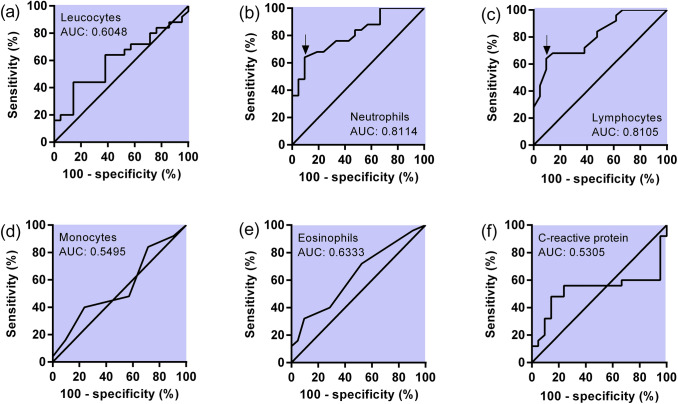
The analysis of ROC curves better explored the differences found in differential white blood cell count. The counting of lymphocytes and neutrophils were able to differentiate both groups (reactive IgG/IgM and non-reactive IgG/IgM) with good accuracy. The ROC curve for lymphocytes showed an area under the curve (AUC) value of 0.8105, whereas for neutrophils the AUC value was 0.8114. The counting of the other white cells showed AUC values slightly higher than 0.50; thus, these parameters demonstrated a smaller efficacy for groups’ differentiation. Figure 2a–e summarizes the ROC analyses considering white blood cell count. For CRP analyses, the ROC analysis yielded an AUC value of 0.5305 (Fig. 2f).
Fig. 2.
ROC analyses for white cells counting (a–e) and CRP (f). The arrow in figure (b) and (c) shows the cut-off value optimized by the Youden’s J statistic
A deeper analysis of ROC curves through Youden’s J statistic showed that for lymphocytes the optimized cut-off of > 32.00% yielded a sensibility of 64.06% and a specificity of 90.48%. According to the same analysis for neutrophils, the cut-off value < 57.50% produced a sensibility of 64.00%, and a specificity of 90.48%. Thus, the use of the information obtained from white cell counting may be a parameter able to improve the specificity for T. gondii detection with good specificity values. These values of specificity and sensibility are highlighted by the black arrow in Fig. 2b, c.
The correlation among relative and absolute variables derived from the hematological data was assessed through Pearson’s coefficient (Table 1). A critical point was related to the influence of lymphocytes and neutrophils relative values, which showed a r value of − 0.971, indicating a strong negative relation between the values. The use of absolute values changed the association between the data. The correlation between lymphocytes and neutrophils absolute numbers changed the r value to 0.106. Thus, the choice of using absolute values of the hematological parameters avoided the intercorrelation between the variables, which might overfit the classification.
Table 1.
Variable intercorrelation estimated through Pearson’s coefficient
| Lymphocytes | Neutrophils | Eosinophils | Monocytes | Basophils | |
|---|---|---|---|---|---|
| Relative values of hematological differential | |||||
| Lymphocytes | 1 | ||||
| Neutrophils | − 0.971 | 1 | |||
| Eosinophils | 0.435 | − 0.566 | 1 | ||
| Monocytes | 0.38 | − 0.522 | 0.314 | 1 | |
| Basophils | 0.257 | − 0.345 | 0.29 | 0.366 | 1 |
| Absolute values of hematological differential | |||||
| Lymphocytes | 1 | ||||
| Neutrophils | − 0.106 | 1 | |||
| Eosinophils | 0.439 | − 0.023 | 1 | ||
| Monocytes | 0.354 | 0.397 | 0.246 | 1 | |
| Basophils | 0.255 | − 0.169 | 0.327 | 0.208 | 1 |
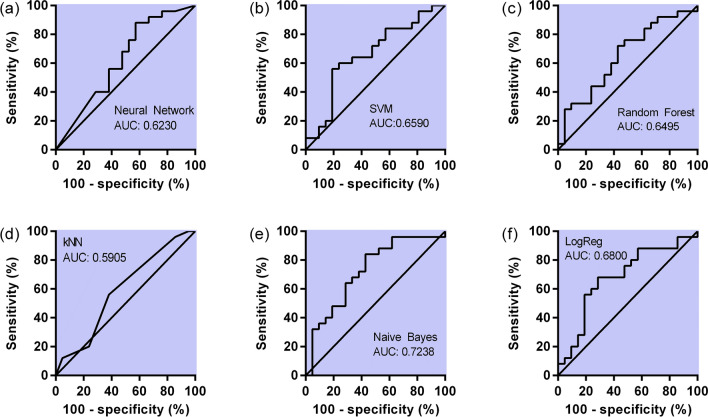
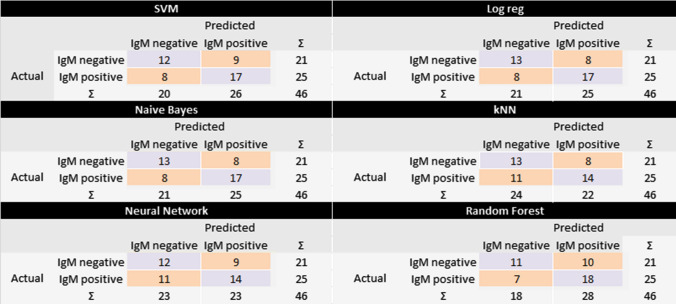
The machine learning techniques employed to classify the outcome into IgM reactive and non-reactive were first tested on the training data (Table 2). According to the table, the learners neural network and random forest had almost a perfect score (AUC ≈ 1.000) in classifying the data, which may be overestimated, whereas the other learners pointed scores between 0.849 and 0.798. In order to evaluate the robustness of the classification, an internal validation method (LOO) was used (Table 3). The validation pointed out that Naïve Bayes was the best scored technique maintaining metrics values closed to 0.700 (Fig. 3) and therefore it may be suitable a classifier, considering the observed confusion matrix (Fig. 4).
Table 2.
Classification metrics of the machine learning techniques into training set for toxoplasmosis
| Method | AUC | CA | F1 | Precision | Recall |
|---|---|---|---|---|---|
| Neural network | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Random forest | 0.996 | 0.935 | 0.935 | 0.935 | 0.935 |
| SVM | 0.798 | 0.761 | 0.761 | 0.768 | 0.761 |
| kNN | 0.798 | 0.761 | 0.758 | 0.763 | 0.761 |
| Naive Bayes | 0.849 | 0.739 | 0.758 | 0.763 | 0.761 |
| Logreg | 0.811 | 0.696 | 0.696 | 0.696 | 0.696 |
Table 3.
Classification metrics using the leave-one-out validation of the machine learning techniques into training set for toxoplasmosis
| Method | AUC | CA | F1 | Precision | Recall |
|---|---|---|---|---|---|
| Neural network | 0.623 | 0.609 | 0.606 | 0.606 | 0.609 |
| Random forest | 0.650 | 0.630 | 0.627 | 0.628 | 0.630 |
| SVM | 0.659 | 0.630 | 0.630 | 0.629 | 0.630 |
| kNN | 0.590 | 0.587 | 0.588 | 0.593 | 0.587 |
| Naive Bayes | 0.724 | 0.652 | 0.652 | 0.652 | 0.652 |
| Logreg | 0.680 | 0.652 | 0.652 | 0.652 | 0.652 |
Fig. 3.
ROC analyses for different classifiers (a–f) using all the white cell counting
Fig. 4.
Confusion matrix of the LOO validation procedures on different classifiers used to predict the toxoplasmosis IgM antibody
Discussion
Infection by T. gondii in immunocompetent hosts produces a complex innate and adaptive immune response, which enables long term parasite persistence (Sasai et al. 2018). The lymphocytes role in T. gondii infection is in restriction of parasite replication during the chronic phase. Amongst the adaptive immune subsets, CD8 T lymphocytes are the primary effector cells while CD4 T cells play an essential helper function to maintain long-term immunity (Khan et al. 2019). The action of lymphocytes in T. gondii infection starts with its activation by macrophages and dendritic cells, followed by induction of Th1 cells and antigen-specific killer CD8 T cells. These lymphocytes contributes for interferon-γ production, yielding cell-autonomous immunity (Sasai et al. 2018).
Despite the neutrophils counting reduction verified in our results, this group of cells shows significant activity in the immune response against T. gondii. Mice that were neutrophils depleted by a monoclonal antibody showed weaker type 1 immune response against T. gondii measured by decreased levels of interferon-γ, interleukin 12 and tumor necrosis factor α. As a result, the parasite lesions in tissues of mice with neutrophil depleted were significantly elevated (Bliss et al. 2001).
There is a lack of robust studies that examine the effect of infections by T. gondii on hematological parameters. In rats infected with T. gondii, a study reported an increase in lymphocyte counting after 10 days of infection. The lymphocyte counting in the control group was 2768 ± 995 cells/µL, while this parameter in the infected group was 4572 ± 748 cells/µL (Tonin et al. 2013). In an investigation performed in 37 patients with the acute phase of toxoplasmosis, four presented low hemoglobin levels (10.8%), six leukopenia (16.2%), one thrombocytopenia (2.7%) and fourteen lymphocytosis (37.8%), which was the most frequent hematological alteration found (Neves et al. 2009). According to these findings, we reported here the increase in lymphocytes counting as a noteworthy implication of toxoplasmosis in individuals with serological tests positives to T. gondii.
Pyrimethamine, a drug used for toxoplasmosis treatment, may produce a reduction in the neutrophils counting (Dardé et al. 2018; Dunay et al. 2018). This collateral effect could be the cause of changes in the white cells counting, however none of the study participants were under treatment with pyrimethamine in the moment of blood samples obtaining. Further investigations with more cases are needed to ensure the diagnostic values of changes in white cells counting.
The data analysis driven by ML is a quite new approach in medical care and complementary diagnosis (Heinrichs and Eickhoff 2020; Sidey-Gibbons and Sidey-Gibbons 2019; Watson et al. 2019); however, in the last years, several studies have reported the use of ML with image data or clinical specimens (blood, stool, urine) to help in diagnose cancer (Kourou et al. 2015; Podnar et al. 2019; Salod and Singh 2019; Wu et al. 2019), diabetes and cardiovascular disease (Dinh et al. 2019; Kavakiotis et al. 2017; Shameer et al. 2018) among other conditions (Ayling et al. 2019; Gunčar et al. 2018; Poostchi et al. 2018; Ullah et al. 2019). Considering the infective diseases, ML techniques were used to identify dengue (Hair et al. 2019), bacterial infections (Rawson et al. 2019) and, more recently, COVID-19 (Banerjee et al. 2020; Brinati et al. 2020). These studies employed mostly Random Forest, Logistic Regression, Naïve Bayes and SVM as learners and included basic blood sample data in their analysis (hematologic data, CRP, alanine and aspartate aminotransferases, among others). Regarding the analysis by ML techniques employing hematological parameters for the clinical investigation of toxoplasmosis, this is the first study to be reported to the best of our knowledge. The Naïve Bayes classifier showed performance metrics values of 0.724 in the AUC and 0.652 for the other metrics at the leave-one-out validation test. In order to evaluate the method’s capacity to classify the data correctly, more patients should be included in the dataset.
Conclusions
The lymphocytes counting was higher in patients with acute toxoplasmosis, and neutrophils counting was lower when compared to the control group. These findings were supported by the analysis of ROC curves, in which AUC values were near of 0.80 for these parameters. Furthermore, the machine learning technique Naïve Bayes was considered a good classifier when analyzing all the white cell population (AUC near to 0.700). Changes in the white cells population were proposed as an additional parameter for clinical investigation in toxoplasmosis diagnostic. The data reported here support that white cells profile may be a useful parameter for be considered in toxoplasmosis diagnostic.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animals participants
All applicable international and institutional guidelines for the use of patient’s data were followed. The study protocol was approved by the ethical committee of the institution (committee of URI-Erechim under the number 2.397.014 and CAAE: 38135014.8.0000.5351). This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbasi FS, et al. Fulminant and diffuse cerebral toxoplasmosis as the first manifestation of HIV infection: a case presentation and review of the literature. Am J Case Rep. 2020;21:e919624. doi: 10.12659/AJCR.919624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre AA, et al. The one health approach to toxoplasmosis: epidemiology, control, and prevention strategies. EcoHealth. 2019;16(2):378–390. doi: 10.1007/s10393-019-01405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayling RM, Lewis SJ, Cotter F. Potential roles of artificial intelligence learning and faecal immunochemical testing for prioritisation of colonoscopy in anaemia. Br J Haematol. 2019;185:311–316. doi: 10.1111/bjh.15776. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Ray S, Vorselaars B, Kitson J, Mamalakis M, Weeks S, Mackenzie LS. Use of machine learning and artificial intelligence to predict SARS-CoV-2 infection from full blood counts in a population. Int Immunopharmacol. 2020;86:106705. doi: 10.1016/j.intimp.2020.106705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj N, Ahmed MZ, Sharma S, Nayak A, Anvikar AR, Pande V. C-reactive protein as a prognostic marker of Plasmodium falciparum malaria severity. J Vector Borne Dis. 2019;56(2):122–126. doi: 10.4103/0972-9062.263727. [DOI] [PubMed] [Google Scholar]
- Bliss SK, Gavrilescu LC, Alcaraz A, Denkers EY. Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect Immun. 2001;69:4898–4905. doi: 10.1128/IAI.69.8.4898-4905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinati D, Campagner A, Ferrari D, Locatelli M, Banfi G, Cabitza F. Detection of COVID-19 infection from routine blood exams with machine learning: a feasibility study. J Med Syst. 2020;44:135. doi: 10.1007/s10916-020-01597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo EL, Póvoa MM, Monteiro NS, Marinho RR, Nascimento JM, Freitas SN, Bichara CNC. Human toxoplasmosis outbreak in the Monte Dourado District, Almeirim municipality, Pará, Brazil. Rev Pan-Amaz Saude. 2010;1:61–66. doi: 10.5123/S2176-62232010000100009. [DOI] [Google Scholar]
- Dardé M-L, FougEre É, Buxeraud J. Les médicaments de la toxoplasmose. Actual Pharm. 2018;57:22–26. doi: 10.1016/j.actpha.2018.09.024. [DOI] [Google Scholar]
- de Melo RPB, et al. Description of an atypical Toxoplasma gondii isolate from a case of congenital toxoplasmosis in northeastern Brazil. Parasitol Res. 2020;119(8):2727–2731. doi: 10.1007/s00436-020-06746-9. [DOI] [PubMed] [Google Scholar]
- Demšar J, et al. Orange: data mining toolbox in Python. J Mach Learn Res. 2013;14:2349–2353. [Google Scholar]
- Dinh A, Miertschin S, Young A, Mohanty SD. A data-driven approach to predicting diabetes and cardiovascular disease with machine learning. BMC Med Inform Decis Mak. 2019;19:211. doi: 10.1186/s12911-019-0918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunay IR, Gajurel K, Dhakal R, Liesenfeld O, Montoya JG. Treatment of toxoplasmosis: historical perspective, animal models, and current clinical practice. Clin Microbiol Rev. 2018;31(4):e00057-17. doi: 10.1128/CMR.00057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escotte-Binet S, et al. A rapid and sensitive method to detect Toxoplasma gondii oocysts in soil samples. Vet Parasitol. 2019;274:108904. doi: 10.1016/j.vetpar.2019.07.012. [DOI] [PubMed] [Google Scholar]
- Greigert V, Di Foggia E, Filisetti D, Villard O, Pfaff AW, Sauer A, Candolfi E. When biology supports clinical diagnosis: review of techniques to diagnose ocular toxoplasmosis. Br J Ophthalmol. 2019;103:1008–1012. doi: 10.1136/bjophthalmol-2019-313884. [DOI] [PubMed] [Google Scholar]
- Greigert V, Pfaff AW, Sauer A, Filisetti D, Candolfi E, Villard O. Biological diagnosis of ocular toxoplasmosis: a nine-year retrospective observational study. mSphere. 2019;4:e00636-19. doi: 10.1128/mSphere.00636-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunčar G, Kukar M, Notar M, Brvar M, Černelč P, Notar M, Notar M. An application of machine learning to haematological diagnosis. Sci Rep. 2018;8:411. doi: 10.1038/s41598-017-18564-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair GM, Nobre FF, Brasil P. Characterization of clinical patterns of dengue patients using an unsupervised machine learning approach. BMC Infect Dis. 2019;19:1–11. doi: 10.1186/s12879-019-4282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs B, Eickhoff SB. Your evidence? Machine learning algorithms for medical diagnosis and prediction. Hum Brain Mapp. 2020;41:1435–1444. doi: 10.1002/hbm.24886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavakiotis I, Tsave O, Salifoglou A, Maglaveras N, Vlahavas I, Chouvarda I. Machine learning and data mining methods in diabetes research. Comput Struct Biotechnol J. 2017;15:104–116. doi: 10.1016/j.csbj.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W, Khan K. Congenital toxoplasmosis: an overview of the neurological and ocular manifestations. Parasitol Int. 2018;67(6):715–721. doi: 10.1016/j.parint.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Khan IA, Hwang S, Moretto M. Toxoplasma gondii: CD8 T Cells Cry for CD4 help. Front Cell Infect Microbiol. 2019;9:1–8. doi: 10.3389/fcimb.2019.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI. Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J. 2015;13:8–17. doi: 10.1016/j.csbj.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L-H, et al. Lymphocyte-C-reactive protein ratio as a novel prognostic index in intrahepatic cholangiocarcinoma: a multicentre cohort study. Liver Int. 2020 doi: 10.1111/liv.14567. [DOI] [PubMed] [Google Scholar]
- Marín JEG, Zuluaga JD, Campo EJP, Triviño J, de-la-Torre A. Polymerase chain reaction (PCR) in ocular and ganglionar toxoplasmosis and the effect of therapeutics for prevention of ocular involvement in South American setting. Acta Trop. 2018;184:83–87. doi: 10.1016/j.actatropica.2018.01.013. [DOI] [PubMed] [Google Scholar]
- Neves E, et al. Acute acquired toxoplasmosis: clinical-laboratorial aspects and ophthalmologic evaluation in a cohort of immunocompetent patients. Mem Inst Oswaldo Cruz. 2009;104(2):393–396. doi: 10.1590/S0074-02762009000200039. [DOI] [PubMed] [Google Scholar]
- Nunes do Rego e Silva G, et al. Toxoplasmosis outbreak in Brazil, 2006. Parasite Epidemiol Control. 2019;7:e00117. doi: 10.1016/j.parepi.2019.e00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraboni MLR, Costa DF, Silveira C, Gava R, Pereira-Chioccola VL, Belfort R, Commodaro AG. A new strain of Toxoplasma gondii circulating in southern Brazil. J Parasit Dis. 2019;44:248–252. doi: 10.1007/s12639-019-01155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E, Liesenfeld O. Clinical disease and diagnostics. In: Weiss LM, Kim K, editors. Toxoplasma Gondii. London: Academic Press; 2007. pp. 81–100. [Google Scholar]
- Podnar S, Kukar M, Gunčar G, Notar M, Gošnjak N, Notar M. Diagnosing brain tumours by routine blood tests using machine learning. Sci Rep. 2019;9:1448. doi: 10.1038/s41598-019-51147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomares C, et al. Validation of IgG, IgM multiplex plasmonic gold platform in French clinical cohorts for the serodiagnosis and follow-up of Toxoplasma gondii infection. Diagn Micr Infect Dis. 2017;87:213–218. doi: 10.1016/j.diagmicrobio.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Poostchi M, Silamut K, Maude RJ, Jaeger S, Thoma G. Image analysis and machine learning for detecting malaria. Transl Res. 2018;194:36–55. doi: 10.1016/j.trsl.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbari AH, Keshavarz H, Shojaee S, Mohebali M, Rezaeian M. IgG avidity ELISA test for diagnosis of acute toxoplasmosis in humans. Korean J Parasitol. 2012;50:99–102. doi: 10.3347/kjp.2012.50.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi-Esboei B, Zarei M, Mohebali M, Valian HK, Shojaee S, Mahmoudzadeh R, Salabati M. Serologic tests of IgG and IgM antibodies and IgG avidity for diagnosis of ocular toxoplasmosis. Korean J Parasitol. 2018;56(2):147–152. doi: 10.3347/kjp.2018.56.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi Esboei B, et al. Evaluation of RE and B1 genes as targets for detection of Toxoplasma gondii by nested PCR in blood samples of patients with ocular toxoplasmosis. Acta Parasitol. 2019;64:384–389. doi: 10.2478/s11686-019-00056-6. [DOI] [PubMed] [Google Scholar]
- Rawson T, et al. Supervised machine learning for the prediction of infection on admission to hospital: a prospective observational cohort study. J Antimicrob Chemother. 2019;74:1108–1115. doi: 10.1093/jac/dky514. [DOI] [PubMed] [Google Scholar]
- Robert-Gangneux F, Dardé M-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25(2):264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salod Z, Singh Y. Comparison of the performance of machine learning algorithms in breast cancer screening and detection: a protocol. J Public Health Res. 2019;8(3):1677. doi: 10.4081/jphr.2019.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai M, Pradipta A, Yamamoto M. Host immune responses to Toxoplasma gondii. Int Immunol. 2018;30(3):113–119. doi: 10.1093/intimm/dxy004. [DOI] [PubMed] [Google Scholar]
- Shameer K, Johnson KW, Glicksberg BS, Dudley JT, Sengupta PP. Machine learning in cardiovascular medicine: are we there yet? Heart (Br Card Soc) 2018;104(14):1156–1164. doi: 10.1136/heartjnl-2017-311198. [DOI] [PubMed] [Google Scholar]
- Sidey-Gibbons JA, Sidey-Gibbons C. Machine learning in medicine: a practical introduction. BMC Med Res Methodol. 2019;19:1–18. doi: 10.1136/heartjnl-2017-311198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroka J, et al. Toxoplasma gondii infection in slaughtered pigs and cattle in Poland: seroprevalence, molecular detection and characterization of parasites in meat. Parasit Vectors. 2020;13:223. doi: 10.1186/s13071-020-04106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanriverdi Z, Gungoren F, Tascanov MB, Besli F, Altiparmak IH. Comparing the diagnostic value of the C-reactive protein to albumin ratio with other inflammatory markers in patients with stable angina pectoris. Angiology. 2020;71:360–365. doi: 10.1177/0003319719897490. [DOI] [PubMed] [Google Scholar]
- Tonin AA, et al. Influence of Toxoplasma gondii acute infection on cholinesterase activities of Wistar rats. Korean J Parasitol. 2013;51(4):421–426. doi: 10.3347/kjp.2013.51.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah R, Khan S, Ali H, Chaudhary II, Bilal M, Ahmad I. A comparative study of machine learning classifiers for risk prediction of asthma disease Photodiagnosis. Photodiagnosis Photodyn Ther. 2019;28:292–296. doi: 10.1016/j.pdpdt.2019.10.011. [DOI] [PubMed] [Google Scholar]
- Watson DS, Krutzinna J, Bruce IN, Griffiths CE, McInnes IB, Barnes MR, Floridi L. Clinical applications of machine learning algorithms: beyond the black box. Br Med J. 2019;364:l886. doi: 10.1136/bmj.l886%JBMJ. [DOI] [PubMed] [Google Scholar]
- Wu J, et al. A machine learning method for identifying lung cancer based on routine blood indices: qualitative feasibility study. JMIR Med Inform. 2019;7(3):e13476. doi: 10.2196/13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybañez RHD, Ybañez AP, Nishikawa Y. Review on the current trends of toxoplasmosis serodiagnosis in humans. Front Cell Infect Microbiol. 2020;8:5. doi: 10.3389/fcimb.2020.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]