Abstract
Influenza virus is the major cause of seasonal and pandemic flu. Currently, oseltamivir, a potent and selective inhibitor of neuraminidase of influenza A and B viruses, is the drug of choice for treating patients with influenza virus infection. However, recent emergence of oseltamivir-resistant influenza viruses has limited its efficacy. Morin hydrate (3,5,7,2′,4′-pentahydroxyflavone) is a flavonoid isolated from Morus alba L. It has antioxidant, anti-inflammatory, neuroprotective, and anticancer effects partly by the inhibition of the NF-кB signaling pathway. However, its effects on influenza virus have not been studied. We evaluated the antiviral activity of morin hydrate against influenza A/Puerto Rico/8/1934 (A/PR/8; H1N1) and oseltamivir-resistant A/PR/8 influenza viruses in vitro. To determine its mode of action, we carried out time course experiments, and time of addition, hemolysis inhibition, and hemagglutination assays. The effects of the co-administration of morin hydrate and oseltamivir were assessed using the murine model of A/PR/8 infection. We found that morin hydrate reduced hemagglutination by A/PR/8 in vitro. It alleviated the symptoms of A/PR/8-infection, and reduced the levels of pro-inflammatory cytokines and chemokines, such as TNF-α and CCL2, in infected mice. Co-administration of morin hydrate and oseltamivir phosphate reduced the virus titers and attenuated pulmonary inflammation. Our results suggest that morin hydrate exhibits antiviral activity by inhibiting the entry of the virus.
Keywords: Morin hydrate, Influenza A Virus, Oseltamivir, Antivirals, Hemagglutinin
INTRODUCTION
Influenza virus causes the flu, killing several thousand people every year worldwide, and in the 3 pandemic flu outbreaks occurred in the 20th century. The most recent influenza pandemic was of the H1N1 subtype, which began in Mexico in 2009 (1,2,3). Currently, for influenza prevention, vaccines against H1N1 and H3N2 subtypes of the influenza A virus, and 2 antigenically distinct virus lineages called the Yamagata and Victoria lineages are used as antigens, but these vaccines are not very effective.
Influenza A has two major antigenic membrane proteins, viz., hemagglutinin (HA) and neuraminidase (NA), and the virus is sub-classified by the subtypes of HA (H1–H16) and NA (N1–N9) (4,5). Sialic acid residues of the target cell membrane receptors, to which the influenza HA binds, is cleaved by the viral NA, allowing the release of the progeny viruses that can infect new cells (6). Although oseltamivir, which inhibits viral NA, is widely used as an antiviral agent to control the influenza virus infection, the incidences of infections with new oseltamivir-resistant viral strains have been recently reported (7). Thus, development of novel antiviral drugs is urgently needed to contain these mutant viruses.
During infection, the HA of the influenza virus binds to the sialic acid-containing receptors of the target cells, and also participates in the fusion of the virus with the endosomal membranes to mediate endocytosis (8). This makes the HA one of the most promising targets for the development of inhibitors of influenza virus entry. As inhibition of HA can blunt the initial step of virus infection, conserved sites in HA can be novel targets for the broad-spectrum anti-influenza drugs (9).
Morin hydrate (2′,3,4′,5,7-petahydroxyflovone) is a natural flavonoid from Morus alba L. (10), with anti-oxidant (11), anti-inflammatory (12), anti-apoptotic (13), and anti-cancer (10) activities. In addition, it showed neuroprotective effects in Parkinson disease (14). Although it is known that morin hydrate inhibits NF-кB signaling pathway and autophagy pathway (14,15), the detailed molecular mechanism of the compound has not been reported.
Recently, morin hydrate was shown to have antiviral activity against influenza virus (16), but this was not assessed in vivo. In the current study, we showed the significant antiviral effect of morin hydrate by its ability to inhibit the endocytosis of influenza A/Puerto Rico/8/1934 (A/PR/8; H1N1) into host cells. Also, it reduced the A/PR/8-associated pulmonary inflammation and viral replication in the lungs of the infected mice. Furthermore, the combined treatment with oseltamivir phosphate and morin hydrate reduced the A/PR/8-associated pulmonary inflammation as well as inhibiting the virus replication, as compared to those treated with oseltamivir phosphate alone. We proposed morin hydrate as a complementary antiviral agent to contain the oseltamivir-resistant influenza virus and reduce the pulmonary inflammation induced by the influenza infection.
MATERIALS AND METHODS
Virus, cells, and reagents
Influenza A/Puerto Rico/8 H1N1 (A/PR/8) and A549 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and oseltamivir-resistant A/PR/8 was selected from A549 cells following several rounds of culture with oseltamivir carboxylate (Santa Cruz Biotechnology, Dallas, TX, USA). After that, amino acid sequence analysis of oseltamivir-resistant A/PR/8 confirmed that the amino acid number 272 of the HA protein was converted from proline to serine. A549 cells were maintained in DMEM supplemented with 10% FBS and 1% antibiotic-antimycotic solution. Antibiotic-antimycotic solution, FBS, and DMEM were supplied by Gibco BRL (Invitrogen Life Technologies, Karlsruhe, Germany). TPCK-Trypsin was purchased from Pierce (Thermo Fisher Scientific, Rockford, IL, USA). Sulforhodamine B (SRB) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The tissue culture plates were purchased from Falcon (BD Biosciences, San Jose, CA, USA).
In vitro assessment of antiviral activity
In vitro antiviral activity of morin hydrate was determined based on the inhibition of viral cytopathy, and the extent of cell survival was measured using SRB assay (5). For this, A549 cells were used as hosts for A/PR/8 and oseltamivir-resistant A/PR/8 viruses.
Time course experiment
A549 cells infected with 1×103 plaque forming units (pfu)/90 μl of A/PR/8 were harvested at the indicated time points including 8, 10, 12, 14, and 16 h post-infection. Morin hydrate (Sigma-Aldrich, St. Louis, MO, USA) and oseltamivir carboxylate (Santa Cruz Biotechnology, Dallas, TX, USA) were added at the time of infection. Total RNA was isolated at the indicated time points post-infection, and the level of polymerase acidic protein (PA) gene of A/PR/8 was analyzed using real time-PCR.
Time-of-addition assay
Morin hydrate and oseltamivir carboxylate were added to A549 cells either at the time of (0 h), or after A/PR/8 infection (1, 2, 4, 6, 8, 10, and 12 h). For all in vitro experiments, oseltamivir carboxylate, the active metabolite of the drug oseltamivir phosphate, was used. At 14 h post-infection, the virus-specific PA gene expression was analyzed by real-time PCR, using Thunderbird SYBR quantitative PCR (qPCR) Mix (Toyobo, Japan).
Detection of influenza A nucleoprotein (NP)
A549 cells cultured for 24 h were treated with 1×103 pfu of A/PR/8 and 250 μM morin hydrate. After 4, 6, and 8 h, the cells were washed thrice with PBS for 5 min and fixed in 100% methanol. Influenza A virus particles were detected though immunofluorescence of NA as previously reported (17). Anti-influenza A virus NP antibody and Alexa Fluor 647-labeled goat-anti mouse IgG H&L antibody (Abcam, Cambridge, MA, USA) were used. Nuclei were counterstained with DAPI and analyzed using a fluorescence microscope at the Central Laboratory of Kangwon National University.
Hemagglutination assay
Hemagglutination inhibition assay was used to measure the effect of morin hydrate on virus adsorption to cells. First, 40 µl of influenza virus suspension was incubated with 10 µl morin hydrate for 1 h at room temperature, and added to an equal volume of 2.5% chicken erythrocyte suspension in PBS in a U round-bottom 96-well plate (Thermo Fisher Scientific). The mixture was incubated for 2 h at room temperature, before measuring the extent of erythrocyte aggregation.
Hemolysis inhibition assay
The inhibitory effect of morin hydrate on A/PR/8 fusion with host cells was analyzed using hemolysis inhibition assay. Briefly, 100 µl (100 µM) of morin hydrate was mixed with an equal volume of virus and incubated at room temperature for 30 min, followed by the addition of 200 µl of 2% chicken erythrocytes and incubation at 37°C for another 30 min. Next, 100 µl of 0.5 M sodium acetate at different pHs was added to this mixture, followed by incubation at 37°C for 30 min. The mixtures were centrifuged at 1,200 rpm for 6 min to separate the intact erythrocytes, and the hemoglobin concentration in the supernatant was spectrophotometrically analyzed (2,18).
Western blot analysis
Total protein lysates from A549 cells were prepared by sonicating cells in lysis buffer (iNtRON Biotechnology, Inc., Seongnam, Korea). Equal amounts of lysates were analyzed using western blot. Primary antibodies against NP (SinoBiological, 11675-RP01) and β-actin (Cell Signaling Technology, Danvers, MA, USA) were used, and these were labeled with goat-anti-rabbit and goat-anti-mouse antibodies conjugated with horseradish peroxidase (HRP), respectively (Cell Signaling Technology). The HRP was developed using the enhanced chemiluminescence method with femtoLUCENT™ PLUS (G-biosciences, St. Louis, MO, USA).
Quantitative real-time PCR
Tissue RNA was extracted using the RNA Extraction Mini kit (Qiagen, Hilden, Germany) and reverse transcription was performed using the cDNA Synthesis Mini kit (TAKARA, Shiga, Japan). Quantitative real-time PCR was carried out using THUNDERBIRD™ SYBR QPCR Mix (Toyobo Co., Ltd., Osaka, Japan). The detection of A/PR/8 PA gene was performed using qRT-PCR. The following primers were used: PA forward primer: 5′-CGGTCCAAATTCCTGCTGAT-3′, PA reverse primer: 5′-CATTGGGTTCCTTCCATCCA-3′. The following β-actin primers were used: Forward Primer: 5′-CCTAGGCACCAGGGTGTGAT-3′ and Reverse Primer: 5′-TCTCCATGTCGTCCCAGTTG-3′. The cycling conditions were as follows: 95°C for 3 min, followed by 40 cycles of 95°C for 30 s, 61°C for 30 s, and 72°C for 30 s. The results were analyzed with real-time system AB 7900 HT software (Life Technologies), and all values were normalized to the levels of β-actin.
Mice and virus infection
Six-wk-old female C57BL/6 mice were purchased from Orent-Bio (Orient Bio Inc., Seongnam, Korea). They were anesthetized with intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (20 mg/kg) and intranasally infected with 5×103 pfu/30 μl of virus. They were orally administered with oseltamivir phosphate (5 mg/kg), or morin hydrate (10 mg/kg), or both once a day for 5 days; their body weight and survival were monitored. All animal experiments were approved by the Institutional Animal Care and Use Committees (IACUC) of Kangwon National University (Permit Number: KW-161101-2).
Histology and histopathology analysis
Lung tissues were fixed overnight in 4% formaldehyde. The tissues were dehydrated by gradually soaking them in alcohol and xylene and then embedded in paraffin. Sections (10 μm) were stained with H&E and mounted on glass slides. The stained tissues were examined by a pathologist under a light microscope (Olympus CX41; Olympus, Tokyo, Japan). For histomorphometric analyses, inflammation in the lungs was examined, and if there were inflammations, they were classified into minimal, mild, moderate, and severe according to their extent and severity. Their sizes were measured using an image analyzer (i-Solution Lite; IMT i-Solution Inc., Burnaby, Canada).
Cytokine and chemokine analysis
The levels of CCL2, IL-1β, and TNF-α were measured using mouse ELISA Ready-SET-GO kit (eBioscience, Thermo Fisher Scientific), following the manufacturer's instructions. The absorbance was then measured at 450 nm using a SpectraMax 340 instrument (Molecular Devices, San Jose, CA, USA).
RESULTS
Antiviral activity of morin hydrate against A/PR/8 and oseltamivir-resistant influenza A virus in vitro
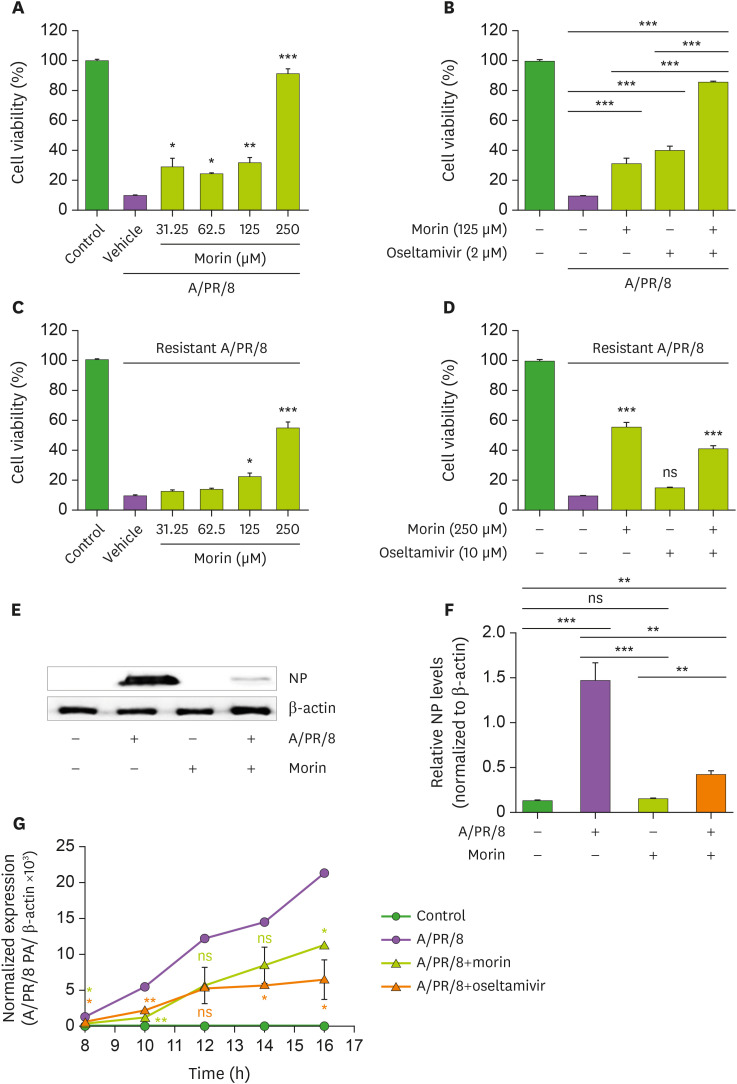
Morin hydrate (250 μM) significantly reduced A/PR/8 virus-induced cytotoxicity, while at a 125 μM concentration, it was marginally effective (Fig. 1A). Positive control with >2 μM oseltamivir carboxylate protected the cells from the virus-induced cytotoxicity, but about 40% of cells died even with 10 μM oseltamivir treatment (Supplementary Fig. 1A). When treated with 125 μM morin hydrate and 2 μM oseltamivir carboxylate together, viability of the A/PR/8-infected cells improved significantly (Fig. 1B). At these concentrations, oseltamivir carboxylate was not effective against oseltamivir-resistant influenza A virus (Supplementary Fig. 1B), but 250 μM morin hydrate protected the cells from the cytotoxicity induced by this oseltamivir-resistant A/PR/8 (Fig. 1C and D); oseltamivir did not show increased antiviral activity against this virus even combined with morin hydrate (Fig. 1D). We also confirmed the there was no significant cytotoxicity of morin hydrate and oseltamivir carboxylate alone (Supplementary Fig. 2).
Figure 1. Antiviral activity of morin hydrate against A/PR/8 and oseltamivir-resistant influenza A virus in vitro. A549 cells were infected with 1×103 pfu of A/PR/8 (A, B) or oseltamivir-resistant influenza A virus (C, D) and incubated with oseltamivir carboxylate and/or morin hydrate at the indicated concentrations for 48 h. Cell viability was determined using SRB assay. (E) Western blotting to detect NP in A/PR/8-infected and non-infected A549 cells after 24 h-treatment with vehicle or 250 μM of morin hydrate. (F) The ratios of NP to β-actin levels based on the quantification of the bands in the immunoblot shown in (E). (G) A549 cells were infected with 1×103 pfu of A/PR/8, and harvested at the indicated time points after treatment with 250 µM morin hydrate or 10 µM oseltamivir carboxylate. Total RNA was isolated and expression levels of viral PA RNA was analyzed using RT-qPCR. Data are expressed as the mean±SEM of the values from three independent experiments.
*p<0.05, **p<0.01 and ***p<0.001 based on the ANOVA with Bonferroni's multiple comparison test.
Viral NP protein was detected in A549 cells 24 h after the infection with A/PR/8, and 250 µM morin hydrate dramatically reduced the NP levels (Fig. 1E and F). Significant amounts of viral RNA were seen in the cells 10 h post-infection; treatment with morin hydrate or oseltamivir carboxylate significantly reduced the viral RNA levels (Fig. 1G). Thus, morin hydrate showed antiviral activity against A/PR/8 in vitro.
Effect of morin hydrate on the intracellular entry of A/PR/8 virus
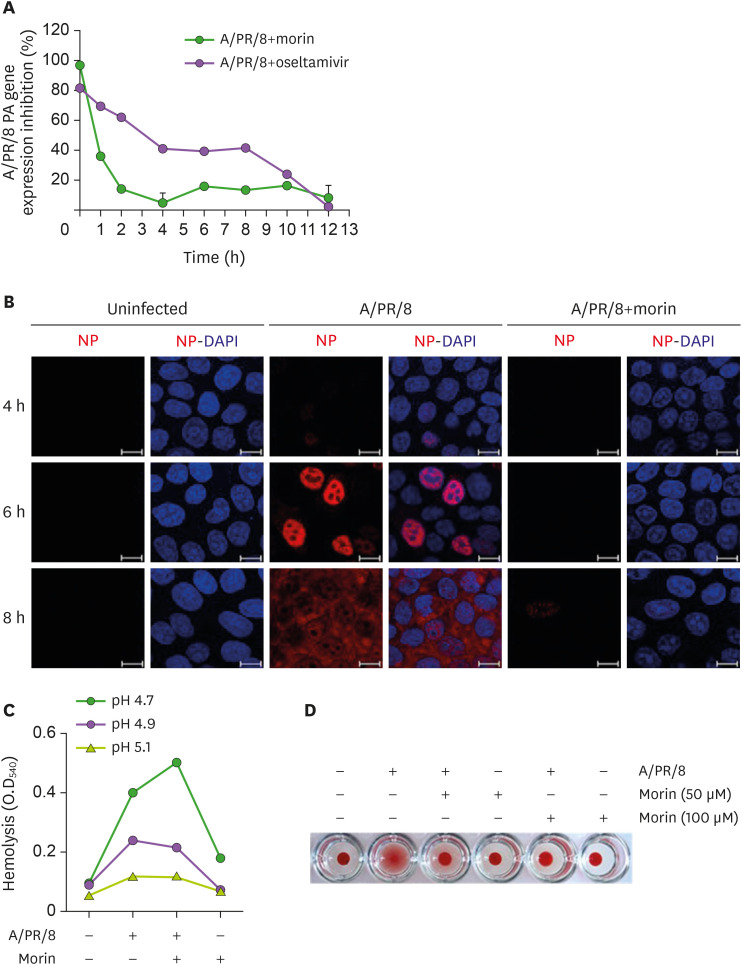
Morin hydrate exhibited bi-phasic antiviral response against influenza infection; it was the most effective when added at the time of infection and marginally effective at 8 h after infection, suggesting that its mode of action was distinct from that of oseltamivir (Fig. 2A).
Figure 2. Restriction of entry of influenza virus into host cells by morin hydrate. (A) 250 µM morin hydrate or 10 µM oseltamivir carboxylate was added to A/PR/8-infected A549 cells at the indicated time points. The percentage of viable cells was calculated at 14 h post-infection. Viral RNA was analyzed using RT-PCR. (B) A549 cells were infected with 1×103 pfu of A/PR/8 and treated with 250 µM morin hydrate. Cells were harvested at 4, 6, and 8 h post-infection, and stained to detect NP under a fluorescence microscope. (C) For the hemolysis inhibition assay, a mixture of 100 µM morin hydrate and 1×103 pfu of A/PR/8 were added to freshly obtained chicken erythrocytes and incubated at pHs 4.7, 4.9, and 5.1 at 37°C for 30 min. After a brief centrifugation, the released hemoglobin in the supernatants was estimated by measuring the OD at 540 nm. (D) For hemagglutination assay, chicken erythrocytes were mixed with A/PR/8 virus and 50 µM and 100 µM morin hydrate.
Influenza virus NP encapsulates the viral RNA and forms a viral ribonucleoprotein complex, which is released into the cytoplasm and later translocated to the nucleus (19). NP was seen in the nucleus of the infected cells 4 h post-infection (Fig. 2B), and at 6 h, the NP levels in the nucleus increased. At 8 h post-infection, the NP distribution indicated that virus was present throughout the cell. However, in morin hydrate-treated virus infected cells, NP was not detected until 6 h after infection, and was seen in the nuclei of only a few cells, only at 8 h post-infection. These results suggested that morin hydrate could inhibit A/PR/8 virus entry at the initial stage of infection (Fig. 2B). In the hemolysis inhibition assay, we found that morin hydrate did not change the extent of hemolysis, implying that it was not able to inhibit the viral HA-mediated membrane fusion (Fig. 2C).
In the hemagglutination assay, 50 and 100 μM morin hydrate inhibited the agglutination of RBC, suggesting that it inhibited the binding of virus to host cell membranes (Fig. 2D). In the molecular docking study, morin hydrate could bind to HA1, indicating a possible synergistic antiviral effect of morin hydrate and oseltamivir carboxylate against the A/PR/8 virus (Supplementary Fig. 3). Collectively, these results suggested that morin hydrate inhibited the entry of the virus into host cells.
Effect of morin hydrate on the progression of A/PR/8 virus infection in mice
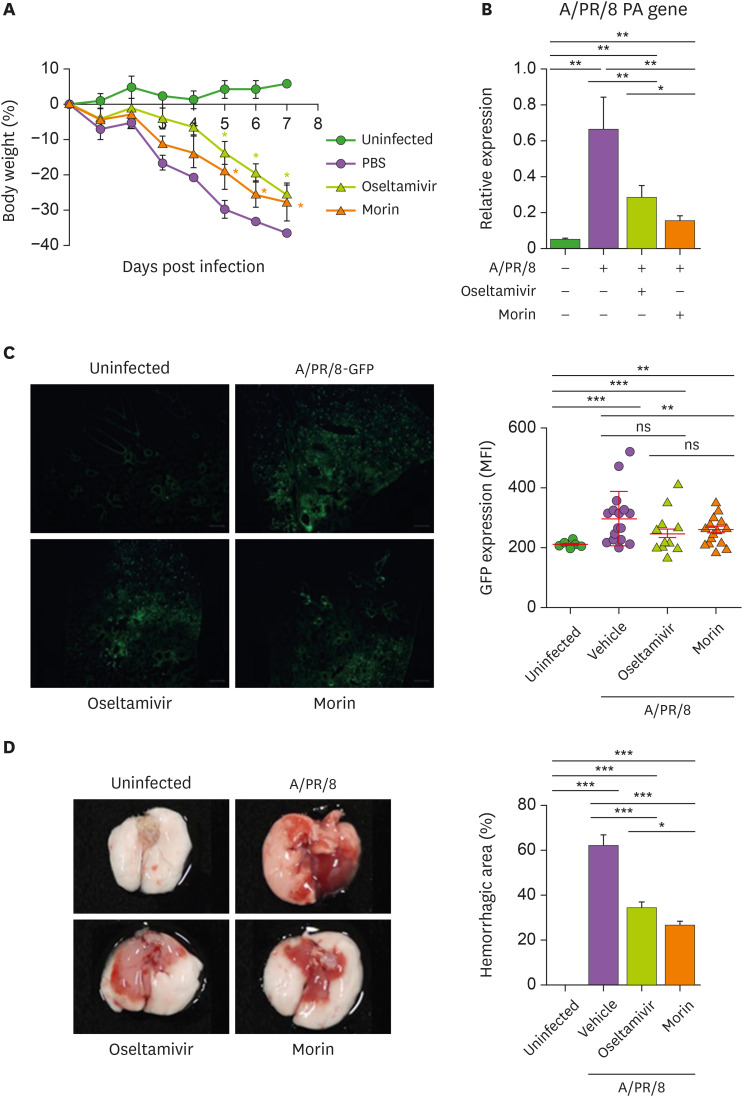
To study the antiviral activity of morin hydrate in vivo, we intranasally infected the mice with sublethal dose of A/PR/8 (5×103 pfu/mouse), followed by administration of 5 mg oseltamivir phosphate and 10 mg morin hydrate/kg body weight. We found that infected mice treated with oseltamivir phosphate and morin hydrate lost lesser weight than those treated with PBS (vehicle) (Fig. 3A). Treatment with morin hydrate significantly inhibited the expression of PA gene 7 days post infection, indicating that inclusion of morin hydrate with oseltamivir phosphate in the treatment resulted in better control of the virus (Fig. 3B). Similar results were seen in mice infected with 5×103 pfu of GFP-expressing A/PR/8 (A/PR/8-GFP) virus (Fig. 3C). The extensive hemorrhagic areas in the lungs of mice infected with A/PR/8, were drastically reduced following treatment with morin hydrate and oseltamivir phosphate (Fig. 3D). These results suggest that the combination of morin hydrate and oseltamivir phosphate formed a very effective antiviral strategy.
Figure 3. Antiviral activity of morin hydrate in A/PR/8-infected mice. Mice were intranasally infected with 5×103 pfu of A/PR/8 or A/PR/8-GFP per mouse and treated with 10 mg/kg morin hydrate or 5 mg/kg oseltamivir phosphate for 5 days. (A) Changes in the body weight of mice. (B) The replication of A/PR/8 in the lungs of infected mice at 7 days post-infection was calculated using RT-qPCR and shown as the mean±SEM after normalization with β-actin. (C) The expression levels of GFP in the lung tissues of mice infected with A/PR/8-GFP, shown as the mean fluorescence intensity±SD. (D) The representative gross pictures of lungs and the proportion of hemorrhagic areas.
*p<0.05, **p<0.01, and ***p<0.001, based on ANOVA with Bonferroni's multiple comparison test.
Status of inflammation in A/PR/8-infected mice after morin hydrate treatment
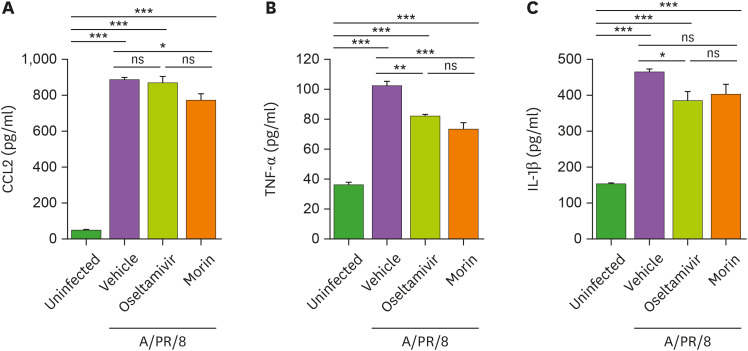
To confirm the ability of morin hydrate to inhibit inflammation, we treated the A/PR/8-infected mice with morin hydrate or oseltamivir phosphate, and found that morin hydrate significantly decreased the levels of CCL2 and TNF-α, whereas oseltamivir phosphate reduced TNF-α and IL-1β in the lungs at 7 days post-infection (Fig. 4). These results suggested that treatment with oseltamivir phosphate and morin hydrate reduced A/PR/8-associated pulmonary inflammation.
Figure 4. Reduction of pulmonary inflammation by morin hydrate in A/PR/8-infected mice. Mice were infected with 5×103 pfu of A/PR/8 per mouse and treated with morin hydrate and/or oseltamivir phosphate for 5 days. (A) CCL-2, (B) TNF-α, and (C) IL-1β levels were estimated in the supernatants of lung tissue homogenates at 7 days post-infection. Data are expressed as the mean±SEM of the values from 3 independent experiments.
*p<0.05, **p<0.01, and ***p<0.001, based on ANOVA with Bonferroni's multiple comparison test.
Effect of morin hydrate on the antiviral activity of oseltamivir
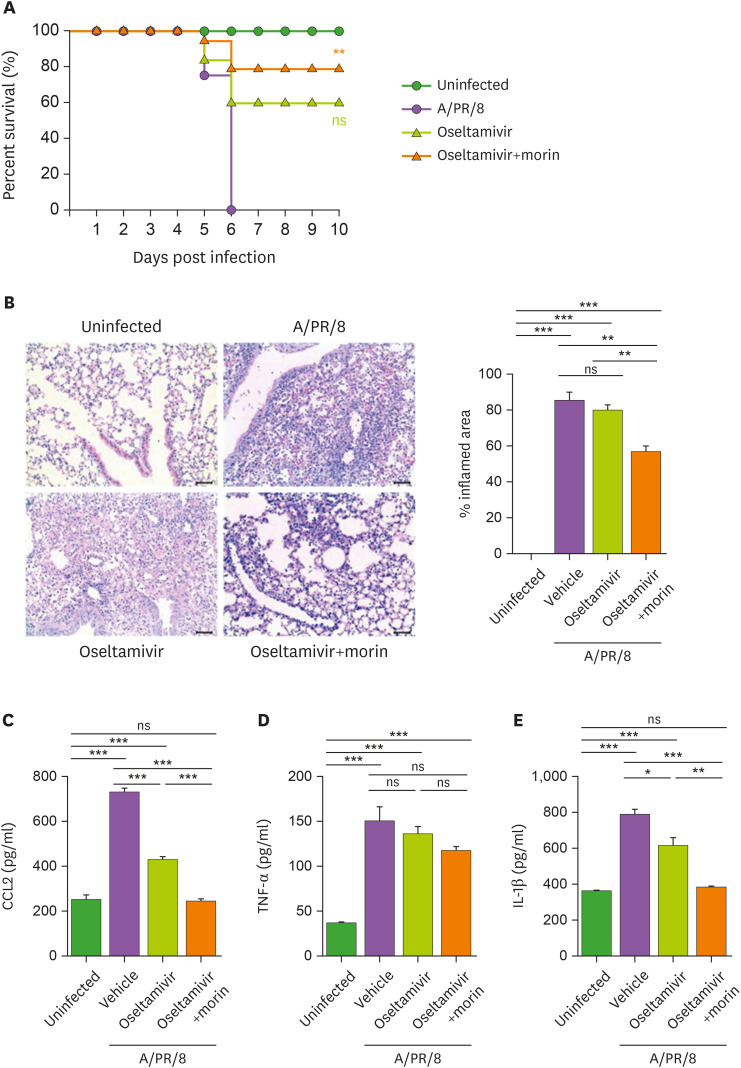
To assess the combined effect of morin hydrate and oseltamivir phosphate, mice infected with a lethal dose of A/PR/8 (105 pfu/mouse) were treated with 5 mg oseltamivir phosphate/kg body weight with or without morin hydrate every day for 5 consecutive days after infection. We found that the combination treatment significantly reduced infection-induced mortality, compared to oseltamivir phosphate treatment alone. This suggested that antiviral activity of oseltamivir phosphate was enhanced by morin hydrate (Fig. 5A). Pulmonary inflammation was also attenuated in infected mice after treatment with morin hydrate and oseltamivir phosphate. Reduced pulmonary infiltration by immune cells and very limited lung inflammation was seen in infected mice after treatment with oseltamivir phosphate and morin hydrate together (Fig. 5B). In these mice, CCL2 and IL-1β levels in the lungs also reduced to a great extent (Fig. 5C). These results suggested that the inflammation caused by influenza infection were mitigated by the combined treatment with morin hydrate and oseltamivir phosphate.
Figure 5. Improved survival of virus-infected mice after treatment with a combination of oseltamivir phosphate and morin hydrate. Mice were infected with 105 pfu of A/PR/8 virus per mouse and treated with oseltamivir phosphate alone or in combination with morin hydrate. (A) Survival of mice was monitored for 10 days following infection. Log-rank test for comparison with A/PR/8-infected group. (B) Lung histology of mice infected with A/PR/8 and treated with oseltamivir phosphate, with or without morin hydrate, on day 5 post-infection. (C) CCL-2, TNF-α, and IL-1β levels in the lung homogenates. The data are expressed as the mean±SEM of the values from three independent experiments.
*p<0.05, **p<0.01, and ***p<0.001, based on ANOVA with Bonferroni's multiple comparison test.
DISCUSSION
RNA genome of the influenza virus has high rate of mutations, and this can make antiviral drugs less effective or completely ineffective in alleviating the infection. Currently, there are several antiviral drugs for the treatment of influenza infection. They can be classified into three categories, depending on their mechanisms of action, as neuraminidase inhibitors (oseltamivir, zanamivir, peramivir, and lanaminivir) (20), ion channel blockers (rimantadine and amantadine) (21), and cap-dependent endonuclease inhibitors (baloxavir marboxil) (22). Four of these, namely, oseltamivir, zanamivir, peramivir, and baloxavir marboxil, are approved by the Food and Drug Administration, and are currently used in the United States. However, the emergence of antiviral drug-resistant influenza viruses either during antiviral treatment, or spontaneously, is a serious problem. Oseltamivir, the most commonly prescribed anti-influenza drug, inhibits the viral NA, and prevents its propagation. Oseltamivir resistance of the 2009 pandemic H1N1 A (H1N1pdm09) virus occurred due to the genetic change of H275Y mutation in (23,24), which also reduced the effect of peramivir (25), while zanamivir (26) remained effective. More seriously, there can be other mutations, in addition to H275Y, which could result in resistance to the currently available antiviral drugs. Therefore, to negate the resistance of influenza viruses to antiviral drugs, it is necessary to expand the pool of new drug candidates with novel modes of action.
Medicinal plant extracts are increasingly being projected as alternative sources of antiviral agents, because of their multiple targets, minor side effects, and lower chance of development of resistance by the target viruses (27,28,29,30). Previously, we showed the antiviral activities of betulinic acid from Zizyphus jujuba, and hederasaponin F isolated from Hedera helix L. against influenza virus (5,31). Previous studies also suggested that pterodontic acid from Laggera pterodonta (32), silymarin (33), and brevilin A (34) inhibited the replication of influenza virus. More interestingly, quercetin, which has a chemical structure similar to morin hydrate, inhibited the influenza virus from entering the host cells (35).
Although the details of the anti-inflammatory mechanisms of morin hydrate are still not clear, it is reported to have anti-inflammatory effect in inflammatory bowel disease through the inhibition of nitric oxide synthase activity and in ameliorating Alzheimer's disease by reducing oxidative stress (36,37). In addition, morin hydrate inhibits TREM-1/TLR4-mediated inflammatory responses in the mouse model of carbon tetrachloride-induced liver injury (38). Besides, morin hydrate exerts its anti-inflammatory effects in atherosclerosis through autophagy regulation and reduces TNF-α expression mediated by cAMP-PKA-AMPK-SIR1 signaling pathway (12). Morin hydrate also regulates NF-κB pathway, leading to reduced production of pro-inflammatory cytokines, such as TNF-α, IL-6, cyclooxygenase-2, prostaglandin E2, in animal model of colon cancer (36), and inhibits IL-1β-induced inflammation in osteoarthritis model (37). In this study, we observed that mice treated with morin hydrate showed decreased levels of pro-inflammatory cytokines in lungs during influenza infection, which may be associated with its effects on immune cells, in addition to direct inhibition of influenza virus entry. It reduced the expression of LPS-induced TNF-α and IL-1β in RAW264.7 macrophage cell line (10) and also inhibited TREM-1, which is highly expressed receptor in neutrophils, monocytes and macrophages (38,39,40). However, further studies are needed to understand whether the anti-viral effects of morin hydrate are mediated through modulation of immune cell activation, or solely through the inhibition of influenza virus entry.
Influenza virus infects millions, causing severe illness and with about 250,000 to 500,000 casualties every year (41). The risk comes from uncontrolled pro-inflammatory responses, called as ‘cytokine storm’ (41). When influenza virus invades lungs, infected epithelial cells express CCL2, and other pro-inflammatory cytokines to recruit innate immune cells, such as alveolar macrophages, monocytes and neutrophils, and (42). In the host cells, viral RNA is sensed by RIG-I receptors that initiates NOD-like receptor protein 3-induced inflammasome to produce IL-1β in both, immune cells and non-immune cells, like epithelial cells (43). Excessive production of those cytokines, which are closely associated with cytokine storm, sometimes causes detrimental effects in patients (41). In this context, we propose that the use of morin hydrate may prove to be one of the promising approaches to control the excessive inflammation in virus-mediated cytokine storm, by reducing the production of CCL2 and IL-1β.
Considering the effectiveness of morin hydrate on oseltamivir-resistant influenza virus, we thought that the mechanism of action of morin hydrate is probably different from that of oseltamivir. The stage of viral infection (entry, fusion and uncoating, nuclear import of viral RNA, replication, translation, packaging, and release) that was targeted by morin hydrate is not known. The results of our time-course and time-of-addition experiments suggested that morin hydrate had its effect at the early phase of viral life cycle. Next, we tried to find out whether morin hydrate inhibits the entry of influenza virus into cells or prevents the fusion of the viral envelope with the endosomal membrane. The results of hemolysis inhibition and hemagglutination assays suggested that morin hydrate blunted the endocytosis of influenza virus, but did not inhibit the fusion step.
Combination therapy involving 2 or more antiviral drugs may increase the antiviral efficacy and, the combination of compounds that interfere with different steps in the viral life cycle may be more useful in suppressing the emergence of drug-resistant variants, compared to monotherapy (44). For example, a combination of ribavirin, an inhibitor of viral RNA synthesis and capping, and neuraminidase inhibitors is known to exhibit synergistic activity against H1N1 virus in vitro (45). Therefore, the combined treatment with three antiviral drugs, amantadine, an inhibitor of viral shedding, ribavirin, and oseltamivir, showed synergistic activity in vitro even against the adamantanes- or oseltamivir-resistant 2009 pandemic H1N1 virus.
In the current study, we found that morin hydrate alone, or in combination with oseltamivir phosphate, showed antiviral activity against A/PR/8 in vitro and in mice. The molecular docking study suggested that morin hydrate binds to HA1, showing the possibility of an additive effect of the combination of morin hydrate with oseltamivir, which is an inhibitor of viral NA. Moreover, morin hydrate was found to be effective against oseltamivir-resistant influenza A viruses. It reduced the loss of body weight in mice infected with A/PR/8, and inhibited the viral replication in the lungs. Furthermore, the combination therapy of morin hydrate and oseltamivir phosphate reduced mortality and remarkably reduced lung inflammation in infected, than the oseltamivir phosphate single treatment. Together, our results suggest that morin hydrate demonstrates an antiviral effect that is further enhanced when administered in combination with oseltamivir, the currently used antiviral drug.
ACKNOWLEDGEMENTS
This study was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science and Ministry of Education (grant numbers: NRF-2020R1A2B5B02001552, NRF-2019R1I1A1A01060238, and NRF-2017R1A6A3A11031757). This study has been worked with the support of a research grant of Kangwon National University in 2017.
Abbreviations
- A/PR/8
influenza A/Puerto Rico/8/1934 (H1N1)
- HA
hemagglutinin
- NA
neuraminidase
- NP
nucleoprotein
- ns
not significant
- PA
polymerase acidic protein
- pfu
plaque forming units
- qPCR
quantitative PCR
- SRB
Sulforhodamine B
Footnotes
Conflicts of Interest: We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
- Data curation: Hong EH, Song JH, Kim SR, Jeong B, Yang H, Ahn JH, Jeong H, Chang SY, Ko HJ.
- Funding acquisition: Hong EH, Song JH, Ko HJ.
- Investigation: Hong EH, Song JH, Ko HJ.
- Methodology: Hong EH, Song JH, Kim SR, Cho J, Jeong H, Kim SE.
- Project administration: Ko HJ.
- Validation: Hong EH, Song JH, Kim SR.
- Visualization: Hong EH, Song JH, Jeong B, Chang SY.
- Writing - original draft: Hong EH, Song JH, Cho J, Chang SY, Ko HJ.
- Writing - review & editing: Jeong JH.
SUPPLEMENTARY MATERIALS
Antiviral activity of oseltamivir carboxylate against A/PR/8 and oseltamivir-resistant A/PR/8 in vitro. A549 cells were infected with 1×103 pfu of (A) A/PR/8 and (B) oseltamivir-resistant A/PR/8, and treated with different concentrations of oseltamivir carboxylate. Cell viability was analyzed by the SRB assay, and determined based on the OD of the samples at 524 nm. Bar graphs show the mean±SD.
3×104 A549 cells/well were seeded in a 96-well culture plate. On the next day, they were treated with (A) morin hydrate, (B) oseltamivir carboxylate, (C) 125 μM morin hydrate with oseltamivir carboxylate, and (D) 250 μM morin hydrate with oseltamivir carboxylate for 2 days. Results are presented as the mean percentage values from 3 independent experiments, carried out in triplicates.
Docking of morin hydrate to HA 1 protein (PDB: 1RU7). The analysis was performed using the PyRx software (ver. 0.8); results were visualized using PyMol software (ver. 2.3). (A) The binding sites of morin hydrate (red) to the HA protein. (B) The anchoring residues between morin hydrate and stem residues of HA are denoted in cyan color. Hydrogen bonding interaction is denoted by dashed green-colored lines.
References
- 1.Neumann G, Kawaoka Y. The first influenza pandemic of the new millennium. Influenza Other Respir Viruses. 2011;5:157–166. doi: 10.1111/j.1750-2659.2011.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JI, Lee S, Lee GY, Park S, Bae JY, Heo J, Kim HY, Woo SH, Lee HU, Ahn CA, et al. Novel small molecule targeting the hemagglutinin stalk of influenza viruses. J Virol. 2019;93:e00878-19. doi: 10.1128/JVI.00878-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palese P. Influenza: old and new threats. Nat Med. 2004;10:S82–S87. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 4.Ward P, Small I, Smith J, Suter P, Dutkowski R. Oseltamivir (Tamiflu) and its potential for use in the event of an influenza pandemic. J Antimicrob Chemother. 2005;55 Suppl 1:i5–i21. doi: 10.1093/jac/dki018. [DOI] [PubMed] [Google Scholar]
- 5.Hong EH, Song JH, Kang KB, Sung SH, Ko HJ, Yang H. Anti-influenza activity of betulinic acid from Zizyphus jujuba on influenza A/PR/8 virus. Biomol Ther (Seoul) 2015;23:345–349. doi: 10.4062/biomolther.2015.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353:1363–1373. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
- 7.Ison MG. Antivirals and resistance: influenza virus. Curr Opin Virol. 2011;1:563–573. doi: 10.1016/j.coviro.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Isin B, Doruker P, Bahar I. Functional motions of influenza virus hemagglutinin: a structure-based analytical approach. Biophys J. 2002;82:569–581. doi: 10.1016/S0006-3495(02)75422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng LY, Yang J, Liu S. Investigational hemagglutinin-targeted influenza virus inhibitors. Expert Opin Investig Drugs. 2017;26:63–73. doi: 10.1080/13543784.2017.1269170. [DOI] [PubMed] [Google Scholar]
- 10.Jakhar R, Paul S, Chauhan AK, Kang SC. Morin hydrate augments phagocytosis mechanism and inhibits LPS induced autophagic signaling in murine macrophage. Int Immunopharmacol. 2014;22:356–365. doi: 10.1016/j.intimp.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Kapoor R, Kakkar P. Protective role of morin, a flavonoid, against high glucose induced oxidative stress mediated apoptosis in primary rat hepatocytes. PLoS One. 2012;7:e41663. doi: 10.1371/journal.pone.0041663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Cao ZQ, Wang HY, Cheng YN, Yu LG, Zhang XK, Sun Y, Guo XL. The anti-inflammatory effects of Morin hydrate in atherosclerosis is associated with autophagy induction through cAMP signaling. Mol Nutr Food Res. 2017;61:1600966. doi: 10.1002/mnfr.201600966. [DOI] [PubMed] [Google Scholar]
- 13.Singh MP, Chauhan AK, Kang SC. Morin hydrate ameliorates cisplatin-induced ER stress, inflammation and autophagy in HEK-293 cells and mice kidney via PARP-1 regulation. Int Immunopharmacol. 2018;56:156–167. doi: 10.1016/j.intimp.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Zhang ZT, Cao XB, Xiong N, Wang HC, Huang JS, Sun SG, Wang T. Morin exerts neuroprotective actions in Parkinson disease models in vitro and in vivo . Acta Pharmacol Sin. 2010;31:900–906. doi: 10.1038/aps.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu M, Zhang Y. Morin inhibits ovarian cancer growth through the inhibition of NF-κB signaling pathway. Anticancer Agents Med Chem. 2019;19:2243–2250. doi: 10.2174/1871521409666191014164742. [DOI] [PubMed] [Google Scholar]
- 16.Kaihatsu K, Kawakami C, Kato N. Potential anti-influenza virus agents based on coffee ingredients and natural flavonols. Nat Prod Chem Res. 2014;2:129. [Google Scholar]
- 17.Kong B, Moon S, Kim Y, Heo P, Jung Y, Yu SH, Chung J, Ban C, Kim YH, Kim P, et al. Virucidal nano-perforator of viral membrane trapping viral RNAs in the endosome. Nat Commun. 2019;10:185. doi: 10.1038/s41467-018-08138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu L, Li Y, Li S, Li H, Qiu Z, Lee C, Lu H, Lin X, Zhao R, Chen L, et al. Inhibition of influenza A virus (H1N1) fusion by benzenesulfonamide derivatives targeting viral hemagglutinin. PLoS One. 2011;6:e29120. doi: 10.1371/journal.pone.0029120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo W, Zhang J, Liang L, Wang G, Li Q, Zhu P, Zhou Y, Li J, Zhao Y, Sun N, et al. Phospholipid scramblase 1 interacts with influenza A virus NP, impairing its nuclear import and thereby suppressing virus replication. PLoS Pathog. 2018;14:e1006851. doi: 10.1371/journal.ppat.1006851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKimm-Breschkin JL. Influenza neuraminidase inhibitors: antiviral action and mechanisms of resistance. Influenza Other Respir Viruses. 2013;7 Suppl 1:25–36. doi: 10.1111/irv.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moorthy NS, Poongavanam V, Pratheepa V. Viral M2 ion channel protein: a promising target for anti-influenza drug discovery. Mini Rev Med Chem. 2014;14:819–830. [PubMed] [Google Scholar]
- 22.Heo YA. Baloxavir: first global approval. Drugs. 2018;78:693–697. doi: 10.1007/s40265-018-0899-1. [DOI] [PubMed] [Google Scholar]
- 23.LeGoff J, Rousset D, Abou-Jaoudé G, Scemla A, Ribaud P, Mercier-Delarue S, Caro V, Enouf V, Simon F, Molina JM, et al. I223R mutation in influenza A(H1N1)pdm09 neuraminidase confers reduced susceptibility to oseltamivir and zanamivir and enhanced resistance with H275Y. PLoS One. 2012;7:e37095. doi: 10.1371/journal.pone.0037095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trebbien R, Pedersen SS, Vorborg K, Franck KT, Fischer TK. Development of oseltamivir and zanamivir resistance in influenza A(H1N1)pdm09 virus, Denmark, 2014. Euro Surveill. 2017;22:30445. doi: 10.2807/1560-7917.ES.2017.22.3.30445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renaud C, Boudreault AA, Kuypers J, Lofy KH, Corey L, Boeckh MJ, Englund JA. H275Y mutant pandemic (H1N1) 2009 virus in immunocompromised patients. Emerg Infect Dis. 2011;17:653–660. doi: 10.3201/eid1704.101429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leang SK, Kwok S, Sullivan SG, Maurer-Stroh S, Kelso A, Barr IG, Hurt AC. Peramivir and laninamivir susceptibility of circulating influenza A and B viruses. Influenza Other Respi Viruses. 2014;8:135–139. doi: 10.1111/irv.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Briskin DP. Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiol. 2000;124:507–514. doi: 10.1104/pp.124.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito S, Kuroda K, Hayashi Y, Sasaki Y, Nagamura Y, Nishida K, Ishiguro I. Preparation of glycyrrhetic acid glycosides having various β(1→2)-linked disaccharides and their cytoprotective effects on carbon tetrachloride-induced hepatic injury. Chem Pharm Bull (Tokyo) 1991;39:2333–2339. doi: 10.1248/cpb.39.2333. [DOI] [PubMed] [Google Scholar]
- 30.Vlietinck AJ, Vanden Berghe DA. Can ethnopharmacology contribute to the development of antiviral drugs? J Ethnopharmacol. 1991;32:141–153. doi: 10.1016/0378-8741(91)90112-q. [DOI] [PubMed] [Google Scholar]
- 31.Hong EH, Song JH, Shim A, Lee BR, Kwon BE, Song HH, Kim YJ, Chang SY, Jeong HG, Kim JG, et al. Coadministration of hedera helix l. Extract enabled mice to overcome insufficient protection against influenza A/PR/8 virus infection under suboptimal treatment with oseltamivir. PLoS One. 2015;10:e0131089. doi: 10.1371/journal.pone.0131089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan W, Li J, Chen Q, Jiang Z, Zhang R, Wang X, Yang Z, Pan X. Pterodontic acid isolated from Laggera pterodonta inhibits viral replication and inflammation induced by influenza a virus. Molecules. 2017;22:1738. doi: 10.3390/molecules22101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song JH, Choi HJ. Silymarin efficacy against influenza A virus replication. Phytomedicine. 2011;18:832–835. doi: 10.1016/j.phymed.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Xia Y, Yang L, He J, Li Y, Xia C. Brevilin A, a sesquiterpene lactone, inhibits the replication of influenza a virus in vitro and in vivo. Viruses. 2019;11:835. doi: 10.3390/v11090835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu W, Li R, Li X, He J, Jiang S, Liu S, Yang J. Quercetin as an antiviral agent inhibits influenza a virus (IAV) entry. Viruses. 2015;8:6. doi: 10.3390/v8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma SH, Kumar JS, Chellappan DR, Nagarajan S. Molecular chemoprevention by morin - A plant flavonoid that targets nuclear factor kappa B in experimental colon cancer. Biomed Pharmacother. 2018;100:367–373. doi: 10.1016/j.biopha.2018.02.035. [DOI] [PubMed] [Google Scholar]
- 37.Qu Y, Wang C, Liu N, Gao C, Liu F. Morin exhibits anti-inflammatory effects on IL-1β-stimulated human osteoarthritis chondrocytes by activating the Nrf2 signaling pathway. Cell Physiol Biochem. 2018;51:1830–1838. doi: 10.1159/000495684. [DOI] [PubMed] [Google Scholar]
- 38.Pelham CJ, Agrawal DK. Emerging roles for triggering receptor expressed on myeloid cells receptor family signaling in inflammatory diseases. Expert Rev Clin Immunol. 2014;10:243–256. doi: 10.1586/1744666X.2014.866519. [DOI] [PubMed] [Google Scholar]
- 39.Tammaro A, Derive M, Gibot S, Leemans JC, Florquin S, Dessing MC. TREM-1 and its potential ligands in non-infectious diseases: from biology to clinical perspectives. Pharmacol Ther. 2017;177:81–95. doi: 10.1016/j.pharmthera.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 40.Tornai D, Furi I, Shen ZT, Sigalov AB, Coban S, Szabo G. Inhibition of triggering receptor expressed on myeloid cells 1 ameliorates inflammation and macrophage and neutrophil activation in alcoholic liver disease in mice. Hepatol Commun. 2018;3:99–115. doi: 10.1002/hep4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Q, Zhou YH, Yang ZQ. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol. 2016;13:3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, Liu S, Goraya MU, Maarouf M, Huang S, Chen JL. Host immune response to influenza a virus infection. Front Immunol. 2018;9:320. doi: 10.3389/fimmu.2018.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuriakose T, Kanneganti TD. Regulation and functions of NLRP3 inflammasome during influenza virus infection. Mol Immunol. 2017;86:56–64. doi: 10.1016/j.molimm.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Govorkova EA, Webster RG. Combination chemotherapy for influenza. Viruses. 2010;2:1510–1529. doi: 10.3390/v2081510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smee DF, Bailey KW, Morrison AC, Sidwell RW. Combination treatment of influenza A virus infections in cell culture and in mice with the cyclopentane neuraminidase inhibitor RWJ-270201 and ribavirin. Chemotherapy. 2002;48:88–93. doi: 10.1159/000057668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antiviral activity of oseltamivir carboxylate against A/PR/8 and oseltamivir-resistant A/PR/8 in vitro. A549 cells were infected with 1×103 pfu of (A) A/PR/8 and (B) oseltamivir-resistant A/PR/8, and treated with different concentrations of oseltamivir carboxylate. Cell viability was analyzed by the SRB assay, and determined based on the OD of the samples at 524 nm. Bar graphs show the mean±SD.
3×104 A549 cells/well were seeded in a 96-well culture plate. On the next day, they were treated with (A) morin hydrate, (B) oseltamivir carboxylate, (C) 125 μM morin hydrate with oseltamivir carboxylate, and (D) 250 μM morin hydrate with oseltamivir carboxylate for 2 days. Results are presented as the mean percentage values from 3 independent experiments, carried out in triplicates.
Docking of morin hydrate to HA 1 protein (PDB: 1RU7). The analysis was performed using the PyRx software (ver. 0.8); results were visualized using PyMol software (ver. 2.3). (A) The binding sites of morin hydrate (red) to the HA protein. (B) The anchoring residues between morin hydrate and stem residues of HA are denoted in cyan color. Hydrogen bonding interaction is denoted by dashed green-colored lines.