Abstract
Objective
Prospective evaluation of intestinal ultrasound (IUS) for disease monitoring of patients with ulcerative colitis (UC) in routine medical practice.
Design
TRansabdominal Ultrasonography of the bowel in Subjects with IBD To monitor disease activity with UC (TRUST&UC) was a prospective, observational study at 42 German inflammatory bowel disease-specialised centres representing different care levels. Patients with a diagnosis of a proctosigmoiditis, left-sided colitis or pancolitis currently in clinical relapse (defined as Short Clinical Colitis Activity Index ≥5) were enrolled consecutively. Disease activity and vascularisation within the affected bowel wall areas were assessed by duplex/Colour Doppler ultrasonography.
Results
At baseline, 88.5% (n=224) of the patients had an increased bowel wall thickness (BWT) in the descending or sigmoid colon. Even within the first 2 weeks of the study, the percentage of patients with an increased BWT in the sigmoid or descending colon decreased significantly (sigmoid colon 89.3%–38.6%; descending colon 83.0%–42.9%; p<0.001 each) and remained low at week 6 and 12 (sigmoid colon 35.4% and 32.0%; descending colon 43.4% and 37.6%; p<0.001 each). Normalisation of BWT and clinical response after 12 weeks of treatment showed a high correlation (90.5% of patients with normalised BWT had symptomatic response vs 9.5% without symptomatic response; p<0.001).
Conclusions
IUS may be preferred in general practice in a point-of-care setting for monitoring the disease course and for assessing short-term treatment response. Our findings give rise to the assumption that monitoring BWT alone has the potential to predict the therapeutic response, which has to be verified in future studies.
Keywords: ulcerative colitis, gastrointestinal ultrasound, inflammatory bowel disease
Significance of this study.
What is already known on this subject?
Intestinal ultrasound (IUS) represents a non-invasive modality to assess disease activity in inflammatory bowel disease and to guide therapy decisions. It is easy to use and offers good repeatability and accuracy and may thus be an obvious tool in the follow-up of patients known to have ulcerative colitis (UC).
Data on using IUS for monitoring treatment success in UC are still limited and most studies are single-centre studies usually performed in centres with a special expertise in IUS.
What are the new findings?
TRansabdominal Ultrasonography of the bowel To monitor disease activity with UC was the largest multicentre study investigating the use of IUS in patients with UC.
Rapid improvement of the IUS pathology was observed as early as 2 weeks after treatment intensification, followed by clinical improvement as determined by Short Clinical Colitis Activity Index.
For 88.5% of patients with a clinical flare enrolled in this study, an increased bowel wall thickness was detected by IUS at baseline. This rate of patients with UC with bowel alterations is higher compared with the same rate in older studies where it ranged from 53% to 84%.
How might it impact on clinical practice in the foreseeable future?
This study clearly supports IUS as a non-invasive monitoring tool for UC.
The combination of non-invasive tools (eg, faecal calprotectin measurement and IUS) in general practice increases the accuracy in determining disease activity in patients with UC.
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are two forms of chronic inflammatory bowel diseases (IBDs) characterised by recurrent episodes of intestinal inflammation and a heterogeneous range of symptoms and clinical courses. In contrast to CD, UC is a mucosal and to some extent submucosal disease restricted to the colon. Depending on the involved colonic segment, UC can be divided into proctitis, left-sided colitis or extensive colitis.1 Even during the periods of clinical remission, subclinical inflammation often persists, and disease progression can cause structural intestinal damage and complications.2 Symptoms often correlate poorly with the endoscopically defined disease in patients with CD, making it challenging to monitor the disease. To some extent, this also applies to UC.3 4 Furthermore, Narula et al in a systemic review and meta-analysis described that many patients still had an abnormal stool frequency despite endoscopic remission.5
Mucosal healing is an important step in IBD treatment. Modern disease management concepts (‘treat to target’) emphasise the need for objective monitoring of disease activity to guide treatment optimisation and to improve long-term outcomes and prevent complications including hospitalisation, stenosis or colectomy.6 7
Hence, reliable tools for frequent measuring of objective parameters during the follow-up are crucial to adjust treatment in a temporal manner. Non-invasive alternatives complementary to endoscopy are needed in clinical practice to assess ‘mucosal response’ to therapy.
The assessment of faecal calprotectin (FC) levels has been proposed as a non-invasive test for the immediate evaluation of intestinal inflammation in patients with IBD.8–10 FC is more specific and sensitive than blood markers, with the main advantage of being unaffected by extraintestinal processes. The need to obtain a faeces sample to quantify calprotectin is obviously a disadvantage compared with serological methods and the fact that FC levels are not immediately available to support therapy decisions.
Intestinal ultrasound (IUS) represents a non-invasive modality to assess disease activity in IBD and to guide therapy decisions.11 12 It is easy to use, inexpensive and can be used repeatedly without limitations and with immediate interaction opportunities with the patient.13 Furthermore, IUS offers good accuracy, therefore, an indication may be in the follow-up of patients with UC. In a nationwide survey in patients with IBD in France, IUS showed very high acceptability and was the preferred imaging method over other diagnostic modalities.14 The typical IUS findings in active UC are: bowel wall thickness (BWT) involving the mucosa and submucosa (>4.0 mm in adults15–18 and >3.0 mm in children15 16 19), sometimes increased echogenicity of the submucosal layer, increased colour Doppler signal, mesenteric fibrofatty proliferation, mesenteric lymphadenopathy and the loss of haustration.17 The stratification of the colonic wall may be disrupted in patients with more severe activity.18
The clinical role of IUS in UC is not as well established as in CD. In some studies, BWT correlates well with clinical activity,17 20–22 C reactive protein (CRP) values16 20 21 and also with endoscopy findings.16 18 20 23
In the context of the evolution of treatment targets in IBD and the increased need for objective monitoring of disease activity, IUS was recently described as an ‘underused resource with potential paradigm-changing application’.11 Since IUS allows the accurate characterisation of both inflammatory infiltration of the bowel wall layers and of peri-intestinal abnormalities, its use should not be limited to diagnostic purposes but may be of great value in disease monitoring and therapy management in the ‘treat to target’ era. IUS is well recognised by international guidelines,24 although there is a lack of standardisation and of general agreement in the definition of the IUS parameters so far.25 26
Numerous studies have evaluated the comparability of IUS to other cross-sectional imaging modalities such as magnetic resonance elastography (MRE) or computed tomography (CT) and a meta-analysis confirmed the non-superiority of both MRE and CT to IUS assessments.27 These data were confirmed by a recent publication highlighting the comparative accuracy of IUS versus MRE in combination with colonoscopy in assessing CD and guiding clinical decision making.28 This study was one of several publications to successfully demonstrate the high interobserver agreement during the assessment of IUS parameters.2
The TRUST initiative (TRansabdominal Ultrasonography of the bowel in Subjects with IBD To monitor disease activity) stands for propagating diagnostic implications of modern therapeutic goals by focusing on the value of tight monitoring of intestinal damage via IUS as a first-line non-invasive modality in IBD. The purpose of the studies TRUST (in CD) and TRUST&UC (in UC) was to analyse and define IUS parameters as surrogate markers for disease activity, which allow for individual monitoring of disease course and treatment response. In TRUST, the role of IUS in monitoring CD treatment response was determined and it was shown that IUS is a useful method to monitor disease activity in CD.29 The primary objective of this TRUST&UC study was the prospective evaluation of IUS in monitoring patients with UC in a large cohort, and under real-world conditions and routine medical follow-up.
Patients and methods
Patients
TRUST&UC is a prospective, observational study, performed from November 2015 to March 2018 at 42 German IBD-specialised centres representing different care levels including both outpatient and inpatient care sites (45.2% IBD-specialised general practices, 38.1% general hospitals and 16.7% university hospitals).
Patients with a proctosigmoiditis, left-sided colitis or pancolitis in clinical relapse (defined as Short Clinical Colitis Activity Index (SCCAI) ≥5)30 and ≥18 years of age at baseline were enrolled consecutively. To generate data on the percentage of patients who had a pathological IUS at time of clinical flare, patients were enrolled before performing the baseline IUS. Patients without increasement in BWT (sigmoid colon ≤4.0 mm, descending colon ≤3.0 mm, transverse colon ≤3.0 mm, ascending colon ≤3.0 mm)31 at baseline were documented, but excluded for further documentation. All patients received standard of care therapy at the discretion of the treating physician according to German guidelines, that is, aminosalicylates, corticosteroids, conventional immunosuppressives and/or biologics.32 At baseline, demographics and characteristics were documented as well as laboratory parameters, changes in UC-specific medication, SCCAI and IUS parameters (at every visit). As a semiquantitative method of determining disease activity, vascularisation within the affected bowel wall areas was assessed by colour Doppler ultrasonography. During the study, up to four visits were scheduled: baseline, an unscheduled visit at week 2 (T2W), week 6 (T1) and week 12 (T2).
The primary endpoint was the proportion of patients with normalisation of BWT in patients with clinical response (decrease in SCCAI by ≥3 points)30 at week 12 as compared with baseline.
Secondary endpoints included (1) the change of the colour Doppler signal (vascularisation) for patients with and without clinical response at week 12 and (2) the correlation of SCCAI with FC and BWT and the correlation of BWT with FC at week 12.
Written informed consent was obtained from each patient enrolled in the study.
IUS examination
At each visit, all large bowel segments were evaluated by IUS. Increased BWT (sigmoid colon >4.0 mm, descending colon >3.0 mm, transverse colon >3.0 mm and ascending colon >3.0 mm) was measured in longitudinal sections.33 Cut-off values were based on data from recent publications. The IUS examinations were performed using different units, ranging from normal to high-end devices using convex (1–7 MHz) and linear (1–15 MHz) probes from various manufacturers. 78.5% of the centres used high-end scanners (class 3, according to ultrasound systems classification of Deutsche Gesellschaft für Ultraschall in der Medizin (DEGUM).34 The IUS examinations at each centre were performed by the same investigator (83.3%) using the same machine (85.7%) throughout the study. Patients were not prepared in a special manner.
Prior to the start of the study, IUS parameters and standards of performing the IUS were discussed at an investigator meeting at which the investigators underwent training with simulators to ensure a consistent investigation procedure.35 Colour Doppler setting for the investigation of vascularity within the affected bowel wall areas had been standardised before starting the assessment and kept stable for each visit. To avoid artefacts of movement or colour noise, colour gain had been set by balancing it using gallbladder or urinary bladder and adjusted to maximise gut wall vessels’ vascularisation but avoiding colour signal to noise artefacts. Due to the slow flow and small dimensions of the vessels, the velocity range of the colour Doppler was set low (from 5 to 7 cm/s). Due to the lack of a validated score for the use of Doppler ultrasound in patients with UC, a simple dichotomous analysis of the data was applied (amplified signal yes/no) based on the investigator’s decision.
All investigators were members of the ‘German IBD Study Group’ and were experienced in the use of IUS for monitoring disease activity in patients with UC in daily practice.
Statistical analysis
For the analysis of the primary endpoint, only patients with assessed BWT at baseline were considered. A reduction of the BWT was defined as ‘no’ in a follow-up visit, a still increased BWT was indicated as ‘yes’ in a subsequent visit. If ‘not assessable’ was documented during the follow-up visits, the patient was excluded from the analysis. The frequencies of BWT normalisation for patients with or without clinical response after 12 weeks in the sigmoid colon and the descending colon were compared by means of X2 tests (significance level adjusted according to Bonferroni-Holm α=0.025).
The differences of parameters were analysed exploratively by Cochran Q test for qualitative parameters and Friedman test for quantitative parameters. For additional explorative analysis and for correlation analysis between independent groups, Mann-Whitney U test was used. All tests in the exploratory analysis were two sided, assuming a 5% error probability (α=0.05). Correlations between parameters were analysed by Spearman correlation coefficient.
Results
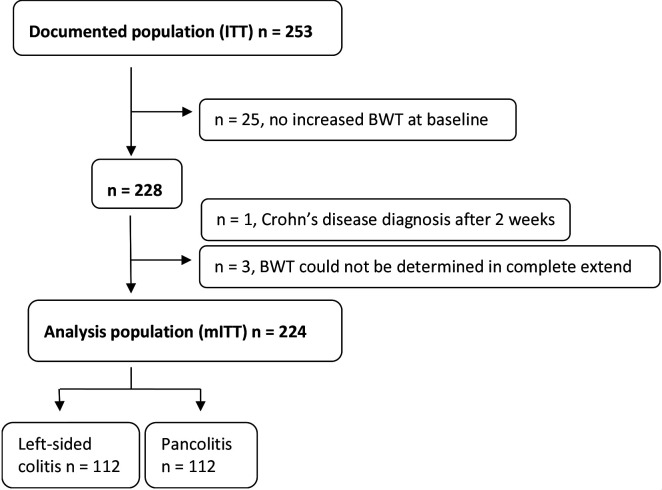
A total of 253 patients with active UC (SCCAI ≥5) were enrolled (intention-to-treat (ITT) population). 9.9% of the patients (n=25) had no increased BWT at baseline as defined above, in 1.2% (n=3), the disease pattern of the BWT could not be determined and for one patient the diagnosis changed from UC to CD after 2 weeks. Therefore, a total of 11.5% (n=29) of the ITT population were excluded from the statistical analysis. 88.5% (n=224) of the documented population were available for the statistical analysis (modified ITT population (mITT)), of which 50.0% (n=112) had a left-sided colitis and 50.0% (n=112) had a pancolitis (see figure 1). Demographic and baseline characteristics of the analysis population (mITT) are shown in table 1.
Figure 1.
Patient disposition and analysis population. BWT, bowel wall thickness; ITT, intention-to-treat; mITT, modified intention-to-treat.
Table 1.
Demographic and baseline characteristics of analysis population at baseline (mITT, n=224)
| Age (years, median and IQR) | 37.7 (29.2–49.9) | |
| Duration of current symptoms (% (n)) | ||
| <7 days | 13.4 (30) | |
| ≥7 days | 86.6 (194) | |
| Sex | ||
| Female | 46.0 (103) | |
| Time since diagnosis (months, median and IQR) | 52.4 (11.1–128.0) | |
| Current medication (% (n)) | Therapy >7 days | Therapy ≤7 days |
| Systemic steroids | 8.5 (19) | 15.2 (34) |
| AZA/6-MP | 2.2 (5) | 10.7 (24) |
| Anti-TNF | 13.8 (31) | 5.8 (13) |
| Anti-integrin | 7.6 (17) | 0.4 (1) |
| Objective signs of inflammation | Normal values for lab results | |
| (Median and IQR) | ||
| SCCAI (n=224) | 9.0 (7.0–10.0) | |
| Haemoglobin (g/L, n=203) | 131 (116–141) | 115–164 (female), 135–180 (male) |
| Platelets (/mm³, n=202) | 338 000 (265 000–430 000) | 150 000–300 000 |
| WCC (/x109/L, n=201) | 8.8 (7.39 – 11.3) | 4.4–11.3 |
| CRP (mg/dL, n=196) | 1.30 (0.58–4.46) | <0.5 |
| Feacal calprotectin | ||
| (µg/g, median and IQR) | 800 (439–1436) | |
| <250 (µg/g) | 9.9% (14) | |
| ≥250 (µg/g) | 90.1% (128) | |
| Bowel wall thickness | ||
| (mm, median and IQR) | ||
| Sigmoid colon (n=218) | 5.05 (4.60–6.20) | |
| Descending colon (n=198) | 5.00 (4.00–6.00) |
AZA, azathioprine; CRP, C reactive protein; mITT, modified intention-to-treat; MP, mercaptopurine; SCCAI, Short Clinical Colitis Activity Index; TNF, tumour necrosis factor; WCC, white cell count.
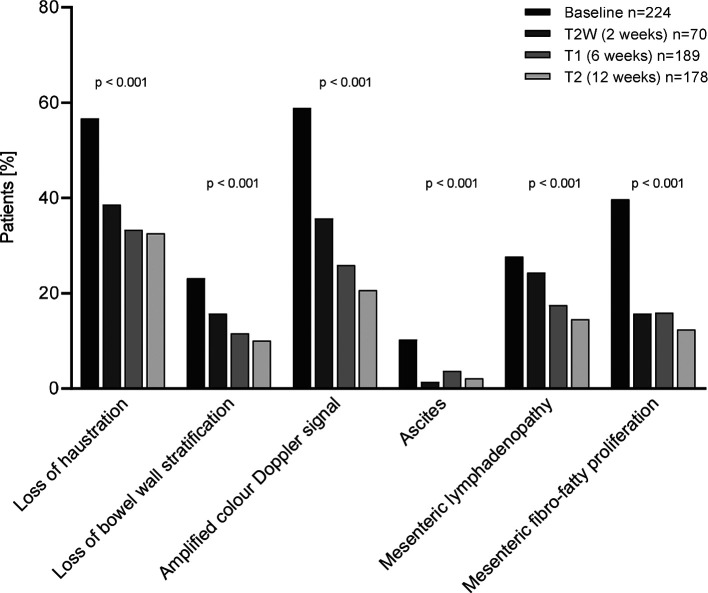
Within 12 weeks the percentage of patients with an increased BWT decreased significantly: sigmoid colon from 89.3% to 38.6% at week 2, 35.4% at week 6 and 32.0% at week 12; descending colon from 83.0% at baseline to 42.9% at week 2, 43.4% at week 6 and 37.6% at week 12 (figure 2A). Improvements in the colour Doppler signal were already observed in week 2 and were maintained up to week 12 (sigmoid colon: 34.8% at baseline to 22.9% at week 2, 15.9% at week 6 and 12.9% at week 12; descending colon: 15.2% at baseline to 7.1% at week 2, 5.3% at week 6 and 7.3% at week 12 (figure 2B). Furthermore, there was a significant reduction in the percentage of patients with additional abnormal IUS parameters 12 weeks after the baseline visit (figure 3): mesenteric fibrofatty proliferation 39.7% at baseline and 12.4% at week 12; mesenteric lymphadenopathies: 27.7% at baseline and 14.6% at week 12; ascites: 10.3% at baseline and 2.2% at week 12; loss of bowel wall stratification: 23.2% at baseline and 10.1% at week 12; loss of haustration: 56.7% at baseline and 32.6% at week 12 (p<0.001 for change during the study course for all parameters).
Figure 2.
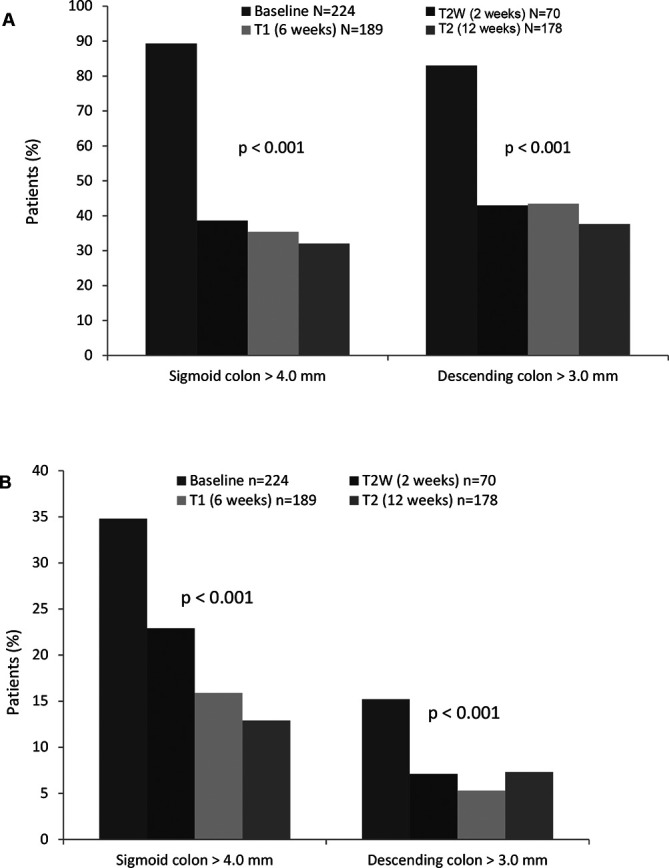
(A) Proportion of patients with increased BWT over the study period. (B) Proportion of patients with increased colour Doppler signal over the study period; comparisons of the values during the study course were performed by Cochran Q test. BWT, bowel wall thickness.
Figure 3.
Percentage of patients with additional IUS parameters at baseline, T2W, T1, T2. IUS, intestinal ultrasound.
In correlation with the improvement of the BWT, the SCCAI decreased from 9.00 (7.00–11.00) points at baseline to 4.00 (2.00–7.00) points after 2 weeks, and to 2.00 (1.00–5.00) and 2.00 (0.00–5.00) points, respectively, at weeks 6 and 12 in response to treatment optimisation (p<0.001).
For the primary endpoint, patients with normalisation of BWT at week 12 and the frequency of clinical response at week 12 were analysed. Table 2 summarises the results for n=178 patients who completed the week 12 visit.
Table 2.
Normalisation of BWT (mm) at T2 (week 12) versus clinical response; X2 test
| Clinical response at T2 | Sigmoid colon | Descending colon | ||
| BWT normalisation | No BWT normalisation | BWT normalisation | No BWT normalisation | |
| % (n) | % (n) | % (n) | % (n) | |
| Yes | 90.5 (95) | 68.5 (50) | 96.4 (80) | 68.4 (65) |
| No | 9.5 (10) | 31.1 (23) | 3.6 (3) | 31.2 (30) |
| P<0.001 | P<0.001 | |||
BWT, bowel wall thickness.
Patients with a normalisation of the BWT in the sigmoid or descending colon demonstrated higher rates of clinical response than patients without normalisation of the BWT (90.5% vs 68.9% and 96.4% vs 68.8%; p<0.001 each). Responders showed a significant reduction in quantitative BWT (mm) in the sigmoid or descending colon from baseline to week 12 compared with the reduction in BWT of non-responders (online supplementary table S1).
gutjnl-2019-319451supp001.pdf (844.7KB, pdf)
In addition, the quantitative analysis of BWT (mm) indicated the same tendency as described above for the T2-population for the mITT population (n=224) (online supplementary table S2).
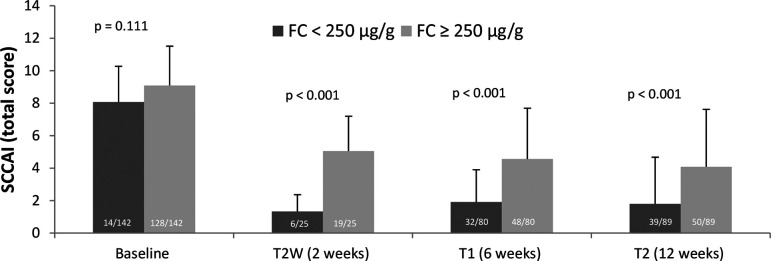
In a subset of patients (n=63, endoscopy date ±7 days from the date of the study visit), we found a highly significant correlation between endoscopic disease activity and increased BWT (online supplementary figure S3). As secondary endpoint, correlation between FC and SCCAI, FC and BWT in the descending/sigmoid colon and between FC and clinical response at baseline, at week 6 and 12 were analysed. For this purpose, the patients were separated into two groups, one group with FC levels of <250 µg/g and another group with FC levels of ≥250 µg/g. In total, FC levels were measured at baseline in n=142 patients, at T2W in n=25 patients, at T1 in n=80 patients and at T2 in n=89 patients.
The results for the SCCAI and FC correlation are depicted in figure 4. At baseline, no significant difference in the SCCAI was present between the two groups (8.07±2.2 vs 9.09±2.42). At weeks 2, 6 and 12, patients with FC levels <250 µg/g had a significantly lower SCCAI (1.33±1.03, 1.91±1.99 and 1.79±2.88) as compared with patients with FC levels ≥250 µg/g (5.05±2.15, 4.56±3.13 and 4.08±3.54).
Figure 4.
Correlation of SCCAI total score and FC for FC group <250 μg/g versus FC group ≥250 μg/g; number in columns represents number of patients. Mann-Whitney U test. FC, faecal calprotectin; SCCAI, Short Clinical Colitis Activity Index.
For the correlation of BWT in the sigmoid colon and FC, patients were divided into two groups, one with increased BWT present at every visit and the other one without increasement of BWT at every visit after baseline. Despite the low number of patients with assessed FC, differences between the FC groups were observed (table 3). At T2, 44.4% of the patients (28/63) with normalised BWT in the sigmoid colon still had FC levels ≥250 µg/g, while 55.6% of the patients with normalised BWT in the sigmoid colon had an FC level of <250 µg/g (55.6%, 35/63). Eighty-four per cent of the patients with a persistent increase in BWT (21/25) had FC levels ≥250 µg/g, while 16.0% of the patients with increased BWT had an FC level of <250 µg/g (4/25). Similar tendencies were detected for T1 and for the descending colon (data not shown).
Table 3.
Correlation of BWT and FC for FC group <250 µg/g versus FC group ≥250 µg/g; X2 test
| Increased BWT | Total % (n) |
FC <250 µg/g % (n) |
FC ≥250 µg/g % (n) |
| Baseline (n=142, p=0.253) | |||
| Yes | 92.3 (131) | 10.7 (14) | 89.3 (117) |
| No | 7.7 (11) | 0.0 (0) | 100.0 (11) |
| T2W (n=25, p=0.080) | |||
| Yes | 28.0 (7) | 0.0 (0) | 100 (7) |
| No | 72.0 (18) | 33.3 (6) | 66.7 (12) |
| T1 (n=80, p=0.014) | |||
| Yes | 31.2 (25) | 20.0 (5) | 80.0 (20) |
| No | 68.8 (55) | 49.1 (27) | 50.9 (28) |
| T2 (n=89, p=0.002) | |||
| Yes | 28.1 (25) | 16.0 (4) | 84.0 (21) |
| No | 70.8 (63) | 55.6 (35) | 44.4 (28) |
| N/A* | 1.1 (1) | 0.0 (0) | 100.0 (1) |
*Could not be determined in complete extend.
BWT, bowel wall thickness; FC, faecal calprotectin.
No differences between the patients of the two FC groups were observed for clinical response (data not shown). The CRP value was not meaningful to confirm an existing inflammation and was therefore not considered for correlation analysis with BWT.
For the mITT population at week 12 (n=178), the normalisation of BWT and the frequency of a normalised FC value (<250 µg/g) were subjected to correlation analysis. A normalised FC value was defined as an FC value ≥250 µg/g at baseline, which was <250 µg/g at week 12. FC analysis was only performed for those patients who experienced FC measurements at baseline and at the 12 week visit.
Normalisation of FC was more frequent in patients with normalisation of the BWT in the sigmoid or descending colon than in patients without normalisation of the BWT after 12 weeks (48.9% vs 22.2%; p=0.023% and 50.0% vs 25.0%; p=0.029, table 4). When only considering patients with BWT normalisation at week 12, only half of the patients with FC measurement had an FC value <250 µg/g (48.9% vs 51.1% sigmoid colon; p=0.884, and 50% vs 50% descending colon; p=1.000).
Table 4.
Normalisation of BWT at T2 (week 12) vs normalised FC; X2 test
| Calprotectin <250 µg/g at T2 | Sigmoid colon | Descending colon | ||
| BWT normalisation | No BWT normalisation | BWT normalisation | No BWT normalisation | |
| % (n) | % (n) | % (n) | % (n) | |
| Yes | 48.9 (23) | 22.2 (6) | 50.0 (21) | 25.0 (8) |
| No | 51.1 (24) | 77.8 (21) | 50.0 (21) | 75.0 (24) |
| P=0.023 | P=0.029 | |||
BWT, bowel wall thickness; FC, faecal calprotectin.
For the BWT of the sigmoid/descending colon, correlation analyses were performed for the mITT population to determine the correlation with the SCCAI as well as the FC value for all visits (table 5).
Table 5.
Correlation of SCCAI, FC and BWT in the sigmoid and descending colon
| Visit | N | rSpearman | ||
| SCCAI | Calprotectin | Baseline | 142 | 0.202 |
| T2 | 89 | 0.408 | ||
| BWT sigmoid colon (mm) | Baseline | 218 | 0.187 | |
| T2 | 140 | 0.547 | ||
| BWT descending colon (mm) | Baseline | 198 | 0.262 | |
| T2 | 115 | 0.5 | ||
| Calprotectin | BWT sigmoid colon (mm) | Baseline | 141 | −0.053 |
| T2 | 73 | 0.344 | ||
| BWT descending colon (mm) | Baseline | 129 | 0.218 | |
| T2 | 67 | 0.358 |
BWT, bowel wall thickness; FC, faecal calprotectin; SCCAI, Short Clinical Colitis Activity Index.
We have also correlated BWT and SCCAI as well as the changes in both parameters on an individual patient basis (online supplementary table S4). Overall, a moderate correlation was observed between the parameters.
During the study, the use of biologics (antitumour necrosis factor or integrin inhibitor therapy) increased from 27.7% (62/224) of the patients at baseline to 43.4% (82/189) at week 6 and 45.5% (81/178) at week 12. The use of systemic steroids doubled in the first 2 weeks as short-term therapy escalation (15.2% (34/224) of the patients at baseline and 30.0% (21/70) at week 2) and then decreased from week 6 to week 12 (25.9% (49/189) at week 6 and 19.7% (35/178) at week 12).
Discussion
It has become evident that treating IBDs solely by clinical symptoms poses the risk of overtreating or undertreating.5 However, as more treatment options and possibilities of optimising therapy via drug monitoring become available, and the IBD treatment algorithm moves towards a more personalised approach, reliable monitoring tools for a ‘treat-to-target’ strategy are required. Ideally, these monitoring tools are non-invasive, non-harmful to the patient, inexpensive and easy to repeat as is true for IUS. In recent years, we and others have demonstrated the potential use of IUS to monitor the therapy of CD in a ‘treat-to-target’ approach.29 36 37
Data on using IUS to monitor treatment success in UC are still limited and most studies are single-centre studies usually performed in centres with a special expertise in IUS. To our knowledge, this study, with more than 40 participating centres, is the largest multicentre study investigating the use of IUS in UC. We were able to demonstrate rapid IUS response to therapy as early as after 2 weeks. The applicability of IUS was shown in a multicentre setting with a broad spectrum of centres with various ultrasound machines. We were also able to clearly demonstrate that IUS for a point-of-care approach is not a specialised method limited to few centres with high-end ultrasonography machines. Comparable results between different gastroenterologists working at various levels of medical care indicate that bowel IUS is a reliable tool to monitor disease courses in patients with UC in daily practice.
Sigmoidoscopy is currently considered the gold standard to monitor treatment in UC. In addition, as UC is defined as a mucosal and to some extent submucosal disease, IUS is often regarded as not being sensitive enough to detect colonic inflammation, and thus only helpful for a small subset of patients. In our study, we were able to demonstrate that in 88.5% of enrolled patients with a clinical flare, an increased BWT was detected at baseline. Thickening of the submucosa probably due to submucosal oedema is likely to contribute to increased BWT in active UC. This clearly supports IUS as a non-invasive monitoring tool for UC. The higher rate of UC patients with bowel alterations as compared with the rate in older studies in the literature ranging from 53% to 84%38 39 is most likely due to the effect of improved ultrasound machines and technology, leading to a better and more sensitive detection of the bowel wall and the underlying pathology during the inflammation.
One of the most striking results, however, was a rapid improvement in IUS pathology as early as 2 weeks after treatment intensification, followed by clinical improvement as determined by SCCAI. Future studies will have to investigate whether IUS as early as 2 weeks can discriminate responders from non-responders and partial responders to facilitate early optimisation of treatment. The most distinct IUS parameter for the detection of inflammatory activity within the intestine was BWT, which mostly correlated well with clinical activity markers such as the Harvey Bradshaw index and the CD activity index.40 41 To our knowledge, for the first time we here demonstrate a moderate correlation between SCCAI and BWT at 12 weeks. Additional IUS parameters, such as mesenteric fibrofatty proliferations, bowel wall stratification as well as haustration and ascites, had significantly improved at the 12-week visit which again highlights the capacity of IUS to very sensitively detect and monitor further IBD-related pathologies. Bowel wall vascularisation normalised significantly in response to anti-inflammatory therapy as early as 2 weeks after treatment intensification. This concurs with the results of recent single-centre studies in UC evaluating potential IUS scoring systems.42 43 While these scores include several parameters, our data suggest that, in UC, measuring BWT for follow-up examinations to determine treatment response by IUS might already be sufficient. This hypothesis requires further validation in future studies. If validated, this would clearly simplify the procedure in a point-of-care setting.44
The combination of IUS and FC are ideal non-invasive tools to monitor disease activity in UC. FC is routinely determined and is also recommended as a surrogate marker for monitoring disease activity in the national as well as international guidelines.24 32 Our study showed that, unlike IUS, FC tests are not performed on a regular basis in Germany, especially not in a non-interventional study design. Therefore, limited FC values were available for the correlation of FC and IUS results or SCCAI. The current data suggest that IUS parameters better correlate with disease activity than FC levels. It can also be concluded that IUS parameters change more quickly after treatment intensification than FC levels, which need to be confirmed in future studies. The combination of IUS and biomarkers might increase the accuracy in determining disease activity and optimise therapy in a patient with UC. In patients with disease limited to the mucosa as well as in patients with proctitis only, the use of FC may be superior to IUS in detecting and monitoring disease activity. Nevertheless, Allocca et al published that a combination of Humanitas Ultrasound Criteria (HUC) with FC did not significantly alter the accuracy of HUC, suggesting that ultrasound parameters alone are sufficient to determine endoscopic activity in UC.28 A huge advantage of IUS over FC is the fact that the results of the measurement are immediately available during examination allowing for rapid treatment decisions.
Our study is subject to several limitations. One is the lack of the currently defined gold standard for UC, namely endoscopy. This limitation may be justified by the objective of our study which was the use of IUS examination in routine monitoring of patients with UC rather than determining the accuracy of IUS compared with other diagnostic procedures. This comparison has already been the subject of several previous studies.15 23 27 41 45 46 However, investigators documented endoscopies, performed as part of clinical routine care, and we found highly significant correlations between the increased BWT and the endoscopic Mayo subscore. These data confirm observations that have recently been demonstrated in a prospective trial.42
As this study was a non-interventional study, medication changes were based on the investigators’ decision which led to small and inhomogeneous patient populations. As the aim of the study was to evaluate the feasibility and benefit of IUS as a monitoring tool for patients with UC, a detailed description of medication changes was not included.
Another limitation may be the potential interobserver and interequipment variability when using IUS examination. We found no differences in the diagnostic quality of IUS measurements between different gastroenterologists.
No significant differences were obtained regarding the results of measurements at different IBD centres or with the use of different US machines. We found no significant differences between the disease duration at different study sites, suggesting a homogeneous patient distribution between the sites. One could also presume a selection bias, but we found remarkable differences between patients with and without increased BWT by IUS at baseline. The latter had significantly longer disease duration, lower SCCAI and enhanced laboratory parameters (lower platelets, white cell count, lower FC, higher haemoglobin), suggesting a milder disease activity for this subpopulation (data not shown).
In conclusion, IUS may be preferred in general practice in a point-of-care setting for monitoring the disease course and for assessing short-term treatment response. Our findings give rise to the assumption that monitoring BWT alone has the potential to predict therapeutic response. The additional monitoring of FC could add further value in monitoring patients with UC. Future prospective studies are necessary to determine the value of IUS in predicting short and long-term response and outcomes of therapies in UC.
Footnotes
Contributors: Study concept and design: CM, TK, UH, AR, SR and GISG (German IBD Study Group). Analysis: IF. Interpretation of data: CM, FP, UH, IF, TK, AR, SR and DL. Drafting of the manuscript: CM, FP, UH, IF, TK, AR, SR and DL. Critical revision of the manuscript for important intellectual content and approval of final version: CM, FP, UH, IF, TK, AR, SR and DL.
Funding: The design, study conduct, and financial support for the study were provided by AbbVie. AbbVie participated in the interpretation of data, review and approval of the study. AbbVie provided funding to the study group for this work.
Competing interests: CM received honorary fees from AbbVie, Biogen, Celgene, Ferring, Falk Foundation, Janssen, MSD Sharp & Dome, Takeda Pharma and Vifor Pharma. UH received lecture and consulting fees from AbbVie, Celltrion, MSD, Ferring, Falk Foundation, Takeda, Mundipharma, Hospira, Pfizer, Amgen, Biogen, Shield, Janssen and Vifor Pharma. IF received consulting and honorary fees from AbbVie. AR, SR and DL are AbbVie employees, and may own AbbVie stock or options. TK received honorary fees from AbbVie, Biogen, Boehringer Ingelheim, Ferring, Hospira, Mundipharma, Falk Pharma GmbH, Janssen, MSD Sharp & Dome and Takeda Pharma.
Patient consent for publication: Not required.
Ethics approval: The protocol was approved by the ethics committee of the Aerztekammer Niedersachsen (Bo/19/2015) on 15 July 2015 and performed in accordance with the principles of the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplementary information.
Contributor Information
On behalf of the German IBD Study Group and TRUST&UC study group:
Martin Faehndrich, Martin Hoffstadt, Stefan Schanz, Klaus Fellermann, Ulf Helwig, Michael Karaus, Henning Kempelmann, Torsten Kucharzik, Tanja Kuehbacher, Diether Ludwig, Andrea Pace, Stefan Schreiber, Carsten Buening, Ulrich Graefe, Harald Gruemmer, Astrid Linde, Stefan Schubert, Martin Wetzel, Ingrid Koenig, Sven Pannach, Ingolf Schiefke, Jens Walldorf, Olaf Engelke, Susanne Erwig, Barbara Lanyi, Axel Naumann, Ron Winograd, Martin Dreier, Theodor Kudlich, Lars Langeloh, Deike Strobel, Dieter Witzemann, Michael Boehmi, Norbert Boerner, Wolfgang Mohl, Gero Moog, Ursula Pohlmann, Benjamin Simonis, Hartmut Steinbrueck, Gerd-Ruediger Franke, Johanna Vogelpohl, and Joachim Weber-Guskar
References
- 1. Silverberg MS, Satsangi J, Ahmad T, et al. . Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working Party of the 2005 Montreal world Congress of gastroenterology. Can J Gastroenterol 2005;19 Suppl A:5A–36. 10.1155/2005/269076 [DOI] [PubMed] [Google Scholar]
- 2. Colombel J-F, Narula N, Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology 2017;152:351–61. 10.1053/j.gastro.2016.09.046 [DOI] [PubMed] [Google Scholar]
- 3. Jharap B, Sandborn WJ, Reinisch W, et al. . Randomised clinical study: discrepancies between patient-reported outcomes and endoscopic appearance in moderate to severe ulcerative colitis. Aliment Pharmacol Ther 2015;42:1082–92. 10.1111/apt.13387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Restellini S, Chao C-Y, Martel M, et al. . Clinical parameters correlate with endoscopic activity of ulcerative colitis: a systematic review. Clin Gastroenterol Hepatol 2019;17:1265–75. 10.1016/j.cgh.2018.12.021 [DOI] [PubMed] [Google Scholar]
- 5. Narula N, Alshahrani A-A, Yuan Y, et al. . Patient-Reported outcomes and endoscopic appearance of ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2019;17:411–8. 10.1016/j.cgh.2018.06.015 [DOI] [PubMed] [Google Scholar]
- 6. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. . Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for Treat-to-Target. Am J Gastroenterol 2015;110:1324–38. 10.1038/ajg.2015.233 [DOI] [PubMed] [Google Scholar]
- 7. Castiglione F, Imperatore N, Testa A, et al. . One-Year clinical outcomes with biologics in Crohn's disease: transmural healing compared with mucosal or no healing. Aliment Pharmacol Ther 2019;49:1026–39. 10.1111/apt.15190 [DOI] [PubMed] [Google Scholar]
- 8. RØseth AG, Fagerhol MK, Aadland E, et al. . Assessment of the neutrophil dominating protein calprotectin in feces: a methodologic study. Scand J Gastroenterol 1992;27:793–8. 10.3109/00365529209011186 [DOI] [PubMed] [Google Scholar]
- 9. Costa F, Mumolo MG, Bellini M, et al. . Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Digestive and Liver Disease 2003;35:642–7. 10.1016/S1590-8658(03)00381-5 [DOI] [PubMed] [Google Scholar]
- 10. Limburg PJ, Ahlquist DA, Sandborn WJ, et al. . Fecal calprotectin levels predict colorectal inflammation among patients with chronic diarrhea referred for colonoscopy. Am J Gastroenterol 2000;95:2831–7. 10.1111/j.1572-0241.2000.03194.x [DOI] [PubMed] [Google Scholar]
- 11. Bryant RV, Friedman AB, Wright EK, et al. . Gastrointestinal ultrasound in inflammatory bowel disease: an underused resource with potential paradigm-changing application. Gut 2018;67:973–85. 10.1136/gutjnl-2017-315655 [DOI] [PubMed] [Google Scholar]
- 12. Novak K, Tanyingoh D, Petersen F, et al. . Clinic-based Point of Care Transabdominal Ultrasound for Monitoring Crohn’s Disease: Impact on Clinical Decision Making. ECCOJC 2015;9:795–801. 10.1093/ecco-jcc/jjv105 [DOI] [PubMed] [Google Scholar]
- 13. Valette P-J, Rioux M, Pilleul F, et al. . Ultrasonography of chronic inflammatory bowel diseases. Eur Radiol 2001;11:1859–66. 10.1007/s003300101065 [DOI] [PubMed] [Google Scholar]
- 14. Buisson A, Gonzalez F, Poullenot F, et al. . Comparative acceptability and perceived clinical utility of monitoring tools: a nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis 2017;23:1425–33. 10.1097/MIB.0000000000001140 [DOI] [PubMed] [Google Scholar]
- 15. Civitelli F, Di Nardo G, Oliva S, et al. . Ultrasonography of the colon in pediatric ulcerative colitis: a prospective, blind, comparative study with colonoscopy. J Pediatr 2014;165:78–84. 10.1016/j.jpeds.2014.02.055 [DOI] [PubMed] [Google Scholar]
- 16. Ruess L, Blask AR, Bulas DI, et al. . Inflammatory bowel disease in children and young adults: correlation of sonographic and clinical parameters during treatment. AJR Am J Roentgenol 2000;175:79–84. 10.2214/ajr.175.1.1750079 [DOI] [PubMed] [Google Scholar]
- 17. Bru C, Sans M, Defelitto MM, et al. . Hydrocolonic sonography for evaluating inflammatory bowel disease. AJR Am J Roentgenol 2001;177:99–105. 10.2214/ajr.177.1.1770099 [DOI] [PubMed] [Google Scholar]
- 18. Haber HP, Busch A, Ziebach R, et al. . Ultrasonographic findings correspond to clinical, endoscopic, and histologic findings in inflammatory bowel disease and other enterocolitides. J Ultrasound Med 2002;21:375–82. 10.7863/jum.2002.21.4.375 [DOI] [PubMed] [Google Scholar]
- 19. Bremner AR, Griffiths M, Argent JD, et al. . Sonographic evaluation of inflammatory bowel disease: a prospective, blinded, comparative study. Pediatr Radiol 2006;36:947–53. 10.1007/s00247-006-0245-8 [DOI] [PubMed] [Google Scholar]
- 20. Arienti V, Campieri M, Boriani L, et al. . Management of severe ulcerative colitis with the help of high resolution ultrasonography. Am J Gastroenterol 1996;91:10:2163–9. [PubMed] [Google Scholar]
- 21. Maconi G, et al. Ultrasonography in the evaluation of extension, activity, and follow-up of ulcerative colitis. Scand J Gastroenterol 1999;34:1103–7. 10.1080/003655299750024904 [DOI] [PubMed] [Google Scholar]
- 22. Sonnenberg A, Erckenbrecht J, Peter P, et al. . Detection of Crohn's disease by ultrasound. Gastroenterology 1982;83:430–4. [PubMed] [Google Scholar]
- 23. Antonelli E, Giuliano V, Casella G, et al. . Ultrasonographic assessment of colonic wall in moderate–severe ulcerative colitis: comparison with endoscopic findings. Digestive and Liver Disease 2011;43:703–6. 10.1016/j.dld.2011.02.019 [DOI] [PubMed] [Google Scholar]
- 24. Maaser C, Sturm A, Vavricka SR, et al. . ECCO-ESGAR guideline for diagnostic assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019;13:144–64. 10.1093/ecco-jcc/jjy113 [DOI] [PubMed] [Google Scholar]
- 25. Nylund K, Maconi G, Hollerweger A, et al. . EFSUMB recommendations and guidelines for gastrointestinal ultrasound. Ultraschall Med 2017;38:e1–15. 10.1055/s-0042-115853 [DOI] [PubMed] [Google Scholar]
- 26. Maconi G, Nylund K, Ripolles T, et al. . EFSUMB recommendations and clinical guidelines for intestinal ultrasound (GIUS) in inflammatory bowel diseases. Ultraschall in Med 2018;39:304–17. 10.1055/s-0043-125329 [DOI] [PubMed] [Google Scholar]
- 27. Horsthuis K, Bipat S, Bennink RJ, et al. . Inflammatory bowel disease diagnosed with us, Mr, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 2008;247:64–79. 10.1148/radiol.2471070611 [DOI] [PubMed] [Google Scholar]
- 28. Allocca M, Fiorino G, Bonifacio C, et al. . Comparative accuracy of bowel ultrasound versus magnetic resonance enterography in combination with colonoscopy in assessing Crohn's disease and guiding clinical decision-making. J Crohns Colitis 2018;12:1280–7. 10.1093/ecco-jcc/jjy093 [DOI] [PubMed] [Google Scholar]
- 29. Kucharzik T, Wittig BM, Helwig U, et al. . Use of intestinal ultrasound to monitor Crohn's disease activity. Clin Gastroenterol Hepatol 2017;15:535–42. 10.1016/j.cgh.2016.10.040 [DOI] [PubMed] [Google Scholar]
- 30. Mannon PJ, Hornung RL, Yang Z, et al. . Suppression of inflammation in ulcerative colitis by interferon-β-1a is accompanied by inhibition of IL-13 production. Gut 2011;60:449–55. 10.1136/gut.2010.226860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roccarina D, Garcovich M, Ainora ME, et al. . Diagnosis of bowel diseases: the role of imaging and ultrasonography. World J Gastroenterol 2013;19:2144–53. 10.3748/wjg.v19.i14.2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kucharzik TDA, Atreya R, Bokemeyer B. Updated S3-Guideline ulcerative colitis. German Society for digestive and metabolic diseases (DGVS). Z Gastroenterol 2019;57:162–241. 10.1055/a-0824-0861 [DOI] [PubMed] [Google Scholar]
- 33. Pascu M, Roznowski AB, Müller H-P, et al. . Clinical relevance of transabdominal ultrasonography and magnetic resonance imaging in patients with inflammatory bowel disease of the terminal ileum and large bowel. Inflamm Bowel Dis 2004;10:373–82. 10.1097/00054725-200407000-00008 [DOI] [PubMed] [Google Scholar]
- 34. Schuler A, Reuss J, Delorme S, et al. . [Costs of clinical ultrasound examinations - an economical cost calculation and analysis]. Ultraschall Med 2010;31:379–86. 10.1055/s-0029-1245283 [DOI] [PubMed] [Google Scholar]
- 35. Bittl M, Petersen F, Maaser C, et al. . P171 reliable assessment of ultrasound parameters during transabdominal ultrasonography in inflammatory bowel disease. J Crohns Colitis 2017;11:S163–4. 10.1093/ecco-jcc/jjx002.297 [DOI] [Google Scholar]
- 36. Fernandes SR, Rodrigues RV, Bernardo S, et al. . Transmural healing is associated with improved long-term outcomes of patients with Crohn's disease. Inflamm Bowel Dis 2017;23:1403–9. 10.1097/MIB.0000000000001143 [DOI] [PubMed] [Google Scholar]
- 37. Dillman JR, Dehkordy SF, Smith EA, et al. . Defining the ultrasound longitudinal natural history of newly diagnosed pediatric small bowel Crohn disease treated with infliximab and infliximab–azathioprine combination therapy. Pediatr Radiol 2017;47:924–34. 10.1007/s00247-017-3848-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parente F, Greco S, Molteni M, et al. . Role of early ultrasound in detecting inflammatory intestinal disorders and identifying their anatomical location within the bowel. Aliment Pharmacol Ther 2003;18:1009–16. 10.1046/j.1365-2036.2003.01796.x [DOI] [PubMed] [Google Scholar]
- 39. Worlicek H, Lutz H, Heyder N, et al. . Ultrasound findings in Crohn's disease and ulcerative colitis: a prospective study. J. Clin. Ultrasound 1987;15:153–63. 10.1002/jcu.1870150302 [DOI] [PubMed] [Google Scholar]
- 40. Calabrese E, Petruzziello C, Onali S, et al. . Severity of postoperative recurrence in Crohn's disease: correlation between endoscopic and sonographic findings. Inflamm Bowel Dis 2009;15:1635–42. 10.1002/ibd.20948 [DOI] [PubMed] [Google Scholar]
- 41. Rigazio C, Ercole E, Laudi C, et al. . Abdominal bowel ultrasound can predict the risk of surgery in Crohn's disease: proposal of an ultrasonographic score. Scand J Gastroenterol 2009;44:585–93. 10.1080/00365520802705992 [DOI] [PubMed] [Google Scholar]
- 42. Allocca M, Fiorino G, Bonovas S, et al. . Accuracy of Humanitas ultrasound criteria in assessing disease activity and severity in ulcerative colitis: a prospective study. J Crohns Colitis 2018;12:1385–91. 10.1093/ecco-jcc/jjy107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bots S, Nylund K, Löwenberg M, et al. . Ultrasound for assessing disease activity in IBD patients: a systematic review of activity scores. J Crohns Colitis 2018;12:920–9. 10.1093/ecco-jcc/jjy048 [DOI] [PubMed] [Google Scholar]
- 44. Kucharzik T, Maaser C, Maconi G. Do we need activity scores or simply clear criteria for intestinal ultrasound in ulcerative colitis? J Crohns Colitis 2018;12:1383–4. 10.1093/ecco-jcc/jjy167 [DOI] [PubMed] [Google Scholar]
- 45. Strobel D, Goertz RS, Bernatik T. Diagnostics in inflammatory bowel disease: ultrasound. World J Gastroenterol 2011;17:3192–7. 10.3748/wjg.v17.i27.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kinoshita K, Katsurada T, Nishida M, et al. . Usefulness of transabdominal ultrasonography for assessing ulcerative colitis: a prospective, multicenter study. J Gastroenterol 2019;54:521–9. 10.1007/s00535-018-01534-w [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2019-319451supp001.pdf (844.7KB, pdf)