ABSTRACT
Background
Whether egg consumption is associated with the risk of type 2 diabetes (T2D) remains unsettled.
Objectives
We evaluated the association between egg consumption and T2D risk in 3 large US prospective cohorts, and performed a systematic review and meta-analysis of prospective cohort studies.
Methods
We followed 82,750 women from the Nurses’ Health Study (NHS; 1980–2012), 89,636 women from the NHS II (1991–2017), and 41,412 men from the Health Professionals Follow-up Study (HPFS; 1986–2016) who were free of T2D, cardiovascular disease, and cancer at baseline. Egg consumption was assessed every 2–4 y using a validated FFQ. We used Cox proportional hazard models to estimate HRs and 95% CIs.
Results
During a total of 5,529,959 person-years of follow-up, we documented 20,514 incident cases of T2D in the NHS, NHS II, and HPFS. In the pooled multivariable model adjusted for updated BMI, lifestyle, and dietary confounders, a 1-egg/d increase was associated with a 14% (95% CI: 7%, 20%) higher T2D risk. In random-effects meta-analysis of 16 prospective cohort studies (589,559 participants; 41,248 incident T2D cases), for each 1 egg/d, the pooled RR of T2D was 1.07 (95% CI: 0.99, 1.15; I2 = 69.8%). There were, however, significant differences by geographic region (P for interaction = 0.01). Each 1 egg/d was associated with higher T2D risk among US studies (RR: 1.18; 95% CI: 1.10, 1.27; I2 = 51.3%), but not among European (RR: 0.99; 95% CI: 0.85, 1.15; I2 = 73.5%) or Asian (RR: 0.82; 95% CI: 0.62, 1.09; I2 = 59.1%) studies.
Conclusions
Results from the updated meta-analysis show no overall association between moderate egg consumption and risk of T2D. Whether the heterogeneity of the associations among US, European, and Asian cohorts reflects differences in egg consumption habits warrants further investigation.
This systematic review was registered at www.crd.york.ac.uk/prospero as CRD42019127860.
Keywords: egg, type 2 diabetes, prospective cohort study, systematic review, meta-analysis
See corresponding editorial on page 503.
Introduction
Eggs are an affordable and low-calorie source of many nutrients, including unsaturated fatty acids, choline, essential amino acids, iron, folate, and other B vitamins (1). Eggs are also among the foods with the highest cholesterol content, with ∼200 mg cholesterol/egg (1). Given the potential impact of dietary cholesterol on serum cholesterol concentrations (2), it has long been recommended to consume no more than 300 mg/d of dietary cholesterol (3). However, in the most recent Dietary Guidelines for Americans (4), the upper limit for dietary cholesterol was not carried forward because current evidence does not support a clear relation between dietary and serum cholesterol, and because dietary cholesterol was no longer considered to be a nutrient of concern for overconsumption. Indeed, substantial evidence from meta-analyses of prospective cohort studies demonstrates no association between egg consumption and risk of coronary artery disease (CAD) or stroke (5), despite recent contradictory data (6). Still, whether egg consumption is associated with type 2 diabetes (T2D) risk remains unsettled. This question is of particular importance because the number of adults living with diabetes worldwide is expected to increase by >200 million by 2045, and T2D represents a major risk factor for cardiovascular morbidity and mortality (7, 8).
Recent meta-analyses of prospective cohort studies were inconclusive regarding the association between egg consumption and T2D risk because they reported positive associations for studies conducted among US populations, but no association among studies conducted in Asia or Europe (9–11). These discrepancies suggest that previous prospective analyses may have been unable to disentangle the effect of egg consumption per se from the background diet (9–11). In that regard, among studies conducted in the United States (12–16), 2 studies did not adjust for intakes of foods commonly consumed with eggs like red and processed meat, refined grains, and sugary beverages (12, 13). This may have created a spurious deleterious association between egg consumption and T2D risk. In this context, the association of egg consumption with T2D risk remains to be thoroughly evaluated.
The current study builds on data from 3 large ongoing US cohort studies, namely the Nurses’ Health Study (NHS), the Nurses’ Health Study II (NHS II), and the Health Professionals’ Follow-up Study (HPFS). In all 3 cohorts, detailed information on egg consumption and diet was collected every 2–4 y for ≤32 y of follow-up. We used these repeated assessments of diet to evaluate the association of long-term egg consumption with risk of T2D among US women and men after adjusting for updated lifestyle and dietary confounders. We also statistically estimated the risk of T2D associated with replacing eggs with other common foods in the diet. Finally, to inform dietary guidelines, we conducted a systematic review and an updated meta-analysis of egg consumption and T2D risk that include these new results.
Methods
Cohort analyses
Study population and design
The NHS was initiated in 1976 with 121,701 female registered nurses between the ages of 30 and 55 y. The NHS II began in 1989 and included 116,430 female registered nurses between the ages of 25 and 44 y. The HPFS included 51,529 male health professionals between the ages of 40 and 75 y at study inception in 1986. In all 3 studies, self-administered validated questionnaires with information on disease diagnoses, health, and lifestyle factors were completed every 2 y. Follow-up rates were ∼90% in the 3 cohorts. Diet was first measured in 1980 for the NHS, 1991 for the NHS II, and 1986 for the HPFS using a validated FFQ (17–19). These time points were used as baseline in the current study.
For the present study, of the participants who completed a baseline FFQ (NHS 1980, n = 98,047; NHS II 1991, n = 97,813; HPFS 1986, n = 51,529), we excluded those with baseline diagnosis of cancer, cardiovascular disease (or history of coronary artery surgery), or T2D. We also excluded participants with missing baseline information on age or egg intake, those who reported unrealistic energy intake (<500 or >3500 kcal/d for women and <800 or >4200 kcal/d for men), those who left >70 items blank in the FFQ, and those who only completed the baseline questionnaire. After exclusions, 82,750 participants in the NHS, 89,636 participants in the NHS II, and 41,412 participants in the HPFS were included in the analyses. Supplemental Figure 1 presents the flowchart of participants. The Institutional Review Board of the Brigham and Women's Hospital (Boston, MA) and Harvard TH Chan School of Public Health approved all study protocols.
Assessment of egg consumption
In the NHS, dietary information was collected through a semiquantitative FFQ with 61 items in 1980 and with 131 items in 1984. Starting in 1986, in both the NHS and HPFS, diet was assessed on a quadrennial basis using the same FFQ. In the NHS II, diet was assessed every 4 y from 1991 using the same questionnaire. The questionnaire is available at https://regepi.bwh.harvard.edu/health/nutrition.html. Participants were asked how often on average they consumed whole eggs with yolk in the past year. Responses could range from <1 time/mo to ≥6 times/d with 1 whole egg as the standard serving size. FFQs were validated against 7-d weighed food records in 127 men from the HPFS (20) and 173 women from the NHS (21). The deattenuated correlation for egg intake was 0.77 in women and 0.80 in men (20, 21). Egg consumption from dishes with whole eggs as the main ingredient like soufflé, quiche, or egg omelet was reported as whole egg intake. However, reported intake excluded eggs in baked goods (e.g., cake), liquid eggs, and egg whites. Consumption of liquid eggs and egg whites was not assessed in the FFQ, but we computed intake of eggs included in baked goods such as cakes, cookies, pancakes, muffins, sweet rolls, and donuts based on standardized recipes.
In the main analyses, we used whole egg intake as the exposure. In a sensitivity analysis, we used total egg consumption (i.e., consumption of whole eggs plus eggs in baked goods) as the exposure.
Assessment of T2D
Our primary outcome was incident confirmed T2D. Cases were first identified by self-report from participants on the main questionnaire completed every 2 y. Diagnoses were confirmed by the completion of a validated supplementary questionnaire on the symptoms, diagnostic tests, and treatment of diabetes (22, 23). Until 1997, the report of ≥1 of the following criteria was used to confirm a case of diabetes: 1) ≥1 classic symptoms (excessive thirst, polyuria, weight loss, hunger, pruritis, or coma) and fasting plasma glucose concentrations ≥7.8 mmol/L or random plasma glucose ≥11.1 mmol/L; 2) ≥2 elevated plasma glucose concentrations on different occasions [fasting concentrations of ≥7.8 mmol/L, random plasma glucose ≥11.1 mmol/L, and/or ≥11.1 mmol/L after ≥2 h on an oral-glucose-tolerance test (OGTT)] in the absence of symptoms; or 3) treatment with hypoglycemic medication (insulin or oral hypoglycemic agent). After 1997, we used the American Diabetes Association cutoffs of fasting plasma glucose ≥7.0 mmol/L, random or ≥2 h on an OGTT plasma glucose ≥11.1 mmol/L, or glycated hemoglobin ≥6.5%, but otherwise the same criteria as aforementioned (24). The current study only includes confirmed T2D cases.
Assessment of covariates
Using the main biennial follow-up questionnaires, we collected and updated information on age, ethnicity (assessed once in 1992 in the NHS, 1989 in the NHS II, 1986 in the HPFS), family history of T2D, body weight, cigarette smoking, physical activity, multivitamin use, menopausal status (NHS and NHS II), use of postmenopausal hormones (NHS and NHS II), oral contraceptive use (NHS II only), and history of hypercholesterolemia and hypertension. Participants were considered to have hypercholesterolemia or hypertension when they reported it on the biennial questionnaire or when they reported use of lipid or blood pressure–lowering medications. Alcohol intake was ascertained through FFQs. Detailed descriptions of the validity and reproducibility of self-reported body weight, physical activity, and alcohol consumption have been published elsewhere (25–27).
Statistical methods for cohort analyses
We followed participants from the date of the return of the first questionnaire (1980 in the NHS, 1991 in the NHS II, and 1986 in the HPFS) to the diagnosis of T2D or return of the last questionnaire before death, loss to follow-up, or the cutoff date (30 June, 2012 in the NHS; 30 June, 2017 in the NHS II; and 31 January, 2016 in the HPFS), whichever came first.
Cox proportional hazards regression models were used to calculate HRs and 95% CIs for incident T2D. To represent long-term diet and reduce within-person variation, a cumulative average update method was used for egg intake and other dietary variables (28). For instance, for the 1999–2001 risk set in the NHS II, egg intakes in 1991, 1995, and 1999 were averaged to predict subsequent T2D risk. A trend across categories of egg consumption was calculated from the median of each category of egg intake (29). Within each cohort, participants were divided into a priori defined categories of egg intake (<1 egg/mo, 1 to <4 eggs/mo, 1 to <3 eggs/wk, 3 to <5 eggs/wk, 5 to <7 eggs/wk, and ≥1 egg/d) with the <1 egg/mo group serving as the reference group. In the main analyses, we used whole egg intake as the exposure.
Analyses were first conducted within each cohort separately, and then by pooling data from the 3 cohorts. Pooled multivariable models included age in months as the time scale, were stratified by calendar year (in 2-y intervals) and by cohort (which allowed concomitant stratification for sex), and were adjusted for race/ethnicity (Caucasian/other), family history of T2D (yes/no), baseline history of hypertension (yes/no), and baseline history of hypercholesterolemia (yes/no). The multivariable models also adjusted for the following time-varying covariates that were updated every 2 y: BMI (<21.0, 21.0–22.9, 23.0–24.9, 25.0–26.9, 27.0–29.9, 30.0–34.9, ≥35.0 kg/m2), smoking status (never, former, current), physical activity level (<3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, ≥27.0 metabolic equivalent of task-h/wk), statin use (yes/no), cumulative average alcohol (ethanol) consumption (g/d; quintiles), multivitamin use (yes/no), postmenopausal status and postmenopausal hormone use (premenopausal, never, former, current; NHS and NHS II only), oral contraceptive use (NHS II only), and history of physical examination in the last 2 y (yes/no). Finally, the multivariable models also included total energy intake (kcal/d; quintiles), and consumption of bacon, red meat, other processed meat, full-fat milk, refined grains, potatoes, fruits, vegetables, coffee, fruit juices, and sugar-sweetened beverages (servings/d; in categories). As with egg intake, a cumulative average update was used for all dietary variables.
We performed several sensitivity analyses. In all multivariable models, we adjusted for the Alternative Healthy Eating Index (AHEI) (30) instead of individual foods included in the main multivariable model (bacon, red meat, other processed meat, refined grains, potatoes, fruits, vegetables, coffee, full-fat milk, fruit juices, and sugar-sweetened beverages). To explore the possibility of confounding due to diagnosis with an intermediate endpoint, we stopped updating egg and dietary data after self-report of hypercholesterolemia, hypertension, angina, a report of statin use, or coronary artery bypass graft procedure. We modeled dietary data in several ways by using the most recent measure of diet, a mean of intakes from the 2 most recent FFQs, and using only baseline diet rather than a cumulative average intake. We also estimated the risk of incident T2D in 7 categories of egg intake, including ≥2 eggs/d as the highest category of intake. We further used total egg intake (i.e., consumption of whole eggs plus eggs in baked goods) as the exposure. Finally, because T2D diagnosis is subject to surveillance bias (i.e., higher-risk individuals are likely to be screened for diabetes and diagnosed more rapidly), we repeated the main analysis by restricting cases to only those with ≥1 symptom of diabetes at diagnosis (31).
We conducted a priori defined stratified analyses by updated age, BMI, and AHEI score. The latter aimed to test the hypothesis of confounding due to background diet quality. Interactions were tested using likelihood ratio tests by including cross-product terms of each stratum and egg consumption in the multivariable models.
We statistically estimated the T2D risk associated with replacing 1 egg/d with 1 daily serving of other foods that are common alternatives (unprocessed red meat, processed red meat, poultry, fish, legumes, nuts, cheese, milk, yogurt, whole grains, and potatoes). To do so, we included continuous variables for both eggs and the substitution food in Cox proportional hazards models. We used the differences in the β-coefficients, variance, and the covariance of eggs and the substituted food to obtain HRs and 95% CIs for incident T2D (32). Substitution analyses were adjusted as per the pooled multivariable model used in the main analysis. In substitution analyses, it is assumed that total consumption of different foods is constrained to a certain level for each individual (the amount of food is held constant), that the association of egg intake with T2D risk is independent of the association between the alternative food intake and T2D risk, and that the intake of other foods in the diet remains constant (33). All P values were 2-sided and statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc.).
Systematic review and meta-analysis on egg consumption and incident T2D
We conducted a systematic review and updated meta-analysis based on the current study and previous prospective cohort studies that evaluated the association between egg consumption and T2D risk using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (34). The study protocol was registered on the international prospective register of systematic reviews (PROSPERO: #CRD42019127860). We searched PubMed, Embase, and Web of Science through 6 April, 2020 using the search strategy presented in Supplemental Table 1. The lists of references of both selected studies and recent meta-analyses on the same topic (9–11) were screened to identify additional relevant studies. Studies were included if they 1) were prospective in nature, 2) were of ≥1-y follow-up duration, and 3) assessed the association between egg consumption and incidence of T2D in people free of diabetes at baseline.
Extracted data included first author name, publication year, cohort name, country where the study was conducted, number of participants, sex, age range at baseline, follow-up duration, method used to assess diet, number of T2D cases, method used to ascertain T2D cases, statistical model used, categories of egg consumption, risk estimates from the maximally adjusted multivariable model, and covariates in the maximally adjusted multivariable model. Study authors were contacted if information was missing. The Newcastle-Ottawa Scale (NOS) was used to assess the risk of bias in included studies (35). We considered age, sex, BMI, smoking status, physical activity, alcohol intake, and energy intake as primary confounders of the association between egg consumption and T2D risk in the assessment of control for confounders using the NOS criteria. Hypertension, dyslipidemia, and intakes of foods associated with eggs (red and processed meat, refined grains, potatoes, coffee, fruit juice, soft drinks, and milk) were considered as secondary confounders. One author (J-PD-C) defined the search terms, screened the literature (title and abstract, then full-text review), and extracted the data. A second author (SC) independently double-checked the literature screening and extracted data. Discrepancies were resolved by mutual consultation.
We used 50 g as the standard weight for 1 egg. RRs were used as the common measure of association across studies. ORs and HRs were considered equivalent to RRs. To determine dose of egg consumption, we used the median of each egg intake category, if available, or the midpoint between the upper and lower bounds. When the highest category was open (e.g., ≥1 egg/d), we multiplied the lower bound of the highest category by 1.75. In studies without dose-response estimates, we calculated the RRs for a 1-egg/d increase using the trend for log RRs, which accounted for correlated estimates (29, 36). In 1 study with only dose-response estimates (37), we used the provided estimates for the meta-analysis on high compared with low egg consumption. For studies that did not publish person-years for each category of egg intake (12–14, 38–42), we imputed person-years based on available data.
We used random-effects models to compute the pooled RR of T2D for a 1-egg/d increase in consumption, and for the highest category of egg intake compared with the lowest category. Heterogeneity was assessed using the I2 statistic and interpreted according to the Cochrane Handbook thresholds (0%–40%: might not be important; 30%–60%: may represent moderate heterogeneity; 50%–90%: may represent substantial heterogeneity; 75%–100%: considerable heterogeneity) (43). We conducted an influence analysis by systematically removing each study from the meta-analysis and calculating the RR to evaluate if any single study caused the heterogeneity. Publication bias was tested using Begg's and Egger's tests and via a visual appreciation of the funnel plot. We conducted univariate meta-regressions by using study-level data to explore potential sources of heterogeneity. Geographic region, sex, number of subjects, risk of bias, dietary assessment method, and adjustment for dietary confounders were identified a priori as potential sources of heterogeneity. Statistical analyses for the meta-analysis were performed using Stata version 15.1 (StataCorp.).
Results
Cohort analyses
We recorded 9226 incident cases of T2D after 32 y of follow-up in the NHS, 7282 cases after 26 y of follow-up in the NHS II, and 4006 cases after 30 y of follow-up in the HPFS. Overall, we documented 20,514 incident cases of T2D during a total of 5,529,959 person-years. Table 1 presents the age-adjusted characteristics of participants in 1998 for the NHS and HPFS and in 1999 for the NHS II (i.e., approximately the midpoint of follow-up). Across the 3 cohorts, higher egg intake was associated with higher BMI and lower prevalence of reported hypercholesterolemia and statin use. Intakes of total calories, red meat, bacon, other processed red meats, refined grains, potatoes, full-fat milk, coffee, and dietary cholesterol were positively associated with egg consumption. In 1998/1999, a total of 2091 participants (1.10%) consumed ≥1 egg/d in the 3 cohorts. Of those, 318 individuals (0.17%) consumed ≥2 eggs/d. Also in 1998/1999, whole egg intake contributed 85%, 71%, and 74% of total egg consumption (i.e., consumption of whole eggs plus eggs in baked goods) in the NHS, NHS II, and the HPFS, respectively.
TABLE 1.
Age-adjusted characteristics of participants in the NHS (1998), the NHS II (1999), and the HPFS (1998) according to whole egg consumption1
| Frequency of egg consumption | ||||||
|---|---|---|---|---|---|---|
| Characteristics | <1/mo | 1 to <4/mo | 1 to <3/wk | 3 to <5/wk | 5 to <7/wk | ≥1/d |
| NHS (1998) | ||||||
| Participants, n | 2477 | 14,769 | 38,484 | 11,533 | 1591 | 836 |
| Age,2 y | 64.8 ± 7.1 | 63.8 ± 7.1 | 63.5 ± 7.1 | 63.2 ± 7.0 | 62.7 ± 6.9 | 63.5 ± 6.8 |
| BMI, kg/m2 | 25.4 ± 4.9 | 25.7 ± 4.7 | 26.5 ± 5.0 | 27.3 ± 5.6 | 27.3 ± 6.2 | 27.6 ± 6.1 |
| Physical activity, MET-h/wk | 20.4 ± 30.8 | 17.9 ± 21.8 | 17.3 ± 20.6 | 17.0 ± 21.4 | 17.0 ± 21.9 | 18.9 ± 27.9 |
| Caucasian, % | 97 | 98 | 98 | 98 | 97 | 98 |
| Current smoker, % | 14 | 12 | 10 | 11 | 13 | 13 |
| Family history of diabetes, % | 27 | 27 | 27 | 26 | 27 | 24 |
| History of hypertension, % | 47 | 48 | 49 | 49 | 50 | 48 |
| History of hypercholesterolemia, % | 57 | 58 | 57 | 51 | 50 | 48 |
| Current statin use, % | 20 | 18 | 16 | 11 | 9 | 9 |
| Current multivitamin use, % | 56 | 58 | 61 | 60 | 58 | 56 |
| Fasting blood glucose screening,3 % | 50 | 52 | 54 | 49 | 47 | 42 |
| Dietary intake | ||||||
| Total energy, kcal/d | 1492 ± 401 | 1547 ± 383 | 1723 ± 395 | 1883 ± 440 | 1922 ± 473 | 1942 ± 489 |
| Alcohol, g/d | 5.6 ± 9.0 | 5.7 ± 8.5 | 5.9 ± 8.5 | 6.3 ± 9.2 | 6.3 ± 9.4 | 6.1 ± 9.8 |
| Egg, units/d | 0.02 ± 0.01 | 0.09 ± 0.03 | 0.27 ± 0.08 | 0.50 ± 0.08 | 0.81 ± 0.07 | 1.38 ± 0.52 |
| Red meat, servings/d | 0.69 ± 0.45 | 0.73 ± 0.40 | 0.81 ± 0.40 | 0.96 ± 0.47 | 1.03 ± 0.62 | 1.02 ± 0.62 |
| Bacon, servings/d | 0.04 ± 0.07 | 0.05 ± 0.06 | 0.08 ± 0.09 | 0.13 ± 0.13 | 0.17 ± 0.22 | 0.19 ± 0.31 |
| Other processed meat, servings/d | 0.12 ± 0.15 | 0.15 ± 0.15 | 0.19 ± 0.17 | 0.23 ± 0.21 | 0.25 ± 0.24 | 0.25 ± 0.29 |
| Refined grains, servings/d | 1.11 ± 0.73 | 1.14 ± 0.69 | 1.28 ± 0.73 | 1.45 ± 0.86 | 1.48 ± 1.02 | 1.52 ± 1.15 |
| Potatoes, servings/d | 0.43 ± 0.29 | 0.43 ± 0.27 | 0.48 ± 0.26 | 0.53 ± 0.29 | 0.53 ± 0.39 | 0.51 ± 0.38 |
| Fruits, servings/d | 1.69 ± 1.10 | 1.68 ± 0.99 | 1.85 ± 0.96 | 1.95 ± 1.05 | 1.99 ± 1.19 | 1.98 ± 1.31 |
| Vegetables, servings/d | 3.20 ± 1.67 | 3.14 ± 1.50 | 3.41 ± 1.51 | 3.49 ± 1.61 | 3.44 ± 1.75 | 3.42 ± 2.01 |
| Full-fat milk, servings/d | 0.11 ± 0.30 | 0.12 ± 0.28 | 0.13 ± 0.29 | 0.19 ± 0.39 | 0.24 ± 0.47 | 0.28 ± 0.54 |
| Coffee, servings/d | 2.11 ± 1.59 | 2.14 ± 1.49 | 2.24 ± 1.47 | 2.36 ± 1.56 | 2.46 ± 1.71 | 2.44 ± 1.74 |
| Fruit juice, servings/d | 0.64 ± 0.57 | 0.66 ± 0.55 | 0.73 ± 0.55 | 0.79 ± 0.59 | 0.76 ± 0.60 | 0.72 ± 0.64 |
| Sugar-sweetened beverages, servings/d | 0.32 ± 0.56 | 0.31 ± 0.51 | 0.30 ± 0.45 | 0.33 ± 0.49 | 0.36 ± 0.57 | 0.34 ± 0.57 |
| Cholesterol, mg | 171 ± 54 | 198 ± 51 | 262 ± 58 | 343 ± 65 | 432 ± 77 | 577 ± 165 |
| NHS II (1999) | ||||||
| Participants, n | 8958 | 35,281 | 31,984 | 8338 | 753 | 416 |
| Age,2 y | 44.3 ± 4.7 | 44.0 ± 4.7 | 44.3 ± 4.6 | 44.6 ± 4.6 | 45.0 ± 4.5 | 45.3 ± 4.6 |
| BMI, kg/m2 | 25.4 ± 5.7 | 26.1 ± 5.8 | 26.7 ± 6.2 | 27.3 ± 6.6 | 27.7 ± 6.6 | 28.8 ± 8.2 |
| Physical activity, MET-h/wk | 22.7 ± 27.2 | 18.8 ± 23.2 | 17.8 ± 21.8 | 17.4 ± 21.8 | 17.5 ± 26.2 | 19.4 ± 28.7 |
| Caucasian, % | 96 | 97 | 97 | 96 | 96 | 95 |
| Current smoker, % | 9 | 9 | 9 | 10 | 9 | 9 |
| Family history of diabetes, % | 31 | 33 | 34 | 34 | 33 | 35 |
| History of hypertension, % | 14 | 14 | 15 | 16 | 17 | 21 |
| History of hypercholesterolemia, % | 25 | 24 | 24 | 22 | 24 | 24 |
| Current statin use, % | 4 | 3 | 3 | 2 | 3 | 2 |
| Current multivitamin use, % | 55 | 55 | 58 | 57 | 60 | 62 |
| Fasting blood glucose screening,3 % | 42 | 44 | 45 | 43 | 44 | 45 |
| Dietary intake | ||||||
| Total energy, kcal/d | 1569 ± 446 | 1700 ± 444 | 1906 ± 464 | 2115 ± 493 | 2056 ± 537 | 2148 ± 520 |
| Alcohol, g/d | 3.1 ± 5.4 | 3.3 ± 5.6 | 3.6 ± 6.1 | 3.7 ± 6.5 | 3.6 ± 6.9 | 2.8 ± 5.3 |
| Egg, units/d | 0.01 ± 0.01 | 0.08 ± 0.02 | 0.23 ± 0.07 | 0.47 ± 0.07 | 0.83 ± 0.08 | 1.32 ± 0.48 |
| Red meat, servings/d | 0.50 ± 0.40 | 0.64 ± 0.40 | 0.75 ± 0.44 | 0.87 ± 0.51 | 0.86 ± 0.54 | 0.92 ± 0.66 |
| Bacon, servings/d | 0.02 ± 0.05 | 0.04 ± 0.05 | 0.07 ± 0.08 | 0.11 ± 0.14 | 0.18 ± 0.26 | 0.21 ± 0.29 |
| Other processed meat, servings/d | 0.09 ± 0.14 | 0.13 ± 0.14 | 0.18 ± 0.17 | 0.22 ± 0.22 | 0.23 ± 0.24 | 0.26 ± 0.32 |
| Refined grains, servings/d | 1.38 ± 0.82 | 1.43 ± 0.78 | 1.62 ± 0.86 | 1.82 ± 0.98 | 1.67 ± 1.06 | 1.92 ± 1.35 |
| Potatoes, servings/d | 0.43 ± 0.31 | 0.49 ± 0.30 | 0.55 ± 0.32 | 0.62 ± 0.35 | 0.58 ± 0.47 | 0.59 ± 0.42 |
| Fruits, servings/d | 1.24 ± 0.98 | 1.17 ± 0.82 | 1.26 ± 0.81 | 1.33 ± 0.87 | 1.34 ± 0.98 | 1.34 ± 0.96 |
| Vegetables, servings/d | 3.41 ± 2.15 | 3.27 ± 1.80 | 3.59 ± 1.85 | 3.81 ± 1.96 | 3.95 ± 2.14 | 4.02 ± 2.27 |
| Full-fat milk, servings/d | 0.03 ± 0.16 | 0.05 ± 0.20 | 0.06 ± 0.23 | 0.09 ± 0.31 | 0.09 ± 0.28 | 0.14 ± 0.43 |
| Coffee, servings/d | 1.44 ± 1.50 | 1.51 ± 1.48 | 1.58 ± 1.49 | 1.64 ± 1.57 | 1.70 ± 1.65 | 1.54 ± 1.61 |
| Fruit juice, servings/d | 0.56 ± 0.69 | 0.59 ± 0.62 | 0.69 ± 0.65 | 0.78 ± 0.71 | 0.68 ± 0.73 | 0.71 ± 0.67 |
| Sugar-sweetened beverages, servings/d | 0.40 ± 0.73 | 0.47 ± 0.73 | 0.50 ± 0.74 | 0.58 ± 0.81 | 0.48 ± 0.77 | 0.52 ± 1.05 |
| Cholesterol, mg | 162 ± 61 | 200 ± 59 | 255 ± 65 | 329 ± 71 | 405 ± 77 | 510 ± 132 |
| HPFS (1998) | ||||||
| Participants, n | 4520 | 9665 | 13,013 | 5514 | 903 | 839 |
| Age,2 y | 63.6 ± 8.8 | 63.4 ± 8.8 | 64.2 ± 9.0 | 64.9 ± 9.0 | 64.5 ± 8.8 | 64.9 ± 8.9 |
| BMI, kg/m2 | 25.4 ± 3.5 | 25.9 ± 3.5 | 26.2 ± 3.5 | 26.7 ± 4.2 | 26.5 ± 3.6 | 26.3 ± 3.8 |
| Physical activity, MET-h/wk | 38.1 ± 41.6 | 36.1 ± 40.5 | 33.7 ± 37.5 | 34.2 ± 39.7 | 34.6 ± 37.4 | 37.9 ± 44.8 |
| Caucasian, % | 95 | 95 | 96 | 95 | 95 | 96 |
| Current smoker, % | 3 | 4 | 6 | 8 | 10 | 12 |
| Family history of diabetes, % | 14 | 14 | 14 | 13 | 13 | 11 |
| History of hypertension, % | 43 | 42 | 42 | 40 | 36 | 35 |
| History of hypercholesterolemia, % | 56 | 51 | 47 | 38 | 36 | 30 |
| Current statin use, % | 24 | 19 | 14 | 8 | 6 | 6 |
| Current multivitamin use, % | 62 | 61 | 60 | 60 | 61 | 60 |
| Fasting blood glucose screening,3 % | 68 | 69 | 67 | 61 | 58 | 55 |
| Dietary intake | ||||||
| Total energy, kcal/d | 1779 ± 482 | 1862 ± 482 | 2022 ± 511 | 2192 ± 571 | 2281 ± 555 | 2365 ± 560 |
| Alcohol, g/d | 8.9 ± 12.0 | 10.1 ± 12.5 | 11.5 ± 13.3 | 12.5 ± 14.3 | 11.4 ± 12.9 | 12.3 ± 15.3 |
| Egg, units/d | 0.01 ± 0.01 | 0.08 ± 0.03 | 0.25 ± 0.08 | 0.48 ± 0.08 | 0.83 ± 0.07 | 1.56 ± 0.52 |
| Red meat, servings/d | 0.47 ± 0.44 | 0.63 ± 0.43 | 0.78 ± 0.46 | 0.94 ± 0.53 | 1.02 ± 0.57 | 1.16 ± 0.69 |
| Bacon, servings/d | 0.02 ± 0.06 | 0.05 ± 0.08 | 0.09 ± 0.10 | 0.16 ± 0.17 | 0.25 ± 0.26 | 0.36 ± 0.40 |
| Other processed meat, servings/d | 0.10 ± 0.18 | 0.16 ± 0.19 | 0.23 ± 0.23 | 0.28 ± 0.28 | 0.32 ± 0.31 | 0.33 ± 0.33 |
| Refined grains, servings/d | 1.19 ± 0.82 | 1.19 ± 0.78 | 1.31 ± 0.83 | 1.44 ± 0.96 | 1.55 ± 1.03 | 1.64 ± 1.16 |
| Potatoes, servings/d | 0.46 ± 0.33 | 0.51 ± 0.32 | 0.57 ± 0.32 | 0.63 ± 0.36 | 0.67 ± 0.39 | 0.69 ± 0.38 |
| Fruits, servings/d | 1.94 ± 1.43 | 1.70 ± 1.18 | 1.62 ± 1.06 | 1.57 ± 1.05 | 1.61 ± 1.12 | 1.45 ± 0.99 |
| Vegetables, servings/d | 3.99 ± 2.32 | 3.69 ± 1.88 | 3.70 ± 1.82 | 3.73 ± 1.81 | 3.87 ± 1.92 | 3.73 ± 1.74 |
| Full-fat milk, servings/d | 0.03 ± 0.15 | 0.06 ± 0.24 | 0.09 ± 0.29 | 0.15 ± 0.38 | 0.23 ± 0.51 | 0.27 ± 0.53 |
| Coffee, servings/d | 1.48 ± 1.45 | 1.74 ± 1.51 | 1.94 ± 1.54 | 2.10 ± 1.60 | 2.12 ± 1.51 | 2.40 ± 1.77 |
| Fruit juice, servings/d | 0.84 ± 0.76 | 0.79 ± 0.67 | 0.80 ± 0.65 | 0.82 ± 0.71 | 0.82 ± 0.70 | 0.82 ± 0.76 |
| Sugar-sweetened beverages, servings/d | 0.28 ± 0.49 | 0.33 ± 0.46 | 0.36 ± 0.50 | 0.42 ± 0.55 | 0.43 ± 0.54 | 0.40 ± 0.55 |
| Cholesterol, mg | 180 ± 64 | 217 ± 65 | 273 ± 72 | 350 ± 81 | 438 ± 83 | 600 ± 144 |
Values are means ± SDs or percentages and are standardized to the age distribution of the study population in 1998/1999 (i.e., approximately the midpoint of follow-up). HPFS, Health Professionals’ Follow-up Study; MET, metabolic equivalent of task; NHS, Nurses’ Health Study.
Value is not age-adjusted.
Values refer to the first assessment of fasting blood glucose examination (1998 for the NHS, 2001 for the NHS II, and 2000 for the HPFS).
Table 2 presents HRs of incident T2D according to categories of whole egg consumption. In the pooled analyses adjusted for age alone (model 1), an increase of 1 whole egg per day was associated with a 52% higher risk of T2D (HR: 1.52; 95% CI: 1.46, 1.59). After adjustment for lifestyle factors (model 2), the association was attenuated but remained significant (HR: 1.26; 95% CI: 1.20, 1.32). Additional adjustment for foods commonly consumed with eggs (model 3) further attenuated the association such that each egg per day was associated with a 14% higher T2D risk (HR: 1.14; 95% CI: 1.07, 1.20).
TABLE 2.
HRs (95% CIs) for incident type 2 diabetes according to whole egg consumption in the NHS, the NHS II, and the HPFS1
| Frequency of egg consumption | P for trend2 | HR (95% CI) per 1-egg/d increase | ||||||
|---|---|---|---|---|---|---|---|---|
| <1/mo | 1 to <4/mo | 1 to <3/wk | 3 to <5/wk | 5 to <7/wk | ≥1/d | |||
| NHS (n = 82,750) | ||||||||
| Cases/person-years | 248/78,663 | 1500/380,448 | 4416/1,024,000 | 2404/600,955 | 394/96,090 | 264/67,968 | ||
| Model 13 | 1.00 (Ref) | 1.09 (0.95, 1.25) | 1.21 (1.06, 1.38) | 1.54 (1.35, 1.76) | 1.69 (1.44, 1.98) | 1.83 (1.53, 2.18) | <0.0001 | 1.52 (1.42, 1.62) |
| Model 24 | 1.00 (Ref) | 1.09 (0.95, 1.25) | 1.11 (0.98, 1.27) | 1.31 (1.15, 1.50) | 1.37 (1.16, 1.61) | 1.43 (1.20, 1.70) | <0.0001 | 1.26 (1.17, 1.36) |
| Model 35 | 1.00 (Ref) | 1.08 (0.94, 1.23) | 1.06 (0.93, 1.21) | 1.22 (1.06, 1.39) | 1.25 (1.06, 1.48) | 1.32 (1.10, 1.58) | <0.0001 | 1.19 (1.10, 1.29) |
| NHS II (n = 89,636) | ||||||||
| Cases/person-years | 531/261,236 | 2362/829,877 | 3189/857,139 | 982/290,016 | 148/27,049 | 70/14,099 | ||
| Model 13 | 1.00 (Ref) | 1.21 (1.10, 1.33) | 1.48 (1.35, 1.62) | 1.79 (1.61, 1.99) | 2.38 (1.98, 2.85) | 2.17 (1.69, 2.79) | <0.0001 | 1.99 (1.82, 2.18) |
| Model 24 | 1.00 (Ref) | 1.08 (0.98, 1.19) | 1.19 (1.08, 1.31) | 1.28 (1.15, 1.43) | 1.48 (1.23, 1.78) | 1.33 (1.03, 1.72) | <0.0001 | 1.35 (1.22, 1.49) |
| Model 35 | 1.00 (Ref) | 1.05 (0.95, 1.15) | 1.09 (0.99, 1.20) | 1.13 (1.01, 1.26) | 1.28 (1.06, 1.55) | 1.14 (0.88, 1.47) | 0.007 | 1.15 (1.02, 1.30) |
| HPFS (n = 41,412) | ||||||||
| Cases/person-years | 340/113,957 | 944/253,241 | 1498/345,060 | 915/218,960 | 165/36,212 | 144/34,989 | ||
| Model 13 | 1.00 (Ref) | 1.23 (1.08, 1.39) | 1.42 (1.26, 1.60) | 1.59 (1.40, 1.80) | 1.70 (1.41, 2.05) | 1.54 (1.26, 1.87) | <0.0001 | 1.29 (1.18, 1.40) |
| Model 24 | 1.00 (Ref) | 1.14 (1.01, 1.30) | 1.25 (1.11, 1.42) | 1.37 (1.21, 1.56) | 1.44 (1.19, 1.75) | 1.36 (1.11, 1.66) | <0.0001 | 1.19 (1.09, 1.30) |
| Model 35 | 1.00 (Ref) | 1.10 (0.96, 1.25) | 1.13 (0.99, 1.28) | 1.18 (1.02, 1.36) | 1.21 (0.98, 1.48) | 1.14 (0.92, 1.42) | 0.15 | 1.07 (0.96, 1.18) |
| Pooled results (n = 213,798) | ||||||||
| Cases/person-years | 1119/453,856 | 4806/1,463,566 | 9103/2,226,199 | 4301/1,109,931 | 707/159,351 | 478/117,056 | ||
| Model 13 | 1.00 (Ref) | 1.18 (1.11, 1.26) | 1.38 (1.29, 1.47) | 1.69 (1.58, 1.80) | 1.90 (1.72, 2.09) | 1.86 (1.67, 2.07) | <0.0001 | 1.52 (1.46, 1.59) |
| Model 24 | 1.00 (Ref) | 1.11 (1.04, 1.19) | 1.19 (1.11, 1.27) | 1.34 (1.26, 1.44) | 1.43 (1.30, 1.58) | 1.41 (1.27, 1.58) | <0.0001 | 1.26 (1.20, 1.32) |
| Model 35 | 1.00 (Ref) | 1.07 (1.00, 1.14) | 1.09 (1.02, 1.16) | 1.18 (1.10, 1.27) | 1.24 (1.12, 1.37) | 1.23 (1.09, 1.37) | <0.0001 | 1.14 (1.07, 1.20) |
HPFS, Health Professionals’ Follow-up Study; NHS, Nurses’ Health Study.
P values for trend were based on a continuous egg variable derived from the median egg intake in each category of consumption.
Cox proportional hazards regression model 1 was stratified by calendar year in 2-y intervals and by cohort (in pooled analysis only), and adjusted for age (mo).
Cox proportional hazards regression model 2 included model 1 and was further adjusted for race/ethnicity (Caucasian/other), family history of diabetes (yes/no), history of hypertension at baseline (yes/no), history of hypercholesterolemia at baseline (yes/no), smoking status (never, former, current), BMI (in kg/m2: <21.0, 21.0–22.9, 23.0–24.9, 25.0–26.9, 27.0–29.9, 30.0–34.9, ≥35.0), physical activity (MET-h/wk: <3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, ≥27.0), oral contraceptive use (never, former, current; in the NHS II only), postmenopausal hormone use (premenopausal, never, former, current; in the NHS and NHS II only), statin use (yes/no), cumulative average alcohol intake (g/d; quintiles), multivitamin use (yes/no), and physical examination during the 2-y cycle (yes/no). All covariates (except race, family history of diabetes, baseline hypercholesterolemia, and baseline hypertension) were updated every 2 y.
Cox proportional hazards regression model 3 included model 2 with updated cumulative average of daily intake of total calories (quintiles), bacon (servings/d; in categories), red meat (servings/d; in categories), other processed red meats (servings/d; in categories), refined grains (servings/d; in categories), potatoes (servings/d; in categories), fruits (servings/d; in categories), vegetables (servings/d; in categories), full-fat milk (servings/d; in categories), coffee (servings/d; in categories), fruit juices (servings/d; in categories), and sugar-sweetened beverages (servings/d; in categories).
In sensitivity analyses (Supplemental Table 2), when we adjusted for AHEI instead of individual foods in the main multivariable model, the association remained unchanged (HR for 1 whole egg per day: 1.17; 95% CI: 1.11, 1.23). An increase of 1 whole egg per day was also associated with a higher risk of T2D when we stopped updating dietary information after diagnosis with an intermediate outcome (hypertension, hypercholesterolemia, angina, beginning statin use, or coronary artery bypass graft procedure; HR: 1.08; 95% CI: 1.02, 1.15), when we used a simple update of diet (HR: 1.13; 95% CI: 1.09, 1.18), and when we used the mean intakes from the 2 most recent FFQs (HR: 1.15; 95% CI: 1.09, 1.20), but not when we used only the baseline diet (HR: 1.02; 95% CI: 0.98, 1.07). The association remained unchanged when we restricted the analysis to symptomatic diabetes cases (HR for 1 egg/d: 1.15; 95% CI: 1.06, 1.25).
We examined the risk of incident T2D associated with the consumption of ≥2 eggs/d (Supplemental Table 3). The pooled HR for ≥2 eggs/d compared with <1 egg/mo was 1.23 (95% CI: 0.97, 1.57). Finally, when we examined total egg intake (i.e., consumption of whole eggs plus eggs in baked goods), we observed similar results to the main analysis (Supplemental Table 2) (HR for 1 egg/d: 1.11; 95% CI: 1.05, 1.17).
In the stratified analyses (Supplemental Table 4), we found no interaction between egg intake and age or BMI, but a significant one with AHEI score (P for interaction = 0.03). The HR for T2D associated with a 1-egg/d increase among individuals with AHEI scores below the median appeared higher (HR: 1.16; 95% CI: 1.08, 1.25) than among individuals with AHEI scores above the median (HR: 1.09; 95% CI: 0.99, 1.20).
We statistically estimated that replacing 1 whole egg per day with 1 serving of yogurt (no information on fat content available), whole grains, nuts, reduced-fat milk (0%–2%), high-fat cheese, or full-fat milk was associated with a 9%–19% lower risk of T2D (Figure 1). Statistical replacement of 1 whole egg per day with 1 serving of legumes, potatoes, refined grains, poultry, low-fat cheese, unprocessed meat, fish, or processed meat was not associated with T2D risk.
FIGURE 1.
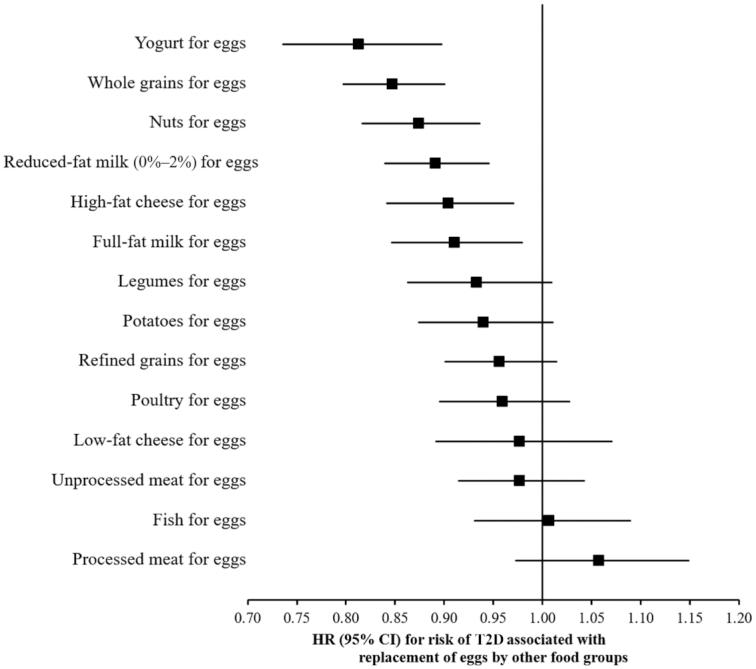
Statistical model–based HRs and 95% CIs for incident T2D associated with replacing 1 egg/d with 1 serving/d of other foods in the NHS, the NHS II, and the HPFS (pooled analysis, n = 213,798). In the substitution analyses, Cox proportional hazards regression models were stratified by calendar time (in 2-y intervals) and cohort, and adjusted for age (mo), race (Caucasian/other), family history of diabetes (yes/no), baseline history of hypercholesterolemia (yes/no), baseline history of hypertension (yes/no), smoking status (never, former, current), BMI (in kg/m2: <21.0, 21.0–22.9, 23.0–24.9, 25.0–26.9, 27.0–29.9, 30.0–34.9, ≥35.0), physical activity (MET-h/wk: <3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, ≥27.0), oral contraceptive use (never, former, current; in the NHS II only), postmenopausal hormone use (premenopausal, never, former, current; in the NHS and NHS II only), statin use (yes/no), cumulative average alcohol intake (g/d; quintiles), multivitamin use (yes/no), and physical examination during the 2-y cycle (yes/no). The model also included updated cumulative average of daily intake of total calories (kcal/d; in quintiles), bacon (servings/d; in categories), unprocessed red meat (servings/d; in categories), other processed red meats (servings/d; in categories), refined grains (servings/d; in categories), fruits (servings/d; in categories), vegetables (servings/d; in categories), potatoes (servings/d; in categories), full-fat milk (servings/d; in categories), coffee (servings/d; in categories), fruit juices (servings/d; in categories), and sugar-sweetened beverages (servings/d; in categories). All covariates (except race, family history of diabetes, baseline hypercholesterolemia, and basline hypertension) were updated every 2 y. HPFS, Health Professionals’ Follow-up Study; NHS, Nurses’ Health Study; T2D, type 2 diabetes.
Systematic review and meta-analysis
Supplemental Figure 2 presents the flow diagram of the literature search. A total of 415 references were identified and reviewed. Fifteen articles (16 including the current study) met the inclusion criteria for the meta-analysis (10, 12–16, 37–42, 44–46). Supplemental Table 5 presents the characteristics of these studies. Six studies were conducted among US cohorts, 8 among European cohorts, and 2 among Asian cohorts. Supplemental Table 6 presents the list of covariates used in the multivariable model of each study. Thirteen studies controlled for all primary confounders, and 8 studies controlled adequately for both primary and secondary confounders. Supplemental Table 7 presents the assessment of risk of bias using the NOS. Ten studies were considered at low risk of bias, having obtained a score ≥7 out of 9. The remaining 6 were considered at unclear or high risk of bias.
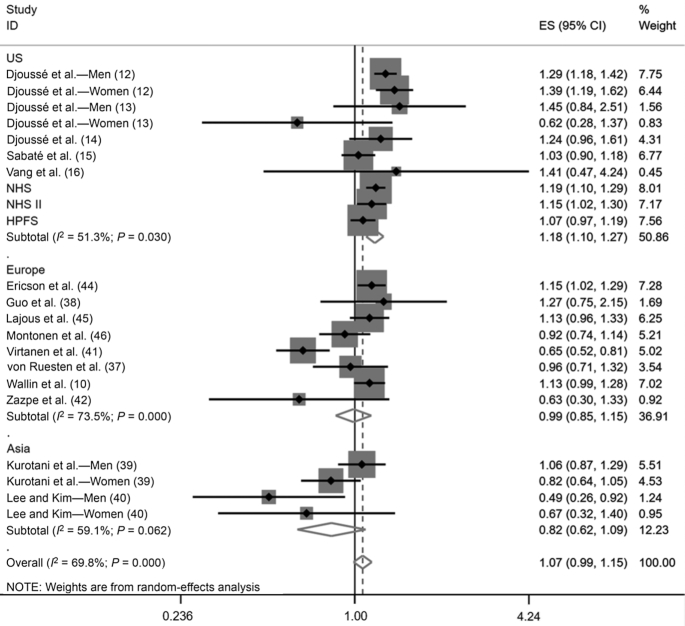
The meta-analysis comprised 22 risk estimates, 589,559 participants, and 41,248 incident cases of T2D. Using random-effects dose-response meta-analysis, the pooled RR of T2D associated with a 1-egg/d increase was 1.07 (95% CI: 0.99, 1.15). There was evidence of substantial heterogeneity between studies (I2 = 69.8%). No single study was found to contribute a significant amount of heterogeneity in the influence analysis (Supplemental Figure 3). However, significant differences between geographic regions were found (Figure 2) (P for interaction = 0.01). We found that a 1-egg/d increase in consumption was associated with a higher risk of T2D among US studies (339,377 participants; 28,528 cases; RR: 1.18; 95% CI: 1.10, 1.27; I2 = 51.3%), but not among European (179,714 participants; 10,698 cases; RR: 0.99; 95% CI: 0.85, 1.15; I2 = 73.5%) or Asian (70,468 participants; 2022 cases; RR: 0.82; 95% CI: 0.62, 1.09; I2 = 59.1%) studies. Still, there was evidence of moderate to substantial heterogeneity within each geographic stratum. Results were similar when we evaluated the association of high compared with low egg intake with the risk of T2D (Supplemental Figure 4). We found no interaction when we stratified analyses according to sex, number of subjects, risk of bias, dietary assessment, or adjustments for dietary confounders (Table 3). None of these stratifications provided pooled RRs with low heterogeneity among each subgroup. Egger's test was suggestive of publication bias (P = 0.03), as was Begg's test (P = 0.06) (Supplemental Figure 5).
FIGURE 2.
Association of egg consumption with T2D risk, for a 1-egg/d increase, stratified by geographic region and using random-effects meta-analysis (589,559 participants; 41,248 incident T2D cases). Weights of each of the studies are represented by the size of the square. Black diamonds represent the individual study effects and black lines represent the 95% CIs. The overall effect estimate and 95% CI are represented by the dotted line and white diamonds respectively. P value for interaction between geographic regions, tested using meta-regression = 0.01. ES, effect size; HPFS, Health Professionals’ Follow-up Study; NHS, Nurses’ Health Study; T2D, type 2 diabetes.
TABLE 3.
Prespecified subgroup meta-analyses for type 2 diabetes risk, per 1-egg/d increase, using random-effects models1
| Stratification, subcategories | Risk estimates, n | Participants, n | Cases, n | Pooled RR (95% CI) | I 2, % | P-interaction |
|---|---|---|---|---|---|---|
| Region | 0.01 | |||||
| United States | 10 | 339,377 | 28,528 | 1.18 (1.10, 1.27) | 51.3 | |
| Europe | 8 | 179,714 | 10,698 | 0.99 (0.85, 1.15) | 73.5 | |
| Asia | 4 | 70,468 | 2022 | 0.82 (0.62, 1.09) | 59.1 | |
| Sex | 0.68 | |||||
| Men | 9 | 153,935 | 11,998 | 1.01 (0.86, 1.19) | 81.4 | |
| Women | 7 | 316,176 | 21,502 | 1.12 (0.99, 1.26) | 66.4 | |
| Both | 6 | 119,448 | 7748 | 1.07 (0.99, 1.17) | 69.8 | |
| Subjects, n | 0.54 | |||||
| <10,000 | 9 | 31,188 | 3179 | 0.90 (0.71, 1.16) | 67.1 | |
| >10,000 | 13 | 558,371 | 38,069 | 1.13 (1.06, 1.20) | 57.6 | |
| Risk of bias2 | 0.78 | |||||
| Low | 15 | 500,718 | 36,793 | 1.08 (0.99, 1.17) | 75.9 | |
| Unclear to high | 7 | 88,841 | 4455 | 1.03 (0.89, 1.19) | 25.8 | |
| Dietary assessment | 0.89 | |||||
| Baseline only | 11 | 279,005 | 16,358 | 1.05 (0.93, 1.18) | 74.1 | |
| Repeated measurements | 11 | 310,554 | 24,890 | 1.09 (0.99, 1.20) | 65.0 | |
| Adjustment for dietary confounders3 | 0.47 | |||||
| Suboptimal | 10 | 108,114 | 9215 | 1.10 (0.94, 1.29) | 79.8 | |
| Sufficient | 12 | 481,445 | 32,033 | 1.06 (0.99, 1.14) | 50.9 |
NOS, Newcastle-Ottawa Scale.
Low: NOS score ≥7; unclear to high risk of bias: NOS score <7.
According to the comparability criteria for control for secondary confounders of the NOS.
Discussion
In these 3 large prospective cohort studies of US women and men, we observed that consumption of each egg per day was associated with a 14% higher risk of T2D, after adjustment for lifestyle factors and foods commonly consumed with eggs. In the updated meta-analysis of 589,559 participants and 41,248 incident cases of T2D, we found no significant association between egg consumption and T2D risk overall. There was, however, evidence of substantial heterogeneity between studies (I2 = 69.8%) and of significant differences between geographic regions (P for interaction = 0.01). Indeed, among US studies, for each egg per day, T2D risk was higher by 18%, but no association was found among European and Asian studies.
The current results are consistent with previous meta-analyses of prospective cohort studies in which a positive association between egg consumption and T2D risk was reported among US cohorts, but not among non-US studies (9–11). One explanation for these discrepancies may be related to egg consumption habits. Indeed, considering the overall low amounts of egg intake observed in our cohorts and in cohorts included in the meta-analysis (most individuals consumed 1 to <5 eggs/wk), it is possible that the association was mainly driven by the egg consumption pattern, rather than by egg consumption per se. In the United States, egg consumption is reflective of adherence to a Western dietary pattern because eggs are often consumed with red or processed meat, refined grains, and sugary beverages (12–15). In Europe, egg consumption is also mainly reflective of adherence to a Western dietary pattern, but this varies between Mediterranean and Northern European countries (10, 38, 41, 42, 45). This may explain the lack of an association and the substantial heterogeneity we observed among European countries in our updated meta-analysis. Among Asian cohorts, the lack of an association we observed needs to be interpreted in the context that, in Asian cultures, eggs are typically incorporated into various cuisines, which differs from Western egg consumption habits. The potential influence of the background diet on the relation between egg consumption and risk of T2D is further exemplified by our AHEI-stratified analysis. In our cohort analyses, we documented a significant positive association even after adjusting for foods associated with both egg intake and T2D risk (red and processed meat, refined grains, sugary beverages, etc.). However, when we stratified our analysis according to AHEI which is an overall measure of diet quality, the positive association of a 1-egg/d increase with T2D risk appeared significant among participants with low diet quality (HR: 1.16; 95% CI: 1.08, 1.25), but not among those with higher diet quality (HR: 1.09; 95% CI: 0.99, 1.20; P for interaction = 0.03). Similarly, Sabaté et al. (15) observed that the relation between egg consumption and T2D risk was modified by concomitant meat consumption in the Adventist Health Study 2. Indeed, egg consumption with low concomitant meat consumption was not associated with a higher risk of T2D, whereas egg consumption with high concomitant meat consumption was associated with a higher risk. This further suggests that even careful adjustments for foods commonly consumed with eggs may not totally eliminate residual confounding related to egg consumption habits. Thus, the higher risk of T2D observed among US individuals needs to be interpreted carefully in the context that it may be a reflection of egg consumption habits.
Biological pathways by which egg consumption may lead to the onset of diabetes are not clearly defined. Eggs provide dietary cholesterol, and a variety of other nutrients such as unsaturated fatty acids, amino acids, and B-vitamins, which are unlikely to have adverse effects on glucose metabolism. However, eggs are also an important source of choline, and egg consumption has been demonstrated to increase plasma concentrations of trimethylamine N-oxide (TMAO), a metabolite of choline (47, 48). Plasma TMAO concentrations have been associated with alterations in glucose homeostasis in mice (49) and T2D risk in humans (50–52), hence it is plausible that choline metabolites are involved in the association between egg consumption and diabetes. On the other hand, TMAO has been linked with CAD risk as strongly as with T2D risk (53), and egg consumption is not consistently associated with CAD risk (54, 55). Also, most randomized dietary interventions that evaluated the impact of high egg consumption on cardiometabolic risk factors reported either neutral or slightly beneficial effects on markers of insulin sensitivity, inflammation, and blood lipids (56, 57). However, in the control arm of these studies, eggs were replaced with yolk-free egg substitute (58–61) or lean animal protein (meat, chicken, or fish) (62), which may influence the results. In our analyses, we statistically estimated that replacing eggs with nuts, whole grains, milk, yogurt, or high-fat cheese was associated with lower T2D risk, whereas consuming red meat, fish, poultry, legumes, refined grains, potatoes, or low-fat cheese in place of eggs was not associated with the risk. Beyond the estimated beneficial association with cardiometabolic health, replacing eggs with nuts or whole grains in the diet may also contribute to a more sustainable environment because of the lower environmental impact of producing nuts and grains than eggs (63).
Our analyses among the NHS, the NHS II, and the HPFS have several strengths including the large sample size, detailed and updated information on diet and lifestyle, high rates of follow-up, the number of T2D cases, as well as the length of follow-up. Also, the detailed information on diet collected on at least a quadrennial basis allowed us to make a better assessment of dietary factors related to both egg intake and T2D. Notably, when we used only the baseline dietary assessment, as has been done in most other studies, we did not find an association between egg intake and risk of diabetes. Lastly, the integration of our cohort analyses in an updated meta-analysis provides a comprehensive overview of evidence pertaining to egg intake and risk of T2D in the United States and globally.
There are limitations to our current analyses. First, in our cohorts, individuals with higher egg intake were generally less healthy in multiple ways. Thus, our results could be affected by unmeasured or residual confounding, even though we were able to account for many lifestyle and dietary covariates, including unprocessed and processed red meats. Second, our study may be less generalizable to other populations owing to the participants being predominantly Caucasians, but the higher education levels and knowledge about health in this population of health professionals enhanced the quality of the data collected. Third, misclassification of long-term dietary intakes assessed by any method is inevitable. However, such measurement errors tend to cause an underestimation of the association between egg intake and T2D because of the prospective design of the study. In addition, the use of cumulative average update of diet minimized random measurement error due to within-person variations. Fourth, our results need to be interpreted in the context that mean egg consumption in our cohorts and in cohorts included in the meta-analysis was relatively low because most individuals consumed 1 to <5 eggs/wk, and few participants consumed ≥1 egg/d. Finally, the substitution analyses were based on a statistical modeling strategy that used data across the whole population, without identifying participants who actually substituted eggs with the replacement food. Therefore, results from the substitution analyses should be interpreted with caution in the context of statistical modeling (33).
Our cohort analyses and updated meta-analysis suggest that daily egg consumption is associated with a modestly higher risk of T2D among US individuals, but not among Europeans or Asians. Given the overall low amount of egg intake observed in the cohorts included in this study, our results need to be interpreted carefully in the context that the higher risk of T2D observed among US individuals may not reflect egg consumption per se but rather egg consumption habits. Indeed, whether the heterogeneity of the associations among US, European, and Asian cohorts reflects differences in egg consumption habits warrants further investigation.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—J-PD-C, ALS, FBH, FMS, BR, MJS, and SNB: designed the research; J-PD-C and YL: performed the statistical analysis; J-PD-C and SC: conducted the systematic review; J-PD-C: conducted the meta-analysis and drafted the paper; SC, ALS, FBH, FMS, BR, JEM, MJS, and WCW: provided comments and edits for the paper; J-PD-C and SNB: had primary responsibility for the final content; and all authors: read and approved the final manuscript. J-PD-C received speaker and consulting honoraria from the Dairy Farmers of Canada in 2016 and 2018, outside the submitted work. YL received grants from the California Walnut Commission, outside the submitted work. All other authors report no conflicts of interest.
Notes
Supported by National Institute of Diabetes and Digestive and Kidney Diseases Career Development Grant K01 DK107804 (to SNB). The Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-up Study cohorts are supported by NIH grants UM1 CA186107, UM1 CA176726, UM1 CA167552, DK112940, U01 HL145386, U01 CA176726, U01 CA167552, and P30 DK46200.
Supplemental Tables 1–7 and Supplemental Figures 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the article, code book, and analytic code will not be made publicly available. Further information including the procedures to obtain and access data from the Nurses’ Health Studies and Health Professionals’ Follow-up Study is described at http://www.nurseshealthstudy.org/researchers (email: nhsaccess@channing.harvard.edu) and http://sites.sph.harvard.edu/hpfs/for-collaborators, respectively.
Abbreviations used: AHEI, Alternative Healthy Eating Index; CAD, coronary artery disease; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; NOS, Newcastle-Ottawa Scale; OGTT, oral-glucose-tolerance test; TMAO, trimethylamine N-oxide; T2D, type 2 diabetes.
Contributor Information
Jean-Philippe Drouin-Chartier, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Centre for Nutrition, Health and Society (NUTRISS), Laval University, Québec, Quebec, Canada; Institute on Nutrition and Functional Foods (INAF), Laval University, Québec, Quebec, Canada; Faculty of Pharmacy, Laval University, Québec, Quebec, Canada.
Amanda L Schwab, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Siyu Chen, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Yanping Li, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Frank M Sacks, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Bernard Rosner, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA; Department of Biostatistics, Harvard TH Chan School of Public Health, Boston, MA, USA.
JoAnn E Manson, Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA; Preventive Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA.
Walter C Willett, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA.
Meir J Stampfer, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA.
Frank B Hu, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA.
Shilpa N Bhupathiraju, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
References
- 1. Clayton ZS, Fusco E, Kern M. Egg consumption and heart health: a review. Nutrition. 2017;37:79–85. [DOI] [PubMed] [Google Scholar]
- 2. Griffin JD, Lichtenstein AH. Dietary cholesterol and plasma lipoprotein profiles: randomized controlled trials. Curr Nutr Rep. 2013;2(4):274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fuller NR, Sainsbury A, Caterson ID, Markovic TP. Egg consumption and human cardio-metabolic health in people with and without diabetes. Nutrients. 2015;7(9):7399–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture. Washington (DC): USDA, Agricultural Research Service; 2015. p. 3. [Google Scholar]
- 5. Drouin-Chartier J-P, Chen S, Li Y, Schwab AL, Stampfer MJ, Sacks FM, Rosner B, Willett WC, Hu FB, Bhupathiraju SN. Egg consumption and risk of cardiovascular disease: three large prospective US cohort studies, systematic review, and updated meta-analysis. BMJ. 2020;368:m513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhong VW, Van Horn L, Cornelis MC, Wilkins JT, Ning H, Carnethon MR, Greenland P, Mentz RJ, Tucker KL, Zhao L et al. Associations of dietary cholesterol or egg consumption with incident cardiovascular disease and mortality. JAMA. 2019;321(11):1081–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. International Diabetes Federation (IDF). IDF diabetes atlas. 8th ed Brussels, Belgium: IDF; 2017. [Google Scholar]
- 9. Schwingshackl L, Hoffmann G, Lampousi AM, Knuppel S, Iqbal K, Schwedhelm C, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(5):363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wallin A, Forouhi NG, Wolk A, Larsson SC. Egg consumption and risk of type 2 diabetes: a prospective study and dose-response meta-analysis. Diabetologia. 2016;59(6):1204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tamez M, Virtanen JK, Lajous M. Egg consumption and risk of incident type 2 diabetes: a dose-response meta-analysis of prospective cohort studies. Br J Nutr. 2016;115(12):2212–18. [DOI] [PubMed] [Google Scholar]
- 12. Djoussé L, Gaziano JM, Buring JE, Lee I-M. Egg consumption and risk of type 2 diabetes in men and women. Diabetes Care. 2009;32(2):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Djoussé L, Kamineni A, Nelson TL, Carnethon M, Mozaffarian D, Siscovick D, Mukamal KJ. Egg consumption and risk of type 2 diabetes in older adults. Am J Clin Nutr. 2010;92(2):422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Djoussé L, Petrone AB, Hickson DA, Talegawkar SA, Dubbert PM, Taylor H, Tucker KL. Egg consumption and risk of type 2 diabetes among African Americans: the Jackson Heart Study. Clin Nutr. 2015;35(3):679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sabaté J, Burkholder-Cooley NM, Segovia-Siapco G, Oda K, Wells B, Orlich MJ, Fraser GE. Unscrambling the relations of egg and meat consumption with type 2 diabetes risk. Am J Clin Nutr. 2018;108(5):1121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vang A, Singh PN, Lee JW, Haddad EH, Brinegar CH. Meats, processed meats, obesity, weight gain and occurrence of diabetes among adults: findings from Adventist Health Studies. Ann Nutr Metab. 2008;52(2):96–104. [DOI] [PubMed] [Google Scholar]
- 17. Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 18. Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338(8765):464–8. [DOI] [PubMed] [Google Scholar]
- 19. Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, Stampfer MJ, Speizer FE, Spiegelman D, Manson JE. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278(13):1078–83. [PubMed] [Google Scholar]
- 20. Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–6. [DOI] [PubMed] [Google Scholar]
- 21. Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–67. [DOI] [PubMed] [Google Scholar]
- 22. Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, Rosner B, Hennekens CH, Speizer FE. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338(8770):774–8. [DOI] [PubMed] [Google Scholar]
- 23. Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161(12):1542–8. [DOI] [PubMed] [Google Scholar]
- 24. The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–97. [DOI] [PubMed] [Google Scholar]
- 25. Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–73. [DOI] [PubMed] [Google Scholar]
- 26. Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, Willett WC. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133(8):810–17. [DOI] [PubMed] [Google Scholar]
- 27. Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7(1):81–6. [DOI] [PubMed] [Google Scholar]
- 28. Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–40. [DOI] [PubMed] [Google Scholar]
- 29. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. [DOI] [PubMed] [Google Scholar]
- 30. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, Hu FB. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26(2):380–4. [DOI] [PubMed] [Google Scholar]
- 32. Pan A, Malik VS, Hao T, Willett WC, Mozaffarian D, Hu FB. Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int J Obes. 2013;37(10):1378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song M, Giovannucci E. Substitution analysis in nutritional epidemiology: proceed with caution. Eur J Epidemiol. 2018;33(2):137–40. [DOI] [PubMed] [Google Scholar]
- 34. Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Internet]. Ottawa: Ottawa Hospital Research Institute; Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [accessed October 2019]. [Google Scholar]
- 36. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6:40–57. [Google Scholar]
- 37. von Ruesten A, Feller S, Bergmann MM, Boeing H. Diet and risk of chronic diseases: results from the first 8 years of follow-up in the EPIC-Potsdam study. Eur J Clin Nutr. 2013;67(4):412–19. [DOI] [PubMed] [Google Scholar]
- 38. Guo J, Hobbs DA, Cockcroft JR, Elwood PC, Pickering JE, Lovegrove JA, Givens DI. Association between egg consumption and cardiovascular disease events, diabetes and all-cause mortality. Eur J Nutr. 2018;57(8):2943–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kurotani K, Nanri A, Goto A, Mizoue T, Noda M, Oba S, Sawada N, Tsugane S. Cholesterol and egg intakes and the risk of type 2 diabetes: the Japan Public Health Center-based Prospective Study. Br J Nutr. 2014;112(10):1636–43. [DOI] [PubMed] [Google Scholar]
- 40. Lee J, Kim J. Egg consumption is associated with a lower risk of type 2 diabetes in middle-aged and older men. Nutr Res Pract. 2018;12(5):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Virtanen JK, Mursu J, Tuomainen TP, Virtanen HE, Voutilainen S. Egg consumption and risk of incident type 2 diabetes in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2015;101(5):1088–96. [DOI] [PubMed] [Google Scholar]
- 42. Zazpe I, Beunza JJ, Bes-Rastrollo M, Basterra-Gortari FJ, Mari-Sanchis A, Martínez-González MÁ; SUN Project Investigators . Egg consumption and risk of type 2 diabetes in a Mediterranean cohort; the SUN project. Nutr Hosp. 2013;28(1):105–11. [DOI] [PubMed] [Google Scholar]
- 43. Higgins JP, Thomas J. Cochrane handbook for systematic reviews of interventions, Version 6. Chichester, UK: John Wiley & Sons; 2019. [Google Scholar]
- 44. Ericson U, Hellstrand S, Brunkwall L, Schulz CA, Sonestedt E, Wallström P, Gullberg B, Wirfält E, Orho-Melander M. Food sources of fat may clarify the inconsistent role of dietary fat intake for incidence of type 2 diabetes. Am J Clin Nutr. 2015;101(5):1065–80. [DOI] [PubMed] [Google Scholar]
- 45. Lajous M, Bijon A, Fagherazzi G, Balkau B, Boutron-Ruault MC, Clavel-Chapelon F. Egg and cholesterol intake and incident type 2 diabetes among French women. Br J Nutr. 2015;114(10):1667–73. [DOI] [PubMed] [Google Scholar]
- 46. Montonen J, Järvinen R, Heliövaara M, Reunanen A, Aromaa A, Knekt P. Food consumption and the incidence of type II diabetes mellitus. Eur J Clin Nutr. 2005;59(3):441–8. [DOI] [PubMed] [Google Scholar]
- 47. Miller CA, Corbin KD, da Costa KA, Zhang S, Zhao X, Galanko JA, Blevins T, Bennett BJ, O'Connor A, Zeisel SH. Effect of egg ingestion on trimethylamine-N-oxide production in humans: a randomized, controlled, dose-response study. Am J Clin Nutr. 2014;100(3):778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118(4):476–81. [DOI] [PubMed] [Google Scholar]
- 50. Shan Z, Sun T, Huang H, Chen S, Chen L, Luo C, Yang W, Yang X, Yao P, Cheng J et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr. 2017;106(3):888–94. [DOI] [PubMed] [Google Scholar]
- 51. Obeid R, Awwad HM, Rabagny Y, Graeber S, Herrmann W, Geisel J. Plasma trimethylamine N-oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am J Clin Nutr. 2016;103(3):703–11. [DOI] [PubMed] [Google Scholar]
- 52. Pan A, Qiu G, Wang H, He M, Yang M, Zhang X, Xiao Y, Li Y, Yuan Y, Wu T et al. Plasma metabolomics identified novel metabolites associated with risk of type 2 diabetes in two prospective cohorts of Chinese adults. Int J Epidemiol. 2016;45(5):1507–16. [DOI] [PubMed] [Google Scholar]
- 53. Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J Am Heart Assoc. 2017;6(7):e004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bechthold A, Boeing H, Schwedhelm C, Hoffmann G, Knüppel S, Iqbal K, De Henauw S, Michels N, Devleesschauwer B, Schlesinger S et al. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. 2019;59(7):1071–90. [DOI] [PubMed] [Google Scholar]
- 55. Rong Y, Chen L, Zhu T, Song Y, Yu M, Shan Z, Sands A, Hu FB, Liu L. Egg consumption and risk of coronary heart disease and stroke: dose-response meta-analysis of prospective cohort studies. BMJ. 2013;346:e8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Richard C, Cristall L, Fleming E, Lewis ED, Ricupero M, Jacobs RL, Field CJ. Impact of egg consumption on cardiovascular risk factors in individuals with type 2 diabetes and at risk for developing diabetes: a systematic review of randomized nutritional intervention studies. Can J Diabetes. 2017;41(4):453–63. [DOI] [PubMed] [Google Scholar]
- 57. Fuller NR, Sainsbury A, Caterson ID, Denyer G, Fong M, Gerofi J, Leung C, Lau NS, Williams KH, Januszewski AS et al. Effect of a high-egg diet on cardiometabolic risk factors in people with type 2 diabetes: the Diabetes and Egg (DIABEGG) Study—randomized weight-loss and follow-up phase. Am J Clin Nutr. 2018;107(6):921–31. [DOI] [PubMed] [Google Scholar]
- 58. Blesso CN, Andersen CJ, Barona J, Volek JS, Fernandez ML. Whole egg consumption improves lipoprotein profiles and insulin sensitivity to a greater extent than yolk-free egg substitute in individuals with metabolic syndrome. Metabolism. 2013;62(3):400–10. [DOI] [PubMed] [Google Scholar]
- 59. Blesso CN, Andersen CJ, Barona J, Volk B, Volek JS, Fernandez ML. Effects of carbohydrate restriction and dietary cholesterol provided by eggs on clinical risk factors in metabolic syndrome. J Clin Lipidol. 2013;7(5):463–71. [DOI] [PubMed] [Google Scholar]
- 60. Mutungi G, Ratliff J, Puglisi M, Torres-Gonzalez M, Vaishnav U, Leite JO, Quann E, Volek JS, Fernandez ML. Dietary cholesterol from eggs increases plasma HDL cholesterol in overweight men consuming a carbohydrate-restricted diet. J Nutr. 2008;138(2):272–6. [DOI] [PubMed] [Google Scholar]
- 61. Ratliff JC, Mutungi G, Puglisi MJ, Volek JS, Fernandez ML. Eggs modulate the inflammatory response to carbohydrate restricted diets in overweight men. Nutr Metab (Lond). 2008;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sainsbury A, Denyer G, Caterson ID, Gerofi J, Baqleh K, Williams KH, Fong M, Lau NS, Markovic TP, Fuller NR. The effect of a high-egg diet on cardiovascular risk factors in people with type 2 diabetes: the Diabetes and Egg (DIABEGG) study—a 3-mo randomized controlled trial. Am J Clin Nutr. 2015;101(4):705–13. [DOI] [PubMed] [Google Scholar]
- 63. Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, Garnett T, Tilman D, DeClerck F, Wood A et al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393(10170):447–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.