Abstract
Mast cells (MCs) are systemically distributed and secrete several allergic mediators such as histamine and leukotrienes to cause type I hypersensitivity. Dasatinib is a type of anti-cancer agent and it has also been reported to inhibit human basophils. However, dasatinib has not been reported for its inhibitory effects on MCs or type I hypersensitivity in mice. In this study, we examined the inhibitory effect of dasatinib on MCs and MC-mediated allergic response in vitro and in vivo. In vitro, dasatinib inhibited the degranulation of MCs by antigen stimulation in a dose-dependent manner (IC50, ~34 nM for RBL-2H3 cells; ~52 nM for BMMCs) without any cytotoxicity. It also suppressed the secretion of inflammatory cytokines IL-4 and TNF-α by antigen stimulation. Furthermore, dasatinib inhibited MC-mediated passive cutaneous anaphylaxis (PCA) in mice (ED50, ~29 mg/kg). Notably, dasatinib significantly suppressed the degranulation of MCs in the ear tissue. As the mechanism of its effect, dasatinib inhibited the activation of Syk and Syk-mediated downstream signaling proteins, LAT, PLCγ1, and three typical MAP kinases (Erk1/2, JNK, and p38), which are essential for the activation of MCs. Interestingly, in vitro tyrosine kinase assay, dasatinib directly inhibited the activities of Lyn and Fyn, the upstream tyrosine kinases of Syk in MCs. Taken together, dasatinib suppresses MCs and PCA in vitro and in vivo through the inhibition of Lyn and Fyn Src-family kinases. Therefore, we suggest the possibility of repositioning the anti-cancer drug dasatinib as a treatment for various MC-mediated type I hypersensitive diseases.
Keywords: Dasatinib, Mast cell, Type I hypersensitivity, Lyn, Fyn
INTRODUCTION
Allergy diseases, emerging as serious health problems worldwide, are caused by an excessive immune response to harmless antigens (Kagan, 2003; Wang et al., 2012). Among them, mast cells (MCs) play an important role in Type I hypersensitive immune responses such as allergic rhinitis, asthma, food allergy, and atopic dermatitis (Wang et al., 2012). The prevalence of these allergy diseases is on the rise in the world. Symptoms of allergy diseases not only significantly reduce the quality of life but can also cause life-threatening symptoms such as anaphylaxis in severe cases (Begin and Nadeau, 2014). Treatment of allergy diseases is very difficult at present and relies on therapies to relieve symptoms using histamine receptor antagonists or steroids. However, the medications have shown significant limitations in their use due to various side effects (Kay, 2001). Therefore, many scientists are currently researching better therapies as cures for allergy diseases (Kay, 2001; Kagan, 2003).
MCs are well known to be distributed systemically and play an important role in the induction of allergic symptoms (Bruhns et al., 2005). MCs express the IgE high affinity receptor (FcεRI) on the surface membrane, and IgE binds to this receptor (Kitanaka et al., 1998). When antigens bind to the IgE/receptor complex, MCs are activated and eventually secrete histamine, prostaglandins, leukotriene, and various inflammatory cytokines (Murakami and Kudo, 2001; Lee et al., 2010) These cytokines activate various immune cells distributed in tissues such as neutrophils and macrophages, and this event causes tissue inflammation (Mitre and Nutman, 2006). For this reason, many scientists are interested in researching therapies that can treat MC-mediated allergy diseases by inhibiting the activation of MCs.
When antigens bind to the complex of IgE and FcεRI on MCs, Src-family kinases Lyn and Fyn are first activated in MCs. Subsequently, the signaling pathways for allergic mediator release are activated by Syk and Syk-dependent downstream signaling proteins (Yamaguchi et al., 1999; Kambayashi and Koretzky, 2007; Masuda and Schmitz, 2008). Therefore, the inhibition of Syk can alleviate the allergic symptoms by preventing the secretion of granules and cytokines that causes allergic symptoms. Therefore, it has been widely accepted that Syk is an important target protein for the development of drugs to treat allergy diseases.
Dasatinib (N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl) piperazin-1-yl]-2-methylpyrimidin-4-yl] amino]-1,3-thiazole-5-carboxamide) is discovered as a drug to inhibit protein tyrosine kinase such as the Breakpoint cluster region protein-Abelson murine leukemia viral oncogene homolog (Bcr-Abl) and Src-kinase families (Kantarjian et al., 2006). Dasatinib is known to be effective for patients with leukemia that is resistant to other anti-cancer drugs. Currently, dasatinib is used as a treatment for chronic myelogenous leukemia (CML) and acute lymphoblastic leukemia (ALL) (Shah et al., 2002; Kantarjian et al., 2006). Dasatinib was also reported to modulate the activity of human basophil by inhibiting Btk, but its detailed mechanism is still unclear (Kneidinger et al., 2008). Furthermore, the effect of dasatinib on MCs and MC-mediated allergic responses in vivo is unknown.
In this study, we examined the effect of dasatinib on activation of MCs and IgE-mediated allergic response. Through various experiments, we found for the first time that dasatinib inhibits MCs and MC-mediated PCA in mice. As a mechanism, dasatinib was found to directly inhibit the activity of Lyn and Fyn during the activation of MCs. We suggest that dasatinib may potentially be a therapeutic agent for various allergy diseases involving MCs based on these results.
MATERIALS AND METHODS
Antibodies and reagents
Dasatinib (Fig. 1A) was purchased from Selleckchem (Houston, TX, USA) and 4-Amino-5-(4-chlorophenyl)-7-(dimethylethyl) pyrazolo[3,4-d] pyrimidine (PP2) was obtained from Calbiochem (La Jolla, CA, USA). Monoclonal dinitrophenol (DNP)-specific IgE, DNP-Human serum albumin (HSA), Evans blue, toluidine blue O and cetrizine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against the phosphorylated forms of Syk (Tyr32), LAT (Tyr191), PLCγ1 (Tyr783), Akt (Ser473), Erk1/2 (Thr202/Try204), JNK (Thr183/Try185) and p38 (Thr180/Tyr182) were purchased from Cell Signaling Technology, Inc (Danvers, MA, USA). All antibodies were used diluted 1:1000, unless otherwise stated. Antibodies against total forms (dilution fold, 1:100) of Lyn (catalog no. sc-7274), Fyn (catalog no. sc-434), Syk, PLCγ1, Erk1/2, JNK and p38 were obtained from Santa Cruz Biotechnology (Dallas, TX, USA) and LAT (dilution fold, 1:1000) and Actin (dilution fold, 1:5000) antibodies were from EMD Millipore Corporation (Billerica, MA, USA). The media and reagents used for cell culture were from Welgene (Gyeongsangbuk-do, Korea) and Gibco/Life Technologies, Inc (Rockville, MD, USA).
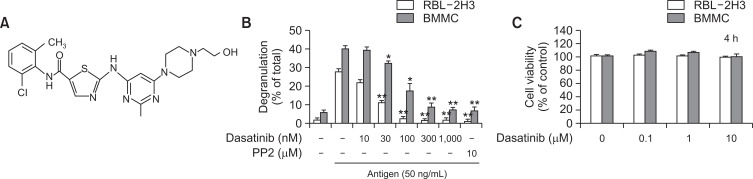
Fig. 1.
Dasatinib inhibits antigen-stimulated degranulation in MCs. (A) The chemical structure of dasatinib. (B) The amount of β-hexosaminidase released from RBL-2H3 and BMMCs. DNP-specific IgE primed RBL-2H3 cells (2.0×105 cells/well) and BMMCs (3.0×105 cells/tube) were stimulated for 15 min by 50 ng/ml antigen with or without the pre-incubating of dasatinib or PP2 for 30 min. (C) Incubation with or without dasatinib for 4 h in MCs and analysis of cytotoxicity by CCK-8. More detail described in the section of “Materials and Methods”. The values represent the mean ± SEM from 3 independent experiments. *p<0.05 and **p<0.01 indicates significant differences compared to antigen only (B). PP2 was used as a typical Src-family kinase inhibitor.
Animals
Mice (Balb/c, 5-week old male mice) were obtained from Orient Bio, Inc (Gyeonggi-do, Korea). The animals were used to prepare bone marrow-derived MCs (BMMCs) and to perform the passive cutaneous anaphylaxis model after 1 week of the adaptation at Konkuk University’s specific pathogen free animal facility. All experiments with mice were conducted according to institutional guidelines after receiving approval from the Institutional Animal Care Committee (IACUC) of Konkuk University (Approval no. KU18127).
Preparation and culture of BMMCs and RBL-2H3 cells
Bone marrow (BM) cells were collected from femurs and tibia of mice and cultured in the Roswell Park Memorial Institute (RPMI) 1640 medium containing 4 mM L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 25 mM HEPES, 10% Fetal bovine serum (FBS) and 10 ng/mL interleukin (IL)-3. The cells were sub-cultured twice a week with fresh complete RPMI medium and used for experiments after 4 weeks of culture. Rat basophilic leukemia (RBL)-2H3 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in complete minimal essential medium (MEM) with Earle’s salts, and supplemented with 4 mM L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, and 15% FBS in a humidified incubator at 37°C and 5% CO2.
Measurements of degranulation in RBL-2H3 cells and BMMCs
The degranulation of MCs was determined by β-hexosaminidase release assay, a granule-marker protein. BMMCs were sensitized overnight with 50 ng/mL DNP-specific IgE. The cells (3.0×105 cells/tube) were washed twice and transferred into the Tyrode buffer (20 mM HEPES, pH7.4, 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 0.1% BSA). Thereafter, BMMCs were incubated with or without dasatinib or PP2 for 30 min before 50 ng/mL of antigen (DNP-HSA) stimulation for 15 min. RBL-2H3 cells on 24-well plates (2.5×105 cells/well) were sensitized with 50 ng/mL of DNP-specific IgE. The next day, the cells were washed twice with PBS and suspended in Siraganian buffer (S-buffer, 25 mM PIPES, pH 7.2, 119 mM NaCl, 5 mM KCl, 0.4 mM MgCl2, 1 mM CaCl2, 5.6 mM glucose, and 0.1% BSA). The cells were incubated for 30 min with or without dasatinib or PP2, and then RBL-2H3 cells were stimulated with 50 ng/mL antigen for 15 min. The degranulation of MCs was determined by calculating the ratio of β-hexosaminidase activity in the supernatant to the total activity from supernatant and cell lysate.
Determination of cell viability
BMMCs and RBL-2H3 cells (both cells 5.0×104 cells/well) were cultured in a 96-well plate for 4 h and subsequently incubated with or without dasatinib. Cell viability was measured by Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s protocol.
Enzyme-linked immunosorbent assay
IgE-primed RBL-2H3 cells (1.0×106 cells/well) were stimulated with 50 ng/mL of DNP-HSA for 3 h with or without dasatinib or PP2. The amount of IL-4 and tumor necrosis factor (TNF)-α in the cultured medium were determined using the BDTM OptEIA ELISA Kit (BD Biosciences, San Jose, CA, USA) following the manufacturer’s protocol.
Immunoblotting analysis
MCs were stimulated with antigen for 15 min with or without dasatinib or PP2 and then placed on ice. The cells were washed 3 times with ice-cold PBS and the cells were lysed using the RIPA buffer (Thermo Fisher scientific, Waltham, MA, USA) in 1 mM phenylmethylsulfonyl fluoride, 2.5 mM p-nitrophenyl phosphate, 0.7 μg/mL pepstatin, and the protease-inhibitor cocktail (Sigma-Aldrich). The cell lysates were centrifuged at 15,000×g for 5 min and the equal amount of protein from the lysates was denatured at 100°C for 5 min with a NuPAGE™LDS sample buffer (4×) (Thermo Fisher Scientific). Proteins were separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Berkeley, CA, USA). The membranes were blocked in 5% BSA for 1 h and then incubated overnight with each primary antibody in the TBS-T buffer (tris-buffered saline with 0.1% tween 20) contained 5% BSA at 4°C. The membranes were washed 3 times with TBS-T buffer and incubated in a horse-radish peroxidase-coupled secondary antibody at room temperature for 1 h. The membranes were washed, and treated with chemiluminescence reagents (Thermo Fisher scientific) according to the manufacturer’s guideline and then visualized and quantified by the ImageQuantTMLAS 4000 system (GE Healthcare Life Sciences, Piscataway, NJ, USA).
In vitro protein tyrosine kinase assay
This experiment was performed as described in the previous study (Nam et al., 2017; Park et al., 2018). Briefly, IgE-sensitized MCs were stimulated with a 50 ng/mL DNP-HSA and were lysed in a NP-40 base lysis buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 1% Nonidet p-40, 10% glycerol, 60 mM octyl-β-glucoside, 10 mM NaF, 1 mM Na3VO4, 1 mM PMSF, 2.5 mM pNPP, 0.7 μg/mL pepstatin, and a protease inhibitor cocktail tablet). Cell lysate was centrifuged at 15,000×g at 4°C for 10 min. The cell lysate containing 1 mg of protein was incubated overnight with 5 µg of a specific antibody against Lyn or Fyn at 4°C and then an addition of 50 µL protein G-agarose was followed. The agarose was washed 5 times with a washing buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 0.1% Nonidet p-40, 10% glycerol, 10 mM NaF, 1 mM Na3VO4, 1 mM PMSF, 2.5 mM pNPP, 0.7 μg/mL pepstatin, and a protease inhibitor cocktail tablet) and the tyrosine kinase activity was analyzed using the Universal Tyrosine Kinase Assay Kit (GenWay Biotech Inc., San Diego, CA, USA) according to the manufacturer’s recommendation.
Passive cutaneous anaphylaxis (PCA)
The formation of PCA was done as previously described (Park et al., 2019). Briefly, mice (Balb/c, 6 weeks of age, male) were injected intradermally with 50 ng DNP-specific IgE in ear. After 24 h, either dasatinib (10, 20, 50 or 100 mg/kg) or cetirizine (50 mg/kg) was diluted in 5% Arabic gum (Sigma-Aldrich) and administered orally to the mice. After 1 h, 250 µL of 5 mg Evans blue/mL PBS with or without 100 μg DNP-HSA was intravenously injected into the mice. The mice were euthanized 1 h after antigen injection, and the ears were removed to measure the amount of dye. The dye was extracted in 1 mL of formamide at 63°C overnight. The absorbance was measured at 620 nm with a microplate reader (Tecan, Männedorf, Switzerland).
Histology
After the PCA, the mice ears were cut and fixed in 4% paraformaldehyde (Biosesang, Gyeonggi-do, Korea). The fixed ear tissue was dehydrated with ethanol and paraffinized. The paraffin tissue was cut into 4-µm thick sections and stained with toluidine blue. To determine the percentage of degranulation, the MCs were calculated as the ratio of degranulated MCs to the total MCs in three sections per ear tissue that was measured.
Statistical analysis
All data were presented as the mean ± SEM from three or more independent experiments. In each animal experiment, five mice were used for the PCA experimental group. For each experiment, the vitro cell experiments were carried out in triplicates. Statistical analysis was performed using the one-way ANOVA and the unpaired student’s t-test. All data analyses were undertaken with the SigmaStat software (Systat Software, Inc., Point Richmond, CA, USA), and the differences that were considered statistically significant set at *p<0.05 and **p<0.01.
RESULTS
Effect of dasatinib on degranulation in antigen-stimulated murine MCs
MCs contain granules in the cells, including histamine, serotonin and platelet activation factors. The secretion of granules by antigen stimulation in MCs is known to play a central role in allergy disorders (Kim et al., 2013). We measured whether dasatinib could inhibit the secretion of intracellular granular β-hexosaminidase, a well-known granule marker. In this experiment, we used RBL-2H3 cells and BMMCs as MCs. Degranulation by antigen stimulation was inhibited by dasatinib in a dose-dependent manner in both RBL-2H3 (IC50, ~34 nM) and BMMCs (IC50, ~52 nM) (Fig. 1B). The inhibitory effect of dasatinib was 30-100 times stronger than that of PP2, a Src-family kinase inhibitor (Fig. 1B). Dasatinib showed no cytotoxicity under our experimental conditions (Fig. 1C).
Dasatinib suppresses pro-inflammatory cytokine production in antigen-stimulated MCs
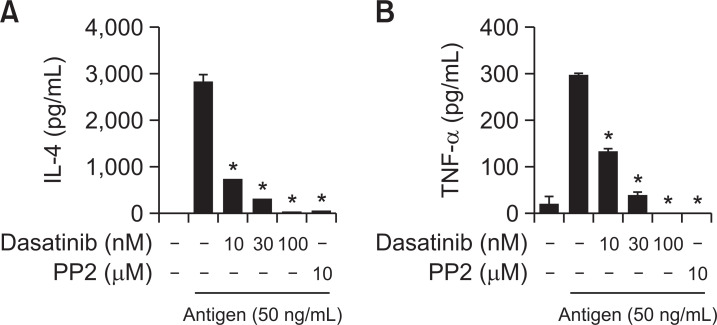
It is commonly known that tumor necrosis factors (TNF)-α and IL-4, which are secreted by antigen stimulation, contribute to the development of allergy diseases (Bradding et al., 2006). TNF-α is one of the major cytokines that can induce late allergic inflammation by attracting various immune cells to the site of inflammation (Bradding et al., 2006; Minai-Fleminger and Levi-Schaffer, 2009). IL-4 is known as a typical Th2 cytokine that plays a critical role in allergic reactions by stimulating the proliferation of MCs in addition to the increase of the FcεRI expression and granule protein in MCs (Bradding et al., 2006; Wedemeyer et al., 2000). We measured the inhibitory effect of dasatinib on cytokine secretion on antigen-stimulated MCs. As a result of ELISA analysis, it was observed that the amount of IL-4 and TNF-α secreted by antigen stimulation was significantly reduced by dasatinib (Fig. 2). It should be noted that even at very low concentrations of dasatinib (100 nM), the inhibitory effect was superior to that of PP2 (10 μM) used as a control.
Fig. 2.
Dasatinib suppresses the secretion of IL-4 and TNF-α from antigen-stimulated MCs. IgE-primed RBL-2H3 cells were pre-treated with dasatinib or PP2 as indicated for 3 h before the addition of 50 ng/ml antigen. The amount of (A) IL-4 and (B) TNF-α were measured using by BDTM ELISA Kit as described in the section for “Materials and Methods”. The values represent the mean ± SEM from three independent experiments. Significant differences with antigen only are indicated, *p<0.01.
Mechanism of action of dasatinib in IgE-mediated MC activation
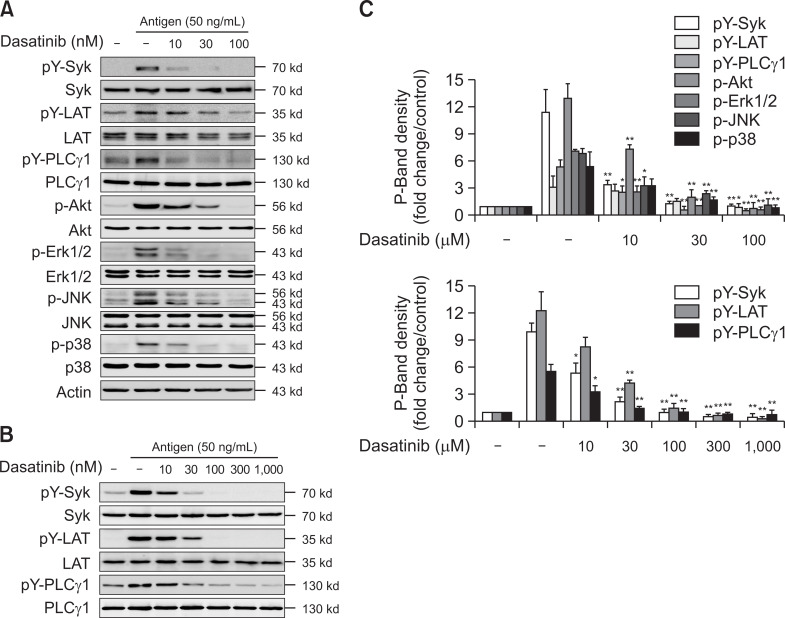
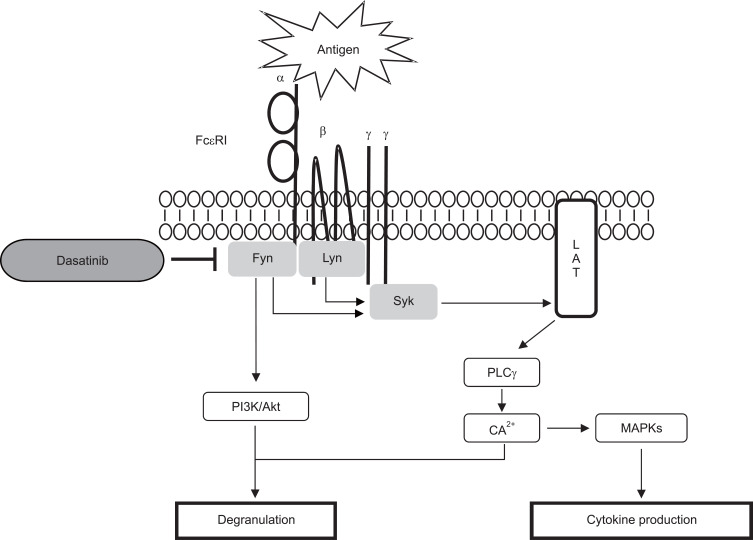
Next, we studied the mechanism of how dasatinib inhibits the activation of MCs by antigen stimulation. Syk is recognized as a protein that plays an essential role in the IgE-mediated signaling pathway in MCs (Kambayashi and Koretzky, 2007). When MCs are stimulated by antigen, Syk is recruited to the phosphorylated ITAM of the γ-subunit of FcεRI and optimally activated (Lin et al., 1996). In RBL-2H3 cells, dasatinib inhibited the phosphorylation of Syk and Syk down-stream proteins, LAT and PLCγ1 in a dose-dependent manner (Fig. 3A). In MCs, Akt and MAP-Kinases (Erk1/2, JNK and p38) are crucial to the synthesis and secretion of cytokines (Qiao et al., 2006). Dasatinib also suppressed the activation of antigen-stimulated Akt, Erk1/2, JNK, and p38 (Fig. 3A). Dasatinib almost completely inhibited Syk and Syk-mediated downstream signaling proteins at the concentration of 30 nM (Fig. 3A, 3C, upper panel). Next, we investigated whether dasatinib suppressed the primary MCs differentiated from bone marrow. As a result, dasatinib inhibited Syk and Syk-mediated signaling proteins in BMMCs, similar to the RBL-2H3 cell line (Fig. 3B, 3C, lower panel). These results suggest that the inhibitory effect of dasatinib may directly act on the upstream tyrosine kinase Lyn or Fyn Src-family kinase that induce Syk activation.
Fig. 3.
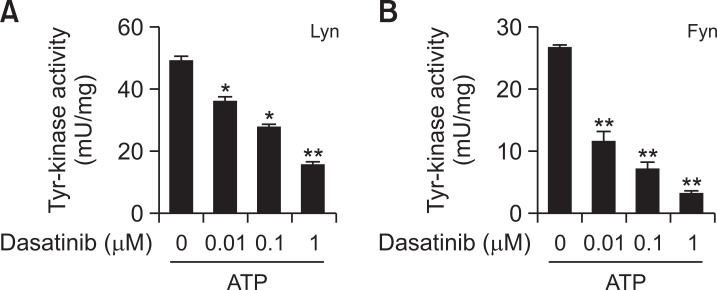
Effects of dasatinib on the tyrosine kinase activity of Lyn and Fyn in vitro. Lyn (A) and Fyn (B) were immunoprecipitated from the cell lysates of MCs as described in the section for “Materials and Methods”. The kinase activity was measured by using the ELISA-based Universal Tyrosine Kinase Assay Kit according to the manufacturer’s instruction. The value shown are mean ± SEM from 3 independent experiments. Significant differences against the values obtained without dasatinib. *p<0.05 and **p<0.01.
Effects of dasatinib on Lyn and Fyn protein kinase activity in vitro
As known, Syk activation by antigen stimulation in MCs is initiated by Src-family kinases such as Lyn and Fyn (Qiao et al., 2006). We tested whether dasatinib directly inhibits the activity of Lyn and Fyn. In vitro tyrosine kinase assay, dasatinib suppressed both Lyn and Fyn activities in a dose-dependent manner (Fig. 4). These results indicate that the inhibitory effect of dasatinib on MC activation is due to the direct inhibition of Lyn and Fyn activity.
Fig. 4.
Dasatinib blockades phosphorylation of Syk and down-signaling protein in antigen-stimulated MCs. (A) RBL-2H3 cells and (B) BMMCs were incubated overnight with antigen-specific IgE. The cells were stimulated with 50 ng/ml antigen for 15 min with or without the pre-treating dasatinib or PP2 for 30 min, as indicated. The cell lysates were used in the Western blot analysis as described in the section for “Materials and Methods”. Representative images (A, B) or the mean ± SEM of band density (C, the upper for panel A; the lower for panel B) from three-independent experiments are shown. Asterisks indicate significant differences between the values obtained following antigen stimulation without dasatinib and those obtained following antigen stimulation with dasatinib or PP2. *p<0.05 and **p<0.01.
Effects of dasatinib on MC-mediated passive cutaneous anaphylaxis (PCA) reaction in mice
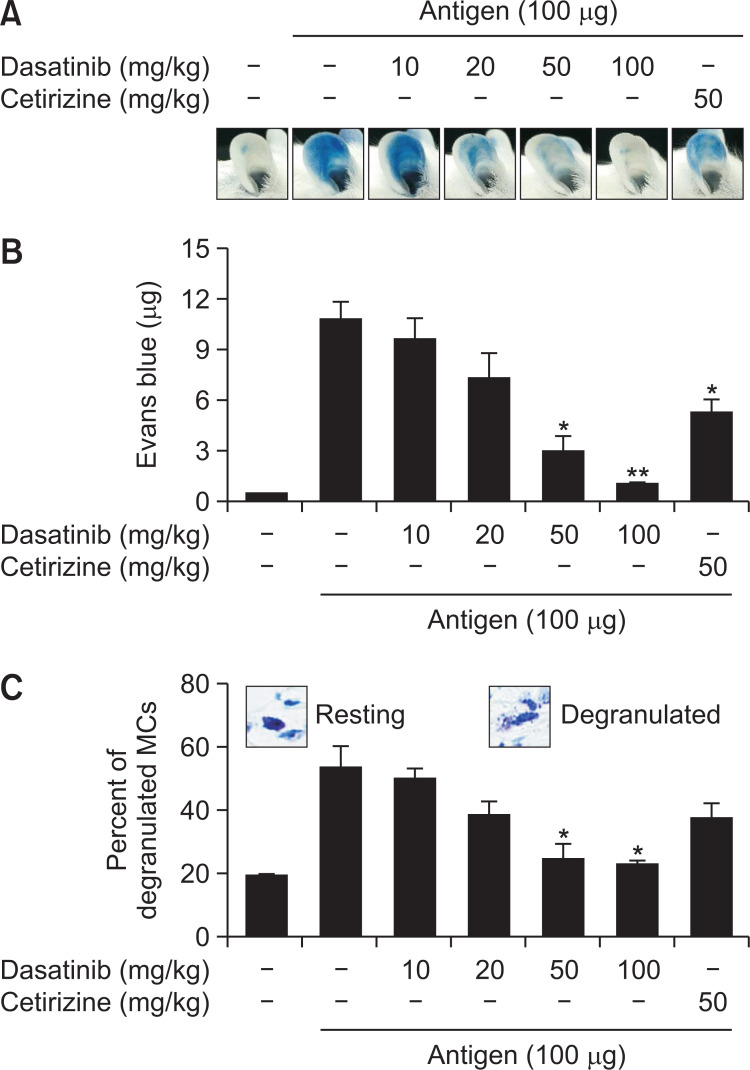
In following, we measured the inhibitory effects of dasatinib using IgE-mediated PCA, a popularly known type I hypersensitive immune response (Kim et al., 2009). After sensitization by injecting IgE into the mouse’s ear, dasatinib was orally administered at the doses of 10, 20, 50 and 100 mg/kg the next day. Then, 100 µg of antigen was injected into the tail of the mouse to induce PCA. In the group injected with antigen only, the ears of mice were heavily stained with Evans blue, whereas the ear staining of mice given oral administration of dasatinib was thinned in a dose-dependent manner (Fig. 5A). As a result of extracting the Evans blue dye from PCA-induced mice ears, the amount of Evans blue dye decreased depending on the dose of dasatinib (Fig. 5B; ED50, ~29 mg/kg). We observed a stronger effect at 50 mg/kg of dasatinib compared to that of cetirizine (50 mg/kg) used as a reference drug (Fig. 5B). After that, we isolated the ear tissue from the PCA-induced mice and measured the degree of degranulation of MCs by toluidine blue staining after the tissue fixation (Fig. 5C). As a result, the percentage of MCs degranulated by antigen was found to be reduced by dasatinib (Fig. 5C). These results indicate that dasatinib inhibits allergic responses by suppressing MCs in vivo.
Fig. 5.
Dasatinib inhibits IgE-mediated passive cutaneous anaphylaxis. DNP-specific IgE was intradermally injected into the mice’s ears. After 24 h, dasatinib or cetirizine in 5% Arabic gum were orally administrated to the mice 1 h before the tail vein injection of 250 µL of Evans blue with or without antigen. After that, mice were euthanized and ears were removed for the extraction of Evans blue dye in formamide. (A) Representative ear images are shown from three independent experiments. (B) Data for the amount of Evans blue dye are expressed as the mean ± SEM of values obtained from three independent experiments (n≥15). (C) The representative images for resting or degranulated ear tissue MC (insets) and the percentage of degranulated MCs per total MCs were presented. Data are expressed as the mean ± SEM of values obtained from three independent experiments (n≥15). Significance differences against the antigen-stimulated group without dasatinib or cetirizine are indicated. *p<0.05 and **p<0.01.
DISCCUSION
Incidences of allergy diseases such as asthma, allergic rhinitis, food allergy and atopic dermatitis have been gradually increasing in countries where industrialization has taken place in the last 50 years (Akin, 2017). MCs are well known as effector cells that play an important role in causing these allergy diseases. MCs are one of the first cells to encounter foreign antigens because they are tissue-resident cells located mainly at the host-environment boundary (Krystel-Whittemore et al., 2016). MCs are activated upon the crosslinking of IgE/IgE-high affinity receptor, FcεRI, by antigen stimulation (Bruhns et al., 2005). Allergy therapies that inhibit the function of MCs are of interest, as they are understood to induce allergy reactions by the release of histamine, serotonin, prostaglandins, leukotrienes, cytokines, and chemokines in MCs (Kambayashi and Koretzky, 2007; Masuda and Schmitz, 2008).
Currently, medicines mainly used to treat allergy diseases are immunosuppressant or antihistamine drugs (Warrington et al., 2018). Most of these medications focus on alleviating symptoms rather than treating the underlying cause of allergy diseases. In addition, drug resistance and side effects of these medicines have great limitations as therapeutic agents (Randall and Hawkins, 2018). Therefore, as an alternative therapeutic approach to treat allergy diseases, scientists have been working on the development of some drugs that inhibit the secretion of the allergic mediators of MCs (Barnes, 1999; Gomez, 2019). In this study, we found that dasatinib inhibited the secretion of MCs and IgE-mediated passive anaphylaxis responses (Fig. 5).
Compounds such as dasatinib and imatinib (Glivec®) inhibit protein tyrosine kinase as anti-cancer agents. Imatinib competitively binds to the adenosine triphosphate-binding site (ATP) in the BCR-ABL fusion protein and thus, imatinib inhibits the tyrosine kinase activity of BCR-ABL to treat chronic myeloid leukemia (Iqbal and Iqbal, 2014). Due to the inhibitory effect of tyrosine kinase, imatinib has been reported to be effective in MC-related diseases. It has a potent therapeutic effect on systemic mytocytosis induced by c-kit mutations affecting the proliferation and activation of MCs (Horny et al., 2007; El-Agamy, 2012). Dasatinib, which has the similar mechanism as imatinib, has been reported to be effective in the treatment of leukemia patients that are resistant to anti-cancer drugs such as imatinib or other anti-cancer drugs (Kantarjian et al., 2006). Dasatinib is a second-generation drug used in the treatment of chronic myelogenous leukemia (CML) and acute lymphoblastic leukemia (ALL) (Shah et al., 2002). It has also been reported that dasatinib improves the clinical symptoms of pulmonary arterial hypertension (Montani et al., 2012). Considering that dasatinib is an inhibitor that suppresses tyrosine kinase, such as Src-family kinase (Shah et al., 2008), it is plausible that dasatinib could inhibit MC secretion by antigen stimulation. However, study on the inhibitory effect of dasatinib on MCs and allergic responses have not been reported. In our studies, dasatinib suppressed MC secretion (Fig. 1) and IgE-mediated allergic reactions in mice (Fig. 5). These results indicate that dasatinib inhibits the activity of MCs, and thereby suppressing allergic response in vivo. We thus suggest that dasatinib is worth developing as a remedy for various allergy diseases.
In this experiment, we measured the degree of degranulation of MCs by antigen stimulation by the rate at which β-hexosaminidase was released from the cells. β-hexosaminidase is a protein contained in granules secreted by MCs. When MCs are stimulated by antigen, it is secreted out of cells when histamine is secreted (Hoffmann et al., 1999). Therefore, many researchers assess the extent of MC degranulation by measuring this protein activity (Mastuda et al., 2002). In our experiment, dasatinib was found to have an inhibitory effect on antigen-induced MC degranulation (Fig. 1B). On the other hand, the late-phase reaction in IgE-mediated Type I allergy occurs within about 4-6 h after the early-phase reaction. TNF-α and IL-4 are critical pro-inflammatory cytokines to stimulate a late-phase allergic responses (Aketani et al., 2001; Bradding et al., 2006). TNF-α can recruit immune cells near the site of inflammation to trigger local inflammation (Hide et al., 1997). IL-4 promotes IgE production from B cells and can cause allergy symptoms through inflammation and the contraction of smooth muscle (Geha et al., 2003). Therefore, the inhibition of cytokine secretion is associated with the suppression of late phase allergic responses. Dasatinib inhibited the amount of TNF-α and IL-4 secreted by antigen stimulation in MCs (Fig. 2). These results suggest that dasatinib has an inhibitory effect on the late-phase allergic responses as well.
When IgE-primed MCs are stimulated by antigen, many signaling proteins are subsequently activated in the cells. Primarily, Lyn, a Src family kinase, phosphorylates ITAMs of FcεRI, and then Syk are recruited to the phosphorylated ITAMs to be activated (Rivera, 2002; Galli et al., 2005). Activated Syk phosphorylates LAT, in turn induces the activation of PLCγ1 (Rivera et al., 2002; Siraganian et al., 2010). Therefore, the inhibition of Syk is a good option to inhibit MC-mediated allergic responses. In this study, dasatinib inhibited the activation of Syk and Syk-mediated signaling proteins in RBL-2H3 cells and BMMCs (Fig. 3). Dasatinib also suppressed the activation of MAP kinases (Erk1/2, p38, and JNK), which are important for cytokine production, in a dose-dependent manner (Fig. 3). As commonly established, Lyn and Fyn are upstream kinase proteins of Syk in the antigen-activated signaling pathway of MCs (Draber et al., 2016). Based on these reports and our results (Fig. 3), we examined whether dasatinib directly inhibits the activity of Lyn and Fyn in vitro. Of note, we observed that Lyn and Fyn were significantly inhibited at concentrations of dasatinib similar to those in cell culture experiments (Fig. 4). It was reported that dasatinib is also an inhibitor of various protein kinases, including platelet-derived growth factor receptor (PDGFR), KIT, Bcr-Abl, and Src-family kinases (Lombardo et al., 2004). In particular, dasatinib inhibits Src and Yes Src-family kinases (Lombardo et al., 2004). Dasatinib was also reported to bind to other Src-family kinases such as Lyn, Fyn, and Fgr (Kneidinger et al., 2008). In addition to Bcr-Abl and Src-family kinases, dasatinib also inhibits various tyrosine kinases such as p38, Her, FGFR, MEK, and Akt (Lombardo et al., 2004). However, since these inhibitory effects are exerted at concentrations of 100-fold or higher, dasatinib is considered suitable for therapeutic use targeting Src-family kinases.
PCA is widely used in animal models with IgE-mediated acute allergic responses. This method was first reported by Ovary, evaluating the degree of increased vascular permeability by MC secretion (Ovary, 1958). Thus, PCA is useful for finding compounds that suppress Type I hypersensitive responses (Lindner et al., 2010). Briefly, after a certain time after injection of IgE into the mouse’s ear, antigen is administered through the tail vein to induce an allergic response in the ear (Feinberg, 1961; Inagaki and Nagai, 2009). We quantified the degree of vascular leakage by extracting Evans blue dye from the ear tissue by the PCA reaction. Dasatinib inhibited the PCA response by antigen in a dose-dependent manner (Fig. 5A, 5B). In histology assays, it was clear that dasatinib also significantly reduced the degranulation of MCs in ear tissues (Fig. 5C). In our in vitro and in vivo study, however, we observed that the ED50 (29 mg/kg, Fig. 5B) in animal models was relatively high compared to IC50 (Fig. 1B) in in vitro cell experiments. This phenomenon has been often reported in experiments with mast cell stabilizers (Weng et al., 2015; Park et al., 2019). We believe that the difference in the activity of dasatinib in vitro and in vivo is due to the bioavailability of dasatinib in vivo. Additionally, in a tumor xenograft mouse experiment to measure the anti-tumor effect of dasatinib, the tumor growth inhibitory rate (ED50) of dasatinib was more than 10 mg/kg when administered 5 times a week for a month (Xiao et al., 2015). Anti-allergic effect in this study were measured after one oral administration of dasatinib. Of note, no adverse events such as death or behavioral abnormalities were observed in our animal experiments. Therefore, our results suggest the ED50 of dasatinib used in this study is comparable to the dose of dasatinib that has an anti-tumor effect.
Although our findings suggest that dasatinib could be used therapeutically in allergic patients, side effects such as pleural effusion, edema, and cytopenia have been observed in cancer patients treated with dasatinib may limit the use of this drug in allergic patients (Atallah et al., 2007). The cause of these side effects is not yet well understood. Strong inhibition by dasatinib on the activity of PDGFR may be responsible for the side effects (Heuchel et al., 1999). Dasatinib also stimulate histamine secretion by antigen in human-derived basophils at low concentrations under 50 nM (Kneidinger et al., 2008). It is unclear whether this phenomenon is observed in the treatment of cancer patients, but it should be very careful to use dasatinib for the patients with allergy. However, the increase of activity in basophil by dasatinib was not observed in our experiments using mast cells (Fig. 1B). This difference may be due to the difference in signaling pathways in mast cells and basophils by antigen. Although the total number of basophils in blood is increased in Lyn-deficient mouse, Lyn is known to be important as a positive signal in basophil histamine secretion by IgE/antigen stimulation (Schroeder et al., 2001; Charles et al., 2009). However, in the mast cell, Lyn can function as a negative role and Fyn stimulates positive signals (Parravicini et al., 2002). To date, the function of Fyn in basophils is unclear, but it is reasonably expected that negative signals by Lyn could also be activated in basophils by antigen as in mast cells. Dasatinib is likely to inhibit the negative role of Lyn in basophils more specifically at low concentrations. However, at higher concentrations, dasatinib inhibits the activation of basophils by inhibiting Fyn or other positive kinases, including Lyn.
In this study, we observed for the first time that dasatinib inhibits the activation of MCs and MC-mediated type I hypersensitive responses in mice. Dasatinib inhibited MC degranulation and cytokines release by antigen stimulation. As a mechanism for suppressing MCs, dasatinib inhibited Lyn and Fyn Src-family kinases (Fig. 6). Given the various side effects observed in the treatment with dasatinib in cancer patients, it seems that dasatinib has some limitations as an allergy treatment in clinic. However, we suggest that dasatinib still has the potential to be used as a therapeutic agent through the study of topical preparations or other derivatives.
Fig. 6.
Proposed diagram for the inhibitory mechanism of dasatinib in MCs. Dasatinib directly inhibits Lyn and Fyn kinases in antigen-stimulated MCs.
ACKNOWLEDGMENTS
This research was supported by the NRF grant (NRF-2017R1A2B4008572) and in part by the National Research Foundation of Korea (NRF) grant (NRF-2016R1A2B3015840) funded by the Korea government.
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interests.
References
- Aketani S., Teshima R., Umezzawa Y., Sawada J. Correlation between cytosolic calcium concentration and degranulation in RBL-2H3 cells in the presence of various concentrations of antigen-specific IgEs. Immunol. Lett. 2001;75:185–189. doi: 10.1016/S0165-2478(00)00311-4. [DOI] [PubMed] [Google Scholar]
- Akin C. Mast cell activation syndromes. J. Allergy Clin. Immunol. 2017;140:349–355. doi: 10.1016/j.jaci.2017.06.007. [DOI] [PubMed] [Google Scholar]
- Atallah E., Kantarjian H., Cortes J. Emerging safety issues with imatinib and other Abl tyrosine kinase inhibitors. Clin. Lymphoma Myeloma. 2007;7:S105–S112. doi: 10.3816/CLM.2007.s.010. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. Therapeutic strategies for allergic diseases. Nature. 1999;402:31–38. doi: 10.1038/35037026. [DOI] [PubMed] [Google Scholar]
- Begin P., Nadeau K. C. Epigenetic regulation of asthma and allergic disease. Allergy Asthma Clin. Immunol. 2014;10:27. doi: 10.1186/1710-1492-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradding P., Walls A. F., Holgate S. T. The role of the mast cell in the pathophysiology of asthma. J. Allergy Clin. Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Bruhns P., Frémont S., Daëron M. Regulation of allergy by Fc receptors. Curr. Opin. Immunol. 2005;17:662–669. doi: 10.1016/j.coi.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Charles N., Watford W. T., Ramos H. L., Hellman L., Oettgen H. C., Gomez G., Ryan J. J., O'Shea J. J., Rivera J. Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity. 2009;30:533–543. doi: 10.1016/j.immuni.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draber P., Halova I., Polakovicova I., Kawakami T. Signal transduction and chemotaxis in mast cells. Eur. J. Pharmacol. 2016;778:11–23. doi: 10.1016/j.ejphar.2015.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agamy D. S. Targeting c-kit in the therapy of mast cell disorders: current update. Eur. J. Pharmacol. 2012;690:1–3. doi: 10.1016/j.ejphar.2012.06.030. [DOI] [PubMed] [Google Scholar]
- Feinberg J. G. Pinnal anaphylaxis: an additional anaphyiactic site. Nature. 1961;191:712. doi: 10.1038/191712a0. [DOI] [PubMed] [Google Scholar]
- Galli S. J., Kalesnikoff J., Grimbaldeston M. A., Piliponsky A. M., Williams C. M., Tsai M. Mast cells as "tunable" effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- Geha R. S., Jabara H. H., Brodeur S. R. The regulation of immunoglobulin E class-switch recombination. Nat. Rev. Immunol. 2003;3:721–732. doi: 10.1038/nri1181. [DOI] [PubMed] [Google Scholar]
- Gomez G. Current strategies to inhibit high affinity FcεRI-mediated signaling for the treatment of allergic disease. Front. Immunol. 2019;10:175. doi: 10.3389/fimmu.2019.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuchel R., Berg A., Tallquist M., Ahlén K., Reed R. K., Rubin K., Claesson-Welsh L., Heldin C. H., Soriano P. Platelet-derived growth factor beta receptor regulates interstitial fluid homeostasis through phosphatidylinositol-3' kinase signaling. Proc. Natl. Acad. Sci. U.S.A. 1999;96:11410–11415. doi: 10.1073/pnas.96.20.11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hide I., Toriu N., Nuibe T., Inoue A., Hide M., Yamamoto S., Nakata Y. Suppression of TNF-alpha secretion by azelastine in a rat mast (RBL-2H3) cell line: evidence for differential regulation of TNF-alpha release, transcription, and degranulation. J. Immunol. 1997;159:2932–2940. [PubMed] [Google Scholar]
- Hoffmann A., Jamin A., Foetisch K., May S., Aulepp H., Haustein D., Vieths S. Determination of the allergenic activity of birch pollen and apple prick test solutions by measurement of β-hexosaminidase release from RBL-2H3 cells. Comparison with classical methods in allergen standardization. Allergy. 1999;54:446–454. doi: 10.1034/j.1398-9995.1999.00917.x. [DOI] [PubMed] [Google Scholar]
- Horny H. P., Sotlar K., Valent P. Mastocytosis: state of the art. Pathobiology. 2007;74:121–132. doi: 10.1159/000101711. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Nagai H. Analysis of the mechanism for the development of allergic skin inflammation and the application for its treatment: mouse models for the development of remedies for human allergic dermatitis. J. Pharmacol. Sci. 2009;110:251–259. doi: 10.1254/jphs.09R01FM. [DOI] [PubMed] [Google Scholar]
- Iqbal N., Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer. Chemother. Res. Pract. 2014;2014:357027. doi: 10.1155/2014/357027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan R. S. Food allergy: an overview. Environ. Health Perspect. 2003;111:223–225. doi: 10.1289/ehp.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambayashi T., Koretzky G. A. Proximal signaling events in Fc epsilon RI-mediated mast cell activation. J. Allergy Clin. Immunol. 2007;119:544–552. doi: 10.1016/j.jaci.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Kantarjian H., Jabbour E., Grimley J., Kirkpatrick P. Dasatinib. Nat. Rev. Drug Discov. 2006;5:717–718. doi: 10.1038/nrd2135. [DOI] [PubMed] [Google Scholar]
- Kay A. B. Allergy allergic diseases. First of two parts. N. Engl. J. Med. 2001;344:30–37. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- Kim J. D., Kim D. K., Kim H. S., Kim A. R., Kim B., Her E., Park K. H., Kim H. S., Kim Y. M., Choi W. S. Morusbombycis extract suppresses mast cell activation and IgE-mediated allergic reaction in mice. J. Ethnopharmacol. 2013;146:287–293. doi: 10.1016/j.jep.2012.12.046. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Lee J. H., Hwang B. Y., Mun S. H., Ko N. Y., Kim D. K., Kim B., Kim H. S., Kim Y. M., Choi W. S. Morin inhibits Fyn kinase in mast cells and IgE-mediated type I hypersensitivity response in vivo. Biochem. Pharmacol. 2009;77:1506–1512. doi: 10.1016/j.bcp.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Kitanaka S., Nakayama T., Shibano T., Ohkoshi E., Takido M. Antiallergic agent from natural sources. Structures and inhibitory effect of histamine release of naphthopyrone glycosides from seeds of Cassia obtusifolia L. Chem. Pharm. Bull. (Tokyo) 1998;46:1650–1652. doi: 10.1248/cpb.46.1650. [DOI] [PubMed] [Google Scholar]
- Kneidinger M., Schmidt U., Rix U., Gleixner K. V., Vales A., Baumgartner C., Lupinek C., Weghofer M., Bennett K. L., Herrmann H., Schebesta A., Thomas W. R., Vrtala S., Valenta R., Lee F. Y., Ellmeier W., Superti-Furga G., Valent P. The effects of dasatinib on IgE receptor-dependent activation and histamine release in human basophils. Blood. 2008;111:3097–3107. doi: 10.1182/blood-2007-08-104372. [DOI] [PubMed] [Google Scholar]
- Krystel-Whittemore M., Dileepan K. N., Wood J. G. Mast cell: a multi-functional master cell. Front. Immunol. 2016;6:620. doi: 10.3389/fimmu.2015.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. S., Kang O. H., Choi J. G., Oh Y. C., Keum J. H., Kim S. B., Jeong G. S., Kim Y. C., Shin D. W., Kwon D. Y. Synergistic effect of emodin in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. Pharm. Biol. 2010;48:1285–1290. doi: 10.3109/13880201003770150. [DOI] [PubMed] [Google Scholar]
- Lin S., Cicala C., Scharenberg A. M., Kinet J. P. The FcεRIβ subunit functions as an amplifier of FcεRIγ-mediated cell activation signals. Cell. 1996;85:985–995. doi: 10.1016/S0092-8674(00)81300-8. [DOI] [PubMed] [Google Scholar]
- Lindner I., Meier C., Url A., Unger H., Grassauer A., Prieschl-Grassauer E., Doerfler P. Beta-escin has potent anti-allergic efficacy and reduces allergic airway inflammation. BMC Immunol. 2010;11:24. doi: 10.1186/1471-2172-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo L. J., Lee F. Y., Chen P., Norris D., Barrish J. C., Behnia K., Castaneda S., Cornelius L. A., Das J., Doweyko A. M., Fairchild C., Hunt J. T., Inigo I., Johnston K., Kamath A., Kan D., Klei H., Marathe P., Pang S., Peterson R., Pitt S., Schieven G. L., Schmidt R. J., Tokarski J., Wen M. L., Wityak J., Borzilleri R. M. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J. Med. Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- Mastuda H., Morikawa T., Ueda K., Managi H., Yoshikawa M. Structural requirements of flavonoids for inhibition of antigen-Induced degranulation, TNF-α and IL-4 production from RBL-2H3 cells. Bioorg. Med. Chem. 2002;10:3123–3128. doi: 10.1016/S0968-0896(02)00227-4. [DOI] [PubMed] [Google Scholar]
- Masuda E. S., Schmitz J. Syk inhibitors as treatment for allergic rhinitis. Pulm. Pharmacol. Ther. 2008;21:461–467. doi: 10.1016/j.pupt.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Minai-Fleminger Y., Levi-Schaffer F. Mast cells and eosinophils: the two key effector cells in allergic inflammation. Inflamm. Res. 2009;58:631–638. doi: 10.1007/s00011-009-0042-6. [DOI] [PubMed] [Google Scholar]
- Mitre E., Nutman T. B. Basophils, basophilia and helminth infections. Chem. Immunol. Allergy. 2006;90:141–156. doi: 10.1159/000088886. [DOI] [PubMed] [Google Scholar]
- Montani D., Bergot E., Günther S., Savale L., Bergeron A., Bourdin A., Bouvaist H., Canuet M., Pison C., Macro M., Poubeau P., Girerd B., Natali D., Guignabert C., Perros F., O'Callaghan D. S., Jaïs X., Tubert-Bitter P., Zalcman G., Sitbon O., Simonneau G., Humbert M. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation. 2012;125:2128–2137. doi: 10.1161/CIRCULATIONAHA.111.079921. [DOI] [PubMed] [Google Scholar]
- Murakami M., Kudo I. Diversity and regulatory functions of mammalian secretory phospholipase A2s. Adv. Immunol. 2001;77:163–194. doi: 10.1016/S0065-2776(01)77017-4. [DOI] [PubMed] [Google Scholar]
- Nam S. T., Park Y. H., Kim H. W., Kim H. S., Lee D., Lee M. B., Kim Y. M., Choi W. S. Suppression of IgE-mediated mast cell activation and mouse anaphylaxis via inhibition of Syk activation by 8-formyl-7-hydroxy-4-methylcoumarin, 4μ8C. Toxicol. Appl. Pharmacol. 2017;332:25–31. doi: 10.1016/j.taap.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Ovary Z. Passive cutaneous anaphylaxis in the mouse. J. Immunol. 1958;81:355–357. [PubMed] [Google Scholar]
- Park Y. H., Kim D. K., Kim H. W., Kim H. S., Lee D., Lee M. B., Min K. Y., Koo J., Kim S. J., Kang C., Kim Y. M., Kim H. S., Choi W. S. Repositioning of anti-cancer drug candidate, AZD7762, to an anti-allergic drug suppressing IgE-mediated mast cells and allergic responses via the inhibition of Lyn and Fyn. Biochem. Pharmacol. 2018;154:270–277. doi: 10.1016/j.bcp.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Park Y. H., Kim H. W., Kim H. S., Nam S. T., Lee D., Lee M. B., Min K. Y., Koo J., Kim S. J., Kim Y. M., Kim H. S., Choi W. S. An anti-cancer drug candidate CYC116 suppresses type I hypersensitive immune responses through the inhibition of Fyn kinase in mast cells. Biomol. Ther. (Seoul) 2019;27:311–317. doi: 10.4062/biomolther.2018.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parravicini V., Gadina M., Kovarova M., Odom S., Gonzalez-Espinosa C., Furumoto Y., Saitoh S., Samelson L. E., O'Shea J. J., Rivera J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat. Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- Qiao H., Andrade M. V., Lisboa F. A., Morgan K., Beaven M. A. FcεRI and toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610–618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall K. L., Hawkins C. A. Antihistamines and allergy. Aust. Prescr. 2018;41:41–45. doi: 10.18773/austprescr.2018.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J. Molecular adapters in FcεRI signaling and the allergic response. Curr. Opin. Immunol. 2002;14:688–693. doi: 10.1016/S0952-7915(02)00396-5. [DOI] [PubMed] [Google Scholar]
- Rivera J., Cordero J. R., Furumoto Y., Luciano-Montalvo C., Gonzalez-Espinosa C., Kovarova M., Odom S., Parravicini V. Macromolecular protein signaling complexes and mast cell responses: a view of the organization of IgE-dependent mast cell signaling. Mol. Immunol. 2002;38:1253–1258. doi: 10.1016/S0161-5890(02)00072-X. [DOI] [PubMed] [Google Scholar]
- Schroeder J. T., MacGlashan D. W., Jr, Lichtenstein L. M. Human basophils: mediator release and cytokine production. Adv. Immunol. 2001;77:93–122. doi: 10.1016/S0065-2776(01)77015-0. [DOI] [PubMed] [Google Scholar]
- Shah N. P., Kasap C., Weier C., Balbas M., Nicoll J. M., Bleickardt E., Nicaise C., Sawyers C. L. Transient potent BCR-ABL inhibition is sufficient to commit chronic myeloid leukemia cells irreversibly to apoptosis. Cancer Cell. 2008;14:485–493. doi: 10.1016/j.ccr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Shah N. P., Nicoll J. M., Nagar B., Gorre M. E., Paquette R. L., Kuriyan J., Sawyers C. L. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/S1535-6108(02)00096-X. [DOI] [PubMed] [Google Scholar]
- Siraganian R. P., de Castro R. O., Barbu E. A., Zhang J. Mast cell signaling: the role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants. FEBS Lett. 2010;584:4933–4940. doi: 10.1016/j.febslet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zhou Q., Liu L., Zou K. Anti-allergic activity of emodin on IgE-mediated activation in RBL-2H3 cells. Pharmacol. Rep. 2012;64:1216–1222. doi: 10.1016/S1734-1140(12)70917-9. [DOI] [PubMed] [Google Scholar]
- Warrington R., Silviu-Dan F., Wong T. Drug allergy. Allergy Asthma Clin. Immunol. 2018;14:60. doi: 10.1186/s13223-018-0289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedemeyer J., Tsai M., Galli S. J. Roles of mast cells and basophils in innate and acquired immunity. Curr. Opin. Immunol. 2000;12:624–631. doi: 10.1016/S0952-7915(00)00154-0. [DOI] [PubMed] [Google Scholar]
- Weng Z., Patel A. B., Panagiotidou S., Theoharides T. C. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J. Allergy Clin. Immunol. 2015;135:1044–1052. doi: 10.1016/j.jaci.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Xu M., Hou T., Huang Y., Yang C., Li J. Dasatinib enhances antitumor activity of paclitaxel in ovarian cancer through Src signaling. Mol. Med. Rep. 2015;12:3249–3256. doi: 10.3892/mmr.2015.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Sayama K., Yano K., Lantz C. S., Noben-Trauth N., Ra C., Costa J. J., Galli S. J. IgE enhances Fc epsilon receptor I expression and IgE-dependent release of histamine and lipid mediators from human umbilical cord blood-derived mast cells: synergistic effect of IL-4 and IgE on human mast cell Fc epsilon receptor I expression and mediator release. J. Immunol. 1999;162:5455–5465. [PubMed] [Google Scholar]