Abstract
It is known that peripheral infections, accompanied by inflammation, represent significant risk factors for the development of neurological disorders by modifying brain development or affecting normal brain aging. The acute effects of systemic inflammation on progressive and persistent brain damage and cognitive impairment are well documented. Anti-inflammatory therapies may have beneficial effects on the brain, and the protective properties of a wide range of synthetic and natural compounds have been extensively explored in recent years. In our previous review, we provided an extensive analysis of one of the most important and widely-used animal models of peripherally induced neuroinflammation and neurodegeneration - lipopolysaccharide (LPS)-treated mice. We addressed the data reproducibility in published research and summarized basic features and data on the therapeutic potential of various natural products, nutraceuticals, with known anti-inflammatory effects, for reducing neuroinflammation in this model. Here, recent data on the suitability of the LPS-induced murine neuroinflammation model for preclinical assessment of a large number of nutraceuticals belonging to different groups of natural products such as flavonoids, terpenes, non-flavonoid polyphenols, glycosides, heterocyclic compounds, organic acids, organosulfur compounds and xanthophylls, are summarized. Also, the proposed mechanisms of action of these molecules are discussed.
Keywords: Neuroinflammation, nutraceuticals, LPS, neurodegeneration, peripheral inflammation, mouse model
1. INTRODUCTION
Neuroinflammation is an important feature in the pathogenesis and progression of neurodegenerative diseases such as Alzheimer´s disease (AD), Parkinson´s disease (PD), frontotemporal dementia, amyotrophic lateral sclerosis and multiple sclerosis [1-6]. Epidemiological studies indicate that AD and PD risk positively correlates with pro-inflammatory conditions such as diabetes mellitus, metabolic syndrome, hypercholesterolemia and atherosclerosis, suggesting that chronic systemic inflammation may influence the development of neurodegenerative diseases [7-9]. It has been demonstrated that peripheral infections accompanied by inflammation represent significant risk factors for the development of neurological disorders by modifying brain development or affecting normal brain aging [10-14]. The acute effects of systemic inflammation on progressive and persistent brain damage and cognitive impairment are well documented [15].
Mice treated intraperitoneally (i.p.) with lipopolysaccharide (LPS), an endotoxin from the outer membrane of bacteria known to be a potent trigger of inflammation, are widely used to study neuroinflammation and neurodegeneration caused by peripheral infection [16-20]. Recently, the suitability of this model for studying inflammation-induced cerebral microhemorrhages (CMH) has been proposed [21]. In our previous review, we provided an extensive analysis of this model and addressed data reproducibility as well as different experimental approaches described in analyzed literature [22]. It has been demonstrated that peripheral administration of LPS causes sustained brain damage and has long-term cognitive consequences [23, 24]. Activated microglia and astrocytes, increased brain levels of pro-inflammatory cytokines (interleukin 1β (IL-1β), interleukin 6 (IL-6), tumor necrosis factor α (TNF-α)), cyclooxygenase-2 (COX-2), reactive oxygen species (ROS), inducible nitric oxide synthase (iNOS), 18 kDa translocator protein TSPO and matrix metalloproteinases (MMP-3, MMP-8 and MMP-9), cerebral mitochondrial dysfunction leading to up-regulation of Bax protein and increased levels of cytochrome C, caspase-9 and caspase-3 as well as decreased levels of brain-derived neurotrophic factor (BDNF) and synaptic failure were observed in response to systemic LPS in the mouse brain [17, 18, 22, 25-29]. Moreover, disruption of the blood-brain barrier followed by the infiltration of NK cells and neutrophils has been documented [30]. It has been proposed that LPS administration activates mitogen-activated protein kinase (MAPK) family protein p38, c-Jun N-terminal kinase (JNK), nuclear factor kappa B (NF-κB) and activator protein 1 (AP-1) pathways leading to neuroinflammation and neuronal apoptosis [31, 32]. Also, it has been demonstrated that nod-like receptor pyrin domain-containing protein 3 (NLRP3) inflammasome activation contributes to long-term behavioral alterations in LPS-exposed mice [33]. Importantly, the time-dependent effects of neuroinflammation after systemic LPS administration are well documented, and future studies should take into account the influence of the dose and timing of the treatment and analysis [34, 35].
Based on current knowledge in the field, suggesting that anti-inflammatory approaches targeting peripheral inflammation may have beneficial effects in the brain, the protective properties of a wide range of synthetic and natural compounds with anti-inflammatory action have been extensively explored in recent years. In our previous review, we summarized basic features and data on the therapeutic potential of various natural products, nutraceuticals, with known anti-inflammatory effects for reducing neuroinflammation in LPS-treated mice (Catorce and Gevorkian, 2016). Here, recent data on the suitability of the LPS-induced murine neuroinflammation model for preclinical assessment of nutraceuticals are discussed (Table 1).
Table 1.
Summary of nutraceuticals tested in LPS-induced mouse neuroinflammation model between 2016 and 2019.
| Classification | Nutraceuticals |
|---|---|
|
Flavonoids (Figure 1) |
Anthocyanins [45-47]; Flavanones: Naringenin [50, 51], Hesperidin [57, 58], Eriodictyol [59, 60]; Flavanonols: Ampelopsin [62]; Flavonols: Kaempferol [64, 65], Quercetin [48, 67]; Icariin [68-71]; Flavanols: Proanthocyanidin [72]; Isoflavones: Tectorigenin [76], Icaritin [71]; Chalcones: Lonchocarpine [79]. |
|
Non-flavonoid Polyphenols (Figure 2) |
Stilbens: Resveratrol [22, 81]; Lignans/Lignins: Honokiol [82], Macranthol [84], Schizandrin [85]; Phenolic acids: Caffeic Acid [86], Chicoric Acid [89, 90]; Tannins: Punicalagin [92]. |
|
Terpenes (Figure 3) |
Triterpenes: Lupeol [31], Glycyrrhizic acid [97], Ginsenoside Rg3 [99], 3-Acetyl-11-Keto-Beta-Boswellic Acid [102], Gypenoside IX [104], Betulinic Acid [107]; Sesquiterpenoids: Aromatic-turmerone [109], Beta-elemene [110]; Tetraterpenes: Lycopene [112-114], Crocin [20]; Diterpenoids: Andrographolide [118, 119]. |
|
Glycosides (Figure 4) |
Saponin glycosides: Cantalasaponin [121], Astragaloside IV [123]; Flavonoid glycosides: Yuglanin [125], Baicalin [127]. |
|
Heterocyclic compounds (Figure 5) |
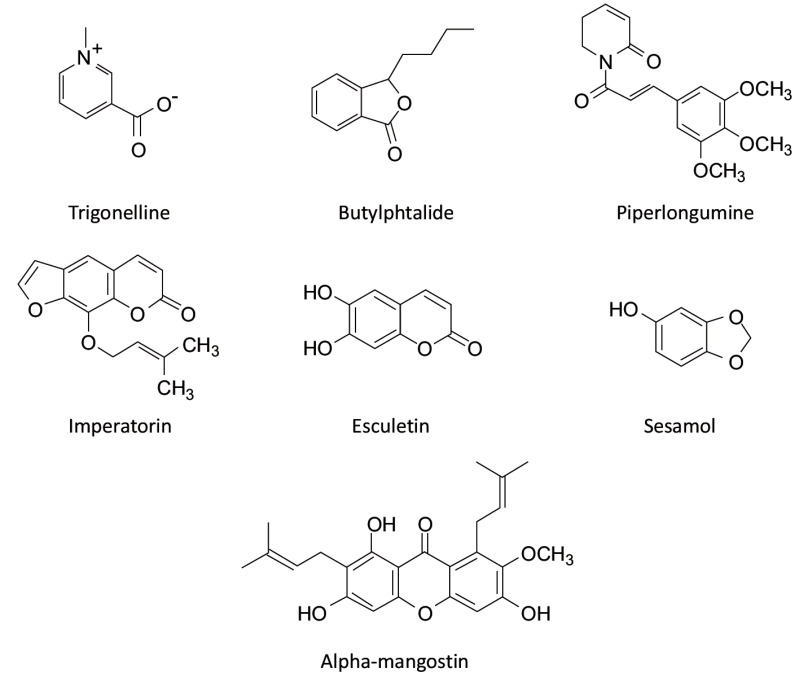
Alcaloids: Trigonelline [129]; Benzopyrans: Imperatorin [132], Esculetin [133,134]; Benzofurans: L-3-n-Butylphthalide [136]; Dioxoles: Sesamol [138]; Dioxolanes: Piperlongumine [140]; Xanthons: Alpha-mangostin [28]. |
|
Other aromatic compounds (Figure 6) |
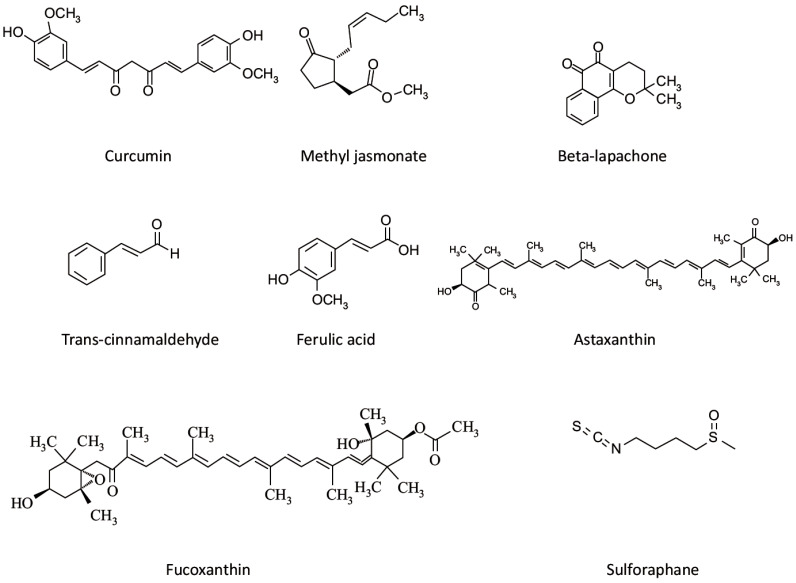
Trans-cinnamaldehyde [144, 145], Curcumine [22, 145, 147], Beta-lapachone [27]. |
|
Organic acids (Figure 6) |
Methyl jasmonate [151-153], Ferulic Acid [155]. |
|
Organosulfur compounds (Figure 6) |
Sulforaphane [157-159] |
| Proteins | Osmotin [160] |
| Lipids | Scallop-derived plasmalogens [163] |
| Other compounds |
Xanthophylls (Fig. 6): Astaxathin [164, 165], Fucoxanthin [167]. Oils: Fish oil [168-170]. |
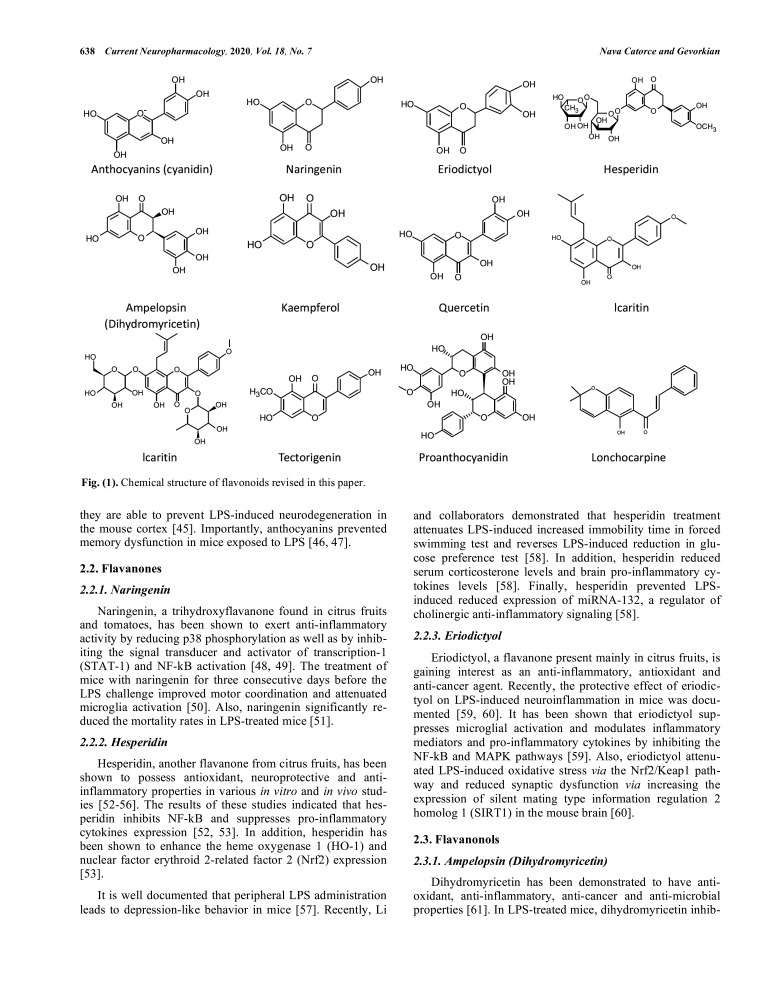
2. FLAVONOIDS (FIG. 1)
Fig. (1).
Chemical structure of flavonoids revised in this paper.
Flavonoids, a large group of polyphenolic compounds found in plants, have been shown to possess antioxidant, anti-inflammatory and anti-carcinogenic properties [36, 37]. LPS-induced murine neuroinflammation model has been used to evaluate the anti-inflammatory potential of a large number of flavonoids belonging to almost all known subgroups: flavonols, flavanones, flavanols, anthocyanins, isoflavones and chalcones. It has been proposed that flavonoids may exert their anti-inflammatory activity by modulating the expression of pro-inflammatory cytokines as well as COX-2 and inducible nitric oxide synthase (iNOS) and by reducing microglial and astrocytes activation [38-40].
2.1. Anthocyanins
Anthocyanins are abundant in flowers, fruits, seeds and plant leaves and have been shown to possess antioxidant, anti-inflammatory and anti-mutagenic properties [41-43]. They have been widely used in studies on the prevention and treatment of many chronic diseases, and it has been proposed that mixtures of anthocyanins found in food rather than their individual anthocyanin components are more beneficial for improving human health [42-44]. Peripheral LPS-induced murine neuroinflammation model has been widely used for the assessment of anti-inflammatory and neuroprotective properties of anthocyanins. It has been demonstrated that anthocyanins attenuate elevated levels of ROS and oxidative stress via reduction of the level of phospho-JNK [45, 46]. Also, they inhibited NF-κB activation and reduced pro-inflammatory cytokines expression, thus leading to inhibition of microglia and astrocytes activation in the brain of LPS-treated mice [45-47]. Furthermore, anthocyanins prevented overexpression of various apoptotic markers (Bax, cytosolic cytochrome C, cleaved caspase 3), indicating that they are able to prevent LPS-induced neurodegeneration in the mouse cortex [45]. Importantly, anthocyanins prevented memory dysfunction in mice exposed to LPS [46, 47].
2.2. Flavanones
2.2.1. Naringenin
Naringenin, a trihydroxyflavanone found in citrus fruits and tomatoes, has been shown to exert anti-inflammatory activity by reducing p38 phosphorylation as well as by inhibiting the signal transducer and activator of transcription-1 (STAT-1) and NF-kB activation [48, 49]. The treatment of mice with naringenin for three consecutive days before the LPS challenge improved motor coordination and attenuated microglia activation [50]. Also, naringenin significantly reduced the mortality rates in LPS-treated mice [51].
2.2.2. Hesperidin
Hesperidin, another flavanone from citrus fruits, has been shown to possess antioxidant, neuroprotective and anti-inflammatory properties in various in vitro and in vivo studies [52-56]. The results of these studies indicated that hesperidin inhibits NF-kB and suppresses pro-inflammatory cytokines expression [52, 53]. In addition, hesperidin has been shown to enhance the heme oxygenase 1 (HO-1) and nuclear factor erythroid 2-related factor 2 (Nrf2) expression [53].
It is well documented that peripheral LPS administration leads to depression-like behavior in mice [57]. Recently, Li and collaborators demonstrated that hesperidin treatment attenuates LPS-induced increased immobility time in forced swimming test and reverses LPS-induced reduction in glucose preference test [58]. In addition, hesperidin reduced serum corticosterone levels and brain pro-inflammatory cytokines levels [58]. Finally, hesperidin prevented LPS-induced reduced expression of miRNA-132, a regulator of cholinergic anti-inflammatory signaling [58].
2.2.3. Eriodictyol
Eriodictyol, a flavanone present mainly in citrus fruits, is gaining interest as an anti-inflammatory, antioxidant and anti-cancer agent. Recently, the protective effect of eriodictyol on LPS-induced neuroinflammation in mice was documented [59, 60]. It has been shown that eriodictyol suppresses microglial activation and modulates inflammatory mediators and pro-inflammatory cytokines by inhibiting the NF-kB and MAPK pathways [59]. Also, eriodictyol attenuated LPS-induced oxidative stress via the Nrf2/Keap1 pathway and reduced synaptic dysfunction via increasing the expression of silent mating type information regulation 2 homolog 1 (SIRT1) in the mouse brain [60].
2.3. Flavanonols
2.3.1. Ampelopsin (Dihydromyricetin)
Dihydromyricetin has been demonstrated to have antioxidant, anti-inflammatory, anti-cancer and anti-microbial properties [61]. In LPS-treated mice, dihydromyricetin inhibited neuroinflammation, enhanced the levels of BDNF in the hippocampus and reduced immobility time in the tail suspension and forced swim tests, indicating its suitability for patients with depression [62].
2.4. Flavonols
2.4.1. Kaempferol
Kaempferol, a flavonol widely distributed in fruits, vegetables and different medicinal and edible plants, has been shown to possess potent anti-inflammatory properties [63]. Suggested mechanisms of action of kaempferol are: inhibition of the NF-kB and MAPK pathways, enhancement of Nrf2 and HO-1 expression and suppression of iNOS, COX-2 and pro-inflammatory cytokines expression [63]. Studies in LPS-treated mice showed that kaempferol inhibits the production of pro-inflammatory cytokines, including IL-1β, IL-6, TNF-α, and reduces the level of monocyte chemotactic protein-1 (MCP-1), iNOS and COX-2 in the brain [64, 65]. Furthermore, kaempferol protected the integrity of the blood-brain barrier (BBB) by reducing the level of ICAM-1 and by increasing the level of tight junction-associated proteins occludin-1 and claudin-1. The authors suggested that kaempferol may suppress the activation of the Myeloid Differentiation Primary-Response Protein 88 (MyD88)/toll-like receptor 4 (TLR4) inflammatory pathway, induced by LPS in the mouse brain [64, 65].
2.4.2. Quercetin
Another flavonol with known anti-inflammatory properties is quercetin [66]. First studies in LPS-treated mice demonstrated that quercetin inhibits the activation of NF-kB and STAT-1, leading to a reduction of iNOS and NO expression [48]. Recently, the protective effect of quercetin against LPS-induced neuroinflammation, neurodegeneration and synaptic/memory dysfunction in adult mice has been documented [67]. Quercetin reduced gliosis as well as inflammatory and apoptotic markers expression in the cortex and hippocampus of adult LPS-treated mice [67]. Moreover, quercetin reversed the LPS-induced synaptic loss in the adult mouse brain and improved memory tasks performance [67].
2.4.3. Icariin
Icariin, a flavonoid with well-documented anti-inflammatory, anti-oxidant and anti-aging properties, has been shown to reduce pro-inflammatory cytokines expression, microglia activation and neuronal death through inhibiting NF-kB and JNK/p38 MAPK pathways and activating Nrf2 signaling pathway in LPS-induced neuroinflammation model [68-70]. A recent study suggested that icariin could attenuate neuroinflammation in the LPS-treated mouse hippocampus via suppressing high mobility group protein box 1 (HMGB1)-receptor for advanced glycation endproducts (RAGE) signaling [71].
2.5. Flavanols
2.5.1. Proanthocyanidin
Proanthocyanidin, obtained mainly from grape seed extract and shown to possess strong anti-inflammatory activity, has been used in LPS-induced neuroinflammation and depressive-like behavior model [72]. Proanthocyanidin reduced the immobility time in forced swimming (FST) and tail suspension (TST) tests in LPS-treated mice [72]. Furthermore, proanthocyanidin reversed LPS-induced overexpression of iNOS, COX-2 and pro-inflammatory cytokines in the hippocampus, prefrontal cortex and amygdala of these mice via modulation of NF-κB [72].
2.6. Isoflavones
2.6.1. Tectorigenin
Tectorigenin, an isoflavone isolated from various medicinal plants, has been shown to inhibit LPS-induced inflammatory responses in vitro and in vivo [73-75]. In the LPS-induced neuroinflammation mouse model, the administration of tectorigenin effectively decreased the levels of iNOS in the hippocampus and reduced the levels of TNF-α and IL-6 in the serum. Also, tectorigenin attenuated microglial activation in LPS-treated mice [76]. Furthermore, pre-treatment of mice with tectorigenin inhibited LPS-activated TLR4/MyD88 inflammatory pathway [76].
2.6.2. Icaritin
Icaritin is an active ingredient of Herba Epimedii, a medicinal herb in Chinese traditional medicine and has been shown to possess a potent anti-inflammatory activity [77]. Like icariin, icaritin also attenuated neuroinflammation in the LPS-treated mouse hippocampus via suppressing HMGB1-RAGE signaling [71].
2.7. Chalcones
2.7.1. Lonchocarpine
Lonchocarpine is a flavonoid with anti-bacterial, antioxidant and anti-inflammatory properties, isolated from the tropical medicinal plant Abrus precatorius [78]. Treatment with lonchocarpine inhibited the expression of iNOS, COX-2, TNF-α, IL-1β, IL-6, TLR2 and TLR4 in LPS-induced neuroinflammation mouse model [79].
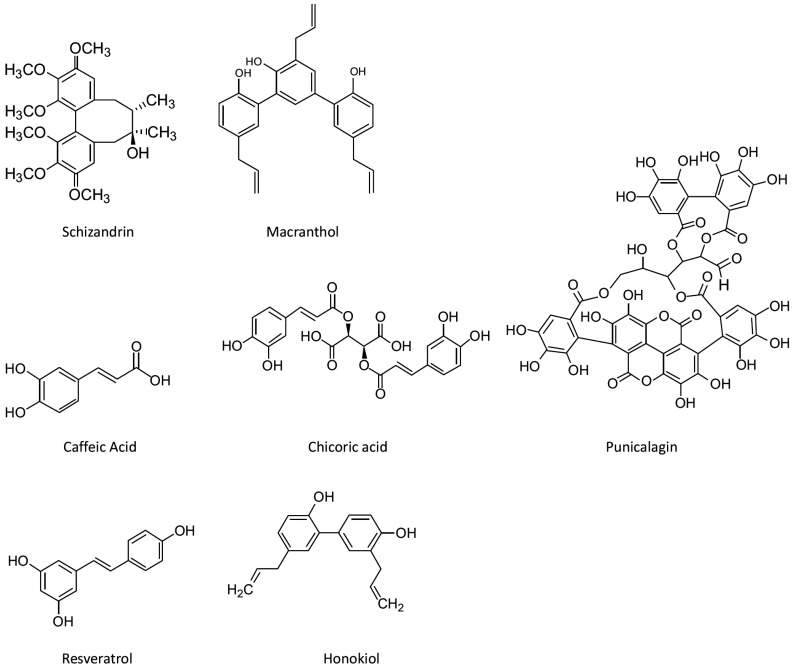
3. NON-FLAVONOID POLYPHENOLS (Fig. 2)
Fig. (2).
Chemical structure of non-flavonoid polyphenols revised in this paper.
3.1. Stilbenes
3.1.1. Resveratrol
Resveratrol, a polyphenol abundant in grape skin and seeds, has been shown to possess potent antioxidant, anti-inflammatory, anti-aging and anti-cancer properties [39, 80]. The protective effect of resveratrol in LPS-induced neuroinflammation mouse model is revised in our previous study [22]. A recent study showed that resveratrol ameliorates LPS-induced sickness behavior in mice, suppresses LPS-evoked pro-inflammatory M1 marker expression in microglia by inhibiting the NF-κB activity, and increases M2 marker expression by activation of the STAT6 and STAT3 pathways [81].
3.2. Lignans/Lignins
3.2.1. Honokiol
Honokiol, a biphenolic neolignan with potent antioxidant and anti-inflammatory properties, has been shown to abrogate LPS-induced depressive-like behavior in mice by inhibiting pro-inflammatory cytokines expression and oxido-nitrosative stress and by increasing BDNF levels [82].
3.2.2. Macranthol
Macranthol, a triphenyl lignan isolated from Illicium dunnianum, has been shown to exhibit anti-depressant properties after chronic but not acute treatment [83]. Recently, Weng and collaborators found that macranthol alleviates depressive-like behaviors in mice induced by peripheral LPS [84]. The authors suggested that the protective effect of macranthol may be mediated, in part, by suppressing microglia-related neuroinflammation in the prefrontal cortex [84].
3.2.3. Schizandrin A
Schizandrin A is a polyphenol lignin demonstrated to possess antioxidant, anti-inflammatory and neuroprotective properties. In peripheral LPS-challenged mice schizandrin A inhibited microglia activation by interfering with the TRAF6-NF-κB and JAK2-STAT3 signaling pathways [85].
3.3. Phenolic Acids
3.3.1. Caffeic Acid
Caffeic acid, a polyphenol with anti-oxidant and anti-inflammatory properties, has been shown to reduce the LPS-induced expression of pro-inflammatory and oxidative stress markers in the brain as well as signs of sickness in peripheral LPS-treated animals [86].
3.3.2. Chicoric Acid
Chicoric acid, the most active compound in Echinacea pupurea, demonstrated antioxidant and anti-inflammatory activities in vitro and in vivo [87, 88]. In a mouse model of peripheral LPS-induced neuroinflammation, chicoric acid ameliorated LPS-induced oxidative stress, inhibited pro-inflammatory cytokines expression and prevented memory impairment and amyloidogenesis [89, 90].
3.4. Tannins
3.4.1. Punicalagin
Punicalagin, a polyphenol found in pomegranate, has been shown to exert antioxidant, anti-inflammatory and anti-apoptotic effects [91]. A recent study demonstrated that in LPS-treated mice, punicalagin inhibited memory impairment via anti-inflammatory and anti-amylogenic mechanisms through inhibition of NF-κB activation [92]. The authors proposed that punicalagin directly binds to NF-κB subunit p50, evidenced by a docking model and pull-down assay [92].
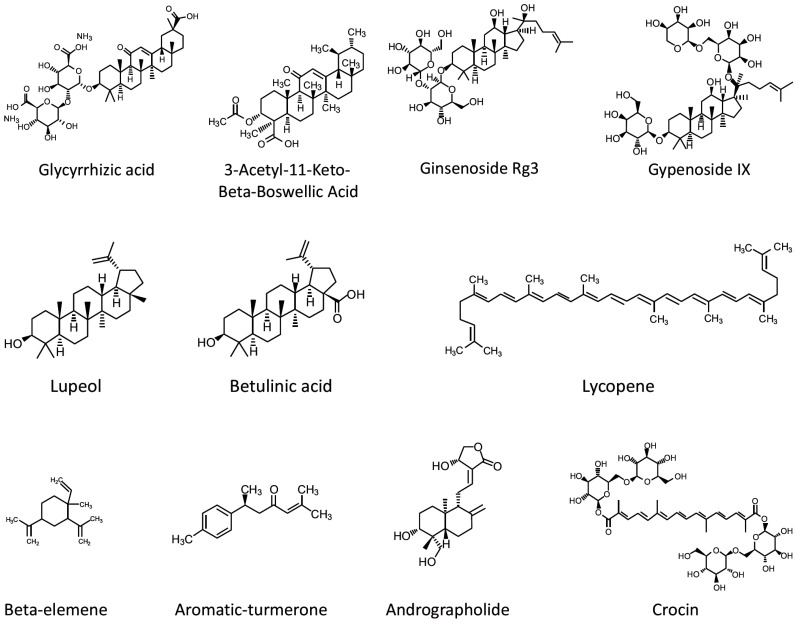
4. TERPENES (Fig. 3)
Fig. (3).
Chemical structure of terpenes revised in this paper.
Terpenes, the most widespread group of natural products found in plants, have been widely used since ancient times. Terpenes are classified into sub-groups based upon the number of isoprene units incorporated in the basic molecular skeleton [93].
4.1. Triterpenes
4.1.1. Lupeol
Lupeol, a pentacyclic triterpene, has been demonstrated to possess anti-inflammatory and anti-tumor activities [94, 95]. The protective effect of lupeol against LPS-induced neuroinflammation has also been documented [31]. It has been observed that lupeol attenuates LPS-induced increase in pro-inflammatory cytokines expression via inhibition of JNK/p38-MAPK pathways [31].
4.1.2. Glycyrrhizic Acid
Glycyrrhizinic acid is a triterpene with anti-inflammatory, antioxidant, anti-tumor and anti-viral properties, found in the roots of licorice [96]. A recent study demonstrated that glycyrrhizinic acid suppresses peripheral LPS-induced pro-inflammatory cytokines expression by inhibiting TLR4 signaling pathway [97]. Also, glycyrrhizinic acid alleviated LPS-induced memory loss and cognitive deficit in mice [97].
4.1.3. Ginsenoside Rg3
Ginsenoside Rg3, a principle active ingredient in Panax ginseng, is a triterpenoid saponin shown to possess anti-inflammatory properties [98]. A recent study demonstrated that ginsenoside Rg3 ameliorates depressive-like behavior induced by peripheral LPS administration [99]. Authors showed that ginsenoside Rg3 decreases the expression of pro-inflammatory cytokines and indoleamine-2,3-dioxygenase (IDO) mRNA in the mouse brain [99]. Also, ginsenoside Rg3 attenuated microglia activation and the disturbed turnover of tryptophan and serotonin in the hippocampus [99].
4.1.4. 3-Acetyl-11-Keto-Beta-Boswellic Acid
Boswellic acids are the main biologically active components of the gum resins of Boswellia serrata, that have been used for a variety of pathological conditions such as cancer, asthma, inflammation, arthritis, colitis, Crohn's disease and hyperlipidaemia [100]. Among different boswellic acids, 3‐Acetyl‐11‐keto‐β‐boswellic acid (AKBA) proved to be the most potent 5‐lipoxygenase inhibitor, and AKBA-loaded polymeric nanomicelles exerted anti-inflammatory and anti-arthritic activity [101]. In a recent study, AKBA exhibited anti-apoptotic and anti-amyloidogenic effects and alleviated the symptoms of neuroinflammation in LPS-treated mice [102].
4.1.5. Gypenoside IX
Gypenoside IX, a dammarane-type triterpene oligoglycoside obtained from the leaves and stems of Panax notoginseng, has been demonstrated to possess anti-inflammatory activities by suppressing LPS-induced NO production and pro-inflammatory cytokines expression [103]. It also alleviated the astrogliosis and decreased the production of inflammatory mediators via inhibition of Akt/p38 MAPK/NFκB signaling pathways in the brain cortex of LPS-treated mice [104]. Authors proposed that gypenoside IX might be a promising drug candidate for neurodegenerative conditions accompanied by neuroinflammation and astrogliosis [104].
4.1.6. Betulinic Acid
Betulinic acid is a naturally occurring pentacyclic triterpenoid with anti-staphylococcal, antimalarial, anti-cancer and anti-inflammatory properties [105, 106]. A recent study showed that betulinic acid enhances AMP-activated protein kinase (AMPK) activation and promotes microglia polarization to the M2 anti-inflammatory phenotype in the cerebral cortex of LPS-treated mice [107].
4.2. Sesquiterpenoids
4.2.1. Aromatic-turmerone
Aromatic-turmerone is one of the main components abundant in turmeric essential oil and has been widely used for diseases caused by chronic inflammation [108]. Chen and collaborators demonstrated that oral administration of aromatic-turmerone reduces LPS-induced microglia activation, brain damage and memory impairment as well as normalizes glucose intake and metabolism in the brains of mice [109]. The authors proposed that aromatic-turmerone targets TLR4-mediated downstream signaling and lowers the release of inflammatory mediators [109].
4.2.2. Beta-elemene
β-Elemene, a sesquiterpenoid with demonstrated anti-inflammatory, antioxidant and anti-tumor properties, has been used recently in peripheral LPS-induced neuroinflammation model by Pan and collaborators [110]. The authors showed that β-elemene improves the learning and memory abilities of mice in the water maze and fear conditioning tests [110]. Moreover, it reduced the expression of the microglial marker Iba-1, significantly increased RAC1 Ser71 phosphorylation and suppressed the RAC1/MLK3/p38 signaling activation and inflammatory response in the hippocampus [110].
4.3. Tetraterpenes
4.3.1. Lycopene
Lycopene, a carotenoid found in tomato and many fruits, has been demonstrated to possess various health benefits [111]. It has been shown that oral administration of lycopene prior to intraperitoneal LPS challenge attenuates LPS-induced expression of pro-inflammatory cytokines in the hippocampus and ameliorates depression-like behavior in mice [112]. Moreover, lycopene inhibited systemic inflammation-induced amyloidogenesis and memory impairment in mice treated intraperitoneally with LPS [113]. Also, lycopene effectively attenuated LPS-caused synapse loss, neuronal damage, insulin resistance and mitochondrial dysfunction in the mouse brain [114].
4.3.2. Crocin
Crocins, the main biologically active constituents of saffron, are water‐soluble carotenoids shown to exert protective effects against various inflammatory conditions [115]. In a recent study, Zhang and collaborators showed that crocin attenuates LPS-induced anxiety and depressive-like behaviors through suppressing NF-kB and NLRP3 signaling pathways [20].
4.4. Diterpenoids
4.4.1. Andrographolide
Andrographolide, a diterpenoid isolated from the medicinal plant Andrographis paniculata, is known to possess immunomodulatory and anti-tumor properties [116, 117]. Andrographolide easily gets through the blood-brain barrier (BBB) and has been reported to have a potent anti-inflammatory effect on leukocytes (neutrophils, macrophages and T-cells) and endothelial cells [118, 119].
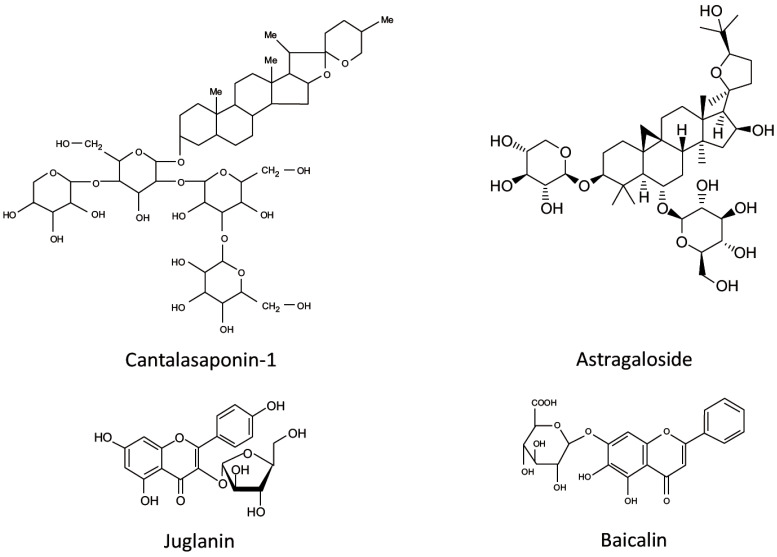
5. GLYCOSIDES (Fig. 4)
Fig. (4).
Chemical structure of glycosides revised in this paper.
5.1. Saponin Glycosides
5.1.1. Cantalasaponin
Cantalasaponin-1is a glycoside with anti-inflammatory and anti-tumor properties, isolated from various agave species [120]. In a mouse model of peripheral LPS-induced neuroinflammation, cantalasaponin-1 reduced brain concentration of pro-inflammatory cytokines IL-6 and TNF-α and increased the brain level of the anti-inflammatory cytokine IL-10 [121].
5.1.2. Astragaloside IV
Astragaloside IV, a glycoside, purified from the Chinese medicinal herbs, demonstrated anti-inflammatory properties in in vitro and in vivo studies [122]. Recently, the protective potential of astragaloside IV was evaluated in the peripheral LPS-induced mouse neuroinflammation model [123]. The authors showed that astragaloside IV reverses LPS-induced increase in TNF-α and IL-1β expression in the mouse hippocampus [123]. Moreover, the administration of astragaloside IV significantly increased PPARγ expression and GSK3β phosphorylation and decreased NF-κB phosphorylation and NLRP3 inflammasome activation [123]. Finally, astragaloside IV ameliorated LPS-induced depressive-like behavior in mice [123].
5.2. Flavonoid Glycosides
5.2.1. Juglanin
Juglanin is a flavonoid glucoside containing the kaempferol moiety and shown to inhibit the inflammatory response and tumor cells growth in vitro and in vivo [124, 125]. In a mouse model of systemic LPS-induced neuroinflammation juglanin treatment attenuated LPS-caused memory impairments, ameliorated synaptic dysfunction through promoting the expression of synaptic markers, such as SYP, PSD-95 and SNAP-25, and significantly reduced LPS-induced production of pro-inflammatory cytokines by inhibiting TLR4/NF-κB pathway in the hippocampus [125].
5.2.2. Baicalin
Baicalin, a flavonoid glycoside isolated from Radix Scutellariae, possesses potent anti-inflammatory, antioxidant and anti-apoptotic properties [126]. A recent study by Guo and collaborators demonstrated that baicalin ameliorates systemic LPS-induced neuroinflammation and depressive-like behavior in mice [127]. The authors suggested that the mechanism of baicalin action may involve the inhibition of TLR4 expression via the PI3K/AKT/FoxO1 pathway [127].
6. HETEROCYCLIC COMPOUNDS (Fig. 5)
Fig. (5).
Chemical structure of heterocyclic compounds revised in this paper.
6.1. Alkaloids
6.1.1. Trigonelline
Trigonelline is a naturally occurring alkaloid, commonly isolated from coffee beans and fenugreek seeds. It has been shown that trigonelline possesses anti-tumor, anti-bacterial, anti-viral, hypoglycemic and neuroprotective properties [128]. Recently, Chowdhury and collaborators demonstrated that trigonelline reverses LPS-induced memory disturbances by significantly decreasing the oxidative stress and acetylcholinesterase (AChE), TNF-α and IL-6 levels in both the hippocampus and cortex [129]. Also, trigonelline pretreatment increased BDNF levels [129].
6.2. Benzopyrans
6.2.1. Imperatorin
Imperatorin, a furanocoumarin derivative found in many fruits and medicinal herbs, has been shown to have anti-tumor, anti-viral, anti-bacterial and anti-inflammatory properties [130, 131]. A recent study demonstrated that imperatorin ameliorates systemic LPS-induced memory impairment, decreases AchE, TNF-α and IL-6 levels in the brain of LPS-treated mice and upregulates BDNF levels [132].
6.2.2. Esculetin
Esculetin, a hydroxycoumarin with potent antioxidant and anti-inflammatory activities has been recently evaluated in a mouse model of neuroinflammation induced by peripheral LPS treatment [133, 134]. It has been shown that esculetin attenuates LPS-induced neuroinflammation and depressive-like behavior in mice [133, 134]. The authors demonstrated that esculetin exhibits an anti-inflammatory effect by inhibiting the NF-kB pathway and by activating BDNF/TrkB signaling [134].
6.3. Benzofurans
6.3.1. L-3-n-butylphthalide
L-3-n-butylphthalide (L-NBP) is a naturally occurring antioxidant isolated from celery oil and found to have potent neuroprotective effects by decreasing oxidative damage, inhibiting inflammatory responses, improving mitochondrial function and reducing neuronal apoptosis [135]. In a mouse model of peripheral LPS-induced neuroinflammation, L-NBP treatment significantly suppressed the expression of pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6, as well as the activation of microglia in the brain [136]. Also, L-NBP inhibited the JNK MAPK-signaling pathway and upregulated the expression of HO-1 in LPS-treated mice, pointing thus to its’ anti-inflammatory and antioxidant potential [136].
6.4. Dioxoles
6.4.1. Sesamol
Sesamol, a predominant active component of sesame seed oil, has previously been demonstrated to possess potent antioxidant, anti-inflammatory, anti-cancer and anti-aging properties [137]. In a recent study, Liu and collaborators demonstrated that dietary supplementation of sesamol prevents systemic LPS-induced neuroinflammation, memory impairment and amyloidogenesis by inhibiting MAPK and NF-kB signaling [138].
6.5. Dioxolanes
6.5.1. Piperlongumine
Piperlongumine, a heterocyclic compound found in long pepper (Piper longum), has been reported to inhibit NF-kB activation [139]. In a murine model of peripheral LPS-induced neuroinflammation, piperlongumine reversed LPS-induced memory impairment and prevented beta-amyloid (Aβ) accumulation by inhibiting β- and γ-secretase activities [140]. Furthermore, piperlongumine decreased pro-inflammatory cytokines expression in LPS-treated mice [140]. To get a further inside into the mechanism of action of piperlongumine, Gu and collaborators carried out a docking model analysis and pull-down assay and found that piperlongumine binds to NF-κB family protein p50 [140].
6.6. Xanthons
6.6.1. Alpha-mangostin
Alpha-mangostin (α-MG), a natural xanthone isolated from Garcinia mangostana, has been shown to have antioxidant and anti-inflammatory activity in numerous in vitro and in vivo studies [141, 142]. We demonstrated that α-MG reduces brain levels of IL-6, COX-2 and TSPO in a mouse model of peripheral LPS-induced neuroinflammation [28].
7. OTHER AROMATIC COMPOUNDS (Fig. 6)
Fig. (6).
Chemical structure of organic acids, organosulfur compounds, xanthophylls and other organic compounds, revised in this paper.
7.1. Trans-cinnamaldehyde
Trans-cinnamaldehyde, an aromatic compound isolated from a medicinal herb Cinnamomum cassia, has been shown
to attenuate cerebral ischemia-induced brain injury by inhibiting iNOS and COX-2 expression and NF-κB activation [143]. Recently, Zhang and collaborators demonstrated that trans-cinnamaldehyde decreases the levels of iNOS and ERK1/2 in the hippocampus of mice challenged with LPS [144]. Moreover, trans-cinnamaldehyde significantly reduced memory deficit and improved synaptic plasticity in LPS-treated mice [144]. In another study, in peripheral LPS-induced neuroinflammation mouse model authors showed that trans-cinnamaldehyde modulates hippocampal Nrf2 and restores levels of anti-oxidant enzymes superoxide dismutase (SOD) and glutathione-S-transferase (GST) in the hippocampus [145]. In addition, cinnamaldehyde attenuated LPS-induced increase in hippocampal content of IL-1β, caspase-3 and malondialdehyde [145]. Finally, trans-cinnamaldehyde was able to inhibit amyloid beta aggregation in LPS-treated mice [145].
7.2. Curcumin
Curcumin, the main ingredient of the Indian spice turmeric, has been shown to display anti-inflammatory, antioxidant, anti-cancer and anti-bacterial properties by multiple mechanisms [146]. The protective effect of curcumin has been
documented in numerous studies in a mouse model of neuroinflammation induced by peripheral LPS [22]. Recent studies demonstrated that curcumin inhibits neuroinflammation and prevents long-term memory impairment in LPS-treated mice, after oral or intraperitoneal administration [145, 147]. Authors suggested that curcumin, shown to be able to enter brain tissue in biologically relevant concentrations, could be of interest in treating the long-term consequences of brain inflammation, such as memory dysfunction and cognitive deficits [147].
7.3. Beta-lapachone
β-Lapachone, a natural naphthoquinone isolated from the lapacho tree, has been used for the treatment of rheumatoid arthritis, infection and cancer [148, 149]. Lee and collaborators demonstrated that β-lapachone inhibits microglia activation and the expression of pro-inflammatory cytokines, iNOS and matrix metalloproteinases MMP-3, MMP-8 and MMP-9 in the brains of peripheral LPS-treated mice [27].
8. ORGANIC ACIDS (Fig. 6)
8.1. Methyl Jasmonate
Methyl jasmonate, a vital cell regulator in plants, has been shown to display antioxidant, anti-inflammatory, anti-tumor and neuroprotective action [150]. In a mouse model of peripheral LPS-induced neuroinflammation, methyl jasmonate reduced brain levels of pro-inflammatory cytokines TNF-α and IL-1β, prostaglandin E2 (PGE2), COX-2 and iNOS, and ameliorated LPS-caused memory deficits [151, 152]. Moreover, methyl jasmonate attenuated LPS-induced depressive-like behavior in mice by suppressing oxidative stress and neuroinflammation [153].
8.2. Ferulic Acid
Ferulic acid, an abundant phenolic phytochemical found in plant cell walls, has been shown to exhibit antioxidant, anti-inflammatory, anti-apoptotic and anti-tumor activities [154]. Recently, Rehman and collaborators reported that ferulic acid rescues peripheral LPS-induced neurodegeneration by inhibiting microglia activation and synaptic dysfunction [155]. The authors demonstrated that ferulic acid interferes with LPS-induced NF-kB activation and mitochondrial apoptotic signaling [155].
9. ORGANOSULFUR COMPOUNDS
9.1. Sulforaphane
Sulforaphane, a known activator of Nrf2 with antioxidant and anti-inflammatory activities, is obtained from cruciferous vegetables [156]. Three recent studies demonstrated that sulforaphane elevates the Nrf2 target genes and synaptic proteins, reduces the expression of pro-inflammatory mediators and regulates the BDNF-mammalian target of rapamycin (mTOR) signaling pathway in the hippocampus in mice after peripheral LPS treatment [157-159]. Also, sulforaphane prevented LPS-induced activation of microglia in the prefrontal cortex [159]. Although LPS-induced sickness behavior was not changed after sulforaphane treatment in one study [157], others showed that sulforaphane does alleviate LPS-caused learning and memory dysfunction and depression-like behavior in mice [158, 159].
10. PROTEINS
10.1. Osmotin
Osmotin is a plant hormone shown to inhibit LPS-induced TLR4 downstream signaling, including activation of NF-κB and the release of inflammatory mediators, such as COX-2, TNF-α, iNOS, and IL-1β [160]. Also, osmotin reduced LPS-caused activation of microglia and astrocytes in the hippocampus after intraperitoneal LPS challenge [160]. In addition, osmotin prevented LPS-induced loss of synaptic function and increased the expression of pre- and post-synaptic markers, like PSD-95 and SNAP-25 [160]. Furthermore, osmotin reduced LPS-induced neuronal apoptosis via inhibition of PARP-1 and caspase-3 [160]. Finally, it has been demonstrated that osmotin reverses LPS-induced behavioral and memory disturbances and attenuates LPS-caused increase in the expression of Aβ, APP, BACE-1 and p-Tau [160].
11. LIPIDS
11.1. Scallop-derived Plasmalogens
In the elderly, the brain and blood levels of glycerophospholipids, known to possess neuroprotective and anti-inflammatory properties, are decreased [161]. It has been demonstrated that oral administration of scallop-derived purified plasmalogens may improve cognitive functions in patients with mild AD [162]. Recently, scallop-derived plasmalogens have been shown to inhibit LPS-induced NF-kB activation and pro-inflammatory cytokines expression by attenuating the increased expression of protein kinase C delta (PKCd) in the brains of peripheral LPS-challenged mice [163].
12. OTHER COMPOUNDS
12.1. Xanthophylls
12.1.1. Astaxanthin
Astaxanthin, a xanthophyll carotenoid compound possessing potent antioxidant, anti-inflammatory and neuroprotective properties, has been shown to cross the blood-brain barrier in rodents, which may be important for its application for various neurological pathologies [164]. Astaxanthin ameliorated peripheral LPS-induced neuroinflammation, oxidative stress, memory dysfunction and depressive-like behavior in mice [164, 165].
12.1.2. Fucoxanthin
Fucoxanthin, another xanthophyll carotenoid found in edible brown seaweeds, has been shown to have antioxidant and anti-inflammatory effects both in vivo and in vitro [166]. Fucoxanthin inhibited LPS-induced overexpression of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α), as well as iNOS and COX-2 in the hippocampus, frontal cortex and hypothalamus, via the modulation of AMPK-NF-κB signaling pathway [167]. Importantly, fucoxanthin prevented LPS-induced depressive-like behavior in mice [167].
12.2. Oils
12.2.1. Fish Oil
Omega-3 polyunsaturated fatty acids (PUFAs), well-known antioxidant and anti-inflammatory agents, have been shown to ameliorate peripheral LPS-induced neuroinflammation and depressive-like behavior in mice [168, 169]. It has been demonstrated that fish oil attenuates LPS-induced dysregulation of the kynurenine pathway and serotoninergic alterations after peripheral LPS challenge [170].
13. PROSPECTS FOR THE USE OF NUTRACEU-TICALS IN HUMANS
In recent years, nutraceuticals have gained growing interest for their medical properties. Many compounds, described in this review, have been tested in healthy individuals and in patients with different pathologies, in order to assess their safety, bioavailability and protective effects [171- 179]. The dose used in clinical trials was close to the dose applied in LPS-treated mice. Thus, 30-100 mg/kg/day dose of anthocyanins, applied during 14 days in mice, attenuated LPS-induced neuroinflammation [45-47]. In clinical trials, anthocyanin supplementation (Medox, 300 mg/day for 3 weeks) decreased the level of pro-inflammatory cytokines [171]. Icariin improved spatial learning and memory after 17 days of administration (30-120 mg/kg/day) in LPS-treated animals. In human studies, icariin decreased depressive symptoms after administration for 8 weeks at a dose of 300 mg/day [174]. Another nutraceutical, naringenin, has been tested in LPS-treated mice and in healthy adults at similar doses (2-12 mg/kg) without any adverse effect [51, 178]. Resveratrol has been shown to be safe and efficient in clinical trials at a dose of up to 5 g [174]. It modulated inflammation and improved anti-oxidant capacity, although a moderate (450 mg/day) but continuing intake was considered to be better than a single, higher dose administration [175]. However, low solubility, poor absorption in the gastrointestinal tract and rapid metabolism of nutraceuticals are important obstacles that we need to overcome. Lyposomal formulations containing quercetin and luteolin or flavonoids nanoparticles with increased oral absorption and bioavailability have been shown to be safe and well-tolerated [172, 173, 179].
An important emerging concept in medicine is that of metabolic endotoxemia, characterized by chronic low-level elevations of gut-derived endotoxin, that was suggested to contribute to the development of a wide range of chronic pathological conditions [180]. Clinical studies demonstrated that optimizing the intake of phytonutrients and using nutritional supplements, such as resveratrol, is beneficial for reducing inflammation and other negative effects caused by metabolic endotoxemia [180, 181]. Findings in the LPS-induced mouse neuroinflammation model may have important implications for further research on the potential use of nutraceuticals in patients with acute (sepsis) or chronic (metabolic endotoxemia) elevated endotoxin levels.
CONCLUSION
It is known that peripheral infections accompanied by inflammation represent significant risk factors for the development of neurological disorders by modifying brain development or affecting normal brain aging [10-14]. The acute effects of systemic inflammation on progressive and persistent brain damage and cognitive impairment are well documented [15]. Anti-inflammatory therapies may have beneficial effects in the brain, and the protective properties of a wide range of synthetic and natural compounds have been extensively explored in recent years.
In the present review, we discussed recent data on the suitability of the LPS-induced murine neuroinflammation model for preclinical assessment of nutraceuticals with known anti-inflammatory action. Many of these compounds have been shown to pass across the BBB and may directly inhibit inflammatory pathways in the brain. Some nutraceuticals do not cross the BBB but rather inhibit the inflammatory response in the periphery, thus leading to the attenuation of neuroinflammation. Researches proposed that anti-inflammatory nutraceuticals may inhibit the activation of JNK/p38 MAPK, NF-kB, STAT-1 and MyD88/TLR4 pathways and interfere with mitochondrial apoptotic signaling [31, 32, 48, 49, 59, 63-65, 68-70, 76, 134, 140, 155]. Also, it has been demonstrated that nutraceuticals are potent activators of SIRT-1 and Nrf2/HO-1 pathway [53, 60, 63, 71, 145, 156]. In addition, nutraceuticals have been shown to increase the expression of BDNF [62, 82, 129, 132, 134, 157-159]. All these mechanisms may explain the ability of nutraceuticals to prevent the synaptic loss, neurodegeneration, memory dysfunction, depression, motor coordination disturbances as well as amyloidogenesis observed in LPS-treated mice. Clinical trials for assessing the safety and efficacy of many nutraceuticals, discussed in this review, are underway.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
Funding was provided to Goar Gevorkian by DGAPAPAPIIT-UNAM (IN203319).
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Eikelenboom P., van Exel E., Hoozemans J.J., Veerhuis R., Rozemuller A.J., van Gool W.A. Neuroinflammation - an early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegener. Dis. 2010;7(1-3):38–41. doi: 10.1159/000283480. [DOI] [PubMed] [Google Scholar]
- 2.Heneka M.T., Kummer M.P., Latz E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014;14(7):463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- 3.Pasqualetti G., Brooks D.J., Edison P. The role of neuroinflammation in dementias. Curr. Neurol. Neurosci. Rep. 2015;15(4):17. doi: 10.1007/s11910-015-0531-7. [DOI] [PubMed] [Google Scholar]
- 4.Calsolaro V., Edison P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. 2016;12(6):719–732. doi: 10.1016/j.jalz.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Ransohoff R.M. How neuroinflammation contributes to neurodegeneration. Science. 2016;353(6301):777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- 6.Nichols M.R., St-Pierre M.K., Wendeln A.C., Makoni N.J., Gouwens L.K., Garrad E.C., Sohrabi M., Neher J.J., Tremblay M.E., Combs C.K. Inflammatory mechanisms in neurodegeneration. J. Neurochem. 2019;149(5):562–581. doi: 10.1111/jnc.14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Exalto L.G., Whitmer R.A., Kappele L.J., Biessels G.J. An update on type 2 diabetes, vascular dementia and Alzheimer’s disease. Exp. Gerontol. 2012;47(11):858–864. doi: 10.1016/j.exger.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Ledesma M.D., Dotti C.G. Peripheral cholesterol, metabolic disorders and Alzheimer’s disease. Front. Biosci. (Elite Ed.) 2012;4:181–194. doi: 10.2741/e368. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira S.T., Clarke J.R., Bomfim T.R., De Felice F.G. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimers Dement. 2014;10(1) Suppl.:S76–S83. doi: 10.1016/j.jalz.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Holmes C., Cotterell D. Role of infection in the pathogenesis of Alzheimer’s disease: implications for treatment. CNS Drugs. 2009;23(12):993–1002. doi: 10.2165/11310910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Su X., Federoff H.J. Immune responses in Parkinson’s disease: interplay between central and peripheral immune systems. BioMed Res. Int. 2014;2014:275178. doi: 10.1155/2014/275178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gale S.D., Erickson L.D., Berrett A., Brown B.L., Hedges D.W. Infectious disease burden and cognitive function in young to middle-aged adults. Brain Behav. Immun. 2016;52:161–168. doi: 10.1016/j.bbi.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Licastro F., Porcellini E. Persistent infections, immune-senescence and Alzheimer’s disease. Oncoscience. 2016;3(5-6):135–142. doi: 10.18632/oncoscience.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McManus R.M., Heneka M.T. Role of neuroinflammation in neurodegeneration: new insights. Alzheimers Res. Ther. 2017;9(1):14. doi: 10.1186/s13195-017-0241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sankowski R., Mader S., Valdés-Ferrer S.I. Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front. Cell. Neurosci. 2015;9:28. doi: 10.3389/fncel.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biesmans S., Meert T.F., Bouwknecht J.A., Acton P.D., Davoodi N., De Haes P., Kuijlaars J., Langlois X., Matthews L.J., Ver Donck L., Hellings N., Nuydens R. Systemic immune activation leads to neuroinflammation and sickness behavior in mice. Mediators Inflamm. 2013;2013:271359. doi: 10.1155/2013/271359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoogland I.C.M., Houbolt C., van Westerloo D.J., van Gool W.A., van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J. Neuroinflammation. 2015;12:114. doi: 10.1186/s12974-015-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreuder L., Eggen B.J., Biber K., Schoemaker R.G., Laman J.D., de Rooij S.E. Pathophysiological and behavioral effects of systemic inflammation in aged and diseased rodents with relevance to delirium: A systematic review. Brain Behav. Immun. 2017;62:362–381. doi: 10.1016/j.bbi.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 19.d’Avila J.C., Siqueira L.D., Mazeraud A., Azevedo E.P., Foguel D., Castro-Faria-Neto H.C., Sharshar T., Chrétien F., Bozza F.A. Age-related cognitive impairment is associated with long-term neuroinflammation and oxidative stress in a mouse model of episodic systemic inflammation. J. Neuroinflammation. 2018;15(1):28. doi: 10.1186/s12974-018-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L., Previn R., Lu L., Liao R.F., Jin Y., Wang R.K. Crocin, a natural product attenuates lipopolysaccharide-induced anxiety and depressive-like behaviors through suppressing NF-kB and NLRP3 signaling pathway. Brain Res. Bull. 2018;142:352–359. doi: 10.1016/j.brainresbull.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Sumbria R.K., Grigoryan M.M., Vasilevko V., Krasieva T.B., Scadeng M., Dvornikova A.K., Paganini-Hill A., Kim R., Cribbs D.H., Fisher M.J. A murine model of inflammation-induced cerebral microbleeds. J. Neuroinflammation. 2016;13(1):218. doi: 10.1186/s12974-016-0693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catorce M.N., Gevorkian G. LPS-induced murine neuroinflammation model: main features and suitability for pre-clinical assessment of nutraceuticals. Curr. Neuropharmacol. 2016;14(2):155–164. doi: 10.2174/1570159X14666151204122017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu H.Q., Yang T., Xiao W., Fan L., Wu Y., Terrando N., Wang T.L. Prolonged neuroinflammation after lipopolysaccharide exposure in aged rats. PLoS One. 2014;9(8):e106331. doi: 10.1371/journal.pone.0106331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson S.T., Commins S., Moynagh P.N., Coogan A.N. Lipopolysaccharide-induced sepsis induces long-lasting affective changes in the mouse. Brain Behav. Immun. 2015;43:98–109. doi: 10.1016/j.bbi.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Skelly D.T., Hennessy E., Dansereau M.A., Cunningham C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1β, [corrected] TNF-α and IL-6 challenges in C57BL/6 mice. PLoS One. 2013;8(7):e69123. doi: 10.1371/journal.pone.0069123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banks W.A. gray, A.M.; Erickson, M.A.; Salameh, T.S.; Damodarasamy, M.; Sheibani, N.; Meabon, J.S.; Wing, E.E.; Morofuji, Y.; Cook, D.G.; Reed, M.J. Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxugenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J. Neuroinflammation. 2015;12:223. doi: 10.1186/s12974-015-0434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee E.J., Ko H.M., Jeong Y.H., Park E.M., Kim H.S. β-Lapachone suppresses neuroinflammation by modulating the expression of cytokines and matrix metalloproteinases in activated microglia. J. Neuroinflammation. 2015;12:133. doi: 10.1186/s12974-015-0355-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nava Catorce M., Acero G., Pedraza-Chaverri J., Fragoso G., Govezensky T., Gevorkian G. Alpha-mangostin attenuates brain inflammation induced by peripheral lipopolysaccharide administration in C57BL/6J mice. J. Neuroimmunol. 2016;297:20–27. doi: 10.1016/j.jneuroim.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Szot P., Franklin A., Figlewicz D.P., Beuca T.P., Bullock K., Hansen K. banks, W.A.; Raskind, M.A.; Peskind, E.R. Multiple lipopolysaccharide (LPS) injections alter interleukin (IL-6), IL-7,IL-10 and IL-7 and IL-7 receptor mRNA in CNS and spleen. Neuroscience. 2017;355:9–21. doi: 10.1016/j.neuroscience.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 30.He H., Geng T., Chen P., Wang M., Hu J., Kang L., Song W., Tang H. NK cells promote neutrophil recruitment in the brain during sepsis-induced neuroinflammation. Sci. Rep. 2016;6:27711. doi: 10.1038/srep27711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badshah H., Ali T. Shafiq-ur Rehman; Faiz-ul Amin; Ullah, F.; Kim, T.H.; Kim, M.O. Protective effect of lupeol against lipopolysaccharide-induced neuroinflammation via the p38/c-Jun N-terminal kinase pathway in the adult mouse brain. J. Neuroimmune Pharmacol. 2016;11(1):48–60. doi: 10.1007/s11481-015-9623-z. [DOI] [PubMed] [Google Scholar]
- 32.Li J., Xu B., Chen Z., Zhou C., Liao L., Qin Y., Yang C., Zhang X., Hu Z., Sun L., Zhu D., Xie P. PI3K/AKT/JNK/p38 signalling pathway-mediated neural apoptosis in the prefrontal cortex of mice is involved in the antidepressant-like effect of pioglitazone. Clin. Exp. Pharmacol. Physiol. 2018;45(6):525–535. doi: 10.1111/1440-1681.12918. [DOI] [PubMed] [Google Scholar]
- 33.Zhu W., Cao F.S., Feng J., Chen H.W., Wan J.R., Lu Q., Wang J. NLRP3 inflammasome activation contributes to long-term behavioral alterations in mice injected with lipopolysaccharide. Neuroscience. 2017;343:77–84. doi: 10.1016/j.neuroscience.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norden D.M., Trojanowski P.J., Villanueva E., Navarro E., Godbout J.P. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64(2):300–316. doi: 10.1002/glia.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopes P.C. LPS and neuroinflammation: a matter of timing. Inflammopharmacology. 2016;24(5):291–293. doi: 10.1007/s10787-016-0283-2. [DOI] [PubMed] [Google Scholar]
- 36.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim H., Heo M.Y., Kim H.P. Flavonoids: broad spectrum agents on chronic inflammation. Biomol. Ther. (Seoul) 2019;27(3):241–253. doi: 10.4062/biomolther.2019.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spencer J.P., Vafeiadou K., Williams R.J., Vauzour D. Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol. Aspects Med. 2012;33(1):83–97. doi: 10.1016/j.mam.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Renaud J., Martinoli M.G. Resveratrol as a protective molecule for neuroinflammation: a review of mechanisms. Curr. Pharm. Biotechnol. 2014;15(4):318–329. doi: 10.2174/1389201015666140617101332. [DOI] [PubMed] [Google Scholar]
- 40.Jaeger B.N., Parylak S.L., Gage F.H. Mechanisms of dietary flavonoid action in neuronal function and neuroinflammation. Mol. Aspects Med. 2018;61:50–62. doi: 10.1016/j.mam.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Morais C.A., de Rosso V.V., Estadella D., Pisani L.P. Anthocyanins as inflammatory modulators and the role of the gut microbiota. J. Nutr. Biochem. 2016;33:1–7. doi: 10.1016/j.jnutbio.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Wallace T.C., Slavin M., Frankenfeld C.L. Systemic review of anthocyanins and markers of cardiovascular disease. Nutrients. 2016;8(1):E32. doi: 10.3390/nu8010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee Y.M., Yoon Y., Yoon H., Park H.M., Song S., Yeum K.J. Dietary anthocyanins against obesity and inflammation. Nutrients. 2017;9(10):E1089. doi: 10.3390/nu9101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Różańska D., Regulska-Ilow B. The significance of anthocyanins in the prevention and treatment of type 2 diabetes. Adv. Clin. Exp. Med. 2018;27(1):135–142. doi: 10.17219/acem/64983. [DOI] [PubMed] [Google Scholar]
- 45.Khan M.S., Ali T., Kim M.W., Jo M.H., Jo M.G., Badshah H., Kim M.O. Anthocyanins protect against LPS-induced oxidative stress-mediated neuroinflammation and neurodegeneration in the adult mouse cortex. Neurochem. Int. 2016;100:1–10. doi: 10.1016/j.neuint.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Khan M.S., Ali T., Kim M.W., Jo M.H., Chung J.I., Kim M.O. Anthocyanins improve hippocampus-dependent memory function and prevent neurodegeneration via JNK/Akt/GSK3β signaling in LPS-treated adult mice. Mol. Neurobiol. 2019;56(1):671–687. doi: 10.1007/s12035-018-1101-1. [DOI] [PubMed] [Google Scholar]
- 47.Carvalho F.B., Gutierres J.M., Bueno A., Agostinho P., Zago A.M., Vieira J., Frühauf P., Cechella J.L., Nogueira C.W., Oliveira S.M., Rizzi C., Spanevello R.M., Duarte M.M.F., Duarte T., Dellagostin O.A., Andrade C.M. Anthocyanins control neuroinflammation and consequent memory dysfunction in mice exposed to lipopolysaccharide. Mol. Neurobiol. 2017;54(5):3350–3367. doi: 10.1007/s12035-016-9900-8. [DOI] [PubMed] [Google Scholar]
- 48.Hämäläinen M., Nieminen R., Vuorela P., Heinonen M., Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007;2007:45673. doi: 10.1155/2007/45673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vafeiadou K., Vauzour D., Lee H.Y., Rodriguez-Mateos A., Williams R.J., Spencer J.P. The citrus flavanone naringenin inhibits inflammatory signalling in glial cells and protects against neuroinflammatory injury. Arch. Biochem. Biophys. 2009;484(1):100–109. doi: 10.1016/j.abb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 50.Wu L.H., Lin C., Lin H.Y., Liu Y.S., Wu C.Y., Tsai C.F., Chang P.C., Yeh W.L., Lu D.Y. Naringenin Suppresses Neuroinflammatory Responses Through Inducing Suppressor of Cytokine Signaling 3 Expression. Mol. Neurobiol. 2016;53(2):1080–1091. doi: 10.1007/s12035-014-9042-9. [DOI] [PubMed] [Google Scholar]
- 51.Liu X., Wang N., Fan S., Zheng X., Yang Y., Zhu Y., Lu Y., Chen Q., Zhou H., Zheng J. The citrus flavonoid naringenin confers protection in a murine endotoxaemia model through AMPK-ATF3-dependent negative regulation of the TLR4 signalling pathway. Sci. Rep. 2016;6:39735. doi: 10.1038/srep39735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu X.X., Yu D.D., Chen M.J., Sun T., Li G., Huang W.J., Nie H., Wang C., Zhang Y.X., Gong Q., Ren B.X. Hesperidin ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting HMGB1 release. Int. Immunopharmacol. 2015;25(2):370–376. doi: 10.1016/j.intimp.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 53.Ren H., Hao J., Liu T., Zhang D., Lv H., Song E., Zhu C. Hesperetin Suppresses Inflammatory Responses in Lipopolysaccharide-Induced RAW 264.7 Cells via the Inhibition of NF-κB and Activation of Nrf2/HO-1 Pathways. Inflammation. 2016;39(3):964–973. doi: 10.1007/s10753-016-0311-9. [DOI] [PubMed] [Google Scholar]
- 54.Tejada S., Pinya S., Martorell M., Capó X., Tur J.A., Pons A., Sureda A. Potential anti-inflammatory effects of hesperidin from the Genus Citrus. Curr. Med. Chem. 2018;25(37):4929–4945. doi: 10.2174/0929867324666170718104412. [DOI] [PubMed] [Google Scholar]
- 55.Qi W., Lin C., Fan K., Chen Z., Liu L., Feng X., Zhang H., Shao Y., Fang H., Zhao C., Zhang R., Cai D. Hesperidin inhibits synovial cell inflammation and macrophage polarization through suppression of the PI3K/AKT pathway in complete Freund’s adjuvant-induced arthritis in mice. Chem. Biol. Interact. 2019;306:19–28. doi: 10.1016/j.cbi.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Ye J., Guan M., Lu Y., Zhang D., Li C., Li Y., Zhou C. Protective effects of hesperetin on lipopolysaccharide-induced acute lung injury by targeting MD2. Eur. J. Pharmacol. 2019;852:151–158. doi: 10.1016/j.ejphar.2019.02.042. [DOI] [PubMed] [Google Scholar]
- 57.Konsman J.P., Veeneman J., Combe C., Poole S., Luheshi G.N., Dantzer R. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur. J. Neurosci. 2008;28(12):2499–2510. doi: 10.1111/j.1460-9568.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- 58.Li M., Shao H., Zhang X., Qin B. Hesperidin Alleviates Lipopolysaccharide-Induced Neuroinflammation in Mice by Promoting the miRNA-132 Pathway. Inflammation. 2016;39(5):1681–1689. doi: 10.1007/s10753-016-0402-7. [DOI] [PubMed] [Google Scholar]
- 59.He P., Yan S., Zheng J., Gao Y., Zhang S., Liu Z., Liu X., Xiao C. Eriodictyol Attenuates LPS-Induced Neuroinflammation, Amyloidogenesis, and Cognitive Impairments via the Inhibition of NF-κB in Male C57BL/6J Mice and BV2 Microglial Cells. J. Agric. Food Chem. 2018;66(39):10205–10214. doi: 10.1021/acs.jafc.8b03731. [DOI] [PubMed] [Google Scholar]
- 60.He P., Yan S., Wen X., Zhang S., Liu Z., Liu X., Xiao C. Eriodictyol alleviates lipopolysaccharide-triggered oxidative stress and synaptic dysfunctions in BV-2 microglial cells and mouse brain. J. Cell. Biochem. 2019;120(9):14756–14770. doi: 10.1002/jcb.28736. [DOI] [PubMed] [Google Scholar]
- 61.Zhang J., Chen Y., Luo H., Sun L., Xu M., Yu J., Zhou Q., Meng G., Yang S. Recent update on the pharmacological effects and mechanisms of dihydromyricetin. Front. Pharmacol. 2018;9:1204. doi: 10.3389/fphar.2018.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ren Z., Yan P., Zhu L., Yang H., Zhao Y., Kirby B.P., Waddington J.L., Zhen X. Dihydromyricetin exerts a rapid antidepressant-like effect in association with enhancement of BDNF expression and inhibition of neuroinflammation. Psychopharmacology (Berl.) 2018;235(1):233–244. doi: 10.1007/s00213-017-4761-z. [DOI] [PubMed] [Google Scholar]
- 63.Devi K.P., Malar D.S., Nabavi S.F., Sureda A., Xiao J., Nabavi S.M., Daglia M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015;99:1–10. doi: 10.1016/j.phrs.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Cheng X., Yang Y.L., Yang H., Wang Y.H., Du G.H. Kaempferol alleviates LPS-induced neuroinflammation and BBB dysfunction in mice via inhibiting HMGB1 release and down-regulating TLR4/MyD88 pathway. Int. Immunopharmacol. 2018;56:29–35. doi: 10.1016/j.intimp.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 65.Yang Y.L., Cheng X., Li W.H., Liu M., Wang Y.H., Du G.H. Kaempferol attenuates LPS-induced striatum injury in mice involving anti-neuroinflammation, maintaining BBB integrity, and down-regulating the HMGB1/TLR4 pathway. Int. J. Mol. Sci. 2019;20(3):E491. doi: 10.3390/ijms20030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y., Yao J., Han C., Yang J., Chaudhry M.T., Wang S., Liu H., Yin Y. Quercetin, inflammation and immunity. Nutrients. 2016;8(3):167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khan A., Ali T., Rehman S.U., Khan M.S., Alam S.I., Ikram M., Muhammad T., Saeed K., Badshah H., Kim M.O. Neuroprotective effect of quercetin against the detrimental effects of LPS in the adult mouse brain. Front. Pharmacol. 2018;9:1383. doi: 10.3389/fphar.2018.01383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo J., Li F., Wu Q., Gong Q., Lu Y., Shi J. Protective effects of icariin on brain dysfunction induced by lipopolysaccharide in rats. Phytomedicine. 2010;17(12):950–955. doi: 10.1016/j.phymed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 69.Zeng K.W., Fu H., Liu G.X., Wang X.M. Icariin attenuates lipopolysaccharide-induced microglial activation and resultant death of neurons by inhibiting TAK1/IKK/NF-kappaB and JNK/p38 MAPK pathways. Int. Immunopharmacol. 2010;10(6):668–678. doi: 10.1016/j.intimp.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 70.Zheng Y., Zhu G., He J., Wang G., Li D., Zhang F. Icariin targets Nrf2 signaling to inhibit microglia-mediated neuroinflammation. Int. Immunopharmacol. 2019;73:304–311. doi: 10.1016/j.intimp.2019.05.033. [DOI] [PubMed] [Google Scholar]
- 71.Liu L., Zhao Z., Lu L., Liu J., Sun J., Wu X., Dong J. Icariin and icaritin ameliorated hippocampus neuroinflammation via inhibiting HMGB1-related pro-inflammatory signals in lipopolysaccharide-induced inflammation model in C57BL/6 J mice. Int. Immunopharmacol. 2019;68:95–105. doi: 10.1016/j.intimp.2018.12.055. [DOI] [PubMed] [Google Scholar]
- 72.Jiang X., Liu J., Lin Q., Mao K., Tian F., Jing C., Wang C., Ding L., Pang C. Proanthocyanidin prevents lipopolysaccharide-induced depressive-like behavior in mice via neuroinflammatory pathway. Brain Res. Bull. 2017;135:40–46. doi: 10.1016/j.brainresbull.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 73.Pan C.H., Kim E.S., Jung S.H., Nho C.W., Lee J.K. Tectorigenin inhibits IFN-gamma/LPS-induced inflammatory responses in murine macrophage RAW 264.7 cells. Arch. Pharm. Res. 2008;31(11):1447–1456. doi: 10.1007/s12272-001-2129-7. [DOI] [PubMed] [Google Scholar]
- 74.Kim Y.P., Yamada M., Lim S.S., Lee S.H., Ryu N., Shin K.H., Ohuchi K. Inhibition by tectorigenin and tectoridin of prostaglandin E2 production and cyclooxygenase-2 induction in rat peritoneal macrophages. Biochim. Biophys. Acta. 1999;1438(3):399–407. doi: 10.1016/S1388-1981(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 75.Ma C.H., Liu J.P., Qu R., Ma S.P. Tectorigenin inhibits the inflammation of LPS-induced acute lung injury in mice. Chin. J. Nat. Med. 2014;12(11):841–846. doi: 10.1016/S1875-5364(14)60126-6. [DOI] [PubMed] [Google Scholar]
- 76.Lim H.S., Kim Y.J., Kim B.Y., Park G., Jeong S.J. The Anti-neuroinflammatory Activity of Tectorigenin Pretreatment via Downregulated NF-κB and ERK/JNK Pathways in BV-2 Microglial and Microglia Inactivation in Mice With Lipopolysaccharide. Front. Pharmacol. 2018;9:462. doi: 10.3389/fphar.2018.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lai X., Ye Y., Sun C., Huang X., Tang X., Zeng X., Yin P., Zeng Y. Icaritin exhibits anti-inflammatory effects in the mouse peritoneal macrophages and peritonitis model. Int. Immunopharmacol. 2013;16(1):41–49. doi: 10.1016/j.intimp.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 78.Jeong Y.H., Park J.S., Kim D.H., Kim H.S. Lonchocarpine Increases Nrf2/ARE-Mediated Antioxidant Enzyme Expression by Modulating AMPK and MAPK Signaling in Brain Astrocytes. Biomol. Ther. (Seoul) 2016;24(6):581–588. doi: 10.4062/biomolther.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeong Y.H., Park J.S., Kim D.H., Kang J.L., Kim H.S. Anti-inflammatory mechanism of lonchocarpine in LPS- or poly(I:C)-induced neuroinflammation. Pharmacol. Res. 2017;119:431–442. doi: 10.1016/j.phrs.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 80.Galiniak S., Aebisher D., Bartusik-Aebisher D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019;66(1):13–21. doi: 10.18388/abp.2018_2749. [DOI] [PubMed] [Google Scholar]
- 81.Yang X., Xu S., Qian Y., Xiao Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav. Immun. 2017;64:162–172. doi: 10.1016/j.bbi.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 82.Sulakhiya K., Kumar P., Jangra A., Dwivedi S., Hazarika N.K., Baruah C.C., Lahkar M. Honokiol abrogates lipopolysaccharide-induced depressive like behavior by impeding neuroinflammation and oxido-nitrosative stress in mice. Eur. J. Pharmacol. 2014;744:124–131. doi: 10.1016/j.ejphar.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 83.Li J., Geng D., Xu J., Weng L.J., Liu Q., Yi L.T. Antidepressant-like effect of macranthol isolated from Illicium dunnianum tutch in mice. Eur. J. Pharmacol. 2013;707(1-3):112–119. doi: 10.1016/j.ejphar.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 84.Weng L., Dong S., Wang S., Yi L., Geng D. Macranthol attenuates lipopolysaccharide-induced depressive-like behaviors by inhibiting neuroinflammation in prefrontal cortex. Physiol. Behav. 2019;204:33–40. doi: 10.1016/j.physbeh.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 85.Song F., Zeng K., Liao L., Yu Q., Tu P., Wang X., Schizandrin A., Schizandrin A. Inhibits Microglia-Mediated Neuroninflammation through Inhibiting TRAF6-NF-κB and Jak2-Stat3 Signaling Pathways. PLoS One. 2016;11(2):e0149991. doi: 10.1371/journal.pone.0149991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Basu Mallik S., Mudgal J., Nampoothiri M., Hall S., Dukie S.A., Grant G., Rao C.M., Arora D. Caffeic acid attenuates lipopolysaccharide-induced sickness behaviour and neuroinflammation in mice. Neurosci. Lett. 2016;632:218–223. doi: 10.1016/j.neulet.2016.08.044. [DOI] [PubMed] [Google Scholar]
- 87.Lee J., Scagel C.F. Chicoric acid: chemistry, distribution, and production. Front Chem. 2013;1:40. doi: 10.3389/fchem.2013.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ding H., Ci X., Cheng H., Yu Q., Li D. Chicoric acid alleviates lipopolysaccharide-induced acute lung injury in mice through anti-inflammatory and anti-oxidant activities. Int. Immunopharmacol. 2019;66:169–176. doi: 10.1016/j.intimp.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 89.Liu Q., Hu Y., Cao Y., Song G., Liu Z., Liu X. Chicoric Acid Ameliorates Lipopolysaccharide-Induced Oxidative Stress via Promoting the Keap1/Nrf2 Transcriptional Signaling Pathway in BV-2 Microglial Cells and Mouse Brain. J. Agric. Food Chem. 2017;65(2):338–347. doi: 10.1021/acs.jafc.6b04873. [DOI] [PubMed] [Google Scholar]
- 90.Liu Q., Chen Y., Shen C., Xiao Y., Wang Y., Liu Z., Liu X. Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-κB. FASEB J. 2017;31(4):1494–1507. doi: 10.1096/fj.201601071R. [DOI] [PubMed] [Google Scholar]
- 91.Yaidikar L., Thakur S. Punicalagin attenuated cerebral ischemia-reperfusion insult via inhibition of proinflammatory cytokines, up-regulation of Bcl-2, down-regulation of Bax, and caspase-3. Mol. Cell. Biochem. 2015;402(1-2):141–148. doi: 10.1007/s11010-014-2321-y. [DOI] [PubMed] [Google Scholar]
- 92.Kim Y.E., Hwang C.J., Lee H.P., Kim C.S., Son D.J., Ham Y.W., Hellström M., Han S.B., Kim H.S., Park E.K., Hong J.T. Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-kappaB. Neuropharmacology. 2017;117:21–32. doi: 10.1016/j.neuropharm.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 93.Sharkey T.D., Yeh S. Isoprene emission from plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:407–436. doi: 10.1146/annurev.arplant.52.1.407. [DOI] [PubMed] [Google Scholar]
- 94.Fernández M.A., de las Heras B., García M.D., Sáenz M.T., Villar A. New insights into the mechanism of action of the anti-inflammatory triterpene lupeol. J. Pharm. Pharmacol. 2001;53(11):1533–1539. doi: 10.1211/0022357011777909. [DOI] [PubMed] [Google Scholar]
- 95.Saleem M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009;285(2):109–115. doi: 10.1016/j.canlet.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ming L.J., Yin A.C. Therapeutic effects of glycyrrhizic acid. Nat. Prod. Commun. 2013;8(3):415–418. doi: 10.1177/1934578X1300800335. [DOI] [PubMed] [Google Scholar]
- 97.Liu W., Huang S., Li Y., Zhang K., Zheng X. Suppressive effect of glycyrrhizic acid against lipopolysaccharide-induced neuroinflammation and cognitive impairment in C57 mice via toll-like receptor 4 signaling pathway. Food Nutr. Res. 2019;63:63. doi: 10.29219/fnr.v63.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park S.M., Choi M.S., Sohn N.W., Shin J.W. Ginsenoside Rg3 attenuates microglia activation following systemic lipopolysaccharide treatment in mice. Biol. Pharm. Bull. 2012;35(9):1546–1552. doi: 10.1248/bpb.b12-00393. [DOI] [PubMed] [Google Scholar]
- 99.Kang A., Xie T., Zhu D., Shan J., Di L., Zheng X. Suppressive Effect of Ginsenoside Rg3 against Lipopolysaccharide-Induced Depression-Like Behavior and Neuroinflammation in Mice. J. Agric. Food Chem. 2017;65(32):6861–6869. doi: 10.1021/acs.jafc.7b02386. [DOI] [PubMed] [Google Scholar]
- 100.Vuddanda P.R., Sing S., Velaga S. Boswellic acid – medicinal use of an ancient herbal remedy. J. Herb. Med. 2016;6:163–170. doi: 10.1016/j.hermed.2016.08.002. [DOI] [Google Scholar]
- 101.Goel A., Ahmad F.J., Singh R.M., Singh G.N. 3-Acetyl-11-keto-beta-boswellic acid loaded-polymeric nanomicelles for topical anti-inflammatory and anti-arthritic activity. J. Pharm. Pharmacol. 2010;62(2):273–278. doi: 10.1211/jpp.62.02.0016. [DOI] [PubMed] [Google Scholar]
- 102.Sayed A.S., Gomaa I.E.O., Bader M., El Sayed N.S.E.D. Role of 3-Acetyl-11-Keto-Beta-Boswellic Acid in Counteracting LPS-Induced Neuroinflammation via Modulation of miRNA-155. Mol. Neurobiol. 2018;55(7):5798–5808. doi: 10.1007/s12035-017-0801-2. [DOI] [PubMed] [Google Scholar]
- 103.Li J., Wang R.F., Zhou Y., Hu H.J., Yang Y.B., Yang L., Wang Z.T. Dammarane-type triterpene oligoglycosides from the leaves and stems of Panax notoginseng and their antiinflammatory activities. J. Ginseng Res. 2019;43(3):377–384. doi: 10.1016/j.jgr.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang X., Yang L., Yang L., Xing F., Yang H., Qin L., Lan Y., Wu H., Zhang B., Shi H., Lu C., Huang F., Wu X., Wang Z., Gypenoside I.X. Suppresses p38 MAPK/Akt/NFκB Signaling Pathway Activation and Inflammatory Responses in Astrocytes Stimulated by Proinflammatory Mediators. Inflammation. 2017;40(6):2137–2150. doi: 10.1007/s10753-017-0654-x. [DOI] [PubMed] [Google Scholar]
- 105.Ríos J.L., Máñez S. New pharmacological opportunities for betulinic acid. Planta Med. 2018;84(1):8–19. doi: 10.1055/s-0043-123472. [DOI] [PubMed] [Google Scholar]
- 106.Chung P.Y. Novel targets of pentacyclic triterpenoids in Staphylococcus aureus: A systematic review. Phytomedicine. 2019;152933 doi: 10.1016/j.phymed.2019.152933. [DOI] [PubMed] [Google Scholar]
- 107.Li C., Zhang C., Zhou H., Feng Y., Tang F., Hoi M.P.M., He C., Ma D., Zhao C., Lee S.M.Y. Inhibitory Effects of Betulinic Acid on LPS-Induced Neuroinflammation Involve M2 Microglial Polarization via CaMKKβ-Dependent AMPK Activation. Front. Mol. Neurosci. 2018;11:98. doi: 10.3389/fnmol.2018.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Murakami A., Furukawa I., Miyamoto S., Tanaka T., Ohigashi H. Curcumin combined with turmerones, essential oil components of turmeric, abolishes inflammation-associated mouse colon carcinogenesis. Biofactors. 2013;39(2):221–232. doi: 10.1002/biof.1054. [DOI] [PubMed] [Google Scholar]
- 109.Chen M., Chang Y.Y., Huang S., Xiao L.H., Zhou W., Zhang L.Y., Li C., Zhou R.P., Tang J., Lin L., Du Z.Y., Zhang K. Aromatic-Turmerone Attenuates LPS-Induced Neuroinflammation and Consequent Memory Impairment by Targeting TLR4-Dependent Signaling Pathway. Mol. Nutr. Food Res. 2018;62(2) doi: 10.1002/mnfr.201700281. [DOI] [PubMed] [Google Scholar]
- 110.Pan C., Si Y., Meng Q., Jing L., Chen L., Zhang Y., Bao H. Suppression of the RAC1/MLK3/p38 Signaling Pathway by β-Elemene Alleviates Sepsis-Associated Encephalopathy in Mice. Front. Neurosci. 2019;13:358. doi: 10.3389/fnins.2019.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Palozza P., Parrone N., Catalano A., Simone R. Tomato Lycopene and Inflammatory Cascade: Basic Interactions and Clinical Implications. Curr. Med. Chem. 2010;17(23):2547–2563. doi: 10.2174/092986710791556041. [DOI] [PubMed] [Google Scholar]
- 112.Zhang F., Fu Y., Zhou X., Pan W., Shi Y., Wang M., Zhang X., Qi D., Li L., Ma K., Tang R., Zheng K., Song Y. Depression-like behaviors and heme oxygenase-1 are regulated by Lycopene in lipopolysaccharide-induced neuroinflammation. J. Neuroimmunol. 2016;298:1–8. doi: 10.1016/j.jneuroim.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 113.Wang J., Li L., Wang Z., Cui Y., Tan X., Yuan T., Liu Q., Liu Z., Liu X. Supplementation of lycopene attenuates lipopolysaccharide-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J. Nutr. Biochem. 2018;56:16–25. doi: 10.1016/j.jnutbio.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 114.Wang J., Zou Q., Suo Y., Tan X., Yuan T., Liu Z., Liu X. Lycopene ameliorates systemic inflammation-induced synaptic dysfunction via improving insulin resistance and mitochondrial dysfunction in the liver-brain axis. Food Funct. 2019;10(4):2125–2137. doi: 10.1039/C8FO02460J. [DOI] [PubMed] [Google Scholar]
- 115.Pashirzad M., Shafiee M., Avan A., Ryzhikov M., Fiuji H., Bahreyni A., Khazaei M., Soleimanpour S., Hassanian S.M. Therapeutic potency of crocin in the treatment of inflammatory diseases: Current status and perspective. J. Cell. Physiol. 2019;23 doi: 10.1002/jcp.28177. [DOI] [PubMed] [Google Scholar]
- 116.Dai Y., Chen S.R., Chai L., Zhao J., Wang Y., Wang Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. 2019. [DOI] [PubMed]
- 117.Tao L., Zhang L., Gao R., Jiang F., Cao J., Liu H. Andrographolide Alleviates Acute Brain Injury in a Rat Model of Traumatic Brain Injury: Possible Involvement of Inflammatory Signaling. Front. Neurosci. 2018;12:657. doi: 10.3389/fnins.2018.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chiou W.F., Chen C.F., Lin J.J. Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells by andrographolide. Br. J. Pharmacol. 2000;129(8):1553–1560. doi: 10.1038/sj.bjp.0703191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chang C.C., Duann Y.F., Yen T.L., Chen Y.Y., Jayakumar T., Ong E.T., Sheu J.R. Andrographolide, a Novel NF-κB Inhibitor, Inhibits Vascular Smooth Muscle Cell Proliferation and Cerebral Endothelial Cell Inflammation. Acta Cardiol Sin. 2014;30(4):308–315. [PMC free article] [PubMed] [Google Scholar]
- 120.Monterrosas-Brisson N., Ocampo M.L., Jiménez-Ferrer E., Jiménez-Aparicio A.R., Zamilpa A., Gonzalez-Cortazar M., Tortoriello J., Herrera-Ruiz M. Anti-inflammatory activity of different agave plants and the compound cantalasaponin-1. Molecules. 2013;18(7):8136–8146. doi: 10.3390/molecules18078136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Herrera-Ruiz M., Jiménez-Ferrer E., Tortoriello J., Zamilpa A., Alegría-Herrera E., Jiménez-Aparicio A.R., Arenas-Ocampo M.L., Martínez-Duncker I., Monterrosas-Brisson N. Anti-neuroinflammatory effect of agaves and cantalasaponin-1 in a model of LPS-induced damage. Nat. Prod. Res. 2019;1-4 doi: 10.1080/14786419.2019.1608537. [DOI] [PubMed] [Google Scholar]
- 122.Zhang W.J., Frei B. Astragaloside IV inhibits NF- κ B activation and inflammatory gene expression in LPS-treated mice. Mediators Inflamm. 2015;2015:274314. doi: 10.1155/2015/274314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Song M.T., Ruan J., Zhang R.Y., Deng J., Ma Z.Q., Ma S.P. Astragaloside IV ameliorates neuroinflammation-induced depressive-like behaviors in mice via the PPARγ/NF-κB/NLRP3 inflammasome axis. Acta Pharmacol. Sin. 2018;39(10):1559–1570. doi: 10.1038/aps.2017.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen L., Xiong Y.Q., Xu J., Wang J.P., Meng Z.L., Hong Y.Q. Juglanin inhibits lung cancer by regulation of apoptosis, ROS and autophagy induction. Oncotarget. 2017;8(55):93878–93898. doi: 10.18632/oncotarget.21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dong Z.W., Yuan Y.F. Juglanin suppresses fibrosis and inflammation response caused by LPS in acute lung injury. Int. J. Mol. Med. 2018;41(6):3353–3365. doi: 10.3892/ijmm.2018.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dinda B., Dinda S., DasSharma S., Banik R., Chakraborty A., Dinda M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017;131:68–80. doi: 10.1016/j.ejmech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 127.Guo L.T., Wang S.Q., Su J., Xu L.X., Ji Z.Y., Zhang R.Y., Zhao Q.W., Ma Z.Q., Deng X.Y., Ma S.P. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J. Neuroinflammation. 2019;16(1):95. doi: 10.1186/s12974-019-1474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou J., Chan L., Zhou S. Trigonelline: a plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012;19(21):3523–3531. doi: 10.2174/092986712801323171. [DOI] [PubMed] [Google Scholar]
- 129.Chowdhury A.A., Gawali N.B., Munshi R., Juvekar A.R. Trigonelline insulates against oxidative stress, proinflammatory cytokines and restores BDNF levels in lipopolysaccharide induced cognitive impairment in adult mice. Metab. Brain Dis. 2018;33(3):681–691. doi: 10.1007/s11011-017-0147-5. [DOI] [PubMed] [Google Scholar]
- 130.Guo W., Sun J., Jiang L., Duan L., Huo M., Chen N., Zhong W., Wassy L., Yang Z., Feng H. Imperatorin attenuates LPS-induced inflammation by suppressing NF-κB and MAPKs activation in RAW 264.7 macrophages. Inflammation. 2012;35(6):1764–1772. doi: 10.1007/s10753-012-9495-9. [DOI] [PubMed] [Google Scholar]
- 131.Wang K.S., Lv Y., Wang Z., Ma J., Mi C., Li X., Xu G.H., Piao L.X., Zheng S.Z., Jin X. Imperatorin efficiently blocks TNF-α-mediated activation of ROS/PI3K/Akt/NF-κB pathway. Oncol. Rep. 2017;37(6):3397–3404. doi: 10.3892/or.2017.5581. [DOI] [PubMed] [Google Scholar]
- 132.Chowdhury A.A., Gawali N.B., Shinde P., Munshi R., Juvekar A.R. Imperatorin ameliorates lipopolysaccharide induced memory deficit by mitigating proinflammatory cytokines, oxidative stress and modulating brain-derived neurotropic factor. Cytokine. 2018;110:78–86. doi: 10.1016/j.cyto.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 133.Sulakhiya K., Keshavlal G.P., Bezbaruah B.B., Dwivedi S., Gurjar S.S., Munde N., Jangra A., Lahkar M., Gogoi R. Lipopolysaccharide induced anxiety- and depressive-like behaviour in mice are prevented by chronic pre-treatment of esculetin. Neurosci. Lett. 2016;611:106–111. doi: 10.1016/j.neulet.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 134.Zhu L., Nang C., Luo F., Pan H., Zhang K., Liu J., Zhou R., Gao J., Chang X., He H., Qiu Y., Wang J., Long H., Liu Y., Yan T. Esculetin attenuates lipopolysaccharide (LPS)-induced neuroinflammatory processes and depressive-like behavior in mice. Physiol. Behav. 2016;163:184–192. doi: 10.1016/j.physbeh.2016.04.051. [DOI] [PubMed] [Google Scholar]
- 135.Abdoulaye I.A., Guo Y.J. A Review of Recent Advances in Neuroprotective Potential of 3-N-Butylphthalide and Its Derivatives. BioMed Res. Int. 2016;2016:5012341. doi: 10.1155/2016/5012341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhao C.Y., Lei H., Zhang Y., Li L., Xu S.F., Cai J., Li P.P., Wang L., Wang X.L., Peng Y. L-3-n-Butylphthalide attenuates neuroinflammatory responses by downregulating JNK activation and upregulating Heme oxygenase-1 in lipopolysaccharide-treated mice. J. Asian Nat. Prod. Res. 2016;18(3):289–302. doi: 10.1080/10286020.2015.1099524. [DOI] [PubMed] [Google Scholar]
- 137.Gao X.J., Xie G.N., Liu L., Fu Z.J., Zhang Z.W., Teng L.Z. Sesamol attenuates oxidative stress, apoptosis and inflammation in focal cerebral ischemia/reperfusion injury. Exp. Ther. Med. 2017;14(1):841–847. doi: 10.3892/etm.2017.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu Z., Chen Y., Qiao Q., Sun Y., Liu Q., Ren B., Liu X. Sesamol supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of nuclear factor kappaB. Mol. Nutr. Food Res. 2017;61(5) doi: 10.1002/mnfr.201600734. [DOI] [PubMed] [Google Scholar]
- 139.Kim N., Do J., Bae J.S., Jin H.K., Kim J.H., Inn K.S., Oh M.S., Lee J.K. Piperlongumine inhibits neuroinflammation via regulating NF-κB signaling pathways in lipopolysaccharide-stimulated BV2 microglia cells. J. Pharmacol. Sci. 2018;137(2):195–201. doi: 10.1016/j.jphs.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 140.Gu S.M., Lee H.P., Ham Y.W., Son D.J., Kim H.Y., Oh K.W., Han S.B., Yun J., Hong J.T. Piperlongumine Improves Lipopolysaccharide-Induced Amyloidogenesis by Suppressing NF-KappaB Pathway. Neuromolecular Med. 2018;20(3):312–327. doi: 10.1007/s12017-018-8495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gupta S.C., Tyagi A.K., Deshmukh-Taskar P., Hinojosa M., Prasad S., Aggarwal B.B. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch. Biochem. Biophys. 2014;559:91–99. doi: 10.1016/j.abb.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 142.Mohan S., Syam S., Abdelwahab S.I., Thangavel N. An anti-inflammatory molecular mechanism of action of α-mangostin, the major xanthone from the pericarp of Garcinia mangostana: an in silico, in vitro and in vivo approach. Food Funct. 2018;9(7):3860–3871. doi: 10.1039/C8FO00439K. [DOI] [PubMed] [Google Scholar]
- 143.Chen Y.F., Wang Y.W., Huang W.S., Lee M.M., Wood W.G., Leung Y.M., Tsai H.Y. Trans-Cinnamaldehyde, An Essential Oil in Cinnamon Powder, Ameliorates Cerebral Ischemia-Induced Brain Injury via Inhibition of Neuroinflammation Through Attenuation of iNOS, COX-2 Expression and NFκ-B Signaling Pathway. Neuromolecular Med. 2016;18(3):322–333. doi: 10.1007/s12017-016-8395-9. [DOI] [PubMed] [Google Scholar]
- 144.Zhang L., Zhang Z., Fu Y., Yang P., Qin Z., Chen Y., Xu Y. Trans-cinnamaldehyde improves memory impairment by blocking microglial activation through the destabilization of iNOS mRNA in mice challenged with lipopolysaccharide. 2016. [DOI] [PubMed]
- 145.Abou El-Ezz D., Maher A., Sallam N., El-Brairy A., Kenawy S. Trans-cinnamaldehyde Modulates Hippocampal Nrf2 Factor and Inhibits Amyloid Beta Aggregation in LPS-Induced Neuroinflammation Mouse Model. Neurochem. Res. 2018;43(12):2333–2342. doi: 10.1007/s11064-018-2656-y. [DOI] [PubMed] [Google Scholar]
- 146.Lee W.H., Loo C.Y., Bebawy M., Luk F., Mason R.S., Rohanizadeh R. Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013;11(4):338–378. doi: 10.2174/1570159X11311040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sorrenti V., Contarini G., Sut S., Dall’Acqua S., Confortin F., Pagetta A., Giusti P., Zusso M. Curcumin Prevents Acute Neuroinflammation and Long-Term Memory Impairment Induced by Systemic Lipopolysaccharide in Mice. Front. Pharmacol. 2018;9:183. doi: 10.3389/fphar.2018.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pardee A.B., Li Y.Z., Li C.J. Cancer therapy with beta-lapachone. Curr. Cancer Drug Targets. 2002;2(3):227–242. doi: 10.2174/1568009023333854. [DOI] [PubMed] [Google Scholar]
- 149.Sitônio M.M., Carvalho Júnior C.H. Campos, Ide.A.; Silva, J.B.; Lima, Mdo.C.; Góes, A.J.; Maia, M.B.; Rolim Neto, P.J.; Silva, T.G. Anti-inflammatory and anti-arthritic activities of 3,4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b]pyran-5,6-dione (β-lapachone). Inflamm. Res. 2013;62(1):107–113. doi: 10.1007/s00011-012-0557-0. [DOI] [PubMed] [Google Scholar]
- 150.Besson J.C.F., de Carvalho Picoli C., Matioli G., Natali M.R.M. Methyl jasmonate: a phytohormone with potential for the treatment of inflammatory bowel diseases. J. Pharm. Pharmacol. 2018;70(2):178–190. doi: 10.1111/jphp.12839. [DOI] [PubMed] [Google Scholar]
- 151.Eduviere A.T., Umukoro S., Adeoluwa O.A., Omogbiya I.A., Aluko O.M. Possible Mechanisms Involved in Attenuation of Lipopolysaccharide-Induced Memory Deficits by Methyl Jasmonate in Mice. Neurochem. Res. 2016;41(12):3239–3249. doi: 10.1007/s11064-016-2050-6. [DOI] [PubMed] [Google Scholar]
- 152.Solomon U., Taghogho E.A. Methyl jasmonate attenuates memory dysfunction and decreases brain levels of biomarkers of neuroinflammation induced by lipopolysaccharide in mice. Brain Res. Bull. 2017;131:133–141. doi: 10.1016/j.brainresbull.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 153.Adebesin A., Adeoluwa O.A., Eduviere A.T., Umukoro S. Methyl jasmonate attenuated lipopolysaccharide-induced depressive-like behaviour in mice. J. Psychiatr. Res. 2017;94:29–35. doi: 10.1016/j.jpsychires.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 154.Ghosh S., Basak P., Dutta S., Chowdhury S., Sil P.C. New insights into the ameliorative effects of ferulic acid in pathophysiological conditions. Food Chem. Toxicol. 2017;103:41–55. doi: 10.1016/j.fct.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 155.Rehman S.U., Ali T., Alam S.I., Ullah R., Zeb A., Lee K.W., Rutten B.P.F., Kim M.O. Ferulic Acid Rescues LPS-Induced Neurotoxicity via Modulation of the TLR4 Receptor in the Mouse Hippocampus. Mol. Neurobiol. 2019;56(4):2774–2790. doi: 10.1007/s12035-018-1280-9. [DOI] [PubMed] [Google Scholar]
- 156.Huang C., Wu J., Chen D., Jin J., Wu Y., Chen Z. Effects of sulforaphane in the central nervous system. Eur. J. Pharmacol. 2019;853:153–168. doi: 10.1016/j.ejphar.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 157.Townsend B.E., Johnson R.W. Sulforaphane reduces lipopolysaccharide-induced proinflammatory markers in hippocampus and liver but does not improve sickness behavior. Nutr. Neurosci. 2017;20(3):195–202. doi: 10.1080/1028415X.2015.1103463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gao J., Xiong B., Zhang B., Li S., Huang N., Zhan G., Jiang R., Yang L., Wu Y., Miao L., Zhu B., Yang C., Luo A. Sulforaphane Alleviates Lipopolysaccharide-induced Spatial Learning and Memory Dysfunction in Mice: The Role of BDNF-mTOR Signaling Pathway. Neuroscience. 2018;388:357–366. doi: 10.1016/j.neuroscience.2018.07.052. [DOI] [PubMed] [Google Scholar]
- 159.Wu Y., Gao M., Wu J., Hu P., Xu X., Zhang Y., Wang D., Chen Z., Huang C. Sulforaphane triggers a functional elongation of microglial process via the Akt signal. J. Nutr. Biochem. 2019;67:51–62. doi: 10.1016/j.jnutbio.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 160.Badshah H., Ali T., Kim M.O. Osmotin attenuates LPS-induced neuroinflammation and memory impairments via the TLR4/NFκB signaling pathway. Sci. Rep. 2016;6:24493. doi: 10.1038/srep24493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lessig J., Fuchs B. Plasmalogens in biological systems: their role in oxidative processes in biological membranes, their contribution to pathological processes and aging and plasmalogen analysis. Curr. Med. Chem. 2009;16(16):2021–2041. doi: 10.2174/092986709788682164. [DOI] [PubMed] [Google Scholar]
- 162.Fujino T., Yamada T., Asada T., Tsuboi Y., Wakana C., Mawatari S., Kono S. Efficacy and Blood Plasmalogen Changes by Oral Administration of Plasmalogen in Patients with Mild Alzheimer’s Disease and Mild Cognitive Impairment: A Multicenter, Randomized, Double-blind, Placebo-controlled Trial. EBioMedicine. 2017;17:199–205. doi: 10.1016/j.ebiom.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sejimo S., Hossain M.S., Akashi K. Scallop-derived plasmalogens attenuate the activation of PKCδ associated with the brain inflammation. Biochem. Biophys. Res. Commun. 2018;503(2):837–842. doi: 10.1016/j.bbrc.2018.06.084. [DOI] [PubMed] [Google Scholar]
- 164.Jiang X., Chen L., Shen L., Chen Z., Xu L., Zhang J., Yu X. Trans-astaxanthin attenuates lipopolysaccharide-induced neuroinflammation and depressive-like behavior in mice. 2016. [DOI] [PubMed]
- 165.Han J.H., Lee Y.S. Im, J.H.; Ham, Y.W.; Lee, H.P.; Han, S.B.; Hong, J.T. Astaxanthin Ameliorates Lipopolysaccharide-Induced Neuroinflammation, Oxidative Stress and Memory Dysfunction through Inactivation of the Signal Transducer and Activator of Transcription 3 Pathway. Mar. Drugs. 2019;17(2):E123. doi: 10.3390/md17020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chuyen H.V., Eun J.B. Marine carotenoids: Bioactivities and potential benefits to human health. Crit. Rev. Food Sci. Nutr. 2017;57(12):2600–2610. doi: 10.1080/10408398.2015.1063477. [DOI] [PubMed] [Google Scholar]
- 167.Jiang X., Wang G., Lin Q., Tang Z., Yan Q., Yu X. Fucoxanthin prevents lipopolysaccharide-induced depressive-like behavior in mice via AMPK- NF-κB pathway. Metab. Brain Dis. 2019;34(2):431–442. doi: 10.1007/s11011-018-0368-2. [DOI] [PubMed] [Google Scholar]
- 168.Shi Z., Ren H., Huang Z., Peng Y., He B., Yao X., Yuan T.F., Su H. Fish Oil Prevents Lipopolysaccharide-Induced Depressive-Like Behavior by Inhibiting Neuroinflammation. Mol. Neurobiol. 2017;54(9):7327–7334. doi: 10.1007/s12035-016-0212-9. [DOI] [PubMed] [Google Scholar]
- 169.Rey C., Delpech J.C., Madore C., Nadjar A., Greenhalgh A.D., Amadieu C., Aubert A., Pallet V., Vaysse C., Layé S., Joffre C. Dietary n-3 long chain PUFA supplementation promotes a pro-resolving oxylipin profile in the brain. Brain Behav. Immun. 2019;76:17–27. doi: 10.1016/j.bbi.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 170.de Gomes M.G., Souza L.C., Goes A.R., Del Fabbro L., Filho C.B., Donato F., Prigol M., Luchese C., Roman S.S., Puntel R.L., Boeira S.P., Jesse C.R. Fish oil ameliorates sickness behavior induced by lipopolysaccharide in aged mice through the modulation of kynurenine pathway. J. Nutr. Biochem. 2018;58:37–48. doi: 10.1016/j.jnutbio.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 171.Karlsen A., Retterstøl L., Laake P., Paur I., Bøhn S.K., Sandvik L., Blomhoff R. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007;137(8):1951–1954. doi: 10.1093/jn/137.8.1951. [DOI] [PubMed] [Google Scholar]
- 172.Theoharides T.C., Asadi S., Panagiotidou S. A case series of a luteolin formulation (NeuroProtek®) in children with autism spectrum disorders. Int. J. Immunopathol. Pharmacol. 2012;25(2):317–323. doi: 10.1177/039463201202500201. [DOI] [PubMed] [Google Scholar]
- 173.Theoharides T.C., Stewart J.M., Hatziagelaki E., Kolaitis G. Brain “fog,” inflammation and obesity: key aspects of neuropsychiatric disorders improved by luteolin. Front. Neurosci. 2015;9:225. doi: 10.3389/fnins.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xiao H., Wignall N., Brown E.S. An open-label pilot study of icariin for co-morbid bipolar and alcohol use disorder. Am. J. Drug Alcohol Abuse. 2016;42(2):162–167. doi: 10.3109/00952990.2015.1114118. [DOI] [PubMed] [Google Scholar]
- 175.Ramírez-Garza S.L., Laveriano-Santos E.P., Marhuenda-Muñoz M., Storniolo C.E., Tresserra-Rimbau A., Vallverdú-Queralt A., Lamuela-Raventós R.M. marhuenda-Muñoz, M.; Storniolo, C. E.; Tresserra-Rimbau, A.; Vallverdu-Queralt, A.; Lamuela-Raventos, R. M. Health effects of resveratrol: results human intervention trials. Nutrients. 2018;10(12):E1892. doi: 10.3390/nu10121892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Khan H., Ullah H., Martorell M., Valdes S. E., Belwal T., Tejada S., Sureda A., Kamal M. A. Flavonoids nanoparticles in cáncer: treatment, prevention and clinical prospects. 2019. [DOI] [PubMed]
- 177.Li S., Wu B., Fu W., Reddivari L. The anti-inflammatory effects of dietary anthocyanins against ulcerative colitis. Int. J. Mol. Sci. 2019;20(10):E2588. doi: 10.3390/ijms20102588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rebello C.J., Beyl R.A. lertora, J. J. L.; Greenway, F. L.; Ravussin, E.; Ribnicky, D. M.; Pouley, A.; Kennedy, B. J.; Castro, H. F.; Campagna, S. R.; Coulter, A. A.; Redman, L. M. Safety and pharmacokinetics of naringenin: a randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2019;22(1):91–98. doi: 10.1111/dom.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vamanu E. Polyphenolic nutraceuticals to combat oxidative stress through microbiota modulation. Front. Pharmacol. 2019;10:492. doi: 10.3389/fphar.2019.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brown B.I. Nutritional management of metabolic endotoxemia: a clinical review. Altern. Ther. Health Med. 2017;23(4):42–54. [PubMed] [Google Scholar]
- 181.McCormick J.K., Yarwood J.M., Schlievert P.M. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]