ABSTRACT
Resection of colorectal liver metastases (CRLM) is a potential curative treatment for patients with metastatic colorectal cancer (mCRC) with liver-limited disease (LLD). Although long-term survival improved considerably within the last decades, high recurrence rates of 50-75% after resection remain a major challenge.Tecemotide (L-BLP25) is an antigen-specific cancer vaccine inducing immunity against mucin-1 (MUC1). The LICC trial aimed to improve survival in patients with mCRC after R0/R1 resection of CRLM. LICC was a binational, randomized, double-blind, placebo-controlled, multicenter phase 2 study including patients with R0/R1 resected CRLM without evidence of metastatic disease outside the liver. Co-primary endpoints were recurrence-free survival (RFS) and 3-year overall survival (OS) rate, secondary endpoints were RFS and OS in subgroups with different MUC1 expression and safety. In total, 121 patients were 2:1 randomized between Oct 2011 and Dec 2014to receive tecemotide (N=79) or placebo (N=42). Baseline characteristics were well balanced. Median RFS was 6.1 months (95% CI 4.5-8.9) and 11.4 months (95% CI 3.7-21.2) (P = .1754), 3-year OS rate 69.1% and 79.1%, median OS 62.8 months and not reached in the tecemotide vs. placebo arm (P = .2141), respectively. Cox regression models revealed no dependence of RFS or OS on MUC1 expression. The most common tecemotide-related grade 3/4 adverse events were diarrhea, injection site reaction, intestinal perforation, peritonitis and tinnitus (1.3% each). The LICC trial failed to meet its primary endpoints of significantly improving RFS and OS with tecemotide. However, both arms showed unexpectedly long OS. MUC1 expression was not associated with outcome.
EudraCT No: 2011–000218-20
Clinical Trial Information: NCT01462513
Financial Support: Merck KGaA, Darmstadt, Germany
Abbreviations: AE: adverse event; CP: cyclophosphamide; CRC: colorectal cancer; CT: computed tomography; ECOG: Eastern Cooperative Oncology Group; FU: follow-up; HR: hazard ratio; IHC: immunohistochemical staining; ITT: intention-to-treat; DSMB: Data Safety Monitoring Board; LLD: liver-limited disease; mCRC: metastatic colorectal cancer; MPLA: monophosphoryl lipid; AMRI: magnetic resonance imaging; MUC1: mucin 1; NA: not applicable; NCI-CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events; NS: normal saline; NSCLC: non-small-cell lung carcinoma; OS: overall surviva; lPP: per protocol; RAS: Rat sarcoma; RFS: recurrence-free survival; TEAE: treatment-emergent adverse event; UICC: Union for International Cancer Control; US: ultrasound; vs.: versus.
KEYWORDS: Tecemotide (L-BLP25), mucin-1 (MUC1), colorectal Neoplasms, liver-limited disease, resection of colorectal liver metastases
Introduction
Patients with metastatic colorectal cancer (mCRC) and liver-limited disease (LLD) have a chance of long-term survival and cure after resection of colorectal liver metastases (CRLM).1–3 However, high recurrence rates of >50% after resection remain a major challenge.4 Five-year survival rates range between 28%–58%,4–11 median overall survival (OS) between 46 and 62 months.10–13
The potential benefit of chemotherapy before and/or after surgery was studied in two large trials in patients with primary resectable CRLM, the EORTC Intergroup trial 40983 reported by Nordlinger et al.10,14 and the FFCD ACHBTH AURC 9002 trial reported by Portier et al.11 Peri- and postoperative chemotherapy had a beneficial effect on RFS compared to surgery alone (20.0 vs. 12.5 months10 and 24.4 vs. 17.6 months11). Though observed differences in OS were not statistically significant (61.3 vs. 54.3 months10 and 62.1 vs. 46.4 months11) they may be of clinical relevance. Patients with primarily unresectable CRLM in the CELIM trial12,15 and a subgroup analysis of the FIRE-3 trial with LLD and metastasectomy showed survival times of 53.9 months12 and 56.2 months,13 respectively.
Tecemotide is an antigen-specific cancer vaccine inducing immunity against mucin-1 (MUC1), a glycoprotein expressed on the cell surface of many normal epithelial tissues and over- or aberrantly expressed on many carcinoma cells, including primary CRC16 and CRLM.17 MUC1 is known to promote tumor cell growth, survival, and metastasis.18,19 Tecemotide incorporates the synthetic, 25 amino acid, non-glycosylated MUC1 lipopeptide (BLP25), and the nonspecific immunoadjuvant monophosphoryl lipid A (MPLA) in a liposomal delivery system.18 Its proposed mode of action is to trigger an antigen-specific T-cell immune response in tumor cells expressing the MUC1 antigen.18 In patients with non-small-cell-lung carcinoma (NSCLC), tecemotide has shown promising results for subgroups but lacked statistically significant survival difference in the total population.20,21
The LICC trial (LICC: L-BLP25 In Colorectal Cancer) was designed to compare the efficacy and safety of adjuvant tecemotide versus placebo in patients with resected CRLM.22
Patients and methods
Study design and patient population
LICC was a randomized, placebo-controlled, multicenter, bi-national, double-blind phase II trial (registered on ClinicalTrials.gov No. NCT01462513). Patients were 2:1 randomized to tecemotide or placebo and stratified by resection status (R0/R1).22 Patients with histologically confirmed adenocarcinoma of the colon or rectum and stage IV mCRC and LLD, aged ≥18 years, with Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 and adequate organ function were included after R0/R1 resection of primary tumor and liver metastases with curative intent within the last 8 weeks before randomization. Key exclusion criteria were metastases other than liver metastases, R2 and Rx hepatic metastasectomy, chemotherapy, and/or immunotherapy within 4 weeks prior to randomization and any known autoimmune disease or immunodeficiency. No evidence of disease after resection of CRLM had to be confirmed in a baseline computed tomography (CT) or magnetic resonance imaging (MRI) scan before randomization.
The study was conducted in accordance with Good Clinical Practice and in accordance with the ethical standards of the national research committee and the 1964 Declaration of Helsinki and its later amendments. The study protocol was approved by the Institutional Review Board at the participating sites and the ethic committee of the Landesärztekammer Rheinland-Pfalz, Germany. All patients provided written informed consent to participate. A Data Safety Monitoring Board (DSMB) held periodic monitoring to ensure subject safety and data validity.
Treatment
For an immune modulation effect, cyclophosphamide (300 mg/m2) (CP) was given intravenously 3 days prior to the first tecemotide dose. The placebo group received a single dose of saline solution (NS) before the subcutaneous administration of placebo. Patients received subcutaneous tecemotide (930 µg) or placebo once weekly for 8 consecutive weeks during the primary treatment phase, followed by a maintenance treatment phase with vaccinations at 6-week intervals for 2 years. A planned total period of 3 years close surveillance and maintenance treatment with 12-week intervals in year 3 was shortened to 2 years (amendment in 2014) due to the development stop of tecemotide and thus limited shelf life of remaining stocks. Maintenance treatment lasted until recurrence or until 2 years after randomization at most. Follow-up (FU) for all patients ended 3 years after the last patient was randomized into the study.
Efficacy and safety assessment
Disease recurrence was followed by CT or MRI (RECIST v 1.1), alternating with ultrasound (US) imaging of the liver performed at six-week intervals during the maintenance treatment phase for two years, starting six weeks after the end of the primary treatment phase. Standard safety assessments were performed during the study, including assessment of adverse events (AEs) according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (NCI-CTCAE v.3.0), laboratory parameters, and physical examinations. Treatment-emergent adverse events (TEAEs) of special interest were thrombocytopenia, hepatic dysfunction, and autoimmune disease. TEAEs were assessed and causality was graded as related or not related to the study drug as determined by the investigator. AEs and SAEs suspected to be related to the investigational medicinal product had to be recorded until 12 weeks after the last treatment or during the whole FU period, respectively. Unrelated TEAEs were recorded until 42 days after the last treatment. For further details see Schimanski et al. 2012.22
Biomarkers
Ninety-six of 121 paraffin-embedded tissue blocks were available for immunohistochemical staining (IHC) of MUC1. The IHC was done as previously described,23 slides were incubated with the primary antibody for MUC1 (214D4, Millipore, 1:100) according to the manufacturer’s instruction. The staining was evaluated by two independent, blinded investigators according to Remmele’s Immunoreactive Score (see supplement 1).24
Statistical analysis
For sample size calculations, the assumed hazard ratio (HR) for RFS was 0.77, corresponding to an increase of 3 months from 10 to 13 months median time to recurrence under tecemotide, with a one-sided alpha of 0.075 (corresponding to a two-sided alpha of 0.15) per test and a total loss to FU rate of 4%. As there were two primary endpoints, adjusting for multiple testing was done using the Bonferroni-Holm procedure. Therefore, in order to keep the overall significance level of 0.15 one-sided, the smaller p-value of the two has to be below 0.075 (one-sided). Considering a 2:1 randomization, 120 patients allowed to achieve a power of 30.7% (3-year OS) and 44.5% (RFS). Notably, the phase II trial LICC was not designed for confirmatory purpose but aimed to explore potential trends of efficacy in the studied population.
The primary analysis population for all efficacy endpoints was the intention-to-treat (ITT) population comprising all randomized patients, according to the treatment assignment. Safety was analyzed for all patients receiving at least one dose of study medication. Co-primary endpoints were recurrence-free survival (RFS) and 3-year OS rate. Secondary endpoints were safety and tolerability as well as RFS and OS in subgroups with low, medium, and high MUC1 expression. MUC1 expression on tumor tissues was analyzed to discover a potential association with progression and survival and to test whether MUC1 is predictive of tecemotide benefit. RFS, based on RECIST v.1.1, was calculated as the period between randomization and date of disease recurrence as determined via standard imaging or death. OS was calculated from the date of randomization until the date of death. Kaplan-Meier estimates were used for the analysis of RFS and OS. Stratified log-rank tests (2-sided, family-wise error rate alpha = 30%, applying the Bonferroni-Holm procedure for multiple testing) were carried out for analysis of RFS and OS, with resection status (R0 vs. R1+ R2+ RX) as stratification factor. For RFS, patients who were lost to follow-up (FU) or who had withdrawn from the study were censored at the last time of recurrence evaluation. For OS, patients lost to FU or who had withdrawn from study were censored at the time of last contact or time of withdrawal. Sensitivity analyses on efficacy endpoints were conducted including per-protocol (PP) analysis and Cox proportional hazards regression models that were applied to the ITT population to test for potential differences in OS and RFS as a function of treatment and one of the variables resection status, age, sex, MUC1 staining, prior systemic therapy, prior radiotherapy, time since the first diagnosis, Fong Score, grading, number of resected metastases or resectability. Statistical analysis was performed with SAS 9.4 TS1 M5.
Results
Patient characteristics
Between Oct 2011 and Dec 2014, 121 patients were randomized at 22 study sites in Germany and Austria to receive either tecemotide (n = 79, 65.3%) or placebo (n = 42, 34.7%) Figure 1. For 12 patients (n = 10 tecemotide arm, n = 2 placebo arm), resection with curative intent was performed more than 56 days (range 58–100 days) before randomization. A centralized radiologic review led to the exclusion of 11 patients (n = 7 tecemotide arm, n = 4 placebo arm) with residual disease at baseline from the PP population. Baseline characteristics (Table 1) were generally balanced between treatment arms except for ECOG status (more patients in the tecemotide arm had ECOG 0, P = .0357). Non-significant differences were found for UICC stage, M0/M1 status, primary tumor site, Fong score, and resection status.
Figure 1.
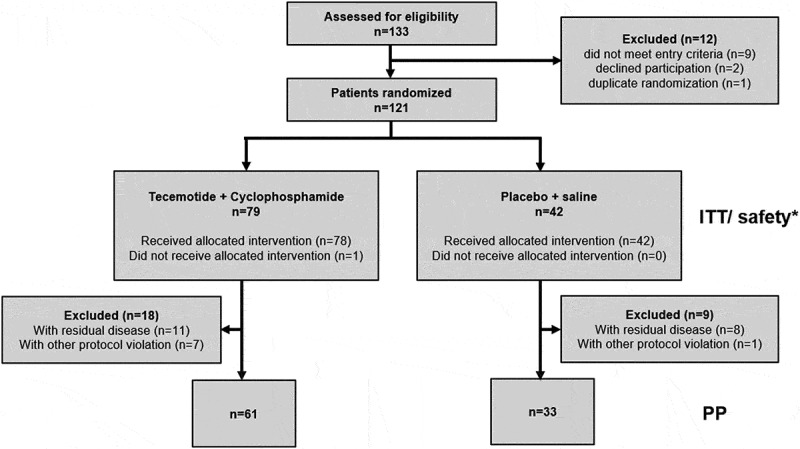
CONSORT flow diagram of the LICC trial. * One patient in the tecemotide arm did not receive vaccination but received CP and was included in the ITT and safety population. In the tecemotide arm, one patient had one R2 resected metastasis besides R0 resected metastases and one patient had one RX metastasis besides R0 and R1 resected metastases. These patients were allocated to the “Non-R0” subgroup in the ITT population and were excluded from the PP population. A centralized radiologic review was conducted for 80 patients, which led to the exclusion of 11 patients (n = 7 tecemotide arm, n = 4 placebo arm) with residual disease at baseline from the per-protocol (PP) population. Abbreviations: ITT: intention-to-treat population, PP: per-protocol population
Table 1.
Patient and tumor characteristics at baseline (ITT population)
| Tecemotide (n = 79) |
Placebo (n = 42) |
All patients (n = 121) |
P-value | ||
|---|---|---|---|---|---|
| Age at start of therapy, years [Median (range)] |
- |
60.0 (24–84) |
58.5 (30–85) |
60.0 (24–85) |
0.5137 b |
| Sex [n (%)] |
Female Male |
30 (38.0) 49 (62.0) |
15 (35.7) 27 (64.3) |
45 (37.2) 76 (62.8) |
0.8456 c |
| Comorbidity a [n (%)] |
No yes |
13 (16.5) 66 (83.5) |
6 (14.3) 36 (85.7) |
19 (15.7) 102 (84.3) |
1.0000 c |
| ECOG performance status a [n (%)] |
0 1 |
61 (77.2) 18 (22.8) |
24 (57.1) 18 (42.9) |
85 (70.2) 36 (29.8) |
0.0357 c |
| Fong score |
0 1 2 3 4 5 |
9 (11.4) 19 (24.1) 28 (35.4) 16 (20.3) 7 (8.9) 0 (0) |
4 (9.5) 12 (28.6) 8 (19.0) 11 (26.2) 6 (14.3) 1 (2.4) |
13 (10.7) 31 (25.6) 36 (29.8) 27 (22.3) 13 (10.7) 1 (0.8) |
0.2971 c |
| Time since primary diagnosis, months [Median (range)] |
- |
20 (1.4–121.3) |
12.6 (0.9–73.6) |
18.3 (0.9–121.3) |
0.1247 d |
| Previous chemotherapy [n (%)] |
No Yes |
24 (30.4) 55 (69.6) |
14 (33.3) 28 (66.7) |
38 (31.4) 83 (68.6) |
0.8375 c |
| Previous immunotherapy [n (%)] |
No Yes |
58 (73.4) 21 (26.6) |
34 (81.0) 8 (19.0) |
92 (76.0) 29 (24.0) |
0.3825 c |
| Primary tumor site [n (%)] |
Colon ascending descending multiple sites transversum unknown Rectum |
7 (8.9) 25 (31.6) 1 (1.3) 3 (3.8) 9 (11.4) 34 (43.0) |
5 (11.9) 18 (42.9) 0 (0) 2 (4.8) 3 (7.1) 14 (33.3) |
12 (9.9) 43 (35.5) 1 (0.8) 5 (4.1) 12 (9.9) 48 (39.7) |
0.3339 c |
| Tumor grading at primary diagnosis [n, %] |
G1 G2 G3 G4 GX |
1 (1.3) 61 (77.2) 11 (13.9) 0 (0) 6 (7.6) |
1 (2.4) 30 (71.4) 9 (21.4) 1 (2.4) 2 (4.8) |
2 (1.7) 91 (75.2) 20 (16.5) 1 (0.8) 7 (5.8) |
0.3134 c |
| UICC stage at primary diagnosis |
I II III IV unknown |
3 (3.8) 9 (11.4) 25 (31.6) 37 (46.8) 5 (6.3) |
0 (0) 2 (4.8) 6 (14.3) 28 (66.7) 6 (14.3) |
3 (2.5) 11 (9.1) 31 (25.6) 65 (53.7) 11 (9.1) |
0.0457 c |
| Resectability [n, %] |
Primary Secondary |
50 (63.3) 29 (36.7) |
30 (71.4) 12 (28.6) |
80 (66.1) 41 (33.9) |
0.4234 c |
| Number of resected metastases [n, %] |
<5 5–10 >10 |
70 (88.6) 8 (10.1) 1 (1.3) |
34 (81.0) 6 (14.3) 2 (4.8) |
104 (86.0) 14 (11.6) 3 (2.5) |
0.3324 c |
| Resection status [n, %] |
R0 R1 R2 |
69 (87.3) 9 (11.4) 1 (1.3) |
38 (90.5) 4 (9.5) 0 (0) |
107 (88.4) 13 (10.7) 1 (0.8) |
0.7687 c |
| MUC1 staining [n (%)] | Low Moderate Strong Not evaluable Missing |
11 (13.9) 30 (38.0) 22 (27.8) 16 (20.3) 0 (0) |
5 (11.9) 18 (42.9) 10 (23.8) 8 (19.0) 1 (2.4) |
16 (13.2) 48 (39.7) 32 (26.4) 24 (19.8) 1 (0.8) |
0.9419 c |
aat enrollment; b two sample t-test (two-sided); c exact Fisher test (two-sided); d Wilcoxon test (two-sided) Abbreviations: ECOG: Eastern Cooperative Oncology Group; UICC: Union for international cancer control
Efficacy
Kaplan-Meier analyses for RFS and OS are shown in Figure 2. Median RFS was 8.6 months (95% CI, 5.9–11.4), 6.1 months (95% CI, 4.5–8.9) and 11.4 months (95% CI, 3.7–21.2) in the total ITT population, tecemotide and placebo arm, respectively. The global null hypothesis for RFS was not rejected (P = .1754). The 3-year RFS rates were 24.5% (95% CI, 17.2–32.6), 20.8% (95% CI, 12.6–30.5) and 31.5% (95% CI, 18.1–45.9) for the total population, tecemotide and placebo, respectively.
Figure 2.
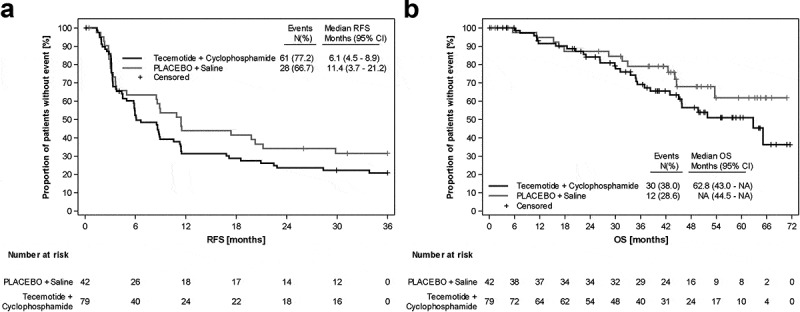
Kaplan-Meier analyses of the ITT population. A) Recurrence-free survival. B) Overall survival
Median OS was 65.1 months (95% CI 45.9-NA) in the total ITT population, 62.8 months (95% CI, 43.0-n.e.) in the tecemotide arm and not yet reached in the placebo arm at the time of analysis. There was no significant difference in OS between treatment arms (P = .2141). The estimated 3-year OS rate was 73% (95% CI, 63.2–80.5), 69.1% (95% CI, 56.1–78.9) and 79.1% (95% CI, 62.5–89.0) for the total population, tecemotide and placebo, respectively.
PP analysis revealed no between-arm differences with respect to RFS and OS according to stratified log-rank tests with stratification factor resection (OS: P = .1004, two-sided; RFS: P = .0741, two-sided) (Supplement 2). Cox proportional hazards regression models revealed no significant effect of treatment on RFS or OS neither with nor without adjusting for potential exploratory variables (Supplement 3). The variables number of resected metastases and resection status showed the most pronounced effect on RFS in multivariate models, with HRs of 2.85 (>5 vs ≤5) and 2.52 (R1 vs R0) and P values of 0.0026 (≥5 vs <5) and 0.0048 (R1 vs R0) respectively (Supplement 4).
Subgroups according to MUC1 expression levels (low, medium, high) were analyzed for RFS and OS for both treatment arms (Figure 3). Cox proportional hazards regression with MUC1 and treatment as covariates revealed neither significant differences between treatment arms (ITT) nor between different levels of MUC1 staining regarding RFS and OS. Also, for the PP population no significant differences were observed (Supplement 5). Subgroup analyses for RFS and OS are presented in Figure 4.
Figure 3.
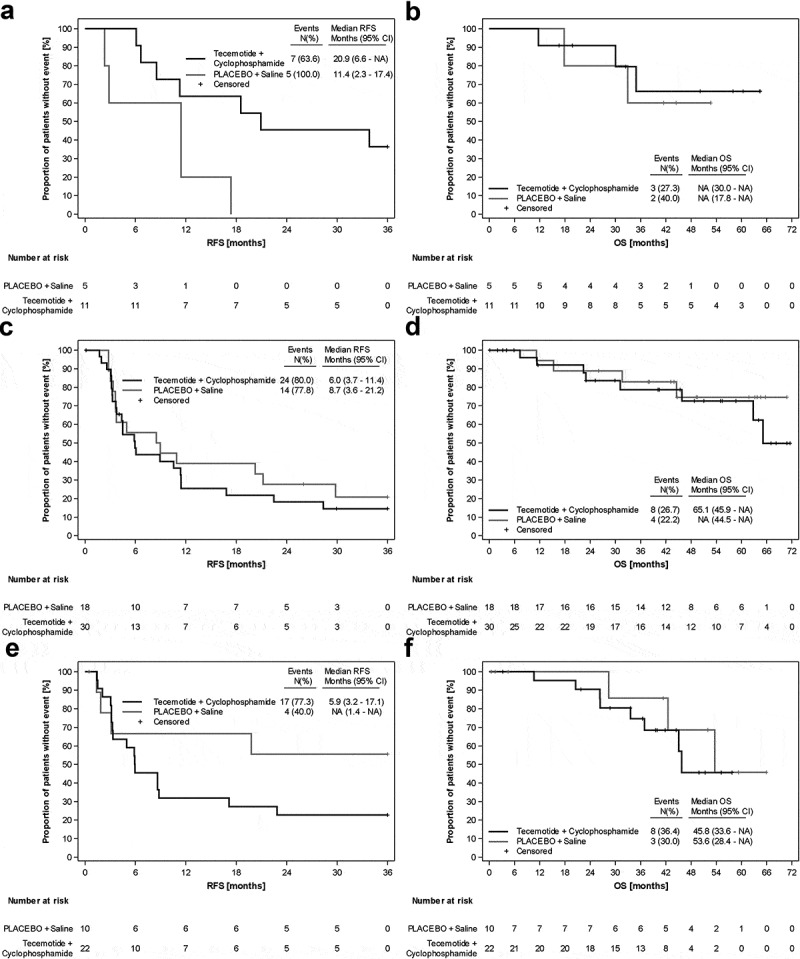
Kaplan-Meier analyses of the ITT population, according to MUC1 expression. A) RFS MUC1 low B) OS MUC1 low C) RFS MUC1 medium D) OS MUC1 medium E) RFS MUC1 high F) OS MUC1 high
Figure 4.
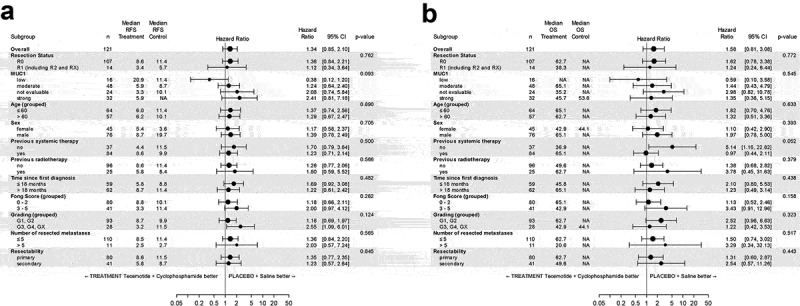
Forest Plots for the ITT population. A) Recurrence-free survival and B) Overall survival. P-values are for testing interaction of treatment with subgroup, thus describing difference in treatment effects across subgroups
Safety
Median vaccination durations were 25 weeks and 49 weeks in the tecemotide and placebo arm, respectively. The corresponding median number of vaccinations was 11 (range 3–27) and 15 (range 2–27). Median FU was 40.1 months (tecemotide arm: 36.5 months, placebo arm: 43.6 months).
An overview of TEAEs can be found in Table 2. TEAEs were recorded for 90.1% of patients (87.3% tecemotide arm, 95.2% placebo arm). Most events were of grade 1/2. Grade 3/4 events were reported for 28.1% of patients (29.1% tecemotide arm, 26.2% placebo arm). The most common TEAEs in the tecemotide and the placebo arm were nausea (27.8%, 19.0%), injection site reaction (29.1%, 14.3%), fatigue (22.8%, 19.0%), diarrhea (17.7%, 16.7%), viral upper respiratory tract infection (17.7%, 9.5%), and abdominal pain (10.1%, 16.7%), respectively. Most frequent grade 3/4 TEAEs were diarrhea, anemia, back pain, injection site reaction, and blood uric acid increased (2 events each, 2.5%) in the tecemotide arm and diarrhea and cholestasis (2 events each, 4.8%) in the placebo arm Table 3. For more details see supplement 6.
Table 2.
Overview of treatment-emergent adverse events (TEAEs) by treatment group – safety population
| TEAE n (%) | Tecemotide + Cyclophosphamide (n = 79) | Placebo + Saline (n = 42) |
|---|---|---|
| Any grade, n (%) | 69 (87.3%) | 40 (95.2%) |
| Grade 3/4, n (%) | 23 (29.1%) | 11 (26.2%) |
| Treatment-related, n (%) | 38 (48.1%) | 20 (47.6%) |
| Treatment-related Grade 3/4, n (%) | 4 (5.1%) | 0 (0%) |
| Fatal TEAE, n (%) | 2 (2.5%) | 0 (0%) |
TEAEs are represented for the number (%) of patients for the two treatment arms respectively (each patient is counted once). Grading according to NCI-CTCAE Version 3.0. Abbreviations: NCI-CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events, TEAE: treatment-emergent adverse event
Table 3.
Incidence and severity of treatment-emergent adverse events (TEAEs) by treatment group and by severity – safety population; Grade1/2 events are listed if ≥10% of patients were affected, grade 3/4 events are listed if at least two patients were affected
| TEAE grade 1/2 (≥10% patients) |
||
|---|---|---|
| Preferred term | Tecemotide + Cyclophosphamide n = 79 |
PLACEBO + Saline n = 42 |
| Nausea | 22 (27.8%) | 8 (19.0%) |
| Injection site reaction | 21 (26.6%) | 6 (14.3%) |
| Fatigue | 18 (22.8%) | 8 (19.0%) |
| Viral upper respiratory tract infection | 14 (17.7%) | 4 (9.5%) |
| Diarrhea | 12 (15.2%) | 5 (11.9%) |
| Abdominal pain | 8 (10.1%) | 7 (16.7%) |
| Hypertension | 7 (8.9%) | 5 (11.9%) |
| Rash | 6 (7.6%) | 5 (11.9%) |
| Arthralgia | 5 (6.3%) | 5 (11.9%) |
| Pruritus | 3 (3.8%) | 6 (14.3%) |
| Flatulence | 2 (2.5%) | 6 (14.3%) |
| Vomiting |
3 (3.8%) |
6 (14.3%) |
| |
TEAE grade 3/4 (≥2 patients) |
|
| Diarrhea | 2 (2.5%) | 2 (4.8%) |
| Cholestasis | 1 (1.3%) | 2 (4.8%) |
| Injection site reaction | 2 (2.5%) | - |
| Back pain | 2 (2.5%) | - |
| Anemia | 2 (2.5%) | - |
| Blood uric acid increased | 2 (2.5%) | - |
| Blood bilirubin increased | 1 (1.3%) | 1 (2.4%) |
| Gastritis | 1 (1.3%) | 1 (2.4%) |
| Intestinal perforation | 1 (1.3%) | 1 (2.4%) |
| Deep vein thrombosis | 1 (1.3%) | 1 (2.4%) |
TEAEs are represented for the number (%) of patients for the two treatment arms respectively. Grading according to NCI-CTCAE Version 3.0., sorted by CTC grades 1/2 and grade 3/4. Patients can be in more than one preferred term category, the highest grade for each patient is counted. Injection site reaction was defined as any event having a preferred term that equals to injection site erythema, injection site reaction, injection site swelling, injection site induration, injection site rash, procedural pain, injection site nodule, injection site pain, injection site pruritus, vaccination site nodule, injection site hematoma or injection site warmth. Abdominal pain was defined as any event having a preferred term that equals to abdominal pain, upper abdominal pain or lower abdominal pain. Intestinal perforation was defined as any event having a preferred term that equals to small intestinal perforation or large intestine perforation. Rash was defined as any event having a preferred term that equals to rash, acne, dermatitis acneiform, rash maculopapular or rash vesicular. Hypertension was defined as any event having a preferred term that equals to hypertension or blood pressure increased. Pruritus was defined as any event having a preferred term that equals to pruritus or pruritus generalized. Deep vein thrombosis was defined as any event having a preferred term that equals to deep vein thrombosis or subclavian vein thrombosis. Abbreviations: NCI-CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events, TEAE: treatment-emergent adverse event
TEAEs considered by the investigator as possibly related to study treatment (tecemotide+CP or placebo+NS) occurred in 48.1% of patients receiving tecemotide and 47.6% of patients receiving placebo, with possibly treatment-related grade 3/4 TEAEs occurring in 5.1% and none of the patients, respectively. In the tecemotide arm, serious TEAE and treatment-related serious TEAE were reported for 27.8% and 5.1% of patients, respectively. In the placebo arm, serious TEAEs were recorded for 31.0% of patients. Most frequent treatment-related (tecemotide+CP) adverse events reported in the tecemotide arm were injection site reaction (25.3%), nausea (16.5%), fatigue (8.9%), and diarrhea (5.1%), in the placebo arm injection site reaction (11.9%), pruritus (9.5%), and diarrhea (7.1%).
Two deaths were reported as TEAEs in the tecemotide arm. One death due to Merkel cell carcinoma was assessed by the investigator as being potentially related to vaccination but rated as not suspected to be related to study drug by the sponsor considering that cancer patients are at an increased risk for secondary malignancies (one patient from the placebo arm developed prostate cancer, which was assessed as not related to study medication, more than two years after the first vaccination). Another patient treated with tecemotide died of respiratory failure, considered not related to tecemotide by both the investigator and the sponsor.
TEAEs of special interest observed in the tecemotide arm were thrombocytopenia (grade 1) in two patients and alkaline phosphatase increased (grade 3) in one patient. One event of thrombocytopenia was assessed as related to the study drug. In the placebo arm, TEAEs of special interest were thrombocytopenia (grade 1 and grade 2, 1 patient each), alkaline phosphatase increased (grade 2, 1 patient) and AST increased (grade 3, 1 patient), which were all assessed as not related to the study drug.
Discussion
The LICC study failed to demonstrate a benefit of tecemotide compared with placebo for the co-primary endpoints RFS and 3-year OS rate in mCRC patients with LLD after resection of CRLM. Remarkably long survival times were observed in both arms which may partly result from favorable baseline patient and tumor characteristics (median age 60 years, 70% ECOG 0, almost 90% R0 resected, >60% with Fong score 0–2). The work by Fong et al.25 attributed median survival times of 74, 51, and 47 months to patients with Fong score classifications of 0, 1, and 2 after resection of liver metastasis.
No standard guidelines exist for the surveillance in stage IV mCRC and especially in patients after secondary hepatic resection. Therefore, in clinical routine surveillance is defined by the treating oncologist. In LICC, clinical evaluations were conducted regularly throughout the whole treatment period, with evaluations every 12 weeks during the maintenance treatment period for two years, including physical examination, ECOG performance status, vital signs, and laboratory. CT scans or MRI measurements were carried out alternating with US measurements every six weeks. This close surveillance may have contributed to the beneficial clinical outcome.
The unexpected better, though not significant outcome for the placebo arm may in part be explained by slightly more favorable patient and tumor characteristics (younger age, higher percentage with ECOG 0 and primary resection). However, patients in the tecemotide arm presented with less metastases and a lower portion with a high-risk Fong score. Moreover, rectal carcinomas were less frequent in the placebo arm.
A potential influence of placebo composition on the study outcome is rated as unlikely. The placebo contained the same carrier lipid matrix as the vaccine but lacked MPLA and MUC1 lipopeptide BLP25. The absence of RAS and BRAF mutation status information may not allow us to see a molecular bias between treatment arms.
Alkylating agents in general, especially cyclophosphamide, can elicit an antitumor immune response in the context of active or adoptive immunotherapy by directly affecting the activity of T lymphocytes and natural killer cells as well as by reducing the number of regulatory T cells (Treg) and their functionality. Cyclophosphamide-induced Treg depletion may trigger a clinically relevant boost in antitumor immunity.26–29 Thus, low-dose cyclophosphamide as administered in the tecemotide arm may have had an effect on the survival outcomes reported in the LICC trial.
Resection status had the most pronounced impact on RFS in Cox proportional hazards regression analysis. This is in accordance with various publications that have listed resection status among the most relevant prognostic factors for mCRC patients with resected CRLM,30–33 and assigned an influence on long-term survival.25 While MUC1 expression status has been described as an indicator of poor prognosis for mCRC patients34-36 and as a predictor of RFS and OS,37 the assessment of its role remains controversial.38–40 The LICC study did not confirm an association between MUC1 expression status and efficacy outcomes.
Many TEAEs assessed as related to study medication concerned injection site reactions and flu-like symptoms, which is consistent with reported safety analyses for the tecemotide formulation.20,41,42
Survival times in LICC equal those of primarily resected patients treated with peri- or postoperative chemotherapy10,11 and are longer than those for secondarily resected patients in the previously published EORTC and FFCD trials.12,13 This may partly be explained by the inclusion of both primarily (66%) and secondarily (34%) resected patients. Naturally, such comparisons are hampered by differences in baseline characteristics (LICC: median age of 60 years; <5 resected metastases 86%, 5–10 resected metastases: 11.6%; EORTC: median age 63 years, up to 4 metastases; FFCD: 1–3 metastases 95.3%).
It is important to consider the limitations of the LICC trial, which was designed as a signal finding study. Thus, all statistical analyses have to be considered as explorative. The relatively high fraction of censored patients and the low number of patients in subgroup analyses limit the value of the Kaplan-Meier survival curves. At study closure, a considerable fraction of patients (45%) was alive. Thus, OS and RFS data must be interpreted with caution. More mature data may be gained in a future long-term analysis. Results from a translational research program on immuno-monitoring parameters that accompanied the LICC trial will be published separately and may provide further insight.
The repeated failure of immunotherapeutic approaches in colorectal cancer, either of vaccination strategies43 or checkpoint inhibitors44 is not well understood. More translational data are urgently needed to enable us to better serve our patients. Trials combining vaccination therapy and checkpoint inhibitors may be an option for future trials.
Conclusion
The LICC trial failed to meet its primary endpoint of significantly improving RFS and 3-year OS rate with tecemotide. MUC1 expression was not associated with the outcome. Nevertheless, patients in both arms showed a better OS compared to historical controls. Next to favorable patient and tumor characteristics, more selective surgery and improved resection techniques due to later study conduct as well as a close surveillance program, which lead to early re-treatment in the case of detected recurrence, may have positively influenced patient survival.
Supplementary Material
Acknowledgments
The authors thank all patients, physicians, and study teams participating in LICC. We thank Dr. Victoria Smith-Machnow (iOMEDICO) for project management, Dr. Annett Maderer, and Dr. Arno Schad for immunohistochemical staining for MUC1 expression analysis of tumor tissue and Peter Eggleton (Merck KGaA, Darmstadt, Germany) for counseling and support. The authors thank Dr. Karin Potthoff and Dr. Bettina Kinkel (iOMEDICO) for the preparation of the manuscript.
The LICC study was designed, managed, and analyzed by the University of Mainz Medical Center, Department of Gastroenterology and iOMEDICO and received financial support from Merck KGaA, Darmstadt, Germany.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Funding Statement
This work was supported by the Merck KGaA [N/A].
Disclosure of interest
F. Kullmann, M. Vöhringer, M. Geissler, H. Bernhard, M.R. Schön, and I. Schmidtmann declare no conflicts of interest. C.C. Schimanski received research funding from Merck KGaA and travel expenses from Janssen-Cilag GmbH; he declares stock ownership of Johnson&Johnson and BioNtech. S. Kasper received research funding from BMS, Roche, Merck Serono, Celgene, Lilly; Honoraria from Merck Serono, BMS, Amgen, Roche, Servier, Sanofi Aventis, AstraZeneca, MSD, and Lilly; declares a consulting/advisory role for Merck Serono, BMS, Amgen, Roche, Servier, Sanofi Aventis, AstraZeneca, MSD, Bayer, and Lilly; and received travel expenses from Roche, BMS, Servier, Merck Serono, Amgen, and Lilly. S. Hegewisch-Becker received research funding from Roche, MDS, Bristol-Myers Squibb; and declares a consulting/advisory role for Merck, Amgen, and Pierre-Fabre. J. Schröder declares a consulting/advisory role for Celgene, Amgen, BMS, Clovis Oncology GmbH, Boehringer Ingelheim, Roche, Novartis, MSD, and AOP. F. Overkamp received honoraria from Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Chugai, Celgene, Eisai, Gilead, Ipsen, Janssen, Merck, MSD, Novartis, Novo Nordisk, Rieurtec, Servier, and Shire; he declares a consulting/advisory role for Amgen, Bayer, BMS, Boehringer, Cellex, Gilead, Hexal, MSD, Novartis, Rieurtec, Roche, Tesaro, and Teva; he declares stock ownership of Onkowissen.de GmbH. WO Bechstein received honoraria from Astellas, Chiesi, Falk Foundation, Gore Deutschland, Medac GmbH, MCI Academy, Novartis, Sanofi Genzyme, Sirtex; he declares a consulting/advisory role for Astellas and Novartis and participated in a speakers’ bureau for Integra. R. Öllinger received research funding from Sanofi, declares a consulting/advisory role for Novartis and Sanofi, and received travel expenses from Astellas. The institution of F. Lordick received research funding by Bristol-Myers Squibb; F. Lordick received honoraria from Lilly, Merck Sharp & Dohme, Bristol-Myers Squibb, AstraZeneca, Elsevier, BioNTech AG, SERVIER, Infomedica, Merck KGaA, Roche, and Medscape; he declares a consulting/advisory role for Lilly, Merck Sharp & Dohme, Bristol-Myers Squibb, Astellas Pharma, SERVIER, Zymeworks, Amgen, and Beigene; he received travel expenses from Bristol-Myers Squibb and Lilly. V. Heinemann received research funding by Merck, Roche, Amgen, Sirtex, Servier, Celgene, Boehringer-Ingelheim, and Shire; he received honoraria from Merck, Roche, Celgene, Amgen, Sanofi, Lilly, Sirtex, Boehringer-Ingelheim, Taiho, and Servier; he declares a consulting/advisory role for Merck, Roche, Amgen, Sanofi, Sirtex, Servier, Celgene, Boehringer-Ingelheim, Halozyme, MSD, and BMS; he received travel Expenses from Merck, Roche, Amgen, Sirtex, Servier, Shire, MSD, and BMS. A. Schulz-Abelius received travel expenses from Lilly and the DGHO. R. Greil received research funding by Celgene, Roche, Merck, Takeda, AstraZeneca, BMS, MSD, Amgen, Novartis, Sandoz, Abbvie, Daiichi S., Gilead, and Janssen; he declares honoraria, a consultant/advisory role and travel expenses from Celgene, Roche, Merck, Takeda, AstraZeneca, BMS, MSD, Amgen, Novartis, Sandoz, Abbvie, Daiichi S., Gilead, and Janssen. P. Galle received research funding by Bayer; he received honoraria from Bayer, BMS, MSD, AstraZeneca, Sirtex, Merck, Lilly, Adaptimmune, Eisai, Roche, Ipsen; he declares a consultant/advisory role for Bayer, BMS, MSD, AstraZeneca, Sirtex, Merck, Lilly, Blueprint, Adaptimmune, Eisai, Roche, and Ipsen; he participated in a speaker’s bureau for Bayer, BMS, AstraZeneca, Sirtex, Lilly, Eisai, Roche, and Ipsen; he received travel expenses from Bayer, BMS, MSD, AstraZeneca, Sirtex, Lilly, Eisai, Roche, and Ipsen. H. Lang declares a consultant/advisory role for the advisory board Humedics and received lecture fees from several companies. The institution of M. Möhler received research funding from Amgen, Leap Therapeutics, Merck Serono, Jennerex, AstraZeneca, and MSD; M. Möhler received honoraria from Taiho Pharmaceutical, Lilly/ImClone, Amgen, Roche/Genentech, Merck Serono, MSD Oncology, Bristol-Myers Squibb, AstraZeneca/MedImmune, and Servier; he declares a consultant/advisory role for Bayer, MSD, Merck Serono, Amgen, Taiho Pharmaceutical, Nordic Group, Pfizer, Yakult, Roche, Lilly, and Servier; he received travel expenses from Amgen, Merck Serono, Roche, Bayer, ASCO, German Cancer Society, MSD, and ESMO.
Disclaimers
The content is solely the responsibility of the authors and does not necessarily represent the official views of the financial supporter Merck KGaA, Darmstadt, Germany.
Financial support
The LICC trial was supported by a medical grant and supply of drugs from Merck KGaA, Darmstadt, Germany.
Prior presentation
The study has been presented in part at the American Society of Clinical Oncology Gastrointestinal Cancer Symposium ASCO-GI, January 17-19, 2019, San Francisco CA, the ASCO Annual Meeting, May 31-June 4, 2019, Chicago, Illinois, and the Conference on Visceral Medicine, October 02-05, 2019, Wiesbaden, Germany.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol Off J Eur Soc Med Oncol. 2016;27(8):1386–10. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Colon cancer (Version 4). (2018). https://www.nccn.org. Accessed August 1st, 20. 20. [Google Scholar]
- 3.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Rectal cancer (Version 3). (2018). https://www.nccn.org. Accessed August 1st, 2020. [Google Scholar]
- 4.de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM.. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250(3):440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 5.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D, Française de Chirurgie A. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association française de Chirurgie. Cancer. 1996;77(7):1254–1262. doi:. [DOI] [PubMed] [Google Scholar]
- 6.Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, Marrero AM, Prasad M, Blumgart LH, Brennan MF. Liver resection for colorectal metastases. J Clin Oncol Off J Am Soc Clin Oncol. 1997;15(3):938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 7.Kato T, Yasui K, Hirai T, Kanemitsu Y, Mori T, Sugihara K, Mochizuki H, Yamamoto J. Therapeutic results for hepatic metastasis of colorectal cancer with special reference to effectiveness of hepatectomy: analysis of prognostic factors for 763 cases recorded at 18 institutions. Dis Colon Rectum. 2003;46(10Suppl):S22–31. doi: 10.1097/01.DCR.0000089106.71914.00. [DOI] [PubMed] [Google Scholar]
- 8.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241(5):715–722. discussion 722-724. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rees M, Tekkis PP, Welsh FKS, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247(1):125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 10.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 11.Portier G, Elias D, Bouche O, Rougier P, Bosset J-F, Saric J, Belghiti J, Piedbois P, Guimbaud R, Nordlinger B, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(31):4976–4982. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 12.Folprecht G, Gruenberger T, Bechstein W, Raab H-R, Weitz J, Lordick F, Hartmann JT, Stoehlmacher-Williams J, Lang H, Trarbach T, et al. Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study). Ann Oncol. 2014;25(5):1018–1025. doi: 10.1093/annonc/mdu088. [DOI] [PubMed] [Google Scholar]
- 13.Holch JW, Ricard I, Stintzing S, Fischer von Weikersthal L, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S-E, Heintges T, Lerchenmüller C, et al. Relevance of liver-limited disease in metastatic colorectal cancer: subgroup findings of the FIRE-3/AIO KRK0306 trial. Int J Cancer. 2018;142(5):1047–1055. doi: 10.1002/ijc.31114. [DOI] [PubMed] [Google Scholar]
- 14.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet Lond Engl. 2008;371(9617):1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folprecht G, Gruenberger T, Bechstein WO, Raab H-R, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11(1):38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Shoda J, Kawamoto T, Shinozaki E, Miyahara N, Hotta S, Iizuka Y, Nakahara A, Tanaka N, Yanaka A, et al. Expression of MUC1 recognized by monoclonal antibody MY.1E12 is a useful biomarker for tumor aggressiveness of advanced colon carcinoma. Clin Exp Metastasis. 2004;21(4):321–329. doi: 10.1023/B:CLIN.0000046133.35133.cc. [DOI] [PubMed] [Google Scholar]
- 17.Zhang K, Tang W, Qu X, Guo Q, Inagaki Y, Seyama Y, Abe H, Gai R, Kokudo N, Sugawara Y, et al. KL-6 mucin in metastatic liver cancer tissues from primary colorectal carcinoma. Hepatogastroenterology. 2009;56(93):960–963. [PubMed] [Google Scholar]
- 18.Sangha R, Butts C. L-BLP25: a peptide vaccine strategy in non-small cell lung cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2007;13(15 Pt 2):s4652–4654. doi: 10.1158/1078-0432.CCR-07-0213. [DOI] [PubMed] [Google Scholar]
- 19.Zeng Y, Zhang Q, Zhang Y, Lu M, Liu Y, Zheng T, Feng S, Hao M, Shi H, Singh PK. MUC1 predicts colorectal cancer metastasis: a systematic review and meta-analysis of case controlled studies. PloS One. 2015;10(9):e0138049. doi: 10.1371/journal.pone.0138049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butts C, Murray N, Maksymiuk A, Goss G, Marshall E, Soulières D, Cormier Y, Ellis P, Price A, Sawhney R, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(27):6674–6681. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 21.Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, Nawrocki S, Ciuleanu T-E, Bosquée L, Trigo JM, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(1):59–68. doi: 10.1016/S1470-2045(13)70510-2. [DOI] [PubMed] [Google Scholar]
- 22.Schimanski CC, Mohler M, Schon M, van Cutsem E, Greil R, Bechstein WO, Hegewisch-Becker S, von Wichert G, Vohringer M, Heike M, et al. LICC: L-BLP25 in patients with colorectal carcinoma after curative resection of hepatic metastases–a randomized, placebo-controlled, multicenter, multinational, double-blinded phase II trial. BMC Cancer. 2012;12(1):144. doi: 10.1186/1471-2407-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moehler M, Maderer A, Ehrlich A, Foerster F, Schad A, Nickolay T, Ruckes C, Weinmann A, Sivanathan V, Marquardt JU, et al. Safety and efficacy of afatinib as add-on to standard therapy of gemcitabine/cisplatin in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, phase I trial with an extensive biomarker program. BMC Cancer. 2019;19(1):55. doi: 10.1186/s12885-018-5223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remmele W, Stegner HE. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue]. Pathol. 1987;8:138–140. [PubMed] [Google Scholar]
- 25.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–318. discussion 318-321. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scurr M, Pembroke T, Bloom A, Roberts D, Thomson A, Smart K, Bridgeman H, Adams R, Brewster A, Jones R, et al. Low-dose cyclophosphamide induces antitumor T-cell responses, which associate with survival in metastatic colorectal cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23(22):6771–6780. doi: 10.1158/1078-0432.CCR-17-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y-J, Fletcher R, Yu J, Zhang L. Immunogenic effects of chemotherapy-induced tumor cell death. Genes Dis. 2018;5(3):194–203. doi: 10.1016/j.gendis.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother CII. 2007;56(5):641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kan S, Hazama S, Maeda K, Inoue Y, Homma S, Koido S, Okamoto M, Oka M. Suppressive effects of cyclophosphamide and gemcitabine on regulatory T-cell induction in vitro. Anticancer Res. 2012;32:5363–5369. [PubMed] [Google Scholar]
- 30.Allard MA, Adam R, Giuliante F, Lapointe R, Hubert C, Ijzermans JNM, Mirza DF, Elias D, Laurent C, Gruenberger T, et al. Long-term outcomes of patients with 10 or more colorectal liver metastases. Br J Cancer. 2017;117(5):604–611. doi: 10.1038/bjc.2017.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tranchart H, Chirica M, Faron M, Balladur P, Lefevre LB, Svrcek M, de Gramont A, Tiret E, Paye F. Prognostic impact of positive surgical margins after resection of colorectal cancer liver metastases: reappraisal in the era of modern chemotherapy. World J Surg. 2013;37(11):2647–2654. doi: 10.1007/s00268-013-2186-3. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Liu YF, Cheng Y, Yi DH, Li P, Song WQ, Fu DZ, Wang X. Prognosis of colorectal cancer with liver metastasis: value of a prognostic index. Braz J Med Biol Res. 2010;43(11):1116–1122. doi: 10.1590/S0100-879X2010007500103. [DOI] [PubMed] [Google Scholar]
- 33.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ, Cameron JL. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235(6):759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duncan TJ, Watson NFS, Al-Attar AH, Scholefield JH, Durrant LG. The role of MUC1 and MUC3 in the biology and prognosis of colorectal cancer. World J Surg Oncol. 2007;5(1):31. doi: 10.1186/1477-7819-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldus SE, Mönig SP, Huxel S, Landsberg S, Hanisch F-G, Engelmann K, Schneider PM, Thiele J, Hölscher AH, Dienes HP. MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin Cancer Res Off J Am Assoc Cancer Res. 2004;10(8):2790–2796. doi: 10.1158/1078-0432.CCR-03-0163. [DOI] [PubMed] [Google Scholar]
- 36.Baldus SE, Mönig SP, Hanisch F-G, Zirbes TK, Flucke U, Oelert S, Zilkens G, Madejczik B, Thiele J, Schneider PM, et al. Comparative evaluation of the prognostic value of MUC1, MUC2, sialyl-Lewis(a) and sialyl-Lewis(x) antigens in colorectal adenocarcinoma. Histopathology. 2002;40(5):440–449. doi: 10.1046/j.1365-2559.2002.01389.x. [DOI] [PubMed] [Google Scholar]
- 37.Khanh DT, Mekata E, Mukaisho K, Sugihara H, Shimizu T, Shiomi H, Murata S, Naka S, Yamamoto H, Endo Y, et al. Transmembrane mucin MUC1 overexpression and its association with CD10+ myeloid cells, transforming growth factor-β1 expression, and tumor budding grade in colorectal cancer. Cancer Sci. 2013;104(7):958–964. doi: 10.1111/cas.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lillehoj EP, Lu W, Kiser T, Goldblum SE, Kim KC. MUC1 inhibits cell proliferation by a beta-catenin-dependent mechanism. Biochim Biophys Acta. 2007;1773(7):1028–1038. doi: 10.1016/j.bbamcr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Díaz Del Arco C, Garré P, Molina Roldán E, Lorca V, Cerón Nieto MÁ, Fernández Aceñero MJ. MUC1 expression in colorectal carcinoma: clinicopathological correlation and prognostic significance. Rev Espanola Patol Publicacion Of Soc Espanola Anat Patol Soc Espanola Citol. 2018;51(4):204–209. doi: 10.1016/j.patol.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Betge J, Schneider NI, Harbaum L, Pollheimer MJ, Lindtner RA, Kornprat P, Ebert MP, Langner C. MUC1, MUC2, MUC5AC, and MUC6 in colorectal cancer: expression profiles and clinical significance. Virchows Arch Int J Pathol. 2016;469(3):255–265. doi: 10.1007/s00428-016-1970-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butts C, Murray RN, Smith CJ, Ellis PM, Jasas K, Maksymiuk A, Goss G, Ely G, Beier F, Soulières D. A multicenter open-label study to assess the safety of a new formulation of BLP25 liposome vaccine in patients with unresectable stage III non-small-cell lung cancer. Clin Lung Cancer. 2010;11(6):391–395. doi: 10.3816/CLC.2010.n.101. [DOI] [PubMed] [Google Scholar]
- 42.Butts C, Anderson H, Maksymiuk A, Vergidis D, Soulières D, Cormier Y, Davis M, Marshall E, Falk M, Goss G. Long-term safety of BLP25 liposome vaccine (L-BLP25) in patients (pts) with stage IIIB/IV non-small cell lung cancer (NSCLC). J Clin Oncol. 2009;27(15_suppl):3055. doi: 10.1200/jco.2009.27.15_suppl.3055. [DOI] [Google Scholar]
- 43.Cunningham D, Salazar R, Sobrero A, Ducreux MP, Van Cutsem E, Scheithauer W, Tournigand C, Molnar V, Starke M, Baumann M, et al. LBA33Lefitolimod vs standard of care (SOC) for patients with metastatic colorectal cancer (mCRC) responding to first-line standard treatment: results from the randomized phase III IMPALA trial. Ann Oncol. 2019;30(Supplement_5):v868–v869. doi: 10.1093/annonc/mdz394.022. [DOI] [Google Scholar]
- 44.Grothey A, Tabernero J, Arnold D, De Gramont A, Ducreux MP, O’Dwyer PJ, Van Cutsem E, Bosanac I, Srock S, Mancao C, et al. LBA19Fluoropyrimidine (FP) + bevacizumab (BEV) + atezolizumab vs FP/BEV in BRAFwt metastatic colorectal cancer (mCRC): findings from Cohort 2 of MODUL – a multicentre, randomized trial of biomarker-driven maintenance treatment following first-line induction therapy. Ann Oncol. 2018;29(suppl_8): doi: 10.1093/annonc/mdy424.020 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


