Abstract
Objectives
Alzheimer’s disease (AD), impaired fasting glucose (IFG), and Type 2 diabetes mellitus (T2DM) were reported associated with smaller brain volumes. Nevertheless, the association of hyperglycemia with brain volume changes in AD patients remains unclear. To investigate this issue, structural magnetic resonance imaging was used to compare brain volumes among AD patients with different fasting glucose levels.
Methods
Eighty-five AD patients were divided into three groups based on their fasting glucose level as suggested by the American Diabetes Association: normal fasting glucose group (AD_NFG, n = 45), AD_IFG group (n = 15), and AD_T2DM group (n = 25). Sagittal 3D T1-weighted images were obtained to calculate the brain volume. Brain parenchyma and 33 brain structures were automatically segmented. Each regional volume was analyzed among groups. For regions with statistical significance, partial correlation analysis was used to evaluate their relationships with fasting glucose level, corrected for Mini-Mental State Examination score, age, education level, cholesterol, triglyceride, and blood pressure.
Results
Compared with the AD_IFG and AD_NFG groups, the volume of pons in AD_T2DM group was significantly smaller. Fasting glucose was negatively correlated with pontine volume.
Conclusions
T2DM may exacerbate pontine atrophy in AD patients, and fasting glucose level is associated with pontine volume.
Keywords: Alzheimer’s disease, Impaired fasting glucose, Type 2 diabetes mellitus, Brain volume, Structural MRI, Brain atrophy, Pons, Fasting glucose level, Hyperglycemia
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder reportedly caused by the abnormal deposition of amyloid β and tau protein. AD is the main cause of dementia (Lane, Hardy & Schott, 2018). Type 2 diabetes mellitus (T2DM) is a phenotype of glucose metabolic disorder caused by insulin resistance (Handelsman et al., 2015). In the past, T2DM was considered as a systemic disease that induces complications in multiple peripheral organs, such as diabetic nephropathy and diabetic retinopathy. Recent studies have indicated that T2DM is closely related to the central nervous system impairment (Degen et al., 2016). AD and T2DM are both chronic non-communicable diseases that are highly prevalent and commonly diagnosed (Lane, Hardy & Schott, 2018; Ogurtsova et al., 2017).
Several previous studies have reported close connections between these two diseases (Greene et al., 2015; Lin, 2008; Rani et al., 2016). T2DM has been strongly associated with an increased risk of developing all types of dementia, including AD (Kadohara, Sato & Kawakami, 2017). Compared with healthy individuals, the incidence of AD in diabetic population is significantly higher (Zhang et al., 2017). Furthermore, prediabetes is an intermediate state between normoglycemia and diabetes, and is also considered to be a risk factor for AD development (Biessels et al., 2014; Roberts et al., 2014). Insulin resistance is a key metabolic disturbance in prediabetes and T2DM. Its features of impaired insulin signaling and inflammation were also observed in AD patients, along with lower levels of insulin in cerebrospinal fluid and reduced activity of brain insulin-receptor (De Felice, 2013; Greene et al., 2015; Lin, 2008; Rani et al., 2016). It is reported that T2DM can promote higher level of β-amyloid protein (plaques) and hyperphosphorylation of Tau protein in cerebrospinal fluid, which resembles the pathological process of AD (Moran et al., 2015). Antidiabetic treatment can ameliorate the cognitive impairment of AD patients and improve their learning and executive processing function (Cai et al., 2018; Infante-Garcia et al., 2018). All those evidence indicate that hyperglycemia affects AD in a variety of forms.
Hippocampus and temporal lobe undergo atrophy in AD patients (Bernard et al., 2014; Gili et al., 2011). Moreover, many studies have indicated that T2DM patients also have brain atrophy (Li et al., 2016). However, the regions involved in different studies seem to be inconsistent and different from the typical AD biomarker-medial temporal atrophy (Bernard et al., 2014; Gili et al., 2011; Guo et al., 2014). In patients with T2DM or impaired fasting glucose (IFG), the volume of the brain was observed to be smaller, including the whole brain, cingulated cortex and temporal gyrus (Hou et al., 2016; Li et al., 2016). McIntyre et al. (2010) observed that individuals with T2DM exhibit volumetric abnormalities in both cortical and subcortical structures. The effect of prediabetes on brain volume is controversial. Some studies showed that prediabetes is associated with cognitive decline and lower brain gray matter volume (Markus et al., 2017), and even a higher plasma glucose level within the normal range is harmful to the brain (Cherbuin, Sachdev & Anstey, 2012). However, Schneider et al. (2017) found that the brain volume in the prediabetes population did not decrease significantly. In short, AD, IFG, and T2DM are all considered to be related to reduced brain volumes. Nevertheless, the association between hyperglycemia and brain volume changes in AD patients remains unclear. To the best of our knowledge, no studies have focused on the combined effects of AD and hyperglycemia (prediabetes or T2DM) on brain volume. In this study, to evaluate the associations between hyperglycemia and brain volume changes in AD patients, structural MRI was used to compare the brain volume among AD patients with different fasting glucose levels.
Material and Methods
Participants
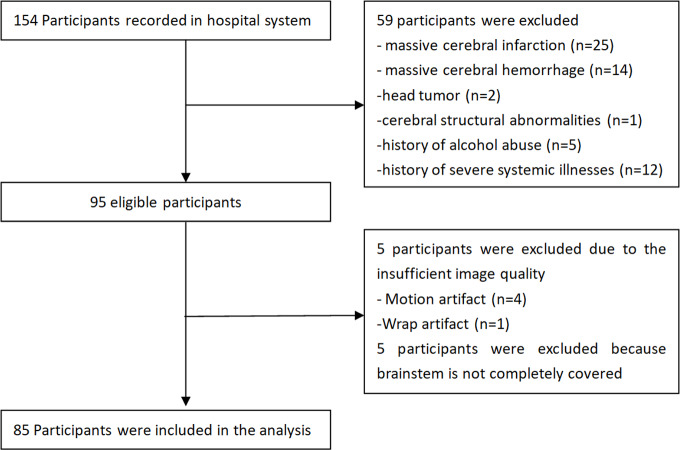
AD patients were included in the study. The diagnosis of AD was confirmed according to the criteria of the National Institute of Neurological Disorders and Stroke - Alzheimer Disease and Related Disorders (Dubois et al., 2007) by neurologists from neurology department. Core diagnostic criteria were met and evidence of medial temporal lobe atrophy was acquired by MRI. Exclusion criteria were as follows: (1) history and clinical features of non - AD dementia; (2) severe cerebrovascular diseases, such as massive cerebral infarction or hemorrhage; head trauma or tumor; (3) cerebral structural abnormalities; (4) history of drug addiction, alcohol abuse, toxic or metabolic abnormalities; (5) histories of psychiatric diseases, such as schizophrenia or depression; (6) history of epilepsy or severe systemic illnesses; and (7) infectious diseases of central nervous system.
Fifty-nine cases were excluded due to the exclusion criteria, five were excluded due to insufficient image quality, and five were excluded due to incomplete coverage of the brainstem during the magnetic resonance image (MRI) scan. Finally, 85 AD patients from outpatient and inpatient wards were included in the study (Fig. 1). These AD patients were divided into three groups based on the fasting glucose (FG) levels, as suggested by the American Diabetes Association (Association American Diabetes, 2010): (1) normal fasting glucose (NFG) group, FG levels <5.6 mmol/L (100 mg/dL); (2) impaired fasting glucose (IFG) group, FG levels of 5.6–6.9 mmol/L (100–125 mg/dL); and (3) T2DM group, FG levels ≥ 7.0 mmol/ L (126 mg/dL).
Figure 1. Flowchart of study participants.
FG, serum total cholesterol, triglyceride, and blood pressure were obtained within 1 week prior to or posterior to the MRI examination. Cognitive performance was assessed using the Mini-Mental State Examination (MMSE). The study was conducted in accordance with the principles of the Declaration of Helsinki. The First Affiliated Hospital of Dalian Medical University has granted ethical approval to carry out the study within its facilities (Ethical Application Ref: YJ-KY-FB-2020-08). Informed consent for inclusion was waived due to the retrospective nature of the study.
MRI data acquisition and analysis
MRI data acquisition was performed on a 3.0 T scanner (GE Signa HDxt) using axial T1WI, axial T2WI, axial T2 FLAIR, and sagittal three-dimensional (3D) T1-weighted gradient-recalled echo sequences. The parameters used for each sequence were as follows: (1) Sagittal 3D T1-weighted images: repetition time (TR) = 10.2 ms, echo time (TE) = 4.2 ms, inversion time (TI) = 450 ms, flip angle (FA) = 12°, FOV = 256 mm × 256 mm, Matrix = 256 × 256, thickness = 1.0 mm, gap = 0 mm, voxel size = 1.0 mm × 1.0 mm × 1.0 mm, totally 188 sagittal slices; (2) Axial T1WI: slice thickness = 6 mm, slice gap = 1 mm, TR = 2250 ms, TE = 24 ms, FOV = 240 mm × 240 mm, Matrix = 320 × 256, NEX = 1, Phase FOV = 0.9; (3) Axial T2WI: slice thickness = 6 mm, slice gap = 1 mm, TR = 5,000 ms, TE = mini, FOV = 240 mm × 240 mm, Matrix = 256 × 256, NEX = 2, Phase FOV = 0.8; (4) Axial T2 FLAIR: slice thickness = 6 mm, slice gap = 1 mm, TR = 9,000 ms, TE = 168 ms, FOV = 24 cm × 24 cm, Matrix = 256 × 192, NEX = 1.
Automatic segmentation and quantification of regional volumes and atrophy were performed using AccuBrain® (BrainNow Research Institute, Shenzhen, Guangdong Province, China) (Abrigo et al., 2018; Yishan, 2017). Regions-of-interest-based segmentation methodology was adopted in AccuBrain®, which allows volume quantification of various anatomically defined brain structures; hence it could provide different types of information and could be more direct and intuitive when quantifying group differences. Multi-atlas-based segmentation method is used to automatically segment individual brain MRI. Preprocessing techniques including noise reduction, bias field correction, and intensity normalization were performed for image quality improvement. Technical aspects of brain segmentation and atrophy evaluation used have been previously described (Abrigo et al., 2018). In summary, brain parenchyma (i.e., gray matter and white matter tissue) and ventricular system were automatically segmented from 3D T1-weighted images by incorporating experienced radiologists’ prior knowledge, in which anatomical information can be transformed and applied to individual brain automatically. Based on the segmentation results, absolute volumes of 33 brain structures were computed. To correct for inter-subject head size variability in the analysis, relative volumes were calculated as the percentage of absolute volume by the total intracranial volume (ICV). Atrophy of cerebral lobes was computed as the ratio of cerebrospinal fluid volume to brain parenchymal volume (i.e., sum of white matter and gray matter volume) in the corresponding region (Yishan, 2017).
White matter hyperintensities (WMH) were automatically segmented from T2 FLAIR and T1-weighted images using an in-house developed pipeline previously published, which is a coarse-to-fine mathematical morphology method based on binary dilation, grayscale closing, binary reconstruction and grayscale reconstruction (Shi et al., 2013).
Statistical analysis
Gender comparison among groups was performed using χ2 test. For other demographics, including physiological indices (serum total cholesterol, triglyceride, and blood pressure) and the cognitive scores, normally distributed variables were compared among three groups using one-way analysis of variance (ANOVA), while non-normally distributed variables were compared using non-parametric test. For regional volumetric measures, one-way ANOVA was used to assess the difference among three groups. Then the p values were corrected by applying the false discovery rate (FDR) correction. Further comparisons were performed using Tukey’s post hoc test to identify regions which two groups showed significant differences. One-way ANOVA was also used to assess WMH differences among the three groups. For regions with statistical significance, partial correlation analysis was used to evaluate their relationships with fasting glucose, controlling for age, education level, MMSE scores, cholesterol, triglyceride, and blood pressure. All the analyses were two-tailed, and p values < 0.05 were considered statistically significant.
Results
Thirty-one male and 54 female AD patients were included with a mean age of 72.718 ± 7.867 years. According to the group division criteria, 45 patients were classified as AD_NFG group, 15 patients as AD_IFG group, and 25 patients as AD_T2DM group. The demographic and physiological indices (fasting glucose, serum total cholesterol, triglyceride, and blood pressure) and cognitive scores of the participants are presented in Table 1. No significant difference in age, gender, education level, and cognitive score were found among all three groups. Except for the fasting glucose level, no statistical difference was found in other physiological indices. All these ensure the comparability among the three groups.
Table 1. Demographics data of the study sample in three groups.
| AD_NFG | AD_IFG | AD_T2DM | p-value | |
|---|---|---|---|---|
| Age, y | 71.844(8.383) | 74.267(9.316) | 73.360(5.816) | 0.527 |
| Male, n(%) | 16(0.356) | 7(0.467) | 8(0.320) | 0.636 |
| Education, y | 9.556(4.467) | 10.033(4.038) | 10.060(4.447) | 0.904 |
| Fasting glucose, mmol/L | 4.966(0.354) | 5.860(0.207) | 8.803(3.691) | 0.000 |
| Total cholesterol, mmol/L | 5.169(1.116) | 4.867(0.721) | 5.138(0.963) | 0.599 |
| Triglyceride, mmol/L | 1.157(0.485) | 1.277(0.414) | 1.174(0.336) | 0.583 |
| SBP, mm Hg | 129.111(16.456) | 137.333(17.512) | 130.800(15.253) | 0.203 |
| DBP, mm Hg | 80.333(7.863) | 86.000(11.832) | 80.600(7.948) | 0.110 |
| MMSE | 14.111(7.334) | 15.400(5.248) | 14.800(7.858) | 0.818 |
Notes.
- NFG
- Normal fasting glucose
- IFG
- Impaired Fasting Glucose
- T2DM
- Type 2 Diabetes mellitus
- SBP
- systolic pressure
- DBP
- diastolic pressure
- y
- years
The data was presented as mean (SD) except gender.
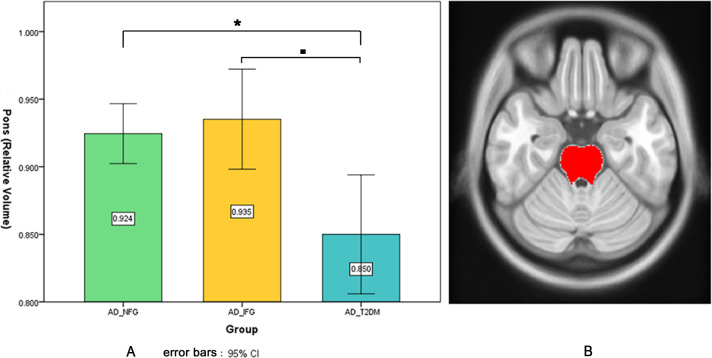
Volumetric results showed no significant difference in ICV among the three groups. When examining regional volumetric measures, we found significant pontine volume difference among the three groups (uncorrected p = 0.001, FDR p = 0.033), but no significant difference in other regions. Post hoc test (Fig. 2) showed that the AD_T2DM group had significantly smaller pons (relative volume = 0.850 ± 0.107) compared with AD_IFG (relative volume = 0.935 ± 0.067, p = 0.003) and AD_NFG group (relative volume = 0.924 ± 0.074, p = 0.001). No significant difference was found between AD_NFG and AD_IFG group. Besides, fasting glucose level was found to have a significant negative correlation with pontine volume (r = − 0.379, p = 0.001) after correcting for age, education level, MMSE, cholesterol, triglyceride, and blood pressure (Fig. 3). No significant difference was found in WMH among the three groups (uncorrected p = 0.683, FDR p = 0.855).
Figure 2. Pontine volumetric measures.
(A) Pontine volumetric measures of AD_NFG, AD_IFG, and AD_T2DM groups. (B) The red area on the axial atlas represents Pons. * ■ FDR p < 0.05.
Figure 3. Scatter plots of pontine volume and fasting glucose level.
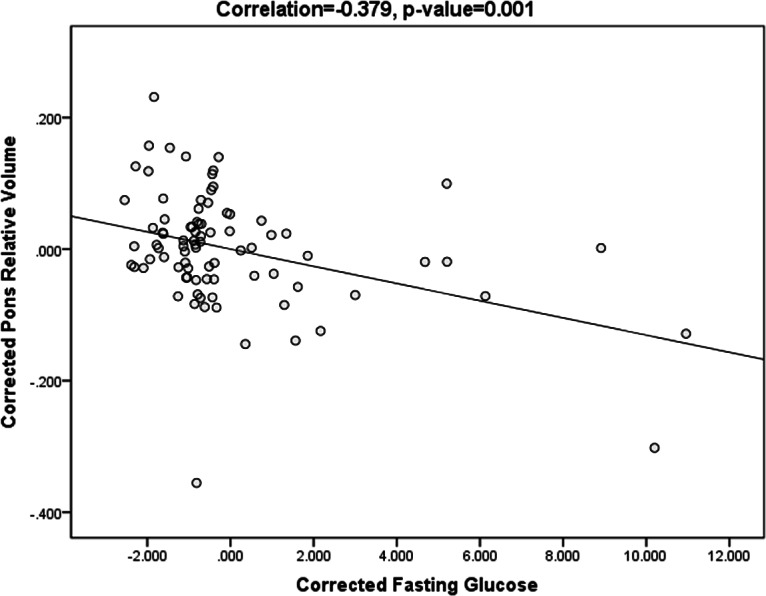
Scatter plots showing the negative correlation between pontine volume and fasting glucose level, after correcting for age, education level, MMSE, cholesterol, triglyceride, and blood pressure (r = − 0.379, p = 0.001).
Discussion
In this study, we examined the heterogeneity of brain regional volume in AD patients with different fasting glucose levels. We found that compared with the AD_IFG and AD_NFG groups, the pontine volume of AD_T2DM patients was smaller. In addition, correlation analysis revealed that fasting glucose level was negatively correlated with pontine volume. These results indicate that T2DM is associated with the brain volume changes in AD patients.
Previous studies have shown that AD, IFG, and T2DM are closely related (Degen et al., 2016; Greene et al., 2015; Kadohara, Sato & Kawakami, 2017; Lin, 2008; Moran et al., 2015; Rani et al., 2016; Zhang et al., 2017). Common pathological processes were found in these diseases. As a progressive neurodegenerative disease, AD is characterized by brain volume loss caused by neurodegeneration and brain cell destruction. Previous studies (Bernard et al., 2014; Matsuda, 2016; Schmidt-Wilcke et al., 2009) have shown that AD patients have more extensive atrophy compared to healthy population, involving both cortex and white matter, such as the hippocampus, corpus callosum, frontal and temporal lobes. Pontine atrophy has also been found in AD population (Mann, 1983; Lee et al., 2015), but it was less reported as compared with medial temporal lobe atrophy. A recent study showed that compared to the control group, bilateral volume loss in the pons was found in the mild AD group (Ji et al., 2020).
In this study, we found that the AD_T2DM group had a smaller pontine volume than the AD_IFG and AD_NFG groups. However, other structures (Matsuda, 2016; Schmidt-Wilcke et al., 2009) that typically show atrophy in the AD population did not demonstrate differences among the three groups. Hence, our findings suggest that for AD patients, their pons might be more susceptible to T2DM related atrophy than other structures. It might associated with the heterogeneous distribution of amyloid plaques (Braak & Braak, 1991) in AD brains. The distribution of amyloid plaques varied within cerebral regions. The pons is known to be relatively unaffected by amyloid deposition compared to other structures (Braak & Braak, 1991; Engler et al., 2006), and it is usually used as the reference structure for the study of cerebral glucose metabolism in AD (Cho et al., 2020; Grothe & Teipel, 2016). It is inferred that compared with structures mostly affected by amyloid deposition, pons could be more vulnerable to hyperglycemia in AD patients. Besides, T2DM is reported related to brainstem dysfunctions (Fuente-Martín et al., 2019), and significant associations were observed between metabolic syndrome (including hyperglycemia) and decreased gray matter volume of brainstem (including the pons) (Kotkowski et al., 2019). Previous studies have shown that FG is a risk predictor for T2DM with brainstem infarction (Ichikawa et al., 2012a; Ichikawa et al., 2010; Lu et al., 2011), and the brainstem is vulnerable to hyperglycemia (Ichikawa et al., 2012b). These evidence suggest the pons is related and vulnerable to hyperglycemia. Overall, our results indicate that among AD patients in different stages of T2DM development, cerebral structures generally presented similar atrophy patterns except for pons. Most cerebral structures, including hippocampus, temporal lobe and other regions previously shown to have atrophy in AD studies remained stable across groups. In the AD population, most regions may not shrink further as dysglycemia progresses, with the exception that the pons is a sensitive and key area associated with hyperglycemia. Pons of AD patients might shrink further as dysglycemia progresses from IFG to T2DM. The results also show that the brain atrophy rate could vary regionally, which is consistent with previous findings that brain pathological changes are regionally different and disease-stage specific (Byun et al., 2015; Tosun et al., 2010). In addition, compared with AD alone, patients with both T2DM and AD would show a distinctive pattern of brain atrophy, and T2DM may accelerate pontine atrophy in AD patients. Meanwhile, the pontine volume of the AD_NFG and AD_IFG groups was not significantly different in this study, which is consistent with the results of Schneider’s study (Schneider et al., 2017). In other words, mild hyperglycemia (IFG) did not cause pontine atrophy that is detectable by MRI, while T2DM is associated with significant pontine atrophy. This suggests that it is necessary to control the progression of hyperglycemia to avoid obvious pontine atrophy in AD patients.
Moreover, it was observed that the volume of pons was significantly negatively correlated with FG level, after correcting for MMSE scores, age, education level, cholesterol, triglyceride, and blood pressure. These results suggest that FG level is associated with pontine volume. This is consistent with existing studies which have shown significant association between hyperglycemia and brain atrophy (Li et al., 2016). Similar to our results, Cherbuin (Cherbuin, Sachdev & Anstey, 2012) showed that FG was positively correlated with hippocampal and amygdalar atrophy. Previous studies also observed that in the default mode network, FG level was positively correlated with the right middle temporal gyrus connection (Chen et al., 2015) and negatively correlated with fine motor skills (Zhang et al., 2018). Compared to non-diabetic participants, aged adults with T2DM showed lower CBF in predilection sites for AD pathology (Bangen et al., 2018). These provide evidence that FG level is related to both brain structure and function. Our results demonstrate that hyperglycemia is associated with a small pontine volume, suggesting that diabetes management is crucial for maintaining the pontine volume for AD patients. As it is known that the FG level of T2DM patients is reversible after medication, the follow-up question is whether the brain atrophy is also reversible.
In this study, there was no significant difference in WMH volume among the three groups. Thus, there was no evidence of a significant relationship between WMH volume and hyperglycemia, which might be attributed to the pathology of AD. WMH is usually deemed as an MRI sign of microvascular diseases. As a progressive neurodegenerative disease, the main pathological cerebral changes in AD are the deposition of β-amyloid protein and hyperphosphorylation of Tau protein. The typical MRI feature of AD is brain atrophy, and previous study has shown that AD is not necessarily associated with WMH volume (Sudre et al., 2017). Although T2DM presents small vessels and microvascular damage, the association between T2DM and WMH remains unclear (Brundel, Kappelle & Biessels, 2014). Some previous findings appear to be inconsistent with our result (Del Bene et al., 2015; Schneider et al., 2017), but De Bresser et al. (2018) also observed that there is no significant difference in WMH volume between T2DM and control group. More evidence is required to elucidate the relationship between WMH volume and hyperglycemia.
Our results have provided new insight into the association between hyperglycemia and brain volume changes in patients with AD. However, this study has several limitations that should be addressed in future research. First, longitudinal follow-up data was not included, so we were restrained from providing more in-depth results under the cross-sectional design. Second, the sample size was relatively small, particularly for the AD_IFG group, which limited the interpretation and generalizability of our results. However, as several studies have associated pontine degeneration with dysglycemia and AD (Ichikawa et al., 2012a; Ji et al., 2020; Lee et al., 2015), it may be possible to extend these findings to patients with comorbid dysglycemia and AD. However, further studies are needed on a larger cohort to confirm these preliminary findings. Third, the study sample only included the Chinese population and lacked ethnic diversity. Last but not least, because of missing data, the potential impact of other factors was not investigated in this study, such as disease duration and diabetes medication, which should be addressed in future research.
Conclusions
AD patients with T2DM showed smaller pontine volume compared to those with normal blood glucose and IFG. Compared with AD alone, patients with both T2DM and AD would show a distinctive pattern of brain atrophy. T2DM may exacerbate pontine atrophy in AD patients, and FG level is associated with pontine volume. No evidence of a significant relationship between WMH volume and hyperglycemia was found.
Supplemental Information
NFG= Normal fasting glucose, IFG= Impaired Fasting Glucose, T2DM= Type 2 Diabetes mellitus, SBP= systolic pressure, DBP= diastolic pressure
Based on the segmentation results, atrophy of cerebral lobes was computed as its atrophic volume of cerebrospinal fluid, which was expressed as a percentage of total brain parenchymal volume. Volume from every other cerebral region was calculated as the percentage of its absolute volume by the total intracranial volume (ICV), where ICV was used to estimate the whole brain volume and correct the inter-subject head size variability.
Acknowledgments
Thanks to Dr. Yang Hu (Shanghai Mental Health Center) for his help with statistical analysis.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant numbers 81801657, 81671646). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Yanwei Miao, Email: ywmiao716@163.com.
Jianlin Wu, Email: cjr.wujianlin@vip.163.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests. Leongtim Wong and Yishan Luo are the employee of BrainNow Research Institute. All other authors report no financial relationships with commercial interests.
Author Contributions
Weiwei Wang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Leongtim Wong and Lin Shi conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Yishan Luo analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Zhanhua Liang, Chunbo Dong, Qingwei Song, Tieli Liu and Qing Zhang performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Ailian Liu, Yanwei Miao and Jianlin Wu conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The First Affiliated Hospital of Dalian Medical University granted Ethical approval to carry out the study within its facilities (YJ-KY-FB-2020-08).
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplementary Files.
References
- Abrigo et al. (2018).Abrigo J, Shi L, Luo Y, Chen Q, Chu WCW, Mok VCT. Standardization of hippocampus volumetry using automated brain structure volumetry tool for an initial Alzheimer’s disease imaging biomarker. Acta Radiologica. 2018;60(6):284185118795327. doi: 10.1177/0284185118795327. [DOI] [PubMed] [Google Scholar]
- Association American Diabetes (2010).Association American Diabetes Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen et al. (2018).Bangen KJ, Werhane ML, Weigand AJ, Edmonds EC, Delano-Wood L, Thomas KR, Nation DA, Evangelista ND, Clark AL, Liu TT, Bondi MW. Reduced regional cerebral blood flow relates to poorer cognition in older adults with type 2 diabetes. Frontiers In Aging Neuroscience. 2018;10:270. doi: 10.3389/fnagi.2018.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard et al. (2014).Bernard C, Helmer C, Dilharreguy B, Amieva H, Auriacombe S, Dartigues JF, Allard M, Catheline G. Time course of brain volume changes in the preclinical phase of Alzheimer’s disease. Alzheimers Dement. 2014;10:143–151. doi: 10.1016/j.jalz.2013.08.279. [DOI] [PubMed] [Google Scholar]
- Biessels et al. (2014).Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2:246–255. doi: 10.1016/s2213-8587(13)70088-3. [DOI] [PubMed] [Google Scholar]
- Braak & Braak (1991).Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/bf00308809. [DOI] [PubMed] [Google Scholar]
- Brundel, Kappelle & Biessels (2014).Brundel M, Kappelle LJ, Biessels GJ. Brain imaging in type 2 diabetes. European Neuropsychopharmacology. 2014;24:1967–1981. doi: 10.1016/j.euroneuro.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Byun et al. (2015).Byun MS, Kim SE, Park J, Yi D, Choe YM, Sohn BK, Choi HJ, Baek H, Han JY, Woo JI, Lee DY. Heterogeneity of regional brain atrophy patterns associated with distinct progression rates in alzheimer’s disease. PLOS ONE. 2015;10:e0142756. doi: 10.1371/journal.pone.0142756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai et al. (2018).Cai HY, Yang JT, Wang ZJ, Zhang J, Yang W, Wu MN, Qi JS. Lixisenatide reduces amyloid plaques, neurofibrillary tangles and neuroinflammation in an APP/PS1/tau mouse model of Alzheimer’s disease. Biochemical and Biophysical Research Communications. 2018;495:1034–1040. doi: 10.1016/j.bbrc.2017.11.114. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2015).Chen Y, Liu Z, Zhang J, Tian G, Li L, Zhang S, Li X, Chen K, Zhang Z. Selectively disrupted functional connectivity networks in type 2 diabetes mellitus. Frontiers in Aging Neuroscience. 2015;7:233. doi: 10.3389/fnagi.2015.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbuin, Sachdev & Anstey (2012).Cherbuin N, Sachdev P, Anstey KJ. Higher normal fasting plasma glucose is associated with hippocampal atrophy: the PATH study. Neurology. 2012;79:1019–1026. doi: 10.1212/WNL.0b013e31826846de. [DOI] [PubMed] [Google Scholar]
- Cho et al. (2020).Cho SH, Choe YS, Kim YJ, Kim HJ, Jang H, Kim Y, Kim SE, Kim SJ, Kim JP, Jung YH, Kim BC, Lockhart SN, Farrar G, Na DL, Moon SH, Seo SW. Head-to-head comparison of 18F-Florbetaben and 18F-Flutemetamol in the cortical and striatal regions. Journal of Alzheimer’s Disease. 2020;76:281–290. doi: 10.3233/jad-200079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bresser et al. (2018).De Bresser J, Kuijf HJ, Zaanen K, Viergever MA, Hendrikse J, Biessels GJ. White matter hyperintensity shape and location feature analysis on brain MRI; proof of principle study in patients with diabetes. Scientific Reports. 2018;8:1893. doi: 10.1038/s41598-018-20084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice (2013).De Felice FG. Alzheimer’s disease and insulin resistance: translating basic science into clinical applications. Journal of Clinical Investigation. 2013;123:531–539. doi: 10.1172/jci64595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen et al. (2016).Degen C, Toro P, Schonknecht P, Sattler C, Schroder J. Diabetes mellitus Type II and cognitive capacity in healthy aging, mild cognitive impairment and Alzheimer’s disease. Psychiatry Research. 2016;240:42–46. doi: 10.1016/j.psychres.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Del Bene et al. (2015).Del Bene A, Ciolli L, Borgheresi L, Poggesi A, Inzitari D, Pantoni L. Is type 2 diabetes related to leukoaraiosis? an updated review. Acta Neurologica Scandinavica. 2015;132:147–155. doi: 10.1111/ane.12398. [DOI] [PubMed] [Google Scholar]
- Dubois et al. (2007).Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurology. 2007;6:734–746. doi: 10.1016/s1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Engler et al. (2006).Engler H, Forsberg A, Almkvist O, Blomquist G, Larsson E, Savitcheva I, Wall A, Ringheim A, Långström B, Nordberg A. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129:2856–2866. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- Fuente-Martín et al. (2019).Fuente-Martín E, Mellado-Gil JM, Cobo-Vuilleumier N, Martín-Montalvo A, Romero-Zerbo SY, Diaz Contreras I, Hmadcha A, Soria B, Martin Bermudo F, Reyes JC, Bermúdez-Silva FJ, Lorenzo PI, Gauthier BR. Dissecting the brain/islet axis in metabesity. Gene. 2019;10(5):350. doi: 10.3390/genes10050350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gili et al. (2011).Gili T, Cercignani M, Serra L, Perri R, Giove F, Maraviglia B, Caltagirone C, Bozzali M. Regional brain atrophy and functional disconnection across Alzheimer’s disease evolution. Journal of Neurology, Neurosurgery and Psychiatry. 2011;82:58–66. doi: 10.1136/jnnp.2009.199935. [DOI] [PubMed] [Google Scholar]
- Greene et al. (2015).Greene A, Ng J, Shepherd L, Carey K. Alzheimer’s disease and type 2 diabetes: what is the connection? Consult Pharm. 2015;30:112–115. doi: 10.4140/TCP.n.2015.112. [DOI] [PubMed] [Google Scholar]
- Grothe & Teipel (2016).Grothe MJ, Teipel SJ. Spatial patterns of atrophy, hypometabolism, and amyloid deposition in Alzheimer’s disease correspond to dissociable functional brain networks. Human Brain Mapping. 2016;37:35–53. doi: 10.1002/hbm.23018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo et al. (2014).Guo Y, Zhang Z, Zhou B, Wang P, Yao H, Yuan M, An N, Dai H, Wang L, Zhang X, Liu Y. Grey-matter volume as a potential feature for the classification of Alzheimer’s disease and mild cognitive impairment: an exploratory study. Neuroscience Bulletin. 2014;30:477–489. doi: 10.1007/s12264-013-1432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelsman et al. (2015).Handelsman Y, Bloomgarden ZT, Grunberger G, Umpierrez G, Zimmerman RS, Bailey TS, Blonde L, Bray GA, Cohen AJ, Dagogo-Jack S, Davidson JA, Einhorn D, Ganda OP, Garber AJ, Garvey WT, Henry RR, Hirsch IB, Horton ES, Hurley DL, Jellinger PS, Jovanovic L, Lebovitz HE, LeRoith D, Levy P, McGill JB, Mechanick JI, Mestman JH, Moghissi ES, Orzeck EA, Pessah-Pollack R, Rosenblit PD, Vinik AI, Wyne K, Zangeneh F. American association of clinical endocrinologists and american college of endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocrine Practice. 2015;21(Suppl 1):1–87. doi: 10.4158/ep15672.gl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou et al. (2016).Hou YC, Lai CH, Wu YT, Yang SH. Gray matter alterations and correlation of nutritional intake with the gray matter volume in prediabetes. Medicine (Baltimore) 2016;95:e3956. doi: 10.1097/md.0000000000003956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa et al. (2012a).Ichikawa H, Kuriki A, Kinno R, Katoh H, Mukai M, Kawamura M. Occurrence and clinicotopographical correlates of brainstem infarction in patients with diabetes mellitus. Journal of Stroke and Cerebrovascular Diseases. 2012a;21:890–897. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Ichikawa et al. (2010).Ichikawa H, Mukai M, Hieda S, Kamiya Y, Akizawa T, Kawamura M. Involvement of the basilar artery in diabetes mellitus: an MRI study of brainstem infarctions. European Neurology. 2010;64:230–235. doi: 10.1159/000319924. [DOI] [PubMed] [Google Scholar]
- Ichikawa et al. (2012b).Ichikawa H, Shimizu Y, Kuriki A, Murakami H, Mukai M, Kawamura M. The brainstem is at high risk for recurrent noncardioembolic cerebral infarction in association with diabetes mellitus: a hospital-based study. European Neurology. 2012b;67:26–32. doi: 10.1159/000333284. [DOI] [PubMed] [Google Scholar]
- Infante-Garcia et al. (2018).Infante-Garcia C, Ramos-Rodriguez JJ, Hierro-Bujalance C, Ortegon E, Pickett E, Jackson R, Hernandez-Pacho F, Spires-Jones T, Garcia-Alloza M. Antidiabetic polypill improves central pathology and cognitive impairment in a mixed model of alzheimer’s disease and type 2 diabetes. Molecular Neurobiology. 2018;55:6130–6144. doi: 10.1007/s12035-017-0825-7. [DOI] [PubMed] [Google Scholar]
- Ji et al. (2020).Ji X, Wang H, Zhu M, He Y, Zhang H, Chen X, Gao W, Fu Y. Brainstem atrophy in the early stage of Alzheimer’s disease: a voxel-based morphometry study. Brain Imaging and Behavior. 2020 doi: 10.1007/s11682-019-00231-3. Epub ahead of print Jan 2 2020. [DOI] [PubMed] [Google Scholar]
- Kadohara, Sato & Kawakami (2017).Kadohara K, Sato I, Kawakami K. Diabetes mellitus and risk of early-onset Alzheimer’s disease: a population-based case-control study. European Journal of Neurology. 2017;24:944–949. doi: 10.1111/ene.13312. [DOI] [PubMed] [Google Scholar]
- Kotkowski et al. (2019).Kotkowski E, Price LR, Franklin C, Salazar M, Woolsey M, DeFronzo RA, Blangero J, Glahn DC, Fox PT. A neural signature of metabolic syndrome. Human Brain Mapping. 2019;40:3575–3588. doi: 10.1002/hbm.24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, Hardy & Schott (2018).Lane CA, Hardy J, Schott JM. Alzheimer’s disease. European Journal of Neurology. 2018;25:59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2015).Lee JH, Ryan J, Andreescu C, Aizenstein H, Lim HK. Brainstem morphological changes in Alzheimer’s disease. Neuroreport. 2015;26:411–415. doi: 10.1097/WNR.0000000000000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2016).Li W, Risacher SL, Huang E, Saykin AJ. Type 2 diabetes mellitus is associated with brain atrophy and hypometabolism in the ADNI cohort. Neurology. 2016;87:595–600. doi: 10.1212/wnl.0000000000002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin (2008).Lin L. Commonality between diabetes and Alzheimer’s disease and a new strategy for the therapy. Clinical Pathology. 2008;1:83–91. doi: 10.4137/cpath.s667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al. (2011).Lu ZQ, Li HY, Hu XQ, Zhang BJ. An evaluation of clinical characteristics and prognosis of brain-stem infarction in diabetics. Zhonghua Nei Ke Za Zhi. 2011;50:27–31. [PubMed] [Google Scholar]
- Mann (1983).Mann DM. The locus coeruleus and its possible role in ageing and degenerative disease of the human central nervous system. Mechanisms of Ageing and Development. 1983;23:73–94. doi: 10.1016/0047-6374(83)90100-8. [DOI] [PubMed] [Google Scholar]
- Markus et al. (2017).Markus MRP, Ittermann T, Wittfeld K, Schipf S, Siewert-Markus U, Bahls M, Bulow R, Werner N, Janowitz D, Baumeister SE, Felix SB, Dorr M, Rathmann W, Volzke H, Grabe HJ. Prediabetes is associated with lower brain gray matter volume in the general population. The Study of Health in Pomerania (SHIP) Nutrition, Metabolism and Cardiovascular Diseases. 2017;27(12):1114–1122. doi: 10.1016/j.numecd.2017.10.007. [DOI] [PubMed] [Google Scholar]
- Matsuda (2016).Matsuda H. MRI morphometry in Alzheimer’s disease. Advances in Therapy. 2016;30:17–24. doi: 10.1016/j.arr.2016.01.003. [DOI] [PubMed] [Google Scholar]
- McIntyre et al. (2010).McIntyre RS, Kenna HA, Nguyen HT, Law CW, Sultan F, Woldeyohannes HO, Adams AK, Cheng JS, Lourenco M, Kennedy SH, Rasgon NL. Brain volume abnormalities and neurocognitive deficits in diabetes mellitus: points of pathophysiological commonality with mood disorders? Advances in Therapy. 2010;27:63–80. doi: 10.1007/s12325-010-0011-z. [DOI] [PubMed] [Google Scholar]
- Moran et al. (2015).Moran C, Beare R, Phan TG, Bruce DG, Callisaya ML, Srikanth V. Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology. 2015;85:1123–1130. doi: 10.1212/wnl.0000000000001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogurtsova et al. (2017).Ogurtsova K, Da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Research and Clinical Practice. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Rani et al. (2016).Rani V, Deshmukh R, Jaswal P, Kumar P, Bariwal J. Alzheimer’s disease: is this a brain specific diabetic condition? Physiology and Behavior. 2016;164:259–267. doi: 10.1016/j.physbeh.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Roberts et al. (2014).Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, Baertlein L, Boeve BF, Tangalos EG, Ivnik RJ, Mielke MM, Petersen RC. Association of diabetes with amnestic and nonamnestic mild cognitive impairment. Alzheimers Dement. 2014;10:18–26. doi: 10.1016/j.jalz.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Wilcke et al. (2009).Schmidt-Wilcke T, Poljansky SF, Hierlmeier S, Hierlmeier SF, Hausner J, Hausner JF, Ibach B, Ibach B. Memory performance correlates with gray matter density in the ento-/perirhinal cortex and posterior hippocampus in patients with mild cognitive impairment and healthy controls–a voxel based morphometry study. Neuroimage. 2009;47:1914–1920. doi: 10.1016/j.neuroimage.2009.04.092. [DOI] [PubMed] [Google Scholar]
- Schneider et al. (2017).Schneider ALC, Selvin E, Sharrett AR, Griswold M, Coresh J, Jack Jr CR, Knopman D, Mosley T, Gottesman RF. Diabetes, prediabetes, and brain volumes and subclinical cerebrovascular disease on MRI: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS) Diabetes Care. 2017;40(11):1514–1521. doi: 10.2337/dc17-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al. (2013).Shi L, Wang D, Liu S, Pu Y, Wang Y, Chu WC, Ahuja AT, Wang Y. Automated quantification of white matter lesion in magnetic resonance imaging of patients with acute infarction. Journal of Neuroscience Methods. 2013;213:138–146. doi: 10.1016/j.jneumeth.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Sudre et al. (2017).Sudre CH, Cardoso MJ, Frost C, Barnes J, Barkhof F, Fox N, Ourselin S. APOE epsilon4 status is associated with white matter hyperintensities volume accumulation rate independent of AD diagnosis. Neurobiology of Aging. 2017;53:67–75. doi: 10.1016/j.neurobiolaging.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Tosun et al. (2010).Tosun D, Schuff N, Truran-Sacrey D, Shaw LM, Trojanowski JQ, Aisen P, Peterson R, Weiner MW. Relations between brain tissue loss, CSF biomarkers, and the ApoE genetic profile: a longitudinal MRI study. Neurobiology of Aging. 2010;31:1340–1354. doi: 10.1016/j.neurobiolaging.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yishan (2017).Yishan L. https://patentsgooglecom/patent/CN107103612A/en. [14 July 2020];Automate the quantitative calculation method of subregion brain atrophy. CN107103612A. 2017
- Zhang et al. (2017).Zhang J, Chen C, Hua S, Liao H, Wang M, Xiong Y, Cao F. An updated meta-analysis of cohort studies: diabetes and risk of Alzheimer’s disease. Diabetes Research and Clinical Practice. 2017;124:41–47. doi: 10.1016/j.diabres.2016.10.024. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2018).Zhang T, Shaw ME, Walsh EI, Sachdev PS, Anstey KJ, Cherbuin N. Higher fasting plasma glucose is associated with smaller striatal volume and poorer fine motor skills in a longitudinal cohort. Psychiatry Research: Neuroimaging. 2018;278:1–6. doi: 10.1016/j.pscychresns.2018.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NFG= Normal fasting glucose, IFG= Impaired Fasting Glucose, T2DM= Type 2 Diabetes mellitus, SBP= systolic pressure, DBP= diastolic pressure
Based on the segmentation results, atrophy of cerebral lobes was computed as its atrophic volume of cerebrospinal fluid, which was expressed as a percentage of total brain parenchymal volume. Volume from every other cerebral region was calculated as the percentage of its absolute volume by the total intracranial volume (ICV), where ICV was used to estimate the whole brain volume and correct the inter-subject head size variability.
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplementary Files.