Abstract
The metalloenzyme soluble methane monooxygenase (sMMO) consists of hydroxylase (sMMOH), regulatory (MMOB), and reductase components. When sMMOH forms a complex with MMOB, the rate constants are greatly increased for the sequential access of O2, protons, and CH4 to an oxygen-bridged diferrous metal cluster located in the buried active site. Here, we report high resolution X-ray crystal structures of the diferric and diferrous states of both sMMOH and the sMMOH:MMOB complex using the components from Methylosinus trichosporium OB3b. These structures are analyzed for O2 access routes enhanced when the complex forms. Previously reported, lower resolution structures of the sMMOH:MMOB complex from the sMMO of Methylococcus capsulatus Bath revealed a series of cavities through sMMOH postulated to serve as the O2 conduit. This potential role is evaluated in greater detail using the current structures. Additionally, a search for other potential O2 conduits in Methylosinus trichosporium OB3b sMMOH:MMOB revealed a narrow molecular tunnel, termed the W308-Tunnel. This tunnel is sized appropriately for O2 and traverses the sMMOH-MMOB interface before accessing the active site. Kinetics of O2 reaction with diferrous sMMOH:MMOB in solution show that use of the MMOB V41R variant decreases the rate constant for O2 binding >25,000-fold without altering component affinity. The location of Val41 near the entrance to the W308-Tunnel is consistent with the tunnel serving as the primary route for O2 transfer into the active site. Accordingly, the crystal structures show that formation of the diferrous sMMOH:MMOB complex restricts access through the chain of cavities while opening the W308-Tunnel.
Graphical Abstract

INTRODUCTION
Methane monooxygenase (MMO) catalyzes the conversion of methane to methanol with the incorporation of one atom of oxygen from O2.1
Two forms of the enzyme have been identified in methanotrophic bacteria. A copper-containing particulate form (pMMO) is present when the soluble copper:biomass ratio is > 5.7 μmol/g protein, while an iron-containing soluble form (sMMO) is present in some methanotrophs at lower copper concentrations.2 Together, the MMOs prevent a significant fraction of the 10 billion tons of biogenic methane produced annually from entering the atmosphere.3, 4
The sMMOs from Methylosinus trichosporium (Mt) OB3b and Methylococcus capsulatus (Mc) Bath are very similar multicomponent metalloenzymes despite significant differences in the cellular structure and metabolism of the two host organisms.1, 5-8 Both sMMOs consist of the following protein components: i) a 40 kDa FAD and Fe2S2 cluster-containing reductase (MMOR), ii) a 245 kDa (αβγ)2 hydroxylase (sMMOH) containing an oxygen-bridged, nonheme diiron cluster, and iii) a 16 kDa regulatory protein termed MMOB.1, 6-12 The catalytic cycle begins with the transfer of two electrons from MMOR to the sMMOH diiron cluster, priming it to react with O2. In the presence of MMOB, a series of intermediates is generated at the diiron cluster during the oxygen activation process (termed O, P*, and P) that culminates in the formation of a diiron(IV) intermediate termed compound Q.1, 8, 9, 13-21 Q is observed to react directly with methane by a hydrogen atom abstraction and hydroxyl radical rebound mechanism.22-25
Regulation of the catalytic cycle by MMOB is a critical aspect of sMMO catalysis. At the beginning of the catalytic cycle, complex formation between reduced sMMOH and MMOB increases the rate constant for the reaction of O2 with the diiron cluster to form P* by at least 1000-fold over that for reduced sMMOH alone.15, 26 Formation of the sMMOH:MMOB complex is also key for maintaining the specificity of sMMO for methane in an environment that may contain numerous alternative substrates, almost all of which have much weaker C-H bond strengths than methane (bond dissociation energy of 105 kcal/mol).6, 27, 28 MMOB apparently alters the reactivity and specificity of Q by two mechanisms.17, 23, 25, 29-32 In the first mechanism, the formation of the sMMOH:MMOB complex opens a route into the active site which is size selective for methane. In the second mechanism, the hydrogen atom abstraction reaction between Q and methane in the sMMOH:MMOB complex is accelerated by quantum tunneling; this mechanism of acceleration is not observed for larger substrates or when MMOB variants are utilized. The structural aspects of the sMMOH:MMOB complex that enforce regulation of O2 and CH4 binding and reaction are poorly understood, but they are essential, because methane is the only growth substrate for the methanotrophs.
A general theme of all of these regulatory events in sMMO is a strict control of the entry and exit of substrates and products, respectively, during the catalytic cycle.7, 8, 29, 31, 33, 34 X-ray crystal structures of sMMOH have provided evidence for two putative routes of substrate entry (Scheme 1). First, a set of three large, internal voids termed cavities have been identified in the α-subunit of sMMOH, one of which (Cavity 1, Cav 1 in Scheme 1) is the active site containing the diiron cluster.10-12, 35 It has been proposed that O2 and methane could traverse from solvent to the buried nonheme diiron active site by filling these cavities.34, 36 However, the passage between Cavities 2 and 1 is greatly restricted in the structure of sMMOH alone.35, 37 The 2.9 Å X-ray crystal structure of the oxidized sMMOH:MMOB complex from Mc Bath showed that a structural change occurs which widens the bottleneck between Cavities 2 and 1.34 This observation could provide an explanation for the dramatic effects of MMOB on O2 and methane binding and reactivity if it can be shown to extend to the reduced sMMOH:MMOB complex and intermediates in the reaction cycle.
Scheme 1.
Proposed Substrate Access Routes in the sMMOH α-Subunit
Another potential route for O2 and methane entry was identified as a short, narrow path termed the Pore, which extends from the sMMOH surface directly into the active site.38-40 However, this route is made less likely by the observation that, in the oxidized Mc Bath sMMOH:MMOB crystal structure, the Pore is blocked by shifts of sMMOH residues and by the fact that MMOB covers the Pore.34
In order to critically examine the potential substrate entry routes, it is vital to obtain a crystal structure of the diferrous sMMOH:MMOB complex, which is the state primed to react with O2. Structural changes in sMMOH and MMOB must also be analyzed in reference to solution transient kinetic studies that depict the influence of MMOB and its variants on substrate regulation at defined points within the catalytic cycle. In the current study, higher resolution crystal structures of Mt OB3b sMMOH alone and in complex with MMOB in both the oxidized and reduced states are used to evaluate the effects of MMOB binding and sMMOH reduction on the proposed substrate entry routes. We also introduce a third potential category of substrate entry route termed a tunnel, which is defined as a narrow, convoluted path that only allows one dimensional substrate migration. One such tunnel that involves the sMMOH-MMOB interface is identified in the complex. Mutagenesis of key MMOB residues lining this tunnel are shown to affect O2 entry in a manner consistent with the kinetics and selectivity of the process.
EXPERIMENTAL PROCEDURES
Reagents, Protein Expression and Purification.
Water used in all experiments was purified with a Millipore Super-Q system. Standard reagents used in this study were purchased from Fisher Scientific. Chemicals for crystallization of proteins including PEG/Ion Screen Reagents 18 and 40, Tacsimate®, PEG6000, and PEG3350 were purchased from Hampton Research unless otherwise stated. Glass rods for crushing crystals and crystal seed bead tubes were also supplied by Hampton Research. Ultra-high purity grades of 3-(N-morpholino)propanesulfonic acid (MOPS) buffer, PEG3350 (alternative source) and sodium phosphate dibasic were obtained from Sigma-Millipore. sMMOH, MMOR, MMOB, and variants of MMOB were produced and purified as previously described. 18, 41
Protein Mutagenesis.
A Thermo Scientific™ Phusion Site-Directed Mutagenesis Kit was used to introduce point mutations at residues 39 and 41 of the pT7-7 derived plasmid pBWJ400 containing the wild type M. trichosporium OB3b MMOB gene.42 The primers shown in Table S1 were synthesized at the University of Minnesota Genomics Center.
Crystallization of sMMOH.
All crystallization was performed at room temperature. Mt sMMOH was crystallized using the hanging drop vapor diffusion method by first mixing 1.5 μl of protein solution containing 45 μM sMMOH (90 μM active sites) and 90 μM MMOB in 25 mM MOPS, pH 7 with 1.5 μl of crystallization solution containing 5% (w/v) PEG6000 in the same buffer. Whisker-like long thin crystals appeared within 2 days. The crystals were pulverized and added to a seed bead tube, brought up to a total volume of 50 μl with mother liquor and vortexed for 3 min in 30 s intervals. The seed stock was used to set up a second hanging drop experiment by first mixing 1.0 μl of protein solution containing 45 μM sMMOH (90 μM active sites) and 90 μM MMOB in 25 mM MOPS, pH 7 with 2.0 μl of crystallization solution containing 0.2 M Na2HPO4 · 2H2O and 20% (w/v) PEG3350. Subsequently, 0.5 μl of seed stock suspension was added to this drop. Over-nucleated crystals formed within a few days. Crystals were pulverized with a glass rod and added to a seed bead tube. They were then brought to a total volume of 50 μl and vortexed for 3 min in 30 s intervals. The seed stock was used to conduct a third hanging drop experiment by first mixing 1.0 μl of protein solution containing 45 μM sMMOH (90 μM active sites) and 90 μM MMOB in 25 mM MOPS, pH 7 with 2.0 μl of crystallization solution containing 8% Tacsimate® and 20% (w/v) PEG3350. Then 0.5 μl of seed stock solution was added to this drop. Disc-shaped crystals formed after 1 week. Diferric sMMOH crystals were cryo-protected with 10% ethylene glycol and frozen in liquid nitrogen. sMMOH crystals were chemically reduced in an anaerobic chamber (Belle Technology) by soaking in a solution containing 8% Tacsimate®, 20% PEG3350 (w/v), 5 mM sodium dithionite and 200 μM methyl viologen for 30 min, cryo-protected with mineral oil, and then frozen in liquid nitrogen. Despite the absence of MMOB in these crystals, the presence of MMOB is vital in the crystallization solution. This crystal form is the same as the Form 2 diferric Mt sMMOH crystals that were described in the original crystallography study for this enzyme.12 The diferric sMMOH structure obtained is nominally the same as that from the Form 1 crystals obtained in the absence of MMOB, albeit with higher resolution.
Crystallization of sMMOH-MMOB Complex.
A Rigaku CrystalMation fully integrated platform for protein crystallization was used to screen for conditions that would produce sMMOH:MMOB crystals. PEG/Ion Screen Reagent 18 (8% v/v Tacsimate® pH 8.0, 20% w/v PEG3350) and Reagent 40 (0.2 M Sodium phosphate dibasic dihydrate, 20% w/v PEG3350) each produced bipyramidal crystals and harvesting trays were made by repeating each condition 96 times using the Rigaku CrystalMation instrument. Oxidized crystals were looped directly from the 96-well plate and transferred to a cryo-solution containing 8% Tacsimate®, 20% (w/v) PEG3350 and 10% (v/v) ethylene glycol, and then they were plunged into liquid nitrogen. Crystals were chemically reduced in an anaerobic chamber by soaking in a solution containing 8% (v/v) Tacsimate®, 20% (w/v) PEG3350, 5 mM sodium dithionite and 200 μM methyl viologen. sMMOH:MMOB crystals were soaked for 2 h, cryo-protected in mineral oil and then frozen in liquid nitrogen.
Crystal growth was scaled up using the sitting-drop vapor diffusion method in 24-well plates (Cryschem M). Both Reagent 18 and 40 solutions were made in lab so that the crystallization conditions could be optimized. A seed stock suspension of the sMMOH:MMOB complex was made from crystals grown in a 96-well plate created by the Rigaku robot. A glass rod crystal crusher was used to pulverize the crystals. The crushed crystals were added to a seed bead tube, brought to a total volume of 50 μl and vortexed for 3 min in 30 s intervals. Sitting-drop experiments were set up by mixing 1.5 μl of protein with 1.5 μl of the well solution and then adding of the 0.5 μl seed stock suspension. The well solution contained 500 μl of precipitant (Reagent 18 or 40). Bipyramidal crystals started to grow in 2 days at room temperature.
Crystal Data Collection, Structure Solution and Refinement.
The crystals were exposed to X-ray radiation at the Advanced Photon Source (Argonne National Laboratory, Lemont, IL) on Beamline 24-ID-C and 24-ID-E at 0.979 Å wavelength at 100 K. A total of 675 frames were collected with an oscillation step of 0.2°. Datasets were subsequently processed using XDS 43 and merged using PHENIX44. Molecular replacement calculation were done with PHASER45 using the X-ray crystal structure of Mt OB3b sMMOH and the NMR structure of Mt OB3b MMOB 12, 42 (PDBID: 1MHZ and 2MOB) as initial search models. The 2Fo-Fc map after the first round of refinement revealed significant structural differences between the sMMOH and the sMMOH:MMOB complex. A structural model was built using COOT46 and refinement was carried out using PHENIX.44 The structures have been deposited in RCSB as detailed below.
PyMol Analysis.
PyMol version 2.3.3 (Schrödinger) was used to visualize, analyze, and design figures of Mt OB3b sMMO crystal structures. Structural alignments were calculated using the align command available via the user interface. In order to find internal cavities in protein structures, the following PyMol settings were used: Display quality = maximum, Surface = cavities and pockets (culled), Cavity detection radius = 3 solvent radii, Cavity detection cutoff = 5 solvent radii, Ignore HETATMs. The solvent radius was additionally set to 1.1 Å instead of the default value of 1.4 Å. The calculated cavities were then manually inspected to determine the amino acids lining the surface of cavities.
The intra-chain hydrogen bonding network of all helices in the α-subunit was determined by the following steps: i) The α-subunit was colored by secondary structure, ii) Each helix was made a separate object, iii) The find polar contacts within selection command was applied to all helices, and iv) Every helix was manually inspected to determine the i and i+x hydrogen bonding pattern for each amino acid.
MOLE Tunnel Calculations.
MOLE 2.5 is an advanced software tool designed to analyze molecular channels and pores.47 The diiron cluster was chosen as the starting point for all sMMOH MOLE 2.5 calculations presented here. The default parameters yielded no tunnels for substrate entry when the diiron cluster is chosen as the starting point. The interior threshold and bottleneck radius had to be then adjusted to discover biologically relevant tunnels. The Interior Threshold (IT) parameter allows the user to identify all voids wider than double the chosen IT value. The Bottleneck Radius (BNR) parameter defines the minimum radius of the tunnel at any point along its length. In order to minimize redundancy, the parameter Cutoff Ratio is adjusted to filter out tunnels that are too similar. The parameter values were used to identify the tunnels in the crystal structure were IT = 0.9 Å, BNR = 0.8 Å and cutoff ratio = 0.5.
Steady-State Experiments.
A Hansatech Instruments Oxytherm+ system equipped with an S1 Clark-type oxygen electrode was used to measure O2 consumption over time. The total volume of the reactions was 1 ml. The reaction components were: 0.2 μM sMMOH (0.4 μM active sites), 0.4 μM wild-type MMOB (WT-MMOB), 1.2 μM MMOR, 200 μM methane, 250 μM O2, 400 μM NADH, 25 mM MOPS pH 7.5, 23 °C and varying concentrations of the MMOB variant. NADH was used to initiate the reaction. The O2view software package was used to calculate the initial rate of O2 consumption.
Stopped-Flow Experiments.
The Applied Photophysics stopped-flow spectrophotometer model SX.18MV was used to rapidly mix reaction components and observe single wavelength absorbance over time. sMMOH was reduced as previously described.19, 41 One syringe contained 55 μM sMMOHred (110 μM active sites) and 110 μM V41R-MMOB in anaerobic 50 mM MOPS buffer, pH 7.0. The second syringe contained dissolved gaseous O2 in buffer at varying concentrations that were all high enough to establish pseudo first order conditions in the reaction. Solutions from the two syringes were rapidly mixed and single wavelength time-dependent absorbance data at 330 nm were collected. At 330 nm, oxidized sMMOH absorbs more strongly than reduced sMMOH. For other experiments of this type in which a saturated O2 solution was used, the reaction was also monitored at 430 and 700 nm where reaction cycle intermediates Q and P, respectively, absorb.1 All stopped-flow experiments were performed at 4.5 °C. The multi-step first order or pseudo-first order reaction time courses were fit to a multiple summed-exponential expression using the nonlinear regression fitting package in Applied Photophysics Pro-Data Viewer software as previously described.18, 30, 48 The analysis yields the number of phases required to fit the time course as well as the amplitude and reciprocal relaxation time (1/τ) for each exponential phase. Data analysis is described in Supporting Information.
EPR Measurements.
Electron paramagnetic resonance (EPR) spectra were recorded using a Bruker Elexsys E-500 spectrometer equipped with a Bruker dual mode cavity and an Oxford ESR 910 liquid helium cryostat. Experimental conditions are given in the figure legend.
RESULTS
Crystal Forms of Mt OB3b sMMOH and sMMOH:MMOB Complex.
In past studies, most preparations of sMMOH from Mc Bath and Mt OB3b contained a sizable fraction of unreactive enzyme without evidence for misfolding or aberrant diiron cluster structure.17, 18 Although never successfully characterized, it is likely that the unreactive fraction resulted from small sMMOH surface modifications that interfered with the precise interaction between sMMOH and MMOB required for full activity.29 Recently, advances have been made in the Mt OB3b sMMOH purification protocol that double the active fraction.41 We find that the new preparation allows for the development of tractable methods to form a co-crystal of sMMOH and MMOB that diffracts to high resolution. Here, we evaluate the structures of oxidized, diferric Mt sMMOH (sMMOHox, PDB:6VK6, 1.52 Å), chemically reduced diferrous Mt sMMOH (sMMOHred, PDB:6VK7, 2.12 Å), Mt sMMOHox:MMOB (Form 1, PDB:6VK5, 1.86 Å and Form 2, PDB:6VK8, 2.03 Å), and Mt sMMOHred:MMOB (PDB:6VK4, 2.35 Å) (Table S2). These structures are also evaluated in the context of structures of oxidized and reduced Mt sMMOH:MMOB determined using room temperature X-ray free electron laser (RT-XFEL) technology published recently by some of us.49
The 1.52 Å Mt sMMOHox structure (PDB:6VK6) is the highest resolution sMMOH structure reported to date.12, 38 The two αβγ protomers of sMMOH protein in the crystal are related by 2-fold crystallographic symmetry. The experimental data allowed for accurate modeling of the amino acids and aquo/hydroxo ligands coordinated to the diiron cluster (Figure 1A). Interatomic distances are summarized in Table S3. Throughout this report, when referring to sMMOH, the established nomenclature for the secondary structural elements of the α-subunit is used (Figure 1B).10
Figure 1.
A) Image of the active site diiron cluster along with the electron density map (1.9 σ contour). Electron density (2Fo-Fc) is colored gray, the irons are shown as orange spheres, amino acids are represented as sticks (white = carbon, red = oxygen, blue = nitrogen), bridging and terminal aquo/hydroxo ligands are shown as red spheres. In this structure (sMMOHox, PDB:6VK6), the second bridging hydroxo ligand is replaced by a hydroxyl group of ethylene glycol (green = carbon) from the cryo-stabilization solution. B) The α-subunit of MMOH is shown as a black and white cartoon. The principal helices are labeled A-H and 4, and the diiron cluster is shown as orange spheres. The four helices providing ligands to the diiron cluster are B, C, E and F.
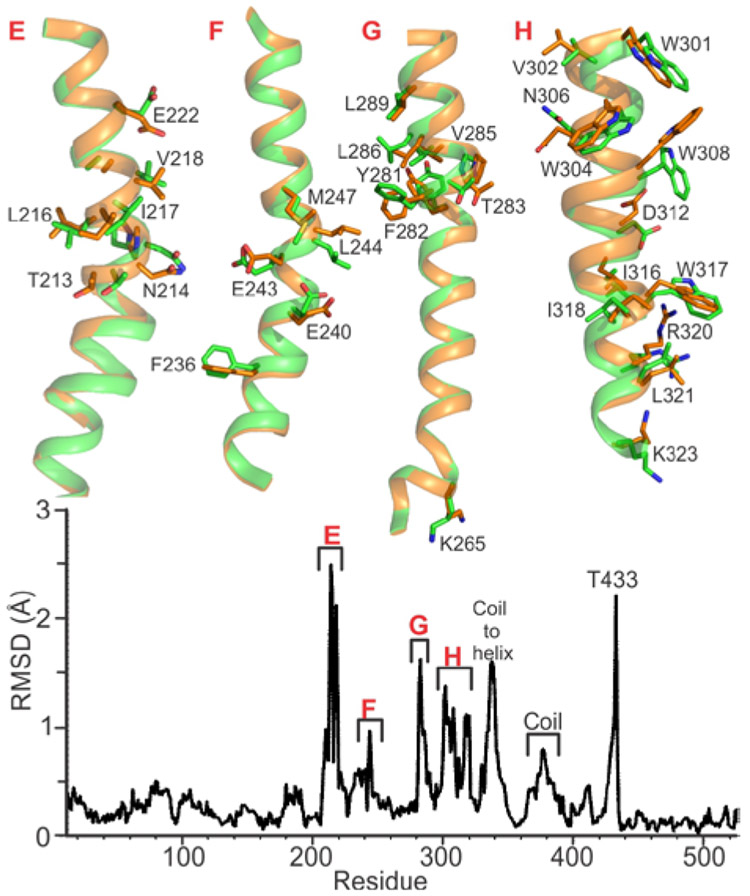
Chemical reduction of the Mt sMMOH crystals caused in crystallo structural rearrangements to occur in the α-subunit. Figure 2 highlights all the pertinent amino acid side chains that change rotometric conformation and/or move ≥1.0 Å in the 2.12 Å structure (PDB:6VK7). For example, the Pore residues N214 and E240 located on Helices E and F change rotameric conformation as well as shift a notable 2.0-2.2 Å upon chemical reduction (Figure 2, Helices E and F, see below). The other regions of the α-subunit and the β and γ subunits of sMMOH remain relatively unchanged. One important change at the diiron cluster is a shift of E243 from a monodentate ligand of Fe2 to a position where it forms a monodentate bridge between the irons and a bidentate coordination to Fe2. The same “carboxylate shift” has been reported for Mc sMMOHred. 11
Figure 2.
Structural rearrangements in Mt sMMOH α-subunit upon chemical reduction. Top: Oxidized sMMOH (6VK6) is colored green and chemically reduced sMMOH (6VK7) is colored orange. Helices E, F, G, and H are labeled and are represented as cartoons. Amino acid side chains that move at least 1 Å upon chemical reduction are represented as sticks (green/orange = carbon, red = oxygen, blue = nitrogen). Bottom: Main chain Cα RMSD of the α-subunit residues upon alignment between the oxidized and reduced states using the CCP4 analysis package.50
The Mt sMMOH:MMOB complex crystallizes with the complete (αβγ)2 sMMOH and 2 bound MMOBs in the asymmetric unit. Form 1 (PDB:6VK5) and Form 2 (PDB:6VK8) Mt sMMOHox:MMOB complex crystal structures (1.86 and 2.03 Å, respectively) are nearly identical except for the exogenous ligands bound to the diiron cluster, as discussed in detail below. The crystal structures exhibit the same overall changes upon complex formation between Mt sMMOH and MMOB. These changes have been detailed in a parallel room temperature XFEL (RT-XFEL) structure study,49 so they are not described further here.
Chemical reduction of Form 1 crystals resulted in a structure (2.35 Å) that has one protomer reduced (diferrous) and the other protomer oxidized (diferric, PDB: 6VK4). The phenomenon of two different oxidation states in the sMMOH homodimer has been observed before in an X-ray crystallographic study of chemically reduced Mc sMMOH crystals.11 The presence of two oxidation states in different protomers facilitates identification of structural changes under otherwise identical conditions and resolution.
Many of the sMMOH α-subunit helices contain regions of π-helix49 and/or 310-helix which increase or decrease flexibility, respectively (see a discussion in the Supporting information, Figure S1 and Table S4). The mechanistic significance of these uncommon secondary structure elements will be described in the discussion.
Exogenous Ligands Bound to the Diiron Cluster of the sMMOH:MMOB Complex Induce Conformational Changes.
Typically, only aquo/hydroxo exogenous molecules are found coordinated to the diiron cluster of sMMOH in crystal structures.10-12, 49 In contrast, the large exogenous ligands benzoate and succinate are found bound to the diiron cluster of the Form 1 and Form 2 Mt sMMOHox:MMOB structures, respectively (Figure 3A and B). The benzoate carboxylate oxygens bridge between the irons of the cluster of Form 1 symmetrically, whereas the succinate carboxylate oxygens bind strongly to Fe1 and weakly to Fe2 in Form 2 (interatomic distances shown in Table S5). The Fe-Fe distance in both structures is an atypically long 3.5 Å, presumably due to the presence of the exogenous ligand carboxylate group. The source of the benzoate in Form 1 was identified as a contaminant in the PEG3350 precipitant present in the mother liquor. Changing the supplier of the PEG eliminated the benzoate in sMMOHox:MMOB Form 2, but it was replaced by succinate from the Tacsimate® mixture of organic acids. We attempted to crystallize the sMMOHox:MMOB complex without succinic acid bound to the diiron cluster by lowering the concentration of Tacsimate® to 2% (v/v), but the structure was unchanged. It is known that larger molecules such as single and double ring aromatic compounds are adventitious substrates of sMMO,6, 51 and thus they can at least slowly access the active site.
Figure 3.
Active site structures of (A) Form 1 (6VK5) and (B) Form 2 (6VK8). Stick colors: green/white = carbon, red = oxygen, blue = nitrogen. Fe atoms shown as orange spheres and aquo/hydroxo are shown as smaller red spheres. Where shown, the 2Fo-Fc electron density is modeled at 1 σ. Interatomic distances can be found in Table S5.
The exogenous ligands bound to the diiron cluster in Form 1 and 2 crystal structures have an effect on the sMMOH α-subunit structure. Inspection of the electron density maps shows that both benzoate and succinate extend to the outer boundary of the active site cavity, causing the side chain of F188 to change rotameric conformation (Figure 4A and B). This conformational change is not observed in the RT-XFEL structures of Mt sMMOHox:MMOB (PDB:6YD0), which have only solvent and possibly a small exogenous molecule near the diiron cluster (Figure 4C).49 A notable observation is that F188 is located adjacent to a π-helix. In both the Mt sMMOHox and Mt sMMOHred crystal structures, K185 and R186 of Helix D form i, i+5 hydrogen bonds while V187 and F188 do not have intra-main chain hydrogen bonds. This region remains unchanged upon MMOB binding in the RT- XFEL structures,49 but it is converted to a coil in the 100 K Mt sMMOHox:MMOB crystal structures reported here (Figure S1, Helix D and Table S4, Helix D). A structural alignment of the substrate-free RT-XFEL sMMOHox:MMOB with the Form 1 sMMOHox:MMOB structure depicts the regions where the α-subunit of sMMOH is reorganized by the presence of a large exogenous substrate in the active site (Figure S2). Apart from the conformational change of F188 and the adjoining residues on Helix D as described above, three of the four helices of the 4-helix bundle (C, E and F) reorganize. This change leads to an alteration in the structure of Helix H along with an unstructured coiled region that interacts with Helices C, E and F. Overall, the structures of sMMOH:MMOB from data at 100 K and at room temperature are very similar, with differences confined to the α-subunits due to the effects of the exogenous ligands in the 100 K structure (see a discussion in the Supplemental Information and Table S6 and Figure S2).
Figure 4.
Exogenous ligands bound to the diiron cluster influence the conformation of F188. In all panels, 2Fo-Fc electron density is shown as mesh contoured at 1 σ, F188 and ligands bound to the diiron cluster are shown as sticks and spheres (Green = carbon, Blue = nitrogen, Red = oxygen). A) Benzoate bound to the diiron cluster of the Mt sMMOHox:MMOB Form 1 (6VK5) structure. B) Succinate bound to the diiron cluster of the Mt sMMOHox:MMOB Form 2 structure (6VK8). C) Active site of RT-XFEL Mt sMMOHox:MMOB structure (6YD0).49 D) Mc sMMOH:MMOB structure from PDB:4GAM. The 2Fo-Fc electron density in the active site extends to the outer edge of Cavity 1, causing a shift in F188 as seen in panels A and B. Two waters were modeled into the density at the active site in the 4GAM structure, but a larger exogenous ligand is possible on the basis of additional unassigned density (see text and Figure S3).
Bottlenecks Between Cavities are Regulated by Flexible Residues.
As noted in the introduction, the current model of substrate binding proposed by Cho and colleagues suggests that O2 and CH4 diffuse from solvent to the active site via a chain of three internal cavities located in the sMMOH α-subunit. 34, 35, 37 The detection of the cavities in the most recent of these studies was conducted using PyMol. The ability to identify cavities is based on PyMol parameters termed cavity detection radii and cutoff values. These parameters are varied in units of a separately entered solvent radius (See Experimental Procedures). The program default values are set for a solvent radius of 1.4 Å (water), and cavity detection radii and cutoff values of 5 and 3 solvent radii, respectively. These parameters values provide a useful, albeit conservative, evaluation of the internal void geometry. However, in the case of sMMOHred, we are seeking a highly restricted substrate access route such that it slows O2 access (see kinetic evaluation below) and can differentiate between the size of methane and ethane.30 Setting the solvent radius to 1.1 Å with concomitant decrease in the cavity detection parameters (estimates for the effective radius of O2 range from 1.17 to 1.7 Å)52-56 allows a more relevant detection of the cavities and the bottlenecks between them (Figure 5).
Figure 5.
Cavity gating residues of Mt sMMOH and sMMOH:MMOB. The internal cavities of A) sMMOHox (6VK6), B) sMMOHred (6VK7), C) sMMOHox:MMOB Form 1 (6VK5), D) sMMOHox:MMOB, Form 2 (6VK8), E) RT-XFEL sMMOHox:MMOB (6YD0),49 and F) RT-XFEL sMMOHred:MMOB (6YDI),49 are shown as a grey colored surface. Amino acids involved in determining the connectivity of the cavities are shown as green sticks. The diiron cluster atoms are represented as orange spheres. Benzoate and succinate are shown bound to the diiron cluster in Forms 1 and 2, respectively.
This analysis shows that a bottleneck regulated by residues V105, F109, V285, and L289 is located between Cavities 3 and 2. Cavity 2 is separated from the active site Cavity 1 by another bottleneck controlled by residues L110, F188, L216, F282 and F286. Cavities 3 and 2 are connected in all of the Mt sMMOH and sMMOH:MMOB crystal structures (Figure 5A-D). However, Cavities 2 and 1 are only connected in the sMMOHox and Form 1 and 2 sMMOHox:MMOB structures (Figure 5A, C-D). These cavities are disconnected in sMMOHred (Figure 5B) along the route used in sMMOHox due to shifts in the gating residues. Importantly, Cavities 1 and 2 are not connected in the RT-XFEL sMMOHox:MMOB and sMMOHred:MMOB crystal structures (PDB:6YD0 and 6YDI) (Figure 5E and F).49 The MMOB binding-induced reorganization of the bottleneck residues L216 and L110 in sMMOH serves to isolate Cavity 1 from Cavity 2 in the complex. While the reorganization of L216 and L110 is also observed in the sMMOHox:MMOB Form 1 and Form 2 crystal structures, the altered position of bottleneck residue F188 due to benzoate or succinate binding in Cavity 1 enables a larger connection with Cavity 2 (Figures 4A and B, 5C and D).
The Pore is Blocked upon Reduction and MMOB Binding.
The Pore is located between Helices E and F and has been proposed to be involved in regulating the access of substrates (e− and H+) and release of products (CH3OH) to and from the active site, respectively.40 The strictly conserved amino acids T213, N214, and E240 are considered the Pore gating residues that regulate these processes (Figure 6). The Pore is a uniquely polar region on the sMMOH surface as it is flanked by hydrophobic amino acids A210, V218, L237, L244, and M247 on Helices E and F. In the Mt sMMOHox crystal structure, the side chains of N214 and E240 are solvent exposed, ~4.0 Å apart, and coordinate 5 water molecules. The side chain of T213 lines the active site cavity and the hydroxyl moiety points towards the diiron cluster. Chemical reduction of the diiron cluster causes the middle of Helix E to twist, resulting in T213 and N214 to shift 2.2 Å and 3.2 Å, respectively. The rotameric conformation of E240 is altered and the distance between this residue and N214 becomes 3.0 Å. The rotameric conformations of the hydrophobic residues V218, L244, and M247 are altered as well, helping to create a chemical environment that does not favor stable binding of water molecules to the region around the Pore.
Figure 6.
Views of the Pore region in Mt sMMOH in various states and in complex with MMOB. Hox = sMMOHox (6VK6), Hred = MMOHred (6VK7), HredB = RT-XFEL sMMOHredMMOB (6YDI). Diiron cluster reduction partially closes the Pore while complex formation with MMOB completely seals off the Pore. Electron density is shown as 2Fo-Fc and modeled at 1 σ.
MMOB binding to sMMOH causes structural rearrangement of the Pore residues as well. The side chain of E240 is no longer solvent exposed, and instead, traverses the width of the Pore. This new conformation blocks the access of substrates through the Pore into the active site cavity. The side chain of T213 is shifted 2.2 Å compared to its position in Mt sMMOHox and rotated ~180° compared to its position in Mt sMMOHred. This new conformation positions the side chain hydroxyl moiety of T213 to face away from the diiron cluster and form a hydrogen bond with E240. It is important to note that MMOB covers the Pore while in complex with sMMOH, further limiting access to the active site by this route.
Binding of MMOB Results in the Optimization of a New Transient Molecular Tunnel into the Active Site.
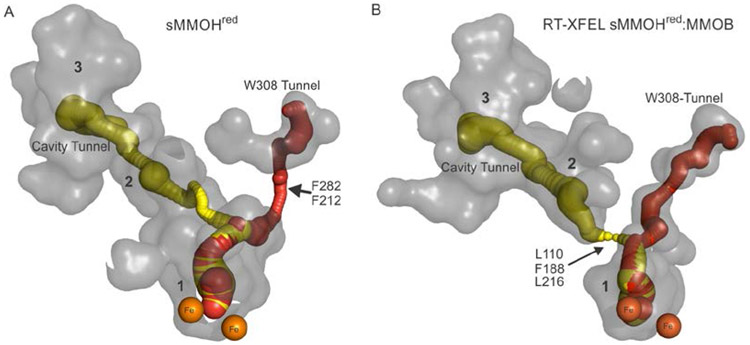
Past kinetic studies have provided a wealth of experimental evidence suggesting that the access of O2 and methane to the active site is precisely regulated by the binding of MMOB.1, 8, 15, 17, 29 The high resolution Mt sMMOH:MMOB crystal structures, especially that of the particularly relevant sMMOHred:MMOB state, afford the opportunity to search for alternative substrate entry routes involving the sMMOH-MMOB interface. Using the program MOLE 2.5, a narrow tunnel (the W308-Tunnel named for the gating tryptophan residue 308, see below) was identified in the Form 1 and Form 2 Mt sMMOHox:MMOB, Mt sMMOHred:MMOB and RT-XFEL Mt sMMOHox/red:MMOB crystal structures (Figures 7 and 8A and B). The W308-Tunnel is primarily lined by hydrophobic residues, which are well-suited to the non-polar nature of sMMO substrates. See Table S7 for a list of the residues lining the W308-Tunnel.
Figure 7.
Starting from bulk solvent, the W308-Tunnel traverses the sMMOH:MMOB interface before reaching the entrance into the sMMOH α-subunit. The α-subunit entrance is gated by sMMOH residues W308 and P215 on Helices H and E respectively. After traveling between Helices H and E the W308-Tunnel curves away from Helix G and turns a corner around Helix E. Finally, the W308-Tunnel goes between Helices E and B into the active site. Colors: sMMOH α-subunit, cyan; W308-Tunnel, black; sMMOH gating residues, red; MMOB, yellow; MMOB “Quad” regulatory residues, green; MMOB W308-Tunnel interface mutated residues, purple; active site diiron cluster, orange. PDB code: 6YDI
Figure 8.
Correlation of voids in the protein identified using PyMol and tunnels computed by MOLE 2.5. A) Tunnels (red and yellow solid spheres) calculated by Mole 2.5 into the active site of sMMOH in the Mt sMMOHred (6VK7) are show superimposed on the cavities computed by PyMol (transparent gray). The diameter of the spheres representing the tunnel depicts the computed width. The significant restriction in the W308-Tunnel is marked with an arrow. The restriction in the computed tunnel between Cavities 2 and 1 is less than in sMMOH:MMOB (see panel B), but rotation around the vertical axis of the view shown reveals that the cavities remain distinct. B) Analogous computations for the Mt sMMOHred:MMOB complex (6YDI). Tunnels are found through the chain of cavities (yellow, severely restricted where marked) and through the W308-Tunnel (red).
Cavity analysis using PyMol with the lower solvent radius parameters described above allows detection of internal voids along the W308-Tunnel route, which correlates very well with the Mole 2.5 prediction (Figure 8B). All the amino acids lining the W308-Tunnel in sMMOH and MMOB are highly conserved in the small hydrocarbon-oxidizing, diiron enzyme family (Table S7).
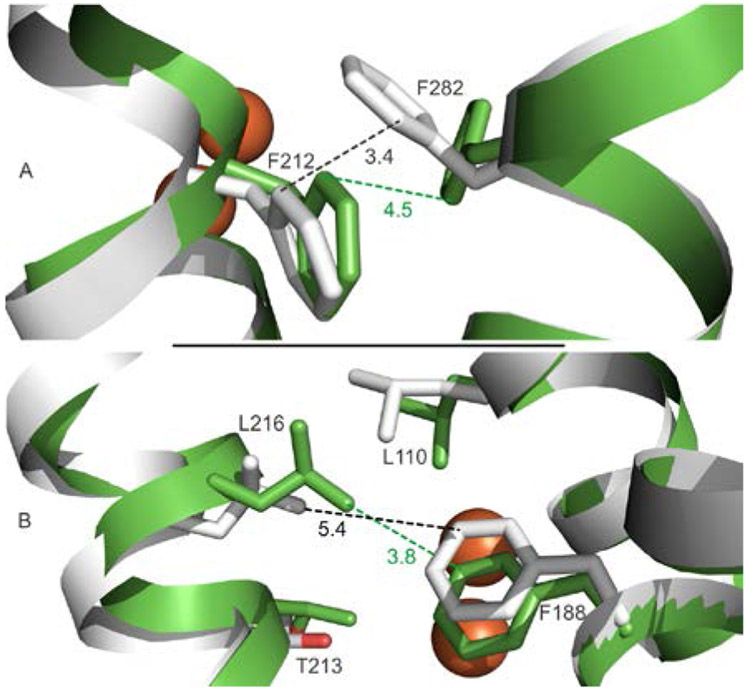
Subtle variations of the W308-Tunnel can also be identified by MOLE 2.5 in the Mt sMMOHox and sMMOHred (Figure 8A) structures. In the sMMOHox structure (Figure S4, top), the entrance into the sMMOH interior is severely constricted by sMMOH residues W308 and P215. Reduction of the diiron cluster leads to structural reorganization of Helix E and H in the sMMOHred structure that partially alleviates this bottleneck (Figure 2 and Figure S4, bottom). However, another significant bottleneck in this tunnel remains within sMMOHred at F282 and F212 (Figure 8A). MMOB binding to sMMOHred removes this constriction, as is apparent in a comparison of the W308-Tunnel in the Mt sMMOHred and RT-XFEL sMMOHred:MMOB structures (Figures 8A and B). Mole 2.5 also allows detection of a route through the chain of cavities to the active site in both of these structures. A constriction between Cavities 2 and 1 is detected by analysis of the sMMOHred structure (Figure 8A). However, in the RT-XFEL sMMOHred:MMOB structure, the changes caused by complex formation result in closure of the Mole 2.5 tunnel between Cavity 2 and 1 (Figure 8B). The same changes occur in the W308-Tunnel found in the 100K sMMOHred:MMOB structure reported here, but the alteration of the active site structure caused by the presence of the large exogenous ligand prevents the complete closure of the Cavity 2 to 1 passageway.
Kinetic Analysis of V41R-MMOB.
Past studies of enzymes such as NiFe hydrogenase that allow selective passage of gases to the active site have shown that small changes in the diameter of the putative gas tunnel via mutagenesis cause large changes in the efficiency of transit.57 Accordingly, the importance of the W308-Tunnel was evaluated by altering access to the tunnel through mutagenesis. Variants of MMOB are easily generated, 29 whereas this is not the case for sMMOH. MMOB residue V41 was chosen as a target for mutagenesis to determine if the W308-Tunnel is involved in trafficking substrates to the active site. It is positioned near the W308-Tunnel entrance into the α-subunit (Figure 9A), and it is a part of a β-sheet involved in the sMMOH-MMOB interaction interface. The only intra/intermolecular polar contacts V41 makes are between its backbone NH and carbonyl oxygen and the backbone atoms of S109 and S110 located on an adjacent β-sheet of MMOB. Residues S109 and S110 have been experimentally shown to strongly influence hydrocarbon substrate selectivity and reactivity.29, 32, 58 Residues with larger side chains were introduced at MMOB position 41 in an attempt to block access into the W308-Tunnel (Figure 9B). The following V41 MMOB mutations were created and biochemically assessed: V41R, V41E, and V41F. In addition, the nearby V39 residue was evaluated by generating the V39R and V39F variants. Each variant nearly halted the reaction of the reconstituted sMMO system (Table S8), but V41R-MMOB exhibited the most dramatic effects, so its reaction will be described in more detail here.
Figure 9.
V41R-MMOB mutation theoretical representation. Panel A depicts the orientation of V39 and V41 in the Form 1 Mt sMMOH:MMOB complex (6VK5) crystal structure. Panel B is the result of using the mutagenesis feature in PyMol to mutate V39 and V41 to R39 and R41, respectively. The new Arg residues have no obvious side chain electrostatic interactions and so a number of rotomeric conformations are possible, of which only one is illustrated. The W308-Tunnel is represented as a tube (black), MMOB (yellow) and sMMOH α-subunit (cyan) are shown as cartoons, and amino acid side chains are represented as sticks (purple = carbon, blue = nitrogen). The nearby “Quad” residues of MMOB are shown in green.
In the absence of MMOB, steady state O2 utilization during CH4 turnover by the reconstituted sMMO system is very slow, but it increases 150-fold when MMOB is added in a 1:1 ratio with the sMMOH diiron sites.9 In contrast, this concentration of V41R-MMOB caused almost no increase in the rate of O2 uptake (Figure 10). The loss of reactivity when using V41R-MMOB could derive either from blocked O2 (or CH4) binding or failure to form a complex with sMMOH. In order to determine if V41R-MMOB can bind to sMMOH, a competitive steady-state experiment was performed in which both MMOB and V41R-MMOB were present. If V41R-MMOB binds to sMMOH with a similar binding affinity as MMOB, then the steady state initial velocity will decrease as V41R-MMOB is added. It was found that the initial velocity of O2 consumption decreased nearly 50% in the presence of equal molar concentrations of V41R-MMOB and MMOB (Figure 10). The initial velocity continued to decrease upon subsequent additions of the MMOB variant. This finding suggests that the mutation does not significantly alter MMOB affinity for sMMOH. The same experiment using V39R-MMOB showed a similar competitive effect, but the affinity of this variant appears to be less than that of WT-MMOB (Figure S5). The other variants tested showed even lower affinity relative to that of WT-MMOB, but perturbation of the integer spin EPR signal of sMMOHred demonstrated that each forms a protein-protein complex (Figure S6).
Figure 10.

Competition between WT-MMOB and V41R-MMOB during steady state turnover. Mt sMMOH was present at the same concentration in all experiments. When WT-MMOB or V41R-MMOB was added alone, it was present at the same concentration as MMOH (active sites). For experiments in which WT-MMOB and V41R were added together in the ratios shown, WT-MMOB was present at the same concentration as sMMOH (active sites). Other conditions for the experiment are given in Experimental Procedures.
Single turnover stopped-flow experiments were performed in which a 1:1 mixture of anaerobic sMMOHred and V41R-MMOB was rapidly mixed with a large excess of O2 in order to determine whether this MMOB variant affects the formation and decay of chemical intermediates in the sMMOH catalytic cycle. MMOB is required to accelerate the start of the reaction cycle so that reaction cycle intermediates P*, P, and Q build up and can be detected.15 In the presence of V41R-MMOB, none of these intermediates were seen. Instead, the diferrous sMMOH decayed slowly to the diferric state.
As sMMOHred oxidizes, a weak chromophore in the near-UV region develops, allowing the kinetics of reoxidation to be monitored. In the case of the Mt sMMOHred:MMOB complex, the initial reaction with O2 is apparently too fast to follow with stopped-flow, because no O2-dependent phase is observed in multi-exponential fits of the time course.15, 26 The lack of O2 concentration dependence in the reciprocal relaxation times of the observed phases implies that the (unobserved) O2 binding is effectively irreversible.26 The reaction following O2 binding in the sMMOHred:MMOB active site, which results in a complex with the diferrous cluster (intermediate P*), occurs with a rate constant of 26 s−1 at 4 °C as monitored by rapid freeze quench EPR and Mössbauer spectroscopies.9, 15, 26, 59 P* then decays at 9 s−1 to the diferric peroxo intermediate P with the resulting chromophoric change.15, 18 The rate constant of 26 s−1 is 1-2 orders of magnitude slower than typically observed for O2 reaction with a metalloenzyme, 60-62 but it is much faster than that observed for O2 reaction with sMMOHred in the absence of MMOB (Figure 11A). The observed reciprocal relaxation time for the latter reaction exhibits a hyperbolic dependence on O2 concentration, suggesting that it occurs in two steps (Figure 11B, inset). Nominally, these steps are reversible binding (KD1 = 0.44 mM ± 0.02 mM) followed by a slower irreversible oxidation step (k = 0.019 ± 0.001 s−1). Interestingly, the reaction of Mt sMMOHred:V41R-MMOB with O2 occurs even slower with a O2-dependent second order rate constant of 1.5 ± 0.2 M−1 s−1 at 4 °C (pseudo first order k ≈ 0.001 s−1 at 720 μM O2) (Figure 11A and B). The linear O2-concentration dependence suggests that this reaction is rate-limited by the initial O2 binding reaction. Thus, the initial O2 binding reaction occurs at least 25,000-fold slower when V41R-MMOB is used in place of WT-MMOB (>26 s−1/0.001 s−1). The fundamentally different reactions with O2 indicated in Figure 11B for sMMOHred:V41R-MMOB versus sMMOHred alone have implications for the mechanism of O2 access as discussed below.
Figure 11.
Reaction after stopped-flow mixing of 28.5 μM sMMOHred (57 μM active sites) with O2 in the presence (red) and absence (blue) of 57 μM V41R-MMOB (after mixing). (A) Time course of the oxidation reaction monitored at 330 nm at the concentration (after mixing) of O2 shown. Three-summed exponential fits to the data are shown superimposed in white on the time courses. All time courses have the same endpoint at infinite time. The faster two, very low amplitude phases are seen in all reactions in the presence or absence of V41R-MMOB and are apparently an artifact of mixing. In the presence of WT-MMOB, the time course would be coincident with the y-axis for the time scale shown. Conditions: 50 mM MOPS buffer pH 7, 4 °C. (B) Plot of observed reciprocal relaxation times of the large amplitude, slowest phase from fits of data like those in panel A for reactions using V41R-MMOB versus O2 concentration. Inset: The same plot from fits of reactions containing MMOHred alone. Data analysis is described in the Supplemental Information.
DISCUSSION
Presented here and in another recent study49 are the first X-ray crystallographic structures of the sMMOHox:MMOB and sMMOHred:MMOB complexes using protein components isolated from the Type II methanotroph Methylosinus trichosporium OB3b. These structures complement those previously published of the complex of the oxidized sMMO components from the Type Ia methanotroph63 Methylococcus capsulatus strain Bath.34 While the structures are clearly similar, the much higher current resolution of the Mt OB3b complex structures now allows the effects of complexation and reduction to be evaluated in detail. We also report the highest resolution structure of sMMOHox to date, as well as the first structure of reduced Mt sMMOH. These studies are used in the following discussion to evaluate the changes in the sMMOH structure caused by MMOB that specifically influence O2 (and potentially methane) access to the active site during the catalytic cycle.
Effect of Molecules in the Active Site.
The Mt sMMOHox:MMOB Form 1 (6VK5) and Form 2 (6VK8) structures reported here show benzoate and succinate, respectively, bound in the active site to the diiron cluster (Figure 3). The presence of these molecules is particularly revealing because the structures demonstrate the flexibility of the active site and its potential influence on substrate access. While sMMOH is clearly tailored to facilitate methane oxidation, the enzyme will oxidize hundreds of larger adventitious substrates.6, 51 These reactions are not beneficial to the methanotroph, but they do indicate that the active site can expand to accommodate larger molecules. The metrics of the diiron cluster itself with benzoate bound are essentially unchanged from those of the substrate-free enzyme with the exception of the Fe-Fe distance, which is lengthened by approximately 0.4 Å. In contrast, the active site cavity is significantly altered, which can be seen by comparing the benzoate-bound Mt sMMOHox:MMOB Form 1 structure to the RT-XFEL Mt sMMOHox:MMOB structure, in which water is modeled as a ligand to the diiron cluster in place of benzoate (Figure 4A vs C and Figure 5C vs E, Movie S1). Changes occur in three of the four helices (C, E, and F) that house the nonheme diiron cluster (Figure S2). The ability of Helices E and F to shift is important because they carry with them residues E209, E243, and H246, which are three of the six ligands that coordinate the diiron cluster. The flexibility of these helices permit Fe2 to change its distance from Fe1 during the catalytic cycle, which allows formation of structurally diverse intermediates.20, 21 It is likely that part of this flexibility derives from the π-helical segment in Helix E adjacent to the cluster.
The mechanistic significance of large substrates in the active site of the Mt sMMOH:MMOB complex remains unknown. In addition to the benzoate and succinate demonstrated here, relatively large alcohols have been structurally characterized as ligands to the diiron cluster in Mc sMMOHox crystals in soaking experiments (see for example PDB:1XVG).37, 64 In contrast, no large molecules that perturb the active site are observed in the structures of Mt sMMOHox, Mt sMMOHred, and only a small molecule that causes no change in structure was observed in RT-XFEL Mt sMMOH:MMOB experiments.49 Thus, the presence of larger exogenous molecules in the active site of crystallized enzyme appears to be dependent on both the sMMO protein components present and the crystallization conditions. Past single turnover kinetic experiments have shown that molecules larger than methane such as C2 to C8 hydrocarbons bind slowly to the enzyme after Q is formed in the reaction cycle and are hydroxylated.1, 14, 15, 30 The route of entry of these large molecules into the active site is unclear, but the current results suggest that the variable volume of the active site is likely to be an important factor in the ability of sMMO to oxidize such a large range of substrates.
It is proposed here that the binding of large molecules in the active site is the direct cause of the shift in the position of F188 and coordinated changes in the π-helical region of Helix D (Figure 4A and B). This observation contradicts the conclusion of Lee, et al. that MMOB binding is the cause of this shift.34 The latter conclusion was based on the observation of the shift in F188 in a structure of Mc sMMOH:MMOB (4GAM) thought to be devoid of a substrate. However, close inspection of the 4GAM electron density map shows that there is additional positive density in the Fo-Fc omit map of the active site (Figure 4D and Figure S3) in the position where we model large molecules in Form 1 and Form 2 Mt sMMOH:MMOB. This observation suggests the likely possibility that there is also a substrate-like molecule present in the Mc sMMOH:MMOB crystal, and that this molecule is the cause of the F188 shift. This new insight is important because the shift in the position of F188 completely removes the barrier between Cavities 2 and 1 (compare Figure 5C with 5E, Table 1). The observation of this pathway opening as an effect of MMOB binding in the Mc Bath sMMOH:MMOB complex was proposed as explanation for the regulatory effect of MMOB on O2 and CH4 binding,34 which we believe should be reevaluated. Indeed, the results presented here indicate that MMOB binding constricts the bottleneck between Cavities 1 and 2 due to the reorganization of L110 and L216 and blocks access to the active site through the chain of cavities. This change is only visualized in the absence of adventitious substrate binding in the active site cavity.
Table 1.
Potential Passages into the sMMOH Active Site.
| sMMOHox 6VK6 |
sMMOHred 6VK7 |
sMMOHox:MMOB Form 1 6VK5 |
sMMOHred:MMOB 6VK4 |
sMMOHox:MMOB RT-XFEL 6YD0 |
sMMOHred:MMOB RT-XFEL 6YDI |
|
|---|---|---|---|---|---|---|
| Chain of Cavities |
Cavities 3 to 2 Connected |
Cavities 3 to 2 Connected |
Cavities 3 to 2 Connected |
Cavities 3 to 2 Connected |
Cavities 3 to 2 Connected |
Cavities 3 to 2 Connected |
| Cavities 2 to 1 Connected |
Cavities 2 to 1 Disconnecteda |
Cavities 2 to 1 Connectedb |
Cavities 2 to 1 Connectedb |
Cavities 2 to 1 Disconnected |
Cavities 2 to 1 Disconnected |
|
| W308- Tunnel |
Closed | Closed | Open | Open | Open | Open |
| Pore | Open | Partially closed |
Closed | Closed | Closed | Closed |
The main route between Cavities 2 and 1 is disconnected, but transit through a constricted side channel cannot be ruled out.
This connection is a result of an artifact of adventitious substrate binding to the active site.
The W308-Tunnel as an Alternative Route for O2 Access.
The structures currently available are those of the states leading directly to formation of the O2 complex. As described in the introduction, two routes for the entry of substrates into the active site have been proposed: (i) direct entry through the Pore region, and (ii) transit through a long assembly of three cavities. The structures presented here and in previous studies of the Mc Bath sMMOH:MMOB suggest that the route through the Pore is unlikely because MMOB physically covers the Pore and residues shift within the Pore upon MMOB binding to block access.34 Access through the chain of cavities remains a possibility, but it is made less likely by the structural, kinetic, and mutagenic studies presented here and in our previous studies.15, 26, 29, 58 We suggest here that passage of O2 through the W308-Tunnel is a third possible transit route, which is consistent with all of the available studies. Several observations favor the W308-Tunnel over the chain of cavities. First, the residues that define the W308-Tunnel, are highly conserved in the known sMMOH enzymes as well as in similar diiron cluster-containing enzymes that catalyze oxidation of small hydrocarbons (Table S7). This conservation is not observed for enzymes structurally related to sMMOH that oxidize larger substrates such as the toluene 4-monooxygenase (T4MO), toluene/o-xylene monooxygenase (ToMOH), and phenol hydroxylase (PHH). In the latter enzymes, a relatively large tunnel in the vicinity of the sMMOH W308-Tunnel has been detected.39 However, these tunnels follow a different path to the active site, and close rather than open when complexes are formed with the cognate regulatory proteins.65 Second, the proposed route for O2 through the W308-Tunnel in the sMMOH:MMOB complex would take it past V41 shown here to profoundly affect the rate of diiron cluster oxidation when mutated to a larger residue (Figure 11). Third, the tunnel also passes the sMMOH-MMOB interface region that includes the MMOB “Quad-residues” (N107, S109, S110, T111)29 that control many aspects of the size selectivity of hydrocarbon substrates (Figures 7 and 9). Fourth, the binding of MMOB causes many structural changes in the W308-Tunnel that are likely to affect O2 binding. Specifically, a dome of conserved, hydrophobic residues is organized above the entry to the W308-Tunnel by MMOB (Figure 12). This structural feature would enhance the binding of O2 because the W308-Tunnel entrance is otherwise exposed to solvent and surrounded by polar residues in the sMMOHred structure. Fifth, the bottlenecks in the W308-Tunnel widen in the sMMOH:MMOB complex sufficiently to permit O2 to pass at the critical choke points, whereas the passage through the chain of cavities effectively closes (Figure 8 and Figure 13). The state of the potential passageways into the active site as seen in the crystal structures are summarized in Table 1. Sixth, the W308-Tunnel entrance is positioned in a structural region in sMMOH between two π-helical sections of adjacent Helices E and H (Figure S7). Often π-helices are evolutionary conserved for their functional roles in enzyme activity.66-69 Finally, all structures of the sMMOH:MMOB complex show that the 30-residue unstructured N-terminal region of isolated MMOB becomes ordered as it binds to sMMOH.34, 49, 70-72 Truncation of this region shifts the rate-limiting step of the catalytic cycle to O2 binding.70 The RT-XFEL study of the sMMOH:MMOB complex showed that the MMOB N-terminal residues selectively reorganize sMMOH Helices H and 4.49 Helix H provides many of the residues for the W308-Tunnel once it enters sMMOH. Indeed, both the N-terminal tail of MMOB and the distinctive tryptophan-rich sequence of Helix H in sMMOH are uniquely conserved in sMMO enzymes within the larger family of bacterial multicomponent monooxygenases (Figure S8).
Figure 12.
Hydrophobic entry into the W308-Tunnel results from the formation of the sMMOHred:MMOB complex (6YDI). Colors: sMMOH α-subunit, teal; MMOB, yellow; residues forming the hydrophobic dome, red. The red colored surface representation of the hydrophobic dome at the protein complex interface is visualized more clearly by making one of the two proteins transparent in the structures to the right.
Figure 13.
Structural changes in constriction points in access routes for O2 into the actives site resulting from MMOB binding. Structures for sMMOHred (6VK7, white) and RT-XFEL sMMOHred:MMOB (6YDI, green) are shown at constriction points in (A) the W308-Tunnel and (B) the passage from Cavity 2 to Cavity 1. The large shift in the position of F282 opens the W308-Tunnel, while the shifts in the Cavity 2 to 1 passage greatly restrict that route of entry.
The novelty of the W308-Tunnel entrance is further highlighted by the observation that the MMOB induced reorganization of Helices E, F and H closes the Pore and opens the W308-tunnel. It is perhaps not coincidental that this conformational change opens up a non-polar route into sMMOH at the expense of a polar route in preparation for the start of the catalytic cycle (Movie S2).
Insight from Studies of the Evolution of Bacterial Multicomponent Monooxygenases.
A recent study of the evolution of bacterial multicomponent monooxygenases suggests that sMMO was the last to emerge and is significantly diverged from the monooxygenases that oxidized larger substrates.73 It is interesting to speculate that the chain of cavities in sMMOH has been carried over from an earlier ancestor, but has been functionally replaced by the narrow W308-Tunnel to provide selectivity for small substrates. Also, it is noteworthy that the critical N-terminal region of MMOB that regulates the W308-Tunnel region in the sMMOH:MMOB complex is missing or truncated in the monooxygenases that oxidize larger substrates (Figure S8). Thus, caution must be exercised when using the structures of these enzymes to draw conclusions regarding substrate access into the active site of sMMOH.
Insight from Diiron Cluster Oxidation Kinetics.
Additional support for the importance of the W308-Tunnel derives from an examination of the kinetics of sMMOHred diiron cluster reoxidation under various conditions (Figure 11). Overall, the rate constant for O2 access into the active site is at least 25,000-fold larger when sMMOHred is complexed with WT-MMOB rather than V41R-MMOB. However, the comparison of the reaction without MMOB versus that with V41R-MMOB present also provides insight. The reaction using V41R-MMOB is much slower, and the rate constant for the reaction displays a first order dependence on O2 concentration rather than the hyperbolic dependence seen for that of the isolated sMMOHred reaction (Figure 11B). A possible scenario to account for these observations is based on a change in the rate-limiting step from: (i) O2 gaining access to the protein at a location remote from the diiron cluster in the V41R-MMOB sMMOHred reaction, to (ii) O2 binding to the diiron cluster in the isolated sMMOHred reaction. If V41R-MMOB restricts the W308-Tunnel, the slow step becomes O2 binding from solution, the rate constant for which would exhibit the observed first order dependence on O2 concentration. In the absence of MMOB, the hyperbolic O2 concentration dependence of the observed rate constant suggests that another step following reversible O2 binding is rate-limiting. Our previous magnetic circular dichroism/circular dichroism74 and RT-XFEL studies49 of the Mt sMMOH:MMOB complex show that discrete changes occur in the diiron cluster environment as MMOB binds that are predicted to enhance its ability to bind and activate O2. In the absence of these changes, the binding of O2 to the cluster may be slower than the O2 transit to a position where it would be poised to react with the cluster. This condition would be satisfied if the transit time through the W308-Tunnel is faster than binding to the diiron cluster. However, the severe constriction in the W308-Tunnel and polar residues at its entry into sMMOHred in the absence of MMOB may greatly slow or even block O2 entry via this route (Figure 8). Alternatively, in the absence of MMOB or its V41R variant, O2 might still slowly enter the active site through the constriction in the channel between Cavities 1 and 2 in the chain of cavities. Accumulation of O2 in the chain of cavities prior to binding to the diiron cluster would result in the observed hyperbolic dependence of the rate constant. In this scenario, the route through the cavities is responsible for the slow background rate of cluster reoxidation in the absence of MMOB, which is shut off when MMOB binds. It should be noted that this analysis implies two separate roles for MMOB. The structural changes it engenders both activate the cluster to react with O2, while also opening the W308-Tunnel for size-selective, bottleneck free, one-dimensional rapid transit of O2 to the cluster.
Relevance to Methane Binding.
Past studies have shown that methane and most adventitious substrates of sMMO react directly with intermediate Q in the sMMOH catalytic cycle.14 These substrates do not affect the kinetics of earlier cycle steps, but their reaction with Q is first order in substrate in the accessible concentration range. Our previous transient kinetic studies demonstrate that MMOB is bound to sMMOH when substrates react with Q.29 MMOB variants with smaller residues in the Quad region (Green β-sheet in Figures 7 and 9) adjacent to the W308-Tunnel allow larger substrates to react more rapidly, consistent with easier passage into the active site.29 Accordingly, larger substrates exhibit no deuterium KIE when reacting with Q unless the MMOB variants with smaller residues in the Quad region are used in place of WT-MMOB.31 In contrast, a deuterium KIE of 50 is observed for the methane reaction when WT-MMOB is utilized.23, 30, 58 These results are consistent with the W308-Tunnel also serving as an access route for hydrocarbon substrates. When the substrate is larger than methane, the constraints of the tunnel cause binding to the protein to be rate-limiting rather than C-H bond cleavage, hence giving first order kinetics in substrate concentration with no deuterium KIE. Methane may give both first order kinetics and a KIE because it is small enough to pass efficiently through the MMOB-modified tunnel. The transit of methane might be faster than expected through the narrow tunnel because it can only move in one- rather than three-dimensions. A parallel can be drawn to the accelerated localization of DNA binding factors to consensus sites due to one-dimensional transit on the DNA polymer.75 In essence, methane behaves kinetically as if it does not accumulate in the tunnel but rather collides directly from solution with the diiron cluster in the highly reactive Q intermediate state. The cleavage of the strong bond of methane would then be rate-limiting to give the detectable KIE. The passage of methane through the chain of cavities is also possible, but the cavities are large enough to accumulate many methane molecules. Indeed, we find multiple solvent molecules in the cavities (Figure S9) and the binding of Xe and small hydrophobic molecules have been reported in the cavities of the Mc Bath sMMOH.35 This accumulation of substrates in the cavities would mean that the substrate would react with Q from within the protein rather than from solution, thereby yielding hyperbolic or concentration independent response of the rate constants.
An important caveat is that the asynchronous binding of O2 and hydrocarbon substrates during the catalytic cycle implies a significant structural change that cannot be investigated using the available crystal structures. In particular, a change that allows the binding of molecules slightly larger than O2 at the Q stage of the reaction cycle would account for the existing kinetic data.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the financial support of this work from Grants NIH GM118030 (to J.D.L.), GM118047 (to H.A.), and training grant GM08347 (to J.C.J.). This work is based upon research conducted at the Northeastern Collaborative Access Team beamlines, which are funded by the US National Institutes of Health grant number P30 GM124165. The Pilatus 6M detector on 24-ID-C beamline is funded by a NIH-ORIP HEI grant number S10 RR029205. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. We thank the staff at NE-CAT beamlines, Advanced Photon Source, Argonne National Laboratory for assisting data collection.
Footnotes
Supporting Information
Experimental procedures for analyzing stopped-flow data; Discussion of rare types of helices found in sMMOH; Comparison of structures obtained using data obtained at 100K versus room temperature; Tables S1-S8; Figures S1 to S9. Separate files contain Movies S1 and S2.
UniProt and PDB Accession Codes
Methane monooxygenase hydroxylase component (sMMOH, α, β, γ chains) UniProt P27353, A0A1A6FJQ4 and A0A1A6FHH2; Methane monooxygenase regulatory component B (MMOB) UniProt P27356; Methane monooxygenase reductase component (MMOR) UniProt Q53563.The Protein Data Bank accession codes of the coordinates are 6VK6 (Mt sMMOHox), 6VK7 (Mt sMMOHred), 6VK5 (Mt sMMOHox:MMOB complex with benzoate bound), 6VK8 (Mt sMMOHox:MMOB complex with succinate in one protomer), 6VK4 (Mt sMMOHred:MMOB complex with one of the two diiron clusters reduced).
The authors declare no competing financial interest.
REFERENCES
- [1].Banerjee R, Jones JC, and Lipscomb JD (2019) Soluble methane monooxygenase, Ann. Rev. Biochem 88, 409–431. [DOI] [PubMed] [Google Scholar]
- [2].Ross MO, and Rosenzweig AC (2017) A tale of two methane monooxygenases, J. Biol. Inorg. Chem 22, 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Reay DS, Smith P, Christensen TR, James RH, and Clark H (2018) Methane and global environmental change, Annu. Rev. Environ. Resour 43, 165–192. [Google Scholar]
- [4].Singh BK, Bardgett RD, Smith P, and Reay DS (2010) Microorganisms and climate change: Terrestrial feedbacks and mitigation options, Nat. Rev. Microbiol 8, 779–790. [DOI] [PubMed] [Google Scholar]
- [5].Fox BG, Froland WA, Jollie DR, and Lipscomb JD (1990) Methane monooxygenase from Methylosinus trichosporium OB3b, Methods Enzymol. 188, 191–202. [DOI] [PubMed] [Google Scholar]
- [6].Pilkington SJ, and Dalton H (1990) Soluble methane monooxygenase from Methylococcus capsulatus (Bath), Methods Enzymol. 188, 181–190. [Google Scholar]
- [7].Wallar BJ, and Lipscomb JD (1996) Dioxygen activation by enzymes containing binuclear non-heme iron clusters, Chem. Rev 96, 2625–2657. [DOI] [PubMed] [Google Scholar]
- [8].Tinberg CE, and Lippard SJ (2011) Dioxygen activation in soluble methane monooxygenase, Acc. Chem. Res 44, 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fox BG, Froland WA, Dege JE, and Lipscomb JD (1989) Methane monooxygenase from Methylosinus trichosporium OB3b. Purification and properties of a three-component system with high specific activity from a type II methanotroph, J. Biol. Chem 264, 10023–10033. [PubMed] [Google Scholar]
- [10].Rosenzweig AC, Frederick CA, Lippard SJ, and Nordlund P (1993) Crystal structure of a bacterial non-haem iron hydroxylase that catalyses the biological oxidation of methane, Nature 366, 537–543. [DOI] [PubMed] [Google Scholar]
- [11].Rosenzweig AC, Nordlund P, Takahara PM, Frederick CA, and Lippard SJ (1995) Geometry of the soluble methane monooxygenase catalytic diiron center in two oxidation states, Chem. Biol 2, 409–418. [PubMed] [Google Scholar]
- [12].Elango N, Radhakrishnan R, Froland WA, Wallar BJ, Earhart CA, Lipscomb JD, and Ohlendorf DH (1997) Crystal structure of the hydroxylase component of methane monooxygenase from Methylosinus trichosporium OB3b, Protein Sci. 6, 556–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fox BG, Hendrich MP, Surerus KK, Andersson KK, Froland WA, Lipscomb JD, and Münck E (1993) Mössbauer, EPR, and ENDOR studies of the hydroxylase and reductase components of methane monooxygenase from Methylosinus trichosporium OB3b, J. Am. Chem. Soc 115, 3688–3701. [Google Scholar]
- [14].Lee SK, Fox BG, Froland WA, Lipscomb JD, and Münck E (1993) A transient intermediate of the methane monooxygenase catalytic cycle containing a FeIVFeIV cluster, J. Am. Chem. Soc 115, 6450–6451. [Google Scholar]
- [15].Lee SK, Nesheim JC, and Lipscomb JD (1993) Transient intermediates of the methane monooxygenase catalytic cycle, J. Biol. Chem 268, 21569–21577. [PubMed] [Google Scholar]
- [16].Shu L, Nesheim JC, Kauffmann K, Münck E, Lipscomb JD, and Que L Jr. (1997) An FeIV2O2 diamond core structure for the key intermediate Q of methane monooxygenase, Science 275, 515–518. [DOI] [PubMed] [Google Scholar]
- [17].Tinberg CE, and Lippard SJ (2009) Revisiting the mechanism of dioxygen activation in soluble methane monooxygenase from M. capsulatus (Bath): Evidence for a multi-step, proton-dependent reaction pathway, Biochemistry 48, 12145–12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Banerjee R, Meier KK, Münck E, and Lipscomb JD (2013) Intermediate P* from soluble methane monooxygenase contains a diferrous cluster, Biochemistry 52, 4331–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Banerjee R, Proshlyakov Y, Lipscomb JD, and Proshlyakov DA (2015) Structure of the key species in the enzymatic oxidation of methane to methanol, Nature 518, 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Castillo RG, Banerjee R, Allpress CJ, Rohde GT, Bill E, Que L Jr., Lipscomb JD, and DeBeer S (2017) High-energy-resolution fluorescence-detected X-ray absorption of the Q intermediate of soluble methane monooxygenase, J. Am. Chem. Soc 139, 18024–18033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cutsail GE, Banerjee R, Zhou A, Que L, Lipscomb JD, and DeBeer S (2018) High-resolution extended x-ray absorption fine structure analysis provides evidence for a longer Fe⋯Fe distance in the Q intermediate of methane monooxygenase, J. Am. Chem. Soc 140, 16807–16820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Priestley ND, Floss HG, Froland WA, Lipscomb JD, Williams PG, and Morimoto H (1992) Cryptic stereospecificity of methane monooxygenase, J. Am. Chem. Soc 114, 7561–7562. [Google Scholar]
- [23].Nesheim JC, and Lipscomb JD (1996) Large isotope effects in methane oxidation catalyzed by methane monooxygenase: Evidence for C─H bond cleavage in a reaction cycle intermediate, Biochemistry 35, 10240–10247. [DOI] [PubMed] [Google Scholar]
- [24].Brazeau BJ, Austin RN, Tarr C, Groves JT, and Lipscomb JD (2001) Intermediate Q from soluble methane monooxygenase hydroxylates the mechanistic substrate probe norcarane: evidence for a stepwise reaction, J. Am. Chem. Soc 123, 11831–11837. [DOI] [PubMed] [Google Scholar]
- [25].Gherman BF, Lippard SJ, and Friesner RA (2005) Substrate hydroxylation in methane monooxygenase: quantitative modeling via mixed quantum mechanics/molecular mechanics techniques, J. Am. Chem. Soc 127, 1025–1037. [DOI] [PubMed] [Google Scholar]
- [26].Liu Y, Nesheim JC, Lee S-K, and Lipscomb JD (1995) Gating effects of component B on oxygen activation by the methane monooxygenase hydroxylase component, J. Biol. Chem 270, 24662–246625. [DOI] [PubMed] [Google Scholar]
- [27].Leak DJ, and Dalton H (1987) Studies on the regioselectivity and stereoselectivity of the soluble methane monooxygenase from Methylococcus capsulatus (Bath), Biocatalysis 1, 23–36. [Google Scholar]
- [28].Green J, and Dalton H (1989) Substrate specificity of soluble methane monooxygenase. Mechanistic implications, J. Biol. Chem 264, 17698–17703. [PubMed] [Google Scholar]
- [29].Wallar BJ, and Lipscomb JD (2001) Methane monooxygenase component B mutants alter the kinetics of steps throughout the catalytic cycle, Biochemistry 40, 2220–2233. [DOI] [PubMed] [Google Scholar]
- [30].Brazeau BJ, and Lipscomb JD (2000) Kinetics and activation thermodynamics of methane monooxygenase compound Q formation and reaction with substrates, Biochemistry 39, 13503–13515. [DOI] [PubMed] [Google Scholar]
- [31].Brazeau BJ, Wallar BJ, and Lipscomb JD (2001) Unmasking of deuterium kinetic isotope effects on the methane monooxygenase compound Q reaction by site-directed mutagenesis of component B, J. Am. Chem. Soc 123, 10421–10422. [DOI] [PubMed] [Google Scholar]
- [32].Zheng H, and Lipscomb JD (2006) Regulation of methane monooxygenase catalysis based on size exclusion and quantum tunneling, Biochemistry 45, 1685–1692. [DOI] [PubMed] [Google Scholar]
- [33].Zhang J, Wallar BJ, Popescu CV, Renner DB, Thomas DD, and Lipscomb JD (2006) Methane monooxygenase hydroxylase and B component interactions, Biochemistry 45, 2913–2926. [DOI] [PubMed] [Google Scholar]
- [34].Lee SJ, McCormick MS, Lippard SJ, and Cho U-S (2013) Control of substrate access to the active site in methane monooxygenase, Nature 494, 380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Whittington DA, Rosenzweig AC, Frederick CA, and Lippard SJ (2001) Xenon and halogenated alkanes track putative substrate binding cavities in the soluble methane monooxygenase hydroxylase, Biochemistry 40, 3476–3482. [DOI] [PubMed] [Google Scholar]
- [36].Song WJ, Gucinski G, Sazinsky MH, and Lippard SJ (2011) Tracking a defined route for O2 migration in a dioxygen-activating diiron enzyme, Proc. Natl. Acad. Sci. USA 108, 14795–14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sazinsky MH, and Lippard SJ (2005) Product bound structures of the soluble methane monooxygenase hydroxylase from Methylococcus capsulatus (Bath): Protein motion in the α-subunit, J. Am. Chem. Soc 127, 5814–5825. [DOI] [PubMed] [Google Scholar]
- [38].Rosenzweig AC, Brandstetter H, Whittington DA, Nordlund P, Lippard SJ, and Frederick CA (1997) Crystal structures of the methane monooxygenase hydroxylase from Methylococcus capsulatus (Bath): implications for substrate gating and component interactions, Proteins 29, 141–152. [PubMed] [Google Scholar]
- [39].McCormick MS, and Lippard SJ (2011) Analysis of substrate access to active sites in bacterial multicomponent monooxygenase hydroxylases: X-ray crystal structure of xenon-pressurized phenol hydroxylase from Pseudomonas sp. OX1, Biochemistry 50, 11058–11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Liang AD, Wrobel AT, and Lippard SJ (2014) A flexible glutamine regulates the catalytic activity of toluene o-xylene monooxygenase, Biochemistry 53, 3585–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Banerjee R, Komor AJ, and Lipscomb JD (2017) Use of isotopes and isotope effects for investigations of diiron oxygenase mechanisms, Methods Enzymol. 596, 239–290. [DOI] [PubMed] [Google Scholar]
- [42].Chang SL, Wallar BJ, Lipscomb JD, and Mayo KH (1999) Solution structure of component B from methane monooxygenase derived through heteronuclear NMR and molecular modeling, Biochemistry 38, 5799–5812. [DOI] [PubMed] [Google Scholar]
- [43].Kabsch W (2010) XDS, Acta Crystallogr. Sect. D: Biol. Crystallogr 66, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, and Zwart PH (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution., Acta Crystallogr. Sect. D: Biol. Crystallogr D66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, and Read RJ (2007) Phaser crystallographic software, J. Appl. Crystallogr 40, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Emsley P, and Cowtan K (2004) Coot: Model-building tools for molecular graphics, Acta Crystallogr E E60, 2126–2132. [DOI] [PubMed] [Google Scholar]
- [47].Sehnal D, Svobodová Vařeková R, Berka K, Pravda L, Navrátilová V, Banáš P, Ionescu C-M, Otyepka M, and Koča J MOLE 2.0: advanced approach for analysis of biomacromolecular channels J. Cheminformatics, 2013, 5, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rogers MS, and Lipscomb JD (2019) Salicylate 5-hydroxylase: intermediates in aromatic hydroxylation by a Rieske monooxygenase, Biochemistry 58, 5305–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Srinivas V, Banerjee R, Lebrette H, Jones JC, Aurelius O, Kim I-S, Pham CC, Gul S, Sutherlin KD, Bhowmick A, John J, Bozkurt E, Fransson T, Aller P, Butryn A, Bogacz I, Simon P, Keable S, Britz A, Tono K, Kim KS, Park S-Y, Lee SJ, Park J, Alonso-Mori R, Fuller FD, Batyuk A, Brewster AS, Bergmann U, Sauter NK, Orville AM, Yachandra VK, Yano J, Lipscomb JD, Kern J, and Högbom M (2020) High resolution XFEL structure of the soluble methane monooxygenase hydroxylase complex with its regulatory component at ambient temperature in two oxidation states, J. Am. Chem. Soc 142, 10.1021/jacs.1020c05613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, and Evans PR (2011) Overview of the CCP4 suite and current developments, Acta Crystallogr. Sect. D: Biol. Crystallogr 67, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Froland WA, Andersson KK, Lee S-K, Liu Y, and Lipscomb JD (1992) Methane monooxygenase component B and reductase alter the regioselectivity of the hydroxylase component-catalyzed reactions. A novel role for protein-protein interactions in an oxygenase mechanism, J. Biol. Chem 267, 17588–17597. [PubMed] [Google Scholar]
- [52].Bondi A (1964) van der Waals volumes and radii, J. Phys. Chem 68, 441–451. [Google Scholar]
- [53].Batsanov SS (2001) Van der Waals radii of elements, Inorg. Mater 37, 871–885. [Google Scholar]
- [54].Kammeyer CW, and Whitman DR (1972) Quantum mechanical calculation of molecular radii. I. Hydrides of elements of periodic Groups IV through VII, J. Chem. Phys 56, 4419–4421. [Google Scholar]
- [55].Murphy KI Graham’s law explained: The difference between effusion and permeation 2007, https://www.getnitrogen.org/pdf/graham.pdf
- [56].Mehio N, Dai S, and Jiang D.-e. (2014) Quantum mechanical basis for kinetic diameters of small gaseous molecules, J. Phys. Chem. A 118, 1150–1154. [DOI] [PubMed] [Google Scholar]
- [57].Liebgott PP, Leroux F, Burlat B, Dementin S, Baffert C, Lautier T, Fourmond V, Ceccaldi P, Cavazza C, Meynial-Salles I, Soucaille P, Fontecilla-Camps JC, Guigliarelli B, Bertrand P, Rousset M, and Léger C (2010) Relating diffusion along the substrate tunnel and oxygen sensitivity in hydrogenase, Nat. Chem. Biol 6, 63–70. [DOI] [PubMed] [Google Scholar]
- [58].Brazeau BJ, and Lipscomb JD (2003) Key amino acid residues in the regulation of soluble methane monooxygenase catalysis by component B, Biochemistry 42, 5618–5631. [DOI] [PubMed] [Google Scholar]
- [59].Hendrich MP, Münck E, Fox BG, and Lipscomb JD (1990) Integer-spin EPR studies of the fully reduced methane monooxygenase hydroxylase component, J. Am. Chem. Soc 112, 5861–5865. [Google Scholar]
- [60].Tyson CA, Lipscomb JD, and Gunsalus IC (1972) The role of putidaredoxin and P450cam in methylene hydroxylation, J. Biol. Chem 247, 5777–5784. [PubMed] [Google Scholar]
- [61].Rivard BS, Rogers MS, Marell DJ, Neibergall MB, Chakrabarty S, Cramer CJ, and Lipscomb JD (2015) Rate-determining attack on substrate precedes Rieske cluster oxidation during cis-dihydroxylation by benzoate dioxygenase, Biochemistry 54, 4652–4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Berger RL, Antonini E, Brunori M, Wyman J, and Rossi-Fanelli A (1967) Observations on the kinetics of the reaction of hemoglobin with oxygen, J. Biol. Chem 242, 4841–4843. [PubMed] [Google Scholar]
- [63].Bowman JP, Sly LI, Nichols PD, and Hayward AC (1993) Revised taxonomy of the Methanotrophs: Description of Methylobacter gen. nov., emendation of Methylococcus, validation of Methylosinus and Methylocystis species, and a proposal that the family Methylococcaceae includes only the group I methanotrophs, Int. J. Syst. Evol. Microbiol 43, 735–753. [Google Scholar]
- [64].Whittington DA, Sazinsky MH, and Lippard SJ (2001) X-ray crystal structure of alcohol products bound at the active site of soluble methane monooxygenase hydroxylase, J. Am. Chem. Soc 123, 1794–1795. [DOI] [PubMed] [Google Scholar]
- [65].Bailey LJ, McCoy JG, Phillips GN Jr., and Fox BG (2008) Structural consequences of effector protein complex formation in a diiron hydroxylase, Proc. Natl. Acad. Sci. USA 105, 19194–19198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cooley RB, Arp DJ, and Karplus PA (2010) Evolutionary origin of a secondary structure: pi-helices as cryptic but widespread insertional variations of alpha-helices that enhance protein functionality, J. Mol. Biol. 404, 232–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Bernard AR, Jessop TC, Kumar P, and Dickenson NE (2017) Deoxycholate-enhanced Shigella virulence Is regulated by a rare π-helix in the type three secretion system tip protein IpaD, Biochemistry 56, 6503–6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Riek RP, and Graham RM (2011) The elusive π-helix, J. Struct. Biol 173, 153–160. [DOI] [PubMed] [Google Scholar]
- [69].Weaver TM (2000) The π-helix translates structure into function, Protein Sci. 9, 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chang SL, Wallar BJ, Lipscomb JD, and Mayo KH (2001) Residues in Methylosinus trichosporium OB3b methane monooxygenase component B involved in molecular interactions with reduced- and oxidized-hydroxylase component: A role for the N-terminus, Biochemistry 40, 9539–9551. [DOI] [PubMed] [Google Scholar]
- [71].Walters KJ, Gassner GT, Lippard SJ, and Wagner G (1999) Structure of the soluble methane monooxygenase regulatory protein B, Proc. Natl. Acad. Sci. USA 96, 7877–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Matsuo H, Walters KJ, Teruya K, Tanaka T, Gassner GT, Lippard SJ, Kyogoku Y, and Wagner G (1999) Identification by NMR spectroscopy of residues at contact surfaces in large, slowly exchanging macromolecular complexes, J. Am. Chem. Soc 121, 9903–9904. [Google Scholar]
- [73].Osborne CD, and Haritos VS Beneath the surface: Evolution of methane activity in the bacterial multicomponent monooxygenases Mol. Phylogenet. Evol, 2019, 139, 106527. [DOI] [PubMed] [Google Scholar]
- [74].Mitić N, Schwartz JK, Brazeau BJ, Lipscomb JD, and Solomon EI (2008) CD and MCD studies of the effects of component B variant binding on the biferrous active site of methane monooxygenase, Biochemistry 47, 8386–8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Gorman J, Chowdhury A, Surtees JA, Shimada J, Reichman DR, Alani E, and Greene EC (2007) Dynamic basis for one-dimensional DNA scanning by the mismatch repair complex Msh2-Msh6, Mol. Cell 28, 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.