ABSTRACT
Background
Therapeutic strategies with immune checkpoint inhibitors (ICIs) counteract the immunosuppressive effects of programmed cell death protein-1 (PD-1) and ligand-1 (PD-L1). ICI treatment has emerged in first- and second-line therapy of non–small cell lung cancer (NSCLC). As immunotherapeutic treatment with ICIs is a dynamic field where new drugs and combinations are constantly evaluated, we conducted an up-to-date systematic review on comparative efficacy and safety in patients with advanced NSCLC.
Methods
We searched PubMed up to February 2020 and Embase, CENTRAL, and clinical trial registries up to August 2018. Additionally, we checked reference lists. We dually screened titles, abstracts and, subsequently, full-texts for eligibility. Two reviewers assessed the risk of bias and graded the certainty of evidence following GRADE (Grading of Recommendations Assessment, Development and Evaluation). For second-line therapy, we performed random-effects meta-analyses. Due to considerable clinical heterogeneity, we reported first-line results narratively.
Results
Of 1497 references, we identified 22 relevant publications of 16 studies. For first-line therapy, a combination of an ICI with chemotherapy improved progression-free survival and overall survival compared to chemotherapy but increased the risk of serious adverse events. Single-agent pembrolizumab increased overall and progression-free survival in patients with PD-L1 expression of ≥50% and resulted in less TRAE than chemotherapy. Compared to placebo, maintenance therapy with durvalumab increased overall and progression-free survival at the downside of higher risk of TRAE. For second-line therapy, a random-effects meta-analysis yielded a statistically significantly improved overall survival (OS) and progression-free survival (PFS) for ICIs compared to docetaxel (HR 0.69; 95% CI: 0.63–0.75 for OS; HR 0.85; 95% CI: 0.77 − 0.93 for PFS; 6 studies, 3478 patients; median OS benefit in months: 2.4 to 4.2). In meta-analysis, risk of any treatment-related adverse events of any grade was lower for ICI than docetaxel as second-line therapy (RR 0.76, 95% CI: 0.73–0.79; 6 studies, 3763 patients).
Conclusion
In first-line therapy of patients with advanced NSCLC, ICI is effective when combined with chemotherapy not depending on PD-L1 expression, or as monotherapy in high PD-L1 expressing tumors. For second-line therapy, single-agent ICI improves efficacy and safety compared to docetaxel.
KEYWORDS: Immune checkpoint inhibitors, advanced non-small cell lung cancer, nsclc, systematic review, meta-analysis, pd-1, pd-L1
Introduction
Lung cancer is the most frequent type of cancer worldwide, with almost 2.1 million estimated new cases in 2018, according to the World Health Organization (WHO).1 In 2018, more than 1.7 million people died of lung cancer, comprising 18.4% of all cancer-related deaths.1 The two main histological types of lung cancer are small cell lung cancer (SCLC), which accounts for approximately 15% of all lung cancers, and non–small cell lung cancer (NSCLC), the remaining 85%. NSCLC can further be subdivided into squamous cell carcinoma, adenocarcinoma, and large cell carcinoma.2,3
Since most patients with lung cancer are not diagnosed until an advanced stage, the prognosis is usually poor. The 5-year survival rate depends on the stage of the tumor, the time of diagnosis, and the histological subtype.4 Conventional chemotherapy protocols for NSCLC comprise 4 to 6 cycles of platinum-based doublet chemotherapy in first-line treatment and 6 cycles of docetaxel as a second-line regimen.5 Both regimens employ unspecific cytotoxic agents, which display numerous side effects.
Thus, for decades, cancer research has aimed to find driver mutations of malignant cells, which could be targeted for more selective and effective therapy.6–10 Currently, upon NSCLC diagnosis, mutational testing for epidermal growth factor receptor-1 (EGFR), anaplastic lymphoma kinase (ALK), proto-oncogene tyrosine-protein kinase (ROS-1), and serine/threonine-protein kinase B-Raf (BRAF) should be performed in order to start first-line therapy with a targeted agent instead of chemotherapy.5
Another breakthrough in recent years has been immunotherapeutic treatment with immune checkpoint inhibitors, which were developed to counteract the immunosuppressive effects of the programmed cell death protein-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) pathway and to activate the immune system for defense against malignant cells.11
Immune checkpoint inhibitors are monoclonal antibodies, targeting either the programmed cell death receptor PD-1 or its ligand PD-L1. The substances that are already approved for clinical use in NSCLC are: atezolizumab,12 durvalumab,13 nivolumab,14 and pembrolizumab15 (see Supplementary Table S1). Recent systematic reviews demonstrated the beneficial effects of immune checkpoint inhibitors on overall survival and progression-free survival for first-16,17 and second-line treatments18,19 compared to chemotherapy in NSCLC patients. International clinical practice guidelines now recommend the use of immune checkpoint inhibitors for first- and second-line therapy of patients with stage IV NSCLC without driver alterations.5,20,21
Immunotherapeutic treatments with checkpoint inhibition, however, is a dynamic field, with new drugs constantly being evaluated in clinical trials. Recent studies have focused on novel compounds such as the abovementioned antibody durvalumab,22 different combination treatments23,24 and assessment of long-term data.23,25–27 Therefore, including these novel aspects we conducted an up-to-date systematic review regarding the comparative efficacy and safety of approved immune checkpoint inhibitors compared with other treatment interventions in patients with advanced NSCLC (stage III or IV).
Methods
We registered our systematic review in the international prospective register of systematic reviews (PROSPERO) under CRD42018104751.28 For this publication, we adhered to the guidance of the Preferred Items for Systematic Reviews and Meta-Analyses (PRISMA).29
Literature searches and information sources
An experienced information specialist (IK) designed and conducted the database searches. The most recent update search was conducted in February 2020 in PubMed. We initially searched PubMed, Embase.com (Elsevier), CENTRAL (Cochrane Library/Wiley) as well as in clinical trial registries (ClinicalTrials.gov and the World Health Organization’s (WHO) International Clinical Trials Registry Platform) in August 2018. The update search was limited to PubMed, because all of the initially included studies had been retrieved by this search. In addition, we checked the reference lists of relevant review articles and all the included studies to detect relevant articles potentially missed by searches in electronic databases. For bibliographic database searches we used both free-text and controlled vocabulary (e.g., Medical Subject Headings). We restricted our search to randomized controlled trials (RCTs) as well as to the English and German languages. We provide the detailed search strategy in the Supplemental material.
Eligibility criteria and study selection
To identify studies that meet our eligibility criteria presented in Table 1, two investigators independently screened titles and abstracts. The included abstracts underwent a subsequent dual full-text screening. For both the abstract and full-text screening, we used pilot-tested review forms. We screened the literature with the web-based systematic review software Covidence.30 Investigators resolved discrepancies of inclusion or exclusion decisions by consensus or by the involvement of a third, senior reviewer.
Table 1.
Eligibility criteria.
| Inclusion | Exclusion | |
|---|---|---|
| Population: | Adults with histologically confirmed unresectable NSCLC (stages IIIA, IIIB, and IV) | Adults with NSCLC stage I–II or SCLC |
| Interventions: |
|
Other treatments |
| Comparators: |
|
Other treatment, no treatment |
| Outcomes: |
|
Other outcomes |
| Study design: |
|
All other study designs |
|
Publication language: |
|
Other languages |
| Search period: | 2000–beginning of search | Before 2000 |
Abbreviations: ALK = anaplastic lymphoma kinase; EGFR = epidermal growth factor receptor; MET = mesenchymal–epithelial transition factor; NSCLC = non–small cell lung cancer; PD-1 = programmed cell death protein-1; PD-L1 = programmed cell death ligand-1; ROS1 = proto-oncogene tyrosine-protein kinase; SCLC = small cell lung cancer
Data collection
For the studies that met our inclusion criteria, we extracted relevant information into pilot-tested data abstraction tables. Items included study and patient characteristics and the description of the intervention and control as well as results for the outcomes of interest for each individual study. A second person checked the extracted data for accuracy and completeness.
Assessment of risk of bias and certainty of evidence
Two persons independently assessed the included RCTs’ risk of bias with the Cochrane Risk of Bias tool.31 The risk of bias for each domain was rated as low, high, or unclear. All ratings on risk of bias decisions were documented in tables, and disagreements were solved by consensus. We applied the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach to assess the certainty of evidence for each outcome considered relevant for decision-making.32,33
Data synthesis and analysis
If the data were sufficient, we conducted meta-analyses of efficacy and safety outcomes of interest. Otherwise, we described the results narratively. For efficacy, we performed meta-analyses by pooling hazard ratios (HRs) with 95% confidence intervals (CIs) using the random-effects inverse-variance model with the DerSimonian–Laird estimate of tau2.34 We preferred HRs, since they summarize the treatment effect for the entire study duration rather than the median survival solely reflecting one time point on the Kaplan–Meier curve. To achieve comparability, we converted CIs to 95%, if authors reported 97% or 99% CIs. For safety, we conducted random-effects meta-analyses by pooling risk ratios with 95% CIs, calculated from the number of events and the number of patients at risk. We evaluated the studies’ statistical heterogeneity by visually inspecting the forest plots and calculating the I2 statistics.35 For the meta-analyses, we used Stata 14.2 (Stata Corp, College Station, TX, USA).
Results
Study selection
We screened 1497 titles and abstracts of 210 were retrieved as full-texts. Ultimately, 16 RCTs (published in 22 articles) met our inclusion criteria.22–27,36-51 The PRISMA flowchart (see Figure 1) illustrates the study selection process in detail.
Figure 1.
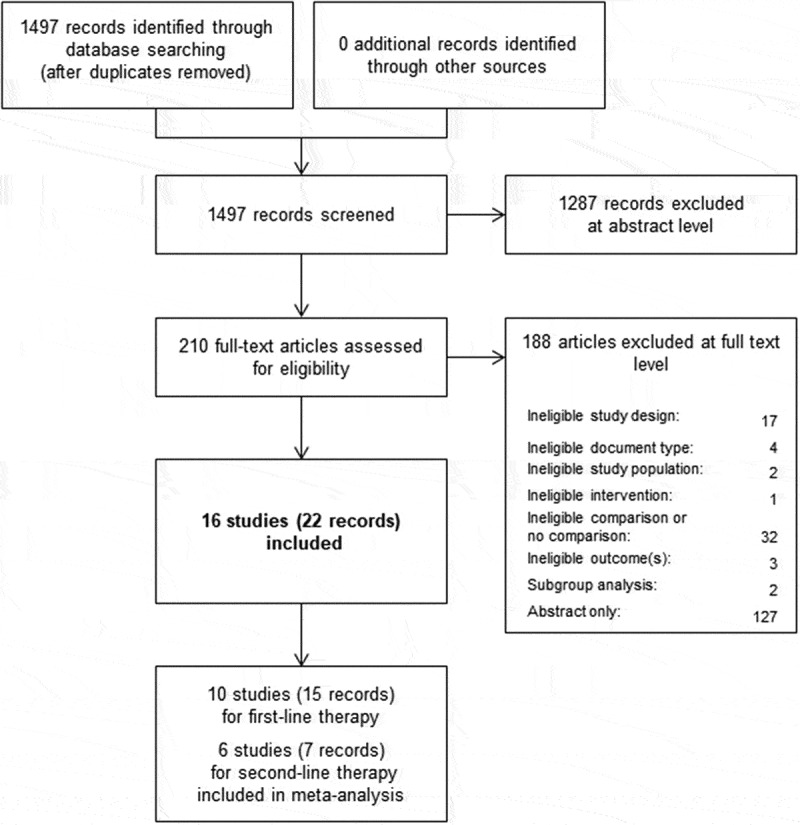
PRISMA flow diagram modified from Moher et al.29
Risk of bias and certainty of evidence
We rated all the domains for each study as a low risk of bias. However, for open-label trials, we rated blinding as a high risk of bias except for overall survival and progression-free survival if assessed by a blinded central review. We present detailed risk of bias assessments and certainty of evidence ratings for each outcome in Supplementary Tables S2 and S3.
Study characteristics
Seven open-label24,39,44,45,47,49,50 and three double-blind RCTs22,42,46 evaluated the PD-1/PD-L1 immune checkpoint-inhibitors in patients treated with first-line therapy. The median follow-up of the patients in the included studies ranged from 7.8 to 33.3 months. Six studies compared PD-1/PD-L1 immune checkpoint inhibitors with docetaxel as a second-line therapy in patients with advanced NSCLC and previous treatment.37,38,40,43,48,51 The median follow-up of patients in the included studies ranged from 8.8 to 21 months. All studies were conducted as international multicenter RCTs and were funded or supported by pharmaceutical companies.22,24,37–40,42-51 Table 2 summarizes the characteristics of the included studies for first- and second-line immune checkpoint inhibitor therapy.
Table 2.
Characteristics of included studies.
| Author, year, trial name, NCT Therapy line |
Study design, phase Funding/Support |
Recruiting Period |
Countries | Follow-up | N total randomized | Key inclusion criteria Primary outcome(s) |
Intervention N randomized |
Comparison N randomized |
|---|---|---|---|---|---|---|---|---|
| ATEZOLIZUMAB | ||||||||
| Fehrenbacher et al. 201640 POPLAR NCT01903993 Second-line therapy |
Randomized, controlled, open-label, phase 2 F. Hoffmann-La Roche/Genentech |
August 2013 – March 2014 | 61 academic medical centers, 13 countries, Europe and North America |
Median: Atezolizumab: 14.8 months Docetaxel: 15.7 months |
287 |
Key inclusion criteria:
Primary endpoints:
|
Atezolizumab 1200 mg every 3 weeks N = 144 |
Docetaxel 75 mg/m2 of BSA every 3 weeks N = 143 |
| Rittmeyer et al. 201748 Fehrenbacher et al. 201841 OAK NCT02008227 Second-line therapy |
Randomized, controlled, open-label, phase 3 F. Hoffmann-La Roche/Genentech |
March 2014 – April 2015 |
194 academic or community oncology centers, 31 countries, Europe, North and South America, New Zealand, and Asia | Median: 21 months |
1225 (secondary efficacy population) 850 (primary efficacy population) |
Key inclusion criteria:
Primary endpoints:
|
Atezolizumab 1200 mg every 3 weeks N = 425 (primary efficacy population) N = 613 (secondary efficacy population) |
Docetaxel 75 mg/m2 of BSA every 3 weeks N = 425 (primary efficacy population) N = 612 (secondary efficacy population) |
| West et al.201950 IMpower130 NCT02367781 First-line therapy |
Randomized, controlled, open-label, phase 3 F. Hoffmann-La Roche/Genentech |
April 2015 – February 2017 |
131 centers, 8 countries, North America, Europe and Israel |
Median: (wild-type population)a Atezolizumab plus Chemotherapy: 18.5 months Chemotherapy: 19.2 months |
724 Wild-type population:a 679 |
Key inclusion criteria:
Primary endpoints:
|
Atezolizumab 1200 mg every 3 weeks + carboplatin at an AUC of 6 mg/mL per minute every 3 weeks + nab-paclitaxel 100 mg/m2 of BSA every week for four or six 21-day cycles N = 483 N = 451 (wild-type population)a |
Carboplatin at an AUC of 6 mg/mL per minute every 3 weeks + nab-paclitaxel 100 mg/m2 of BSA every week for four or six 21-day cycles N = 240 N = 228 (wild-type population) a |
| Socinski et al. 201849 IMpower150 NCT02366143 First-line therapy |
Randomized, controlled, open-label, phase 3 F. Hoffmann-La Roche/Genentech |
March 2015 – December 2016 |
240 sites, 26 countries, Australia, Asia, Europe, and North and South America | Median: (wild-type population)a ABCP: 15.4 months BCP: 15.5 months |
1202 Wild-type population:a 1040 |
Key inclusion criteria:
Primary endpoints:
|
ABCP: Atezolizumab 1200 mg + bevacizumab 15 mg/kg BW + carboplatin at an AUC of 6 mg/mL per minute + paclitaxel 200 mg/m2 of BSA (175 mg/m2 for Asian patients) for four or six 21-day cycles N = 400 N = 356 (wild-type population)a ACP: Atezolizumab 1200 mg + carboplatin at an AUC of 6 mg/mL per minute + paclitaxel 200 mg per m2 of BSA (175 mg per m2 for Asian patients) for four or six 21-day cycles N = 402 N = 348 (wild-type population)a |
BCP: Bevacizumab 15 mg/kg BW + carboplatin at an AUC of 6 mg/mL per minute for four or six 21-day cycles + paclitaxel 200 mg per m2 of BSA (175 mg/m2 for Asian patients) N = 400 N = 336 (wild-type population)a |
| DURVALUMAB | ||||||||
| Antonia et al. 201722 Antonia et al. 201836 Gray et al. 201926 PACIFIC NCT02125461 First-line therapy (consolidation after radiochemotherapy) |
Randomized, double-blind, placebo-controlled, phase 3 Astra Zeneca |
May 2014 – April 2016 |
Australia, Asia, Europe, North and South America, and South Africa | Antonia et al. 2017:22 Median: 14.5 months Antonia et al. 2018:36 Median: 25.2 months Gray et al. 2019:26 Median: 33.3 months |
713 |
Key inclusion criteria:
Primary endpoints:
|
Durvalumab 10 mg/kg BW every 2 weeks for up to 12 months N = 476 ( |
Placebo every 2 weeks for up to 12 months N = 237 |
| NIVOLUMAB | ||||||||
| Wu et al. 201951 CheckMate 078 NCT02613507 Second-line therapy |
Randomized, controlled, open-label, phase 3 Bristol-Myers Squibb |
December 2015 – November 2016 | 32 hospitals and cancer/medical centers, China, Russia, Singapore |
Median: Nivolumab: 10.4 months Docetaxel: 8.8 months |
504 |
Key inclusion criteria:
Primary endpoint:
|
Nivolumab 3 mg/kg BW every 2 weeks N = 338 |
Docetaxel 75 mg/m2 of BSA every 3 weeks N = 166 |
| Borghaei et al. 201537 CheckMate 057 NCT01673867 Second-line therapy |
Randomized, controlled, open-label, phase 3 Bristol-Myers Squibb |
November 2012 – December 2013 | Europe and North and South America | Minimum: 13.2 months |
582 |
Key inclusion criteria:
Primary endpoint:
|
Nivolumab 3 mg/kg BW every 2 weeks N = 292 |
Docetaxel 75 mg/m2 of BSA every 3 weeks N = 290 |
| Brahmer et al. 201538 CheckMate 017 NCT01642004 Second-line therapy |
Randomized, controlled, open-label, phase 3 Bristol-Myers Squibb |
October 2012 – December 2013 | Australia, Europe, and North and South America | Minimum: 11 months |
272 |
Key inclusion criteria:
Primary endpoint:
|
Nivolumab 3 mg/kg BW every 2 weeks N = 135 |
Docetaxel 75 mg/m2 of BSA every 3 weeks N = 137 |
| Carbone et al. 201739 CheckMate 026 NCT02041533 First-line therapy |
Randomized, controlled, open-label, phase 3 Bristol-Myers Squibb |
March 2014 – April 2015 |
Australia, Asia, Europe, and North and South America | Median: 13.5 months |
541 |
Key inclusion criteria:
Primary endpoint:
|
Nivolumab 3 mg/kg BW every 2 weeks N = 271 |
Platinum-based doublet CHT every 3 weeks for 4 to 6 cycles: carboplatin + pemetrexed, cisplatin + pemetrexed, carboplatin + gemcitabine, cisplatin + gemcitabine, or carboplatin + paclitaxel N = 270 |
| Hellmann et al. 201824 Hellmann et al. 201923 CheckMate 227 NCT02477826 First-line therapy |
Randomized, controlled, open-label, phase 3 Bristol-Myers Squibb |
August 2015 – November 2016 | Europe, North and South America, Australia, Asia, and Africa | Minimum: 29.3 months |
1739 PD-L1 expression of ≥1%: 1189 PD-L1 expression of <1%: 550 |
Key inclusion criteria:
Primary endpoints:
|
PD-L1 expression of ≥1%: Nivolumab 3 mg/kg BW every 2 weeks + ipilimumab 1 mg/kg every 6 weeks N = 396 or Nivolumab 240 mg every 2 weeks N = 396 PD-L1 expression of <1%: Nivolumab 3 mg/kg BW every 2 weeks + ipilimumab 1 mg/kg BW every 6 weeks N = 187 or Nivolumab 360 mg every 3 weeks + platinum-doublet CHT based on histologic tumor type every 3 weeks or up to four cycles N = 177 |
PD-L1 expression of ≥1%: Platinum-doublet CHT based on histologic tumor type every 3 weeks for up to four cycles N = 397 PD-L1 expression of <1%: Platinum-doublet CHT based on histologic tumor type every 3 weeks for up to four cycles N = 186 |
| PEMBROLIZUMAB | ||||||||
| Herbst et al. 201643 KEYNOTE-010 NCT01905657 Second-line therapy |
Randomized, controlled, open-label, phase 2/3 Merck & Co. |
August 2013 – February 2015 | 202 academic centers, 24 countries, Africa, Australia, Asia, Europe, and North and South America | Median: 13.1 months |
1034 |
Key inclusion criteria:
Primary endpoints:
|
Pembrolizumab 2 mg/kg BW every 3 weeks N = 345 Pembrolizumab 10 mg/kg BW every 3 weeks N = 346 |
Docetaxel 75 mg per m2 of BSA every 3 weeks N = 343 |
| Mok et al. 201945 KEYNOTE-042 NCT02220894 First-line therapy |
Randomized, controlled, open-label, phase 3 Merck Sharp & Dohme |
December 2014 – March 2017 | 213 centers, 32 countries, North and South America, Europe, Asia and South Africa |
Median: 12.8 months |
1274 |
Key inclusion criteria:
Primary endpoints:
|
Pembrolizumab 200 mg every 3 weeks for up to 35 cycles N = 637 |
Platinum-based CHT of the investigator’s choice for 4–6 cycles: carboplatin at an AUC of 5–6 mg/mL/min + paclitaxel 200 mg/m2 of BSA or pemetrexed 500 mg/m2 N = 637 |
| Reck et al. 201647 Reck et al. 201927 KEYNOTE-024 NCT2142738 First-line therapy |
Randomized, controlled, open-label, phase 3 Merck & Co. |
September 2014 – October 2015 | 142 sites, 16 countries, Australia, Europe, and North America | Reck et al. 2016: Median: 11.2 months Reck et al. 2019: Median: 25.2 months |
305 |
Key inclusion criteria:
Primary endpoint:
|
Pembrolizumab 200 mg every 3 weeks for 35 cycles N = 154 |
Platinum-based CHT for 4 to 6 cycles: carboplatin + pemetrexed, cisplatin + pemetrexed, carboplatin + gemcitabine, cisplatin + gemcitabine, or carboplatin + paclitaxel N = 151 |
| Paz-Ares et al. 201846 KEYNOTE-407 NCT02775435 First-line therapy |
Randomized, controlled, double-blind, phase 3 Merck Sharp & Dohme |
August 2016 – December 2017 | 125 sites, 17 countries, Australia, Europe, and North and Central America, Asia | Median: 7.8 months |
559 |
Key inclusion criteria:
Primary endpoints:
|
Pembrolizumab 200 mg every 3 weeks for up to 35 cycles + carboplatin at an AUC of 6 mg/mL + paclitaxel 200 mg/m2 of BSA or nab-paclitaxel 100 mg/m2 of BSA for the first 4 cylces N = 278 |
Placebo for up to 35 cycles + carboplatin at an AUC of 6 mg/mL + paclitaxel 200 mg/m2 of BSA or nab-paclitaxel 100 mg/m2 of BSA for the first 4 cylces N = 281 |
| Langer et al. 201644 Borghaei et al. 201925 KEYNOTE-021 NCT02039674 First-line therapy |
Randomized, controlled, open-label, phase 2 Merck & Co. |
November 2014 – January 2016 | 26 medical centers, 2 countries, Taiwan and USA | Langer et al. 2016: Median: 10.6 months Borghaiei et al. 2019: Median: 23.9 months |
123 |
Key inclusion criteria:
Primary endpoint:
|
Pembrolizumab 200 mg + Platinum-based doublet CHT: carboplatin + pemetrexed every 3 weeks for 4 cycles N = 60 |
Platinum-based doublet CHT: carboplatin + pemetrexed for 4 cycles N = 63 |
| Gandhi et al. 201842 KEYNOTE-189 NCT02578680 First-line therapy |
Randomized, controlled, double-blind, phase 3 Merck & Co. |
February 2016 – March 2017 | 126 sites, 16 countries, Europe, North America, Australia, Asia, and Japan | Median: 10.5 months |
616 |
Key inclusion criteria:
Primary endpoints:
|
Pembrolizumab 200 mg every 3 weeks for 35 cycles + Platinum-based CHT: four cycles of the investigator’s choice of IV cisplatin (75 mg/m2 of BSA) or carboplatin (AUC, 5 mg per ml per minute) plus pemetrexed (500 mg/m2), all administered IV every 3 weeks, N = 410 |
Placebo every 3 weeks for up to 35 cycles + Platinum-based CHT: four cycles of the investigator’s choice of IV cisplatin (75 mg/m2 of BSA) or carboplatin (AUC, 5 mg per ml per minute) plus pemetrexed (500 mg per m2), all administered IV every 3 weeks, N = 206 |
Abbreviations: ABCP = atezolizumab plus bevacizumab plus carboplatin plus paclitaxel; ACP = atezolizumab plus carboplatin plus paclitaxel; ALK = anaplastic lymphoma kinase; AUC = area under the concentration−time curve; BSA = body surface area; BCP = bevacizumab plus carboplatin plus paclitaxel; BW = body weight; CHT = chemotherapy; ECOG = Eastern Cooperative Oncology Group; EGFR = epidermal growth factor receptor; IV = intravenously; ITT = intention-to-treat; N = number of patients; NCT = National Clinical Trial; NR = not reported; NSCLC = non–small cell lung cancer; PD-L1 = programmed death ligand-1; mg = milligram; ml = milliliter; kg = kilogram; TPS = tumor proportion score; WHO = World Health Organization
aWild-type genotype: patients with no EGFR or ALK genomic alterations
Study participants
The number of randomized patients in the first-line therapy studies22,24,39,42,44–47,49,50 ranged from 123 to 1739. The study patients’ median age ranged from 62.5 to 66.0 years. The majority were men, except in one study.44 In the studies assessing second-line therapies,37,38,40,43,48,51 the total number of randomized patients ranged from 272 to 1225. The median age ranged from 60 to 64 years. In all the studies, the majority of participants were men. Supplementary Table S4 provides a detailed summary of the participants’ baseline characteristics and the outcomes that were assessed in each included study.
Overall survival and progression-free survival
First-line therapy
We included 10 RCTs that compared immune checkpoint inhibitors alone or in combination with other treatments to various control regimens.22,24,39,42,44–47,49,50 Three trials compared nivolumab39 or pembrolizumab45,47 to chemotherapy; 6 trials assessed combination treatments of atezolizumab,49,50 nivolumab23 or pembrolizumab42,44,46 plus chemotherapy relative to chemotherapy alone; one RCT compared a combination of nivolumab plus ipilimumab23 to chemotherapy; and one trial assessed the efficacy of durvalumab22 compared to placebo in patients with 1 to 42 days after chemoradiotherapy (see Table 2).
Figure 2(a) presents the hazard ratios for overall survival from each trial. Seven studies found a statistically significantly improved overall survival for treatment with immune checkpoint inhibition.23,42,45–47,49,50 Figure 2(b) shows the treatment effects regarding progression-free survival. Except for two studies comparing nivolumab39 or pembrolizumab45 with platinum-based chemotherapy, all the trials showed a statistically significant improvement of progression-free survival in patients treated with immune checkpoint inhibitors.22,23,42,44,46,47,49,50 The absolute time difference in the median overall and progression-free survival is depicted in Supplementary Table S5. In the following sections, we summarize comparisons of immune checkpoint inhibitors with different control regimens in more detail.
Figure 2.
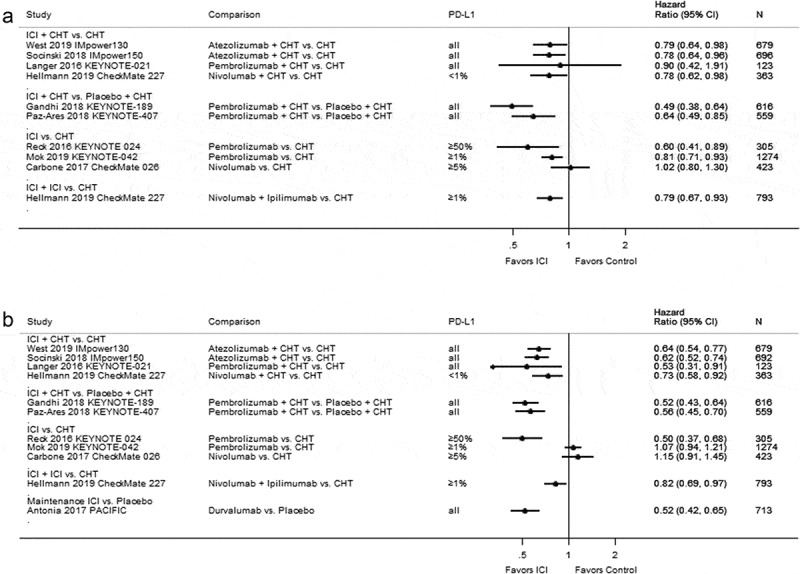
Forest plots for (a) overall survival and (b) progression-free survival in studies assessing immune checkpoint inhibitors as first-line therapy.
Hellmann 2019 CheckMate 227, Nivolumab + CHT vs. CHT: We converted 97.72% CI (0.60–1.02) for overall survival and 97.72% CI (0.56–0.95) for progression free survival to 95% CI. Hellmann 2019 CheckMate 227, Nivolumab + Ipilimumab vs. CHT: We converted 97.72% CI (0.65–0.96) for overall survival to 95% CI. Abbreviations: CHT = chemotherapy; CI = confidence interval; ICI = immune checkpoint inhibitor; N = number of patients; PD-L1 = programmed cell death ligand-1.
Nivolumab or pembrolizumab versus chemotherapy
The multicenter, open-label, phase 3 CheckMate 026 trial39 enrolled 541 patients with recurrent or stage IV NSCLC without prior treatment and a PD-L1 tumor-expression level of ≥1%. Investigators randomized patients to either nivolumab or platinum-based chemotherapy. In patients with a PD-L1 expression level of ≥5% (primary efficacy analysis population, N = 423), overall survival was similar between single-agent nivolumab (n = 211) treatment and platinum-doublet chemotherapy (n = 212) (median 14.4 versus 13.2 months; HR 1.02; 95% CI: 0.80–1.30; see Figure 2(a)). This study also reported no statistically significant difference for progression-free survival in patients with a PD-L1 expression level ≥5% (median 4.2 versus 5.9 months; HR 1.15; 95% CI: 0.91–1.45; see Figure 2(b)).39 Analyses of all randomized patients revealed similar results regarding progression-free survival and overall survival (see Supplementary Table S4).
Two multicenter, open-label, phase 3 RCTs compared pembrolizumab with chemotherapy in patients with previously untreated locally advanced or metastatic NSCLC.45,47 In the KEYNOTE-024 trial,27,47 305 patients with a PD-L1 expression level of ≥50% received either pembrolizumab or chemotherapy. At a median follow-up of 11.2 months, overall survival (median survival not reached in both groups; HR 0.60; 95% CI: 0.41–0.89; see Figure 2(a)) and progression-free survival (median 10.3 versus 6.0 months; HR 0.50; 95% CI: 0.37–0.68; see Figure 2(b)), were statistically significantly longer in the pembrolizumab group than in the chemotherapy group.47 In a later publication with a longer follow-up (median 25.2 months), median overall survival was 30.0 versus 14.2 months (HR 0.63; 95% CI: 0.47–0.86; see Supplementary Figure S1A).27
The second trial, KEYNOTE-42,45 randomized 1274 patients with PD-L1 expression of ≥1% to either pembrolizumab or platinum-based chemotherapy. The median follow-up of this study was 12.8 months. Overall survival was statistically significantly longer with pembrolizumab than with chemotherapy in patients with PD-L1 expression of ≥50% (median 20.0 versus 12.2 months; HR 0.69; 95% CI: 0.56–0.85), ≥20% (median 17.7 versus 13.0 months; HR 0.77; 95% CI: 0.64–0.92) and ≥1% (median 16.7 versus 12.1 months 0.81; 95% CI: 0.71–0.93; see Figure 2(a)). Progression-free survival, however, was only statistically significantly longer with pembrolizumab than with chemotherapy in patients with a PD-L1 expression of ≥50% (median 7.1 versus 6.4 months; HR 0.81; 95% CI: 0.67–0.99). In study participants with PD-L1 of ≥20% (median 6.2 versus 6.6 months; HR 0.94; 95% CI: 0.80–1.11) and ≥1% (median 5.4 versus 6.5 months; HR 1.07; 95% CI: 0.94–1.21; see Figure 2(b)) this effect could not be observed.45
Atezolizumab, pembrolizumab or nivolumab plus chemotherapy versus chemotherapy
Six multicenter RCTs evaluated the comparative efficacy and safety of a combination of atezolizumab, pembrolizumab or nivolumab plus chemotherapy and platinum-based chemotherapy alone.23,42,44,46,49,50
In the 3-armed, open-label, phase 3 IMpower150 trial,49 1202 patients with metastatic nonsquamous NSCLC were randomized to receive either atezolizumab plus bevacizumab plus carboplatin plus paclitaxel (ABCP), or atezolizumab plus carboplatin plus paclitaxel (ACP), or bevacizumab plus carboplatin plus paclitaxel (BCP). The efficacy was only reported for the ABCP and BCP groups. The median overall survival was statistically significantly longer in the ABCP group than in the BCP group (19.2 versus 14.7 months; HR 0.78; 95% CI: 0.64–0.96; Figure 2(a)). Likewise, the progression-free survival (8.3 versus 6.8 months; HR 0.62; 95% CI: 0.52–0.74; see Figure 2(b)) was also statistically significantly longer for the ABCP group.49
The open-label, phase 3 IMpower130 trial50 randomized 724 participants with stage IV nonsquamous NSCLC to atezolizumab plus chemotherapy (carboplatin plus nab-paclitaxel) or chemotherapy alone. In patients with no EGFR and ALK mutations (N = 679) combination of atezolizumab and chemotherapy resulted in statistical significant improvement of overall survival (median 18.6 versus 13.9 months; HR 0.79; 95% CI: 0.64–0.98) progression-free survival (median 7.0 versus 5.5 months; HR 0.64; 95% CI: 0.54–0.77) as compared with chemotherapy alone.50
In the multicenter, open-label, phase 2 KEYNOTE-021 trial,25,44 123 patients were randomized to either pembrolizumab plus platinum-based chemotherapy or to platinum-based chemotherapy alone. After a median follow-up of 10.6 months, primary analysis of overall survival yielded no statistically significant difference between the two groups (median survival not reached in both groups; HR 0.90; 95% CI: 0.42–1.91; see Figure 2(a)).44 The progression-free survival was statistically significantly prolonged for patients treated with pembrolizumab plus chemotherapy (median 13.0 versus 8.9 months; HR 0.53; 95% CI: 0.31–0.91; see Figure 2(b)).44 Improvement in progression-free survival maintained in an updated analysis with a median follow-up of 23.9 months (median 24.0 versus 9.3 months; HR 0.53; 95% CI; 0.33–0.86; see Supplementary Figure S1B).25 Regarding overall survival, this analysis showed a benefit for the pembrolizumab plus chemotherapy group compared with chemotherapy alone (HR 0.56; 95% CI: 0.32–0.95; see Supplementary Figure S1A).25
The open-label, phase 3 CheckMate 22723 randomized patients with PD-L1 expression level of <1% (N = 550) to either nivolumab plus ipilimumab, nivolumab plus chemotherapy, or chemotherapy alone. Overall survival was longer with nivolumab plus chemotherapy than with chemotherapy alone, but the difference did not reach statistical significance (median 15.2 versus 12.2 months; HR 0.78; 97.72% CI: 0.60–1.02, see Figure 2(a)). Compared to chemotherapy alone, progression-free survival was statistically significant longer if chemotherapy was combined with nivolumab (median 5.6 versus 4.7 months; HR 0.73; 97.72% CI, 0.56–0.95; see Figure 2(b)).23
The phase 3, double-blinded RCT KEYNOTE-40746 enrolled 559 participants with untreated metastatic squamous NSCLC. This study randomized participants to pembrolizumab or saline placebo both in combination with chemotherapy (carboplatin and either paclitaxel or nanoparticle albumin-bound [nab]-paclitaxel). The median follow-up was 7.8 months. Overall survival (median 15.9 versus 11.3 months; HR 0.64; 95% CI: 0.49–0.85; see Figure 2(a)) and progression-free survival (median 6.4 versus 4.8 months; HR 0.56; 95% CI: 0.45–0.70; see Figure 2(b)) were statistically significantly longer in patients treated with pembrolizumab in addition to chemotherapy than placebo and chemotherapy.46
Similar results could be observed in the double-blind, phase 3 KEYNOTE-189 trial,42 where patients with metastatic nonsquamous NSCLC without sensitizing EGFR or ALK mutations were randomized to either pembrolizumab plus platinum-based chemotherapy or to placebo plus platinum-based chemotherapy. Overall survival (median survival not reached versus 11.3 months; HR 0.49; 95% CI: 0.38–0.64; see Figure 2(a)) and median progression-free survival were statistically significantly longer in the pembrolizumab group (8.8 versus 4.9 months; HR 0.52; 95% CI: 0.43–0.64; see Figure 2(b)).42
Nivolumab plus ipilimumab versus chemotherapy
The open-label, phase 3 CheckMate 22723 trial enrolled patients with stage IV or recurrent NSCLC without previous chemotherapy. Patients with PD-L1 expression level of ≥1% were randomized to nivolumab plus ipilimumab, nivolumab monotherapy, or chemotherapy. Patients with PD-L1 expression level <1% were randomized to nivolumab plus ipilimumab, nivolumab plus chemotherapy, or chemotherapy alone. As compared with chemotherapy, in patients with PD-L1 expression level of ≥1% (N = 793) overall survival (median 17.1 versus 14.9 months; HR 0.79; 97.72% CI: 0.65–0.96; see Figure 2(a)) and progression-free survival (HR 0.82; 95% CI: 0.69–0.97; see Figure 2(b)) was significantly immmproved with nivolumab plus ipilimumab.23 Results for patients with PD-L1 expression level of <1% and all randomized patients are shown in Supplementary Table S4. Progression-free survival was longer with nivolumab plus ipilimumab as compared with chemotherapy in all randomized patients with a high tumor mutational burden (TMB, defined as ≥10 mutations per megabase; N = 299, median 7.2 versus 5.5 months; HR 0.58; 97.5% CI: 0.41–0.81), irrespective of PD-L1 expression.24
Durvalumab versus placebo
The multicenter, double-blind, phase 3 PACIFIC study22,26,36 randomized 713 patients with stage III locally advanced, unresectable NSCLC without disease progression after previous chemoradiotherapy to either durvalumab or placebo as consolidation therapy. For progression-free survival after a median follow-up of 14.5 months, a statistically significant improvement could be seen in patients treated with durvalumab (median 16.8 versus 5.6 months; HR 0.52; 95% CI: 0.42–0.65; see Figure 2(b)).22 Updated analysis after a median follow-up of 25.2 months, showed similar findings for progression free survival (median 17.2 versus 5.6 months; HR 0.51; 95% CI: 0.41–0.63; see Supplementary Figure S1B).36 Overall survival was statistically significantly prolonged for durvalumab-treated patients as well (HR 0.68; 99.73% CI: 0.47–0.997).36 In addition, a post-hoc, exploratory analysis found consistent benefit after median follow-up of 33.3 months (HR 0.69; 95% CI: 0.55–0.86; see Supplementary Figure S1A).26
Second-line therapy
For second-line therapy, a random-effects meta-analysis of 6 RCTs37,38,40,43,48,51 (one with two dosing arms43) including 3478 patients yielded a statistically significantly improved overall survival for participants treated with immune checkpoint inhibitors as compared to patients treated with single-agent chemotherapy (HR 0.69; 95% CI: 0.63–0.75; I2 = 0.0%, see Figure 3(a)). In a meta-analysis based on the results from the same 6 trials and 3478 patients, progression-free survival was statistically significantly improved for patients treated with atezolizumab, nivolumab, or pembrolizumab as compared to patients treated with a taxane-based chemotherapy (HR 0.85; 95% CI: 0.77–0.93; I2 = 41.3%, see Figure 3(b)).
Figure 3.
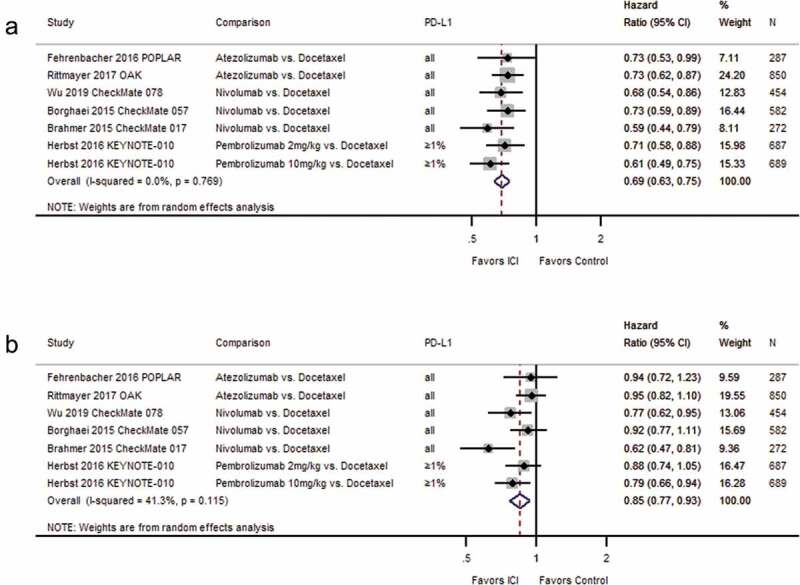
Forest plots for (a) overall survival and (b) progression-free survival in studies assessing immune checkpoint inhibitors as second-line therapy.
Wu 2019 CheckMate 078: We converted 97.7% CI (0.52–0.90) for overall survival to 95% CI. Herbst 2016 KEYNOTE-010:intention-to-treat N = 1033 including all three study arms (pembrolizumab 2 mg, N = 344, pembrolizumab 10 mg, N = 346, docetaxel 75 mg, N = 343). Percentage of randomized patients with quantifiable tumor PD-L1 expression: Borghaei 2015 CheckMate 057: 78%; Brahmer 2015 CheckMate 017: 83%; Wu 2019 CheckMate 078: 9%. Abbreviations: CI = confidence interval; D + L = DerSimonian and Laird method; ICI = immune checkpoint inhibitor; kg = kilogram; mg = milligram; N = number of patients; PD-L1 = programmed cell death ligand-1.
Again, the differences in median overall and progression-free survival in months are depicted in Supplemental Table S6. Concerning median progression-free survival, treatment with a single-agent immune checkpoint blockade compared to docetaxel resulted in net differences of −1.9 to +0.7 months. With regard to overall survival, the differences in median overall survival ranged from 2.4 to 4.2 months longer than in patients treated with chemotherapy.
Safety
First-line therapy
The proportion of patients with adverse events in each study is depicted in Figure 4(a) and Supplementary Table S4. Eight RCTs provided data on treatment-related adverse events (TRAE).22,23,39,44,45,47,49,50 In four studies that compared either nivolumab or pembrolizumab39,45,47 or nivolumab plus ipilimumab23 to chemotherapy, the proportion of patients with TRAE was higher in the chemotherapy control group than in the immune checkpoint inhibitor treatment groups (see Figure 4(a)). One study comparing durvalumab with placebo22 showed a statistically significantly higher incidence of TRAE in patients treated with immune checkpoint inhibitors (see Figure 4(a)). However, immune checkpoint inhibition was used as consolidation therapy 1 to 42 days after chemoradiotherapy and compared with placebo.22 In four studies that compared immune checkpoint inhibitors in combination with chemotherapy to chemotherapy alone, the proportion of patients with TRAE were either similar between the groups44,49,50 or lower in patients receiving only chemotherapy.23 Regarding serious adverse events, the IMpower150 study published by Socinski et al.49 found a statistically significant higher incidence in patients treated with atezolizumab plus bevacizumab, carboplatin and paclitaxel (ABCP) compared with patients receiving bevacizumab, carboplatin and paclitaxel (BCP; 42.0% versus 34.0%, risk ratio [RR] 1.23; 95% CI: 1.03–1.48, see Figure 4(a)). Likewise, the IMpower130 study50 found significant more serious adverse events in patients receiving atezolizumab in combination with chemotherapy (carboplatin and nab-paclitaxel) than those receiving chemotherapy alone (50.7% versus 37.9%, RR 1.34; 95% CI: 1.11–1.61, see Figure 4(a)) In the PACIFIC trial by Antonia et al.22 serious adverse events were also more frequent in patients treated with durvalumab than in those treated with placebo, but the difference did not reach statistical significance (28.6% versus 22.6%, RR 1.26; 95% CI: 0.96–1.67).
Figure 4.
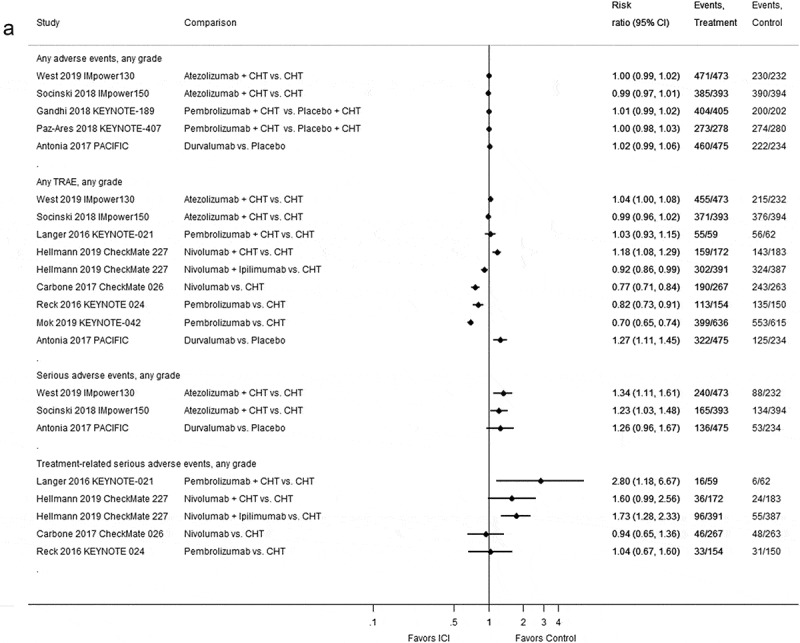
Forest plots for adverse events in studies assessing immune checkpoint inhibitors as (a) first-line and (b) second-line therapy.
Herbst 2016 KEYNOTE-010: docetaxel arm (N = 343) was split upAbbreviations: CHT = chemotherapy; CI = confidence interval; ICI = immune checkpoint inhibitor; N = number of patients; TRAE = treatment-related adverse events.
Compared to chemotherapy, the risk of treatment-related serious adverse events was higher in patients treated with a combination of two immune checkpoint inhibitors23 or the combination of an immune checkpoint inhibitor on top of chemotherapy23,44 (see Figure 4(a)). For single-agent immune checkpoint inhibitor therapy compared to chemotherapy, similar risks of treatment-related serious adverse events were observed39,47 (see Figure 4(a)). Supplementary Figure S2 shows extended follow-up data on adverse events of three studies that are consistent with prior findings.25,27,36
Second-line therapy
Based on a random-effects meta-analysis, the risk of overall adverse events was similar between second-line treatment groups with an immune checkpoint inhibitor or docetaxel (3 RCTs, 2019 patients; RR 0.98; 95% CI: 0.97–1.00; I2 = 0.0%, see Figure 4(b)). A random-effects meta-analysis of 6 RCTs (one with two dosing arms43) including 3763 patients showed statistically significantly fewer TRAE for patients treated with immune checkpoint inhibitors than for those treated with chemotherapy (RR 0.76; 95% CI: 0.73–0.79; I2 = 0.0%, see Figure 4(b)). Serious adverse events were similar in patients treated with atezolizumab compared to those receiving docetaxel40,48 and lower in patients treated with nivolumab compared to docetaxel51 (see Figure 4(b)). In two RCTs, the risk of treatment-related serious adverse events was lower in the nivolumab than in the docetaxel groups37,38 (see Figure 4(b)).
Figure 4.
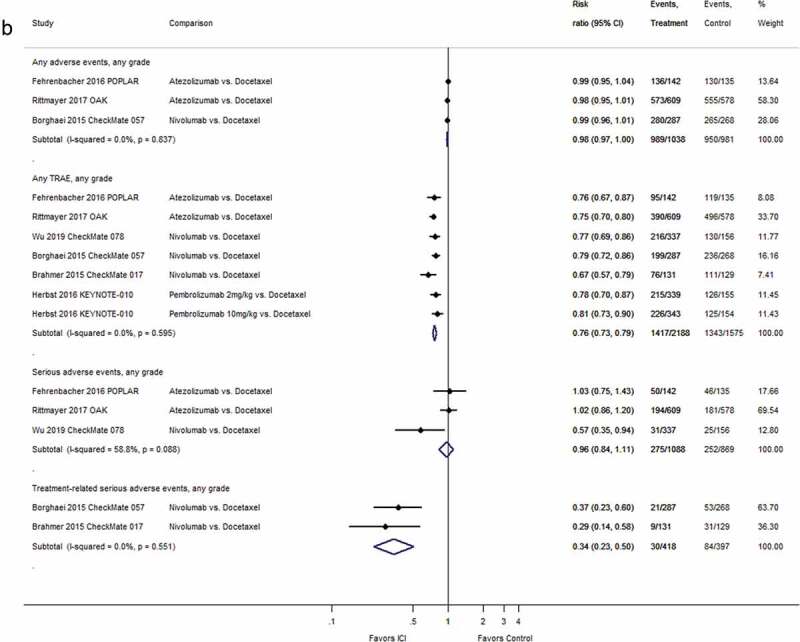
(Continued).
Discussion
Our systematic review shows for first-line therapy of patients with advanced NSCLC that a combination of an immune checkpoint inhibitor (atezolizumab, nivolumab or pembrolizumab) with platinum-based chemotherapy or nivolumab combined with ipilimumab improved progression-free survival and overall survival compared to chemotherapy. However, combination therapies increased the risk of serious adverse events. For single-agent nivolumab or pembrolizumab compared to chemotherapy alone, we observed different effects depending on the PD-L1 expression level. Pembrolizumab increased overall and progression-free survival in patients with PD-L1 expression of ≥50% and resulted in less TRAE than chemotherapy. Compared to placebo, maintenance therapy with durvalumab increased overall and progression-free survival at the downside of higher risk of TRAE. Meta-analyses of second-line therapy trials yield statistically significantly improved progression-free survival and overall survival for immune checkpoint inhibitor compared to docetaxel. Immune checkpoint inhibition resulted in lower risk of any and serious TRAE than docetaxel.
As mentioned above PD-L1 expression level is an important factor that decides the choice of therapy in patients with advanced NSCLC without driver mutations. Two first-line therapy trials compared single-agent immunotherapy with pembrolizumab to chemotherapy and resulted in a longer progression-free survival,45,47 while nivolumab single-agent treatment displayed no significant benefit for progression-free survival.39 These two studies differ greatly concerning PD-L1 expression: in the nivolumab study,39 PD-L1 positivity was defined as ≥5% of tumor cells, while Reck et al.47 defined the PD-L1 threshold at ≥50%. The progression-free survival findings also translated to overall-survival, which was longer in the pembrolizumab study while, for nivolumab, no significant benefit could be displayed in this setting. The third single-agent immunotherapy trial45 underlined the importance of PD-L1 status; only in the patients with tumor PD-L1 expression of ≥50%, statistically significant progression-free survival benefit could be seen. In patients with PD-L1 expression of ≥20% or ≥1% this effect could not be observed.45 Consequently, pembrolizumab was initially approved by the Food and Drug Administration (FDA) as a single agent in first and later therapy lines for patients whose tumors express PD-L1 ≥ 50%. This was later expanded in April 2019 to tumors expressing PD-L1 ≥ 1%. However, in tumors with PD-L1 ≥ 1%, pembrolizumab is applied in combination with chemotherapy.5 Nivolumab, is not approved for first-line treatment of NSCLC patients, but for progression during or after platinum-based chemotherapy (i.e. second-line), regardless of PD-L1 expression. This is due to the fact, that in the second-line CheckMate 017 trial, both patient groups, positive and negative for PD-L1 expression, benefited with regard to overall survival from nivolumab treatment.38 The results of our meta-analyses underline the rationale for this approval status.
Further patient stratification strategies seem to be important in identifying which patients will benefit from immune checkpoint inhibition in first-line therapy of NSCLC. An interesting biomarker was used in one part of the Checkmate-227 trial: tumor mutational burden (TMB).23,24 This is based on the hypothesis that tumors with high TMB have a higher likelihood to display neo-antigens on their surface, which can be subsequently recognized and targeted by T-cells.52 In CheckMate 227, patients were treated with a combinatorial immune checkpoint blockade with nivolumab and ipilimumab.23,24 In the TMB-high patient cohort, a double immune checkpoint blockade resulted in significantly longer progression-free survival than chemotherapy.24 This trial, which had also other study arms, was later analyzed based on the original stratification of PD-L1 negativity (<1%) or positivity (≥1%). Here, an overall survival benefit of double immune checkpoint blockade could be observed in both groups.23 It was later also demonstrated, that the relative benefit of double immune checkpoint blockade compared to chemotherapy was also seen in patients with low TMB.23 Thus, TMB has not yet emerged as a biomarker for treatment stratification in NSCLC, in contrast to PD-L1.
Previous systematic reviews have indicated that atezolizumab, nivolumab and pembrolizumab improve outcomes in the second-line treatment of patients with advanced NSCLC as compared to chemotherapy.18,19,53,54 These results are in line with the findings of our systematic review, which includes data of more recent trials.
With respect to other PD-L1 antibodies, also avelumab was investigated as treatment for NSCLC. Avelumab is currently applied for treatment of metastatic Merkel Cell Carcinoma,55 Renal Cell Cancer56 and Urothelial Carcinoma.57 With respect to NSCLC, in a large open-label, phase III clinical trial enrolling 792 patients, avelumab treatment did not improve overall survival in patients with platinum-treated PD-L1-positive tumors when compared to docetaxel.58 Thus, avelumab failed approval status as a therapy for NSCLC.
Our systematic review has several limitations. First, we limited the eligible studies to those in English and German language. Second, we did not include trials investigating antibodies as single agents against other immune checkpoints (e.g., CTLA-4). Third, potential publication bias and selective outcome reporting are other potential limitations of this review. Moreover, the included studies were, especially for first-line therapy, very heterogeneous concerning the interventions and controls and used different cutoff values for PD-L1 expression.
Conclusion
In first-line therapy of patients with advanced NSCLC, ICI is effective when combined with chemotherapy not depending on PD-L1 expression, or as monotherapy in high PD-L1 expressing tumors. For second-line therapy, single-agent ICI improves efficacy and safety compared with docetaxel.
Supplementary Material
Funding Statement
There was no funding for this work. JS declares honorarium payments from Janssen and Roche as an invited speaker and from Amgen, Janssen, Merck, Roche and Pfizer for expert consulting on other topics than immune checkpoint inhibition and NSCLC. MP declares financial support from Roche for research projects other than immune checkpoint inhibition and NSCLC.
Abbreviations
- ABCP
atezolizumab plus bevacizumab plus carboplatin plus paclitaxel
- ALK
anaplastic lymphoma kinase
- BCP
bevacizumab plus carboplatin plus paclitaxel
- CHT
chemotherapy
- CI
confidence interval
- CTLA-4
cytotoxic T-lymphocyte–associated protein 4
- ECOG
Eastern Cooperative Oncology Group
- EGFR
epidermal growth factor receptor
- FDA
US Food and Drug Administration
- Gy
grays
- ICIs
immune checkpoint inhibitors
- MET
mesenchymal–epithelial transition factor
- NCT
National Clinical Trial
- NSCLC
non–small cell lung cancer
- N
number of patients
- OS
overall survival
- PD-1
programmed cell death protein-1
- PD-L1
programmed cell death ligand-1
- PFS
progression-free survival
- RCT
randomized controlled trial
- ROS1
proto-oncogene tyrosine-protein kinase
- RR
risk ratio
- SCC
squamous cell carcinoma
- SCLC
small cell lung cancer
- SD
standard deviation
- TMB
tumor mutational burden
- TNM
classification system for malignant tumors (tumor, nodus, metastasis)
- TRAE
treatment-related adverse events
- WHO
World Health Organization
Authors’ contributions
GW and JS conceptualized this work, conducted literature screening, data extraction, risk of bias assessment and wrote the manuscript. GW performed statistical analysis. HKS conducted literature screening, data extraction, risk of bias assessment, and critically revised the manuscript. IK developed the search strategy, conducted electronic literature searches, and critically revised the manuscript. MP and GG advised this project and critically revised the manuscript. All authors read and approved the final manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.GLOBOCAN . Global cancer observatory. GLOBOCAN; 2018. [accessed 2019 Mar 07]. https://gco.iarc.fr.
- 2.Hammerschmidt S, Wirtz H.. Lung cancer: current diagnosis and treatment. Dtsch Arztebl Int. 2009;106(49):809–16. quiz 819-820. doi: 10.3238/arztebl.2009.0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kashima J, Kitadai R, Okuma Y. Molecular and morphological profiling of lung cancer: a foundation for “Next-Generation” pathologists and oncologists. Cancers (Basel). 2019;11(5):599. doi: 10.3390/cancers11050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, Nicholson AG, Groome P, Mitchell A, Bolejack V, et al. The IASLC lung cancer staging project: proposals for revision of the tnm stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thoracic Oncol. 2016;11(1):39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Ettinger DS, Aisner DL, Wood DE, Akerley W, Bauman J, Chang JY, Chirieac LR, D’Amico TA, Dilling TJ, Dobelbower M, et al. NCCN guidelines insights: non–small cell lung cancer, Version 5.2018. J Natl Compr Canc Netw. 2018;16(7):807–821. doi: 10.6004/jnccn.2018.0062. [DOI] [PubMed] [Google Scholar]
- 6.Russo A, Franchina T, Ricciardi GRR, Smiroldo V, Picciotto M, Zanghì M, Rolfo C, Adamo V. Third generation EGFR TKIs in EGFR-mutated NSCLC: where are we now and where are we going. Crit Rev Oncol Hematol. 2017;117:38–47. doi: 10.1016/j.critrevonc.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Vestergaard HH, Christensen MR, Lassen UN. A systematic review of targeted agents for non-small cell lung cancer. Acta Oncol. 2018;57(2):176–186. doi: 10.1080/0284186X.2017.1404634. [DOI] [PubMed] [Google Scholar]
- 8.Chin LP, Soo RA, Soong R, Ou SH. Targeting ROS1 with anaplastic lymphoma kinase inhibitors: a promising therapeutic strategy for a newly defined molecular subset of non-small-cell lung cancer. J Thoracic Oncol. 2012;7(11):1625–1630. doi: 10.1097/JTO.0b013e31826baf83. [DOI] [PubMed] [Google Scholar]
- 9.Rosas G, Ruiz R, Araujo JM, Pinto JA, Mas L. ALK rearrangements: biology, detection and opportunities of therapy in non-small cell lung cancer. Crit Rev Oncol Hematol. 2019;136:48–55. doi: 10.1016/j.critrevonc.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Gkolfinopoulos S, Mountzios G. Beyond EGFR and ALK: targeting rare mutations in advanced non-small cell lung cancer. Ann Transl Med. 2018;6(8):142. doi: 10.21037/atm.2018.04.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jardim DL, de Melo Gagliato D, Kurzrock R. Lessons from the development of the immune checkpoint inhibitors in oncology. Integr Cancer Ther. 2018;17(4):1012–1015. doi: 10.1177/1534735418801524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinstock C, Khozin S, Suzman D, Zhang L, Tang S, Wahby S, Goldberg KB, Kim G, Pazdur R. U.S. Food and drug administration approval summary: atezolizumab for metastatic non-small cell lung cancer. Clin Cancer Res. 2017;23(16):4534–4539. doi: 10.1158/1078-0432.CCR-17-0540. [DOI] [PubMed] [Google Scholar]
- 13.Mezquita L, Planchard D. Durvalumab for the treatment of non-small cell lung cancer. Expert Rev Respir Med. 2018;12(8):627–639. doi: 10.1080/17476348.2018.1494575. [DOI] [PubMed] [Google Scholar]
- 14.Kazandjian D, Suzman DL, Blumenthal G, Mushti S, He K, Libeg M, Keegan P, Pazdur R. FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist. 2016;21(5):634–642. doi: 10.1634/theoncologist.2015-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pai-Scherf L, Blumenthal GM, Li H, Subramaniam S, Mishra‐Kalyani PS, He K, Zhao H, Yu J, Paciga M, Goldberg KB. FDA approval summary: pembrolizumab for treatment of metastatic non-small cell lung cancer: first-line therapy and beyond. Oncologist. 2017;22(11):1392–1399. doi: 10.1634/theoncologist.2017-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen R, Hou X, Yang L, Zhao D. Comparative efficacy and safety of first-line treatments for advanced non-small cell lung cancer with immune checkpoint inhibitors: A systematic review and meta-analysis. Thorac Cancer. 2019;10(4):607–623. doi: 10.1111/1759-7714.12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tun AM, Thein KZ, Thein WL, Guevara E. Checkpoint inhibitors plus chemotherapy for first-line treatment of advanced non-small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Future Sci OA. 2019;5(9):Fso421. doi: 10.2144/fsoa-2019-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos-Esquivel A, van der Laat A, Rojas-Vigott R, Juarez M, Corrales-Rodriguez L. Anti-PD-1/anti-PD-L1 immunotherapy versus docetaxel for previously treated advanced non-small cell lung cancer: a systematic review and meta-analysis of randomised clinical trials. ESMO Open. 2017;2(3):e000236. doi: 10.1136/esmoopen-2017-000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Zhong Y, Peng S, Zhou X, Gan X. Efficacy and safety of PD1/PDL1 blockades versus docetaxel in patients with pretreated advanced non-small-cell lung cancer: a meta-analysis. Onco Targets Ther. 2018;11:8623–8632. doi: 10.2147/OTT.S181413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanna NH, Schneider BJ, Temin S, Baker Jr S, Brahmer J, PM Ellis, Gaspar LE, Haddad RY, Hesketh PJ, Jain D, et al. Therapy for Stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2020;38(14):1608–1632. [DOI] [PubMed] [Google Scholar]
- 21.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):863–870. doi: 10.1093/annonc/mdy474. [DOI] [PubMed] [Google Scholar]
- 22.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et al. Durvalumab after chemoradiotherapy in Stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 23.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S-W, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora Jimenez E, et al. Nivolumab plus Ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 24.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borghaei H, Langer CJ, Gadgeel S, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, et al. 24-month overall survival from KEYNOTE-021 Cohort G: pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous non-small cell lung cancer. J Thoracic Oncol. 2019;14(1):124–129. doi: 10.1016/j.jtho.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, Cho BC, et al. Three-Year overall survival with durvalumab after chemoradiotherapy in Stage III NSCLC-update from PACIFIC. J Thoracic Oncol. 2019;15(2):288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 28.York Uo. PROSPERO International prospective register of systeatic reviews. University of York; [accessed 2019 Apr 16]. https://www.crd.york.ac.uk/prospero/. [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Innovation VH. Covidence systematic review software. Veritas Health Innovation; [accessed Feb 06 2019]. www.covidence.org.
- 31.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed). 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Research Ed). 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group Web site. 2013. [accessed 2020 Feb 25]. www.guidelinedevelopment.org/handbook.
- 34.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed). 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M. Overall survival with durvalumab after chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 37.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu T-E, Badin F, et al. First-line nivolumab in Stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet (London, England). 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 41.Fehrenbacher L, von Pawel J, Park K, Rittmeyer A, Gandara DR, Ponce Aix S, Han J-Y, Gadgeel SM, Hida T, Cortinovis DL, et al. Updated efficacy analysis including secondary population results for OAK: a randomized Phase III study of atezolizumab versus docetaxel in patients with previously treated advanced non–small cell lung cancer. J Thoracic Oncol. 2018;13(8):1156–1170. doi: 10.1016/j.jtho.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 42.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 43.Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, Molina J, Kim J-H, Arvis CD, Ahn M-J, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (London, England). 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 44.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Srimuninnimit V, Laktionov KK, Bondarenko I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (London, England). 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 46.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 47.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 48.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (London, England). 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 50.West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp H-G, Daniel D, McCune S, Mekhail T, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 51.Wu Y-L, Lu S, Cheng Y, Zhou C, Wang J, Mok T, Zhang L, Tu H-Y, Wu L, Feng J, et al. nivolumab versus docetaxel in a predominantly chinese patient population with previously treated advanced NSCLC: checkMate 078 randomized Phase III clinical trial. J Thoracic Oncol. 2019;14(5):867–875. doi: 10.1016/j.jtho.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Greillier L, Tomasini P, Barlesi F. The clinical utility of tumor mutational burden in non-small cell lung cancer. Transl Lung Cancer Res. 2018;7(6):639–646. doi: 10.21037/tlcr.2018.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crequit P, Chaimani A, Yavchitz A, Attiche N, Cadranel J, Trinquart L, Ravaud P. Comparative efficacy and safety of second-line treatments for advanced non-small cell lung cancer with wild-type or unknown status for epidermal growth factor receptor: a systematic review and network meta-analysis. BMC Med. 2017;15(1):193. doi: 10.1186/s12916-017-0954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barnfield PC, Ellis PM. Second-line treatment of non-small cell lung cancer: new developments for tumours not harbouring targetable oncogenic driver mutations. Drugs. 2016;76(14):1321–1336. doi: 10.1007/s40265-016-0628-6. [DOI] [PubMed] [Google Scholar]
- 55.Kim ES. Avelumab: first Global Approval. Drugs. 2017;77(8):929–937. doi: 10.1007/s40265-017-0749-6. [DOI] [PubMed] [Google Scholar]
- 56.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zarrabi K, Paroya A, Wu S. Emerging therapeutic agents for genitourinary cancers. J Hematol Oncol. 2019;12(1):89. doi: 10.1186/s13045-019-0780-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, Özgüroğlu M, Szczesna A, Polychronis A, Uslu R, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19(11):1468–1479. doi: 10.1016/S1470-2045(18)30673-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


