Abstract
Purpose
The impact of glycemic control on macrovascular complications and arterial stiffness in type II diabetes (T2D), as well as the extent of additive effect of hypertension, is unclear. The aims of this study were to investigate the impact of glycemic control on the cardio-ankle vascular index (CAVI), an indicator of arterial stiffness, and to determine the relative risk of concomitant diabetes and hypertension with arterial stiffness.
Methods
One hundred and nine participants were enrolled and classified as non-diabetes (n= 37) and diabetes (n=72); the diabetic group was further identified as controllable and uncontrollable T2D depending on their hemoglobin A1c (HbA1c) levels. Univariate and multiple regression analyses were used to assess the association between CAVI and glycemic control status and hypertension. Relative risk analysis for abnormal CAVI with exposure to diabetes and hypertension was investigated.
Results
In all participants, age, systolic blood pressure, body mass index, and fasting blood sugar were independent predictors of CAVI. In diabetic participants, glycemic control status or HbA1c levels did not significantly correlate with CAVI. Systolic blood pressure was an independent predictor for CAVI with β = 0.26. In addition, the coexistence of diabetes together with hypertension was significantly associated with a 2.4-fold increase in the risk of abnormal CAVI (95% CI, 1.410–4.184; p <0.001).
Conclusion
This study demonstrates that HbA1c as well as fasting blood sugar levels in diabetic participants do not correlate with arterial stiffness. Concomitant diabetes and hypertension significantly increase the risk of arterial stiffness.
Keywords: arterial stiffness, cardio-ankle vascular index, diabetes, glycemic control, hypertension
Introduction
Type 2 diabetes (T2D) is a major health problem worldwide with a substantially increasing incidence.1 T2D increases risk of cardiovascular disease by two to four times, and its impact is as equivalent to that of coronary heart disease.2–4 The major cause of death in T2D patients is cardiovascular disorders, ie coronary heart disease and stroke, which are related to macrovascular dysfunction, a crucial complication of diabetes.5,6 The assessment of vascular function in diabetic patients is recommended as class IIa, for monitoring vascular complications and predicting cardiovascular events.7 In the past, brachial-ankle pulse wave velocity (baPWV) was widely used as a gold standard for assess atherosclerosis and arterial stiffness. Recently, the cardio-ankle vascular index (CAVI) was developed and reported as an equivalent to baPWV for the assessment of arterial stiffness, with blood pressure-independent characteristics.8 Recent studies have demonstrated that CAVI is associated with plasma glucose levels. It is higher in diabetic when compared with non-diabetic subjects.9,10 According to the joint statement between the American Heart Association and the American College of Cardiology on the prevention of cardiovascular disease in diabetes, glycated hemoglobin (HbA1c) has been added as a criterion for diagnosis and monitoring of diabetes.11 The goal of diabetes care is, generally, to keep HbA1c < 7%. However, HbA1c can be kept at < 6.5% or < 8.0% depending on the characteristics of the patient.11 Despite the fact that hyperglycemia is associated with cardiovascular diseases, studies have shown that the link between HbA1c and macrovascular complications is weaker than that of microvascular complications, and lowering HbA1c has little or no effect on cardiovascular risk.7,12 Likewise, the impact of blood sugar control on arterial stiffness is controversial. Ibata et al reported the improvement of CAVI after two weeks of hospitalized hyperglycemia control.10 Elias et al also demonstrated a higher PWV in T2D patients and found that the risk of arterial stiffness was over nine times higher in uncontrolled T2D compared to non-diabetic patients.13 On the other hand, Chang et al did not observe any differences in CAVI between controlled and uncontrolled diabetes patients, when HbA1c at 7.5 was used as a cut-off value.14 Similarly, Tian et al found that age, not HbA1c levels, is an independent predictor for CAVI in T2D.15
One major confounder influencing vascular function in T2D is hypertension. The coexistence of hypertension and T2D presents in 30–80% of patients and increases the risk of cardiovascular disease.16 There is evidence showing a correlation between HbA1c levels and arterial stiffness in people with resistance hypertension.17 The extent of the association between glucose levels, blood pressure, and arterial stiffness parameters is unclear. Tedesco et al found the highest carotid-femoral PWV in the concomitant group compared to the diabetes and hypertension alone groups. The multivariate regression analysis showed that mean arterial blood pressure affected arterial stiffness less than the blood glucose level did.18 The additive effect of hypertension has been shown in another study showing a weaker effect of diabetes.19 Therefore, the objectives of this study were to investigate the impact of blood sugar control, determined by HbA1c, and hypertension on arterial stiffness in T2D participants.
Materials and Methods
Study Design and Participants
This study was a cross-sectional study approved by the Naresuan University Institutional Review Board (COA No. 360/2016). The study was performed in agreement with the principles of the Declaration of Helsinki. Data were collected at Wang I-Thok Health Promoting Hospital, which is a community hospital located in Wang I-Thok sub-district, Bang Rakam district, Phitsanulok province, Thailand between September and December 2016. One hundred and nine participants aged over 18 years old were recruited. Written informed consent was obtained from all participants. The participants who had renal failure, arrhythmia, alcohol or drug addiction, cerebrovascular disease or peripheral vascular disease were excluded. Participants who had no history of diabetes and blood glucose levels were < 126 mg/dL were classified as non-diabetic participants. The diabetic group comprised of the participants who were diagnosed as T2D by physicians. Type 2 diabetic participants then were divided into two groups including 1) controllable diabetes (HbA1c < 6.5%) and 2) uncontrollable diabetes (HbA1c ≥ 6.5%).20
Clinical Variables
Medical history and medications used were obtained by interviewing. Body weight, percentage of body fat, and percentage of visceral fat were measured using a body composition monitor (Omron Karada Scan Body Composition Monitor HBF-214, Japan). Body mass index (BMI) was calculated as body weight in kilograms divided by the square of height in meters. Waist circumference was measured at the approximate midpoint between the lower margin of the last rib and the top of the iliac crest and hip circumference was measured at the widest point of the buttocks in the standing position.21 Blood pressure and heart rate were measured twice using an automatic brachial sphygmomanometer (HEM-7130, Omron, Japan). The participants were seated and relaxed for 5 minutes before the measurement. Blood tests were performed after a 12-hour fast including the lipid profile, creatinine, fasting blood sugar, and HbA1c. Estimated glomerular filtration rate was calculated from creatinine using the CKD-EPI creatinine equation. The blood test was performed according to the manufacturer’s protocols (Human Diagnostics Worldwide, Germany).
CAVI Measurements
CAVI was measured using a vascular screening device (VaSera1500, Fukuda Denshi Co. Ltd, Tokyo). The measurement was performed with the participants lying in the supine position by applying four blood pressure cuffs to the bilateral upper arms and ankles, placing two electrocardiogram electrodes on both wrists and a microphone on the sternum between the second ribs to detect heart sounds. The examination was performed after 15 minutes of resting in a quiet and temperature-controlled room (25 ± 1°C). CAVI was automatically calculated using Equation 1 based on the stiffness parameter β and Bramwell-Hill equation.8 CAVI < 8 is classified as normal, 8 ≤ CAVI < 9 is classified as borderline arterial stiffness, and CAVI ≥ 9 is considered as suspected arterial stiffness. In this study, an abnormal CAVI was defined as CAVI ≥ 8, which included borderline and suspected arterial stiffness.
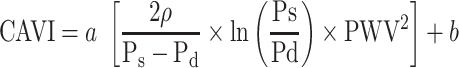 |
Equation 1 |
where ρ is the blood density of 1.05 g/mL; Ps and Pd are systolic and diastolic blood pressure, respectively; PWV is pulse wave velocity; and a and b are constants.
Statistical Analysis
The normal distribution of continuous data was tested by the Kolmogorov–Smirnov test. Continuous data are expressed as mean ± standard deviation (SD) for normally distributed data and median (interquartile range, IQR) for non-normally distributed data. In order to compare two independent means, Student’s t-test and the Mann–Whitney U-test were performed for normally distributed data and non-normally distributed data, respectively. Categorical data are expressed as numbers and percentages and the differences between the two groups were analyzed using the chi-squared test. Pearson correlation analysis was performed to evaluate the association between CAVI and other clinical variables. Significant potential variables in the Pearson correlation analysis were further analyzed with a stepwise multiple regression analysis to identify independent variables associated with CAVI. To demonstrate the association between risk factors and abnormal CAVI, relative risk with 95% confidence intervals is presented. All data were analyzed using SPSS 23.0. A p-value <0.05 was considered statistically significant.
Results
Impact of Hyperglycemia on CAVI
Out of 109 participants enrolled in this study, 37 were non-diabetic and 72 were diabetic participants. Table 1 shows the demographic characteristics of all participants. There was no significant difference in the gender balance between the two groups. Average age, systolic blood pressure, and heart rate of the diabetic subjects were significantly higher than those of the non-diabetic subjects. Regarding body composition, the body fat percentage and waist-hip ratio of the diabetic subjects were significantly higher than those of the non-diabetic subjects. The mean CAVI of the diabetic subjects (8.99 ± 1.23) was significantly higher than that of the non-diabetic subjects (7.89 ± 0.87) at p <0.001.
Table 1.
Demographic Characteristics of Participants Between Non-Diabetes and Diabetes
| Variables | Participants (n=109) | P-value | |
|---|---|---|---|
| Non-Diabetes (n=37) | Diabetes (n=72) | ||
| Sex | 0.164 | ||
| Male (n (%)) | 11 (29.7) | 13 (18.1) | |
| Female (n (%)) | 26 (70.3) | 59 (81.9) | |
| Age (years) | 49.5 ± 9.8 | 61.0 ± 9.9 | < 0.001* |
| Heart rate (bpm) | 73.00 (65.00–78.25) | 95.50 (77.00–114.25) | < 0.001* |
| Systolic blood pressure (mmHg) | 124.00 (113.25–139.75) | 143.75 (126.63–155.75) | < 0.001* |
| Diastolic blood pressure (mmHg) | 79.77 ± 9.50 | 81.58 ± 12.53 | 0.401 |
| Body composition | |||
| BMI (kg/m2) | 25.00 ± 3.78 | 25.98 ± 4.39 | 0.250 |
| Body fat (%) | 29.90 (24.25–35.20) | 33.40 (29.18–35.95) | 0.024* |
| Visceral fat (%) | 9.00 (5.50–11.50) | 9.50 (7.00–13.25) | 0.118 |
| Waist-hip ratio | 0.87 ± 0.05 | 0.92 ± 0.06 | < 0.001* |
| Lipid profile | |||
| Total cholesterol (mmol/L) | 6.23 (5.57–6.66) | 4.40 (3.96–5.50) | < 0.001* |
| Triglyceride (mmol/L) | 1.04 (0.66–1.52) | 1.63 (1.27–2.41) | < 0.001* |
| HDL-C (mmol/L) | 1.59 ± 0.34 | 1.74 ± 0.48 | 0.092 |
| LDL-C (mmol/L) | 3.70 (3.13–4.36) | 1.91 (1.29–2.82) | < 0.001* |
| Medical History | |||
| Hypertension | 9 (24.3) | 59 (81.9) | < 0.001* |
| Hyperlipidemia | 3 (8.1) | 50 (69.4) | < 0.001* |
| Smoking | 5 (13.5) | 16 (22.2) | 0.204 |
| Mean CAVI | 7.89 ± 0.87 | 8.99 ± 1.23 | < 0.001* |
Notes: Values are displayed as n (%), mean ± SD, and median (IQR). * represents statistical significance.
Abbreviations: BMI, body mass index; CAVI, cardio-ankle vascular index; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol.
To investigate the factors affecting CAVI, the participants were classified as normal CAVI or abnormal CAVI with a cut-off value of 8 and their basic characteristics were compared. It was found that age, systolic blood pressure, diabetes, and hypertension were significantly different between the two groups. Further analysis by univariate and multiple regression analysis demonstrated that age, systolic blood pressure, BMI, and fasting blood sugar were independent predictors of CAVI. The details are presented in Tables 2 and 3.
Table 2.
Comparison of Clinical Variables Between Normal and Abnormal CAVI
| Variables | Participants (n=109) | P-value | |
|---|---|---|---|
| Normal CAVI (n=34) | Abnormal CAVI (n=75) | ||
| Sex | 0.808 | ||
| Male | 7 (20.6) | 17 (22.7) | |
| Female | 27 (79.4) | 58 (77.3) | |
| Age (years) | 50.5 ± 10.4 | 60.1 ± 10.3 | < 0.001* |
| Heart rate (bpm) | 78.75 (73.00–94.63) | 82.00 (70.00–112.50) | 0.617 |
| Systolic blood pressure (mmHg) | 124.75 (116.87–140.00) | 143.50 (125.50–154.00) | 0.001* |
| Diastolic blood pressure (mmHg) | 80.34 ± 11.53 | 81.25 ± 11.67 | 0.704 |
| Body composition | |||
| BMI (kg/m2) | 26.26 ± 3.91 | 25.37 ± 4.32 | 0.306 |
| Body fat (%) | 32.00 (28.63–35.43) | 33.10 (28.50–35.70) | 0.857 |
| Visceral fat (%) | 9.50 (6.50–11.63) | 9.00 (6.00–12.00) | 0.950 |
| Waist-hip ratio | 0.89 ± 0.05 | 0.91 ± 0.06 | 0.235 |
| Lipid profile | |||
| Total cholesterol (mmol/L) | 5.75 (4.57–6.79) | 5.07 (4.06–5.90) | 0.006* |
| Triglyceride (mmol/L) | 1.30 (0.95–1.70) | 1.58 (1.14–2.31) | 0.065 |
| HDL-C (mmol/L) | 1.64 ± 0.39 | 1.71 ± 0.46 | 0.482 |
| LDL-C (mmol/L) | 2.75 (1.80–4.05) | 2.20 (1.50–3.57) | 0.165 |
| Diabetes | 13 (38.2) | 59 (78.7) | < 0.001* |
| Hypertension | 11 (32.4) | 44 (58.7) | 0.011* |
| Mean CAVI | 7.29 ± 0.62 | 9.22 ± 0.93 | < 0.001* |
Notes: Values are displayed as n (%), mean ± SD, and median (IQR). * represents statistical significance. Abnormal CAVI is defined as CAVI ≥ 8.
Abbreviations: BMI, body mass index; CAVI, cardio-ankle vascular index; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol.
Table 3.
Pearson Correlation and Multiple Linear Regression Analysis of CAVI and Other Clinical Variables in All Participants
| Variables | Pearson Correlation | Multiple Regression | ||||
|---|---|---|---|---|---|---|
| r | P-value | β | 95% CI | P-value | ||
| Lower | Upper | |||||
| Age | 0.509 | < 0.001* | 0.332 | 0.018 | 0.054 | < 0.001* |
| Sex | 0.046 | 0.632 | ||||
| Systolic blood pressure | 0.392 | < 0.001* | 0.261 | 0.006 | 0.026 | 0.002* |
| Diastolic blood pressure | 0.015 | 0.880 | ||||
| Heart rate | 0.223 | 0.020* | ||||
| BMI | −0.296 | 0.002* | −0.241 | −0.116 | −0.025 | 0.003* |
| Body fat | −0.047 | 0.625 | ||||
| Visceral fat | −0.182 | 0.059 | ||||
| Waist-hip ratio | 0.022 | 0.818 | ||||
| Fasting blood sugar | 0.319 | 0.001* | 0.215 | 0.002 | 0.013 | 0.006* |
| Total cholesterol | −0.265 | 0.005* | ||||
| Triglyceride | 0.183 | 0.056 | ||||
| HDL-C | 0.089 | 0.356 | ||||
| LDL-C | −0.161 | 0.095 | ||||
| Diabetes | 0.424 | < 0.001* | ||||
| Hypertension | 0.352 | < 0.001* | ||||
Notes: * Represents statistical significance. Adjusted R2 = 0.388. Adjusting for sex, heart rate, and total cholesterol.
Abbreviations: BMI, body mass index; CAVI, cardio-ankle vascular index; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; CI, confidence interval; r, Pearson correlation coefficient; β, standardized coefficients.
Impact of Glycemic Control on CAVI
The comparison of demographic characteristics and clinical variables between controllable diabetes and uncontrollable diabetes showed that only fat-related parameters (BMI and percentage of body fat) were significantly different (Table 4). We further assessed the factors involved in arterial stiffness in T2D participants and found that CAVI was significantly positively correlated with age, systolic blood pressure, and hypertension and significantly negatively correlated with BMI, body fat, and visceral fat. The stepwise multiple linear regression analysis showed that systolic blood pressure (β = 0.262, p = 0.013) and BMI (β = −0.443, p < 0.001) were independent predictors of CAVI with adjusted R2 = 0.247. The details are presented in Table 5.
Table 4.
Demographic Characteristics of Diabetic Participants Between Controllable Diabetes and Uncontrollable Diabetes
| Variables | Diabetes (n=72) | P-value | |
|---|---|---|---|
| Controllable Diabetes (n=23) | Uncontrollable Diabetes (n=49) | ||
| Sex | 0.449 | ||
| Male | 3 (13) | 10 (20.4) | |
| Female | 20 (87) | 39 (79.6) | |
| Age (years) | 62.2 ± 9.8 | 60.5 ± 10.0 | 0.495 |
| Heart rate (bpm) | 113.50 (103.25–125.00) | 82.50 (72.75–100.50) | < 0.001* |
| Systolic blood pressure (mmHg) | 147.00 (136.00–156.50) | 143.00 (125.50–154.00) | 0.227 |
| Diastolic blood pressure (mmHg) | 81.65 ± 13.26 | 81.55 ± 12.31 | 0.975 |
| Body composition | |||
| BMI (kg/m2) | 24.48 ± 3.52 | 26.69 ± 4.61 | 0.045* |
| Body fat (%) | 31.70 (28.60–35.70) | 33.50 (29.40–36.80) | 0.499 |
| Visceral fat (%) | 8.00 (5.50–11.30) | 10.00 (7.65–15.00) | 0.031* |
| Waist-hip ratio | 0.93 ± 0.05 | 0.93 ± 0.06 | 0.074 |
| Lipid profile | |||
| Total cholesterol (mmol/L) | 4.24 (3.96–4.84) | 4.58 (3.90–5.69) | 0.229 |
| Triglyceride (mmol/L) | 1.70 (1.14–2.07) | 1.61 (1.27–2.53) | 0.574 |
| HDL-C (mmol/L) | 1.75 ± 0.48 | 1.74 ± 0.48 | 0.907 |
| LDL-C (mmol/L) | 1.86 (1.29–2.30) | 2.04 (1.29–3.09) | 0.316 |
| Fasting blood sugar (mmol/L) | 6.38 (5.94–6.94) | 7.05 (5.99–8.82) | 0.022* |
| HbA1c (%) | 6.00 (5.80–6.20) | 7.60 (7.00–8.35) | < 0.001* |
| Creatinine (μmol/L) | 82.23 (69.85–91.96) | 84.00 (71.62–101.68) | 0.677 |
| eGFR (mL/s) | 1.21 ± 0.40 | 1.18 ± 0.29 | 0.708 |
| Medical History | |||
| Hypertension | 19 (82.6) | 40 (81.6) | 0.920 |
| Hyperlipidemia | 14 (60.9) | 36 (73.5) | 0.279 |
| Smoking | 5 (21.7) | 11 (22.4) | 0.946 |
| Medications | |||
| Antihypertensive drugs | 15 (65.2) | 32 (65.3) | 0.994 |
| Lipid lowering drugs | 9 (39.1) | 27 (55.1) | 0.206 |
| Mean CAVI | 8.83 ± 1.40 | 9.06 ± 1.15 | 0.468 |
Notes: Values are displayed as n (%), mean ± SD, and median (IQR). * represents statistical significance.
Abbreviations: BMI, body mass index; CAVI, cardio-ankle vascular index; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; HbA1c, hemoglobin A1c; eGFR, estimated glomerular filtration rate.
Table 5.
Pearson Correlation and Multiple Linear Regression Analysis of CAVI and Other Clinical Variables in Diabetic Participants
| Variables | Pearson | Multiple Regression | ||||
|---|---|---|---|---|---|---|
| r | P-value | β | 95% CI | P-value | ||
| Lower | Upper | |||||
| Age | 0.377 | 0.001* | ||||
| Sex | 0.005 | 0.968 | ||||
| Systolic blood pressure | 0.269 | 0.022* | 0.262 | 0.004 | 0.031 | 0.013* |
| Diastolic blood pressure | 0.014 | 0.909 | ||||
| Heart rate | 0.056 | 0.639 | ||||
| BMI | −0.447 | < 0.001* | −0.443 | −0.182 | −0.066 | < 0.001* |
| Body fat | −0.265 | 0.024* | ||||
| Visceral fat | −0.352 | 0.002* | ||||
| Waist-hip ratio | −0.208 | 0.080 | ||||
| Fasting blood sugar | 0.138 | 0.249 | ||||
| HbA1c | 0.087 | 0.469 | ||||
| Total cholesterol | 0.054 | 0.651 | ||||
| Triglyceride | 0.080 | 0.504 | ||||
| HDL-C | 0.032 | 0.792 | ||||
| LDL-C | 0.011 | 0.924 | ||||
| Hypertension | 0.263 | 0.025* | ||||
Notes: * Represents statistical significance. Adjusted R2 = 0.247. Adjusting for age and sex.
Abbreviations: BMI, body mass index; CAVI, cardio-ankle vascular index; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; HbA1c, hemoglobin A1c; CI, confidence interval; r, Pearson correlation coefficient; β, standardized coefficients.
Additional Effects of Hypertension and Diabetes on Abnormal CAVI
The relative risk analysis was performed according to diabetes and hypertension exposure. It was shown that diabetes was significantly associated with a 2.27-fold increase in the risk of abnormal CAVI (95% CI, 1.293–3.984; p =0.002). Furthermore, diabetes and hypertension were significantly associated with a 2.43-fold increase in the risk of abnormal CAVI (95% CI, 1.410–4.184; p <0.001) (Table 6).
Table 6.
Association Between Clinical Risk Factors and Abnormal CAVI
| Clinical Risk Factors | Relative Risk | 95% CI | P-value | ||
|---|---|---|---|---|---|
| Diabetes | Hypertension | Lower | Upper | ||
| Yes | No | 2.270 | 1.293 | 3.984 | 0.002* |
| No | Yes | 1.838 | 0.920 | 3.672 | 0.151 |
| Yes | Yes | 2.429 | 1.410 | 4.184 | < 0.001* |
Notes: * Represents statistical significance. Abnormal CAVI is defined as CAVI ≥ 8.
Abbreviations: CAVI, cardio-ankle vascular index; CI, confidence interval.
Discussion
The main findings of our study were that having diabetes or hypertension was associated with arterial stiffness; however, HbA1c as well as fasting blood sugar levels did not correlate to CAVI in T2D participants. Being hypertensive increased the risk of abnormal CAVI in T2D participants with a relative risk of 2.43-fold compared to healthy participants.
In this study, we used CAVI to assess arterial stiffness. It is a non-invasive, simple, reproducible measurement, independent of blood pressure, which is thought to be superior to baPWV.22 CAVI determines the majority of arteries, ie all of the arterial segments from the heart to ankle, and shows a significant correlation with cardiovascular disease. CAVI was positively correlated with intima media thickness and 10-year atherosclerotic cardiovascular disease risk in T2D patients.23 Thus, CAVI is a simple but effective marker of arterial stiffness and cardiovascular disease. In this study, an abnormal CAVI was defined as CAVI ≥ 8, which included borderline and suspected arterial stiffness. As defined by the manufacturer, CAVI < 8 is classified as normal, 8 ≤ CAVI < 9 is classified as borderline arterial stiffness, and CAVI ≥ 9 is considered as suspected arterial stiffness. However, there were previous reports showing the clinical significance of CAVI value about 8 in cardiovascular disease, especially for Asians. Kabutoya and Kazuomi compared CAVI and baPWV measured in the same Japanese individuals in a large-scale clinical study (n = 4,545 patients). They reported that the CAVI value about 8.3 corresponded to baPWV of 14 m/s which related to moderate risk of cardiovascular events based on the Framingham risk score.24 For Thais, Yingchoncharoen and Sritara reported that the CAVI value of 8 was an optimal cutoff to predict coronary artery disease with sensitivity 92%, specificity 63%, and accuracy 70% compared to the presence of coronary artery disease as assessed by 64-slice coronary computed tomography angiography from a large cross-sectional study in Thais (n = 1,391 patients).25
In the present study, the results show that the mean CAVI and systolic blood pressure of the diabetic participants were significantly higher than those of the non-diabetic participants. Furthermore, correlation analysis demonstrated that age, systolic blood pressure, and fasting blood sugar were independent variables associated with CAVI. Similarly, Namekata et al reported that systolic and diastolic blood pressure and mean CAVI of diabetes and prediabetic subjects were significantly higher than those of non-diabetic subjects. The prevalence of abnormally high CAVI (mean CAVI + one standard deviation) was significantly higher in a group of ≥40-year-old participants with diabetes and had an increasing trend with age in all participants. It was also found that abnormally high CAVI was significantly associated with diabetes (odds ratios of 2.41 times in men and 2.52 times in women) compared to participants with normal CAVI scores.9 In the present study, we found that BMI as an independent variable was inversely correlated with CAVI, which supports previous reports.26,27
The goals of glycemic control in T2D patients are to reduce microvascular complications and improve cardiovascular outcomes.12,28 The impact of glycemic control on macrovascular complications is still controversial.12,29 The present study demonstrates that HbA1c levels, or controlled vs uncontrolled DM, are not related to arterial stiffness in T2D patients. These data support previous studies that found no differences in PWV between controlled and uncontrolled DM.14,15 Our results are also similar to the findings of large clinical trials, ie The Action to Control Cardiovascular Risk in Diabetes (ACCORD) and The Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE), which found that intensified glycemic control did not significantly reduce macrovascular events.30,31 However, there is some evidence claiming an effect of glycemic control on arterial stiffness.10,13 This discrepancy may be due to the differences in the duration and the level of glycemic control and other potential parameters such as obesity, hyperlipidemia, hypertension, and hyperinsulinemia. Particularly, insulin level, apart from hyperglycemia, has been found to affect arterial stiffness.32,33
The coexistence of diabetes and hypertension is common since they share some pathophysiological aspects, ie obesity and insulin resistance. Our study shows that being hypertensive is an independent predictor of CAVI in all participants and T2D patients. The coexistence of diabetes and hypertension gave a relative risk of 2.43-fold increase in the risk of abnormal CVAI when compared to that of healthy participants, which was higher than that of diabetic or hypertensive alone participants. The result is related to the studies of Tedesco et al and de Oliveira Alvim et al, who used PWV as an indicator of arterial stiffness and also found the worst arterial stiffness in diabetic participants with hypertension.18,19 The negative influence of diabetes and hypertension on arterial stiffness may occur through structural and functional changes to the vascular wall via both separate mechanisms and shared mechanisms. In diabetes, the main mechanism of increased arterial stiffness is the enhanced generation and accumulation of advanced glycation end products (AGEs) in the vascular wall, causing excessive crosslinking between AGEs and collagen molecules of the extracellular matrix (ECM) and resulting in intimal medial thickening and stiffening of arterial walls.34 AGEs and their receptors also affect the arterial wall stiffness via a receptor-mediated endothelial dysfunction and inflammation process.35,36 Additionally, oxidative stress is closely related to increased arterial stiffness. Chronic hyperglycemia can increase free radicals through glucose autooxidation, protein glycation, and activation of polyol pathway resulting in lipid peroxidation and protein oxidation of cellular structures.37 These adverse events lead to progressive endothelial cell injury and it is implicated in accelerated arterial stiffening. There was evidence indicated the upregulation of metalloproteinases (MMPs) in diabetes, especially MMP-9 which mediated elastin fragmentation as well as medial arterial calcification.38 MMP-12 is also associated with greater arterial stiffness.39 Another mechanism is related to endothelial dysfunction in diabetes through alterations to many vasoactive substances including reduced nitric oxide (NO) bioavailability and activation of vascular renin-angiotensin-aldosterone-system.40 In hypertension, arterial stiffening occurs as a result of increased intraluminal pressure causes augment pulsatile stress resulting in elastin degradation and subsequent stimulation of collagen production.41–43 Moreover, it has been found a possible link between increased levels of MMP-9 and arterial stiffness in hypertension.44 Levels of MMP-9 associate with aortic PWV in hypertensive patients. Calcification and low-grade inflammation in the arterial wall also cause increased arterial stiffness in hypertension.45,46 Furthermore, the elevation of angiotensin II and aldosterone levels in hypertension have been shown to be associated with collagen turnover and arterial wall fibrosis and subsequent vascular damage along with increased arterial stiffness.47 On the other hand, increased arterial stiffness has been shown to precede the development of hypertension.48
Our limitation is that this cross-sectional study design did not allow us to follow the development of arterial stiffness and other vascular complications. Therefore, a more large-scale longitudinal study should be carried out to determine the complex cause-effect relationship between diabetes and hypertension on arterial stiffness. In this study, we did not measure HbA1c levels in healthy subjects. Therefore, only fasting blood sugar was used to study the relationship between glycemic status and CAVI in all participants. However, the close relationship between HbA1c and fasting blood sugar may imply the association between HbA1c and CAVI.49 Further studies are needed to analyze some clinical aspects, especially drug therapy, disease duration, health-related quality of life, postprandial glucose tests, and insulin levels. These data might explain how some hypoglycemic agents and antihypertensive drugs differentially affect vascular function and arterial stiffness.
In the past decade, the management of diabetes has been studied and updated extensively. It is recommended that the treatment of comorbidities such as hyperlipidemia and hypertension is necessary and might be more effective than lowering blood sugar alone in the prevention/reduction of cardiovascular events.6 The dissociation between glycemic control and arterial stiffness and the additive effect of hypertension found in our study may be useful for clinical considerations in the monitoring and management of T2D patients, especially in individualized glycemic control. However, issues about intensified glucose control and the effects of insulin resistance should be clarified for better management and the search for novel anti-diabetic agents.
Conclusion
This study demonstrated that HbA1c as well as fasting blood sugar levels in diabetic participants do not correlate with arterial stiffness. Concomitant diabetes and hypertension significantly increase the risk of arterial stiffness.
Funding Statement
This research was supported by the Naresuan University Research Grant under the grant number R2560C098.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Standl E, Khunti K, Hansen TB, Schnell O. The global epidemics of diabetes in the 21st century: current situation and perspectives. Eur J Prev Cardiol. 2019;26(2_suppl):7–14. doi: 10.1177/2047487319881021 [DOI] [PubMed] [Google Scholar]
- 2.Goff DC Jr., Gerstein HC, Ginsberg HN, et al. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99(12a):4i–20i. doi: 10.1016/j.amjcard.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 3.Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Type 2 diabetes as a “coronary heart disease equivalent”: an 18-year prospective population-based study in Finnish subjects. Diabetes Care. 2005;28(12):2901–2907. doi: 10.2337/diacare.28.12.2901 [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127(1):143–152. doi: 10.1161/CIR.0b013e318282ab8f [DOI] [PubMed] [Google Scholar]
- 6.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–584. doi: 10.1016/j.cjca.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox CS, Golden SH, Anderson C, et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the american heart association and the american diabetes association. Diabetes Care. 2015;38(9):1777–1803. doi: 10.2337/dci15-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Namba T, Masaki N, Takase B, Adachi T. Arterial stiffness assessed by cardio-ankle vascular index. Int J Mol Sci. 2019;20(15):3664. doi: 10.3390/ijms20153664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namekata T, Shirai K, Tanabe N, et al. Estimating the extent of subclinical arteriosclerosis of persons with prediabetes and diabetes mellitus among Japanese urban workers and their families: a cross-sectional study. BMC Cardiovasc Disord. 2016;16:52. doi: 10.1186/s12872-016-0230-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibata J, Sasaki H, Hanabusa T, et al. Increased arterial stiffness is closely associated with hyperglycemia and improved by glycemic control in diabetic patients. J Diabetes Investig. 2013;4(1):82–87. doi: 10.1111/j.2040-1124.2012.00229.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(Supplement 1):S1–93. [Google Scholar]
- 12.Abdul-Ghani M, DeFronzo RA, Del Prato S, Chilton R, Singh R, Ryder REJ. Cardiovascular disease and Type 2 diabetes: has the dawn of a new era arrived? Diabetes Care. 2017;40(7):813–820. doi: 10.2337/dc16-2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias MF, Crichton GE, Dearborn PJ, Robbins MA, Abhayaratna WP. Associations between Type 2 diabetes mellitus and arterial stiffness: a prospective analysis based on the maine-syracuse study. Pulse (Basel). 2018;5(1–4):88–98. doi: 10.1159/000479560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang S, Kim J, Sohn T, Son H, Lee J. Effects of glucose control on arterial stiffness in patients with type 2 diabetes mellitus and hypertension: an observational study. J Int Med Res. 2018;46(1):284–292. doi: 10.1177/0300060517722697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian G, Wei W, Zhang W, et al. Increasing age associated with elevated cardio-ankle vascular index scores in patients with type 2 diabetes mellitus. J Int Med Res. 2013;41(2):435–444. doi: 10.1177/0300060513477290 [DOI] [PubMed] [Google Scholar]
- 16.Sunkara N, Ahsan CH. Hypertension in diabetes and the risk of cardiovascular disease. Cardiovasc Endocrinol. 2017;6(1):33–38. doi: 10.1097/XCE.0000000000000114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno B, de Faria AP, Ritter AMV, et al. Glycated hemoglobin correlates with arterial stiffness and endothelial dysfunction in patients with resistant hypertension and uncontrolled diabetes mellitus. J Clin Hypertension. 2018;20(5):910–917. doi: 10.1111/jch.13293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tedesco MA, Natale F, Di Salvo G, Caputo S, Capasso M, Calabró R. Effects of coexisting hypertension and type II diabetes mellitus on arterial stiffness. J Hum Hypertens. 2004;18(7):469–473. doi: 10.1038/sj.jhh.1001690 [DOI] [PubMed] [Google Scholar]
- 19.de Oliveira Alvim R, Santos P, Musso MM, et al. Impact of diabetes mellitus on arterial stiffness in a representative sample of an urban Brazilian population. Diabetol Metab Syndr. 2013;5(1):45. doi: 10.1186/1758-5996-5-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarro-Pérez J, Orozco-Beltran D, Gil-Guillen V, et al. Mortality and cardiovascular disease burden of uncontrolled diabetes in a registry-based cohort: the ESCARVAL-risk study. BMC Cardiovasc Disord. 2018;18(1):180. doi: 10.1186/s12872-018-0914-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Waist circumference and waist-hip ratio: report of a WHO expert consultation. Geneva, 8-11 December 2008; 2011. [Google Scholar]
- 22.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13(2):101–107. doi: 10.5551/jat.13.101 [DOI] [PubMed] [Google Scholar]
- 23.Park SY, Chin SO, Rhee SY, et al. Cardio-ankle vascular index as a surrogate marker of early atherosclerotic cardiovascular disease in Koreans with Type 2 diabetes mellitus. Diabetes Metab J. 2018;42(4):285–295. doi: 10.4093/dmj.2017.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabutoya T, Kario K. Comparative assessment of cutoffs for the cardio-Ankle vascular index and brachial-ankle pulse wave velocity in a nationwide registry: a cardiovascular prognostic coupling study. Pulse (Basel). 2019;6(3–4):131–136. doi: 10.1159/000489604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yingchoncharoen T, Sritara P. Cardio-Ankle vascular index in a Thai population. Pulse. 2017;4(Suppl 1):8–10. doi: 10.1159/000448490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagayama D, Imamura H, Sato Y, et al. Inverse relationship of cardioankle vascular index with BMI in healthy Japanese subjects: a cross-sectional study. Vasc Health Risk Manag. 2017;13:1–9. doi: 10.2147/VHRM.S119646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thitiwuthikiat P, Siriwittayawan D, Nuamchit T. Prehypertension and high serum uric acid increase risk of arterial stiffness. Scand J Clin Lab Invest. 2017;77(8):673–678. doi: 10.1080/00365513.2017.1397287 [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes Association. Standards of medical care in diabetes-2019 abridged for primary care providers. Clin Diabetes. 2019;37(1):11–34. doi: 10.2337/cd18-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duckworth WC. Hyperglycemia and cardiovascular disease. Curr Atheroscler Rep. 2001;3(5):383–391. doi: 10.1007/s11883-001-0076-x [DOI] [PubMed] [Google Scholar]
- 30.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. [DOI] [PubMed] [Google Scholar]
- 32.Boyne MS, Saudek CD. Effect of insulin therapy on macrovascular risk factors in type 2 diabetes. Diabetes Care. 1999;22(Suppl 3):C45–53. [PubMed] [Google Scholar]
- 33.Sengstock DM, Vaitkevicius PV, Supiano MA. Arterial stiffness is related to insulin resistance in nondiabetic hypertensive older adults. J Clin Endocrinol Metab. 2005;90(5):2823–2827. doi: 10.1210/jc.2004-1686 [DOI] [PubMed] [Google Scholar]
- 34.Llauradó G, Ceperuelo-Mallafré V, Vilardell C, et al. Advanced glycation end products are associated with arterial stiffness in type 1 diabetes. J Endocrinol. 2014;221(3):405–413. doi: 10.1530/JOE-13-0407 [DOI] [PubMed] [Google Scholar]
- 35.Prasad K, Mishra M. Do advanced glycation end products and its receptor play a role in pathophysiology of hypertension? Int J Angiol. 2017;26(1):1–11. doi: 10.1055/s-0037-1598183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stehouwer CDA, Henry RMA, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51(4):527. doi: 10.1007/s00125-007-0918-3 [DOI] [PubMed] [Google Scholar]
- 37.Ramakrishna V, Jailkhani R. Oxidative stress in non-insulin-dependent diabetes mellitus (NIDDM) patients. Acta Diabetol. 2008;45(1):41–46. doi: 10.1007/s00592-007-0018-3 [DOI] [PubMed] [Google Scholar]
- 38.Chung AW, Yang HH, Sigrist MK, et al. Matrix metalloproteinase-2 and −9 exacerbate arterial stiffening and angiogenesis in diabetes and chronic kidney disease. Cardiovasc Res. 2009;84(3):494–504. doi: 10.1093/cvr/cvp242 [DOI] [PubMed] [Google Scholar]
- 39.Goncalves I, Bengtsson E, Colhoun HM, et al. Elevated plasma levels of MMP-12 are associated with atherosclerotic burden and symptomatic cardiovascular disease in subjects with Type 2 diabetes. Arterioscler Thromb Vasc Biol. 2015;35(7):1723–1731. doi: 10.1161/ATVBAHA.115.305631 [DOI] [PubMed] [Google Scholar]
- 40.Aroor A, DeMarco V, Jia G, et al. The role of tissue renin-angiotensin-aldosterone system in the development of endothelial dysfunction and arterial stiffness. Front Endocrinol (Lausanne). 2013;4:161. doi: 10.3389/fendo.2013.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu C, Zarins CK, Pannaraj PS, Bassiouny HS, Glagov S. Hypercholesterolemia superimposed by experimental hypertension induces differential distribution of collagen and elastin. Arterioscler Thromb Vasc Biol. 2000;20(12):2566–2572. doi: 10.1161/01.ATV.20.12.2566 [DOI] [PubMed] [Google Scholar]
- 42.Harvey A, Montezano AC, Lopes RA, Rios F, Touyz RM. Vascular fibrosis in aging and hypertension: molecular mechanisms and clinical implications. Can J Cardiol. 2016;32(5):659–668. doi: 10.1016/j.cjca.2016.02.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ecobici M, Stoicescu C. Arterial stiffness and hypertension - which comes first? Maedica (Buchar). 2017;12(3):184–190. [PMC free article] [PubMed] [Google Scholar]
- 44.Yasmin MCM, Wallace S, Dakham Z, et al. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(2):372. doi: 10.1161/01.ATV.0000151373.33830.41 [DOI] [PubMed] [Google Scholar]
- 45.Lyle AN, Raaz U. Killing me unsoftly: causes and mechanisms of arterial stiffness. Arterioscler Thromb Vasc Biol. 2017;37(2):e1–e11. doi: 10.1161/ATVBAHA.116.308563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pietri P, Vyssoulis G, Vlachopoulos C, et al. Relationship between low-grade inflammation and arterial stiffness in patients with essential hypertension. J Hypertens. 2006;24(11):2231–2238. doi: 10.1097/01.hjh.0000249701.49854.21 [DOI] [PubMed] [Google Scholar]
- 47.Mahmud A, Feely J. Arterial stiffness and the renin-angiotensin-aldosterone system. J Renin Angiotensin Aldosterone Syst. 2004;5(3):102–108. doi: 10.3317/jraas.2004.025 [DOI] [PubMed] [Google Scholar]
- 48.Oh YS. Arterial stiffness and hypertension. Clin Hypertension. 2018;24:17. doi: 10.1186/s40885-018-0102-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghazanfari Z, Haghdoost AA, Alizadeh SM, Atapour J, Zolala F. A comparison of hba1c and fasting blood sugar tests in general population. Int J Prev Med. 2010;1(3):187–194. [PMC free article] [PubMed] [Google Scholar]


