ABSTRACT
Background
Symptomatic gallstones cause high financial and disease burden for public health systems. The combined role of diet and other lifestyle factors has not been studied so far.
Objectives
We aimed to investigate the association between an a priori defined healthy lifestyle score (HLS, including healthy diet, moderate alcohol and regular coffee intakes, never smoking, physical activity, and normal weight) and the risk of symptomatic gallstone disease, and to estimate the proportion of cases potentially preventable by lifestyle modification.
Methods
We followed 60,768 women from the Nurses’ Health Study (NHS) and 40,744 men from the Health Professionals Follow-up Study (HPFS), both ongoing prospective cohort studies, from baseline (1986) until 2012. Symptomatic gallstone disease was self-reported and validated by review of medical records. The association between the HLS and the risk of symptomatic gallstone disease was investigated using Cox proportional hazards regression.
Results
During 1,156,079 and 769,287 person-years of follow-up, respectively, 6946 women and 2513 men reported symptomatic gallstone disease. Comparing 6 with 0 points of the HLS, the multivariable HR of symptomatic gallstone disease was 0.26 (95% CI: 0.15, 0.45) for women, and 0.17 (95% CI: 0.07, 0.43) for men. For individual lifestyle factors, multivariable and mutually adjusted partial population attributable risks (women and men) were 33% and 23% for BMI <25 kg/m2, 10% and 18% for ≥2 cups of coffee per day, 13% and 7% for moderate alcohol intake, 8% and 11% for a high Alternate Healthy Eating Index 2010, 9% and 5% for being physically active, and 1% and 5% for never smoking. The full population attributable risk percentage for all factors combined was 62% and 74%, respectively.
Conclusions
Findings from these large prospective studies indicate that adopting a healthy lifestyle, especially maintaining a healthy weight, can help to prevent a considerable proportion of symptomatic gallstone diseases.
Keywords: gallstones, cholecystectomy, cholelithiasis, lifestyle factors, diet, epidemiology
Introduction
Gallstone disease is a common and costly disease that occurs in 10–25% of adults in Westernized countries (1–3). Despite a low mortality rate of symptomatic gallstone disease, serious complications (e.g., cholecystitis, cholangitis, and pancreatitis) and the recurrence of gallstones—even after cholecystectomy (gallbladder removal)—have been reported (4, 5). More than 600,000 hospitalizations, and ∼1.8 million ambulatory care visits result in more than USD 6.5 billion/y attributed to symptomatic gallstone disease and its treatment (1–3, 6). Furthermore, gallstones have been associated with mortality and increased risks of major chronic diseases, such as diabetes, cardiovascular diseases, and cancer, most likely due to shared pathways and common risk factors (7–10). As the incidence of symptomatic gallstone disease is escalating with a shift toward younger age at onset, so will gallstone-related complications and related diseases (11).
Abdominal obesity (12), smoking (13), and dietary factors such as saturated fat, trans fat, and heme iron (14–16) have been identified as risk factors for symptomatic gallstone disease. However, physical activity (17), moderate alcohol drinking (18), coffee consumption (19), and the intakes of some plant foods, such as nuts, fruits, and vegetables, as well as fiber and polyunsaturated and monounsaturated fats (20–23), have been associated with lower gallstone risk. In addition, our research group has recently shown that healthy dietary patterns expressed by 3 diet quality scores—Alternate Healthy Eating Index (AHEI-2010), the Dietary Approaches to Stop Hypertension diet score, and the alternate Mediterranean diet score—were inversely associated with risk of symptomatic gallstone disease in men (24).
Yet, little is known about the combined effects on risk of symptomatic gallstone disease and the number of cases that could be prevented by adopting a healthy lifestyle. Therefore, we investigated the association between a healthy lifestyle score (HLS) and risk of symptomatic gallstone disease in 2 large prospective cohort studies.
Methods
Study population
The Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS) are ongoing prospective cohort studies among US health professionals. The NHS was established in 1976 with 121,701 female registered nurses aged 30 to 55 y (25, 26). The HPFS comprised 51,529 men aged 40 to 75 y at time of recruitment (1986). Follow-up questionnaires were mailed biennially to update medical history, lifestyle, and other health-related data. Validated semiquantitative FFQs were mailed every 4 y to assess habitual dietary intake. Follow-up rates have exceeded 94% cumulatively, and the studies were approved by the Institutional Review Board at the Harvard T.H. Chan School of Public Health and the Brigham and Women's Hospital.
For this analysis, data from 1986 were used as baseline for both NHS (n = 73,580) and HPFS (n = 51,529), because nutritional data were first assessed with the same expanded FFQ. We excluded participants with missing exposure information and prevalent/asymptomatic gallstone disease. Because a diagnosis of diabetes might cause changes in lifestyle habits, and diabetes and insulin resistance have been linked to development of gallstones, we further excluded men and women with a history of diabetes at baseline and prior to each biennial follow-up cycle (8, 27, 28). Our final study populations comprised 60,768 women (NHS) and 40,744 men (HPFS) (Supplemental Figure 1).
Assessment of exposure and covariates
Medication use, medical history (including diabetes, hypercholesterolemia, and hypertension), and lifestyle and other health-related data were measured at baseline and biennially using self-administered questionnaires.
We collected detailed information on smoking history at baseline (29). On each biennial questionnaire participants provided updated information about their smoking status.
BMI was calculated by dividing self-reported weight (in kilograms, updated biennially) by the square of height (in meters: kg/m2). Validity of anthropometric measures has been tested by comparing self-reports with technician-measured data (Pearson correlation coefficient for weight = 0.97 in both men and women) (30).
Physical activity was calculated as metabolic equivalent hours per week (MET-h/wk) from questions on a variety of recreational and leisure-time activities (e.g., running) (17, 31). Our physical activity questionnaires were validated previously (32, 33).
Habitual dietary information during the previous year (including alcohol and coffee intakes) was obtained at baseline and every 4 y using semiquantitative FFQs including 131–148 food items. The 9 answers that covered consumption quantity ranged from “never or less than once per month” to “more than six times per day” and were based on US standard portion sizes. The FFQs have been validated by comparisons with multiple dietary records and biochemical indicators (34, 35). The observed Pearson correlation coefficients between the FFQ and the diet records ranged from 0.13 for nuts (component of AHEI-2010) to 0.90 for coffee in men (mean = 0.63), and from an average of 0.35 for legumes (component of AHEI-2010) to a Spearman correlation coefficient of 0.90 for alcohol in women (mean = 0.55) (34–36).
Definition of the HLS
Six factors were considered to define the HLS based on their previous associations with symptomatic gallstone disease: 1) following a healthy diet (24); 2) consuming a moderate amount of alcohol (18); 3) having a normal body weight (12); 4) being physically active (17); 5) consuming coffee regularly (19); and 6) being a nonsmoker (13).
A healthy diet was defined using the AHEI-2010. We previously observed an inverse association between the AHEI-2010 score and the risk of symptomatic gallstone disease in men (24). The AHEI-2010 (37, 38) is a diet quality score developed to measure adherence to the USDA Dietary Guidelines for Americans. The 2010 version included 7 favorable foods [vegetables (except potatoes), fruits, nuts and legumes, whole grains, ω-3 (n–3) fatty acids, PUFAs, and moderate alcohol consumption] and 4 unfavorable foods/nutrients (red and processed meat, sodium, sugar-sweetened beverages, and trans fat). We excluded alcohol from the AHEI-2010 score and used it as a separate component of the HLS. A scoring system based on predefined cut-points assigned 0 to 10 points for each food component to the AHEI-2010, so that the total score could range from 0 to 100 possible points (higher scores representing a healthier diet). For the present analysis, participants in the upper 2 quintiles of the cohorts’ AHEI-2010 were considered to be consuming a healthy diet. We used the cut-points of the original AHEI-2010 score to define moderate alcohol consumption: 0.5–2 drinks/d for men and 0.5–1.5 drinks/d for women (37). This equals 7–28 g alcohol for men and 7–21 g alcohol for women (US standard drink = 14 g alcohol). Coffee consumption of ≥2 cups of caffeinated coffee per day [we did not include decaffeinated coffee because there is insufficient evidence supporting a role of decaffeinated coffee in the formation of gallstones (19)], physical activity of ≥150 vigorous-intensity or ≥300 moderate-intensity minutes per week [equivalent to 15 MET-h/wk, defined as high activity level by the Physical Activity Guidelines for Americans (39)], having a normal weight based on BMI between 18.5 and 24.9 (40), and being a never-smoker each contributed 1 point to the HLS, which could range from 0 to 6 points.
Ascertainment of symptomatic gallstone disease
At baseline and on each biennial follow-up questionnaire, participants were asked whether they had undergone a cholecystectomy or had been diagnosed as having gallstones by a physician. Participants were also asked whether the gallstone diagnosis had been confirmed by radiographic procedures or surgery and whether their gallstones were symptomatic. From 1988 (NHS) or 2002 (HPFS) onwards participants only reported whether they had undergone a cholecystectomy. To verify self-reports of surgical cholecystectomy and diagnosed but unremoved gallstones, a random sample of 441 medical records of men who reported a cholecystectomy or gallstones were reviewed. Of these, the diagnosis was confirmed in all but 5 (99%). Medical chart review confirmed all self-reported symptoms. Furthermore, a random sample of 50 women was drawn, 43 of whom responded and on their consent form reiterated their initial self-report of cholecystectomy. In addition, for these 43 women, 36 medical records could be obtained, all confirming the surgery. Our primary outcome was incident symptomatic gallstone disease with or without performed cholecystectomy. Our secondary outcome was symptomatic gallstone disease with performed cholecystectomy. For more detail, refer to our previous publications (12, 17, 31). For women, the primary and secondary outcomes are identical due to the nature of the assessment from 1988 onwards.
Statistical analyses
Person-years of follow-up were calculated for each participant from the age at the baseline questionnaire's return date until the age at symptomatic gallstone diagnosis or cholecystectomy, death, loss to follow-up, or end of the 13th follow-up cycle (2012), whichever came first. We stratified by age (continuous) and time period to finely control for confounding. Covariate information was carried forward from prior questionnaire cycles to replace missing values. For covariates that still had missing data, we created and included missing value indicators in the statistical model. HRs and 95% CIs for the association between the HLS and symptomatic gallstone disease were estimated using Cox proportional hazards regression analyses further adjusted for regular use of statins and thiazide diuretics (yes/no), regular use of nonsteroidal anti-inflammatory drugs (NSAIDs; ≥2 times/wk; yes/no), postmenopausal hormone use (pre-/missing menopause, never used, current user, past user; only in NHS), and history of hypercholesterolemia and hypertension (yes/no). The proportional hazard assumption was validated by including cross-product terms between the HLS and the questionnaire cycle to the multivariable model and performing a Wald test. The assumption was satisfied for the HPFS but violated for the NHS. Therefore, in the NHS we also examined associations for 3 time periods separately: 1986–1994, 1994–2002, and 2002–2012.
Furthermore, we calculated the population attributable risk percentage (PAR%) for all 6 healthy lifestyle factors, separately (partial PAR%) and combined (full PAR%), to estimate the percentage of symptomatic gallstone cases that could be prevented if 1 or all risk factors were eliminated from the analysis population. The PAR% and its 95% CI were calculated using the methods and macro developed by Spiegelman et al. (41). Briefly, HRs from the multivariable Cox models were estimated and combined with the observed prevalence rates of each healthy lifestyle factor or all of them combined, respectively (41).
In our main analysis based on our recent investigation on AHEI-2010 and gallstones (24), exposure represents the intake reported on the most recent FFQ before each follow-up interval (simple update, every 4 y) because our a priori expectation was that the strongest effects would be with relatively short-term induction periods (42). For nondietary covariates, information was updated biennially. In secondary analyses, cumulative average HLS scores were calculated by averaging all available measurements starting from baseline until the beginning of each biennial follow-up interval (42). Cumulative updating reduces variability related to random error and true changes over time and has been used previously to assess the role of long-term exposure in disease risk. We also studied associations including diabetics, and after exclusion of symptomatic gallstone cases who did not undergo cholecystectomy in the HPFS (n = 415).
To investigate the associations between each individual lifestyle factor and the risk of symptomatic gallstone disease separately, we used Cox proportional hazards regression with lifestyle factors being mutually adjusted.
All statistical analyses were conducted using SAS release 9.4 (SAS Institute Inc), and a 2-sided P value <0.05 was considered statistically significant.
Results
In the NHS, we documented 6946 cases of symptomatic gallstone disease during 1,156,079 person-years of follow-up (1986–2012), while 2513 symptomatic gallstone cases were recorded during 769,287 person-years of follow-up (1986–2012) in the HPFS.
Participants with a higher HLS were older than those with lower scores. Consistent with the composition of the score, they were also less likely to smoke, had a lower BMI, a higher coffee intake, and a higher AHEI-2010 than those with a lower HLS (Tables 1 and 2). Furthermore, participants with a higher HLS were considerably more physically active than those with lower scores, especially men. For alcohol intake, the data suggested a linear relation for women and a U-shaped relation for men. Both, men and women with higher scores had less hypercholesterolemia and hypertension, but men and not women with higher scores were more likely to use statins and NSAIDs.
TABLE 1.
Selected age-standardized characteristics according to the healthy lifestyle score of women in the Nurses’ Health Study 1986–20121
| Healthy lifestyle score | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total | 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Person-years | 1,156,079 | 55,378 | 222,523 | 346,862 | 311,695 | 166,479 | 47,327 | 5816 |
| Age, y | 62.2 ± 9.8 | 62.9 ± 9.9 | 62.2 ± 9.9 | 62.0 ± 9.9 | 62.1 ± 9.7 | 62.5 ± 9.6 | 62.6 ± 9.3 | 62.9 ± 9.1 |
| BMI, kg/m2 | 25.8 ± 4.6 | 30.1 ± 4.6 | 28.4 ± 4.8 | 26.2 ± 4.5 | 24.5 ± 3.8 | 23.3 ± 2.8 | 22.7 ± 2.1 | 22.3 ± 1.6 |
| Smoking status | ||||||||
| Never, % | 45.5 | 0.0 | 30.2 | 43.4 | 52.4 | 61.7 | 74.3 | 100.0 |
| Past (≥10 y) | 33.5 | 63.9 | 42.2 | 33.7 | 29.7 | 24.6 | 16.9 | 0.0 |
| Past (<10 y) | 9.5 | 18.8 | 12.8 | 9.7 | 7.9 | 6.6 | 4.7 | 0.0 |
| Current smoking, % | 11.5 | 17.3 | 14.8 | 13.2 | 10.0 | 7.1 | 4.1 | 0.0 |
| Pack-years of smoking2 | 39.9 ± 20.4 | 39.7 ± 21.5 | 41.2 ± 21.1 | 40.7 ± 20.3 | 38.9 ± 19.7 | 36.6 ± 19.2 | 35.2 ± 19.0 | 0.0 |
| Physical activity,3 MET-h/wk | 18.4 ± 22.8 | 4.8 ± 4.1 | 8.1 ± 12.0 | 14.2 ± 18.7 | 22.9 ± 24.8 | 30.9 ± 26.9 | 36.3 ± 28.3 | 39.6 ± 28.9 |
| History of hypercholesterolemia, % | 48.8 | 56.3 | 53.0 | 49.5 | 47.3 | 44.5 | 42.7 | 40.3 |
| History of hypertension, % | 39.5 | 53.6 | 47.8 | 41.5 | 35.6 | 30.7 | 28.0 | 25.7 |
| Current medication and hormone use, % | ||||||||
| NSAIDs4 | 37.9 | 41.2 | 39.4 | 38.1 | 37.6 | 36.0 | 35.5 | 36.3 |
| Thiazide diuretics | 12.8 | 18.0 | 15.7 | 13.4 | 11.6 | 9.9 | 8.7 | 7.5 |
| Statins | 23.1 | 31.0 | 27.1 | 23.7 | 21.4 | 19.1 | 18.4 | 16.7 |
| Postmenopausal hormones | 29.2 | 25.8 | 26.5 | 28.4 | 30.4 | 32.4 | 32.6 | 35.0 |
| Diet | ||||||||
| Total energy intake, kcal/d | 1732 ± 527 | 1694 ± 525 | 1708 ± 531 | 1722 ± 528 | 1736 ± 522 | 1760 ± 525 | 1805 ± 522 | 1838 ± 527 |
| AHEI-2010 score5 | 49.9 ± 11.1 | 42.4 ± 6.7 | 44.7 ± 9.0 | 48.2 ± 10.6 | 52.1 ± 11.0 | 56.0 ± 10.4 | 58.5 ± 8.9 | 60.4 ± 7.2 |
| Coffee intake, servings/d | 1.3 ± 1.4 | 0.4 ± 0.4 | 0.9 ± 1.2 | 1.2 ± 1.4 | 1.5 ± 1.4 | 1.8 ± 1.4 | 2.3 ± 1.1 | 2.7 ± 0.6 |
| Alcohol intake, g/d | 6.1 ± 10.4 | 4.7 ± 1.7 | 4.8 ± 1.2 | 5.5 ± 10.8 | 6.4 ± 10.2 | 7.6 ± 9.0 | 9.7 ± 7.0 | 11.6 ± 3.8 |
| Fruits,5 servings/d | 1.6 ± 1.1 | 1.2 ± 0.9 | 1.3 ± 1.0 | 1.5 ± 1.1 | 1.8 ± 1.2 | 2.0 ± 1.3 | 2.1 ± 1.2 | 2.2 ± 1.2 |
| Vegetables,5 servings/d | 3.5 ± 1.9 | 2.8 ± 1.6 | 3.0 ± 1.7 | 3.3 ± 1.9 | 3.7 ± 2.0 | 4.0 ± 2.1 | 4.3 ± 2.1 | 4.5 ± 2.1 |
| Red and processed meat,5 servings/d | 0.8 ± 0.5 | 0.9 ± 0.6 | 0.9 ± 0.6 | 0.8 ± 0.6 | 0.7 ± 0.5 | 0.7 ± 0.5 | 0.6 ± 0.5 | 0.6 ± 0.4 |
| Nuts,5 servings/d | 0.4 ± 0.5 | 0.3 ± 0.3 | 0.3 ± 0.4 | 0.4 ± 0.5 | 0.5 ± 0.6 | 0.6 ± 0.7 | 0.6 ± 0.7 | 0.7 ± 0.7 |
| Sugar-sweetened beverages,5 servings/d | 1.0 ± 1.0 | 1.2 ± 1.1 | 1.1 ± 1.1 | 1.0 ± 1.0 | 1.0 ± 0.9 | 0.9 ± 0.9 | 0.8 ± 0.9 | 0.8 ± 0.8 |
| Whole grains,5 g/d | 23.7 ± 18.1 | 19.0 ± 15.2 | 20.5 ± 16.3 | 22.7 ± 17.6 | 25.2 ± 18.7 | 27.6 ± 19.3 | 28.1 ± 18.3 | 28.0 ± 16.9 |
| Polyunsaturated fat,5 g/d | 11.5 ± 5.2 | 10.8 ± 4.7 | 11.0 ± 4.9 | 11.3 ± 5.1 | 11.7 ± 5.3 | 12.1 ± 5.7 | 12.6 ± 5.8 | 13.1 ± 6.3 |
| Trans fat,5 g/d | 2.2 ± 1.3 | 2.5 ± 1.4 | 2.4 ± 1.4 | 2.3 ± 1.4 | 2.1 ± 1.3 | 1.9 ± 1.2 | 1.8 ± 1.1 | 1.8 ± 1.0 |
| Marine ω-3 fat,5 mg/d | 253 ± 260 | 180 ± 203 | 201 ± 216 | 234 ± 248 | 273 ± 271 | 316 ± 293 | 346 ± 289 | 376 ± 282 |
| Sodium,5 mg/d | 2218 ± 1021 | 2248 ± 1033 | 2246 ± 1041 | 2236 ± 1040 | 2204 ± 1008 | 2175 ± 994 | 2171 ± 958 | 2161 ± 918 |
Updated information throughout all follow-up periods was used to calculate the mean ± SD for continuous variables and percentages for categorical variables. All characteristics (except for age) and SDs are age-standardized using the following 5 age groups: <49 y, 50–54 y, 55–59 y, 60–64 y, and ≥65 y. MET, metabolic equivalent; NSAID, nonsteroidal anti-inflammatory drug.
Pack-years of smoking are calculated among current smokers.
MET-h/wk from recreational and leisure-time activities are calculated by multiplying the amount of time spent on a specific activity (e.g., running) per week with its energy-expenditure requirements. One MET is defined as the energy expended in sitting quietly.
Regular users are defined as taking medication ≥2 times/wk.
The Alternate Healthy Eating Index 2010 (AHEI-2010) comprises 7 favorable and 4 unfavorable food items, each contributing 0–10 points to the overall score.
TABLE 2.
Selected age-standardized characteristics according to the healthy lifestyle score for men in the Health Professionals Follow-up Study 1986–20121
| Healthy lifestyle score | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total | 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Person-years | 769,287 | 23,007 | 110,180 | 211,751 | 226,521 | 145,333 | 46,143 | 6354 |
| Age, y | 62.3 ± 10.8 | 62.4 ± 10.8 | 61.8 ± 10.8 | 61.9 ± 10.8 | 62.4 ± 10.9 | 62.7 ± 10.8 | 63.1 ± 10.8 | 63.0 ± 10.4 |
| BMI, kg/m2 | 25.8 ± 3.3 | 28.6 ± 3.5 | 27.6 ± 3.3 | 26.5 ± 3.3 | 25.4 ± 3.1 | 24.3 ± 2.6 | 23.6 ± 2.1 | 23.1 ± 1.3 |
| Smoking status | ||||||||
| Never, % | 49.2 | 0.0 | 24.5 | 40.2 | 53.4 | 68.6 | 83.5 | 100.0 |
| Past (≥10 y), % | 38.0 | 69.8 | 53.5 | 44.0 | 36.0 | 25.3 | 13.9 | 0.0 |
| Past (<10 y), % | 6.8 | 16.6 | 11.4 | 8.2 | 5.6 | 3.3 | 1.6 | 0.0 |
| Current, % | 6.1 | 13.6 | 10.5 | 7.6 | 5.0 | 2.7 | 1.0 | 0.0 |
| Pack-years of smoking2 | 37.5 ± 22.7 | 36.3 ± 23.3 | 38.7 ± 22.7 | 38.5 ± 22.7 | 37.1 ± 22.5 | 34.5 ± 22.5 | 31.0 ± 21.1 | 0.0 |
| Physical activity,3 MET-h/wk | 35.9 ± 40.8 | 5.7 ± 4.4 | 16.7 ± 27.2 | 30.1 ± 37.6 | 41.0 ± 42.0 | 49.2 ± 43.8 | 53.7 ± 43.4 | 58.3 ± 43.7 |
| History of hypercholesterolemia, % | 42.0 | 46.3 | 42.3 | 42.8 | 41.5 | 41.0 | 41.3 | 40.7 |
| History of hypertension, % | 34.9 | 45.7 | 41.4 | 37.7 | 33.4 | 29.6 | 26.6 | 23.1 |
| Medication use, % | ||||||||
| NSAIDs4 | 54.7 | 53.6 | 53.5 | 54.8 | 54.5 | 54.7 | 57.8 | 60.1 |
| Thiazide diuretics | 7.2 | 10.1 | 9.1 | 8.0 | 6.6 | 5.7 | 5.3 | 5.1 |
| Statins | 15.6 | 14.9 | 14.4 | 15.6 | 15.5 | 16.1 | 17.1 | 16.6 |
| Diet | ||||||||
| Total energy intake, kcal/d | 1994 ± 622 | 1906 ± 619 | 1959 ± 629 | 1983 ± 629 | 1999 ± 621 | 2018 ± 610 | 2054 ± 606 | 2081 ± 595 |
| AHEI-2010 score5 | 49.9 ± 11.4 | 41.3 ± 7.0 | 43.5 ± 8.8 | 46.7 ± 10.3 | 51.0 ± 11.1 | 55.5 ± 10.9 | 58.5 ± 9.5 | 60.8 ± 7.1 |
| Coffee intake, servings/d | 1.3 ± 1.5 | 0.4 ± 0.4 | 0.9 ± 1.3 | 1.1 ± 1.5 | 1.3 ± 1.5 | 1.5 ± 1.5 | 2.0 ± 1.5 | 2.9 ± 0.9 |
| Alcohol intake, g/d | 11.8 ± 15.3 | 13.4 ± 21.2 | 12.0 ± 19.1 | 11.4 ± 16.7 | 11.4 ± 14.3 | 11.8 ± 11.8 | 14.1 ± 8.5 | 14.9 ± 5.1 |
| Fruits,5 servings/d | 1.7 ± 1.3 | 1.1 ± 0.9 | 1.3 ± 1.1 | 1.5 ± 1.2 | 1.8 ± 1.3 | 2.1 ± 1.5 | 2.2 ± 1.4 | 2.3 ± 1.4 |
| Vegetables,5 servings/d | 3.3 ± 2.0 | 2.6 ± 1.6 | 2.8 ± 1.7 | 3.1 ± 1.9 | 3.4 ± 2.0 | 3.8 ± 2.2 | 4.0 ± 2.1 | 4.2 ± 2.2 |
| Red and processed meat,5 servings/d | 1.1 ± 0.7 | 1.3 ± 0.7 | 1.3 ± 0.7 | 1.2 ± 0.7 | 1.0 ± 0.7 | 0.9 ± 0.7 | 0.8 ± 0.6 | 0.7 ± 0.5 |
| Nuts,5 servings/d | 0.6 ± 0.7 | 0.4 ± 0.4 | 0.4 ± 0.5 | 0.5 ± 0.6 | 0.6 ± 0.7 | 0.7 ± 0.8 | 0.8 ± 0.8 | 0.9 ± 0.9 |
| Sugar-sweetened beverages,5 servings/d | 1.2 ± 1.1 | 1.4 ± 1.2 | 1.3 ± 1.1 | 1.3 ± 1.1 | 1.2 ± 1.1 | 1.1 ± 1.0 | 1.1 ± 1.0 | 1.0 ± 1.1 |
| Whole grains,5 g/d | 29.9 ± 21.9 | 20.4 ± 16.9 | 23.4 ± 18.3 | 26.8 ± 20.4 | 31.3 ± 22.1 | 35.9 ± 23.6 | 38.0 ± 22.5 | 39.4 ± 21.5 |
| Polyunsaturated fat,5 g/d | 13.1 ± 6.1 | 11.9 ± 5.3 | 12.4 ± 5.5 | 12.8 ± 5.8 | 13.2 ± 6.1 | 13.6 ± 6.6 | 14.0 ± 6.7 | 14.5 ± 7.0 |
| Trans fat,5 g/d | 2.7 ± 1.6 | 3.1 ± 1.7 | 3.0 ± 1.7 | 2.9 ± 1.7 | 2.6 ± 1.6 | 2.3 ± 1.5 | 2.1 ± 1.3 | 2.0 ± 1.1 |
| Marine ω-3 fat,5 g/d | 342 ± 345 | 250 ± 265 | 269 ± 288 | 305 ± 315 | 355 ± 357 | 405 ± 376 | 452 ± 390 | 465 ± 353 |
| Sodium,5 mg/d | 2439 ± 1114 | 2553 ± 1274 | 2537 ± 1213 | 2483 ± 1152 | 2418 ± 1087 | 2356 ± 1026 | 2325 ± 976 | 2289 ± 880 |
Updated information throughout all follow-up periods was used to calculate the mean ± SD for continuous variables and percentages for categorical variables. All characteristics (except for age) and SDs are age-standardized using the following 5 age groups: <49 y, 50–54 y, 55–59 y, 60–64 y, and ≥65 y. MET, metabolic equivalent; NSAID, nonsteroidal anti-inflammatory drug.
Pack-years of smoking are calculated among current smokers.
MET-h/wk from recreational and leisure-time activities are calculated by multiplying the amount of time spent on a specific activity (e.g., running) per week with its energy-expenditure requirements. One MET is defined as the energy expended in sitting quietly.
Regular users are defined as taking medication ≥2 times/wk.
The Alternate Healthy Eating Index 2010 (AHEI-2010) comprises 7 favorable and 4 unfavorable food items, each contributing 0–10 points to the overall score.
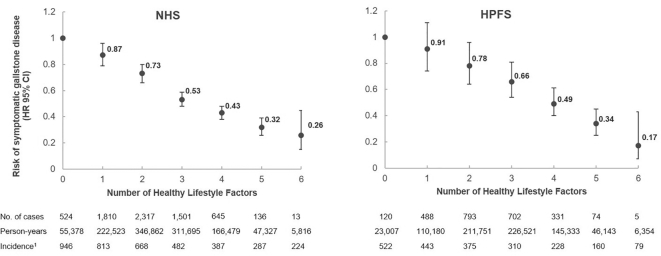
HRs and 95% CIs for symptomatic gallstone disease according to the number of healthy lifestyle factors are presented in Figure 1. In multivariable adjusted analyses, adhering to all healthy lifestyle factors when compared with none was associated with an HR of 0.26 (95% CI: 0.15, 0.45) for women, and 0.17 (95% CI: 0.07, 0.43) for men. When we investigated associations separately by time period, they appeared to be slightly attenuated over time [multivariable HR for women comparing highest with lowest HLS score for 1986–1994: 0.22 (95% CI: 0.08, 0.58); 1994–2002: 0.32 (95% CI: 0.15, 0.68); and 2002–2012: 0.30 (95% CI: 0.10, 0.97)].
FIGURE 1.
Number of healthy lifestyle factors and the risk of symptomatic gallstone disease in the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS). Healthy lifestyle factors were defined as the upper 2 quintiles of the cohort's Alternate Healthy Eating Index 2010 (excluding alcohol), consuming ≥2 cups of caffeinated coffee per day, performing ≥150 vigorous-intensity or ≥300 moderate-intensity minutes of physical activity per week (15 MET-h/wk), having a BMI <25 kg/m2, being a never smoker, and consuming a moderate amount of alcohol (men: 0.5–2 drinks/d; women: 0.5–1.5 drinks/d). HRs and 95% CIs from Cox regression analyses are adjusted for age, questionnaire cycle, regular use of statins and thiazide diuretics (yes/no), regular use of NSAIDs (≥2 times/wk; yes/no), postmenopausal hormone use (pre-/missing menopause, never used, current user, past user; only in NHS), and history of hypercholesterolemia and hypertension (yes/no). 1Incidence is per 100,000 person-years. HPFS, Health Professionals Follow-up Study; MET, metabolic equivalent; NHS, Nurses’ Health Study; NSAID, nonsteroidal anti-inflammatory drug.
When we excluded symptomatic gallstone cases without cholecystectomy in the HPFS (n = 415), we observed similar results (multivariable HRs comparing highest with lowest HLS: 0.14; 95% CI: 0.05, 0.39). Including diabetics in the analyses slightly altered the findings in men but not women [multivariable HRs for highest compared with lowest HLS: women 0.25 (95% CI: 0.15, 0.44); men: 0.21 (95% CI: 0.10, 0.45)].
The association was weaker when we used cumulative averages of lifestyle factors to generate the HLS [multivariable HRs comparing highest with lowest HLS: women 0.36 (95% CI: 0.23, 0.57); men: 0.32 (95% CI: 0.16, 0.67)].
Partial PAR% for each individual healthy lifestyle factor and full PAR% for all factors combined are shown in Tables 3 and 4. After controlling for potential confounders (see footnotes), having a BMI between 18.5 and 24.9 was the factor with the highest partial PAR% in both women and men (33% and 23%, respectively), followed by moderate alcohol intake (13%), coffee consumption (10%), physical activity (9%), AHEI (8%), and never-smoking (1%, nonsignificant) in women; and coffee consumption (18%), AHEI-2010 (11%), moderate alcohol (7%), physical activity (5%), and never-smoking (5%) in men. The combined full PAR% was 62% for women and 74% for men.
TABLE 3.
Partial and full population attributable risk percentage (PAR%) of different lifestyle factors on gallstone disease for women in the Nurses’ Health Study (NHS)1
| Lifestyle factor | Healthy definition | Person-years with healthy factor, % | Incidence, no. per 100,000 person-years: nonhealthy/healthy | PAR% (95% CI): individual factor | PAR% (95% CI): combined |
|---|---|---|---|---|---|
| Smoking | Never smoking | 45 | 595/607 | 1 (−1, 4) | 62 (37, 79) |
| AHEI-2010 | Upper 2 quintiles | 41 | 639/547 | 8 (5, 11) | |
| BMI, kg/m2 | 18.5–24.9 | 51 | 820/394 | 33 (30, 36) | |
| Physical activity | ≥15 METs/wk | 42 | 662/518 | 9 (6, 12) | |
| Coffee consumption | ≥2 cups/d | 39 | 645/530 | 10 (7, 13) | |
| Moderate alcohol intake | 0.5–1.5 drinks/d | 22 | 634/483 | 13 (8, 17) |
The PAR% is dependent on the magnitude of the association and the prevalence of exposure and describes the proportion of symptomatic gallstone disease that could be prevented if 1 or all of the mentioned exposures were to be eliminated from the population. Briefly, the PAR% was calculated by combining the observed prevalence rates of each healthy lifestyle factor with the estimated HR from the multivariable Cox proportional hazards model, which was adjusted for age, questionnaire cycle, regular use of statins and thiazide diuretics (yes/no), regular use of NSAIDs (≥2 times/wk; yes/no), postmenopausal hormone use (pre-/missing menopause, never used, current user, past user), history of hypercholesterolemia and hypertension (yes/no), and mutually adjusted for each of the healthy lifestyle factors. AHEI-2010, Alternate Healthy Eating Index 2010; MET, metabolic equivalent; NSAID, nonsteroidal anti-inflammatory drug; PAR%, population attributable risk percentage.
TABLE 4.
Partial and full population attributable risk percentage (PAR%) of different lifestyle factors on gallstone disease for men in the Health Professionals Follow-up Study (HPFS)1
| Lifestyle factor | Healthy definition | Person-years with healthy factor, % | Incidence, no. per 100,000 person-years: nonhealthy/healthy | PAR% (95% CI): individual factor | PAR% (95% CI): combined |
|---|---|---|---|---|---|
| Smoking | Never smoking | 49 | 357/295 | 5 (1, 10) | 74 (43, 90) |
| AHEI-2010 | Upper 2 quintiles | 42 | 349/295 | 11 (7, 16) | |
| BMI, kg/m2 | 18.5–24.9 | 43 | 385/250 | 23 (18, 27) | |
| Physical activity | ≥15 METs/wk | 66 | 378/300 | 5 (2, 8) | |
| Coffee consumption | ≥2 cups/d | 33 | 354/270 | 18 (12, 23) | |
| Moderate alcohol intake | 0.5–2 drinks/d | 35 | 343/297 | 7 (2, 13) |
The PAR% is dependent on the magnitude of the association and the prevalence of exposure and describes the proportion of symptomatic gallstone disease that could be prevented if 1 or all of the mentioned exposures were to be eliminated from the population. Briefly, the PAR% was calculated by combining the observed prevalence rates of each healthy lifestyle factor with the estimated HR from the multivariable Cox proportional hazards model, which was adjusted for age, questionnaire cycle, regular use of statins and thiazide diuretics (yes/no), regular use of NSAIDs (≥2 times/wk; yes/no), history of hypercholesterolemia and hypertension (yes/no), and mutually adjusted for each of the healthy lifestyle factors. AHEI-2010, Alternate Healthy Eating Index 2010; MET, metabolic equivalent; NSAID, nonsteroidal anti-inflammatory drug; PAR%, population attributable risk percentage.
Analyses of single lifestyle components showed that with mutual adjustment, all single lifestyle factors were associated with risk of symptomatic gallstone disease, except for smoking status in women. For men, only the highest category (>30 MET-h/wk) of physical activity was associated with a lower risk of symptomatic gallstone disease (Supplemental Tables 1 and 2).
Discussion
In these 2 large prospective cohort studies of male and female health professionals, a healthy lifestyle including a healthy diet (reflected by the highest 40% of the cohorts’ AHEI-2010), moderate alcohol intake (women: 0.5–1.5 drinks/d; men: 0.5–2 drinks/d), regular consumption of caffeinated coffee (≥2 cups/d), never-smoking, physical activity (≥15 MET-h/wk), and normal body weight (BMI between 18.5 and 24.9), was inversely associated with the risk of symptomatic gallstone disease. Women and men with the highest HLS had a 74% and an 83% lower risk of symptomatic gallstone disease, respectively, than those with no healthy lifestyle factor. The combined PAR% was 64% for women and 74% for men. Thus, assuming causal relations, the incidence of symptomatic gallstone disease might have been reduced by two-thirds to three-quarters if all participants had adhered to the lowest-risk lifestyle. All selected lifestyle factors contributed to the population risk, except for smoking in women. For both women and men, being normal weight was associated with the highest PAR% (33% and 23%, respectively).
Although the etiology of gallstones remains unclear, biologically plausible mechanisms suggested to play a role in gallstone development include insulin resistance and alterations of lipid metabolism (28, 43). Lifestyle factors associated with gallstone formation are also related to insulin resistance, including obesity (12, 44), smoking (13, 45), the AHEI-2010 (24, 46), physical activity (17, 31, 47), alcohol (18, 48) and coffee consumption (19, 49). Furthermore, the investigated lifestyle factors have been shown to favorably alter the lipid profile and, thus, could counteract pathways related to cholesterol supersaturation in the bile (5, 50–55).
Overall, the association between the HLS and symptomatic gallstone disease appeared to be more pronounced in men than in women. Differences in the distribution/prevalence of single lifestyle factors might have contributed to these disparities; for example, men in the highest HLS group were substantially more physically active than women in the highest HLS group (58.3 compared with 39.6 MET-h/wk). Furthermore, the BMI range between the lowest and highest HLS group was wider in women than in men, which can also explain the higher partial PAR% for being normal weight found in the NHS compared with the HPFS. Second, underlying mechanisms in the development of gallstones might differ between men and women. Especially worth mentioning is the increase of estrogen by BMI (56), which, in turn, increases biliary cholesterol secretion, the first step to gallstone development (57). Although the overall PAR% was higher in men than in women, the absolute number of potentially preventable cases of symptomatic gallstone disease is likely higher in women, due to the 2–3-fold higher incidence of this disease in women (1).
This large prospective study including >100,000 participants with almost 10,000 symptomatic gallstone cases provides further support for a role of lifestyle modification in the primary prevention of symptomatic gallstone disease. To the best of our knowledge, this is the first study to examine associations between both individual healthy lifestyle factors and joint effects within a healthy lifestyle pattern and the risk of symptomatic gallstone disease. Other studies relating several lifestyle factors and dietary patterns to gallstone disease are scarce and often limited by inadequate assessment, inadequate control of confounding, or small numbers of cases (58).
A small prospective cohort study (n = 2848, gallstone cases n = 256) has investigated the association between different lifestyle factors and the risk of gallstones (58). In agreement with our results, the authors observed a positive association between BMI and the risk of gallstones but saw no associations with smoking, coffee intake, alcohol consumption, or healthy diet. Although ultrasonography was used to determine gallstone status, which also captures asymptomatic cases and allows a more accurate conclusion about gallstone formation, other reasons could be responsible for the deviation from our findings, including simplified assessment of covariates and adjustment. In a subsequent meta-analysis (58) of prospective studies using ultrasonography to assess gallstone status, the same authors observed positive associations with gallstone disease for smoking and BMI but not for alcohol consumption. However, they did not include results from 2 of their identified studies, which investigated alcohol consumption and found negative associations with gallstone disease.
Strengths of our study include its large sample size and the availability of prospective detailed lifestyle and outcome data that were regularly updated over almost 30 y of follow-up, generally not available when studying these associations in a clinical setting. However, some limitations should be considered. First, exposure and outcome were self-reported, which could have led to measurement errors. However, lifestyle factors have been rigorously tested in several validation studies showing reasonably high validity and reproducibility (32–34). Recall bias, if any, might be nondifferential, which would have attenuated the results toward a null association in most but not all circumstances. Furthermore, we performed sensitivity analyses to test the robustness of our findings, including the use of cumulative averages of lifestyle information to generate the HLS. Cumulative averaging reduces the impact of random error and reflects long-term exposures. In our analysis, it yielded weaker results, suggesting that risk factors in the recent past might be more etiologically relevant than remote exposure. Moreover, we showed that self-reports of the outcome had excellent validity (12), and results from sensitivity analyses restricted to participants who had undergone cholecystectomy were similar. In our study population it was not possible to perform systematic screening for gallstones using ultrasonography. However, possible misclassification is expected to be nondifferential, because both cases and noncases might have had silent gallstones. Furthermore, we examined symptomatic gallstone disease, which is the most clinically relevant outcome and probably less prone to detection bias. As described in our Methods section, based on our validation studies >95% of those who reported gallstone disease were confirmed (12, 17, 31). Another limitation is that the proportional hazards assumption was violated in the NHS. In additional analyses, we observed slight attenuations of the effect size in later follow-up periods. Thus, our overall result can be interpreted as an average association over time (59). Furthermore, our study population consisted of mainly white health professionals, limiting the generalizability to populations with other ethnic and socioeconomic backgrounds. Finally, even though we adjusted for a variety of potential confounders, as with all observational studies, we cannot exclude the possibility of residual or unmeasured confounding.
In conclusion, our results indicate that a considerable proportion of symptomatic gallstone disease cases might be prevented by maintaining a healthy lifestyle including following a healthy diet, not smoking, moderate alcohol intake, regular coffee consumption, being normal weight, and exercising regularly. Recommendations regarding the benefit of moderate alcohol intake should be weighed against potential health hazards of alcohol consumption. Alcohol should be consumed carefully, and according to current USDA dietary guidelines no more than 7 drinks/wk should be consumed by women. Moreover, nondrinkers should not start alcohol consumption just to avoid the development of gallstones. Given the high burden of symptomatic gallstone disease in Western populations, broader adoption of a healthy lifestyle could have a substantial impact on the health care systems. The HLS is easy to communicate in both public and clinical settings and could serve as a tool for improved precision medicine. Further studies are warranted to explore the utility of the HLS to investigate the joint effect of these lifestyle factors in other less health-conscious populations.
Supplementary Material
Acknowledgments
We acknowledge the participants and staff of the Health Professionals Follow-up Study for their valuable contributions as well as the following US state cancer registries for their help: AL, AR, AZ, CA, CO, CT, DE, FL, GA, IA, ID, IL, IN, KY, LA, MA, MD, ME, MI, NC, ND, NE, NH, NJ, NY, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data. We are, furthermore, indebted to Scott G Smith for his support in statistical analysis.
The authors’ responsibilities were as follows—CW, EG, JW, KW, ML: designed the research; JW, KW, MS: conducted the research; JW: created the database and analyzed the data; WCW, KW, EG, JW, ATC, TTF, FKT, MS and DHL: interpreted results; ADJ: performed a technical review of the manuscript; JW: wrote the manuscript; JW, KW: had primary responsibility for final content; and all authors: critically reviewed the manuscript and read and approved the final version. The authors report no conflicts of interest.
Notes
This work was supported by grants from the NIH (U01 167552, UM1 CA186107, UM1 CA167552, K01 DK110267). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The current study was supported by the German Research Foundation (grant number WI 4568/2-2 to JW). The funders had no role in the study design; in the collection, analysis, and interpretation of the data; in the preparation, review, or approval of the manuscript; or in the decision to submit the article for publication.
Further information including the procedures to obtain and access data from the Nurses’ Health Study and Health Professionals Follow-up Study is described at https://www.nurseshealthstudy.org/researchers (contact email: nhsaccess@channing.harvard.edu and https://sites.sph.harvard.edu/hpfs/for-collaborators/).
Supplemental Figure 1 and Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AHEI-2010, Alternate Healthy Eating Index 2010; HLS, healthy lifestyle score; HPFS, Health Professionals Follow-up Study; MET, metabolic equivalent; NHS, Nurses’ Health Study; NSAID, nonsteroidal anti-inflammatory drug; PAR%, population attributable risk percentage.
Contributor Information
Janine Wirth, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; University College Dublin (UCD) School of Agriculture and Food Science, UCD Institute of Food and Health, University College Dublin, Dublin, Ireland.
Amit D Joshi, Clinical and Translational Epidemiology Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Mingyang Song, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Clinical and Translational Epidemiology Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Dong Hoon Lee, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Fred K Tabung, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Division of Medical Oncology, The Ohio State University Wexner Medical Center, Columbus, OH, USA; The Ohio State University Comprehensive Cancer Center—James Cancer Hospital and Solove Research Institute, Columbus, OH, USA.
Teresa T Fung, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Nutrition, Simmons College, Boston, MA, USA.
Andrew T Chan, Clinical and Translational Epidemiology Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Harvard Medical School, Boston, MA, USA; Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Cornelia Weikert, Federal Institute of Risk Assessment, Department of Food Safety, Berlin, Germany.
Michael Leitzmann, Department of Epidemiology and Preventive Medicine, Regensburg University, Regensburg, Germany.
Walter C Willett, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Boston, MA, USA.
Edward Giovannucci, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Boston, MA, USA.
Kana Wu, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
References
- 1. Kratzer W, Mason RA, Kachele V. Prevalence of gallstones in sonographic surveys worldwide. J Clin Ultrasound. 1999;27(1):1–7. [DOI] [PubMed] [Google Scholar]
- 2. Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117(3):632–9. [DOI] [PubMed] [Google Scholar]
- 3. Stokes CS, Krawczyk M, Lammert F. Gallstones: environment, lifestyle and genes. Dig Dis. 2011;29(2):191–201. [DOI] [PubMed] [Google Scholar]
- 4. Chowbey P, Sharma A, Goswami A, Afaque Y, Najma K, Baijal M, Soni V, Khullar R. Residual gallbladder stones after cholecystectomy: a literature review. J Minim Access Surg. 2015;11(4):223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. 2012;6(2):172–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136(4):1134–44. [DOI] [PubMed] [Google Scholar]
- 7. Wirth J, Giuseppe RD, Wientzek A, Katzke VA, Kloss M, Kaaks R, Being H, Weikert C. Presence of gallstones and the risk of cardiovascular diseases: the EPIC-Germany cohort study. Eur J Prev Cardiol. 2015;22(3):326–34. [DOI] [PubMed] [Google Scholar]
- 8. Weikert C, Weikert S, Schulze MB, Pischon T, Fritsche A, Bergmann MM, Willich SN, Boeing H. Presence of gallstones or kidney stones and risk of type 2 diabetes. Am J Epidemiol. 2010;171(4):447–54. [DOI] [PubMed] [Google Scholar]
- 9. Ruhl CE, Everhart JE. Gallstone disease is associated with increased mortality in the United States. Gastroenterology. 2011;140(2):508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fan Y, Hu J, Feng B, Wang W, Yao G, Zhai J, Li X. Increased risk of pancreatic cancer related to gallstones and cholecystectomy: a systematic review and meta-analysis. Pancreas. 2015;45(4):503–9. [DOI] [PubMed] [Google Scholar]
- 11. Feneberg A, Malfertheiner P. Epidemic trends of obesity with impact on metabolism and digestive diseases. Dig Dis. 2012;30(2):143–7. [DOI] [PubMed] [Google Scholar]
- 12. Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Central adiposity, regional fat distribution, and the risk of cholecystectomy in women. Gut. 2006;55(5):708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aune D, Vatten LJ, Boffetta P. Tobacco smoking and the risk of gallbladder disease. Eur J Epidemiol. 2016;31(7):643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Long-chain saturated fatty acids consumption and risk of gallstone disease among men. Ann Surg. 2008;247(1):95–103. [DOI] [PubMed] [Google Scholar]
- 15. Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Long-term intake of trans-fatty acids and risk of gallstone disease in men. Arch Intern Med. 2005;165(9):1011–5. [DOI] [PubMed] [Google Scholar]
- 16. Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Heme and non-heme iron consumption and risk of gallstone disease in men. Am J Clin Nutr. 2007;85(2):518–22. [DOI] [PubMed] [Google Scholar]
- 17. Leitzmann MF, Rimm EB, Willett WC, Spiegelman D, Grodstein F, Stampfer MJ, Coldiz GA, Giovannucci E. Recreational physical activity and the risk of cholecystectomy in women. N Engl J Med. 1999;341(11):777–84. [DOI] [PubMed] [Google Scholar]
- 18. Leitzmann MF, Tsai CJ, Stampfer MJ, Rimm EB, Colditz GA, Willett WC, Giovannucci EL. Alcohol consumption in relation to risk of cholecystectomy in women. Am J Clin Nutr. 2003;78(2):339–47. [DOI] [PubMed] [Google Scholar]
- 19. Zhang YP, Li WQ, Sun YL, Zhu RT, Wang WJ. Systematic review with meta-analysis: coffee consumption and the risk of gallstone disease. Aliment Pharmacol Ther. 2015;42(6):637–48. [DOI] [PubMed] [Google Scholar]
- 20. Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Fruit and vegetable consumption and risk of cholecystectomy in women. Am J Med. 2006;119(9):760–7. [DOI] [PubMed] [Google Scholar]
- 21. Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. The effect of long-term intake of cis unsaturated fats on the risk for gallstone disease in men: a prospective cohort study. Ann Intern Med. 2004;141(7):514–22. [DOI] [PubMed] [Google Scholar]
- 22. Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Long-term intake of dietary fiber and decreased risk of cholecystectomy in women. Am J Gastroenterol. 2004;99(7):1364–70. [DOI] [PubMed] [Google Scholar]
- 23. Tsai CJ, Leitzmann MF, Hu FB, Willett WC, Giovannucci EL. Frequent nut consumption and decreased risk of cholecystectomy in women. Am J Clin Nutr. 2004;80(1):76–81. [DOI] [PubMed] [Google Scholar]
- 24. Wirth J, Song M, Fung TT, Joshi AD, Tabung FK, Chan AT, Weikert C, Leitzmann M, Willett WC, Giovannucci E et al. Diet-quality scores and the risk of symptomatic gallstone disease: a prospective cohort study of male US health professionals. Int J Epidemiol. 2018;47(6):1938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 26. Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–8. [DOI] [PubMed] [Google Scholar]
- 27. Biddinger SB, Haas JT, Yu BB, Bezy O, Jing E, Zhang W, Unterman TG, Carey MC, Kahn CR. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med. 2008;14(7):778–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aune D, Vatten LJ. Diabetes mellitus and the risk of gallbladder disease: a systematic review and meta-analysis of prospective studies. J Diabetes Complications. 2016;30(2):368–73. [DOI] [PubMed] [Google Scholar]
- 29. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Kearney J, Willett WC. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. men. J Natl Cancer Inst. 1994;86(3):183–91. [DOI] [PubMed] [Google Scholar]
- 30. Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–73. [DOI] [PubMed] [Google Scholar]
- 31. Leitzmann MF, Giovannucci EL, Rimm EB, Stampfer MJ, Spiegelman D, Wing AL, Willett WC. The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med. 1998;128(6):417–25. [DOI] [PubMed] [Google Scholar]
- 32. Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7(1):81–6. [DOI] [PubMed] [Google Scholar]
- 33. Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–9. [DOI] [PubMed] [Google Scholar]
- 34. Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–6. [DOI] [PubMed] [Google Scholar]
- 35. Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–67. [DOI] [PubMed] [Google Scholar]
- 36. Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, Willett WC. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133(8):810–7. [DOI] [PubMed] [Google Scholar]
- 37. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public Health Nutr. 2006;9(1A):152–7. [DOI] [PubMed] [Google Scholar]
- 39. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The Physical Activity Guidelines for Americans. JAMA. 2018;320(19):2020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. [DOI] [PubMed] [Google Scholar]
- 41. Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18(5):571–9. [DOI] [PubMed] [Google Scholar]
- 42. Willett W. Nutritional epidemiology. 3rd ed Oxford University Press; 2013. [Google Scholar]
- 43. Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Macronutrients and insulin resistance in cholesterol gallstone disease. Am J Gastroenterol. 2008;103(11):2932–9. [DOI] [PubMed] [Google Scholar]
- 44. Barazzoni R, Gortan Cappellari G, Ragni M, Nisoli E. Insulin resistance in obesity: an overview of fundamental alterations. Eat Weight Disord. 2018;23(2):149–57. [DOI] [PubMed] [Google Scholar]
- 45. Haj Mouhamed D, Ezzaher A, Neffati F, Douki W, Gaha L, Najjar MF. Effect of cigarette smoking on insulin resistance risk. Ann Cardiol Angeiol (Paris). 2016;65(1):21–5. [DOI] [PubMed] [Google Scholar]
- 46. Jacobs S, Boushey CJ, Franke AA, Shvetsov YB, Monroe KR, Haiman CA, Kolonel LN, Marchand LL, Maskarinec G. A priori-defined diet quality indices, biomarkers and risk for type 2 diabetes in five ethnic groups: the Multiethnic Cohort. Br J Nutr. 2017;118(4):312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang D, Liu X, Liu Y, Sun X, Wang B, Ren Y, Zhao Y, Zhou J, Han C, Yin L et al. Leisure-time physical activity and incident metabolic syndrome: a systematic review and dose-response meta-analysis of cohort studies. Metabolism. 2017;75:36–44. [DOI] [PubMed] [Google Scholar]
- 48. Schrieks IC, Heil AL, Hendriks HF, Mukamal KJ, Beulens JW. The effect of alcohol consumption on insulin sensitivity and glycemic status: a systematic review and meta-analysis of intervention studies. Diabetes Care. 2015;38(4):723–32. [DOI] [PubMed] [Google Scholar]
- 49. Loureiro LMR, Reis CEG, da Costa THM. Effects of coffee components on muscle glycogen recovery: a systematic review. Int J Sport Nutr Exerc Metab. 2018;28(3):284–93. [DOI] [PubMed] [Google Scholar]
- 50. Rimm EB, Williams, Fosher P, Criqui K, Stampfer MJM. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319(7224):1523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mattei J, Sotres-Alvarez D, Daviglus ML, Gallo LC, Gellman M, Hu FB, Tucker KL, Willett WC, Siega-Riz AM, Van Horn L et al. Diet quality and its association with cardiometabolic risk factors vary by Hispanic and Latino ethnic background in the Hispanic Community Health Study/Study of Latinos. J Nutr. 2016;146(10):2035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Silva RC, Diniz Mde F, Alvim S, Vidigal PG, Fedeli LM, Barreto SM. Physical activity and lipid profile in the ELSA-Brasil Study. Arq Bras Cardiol. 2016;107(1):10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Willett W, Hennekens CH, Castelli W, Rosner B, Evans D, Taylor J, Kass EH. Effects of cigarette smoking on fasting triglyceride, total cholesterol, and HDL-cholesterol in women. Am Heart J. 1983;105(3):417–21. [DOI] [PubMed] [Google Scholar]
- 54. Cai L, Ma D, Zhang Y, Liu Z, Wang P. The effect of coffee consumption on serum lipids: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2012;66(8):872–7. [DOI] [PubMed] [Google Scholar]
- 55. Ebbert JO, Jensen MD. Fat depots, free fatty acids, and dyslipidemia. Nutrients. 2013;5(2):498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oh H, Coburn SB, Matthews CE, Falk RT, LeBlanc ES, Wactawski-Wende J, Sampson J, Pfeiffer RM, Brinton LA, Wentzensen N et al. Anthropometric measures and serum estrogen metabolism in postmenopausal women: the Women's Health Initiative Observational Study. Breast Cancer Res. 2017;19(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Novacek G. Gender and gallstone disease. Wien Med Wochenschr. 2006;156(19-20):527–33. [DOI] [PubMed] [Google Scholar]
- 58. Shabanzadeh DM, Sorensen LT, Jorgensen T. Determinants for gallstone formation – a new data cohort study and a systematic review with meta-analysis. Scand J Gastroenterol. 2016;51(10):1239–48. [DOI] [PubMed] [Google Scholar]
- 59. Hancock GR, Stapleton LM, Mueller RO. The reviewer's guide to quantitative methods in the social sciences. 2nd ed Routledge; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.