ABSTRACT
Background
Maternal iron absorption during pregnancy can be evaluated using RBC incorporation of orally administered stable iron isotope. This approach underestimates true maternal absorption of iron as it does not account for absorbed iron that is transferred to the fetus or retained within the placenta.
Objective
Our objective was to re-evaluate maternal iron absorption after factoring in these losses and identify factors associated with iron partitioning between the maternal, neonatal, and placental compartments.
Methods
This study utilized data from stable iron isotope studies carried out in 68 women during the third trimester of pregnancy. Iron status indicators and stable iron isotopic enrichment were measured in maternal blood, umbilical cord blood, and placental tissue when available. Factors associated with iron isotope partitioning between the maternal, neonatal, and placental compartments were identified.
Results
On average, true maternal absorption of iron increased by 10% (from 19% to 21%) after accounting for absorbed iron present in the newborn (P < 0.001), and further increased by 7%, (from 39% to 42%, P < 0.001) after accounting for iron retained within the placenta. On average, 2% of recovered tracer was present in the placenta and 6% was found in the newborn. Net transfer of iron to the neonate was higher in women with lower total body iron (standardized β = −0.48, P < 0.01) and lower maternal hepcidin (standardized β = −0.66, P < 0.01). In women carrying multiple fetuses, neonatal hepcidin explained a significant amount of observed variance in net placental transfer of absorbed iron (R = 0.95, P = 0.03).
Conclusions
Maternal RBC iron incorporation of an orally ingested tracer underestimated true maternal iron absorption. The degree of underestimation was greatest in women with low body iron. Maternal hepcidin was inversely associated with maternal RBC iron utilization, whereas neonatal hepcidin explained variance in net transfer of iron to the neonatal compartment.
These trials were registered at clinicaltrials.gov as NCT01019096 and NCT01582802.
Keywords: pregnancy, iron absorption, newborn, iron partitioning, hepcidin, stable isotope
Introduction
Maternal iron deficiency anemia is associated with a higher incidence of preterm birth, low birth weight (LBW), and intrauterine growth restriction (1–3); and an adequate iron supply is essential for normal fetal brain development (4). To prevent anemia, the Institute of Medicine recommends universal iron supplementation for all pregnant women, regardless of maternal age or the number of fetuses carried (5). The US Preventative Services Task Force has concluded that there is insufficient evidence to document the benefits and/or risks of universal iron supplementation throughout pregnancy (6, 7). Determination of risks and benefits of universal iron supplementation is constrained by insufficient knowledge regarding gestational adaptions in iron physiology and lack of guidelines for the interpretation of iron status indicators as a function of gestational stage (6).
Iron balance is largely controlled at the enterocyte by the hormone hepcidin. Iron absorption must be tightly regulated as humans have no physiologic means of excreting excess iron. During pregnancy, iron requirements increase to support increased erythropoiesis, given plasma volume expansion, as well as fetal/placental iron needs. Early iron radioisotope studies (8, 9) and subsequent stable iron isotope studies (10–12) have provided key data on maternal iron utilization across gestation. These studies quantified maternal iron absorption based on the amount of orally administered iron tracer that was incorporated into maternal red blood cells (RBCs), assuming a fixed fraction of absorbed tracer is incorporated into RBCs. If a second iron isotope is administered intravenously, the fraction of iron incorporated into maternal RBCs can be directly measured and used to improve estimates of maternal iron absorption (8, 12–14). In nonpregnant populations this approach likely captures the majority of absorbed iron, but in pregnant women this methodology fails to account for maternally absorbed iron that is transferred to the fetus or retained within the placenta, and thus underestimates true maternal iron absorption. At this time, the degree of underestimation as well as the factors that impact relative utilization of absorbed iron between the maternal, fetal, or placental compartments are unknown.
A more accurate understanding of iron utilization by the mother, fetus, and placenta is needed. To address this issue, we pooled existing and new data from stable iron isotope studies conducted in maternal–neonatal dyads to obtain novel data on relative maternal, neonatal, and placental iron utilization. The primary objective of this study was to obtain a more accurate estimation of true maternal iron absorption during pregnancy. A secondary objective was to evaluate the impact of maternal and neonatal iron status and iron regulatory hormones on the observed variability in partitioning of absorbed iron between the mother, placenta, and fetus.
Methods
Participants
Stable iron isotope studies were undertaken in a total of 68 women studied during the third trimester of pregnancy. The population of 68 women included 63 women carrying singletons (10, 14), of whom 43 women (age 18–30 y) were recruited from the community of Villa El Salvador, Peru (studied between 1995 and 1997), and 20 women (age 16–32 y) were recruited from Rochester, New York (studied between 2008 and 2009) (Supplemental Figure 1). Data on iron transfer to the fetus in these women have been published (15, 16). A third data set comprised unpublished data from 5 women carrying multiple fetuses (n = 4 carrying twins, n = 1 carrying triplets) who participated in iron absorption studies in Rochester, New York, between 2013 and 2014. All women were healthy with no pre-existing conditions known to be associated with alterations in iron physiology. Informed consent was obtained from all participants. The studies undertaken in Rochester, New York, were approved by the Institutional Review Boards of the University of Rochester and Cornell University, and the study undertaken in Peru was approved by the Instituto de Investigación Nutricional and the Committee on Human Research at The Johns Hopkins School of Hygiene and Public Health.
Iron isotope preparation
Oral stable iron isotope for all studies was purchased as the metal (57Fe at 94.67–94.69%) and converted into ferrous sulfate following published procedures (17). Metal tracer was converted to ferrous sulfate and administered orally as an aqueous solution. In the Peru study, the ferrous sulfate was administered without ascorbic acid, and in the Rochester studies the ferrous sulfate solution contained ascorbic acid in a 2:1 molar ratio of ascorbic acid to iron. All stable iron isotopic analyses were completed using a magnetic sector thermal ionization mass spectrometer [MAT 261 (Finnigan) or Triton TI (Thermo Fisher Scientific)].
Study design and isotope dosing
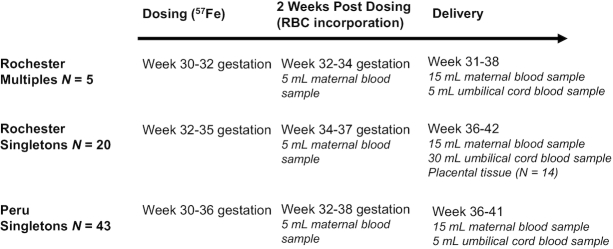
The study design is presented in Figure 1. Fasting women received an oral dose of 57Fe averaging 9.32 ± 1.03 mg (ranging from 6.08 to 10.22 mg). Two-thirds of the Peruvian women also consumed an additional 50 mg supplemental Fe (as ferrous sulfate) with the tracer because the intent of the study was to evaluate iron absorption from a typical supplemental dose of iron used in Peru at the time this study was undertaken. All women remained fasting for 1.5 h postdosing, and a maternal blood sample was obtained 2 wk postdosing to measure iron isotope incorporation into maternal RBCs. At delivery, an additional sample of maternal blood and 5–15 mL of umbilical cord blood were collected to assess isotope incorporation into neonatal RBCs to quantify net transfer of iron tracer to the fetus. Maternal and umbilical cord serum was isolated and stored at −80°C until utilized to evaluate iron status indicators.
FIGURE 1.
Study design and timing of iron isotope dosing and sample collections in each cohort.
Biochemical assessment of iron status indicators
Within the Peru cohort, hemoglobin (Hb) was measured using the cyanomethemoglobin method, whereas Hb concentrations in the Rochester cohorts were measured using a Cell-Dyn 4000 hematology analyzer (Abbott Diagnostics). Maternal anemia in late gestation was defined as an Hb concentration <11.0 g/dL (18), and neonatal anemia was defined as a cord Hb concentration <13.0 g/dL (19). In the Rochester cohorts, serum ferritin (SF) and serum transferrin receptor (sTfR) were measured by ELISA (Ramco Laboratories), as previously described (20, 21). In the Peru cohort, sTfR was measured by the Quantikine ELISA (R&D Systems) and SF was measured by ELISA (DAKO). Although commercial sTfR kits are not standardized, the strong correlation between R&D Systems method and Ramco (22) has previously been used to justify the use of TfR data from both assays to evaluate total body iron (TBI) as described by Cook et al. (23) and Mei et al. (24). TBI in all women was calculated with the following equation: TBI (mg/kg) = -[log (sTfR/SF) − 2.8229]/0.1207. The use of the TBI estimate has been documented in pregnant women (24). In the Rochester cohorts, folate and vitamin B-12 were analyzed by immunoassay (Siemens Immulite 2000), and in the Peru cohort they were measured by radioimmunoassay (Diagnostic Products Corporation Immulite 2500). Folate deficiency was defined when folate was <6.8 nmol/L and vitamin B-12 deficiency was defined when concentrations were <148 pmol/mL (25, 26). Hepcidin in the multiples cohort was measured using an enzyme immunoassay kit (Bachem) (range: 0–25 ng/mL) or by ELISA in the Rochester singletons cohort (Intrinsic Lifesciences) (range: 2.5–1000 ng/mL). Erythropoietin (EPO) was measured in both Rochester cohorts by an immunoassay system (Siemens Immulite 1000). In statistical analyses, the cohort was controlled for to account for possible differences in assay methodology. When placental samples were obtained, the iron content of the placenta was measured by a atomic absorption spectrophotometry (Perkin-Elmer Analyst 800) (27).
Iron isolation from biological samples and mass spectrometric analysis
Whole blood (1 mL) and ∼1 g placental tissue (a mixture of tissue was pooled from multiple sites sampled across the syncytiotrophoblast) were digested with 5–15 mL Ultrex nitric acid (JT Baker) and evaporated to dryness. The dried residue was reconstituted in 2–4 mL 6N Ultrex hydrochloric acid (JT Baker), extracted using anion exchange chromatography, and the eluate was heated until dry. The iron residue was reconstituted in 30 µL of 3% nitric acid as previously described (14). Extracted iron samples were loaded onto rhenium filaments and isotopic ratios (57/56Fe, 58/56Fe, and 54/56Fe) were measured using magnetic sector thermal ionization MS, as previously described [Triton (ThermoFisher) or MAT 261 (Finnigan)] (14–16).
Approaches used to evaluate maternal iron absorption and iron partitioning
Stable iron isotope enrichment in maternal blood, placental tissue, and umbilical cord blood was expressed as a delta percent excess (Δ% excess), which is the degree to which the measured 57/56Fe ratio was increased over the corresponding natural abundance 57/56Fe ratio. In the maternal compartment, the net 57Fe recovered was also determined using the 57/56Fe enrichment of maternal RBCs 14 d postdosing, maternal weight, Hb concentration, maternal blood volume (70 mL/kg) (14), and the iron content of Hb (3.47 g/kg) (28), and assuming 80% of absorbed iron was incorporated into maternal RBCs.
The net quantity of 57Fe recovered (milligrams) in the neonate was estimated at birth using the same approach and an estimated newborn blood volume of 80 mL/kg (15). In the newborn, it was assumed that 80% of absorbed isotope was incorporated into RBCs (13, 29), based on autopsy data showing that 75–80% of the total iron present in the newborn is found within their RBCs (30, 31). Net 57Fe recovered in placental tissue was calculated using the untrimmed placental wet weight, 57/56Fe enrichment, and the iron content of the placenta. To account for excess weight contributed by the placental membranes and umbilical cord, a factor of 16% was removed from the untrimmed weight (32). Iron recovered in each compartment was expressed as either the net milligram quantity of 57Fe recovered or as the net milligrams of 57Fe recovered per kilogram of placental, or neonatal, birth weight.
Three different approaches were utilized to evaluate maternal iron absorption. The first approach followed standard methodology and evaluated 57Fe incorporation into maternal RBCs 14 d postdosing (M absorption; n = 66). The second approach also factored in the total amount of 57Fe recovered in neonatal RBCs at birth (M+N absorption; n = 60). The third approach included the total amount of 57Fe recovered in placental tissue when available (M + N + P absorption; n = 14).
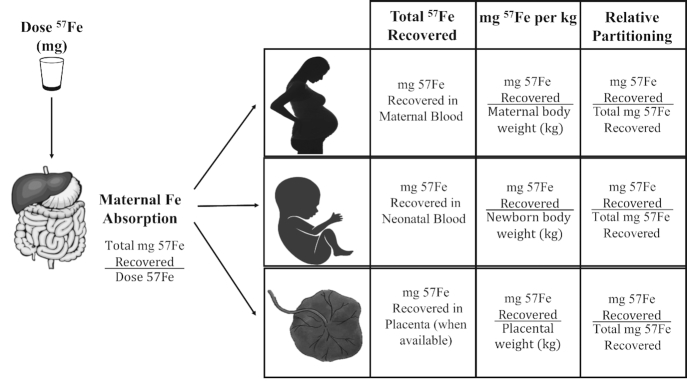
Iron partitioning between the maternal, neonatal, or placental compartment was calculated as the net 57Fe recovered (milligrams) in each compartment as a fraction of the total 57Fe recovered (milligrams) in all 3 compartments. The variable approaches utilized are depicted in Figure 2. To control for the slight differences in dose of stable isotope consumed by each women, all values were dose adjusted to a mean dose of 8.35 mg (9). Significant findings remained significant if nonadjusted doses were utilized.
FIGURE 2.
Diagram depicting calculations used to determine maternal absorption, total iron recovered, milligrams of iron per kilogram of weight, and partitioning of iron between maternal, neonatal, and placental compartments. Maternal absorption was calculated using RBC iron incorporation of 57Fe 2 wk postdosing. Stable iron enrichment in the neonatal and placental compartments was calculated using samples collected at birth.
Statistical analysis
All statistical analyses were completed using JMP 13.0 (SAS Institute). Nonnormally distributed values were transformed prior to statistical analysis. Possible differences by cohort were assessed by ANOVA, chi-square, or Wilcoxon rank sum test. Multiple linear regression analysis was used to assess relations between iron absorption and partitioning with iron status indicators and hormones while controlling for study and with maternal ID as a random effect to control for neonates in the multiples cohort being born from the same mother. Significance was considered P < 0.05. The sβ abbreviation indicates standardized β-coefficients reported for individual correlations between iron regulatory hormones and iron recovery that are controlled for study.
Results
Subject characteristics
Maternal and neonatal characteristics are presented in Table 1. Women carrying multiple fetuses were significantly older than women carrying singletons (P < 0.001), and maternal BMI at entry into the study differed significantly by cohort (P < 0.001). None of the women in this partitioning study developed pre-eclampsia. Twenty-six percent of newborns were small for gestational age (SGA; n = 13/41 Peru and n = 4/31 Rochester). Although the majority of the neonates born to women carrying multiples were LBW, none were classified as SGA. In the multiples cohort, discordant growth was not evident among neonates based on the American College of Obstetricians and Gynecologists threshold of a 15–25% difference in birth weight between siblings (33).
TABLE 1.
Maternal and neonatal characteristics1
| Singletons | ||||
|---|---|---|---|---|
| Variable | Combined | Peru | Rochester | Multiples |
| Maternal, n | 68 | 43 | 20 | N = 5 |
| Age, y | 22.73 ± 5.00 | 23.26 ± 4.38a | 19.55 ± 3.87b | 31.0 ± 2.34c |
| BMI2 | 28.95 ± 5.64 | 26.48 ± 2.66b | 32.52 ± 6.67a | 35.64 ± 7.74a |
| Neonatal, n | 74 | 42 | 19 | 11 |
| GA, wk | 38.63 ± 0.05 | 39.75 ± 1.00a | 39.52 ± 1.60a | 33.81 ± 2.52b |
| PTB, % | 20 | 03a | 16a | 82b |
| Birth weight, g | 2970.96 ± 587.91 | 3104.14 ± 417.56a | 3169.74 ± 548.25a | 2107.00 ± 492.79b |
| LBW, % | 21 | 05a | 21b | 82c |
| SGA, % | 26 | 37a | 21b | 0c |
| Male, % | 52 | 44 | 68 | 45 |
Values are means ± SDs unless otherwise indicated. Data were analyzed by Wilcoxon rank sum test, ANOVA, or chi-square test. Different superscript letters indicate significant differences between cohorts. GA, gestational age; LBW, low birth weight; PTB, preterm birth; SGA, small for gestational age.
The BMI value obtained during the third trimester when dosing occured.
Maternal iron status indicators at delivery are presented in Table 2. At delivery, the prevalence of maternal anemia was 31% and did not significantly differ between the 3 cohorts (P = 0.83). None of the women from the Rochester cohorts were folate deficient (serum folate <6.8 nmol/L); however, 21% of women in the Rochester singletons cohort were vitamin B-12 deficient (serum vitamin B-12 <148 pmol/L). In the Peru cohort, 11% of women were folate deficient and 37% were vitamin B-12 deficient. Data on umbilical cord iron status indicators are also presented in Table 2. In the combined cohort, the prevalence of neonatal anemia was 14%. The prevalence of neonatal anemia was significantly higher in the Rochester singletons cohort (P < 0.01), consistent with other findings in neonates born to adolescents and with animal data showing altered nutrient partitioning in adolescent pregnancies (21, 34). Folate status was adequate in all newborns, but 25% of all newborns studied were vitamin B-12 deficient (12/24 in the Peru cohort and 1/18 in the Rochester singletons cohort).
TABLE 2.
Maternal and neonatal iron status indicators at delivery1
| Singletons | |||
|---|---|---|---|
| Peru | Rochester | Multiples | |
| Maternal indicators, n | 43 | 20 | 5 |
| Hb, g/dL | 11.92 ± 1.77 | 11.15 ± 1.18 | 11.64 ± 2.30 |
| Anemic, % | 30 | 37 | 20 |
| SF, μg/L | 32.49 [23.37, 45.17] | 19.47 [12.19, 23.54] | 27.50 [7.50, 100.93] |
| <20 μg/L | 33 | 40 | 67 |
| <12 μg/L | 17 | 22 | 33 |
| sTfR, mg/L | 3.66 [2.98, 4.49] | 6.26 [5.01, 7.83] | 5.53 [0.61, 49.66] |
| TBI, mg/kg | 3.39 ± 3.72 | 2.30 ± 3.03 | 2.89 ± 5.98 |
| Hepcidin, ng/mL | — | 36.92 ± 58.20 [2.5, 207.1] | 9.59 ± 12.89 [1.2, 24.4] |
| EPO, IU/L | — | 32.61 ± 23.00 [9.4, 109.5] | 44.7 ± 38.24 [10, 185.7] |
| Folate, nmol/L | 12.42b [10.30, 14.97] | 37.40a [30.20, 46.32] | 20.32ab [8.83, 46.81] |
| Deficient, % | 11 | 0 | 0 |
| Vitamin B-12, pmol/L | 178.52 ± 81.57a | 262.21 ± 123.03b | 343 ± 101.06b |
| Deficient, % | 37 | 21 | 0 |
| Neonatal indicators, n | 42 | 20 | 11 |
| Hb, g/dL | 15.71 ± 1.89a | 13.36 ± 3.05b | 15.37 ± 1.13a |
| Anemic, % | 7a | 37b | 0a |
| SF, μg/L | 167.84 ± 77.68 | 202.01 ± 115.97 | 148.46 ± 52.83 |
| sTfR, mg/L | 6.23 ± 1.79 | 8.35 ± 2.15 | 7.68 ± 2.55 |
| TBI, mg/kg | 10.18 ± 2.49 | 9.43 ± 2.80 | 9.10 ± 2.61 |
| Hepcidin, ng/mL | — | 62.08 [8.5, 95.94] | 16.13 [9.58, 27.16] |
| EPO, IU/L | — | 28.13 [17.07, 46.35] | 14.92 [10.42, 21.37] |
| Folate, nmol/L | 24.36 ± 8.50b | 69.44 ± 26.61a | 85.59 ± 23.54a |
| Deficient, % | 0 | 0 | 0 |
| Vitamin B-12, pmol/L | 140.91b [110.72, 179.33] | 510.88a [348.05, 749.89] | 408.58a [298.94, 558.42] |
| Deficient, % | 50b | 6a | 0a |
Values are means ± SDs or geometric means [95% CIs] unless otherwise indicated. Data were analyzed by ANOVA or chi-square test to assess indicator differences between cohorts measured with the same assay. Different superscript letters indicate significant differences between cohorts. EPO, erythropoietin; Hb, hemoglobin; SF, serum ferritin; sTfR, soluble transferrin receptor; TBI, total body iron.
Maternal tracer enrichment and net amount of tracer recovered in maternal RBCs
The net milligrams of 57Fe recovered in the maternal compartment significantly differed by cohort (P < 0.001) (Table 3). Maternal RBC 57Fe incorporation (M absorption) averaged 19% (95% CI: 15%, 22%) and is presented in Table 4. Maternal TBI explained 70% of the observed variance in net 57Fe recovered (milligrams) in maternal RBCs (sβ = −0.19, P = 0.01, n = 60) and M absorption (sβ = −0.19, P = 0.01, n = 60). M absorption was positively associated with maternal EPO (sβ = 0.04, P = 0.05, n = 21) and negatively associated with maternal hepcidin (sβ = −0.48, P = 0.04, n = 21).
TABLE 3.
Iron isotope enrichment in maternal and neonatal blood1
| Observed isotope enrichment, % | Total net 57Fe recovered, mg | Net 57Fe recovered, mg/kg | Partitioning, % | |
|---|---|---|---|---|
| All groups | ||||
| Mothers | 3.27 [2.90, 3.89]a; n = 64 | 1.24 [1.21, 1.55]a; n = 66 | 0.02 [0.01, 0.02]; n = 66 | 91.21 [90.12, 92.31]a; n = 61 |
| Infants | 3.66 [3.06, 4.31]b; n = 61 | 0.11 [0.10, 0.13]b; n = 61 | 0.03 [0.03, 0.4]b; n = 61 | 8.12 [7.11, 9.19]b; n = 61 |
| Total | — | 1.50 [1.24, 1.80]c; n = 61 | — | — |
| Peru singletons | ||||
| Mothers | 2.54 [2.13, 2.98]; n = 43 | 0.72 [0.59, 0.87]a; n = 43 | 0.01 [0.01, 0.01]a; n = 43 | 90.01 [88.48, 91.55]b; n = 37 |
| Infants | 2.47 [2.05, 2.92]; n = 37 | 0.07 [0.06, 0.08]b; n = 37 | 0.02 [0.02, 0.03]b; n = 37 | 9.47 [8.10, 10.94]a; n = 37 |
| Total | — | 0.81 [0.66, 0.97]c; n = 37 | — | — |
| Rochester singletons | ||||
| Mothers | 5.21 [4.19, 6.35]a; n = 18 | 2.62 [2.19, 3.08]a; n = 18 | 0.03 [0.03, 0.04]; n = 18 | 94.14 [92.94, 95.35]b; n = 18 |
| Infants | 6.62 [5.21, 8.20]b; n = 19 | 0.17 [0.12, 0.23]b; n = 19 | 0.05 [0.04, 0.07]; n = 19 | 5.62 [4.52, 6.82]a; n = 18 |
| Total | — | 2.78 [2.32, 3.29]c; n = 18 | — | — |
| Rochester multiples | ||||
| Mothers | 4.22 [2.70, 6.08]a; n = 5 | 2.45 [1.20, 4.13]a; n = 5 | 0.03 [0.02, 0.03]a; n = 5 | 90.53 [87.47, 93.64]b; n = 5 |
| Infant unit | 4.94 [4.08, 5.87]b; n = 5 | 0.21 [0.11, 0.34]b; n = 5 | 0.05 [0.03, 0.06]b; n = 5 | 8.32 [3.84, 14.52]a; n = 5 |
| Total | — | 2.55 [1.84, 3.36]c; n = 5 | — | — |
| Rochester singletons with placenta available | ||||
| Mothers | 5.20 [3.93, 6.48]; n = 14 | 2.62 [2.08, 3.16]; n = 14 | 0.03 [0.02, 0.04]; n = 14 | 92.27 [90.61, 93.93]; n = 14 |
| Infants | 6.59 [4.95, 8.23]; n = 14 | 0.18 [0.11, 0.24]; n = 14 | 0.06 [0.04, 0.07]; n = 14 | 5.91 [4.43, 7.38]; n = 14 |
| Placenta | 5.91[4.58, 7.24]; n = 14 | 0.05 [0.03, 0.06]; n = 14 | 0.10 [0.07, 0.12]; n = 14 | 1.82 [1.44, 2.20]; n = 14 |
| Total | — | 2.82 [2.22, 3.42]; n = 14 | — | — |
Values are means [95% CIs] unless otherwise indicated. Differences between mothers, infants, and/or placenta were analyzed by ANOVA. Different superscript letters indicate significant differences within cohorts between the mother, neonate, and placenta.
TABLE 4.
Percent iron absorption after inclusion of 57Fe recovered from the neonatal and placental compartments1
| Compartments included in analysis | Singletons | |||
|---|---|---|---|---|
| Combined | Peru | Rochester | Multiples | |
| Mother | 19.14 [15.45, 23.24]; n = 66 | 10.84a [8.87, 13.01]; n = 43 | 39.17b [32.81, 46.10]; n = 18 | 36.61b [17.96, 61.83]; n = 5 |
| Mother and neonate | 21.26 [17.22, 25.73]; n = 60 | 12.13a [9.93, 14.55]; n = 37 | 41.68b [34.764, 49.22]; n = 18 | 39.92b [20.53, 65.70]; n = 5 |
| Difference in absorption | 1.56 [1.29, 1.86]; n = 60 | 1.13b [0.96, 1.31]; n = 37 | 2.62a [1.84, 3.41]; n = 18 | 3.26a [1.37, 5.16]; n = 5 |
| n = 14 | ||||
| Mother | 39.19 ± 13.92 | |||
| Mother and neonate | 41.86 ± 15.16 | |||
| Mother, neonate, and placenta | 42.21 ± 15.50 | |||
| Difference in absorption | 3.42 ± 2.10 | |||
Values are means ± SDs or geometric means [95% CIs] unless otherwise indicated. Differences between absorption estimations were analyzed by ANOVA. Different superscript letters indicate significant differences within each cohort.
Neonatal tracer enrichment and net amount of tracer recovered in neonatal umbilical cord RBCs
The net milligrams of 57Fe recovered in the neonatal compartment significantly differed by cohort (P < 0.001) (Table 3). Net 57Fe recovered (milligrams) in the neonate was significantly higher in neonates born to anemic mothers (P = 0.01, n = 60) and in those born to women with depleted iron stores (SF < 20 μg/L; P < 0.001, n = 59). In the combined cohort, there was no significant association between net amount of 57Fe (milligrams) recovered in the neonatal compartment and the number of days that had elapsed between dosing and parturition (across a range of 7–69 d). Net 57Fe recovered (milligrams) in the neonatal compartment was also positively associated with net 57Fe recovered (milligrams) in the maternal compartment (R2 = 0.59 P < 0.001, n = 60). The strongest determinants of net 57Fe recovered (milligrams) in the neonatal compartment were maternal TBI (sβ = −0.48, P < 0.001, n = 52) and neonatal Hb (sβ = 0.37, P < 0.001, n = 52), which together explained 77% of the variance in net 57Fe recovered (milligrams) in neonatal RBCs (P < 0.001). In women with iron regulatory hormone data available, women with higher hepcidin concentrations transferred less iron tracer to their neonates (sβ = −0.66, P < 0.01, n = 25). Similarly, neonates with higher hepcidin concentrations at birth had a lower quantity of tracer present in neonatal RBCs at birth (sβ = −0.42, P = 0.06, n = 29). Maternal EPO was positively associated with iron transfer to the neonatal compartment (sβ = 0.61, P = 0.01, n = 25). Neonates with higher EPO had significantly less iron tracer transferred across the placenta (sβ = −0.44, P = 0.02, n = 29). The only significant correlation between maternal and neonatal hormones was a positive association between maternal hepcidin and neonatal EPO (sβ = 0.44, P = 0.03, n = 25).
As expected, neonates born to women carrying multiple fetuses weighed significantly less at birth and were born at a significantly earlier gestational age. To address the possible impact of variability in neonatal birth weight on the net amount of 57Fe recovered, 57Fe recovery data were expressed per kilograms of birth weight (Table 3). Significant determinants of neonatal 57Fe (milligrams per kilogram) were neonatal Hb and maternal TBI, which explained 73% of the variance in this measure (P < 0.001). In cohorts with iron regulatory hormone data available, net 57Fe mg/kg was significantly inversely associated with maternal hepcidin (sβ = −0.66, P < 0.01, n = 25). Neonates had a significantly greater 57Fe content on a mg/kg basis when maternal EPO concentrations were elevated (sβ = 0.46, P = 0.02, n = 25) or when neonatal EPO concentrations were low (sβ = −0.48, P < 0.01, n = 29). As previously mentioned, maternal and neonatal EPO concentrations were not significantly correlated (sβ = 0.001, P = 0.9, n = 25).
When the total amount of 57Fe recovered in both maternal and neonatal compartments was used to calculate net M + N iron absorption, (n = 60), the estimation of true maternal absorption increased by 10% (P < 0.001)—that is, from 19% (M absorption) to 21% (95% CI: 17%, 26%) (M + N absorption) (Table 4). As expected, anemic mothers (n = 21) absorbed more 57Fe than nonanemic women (P = 0.02, n = 39), regardless of approach used (M or M + N absorption). Additionally, M + N absorption was higher in women with SF <20 μg/L (n = 39) compared with those with SF >20 μg/L (n = 20; P < 0.001 ). Maternal TBI and neonatal Hb together explained 73% of variance found in M + N absorption (P < 0.001). In women with regulatory hormone data available, M + N absorption was associated with neonatal hepcidin (sβ = −0.70, P < 0.01, n = 23), maternal EPO (sβ = 0.56, P = 0.01, n = 21), and maternal hepcidin (sβ = −0.50, P = 0.02, n = 21).
Placental tracer enrichment
In the current study, net placental iron content averaged 39.4 mg (95% CI: 32.6, 46.2 mg) or 74.3 μg/g wet weight (95% CI: 66.2, 82.4 μg/g). Placental iron content did not significantly differ between mothers who did (n = 4) or did not (n = 10) have anemia (P = 0.80). Placental iron content also did not significantly differ between mothers with (n = 5) or without (n = 9) iron depletion (SF <20 μg/L) at delivery (P = 0.92). Placental iron content was lower in women with (n = 9) gestational iron depletion (based on SF <20 μg/L), a difference that approached significance (P = 0.14). Maternal TBI was inversely associated with the net amount of 57Fe recovered in the placental compartment (on both a milligram and a milligram per kilogram basis) and explained 40% and 36% of variance, respectively (both P < 0.02).
Within the group of 14 women who had placental tissue available, M absorption averaged 39% (95% CI: 31%, 47%) and M + N absorption averaged 41% (95% CI: 33%, 51%). After the addition of the net amount of 57Fe recovered in the placental compartment, the estimation of true maternal absorption increased by 7% (P < 0.001) to 42% (95% CI: 33%, 51%) (M + N + P absorption). M + N + P absorption was significantly associated with maternal Hb and TfR and neonatal SF, which together explained 70% of the variance in true maternal absorption (P = 0.002, n = 14).
Tracer enrichment in women carrying multiple fetuses
In women carrying multiple fetuses, each individual neonate accrued a significantly lower amount of tracer compared with the mean accrued by each singleton neonate [0.09 mg (95% CI: 0.07, 0.13) versus 0.17 mg (95% CI: 0.12, 0.23); P = 0.02]. However, if the net 57Fe transferred (milligrams) to all siblings was summed, the net amount of tracer that each women in the multiples cohort transferred to their newborns was comparable to the amount of 57Fe transferred (milligrams) to each singleton neonate (0.21 versus 0.17 mg; P = 0.30) (Table 3). In addition, neonates in both cohorts accrued a similar amount of 57Fe when expressed per kilogram of birth weight (0.054 versus 0.045 mg/kg; P = 0.53).
In the multiples cohort, variability between neonates in the same uterine compartment was calculated as the largest difference observed between siblings at birth. The mean Δ% excess in this group was 5.02% ± 1.39% and the average variability between sibling was 0.62% (95% CI: 0.24%, 1.47%). The mean net 57Fe recovered (milligrams) in this group was 0.10 ± 0.05 mg, and the average variability between siblings was 0.015 mg (95% CI: 0.002, 0.027 mg). The mean net 57Fe recovered (milligrams) per kilogram of birth weight in this group was 0.05 ± 0.01 mg/kg, and the average variability between siblings was 0.01 mg/kg (95% CI: 0.003, 0.0096 mg). The strongest determinant of the observed variability between all 3 measurements of 57Fe transfer to the neonate was the variability in hepcidin between neonates [Δ% excess: R = 0.95 P = 0.03; net 57Fe (mg): R = 0.41, P = 0.60; net 57Fe (mg/kg): R = 0.47, P = 52].
Iron partitioning between compartments
Relative utilization of maternally absorbed iron between the maternal, neonatal, and placental compartments was explored. In the combined cohort, the highest percentage of recovered 57Fe tracer was utilized by the maternal compartment (Table 3, Figure 3). The strongest negative determinant of relative partitioning of absorbed 57Fe to the neonatal compartment was maternal TBI (sβ = −0.31, P = 0.01, n = 58), with lower maternal TBI being associated with significantly greater transfer of maternally absorbed tracer to the fetus. Maternal TBI explained 35% of the variance in partitioning of 57Fe between maternal and neonatal compartments. Partitioning of iron to the neonatal compartment was significantly inversely associated with total 57Fe recovered (milligrams) (sβ = −0.52 P = 0.01, n = 60). Iron partitioning to the neonatal compartment was significantly different by cohort (P = 0.001), with Rochester singletons receiving significantly less absorbed iron. This finding did not appear to be driven by differences in maternal age as partitioning was not significantly associated with maternal age when examined as a continuous variable (P = 0.21) or categorically in pregnant adolescents (<20 y; n = 19) compared with those aged >20 y (n = 41, P = 0.24). In the subset of women with iron regulatory hormone data, relative partitioning of 57Fe to the neonatal compartment was significantly inversely associated with both maternal hepcidin (sβ = −0.55 P < 0.01, n = 24) and neonatal EPO (sβ = −0.52, P < 0.01, n = 22). Partitioning of 57Fe to the neonatal compartment was not significantly associated with maternal EPO or neonatal hepcidin.
FIGURE 3.
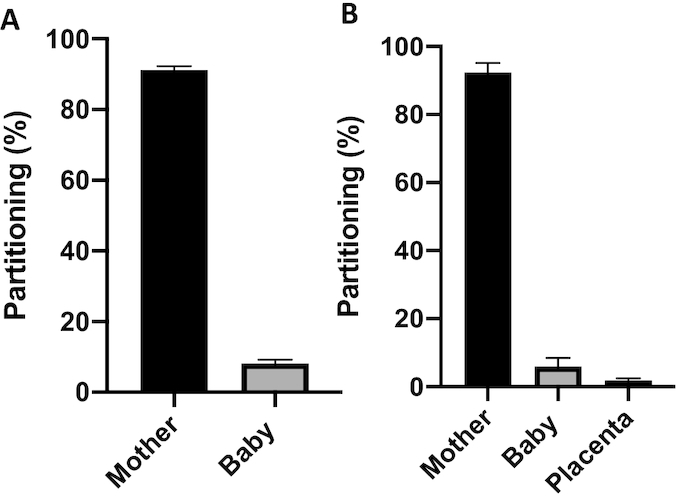
(A) Partitioning of iron between the maternal and neonatal compartment is shown for the combined cohort (n = 60). The bar chart presents the percentage (95% CI) of total iron partitioned to each compartment. In the multiples cohort, the baby component reflects the sum of all tracer recovered in the neonatal compartments. (B) Partitioning of iron between the maternal, neonatal, and placental compartments is shown for women in the Rochester cohort who had placental tissue available for analysis of isotopic enrichment.
Although not statistically significant, there were negative trends between placental partitioning and both maternal vitamin B-12 status (95% CI: −3.12, 0.69), maternal folate status (95% CI: −2.98, 0.10), and neonatal folate status (95% CI: −0.06, 0.01). Total tracer partitioning to the neonatal/placental unit was inversely associated with maternal hepcidin (95% CI: −2.10, −0.23) and maternal TBI (95% CI: −1.05, −0.23).
Discussion
This is the first human study to describe significantly higher maternal iron absorption during pregnancy after accounting for the amount of absorbed tracer that was transferred to the fetus or retained within the placenta. The degree of underestimation significantly varied as a function of maternal iron status and was greatest in women with depleted body iron stores. Both maternal and neonatal hepcidin concentrations were associated with net transfer of absorbed iron to the fetus. The impact of fetal hepcidin on iron utilization was also evident among the multiple-birth neonates, as in this group neonatal hepcidin concentrations captured the largest amount of variability in observed uptake of iron between siblings.
Maternally absorbed iron that is rapidly transferred to the neonatal/placental unit cannot readily be quantified, and therefore maternal absorption estimations used in the current dietary recommendations rely solely on estimates of iron utilized by maternal erythropoiesis (35, 36). Pregnant women rapidly transfer orally ingested radioisotopic iron to the fetus, as radioactivity has been detected in the infant within 40 min of the mother ingesting the iron radioisotope (37). In this group of pregnant women, ∼5% of maternally absorbed iron was present in the newborn at birth and 2% of maternally absorbed iron was retained within placental tissue. As expected, inclusion of the iron recovered within the neonate and placenta at birth resulted in a significant increase in true maternal iron absorption. The degree of underestimation was not constant but was instead impacted by maternal iron status, with iron-insufficient women transferring the largest fraction of absorbed iron to the neonatal/placental compartment.
The placenta mediates nutrient transfer to the fetus and is exposed to both maternal and fetal regulatory signals. Although total placental iron content was not significantly associated with maternal iron status, we found that the net amount of 57Fe retained in the placenta in late gestation (on both a milligram and milligram per kilogram basis) was inversely associated with maternal TBI in our population where 28% of women studied were anemic at delivery. Recent animal data found that acute placental uptake of an intravenous dose of stable iron isotope 6 h postdosing did not significantly differ by the iron status of the dam (38). To our knowledge, this is the first human study to describe human placental retention of an orally ingested iron tracer and to assess relations between placental retention of iron and maternal and neonatal iron status.
The developing fetus accumulates ∼300 mg Fe across the 280-d gestational period, with iron transfer being highest in the third trimester of pregnancy. In the current study, the net amount of 57Fe transferred to the fetus over late gestation and measured within the neonate at birth was inversely associated with maternal TBI. This association remained significant when expressed as net tracer recovered or when expressed as tracer recovered per kilogram of birth weight. Additionally, partitioning of absorbed iron to the neonatal compartment was higher in women with low TBI. While studies have found maternal iron deficiency compromises neonatal iron stores at birth (39–42), our dynamic measures of placental iron transfer and other studies evaluating placental iron trafficking proteins (38, 43–46) indicate that the human placenta responds to iron insufficiency by upregulating iron transfer to the neonatal compartment when maternal iron status is low.
Iron homeostasis and tissue utilization are regulated by 3 hormones; hepcidin, EPO, and erythroferrone (ERFE). A commercial assay for ERFE was not available when these studies were undertaken, but EPO and hepcidin data were available in a subset of these women and neonates. In the current study, maternal EPO concentrations were positively associated with both maternal absorption (both M and M + N absorption) and net transfer of absorbed 57Fe to the neonate. Interestingly, neonatal EPO was negatively associated with net 57Fe recovered in the neonate and partitioning of absorbed 57Fe to the neonatal compartment. Data on determinants of neonatal EPO concentrations at birth are limited. Studies suggest that umbilical cord EPO is increased in neonates who experience hypoxic intrauterine conditions (47, 48), as EPO is inversely correlated with cord blood pH, blood gas values (49, 50), and oxygen tension (51); however, some have not found significant associations between umbilical cord EPO and Hb (39, 52–55). The relation between EPO and iron transfer is supported by other human data showing that neonates with higher umbilical cord EPO concentrations had lower iron status as measured by cord ferritin (56, 57).
Maternal hepcidin concentrations were inversely associated with the net amount of iron transferred to the fetus in late gestation, consistent with other published human pregnancy data using stable iron isotopes (11, 15). Hepcidin concentrations increase to prevent iron overload, but this hormone is also impacted by inflammation and may be elevated in obese pregnant women (58–60) as well as in pregnant women with an increased risk of gestational diabetes (61). Given our observed association between elevated maternal hepcidin concentrations and reduced iron transfer and partitioning of absorbed iron to the neonatal compartment, iron transfer to the fetus may be compromised in these populations and further studies in these groups may be warranted.
Studies in women carrying multiple fetuses allowed us to evaluate the impact of neonatal iron status indicators on variability seen in 57Fe accrual between siblings. Cord hepcidin concentrations were a significant determinant of net 57Fe transfer to the neonate and of the observed 57Fe variability between siblings at birth. This finding is consistent with prior data in a larger cohort of women carrying multiples in whom cord hepcidin concentrations explained ∼60% of the observed intrauterine variability in iron status (62). Together, these new tracer data and our prior indicator data in these women carrying multiples suggest that the fetus has some capacity to regulate its own iron status. This conclusion differs from recent animal studies in which hepcidin knock-out fetuses exhibited no deficits in fetal iron status when compared with wild-type animals (38).
A main strength of this study was the ability to assess iron isotopic enrichment in the maternal, neonatal, and placental compartments and identify factors associated with differential iron partitioning between these compartments. However, there are limitations. The current study included only a small number of women in the multiples group. Women participating in the tracer study were recruited from a larger cohort where 35% of women gave birth to infants who exhibited discordant growth (63). As these women did not give birth to infants with growth discordance, they may not be representative of all women carrying multiple fetuses. Second, we were only able to evaluate maternal and neonatal isotopic enrichment within the RBC compartment and cannot capture iron tracer that was sequestered within other maternal or neonatal tissues. This may be less of a concern in neonates as they prioritize iron in support of RBC iron demands (30).
Values of maternal iron absorption during pregnancy are significantly higher when inclusion of maternally absorbed iron that is recovered in the neonate at birth or within placental tissue at delivery is also quantified. When maternal iron status is compromised, a greater fraction of maternally absorbed iron is transferred to the fetus and retained within the placenta relative to that recovered within maternal RBCs. Both maternal and neonatal hormones are associated with partitioning of absorbed iron tracer. Neonatal hepcidin concentrations were strongly associated with iron uptake by the fetus, indicating that the human fetus has some capacity to regulate placental iron trafficking in a population at higher risk for maternal and neonatal anemia.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—KMD: analyzed and interpreted the data and wrote the manuscript; KOO: designed the research, performed experiments, analyzed and interpreted the data, and wrote the manuscript; RG, EKP, LEC, SAA and NZ: were responsible for the clinical implementation of the studies and assisted with the design of the research and analysis and interpretation of the data and with the preparation of the manuscript and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by Cornell Agricultural Experiment Station Federal Formula Funds (project no. 2006-07-160) received from the USDA National Institutes for Food and Agriculture, General Clinical Research Center grant 5M01-RR 00044 from the National Center for Research Resources (NCRR), the US NIH National Institute of Digestive and Kidney Diseases (NIDDK) grant T32-DK007158, and the Nestlé Foundation.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the manuscript, code book, and analytic code will not be made available because of the pediatric composition of patient population and confidential nature of the data collected.
Abbreviations used: EPO, erythropoietin; ERFE, erythroferrone; Hb, hemoglobin; LBW, low birth weight; RBCs, red blood cells; SF, serum ferritin; SGA, small for gestational age; sTfR, serum transferrin receptor; sβ, standardized β-coefficient; TBI, total body iron; M absorption, iron absorption based on recovery of iron tracer in maternal red blood cells; M+N absorption, iron absorption based on recovery of tiron racer in maternal and neonatal red blood cells; M+N+P absorption, iron absorption based on recovery of iron tracer in maternal and neonatal red blood cells and in placental tissue.
Contributor Information
Katherine M Delaney, Division of Nutritional Sciences, Cornell University, Cornell, NY, USA.
Ronnie Guillet, Division of Neonatology, Department of Pediatrics, University of Rochester School of Medicine, Rochester, NY, USA.
Eva K Pressman, Department of Obstetrics and Gynecology, University of Rochester School of Medicine, Rochester, NY, USA.
Laura E Caulfield, Department of International Health, Johns Hopkins University, Baltimore, MD, USA.
Nelly Zavaleta, Instituto de Investigacion Nutricional, Lima, Peru.
Steven A Abrams, Department of Pediatrics, Dell Medical School, University of Texas at Austin, Austin, TX.
Kimberly O O'Brien, Division of Nutritional Sciences, Cornell University, Cornell, NY, USA.
References
- 1. Rasmussen K. Is there a causal relationship between iron deficiency or iron-deficiency anemia and weight at birth, length of gestation and perinatal mortality?. J Nutr. 2001;131(2s-2):590S–601S.; discussion: S-3S. [DOI] [PubMed] [Google Scholar]
- 2. Scholl TO. Iron status during pregnancy: setting the stage for mother and infant. Am J Clin Nutr. 2005;81(5):1218s–22s. [DOI] [PubMed] [Google Scholar]
- 3. Geltman PL, Meyers AF, Mehta SD, Brugnara C, Villon I, Wu YA, Bauchner H. Daily multivitamins with iron to prevent anemia in high-risk infants: a randomized clinical trial. Pediatrics. 2004;114(1):86–93. [DOI] [PubMed] [Google Scholar]
- 4. Lynch S, Pfeiffer CM, Georgieff MK, Brittenham G, Fairweather-Tait S, Hurrell RF, McArdle HJ, Raiten DJ. Biomarkers of Nutrition for Development (BOND)—iron review. J Nutr. 2018;148(Suppl 1):1001s–67s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Institute of Medicine. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: The National Academies Press, 2001. [PubMed] [Google Scholar]
- 6. Brannon PM, Stover PJ, Taylor CL. Integrating themes, evidence gaps, and research needs identified by workshop on iron screening and supplementation in iron-replete pregnant women and young children. Am J Clin Nutr. 2017;106(Suppl 6):1703s–12s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brannon PM, Taylor CL. Iron supplementation during pregnancy and infancy: uncertainties and implications for research and policy. Nutrients. 2017;9(12). doi: 10.3390/nu9121327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Balfour WM, Hahn PF, Bale WF, Pommerenke WT, Whipple GH. Radioactive iron absorption in clinical conditions: normal, pregnancy, anemia and hemochromatosis. J Exp Med. 1942;76(1):15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hahn PF, Carothers EL, Darby WJ, Martin M, Sheppard CW, Cannon RO, Beam AS, Densen PM, Peterson JC, Mc CG. Iron metabolism in human pregnancy as studied with radioactive isotope, Fe59. Am J Obstet Gynecol. 1951;61(3):477–86. [DOI] [PubMed] [Google Scholar]
- 10. Young MF, Griffin I, Pressman E, McIntyre AW, Cooper E, McNanley T, Harris ZL, Westerman M, O'Brien KO. Utilization of iron from an animal-based iron source is greater than that of ferrous sulfate in pregnant and nonpregnant women. J Nutr. 2010;140(12):2162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Young MF, Glahn RP, Ariza-Nieto M, Inglis J, Olbina G, Westerman M, O'Brien KO. Serum hepcidin is significantly associated with iron absorption from food and supplemental sources in healthy young women. Am J Clin Nutr. 2009;89(2):533–8. [DOI] [PubMed] [Google Scholar]
- 12. Whittaker PG, Lind T, Williams JG. Iron absorption during normal human pregnancy: a study using stable isotopes. Br J Nutr. 1991;65(3):457–63. [DOI] [PubMed] [Google Scholar]
- 13. Whittaker PG, Barrett JF, Lind T. The erythrocyte incorporation of absorbed non-haem iron in pregnant women. Br J Nutr. 2001;86(3):323–9. [DOI] [PubMed] [Google Scholar]
- 14. O'Brien KO, Zavaleta N, Caulfield LE, Yang DX, Abrams SA. Influence of prenatal iron and zinc supplements on supplemental iron absorption, red blood cell iron incorporation, and iron status in pregnant Peruvian women. Am J Clin Nutr. 1999;69(3):509–15. [DOI] [PubMed] [Google Scholar]
- 15. Young MF, Griffin I, Pressman E, McIntyre AW, Cooper E, McNanley T, Harris ZL, Westerman M, O'Brien KO. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J Nutr. 2012;142(1):33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Brien KO, Zavaleta N, Abrams SA, Caulfield LE. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am J Clin Nutr. 2003;77(4):924–30. [DOI] [PubMed] [Google Scholar]
- 17. Kastenmayer P, Davidsson L, Galan P, Cherouvrier F, Hercberg S, Hurrell RF. A double stable isotope technique for measuring iron absorption in infants. Br J Nutr. 1994;71(3):411–24. [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR. 1998;47(RR-3):1–36. [PubMed] [Google Scholar]
- 19. Orkin SH, Nathan DG, Ginsburg D, Look AT, Fisher DE, Lux S. Nathan and Oski's Hematology of Infancy and Childhood. 8th ed, Philadelphia, PA: Saunders Elsevier, 2014. [Google Scholar]
- 20. Ru Y, Pressman EK, Cooper EM, Guillet R, Katzman PJ, Kent TR, Bacak SJ, O'Brien KO. Iron deficiency and anemia are prevalent in women with multiple gestations. Am J Clin Nutr. 2016;104(4):1052–60. [DOI] [PubMed] [Google Scholar]
- 21. Lee S, Guillet R, Cooper EM, Westerman M, Orlando M, Kent T, Pressman E, O'Brien KO. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr Res. 2016;79(1-1):42–8. [DOI] [PubMed] [Google Scholar]
- 22. Wians FH Jr, Urban JE, Kroft SH, Keffer JH. Soluble transferrin receptor (sTfR) concentration quantified using two sTfR kits: analytical and clinical performance characteristics. Clin Chim Acta. 2001;303(1-2):75–81. [DOI] [PubMed] [Google Scholar]
- 23. Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101(9):3359–64. [DOI] [PubMed] [Google Scholar]
- 24. Mei Z, Cogswell ME, Looker AC, Pfeiffer CM, Cusick SE, Lacher DA, Grummer-Strawn LM. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am J Clin Nutr. 2011;93(6):1312–20. [DOI] [PubMed] [Google Scholar]
- 25. Yetley EA, Pfeiffer CM, Phinney KW, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R, Curtin LR, Durazo-Arvizu RA et al. Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr. 2011;94(1):313s–21s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yetley EA, Pfeiffer CM, Phinney KW, Fazili Z, Lacher DA, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R et al. Biomarkers of folate status in NHANES: a roundtable summary. Am J Clin Nutr. 2011;94(1):303s–12s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Angelis P, Miller RK, Darrah TH, Katzman PJ, Pressman EK, Kent TR, O'Brien KO. Elemental content of the placenta: a comparison between two high-risk obstetrical populations, adult women carrying multiples and adolescents carrying singletons. Environ Res. 2017;158:553–65. [DOI] [PubMed] [Google Scholar]
- 28. Fomon SJ, Ziegler EE, Rogers RR, Nelson SE, Edwards BB, Guy DG, Erve JC, Janghorbani M. Iron absorption from infant foods. Pediatr Res. 1989;26(3):250–4. [DOI] [PubMed] [Google Scholar]
- 29. Barrett JF, Whittaker PG, Williams JG, Lind T. Absorption of non-haem iron in normal women measured by the incorporation of two stable isotopes into erythrocytes. Clin Sci (Lond). 1992;83(2):213–9. [DOI] [PubMed] [Google Scholar]
- 30. Rao R, Georgieff MK. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med. 2007;12(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Georgieff MK, Landon MB, Mills MM, Hedlund BE, Faassen AE, Schmidt RL, Ophoven JJ, Widness JA. Abnormal iron distribution in infants of diabetic mothers: spectrum and maternal antecedents. J Pediatr. 1990;117(3):455–61. [DOI] [PubMed] [Google Scholar]
- 32. Ma LX, Levitan D, Baergen RN. Weights of fetal membranes and umbilical cords: correlation with placental pathology. Pediatr Dev Pathol. 2019; doi: 10.1177/1093526619889460. [DOI] [PubMed] [Google Scholar]
- 33. Miller J, Chauhan SP, Abuhamad AZ. Discordant twins: diagnosis, evaluation and management. Am J Obstet Gynecol. 2012;206(1):10–20. [DOI] [PubMed] [Google Scholar]
- 34. Wallace J, Bourke D, Da Silva P, Aitken R. Nutrient partitioning during adolescent pregnancy. Reproduction. 2001;122(3):347–57. [DOI] [PubMed] [Google Scholar]
- 35. Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72(1 Suppl):257s–64s. [DOI] [PubMed] [Google Scholar]
- 36. Barrett JF, Whittaker PG, Williams JG, Lind T. Absorption of non-haem iron from food during normal pregnancy. BMJ. 1994;309(6947):79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pommerenke WT, Hahn PF, Bale WF, Balfour WM. Transmission of radio-active iron to the human fetus. Am J Physiol. 1942;137(1):164–70. [Google Scholar]
- 38. Sangkhae V, Fisher AL, Wong S, Koenig MD, Tussing-Humphreys L, Chu A, Lelic M, Ganz T, Nemeth E. Effects of maternal iron status on placental and fetal iron homeostasis. J Clin Invest. 2019;30(2):625–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scholl TO. Maternal iron status: relation to fetal growth, length of gestation, and iron endowment of the neonate. Nutr Rev. 2011;69(Suppl 1):S23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jaime-Perez JC, Herrera-Garza JL, Gomez-Almaguer D. Sub-optimal fetal iron acquisition under a maternal environment. Arch Med Res. 2005;36(5):598–602. [DOI] [PubMed] [Google Scholar]
- 41. Colomer J, Colomer C, Gutierrez D, Jubert A, Nolasco A, Donat J, Fernandez-Delgado R, Donat F, Alvarez-Dardet C. Anaemia during pregnancy as a risk factor for infant iron deficiency: report from the Valencia Infant Anaemia Cohort (VIAC) study. Paediatr Perinat Epidemiol. 1990;4(2):196–204. [DOI] [PubMed] [Google Scholar]
- 42. Kilbride J, Baker TG, Parapia LA, Khoury SA, Shuqaidef SW, Jerwood D. Anaemia during pregnancy as a risk factor for iron-deficiency anaemia in infancy: a case-control study in Jordan. Int J Epidemiol. 1999;28(3):461–8. [DOI] [PubMed] [Google Scholar]
- 43. Young MF, Pressman E, Foehr ML, McNanley T, Cooper E, Guillet R, Orlando M, McIntyre AW, Lafond J, O'Brien KO. Impact of maternal and neonatal iron status on placental transferrin receptor expression in pregnant adolescents. Placenta. 2010;31(11):1010–4. [DOI] [PubMed] [Google Scholar]
- 44. Best CM, Pressman EK, Cao C, Cooper E, Guillet R, Yost OL, Galati J, Kent TR, O'Brien KO. Maternal iron status during pregnancy compared with neonatal iron status better predicts placental iron transporter expression in humans. FASEB J. 2016;30(10):3541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cornock R, Gambling L, Langley-Evans SC, McArdle HJ, McMullen S. The effect of feeding a low iron diet prior to and during gestation on fetal and maternal iron homeostasis in two strains of rat. Reprod Biol Endocrinol. 2013;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gambling L, Danzeisen R, Gair S, Lea RG, Charania Z, Solanky N, Joory KD, Srai SK, McArdle HJ. Effect of iron deficiency on placental transfer of iron and expression of iron transport proteins in vivo and in vitro. Biochem J. 2001;356(Pt 3):883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Finne PH. Erythropoietin production in fetal hypoxia and in anemic uremic patients. Ann NY Acad Sci. 1968;149(1):497–503. [DOI] [PubMed] [Google Scholar]
- 48. Varvarigou A, Beratis NG, Makri M, Vagenakis AG. Increased levels and positive correlation between erythropoietin and hemoglobin concentrations in newborn children of mothers who are smokers. J Pediatr. 1994;124(3):480–2. [DOI] [PubMed] [Google Scholar]
- 49. Teramo KA, Hiilesmaa VK, Schwartz R, Clemons GK, Widness JA. Amniotic fluid and cord plasma erythropoietin levels in pregnancies complicated by preeclampsia, pregnancy-induced hypertension and chronic hypertension. J Perinat Med. 2004;32(3):240–7. [DOI] [PubMed] [Google Scholar]
- 50. Gun Eryilmaz O, Tavil B, Turan S, Yumusak O, Doganay M, Uzunlar O, Akar S, Eyi EG. Hepcidin and erythropoietin measurements in the cord blood of neonates with meconium-stained amniotic fluid. J Obstet Gynaecol Res. 2013;39(1):175–9. [DOI] [PubMed] [Google Scholar]
- 51. Rollins MD, Maxwell AP, Afrasiabi M, Halliday HL, Lappin TR. Cord blood erythropoietin, pH, PaO2 and haematocrit following caesarean section before labour. Biol Neonate. 1993;63(3):147–52. [DOI] [PubMed] [Google Scholar]
- 52. Erdem A, Erdem M, Arslan M, Yazici G, Eskandari R, Himmetoglu O. The effect of maternal anemia and iron deficiency on fetal erythropoiesis: comparison between serum erythropoietin, hemoglobin and ferritin levels in mothers and newborns. J Matern Fetal Neonatal Med. 2002;11(5):329–32. [DOI] [PubMed] [Google Scholar]
- 53. Briana DD, Boutsikou T, Baka S, Boutsikou M, Stamati L, Hassiakos D, Gourgiotis D, Malamitsi-Puchner A. Perinatal role of hepcidin and iron homeostasis in full-term intrauterine growth-restricted infants. Eur J Haematol. 2013;90(1):37–44. [DOI] [PubMed] [Google Scholar]
- 54. Amarilyo G, Mimouni FB, Oren A, Ochshorn Y, Ballin A, Deutsch V, Mandel D. Prohepcidin concentrations and erythroid progenitors in cord blood of appropriate versus small for gestational age neonates. J Perinatol. 2010;30(6):396–8. [DOI] [PubMed] [Google Scholar]
- 55. Abrams ET, Kwiek JJ, Mwapasa V, Kamwendo DD, Tadesse E, Lema VM, Molyneux ME, Rogerson SJ, Meshnick SR. Malaria during pregnancy and foetal haematological status in Blantyre, Malawi. Malar J. 2005;4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Korlesky C, Kling PJ, Pham DQD, Ovasapyan AA, Leyns CEG, Weber MB, Coe CL. Cord blood erythropoietin and hepcidin reflect lower newborn iron stores due to maternal obesity during pregnancy. Am J Perinatol. 2019;36(5):511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Delaney KM, Guillet R, Fleming RE, Ru Y, Pressman EK, Vermeylen F, Nemeth E, O'Brien KO. Umbilical cord serum ferritin concentration is inversely associated with umbilical cord hemoglobin in neonates born to adolescents carrying singletons and women carrying multiples. J Nutr. 2019;149(3):406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Flores-Quijano ME, Vega-Sanchez R, Tolentino-Dolores MC, Lopez-Alarcon MG, Flores-Urrutia MC, Lopez-Olvera AD, Talavera JO. Obesity is associated with changes in iron nutrition status and its homeostatic regulation in pregnancy. Nutrients. 2019;11(3):693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Flores-Quijano ME, Montalvo-Velarde I, Vital-Reyes VS, Rodriguez-Cruz M, Rendon-Macias ME, Lopez-Alarcon M. Longitudinal analysis of the interaction between obesity and pregnancy on iron homeostasis: role of hepcidin. Arch Med Res. 2016;47(7):550–6. [DOI] [PubMed] [Google Scholar]
- 60. Cao C, Pressman EK, Cooper EM, Guillet R, Westerman M, O'Brien KO. Prepregnancy body mass index and gestational weight gain have no negative impact on maternal or neonatal iron status. Reprod Sci. 2016;23(5):613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rawal S, Hinkle SN, Bao W, Zhu Y, Grewal J, Albert PS, Weir NL, Tsai MY, Zhang C. A longitudinal study of iron status during pregnancy and the risk of gestational diabetes: findings from a prospective, multiracial cohort. Diabetologia. 2017;60(2):249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ru Y, Pressman EK, Guillet R, Katzman PJ, Vermeylen F, O'Brien KO. Umbilical cord hepcidin concentrations are positively associated with the variance in iron status among multiple birth neonates. J Nutr. 2018;148(11):1716–22. [DOI] [PubMed] [Google Scholar]
- 63. Ru YP, Pressman EK, Guillet R, Katzman PJ, Bacak SJ, O'Brien KO. Predictors of anemia at birth in neonates born to women carrying multiple fetuses. Pediatrics. 2018;84(2):199–204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.