Abstract
This study examined the association between early life adversity, in the form of early rearing in an institution (orphanage), and the slope of cortisol in the first thirty minutes after waking in 277 children, aged 7 through 15 years old, who had either been adopted between 6 and 60 months of age into well-resourced homes in the United States or born into similar homes. The adopted youth were divided at the median (age 16 months) into those adopted earlier (earlier-adopted, EA) and later (later-adopted, LA). The purpose of this study was to examine the post-waking slope in cortisol in post-institutionalized youth, predicting that it would be blunted, especially in later-adopted youth, when compared to the non-adopted (NA) youth. A secondary goal was to examine whether there would be some evidence of less blunting of the first 30 min of the cortisol awakening response among the children further along in pubertal development (i.e., Pubertal Recalibration Hypothesis). Pubertal stage was determined by nurse exam. Salivary cortisol was assessed at 0 and 30-min post-awakening on three days. The results showed that LA children had a blunted wake-30 min cortisol slope relative to NA and EA children. Neither the age by group nor pubertal stage by group analyses were significant. However, the majority of the sample were in early stages of puberty (56% in stages 1 & 2), thus the power was low for detecting such an interaction. This is the first year of a cohort-sequential longitudinal study examining early experiences and pubertal influences on the HPA axis, so it will be important to re-examine this question as the sample ages.
Keywords: Early life adversity, Post-waking, Rise in cortisol, Puberty, Post-institutionalized
1. Introduction
Adverse care early in life has been shown to influence the development of the hypothalamic-pituitary-adrenal (HPA) axis in animal models. In rat and mouse pups, maternal separation (Levine, 2005) and manipulations that affect maternal behavior (Ivy et al., 2008) alter reactivity of the HPA axis. In non-human primates, lack of maternal care (i.e., nursery rearing) early in life relates to low basal cortisol levels and blunted reactivity (Capitanio et al., 2005). These data, among other studies, indicate that social experiences early in life have marked effects on the development of this neuroendocrine system (Sanchez et al., 2001; Loman and Gunnar, 2010). In humans, most of the work on adverse early social experiences and HPA axis activity assessed during childhood has focused on basal activity of the axis out of concern for using stress paradigms with abused or neglected children. Both higher and lower set points for the axis have been noted, perhaps partly a function of when, in development, measures of the HPA axis are obtained (Doom and Gunnar, 2013).
One common measure of HPA axis functioning is the cortisol awakening response (CAR; Adam et al., 2015; Stalder et al., 2016). Pruessner was one of the first to test and document the need for multiple cortisol assessments after awakening and strict measurement with respect to waking time (Pruessner et al., 1997). Since that time, much work has been done to clarify adequate sampling methods and investigate state and trait influences on the CAR, defined as the dynamic change in cortisol secretion upon awakening, typically demonstrating an increase for the 30–45 min after awakening (Chida and Steptoe, 2009; Clow et al., 2004; Law et al., 2013; Stalder et al., 2016). This increase is superimposed on the cortisol circadian rhythm, is present early in life, and undergoes changes across development (Bäumler et al., 2013; Stalder et al., 2016). To obtain a more accurate measure of the CAR, frequent sampling is needed across the first 60 min post-waking, including a sample at 0-, 15-, 30-, 45-, and 60-min post-waking. However, while all these samples may be necessary to provide a full picture of the CAR, studies of chronic stress often show alterations in the first 30 min post-awakening.
Studies of chronic stress in adults have revealed both increases and blunting of the initial 30 min of the cortisol awakening response (Chida and Steptoe, 2009). Nonetheless, studies of HPA axis functioning in children exposed to early life stress frequently report low early morning levels (i.e. Bruce et al., 2009; Koss et al., 2016). In these studies, collection of cortisol is often within 20–40 min post-awakening and therefore may reflect a blunted awakening response, as this falls within the expected peak of the CAR in children. Studies specifically assessing the CAR in the context of early life stress are very limited. In one study, adults reported on their childhood trauma and cortisol was sampled at wakeup, 30, 45 and 60 min after awakening. The results showed that mild to severe childhood trauma was associated with a flatter slope in cortisol from wakeup to 30-min with differences in levels maintained for the next hour (Mangold et al., 2010). In the present study we examined the first thirty minutes of the CAR (slope in cortisol from wake to 30-min post-wake) in a relatively large sample of post-institutionalized (PI) children adopted into well-resourced families and youth born and raised in families of comparable education and income. The sample ranged in age from 7 to 15 years. Several studies of HPA axis activity following early institutional rearing have found greater effects for individuals adopted or fostered into families later rather than earlier in development (Gunnar et al., 2009; McLaughlin et al., 2015). Hodel et al., 2015; van IJzendoorn et al., 2011). Therefore, we divided the PI children at the median age for adoption and compared earlier-versus later-adopted children. This was the first year of a multi-year accelerated longitudinal design. We tested the hypothesis that a) PI children would have a significantly blunted or flatter slope from wake to 30-min later compared to NA children and b) the post-waking rise in cortisol would be the most blunted or flatter for PI youth adopted later in childhood who experienced a longer period of institutional deprivation.
Quevedo et al. (2011) investigated the CAR (0-, 30-, and 60-min post-waking) in adolescents adopted from institutional care as infants or toddlers. Results indicated that pubertal stage moderated the association between orphanage care early in life and slope of cortisol from wakeup to 30 min. Specifically, the rise in cortisol to 30 min post-awakening was blunted for youth who were at earlier stages of pubertal development, but not for those who were beyond the midpoint in puberty. This suggests a potential for recalibration of this component of the HPA axis response during the pubertal transition (see also Romeo et al., 2016). The same pattern was reported in a community sample of 9- to 13-year old children who self-reported on their life stress experiences (King et al., 2016). As in the Quevedo et al. (2011) study, greater severity of early life stress (ELS) was associated with a blunted slope of cortisol from wake to 30 min for youth who were earlier, but not later, in puberty. Indeed, ELS adolescents at later stages of puberty exhibited a heightened wake to 30 min cortisol increase. A shift from a blunted to an exaggerated initial 30 min CAR with pubertal development would be consistent with evidence that adult men reporting more ELS experiences prior to age 12 years exhibited an exaggerated initial wake to 30 min CAR compared to those who did not report such experiences (Butler et al., 2017). These results stand in contrast to a recent report on young adults adopted from severely depriving Romanian orphanages over 20 years ago. Here a blunted CAR (measured at 0-, 30-, and 45-min post-waking) was still noted for those adopted after 6 months in these Romanian institutions (Kumsta et al., 2017). Thus, we explored whether PI children who were at later stages of puberty would show a less blunted rise in cortisol from wake to 30-min post-wake, evidenced by a flattened slope. Due to these data being from Year 1 of a 3-year longitudinal study, a majority of the sample was still in early puberty, stronger tests of pubertal recalibration would be allowed in later years of the study. Furthermore, while Quevedo et al. (2011) did observe changes across puberty with only a 0- and 30-minute sample, our ability to observe the full effect is limited by our lack of a 45-minute post-wake sample.
2. Methods
2.1. Participants
The sample for analysis consisted of 277 children (59% girls); of these, 123 children (67% girls) had been adopted from institutions (e.g., orphanages) in other countries (post-institutionalized, PI) and 154 (53% girls) had been born and raised in their families in the United States (non-adopted, NA). The children ranged in age from 7 to 15 years (M = 11.26, SD = 2.31). For analyses, a median split was used in the PI group to create earlier adopted (EA, < 16 months at adoption) and later adopted (LA, ≥ 16 months) groups. Demographic data, including tests of group differences, are shown in Table 1.
Table 1.
Descriptive statistics for participant and family characteristics.
| Non-adopted n = 154 M (SD) or N(%) |
Earlier-Adopted n = 66 M (SD) or N(%) |
Later-Adopted n = 57 M (SD) or N(%) |
|
|---|---|---|---|
| Age (years)* | 11.16 (2.25)a | 11.99 (2.11)a,b | 10.67 (2.49)b |
| Sex (% female)* | 52.6% | 63.6% | 70.2% |
| Tanner Stage* | |||
| 1 | 60 (39%) | 17 (26%) | 24 (42%) |
| 2 | 38 (25%) | 8 (12%) | 9 (16%) |
| 3* | 13 (8%) | 16 (24%) | 13 (23%) |
| 4* | 12 (8%) | 14 (21%) | 4 (7%) |
| 5 | 30 (20%) | 11 (17%) | 7 (12%) |
| Race/Ethnicity1,** | |||
| Native American | 0 | 10 (15.2%) | 3 (5.3%) |
| Asian | 1 (0.6%) | 25 (37.9%) | 22 (38.6%) |
| Black | 2 (1.3%) | 3 (4.5%) | 3 (5.3%) |
| Pacific Islander | 0 | 0 | 0 |
| White | 138 (89.6%) | 26 (39.4%) | 24 (42.1%) |
| Mixed | 12 (7.8%) | 0 | 4 (7.0%) |
| Unknown/Other | 1 (0.6%) | 2 (3.0%) | 1 (1.8%) |
| Wake Time (mean across 3 days) | 6:55 (0:50) | 7:02 (1:03) | 6:42 (0:45) |
| Time Since Wake (wake sample - mean across 3 days) | 1.24 (1.42) | 1.36 (1.78) | (1.38) |
| Time Since Wake (30 m sample - mean across 3 days) | 31.92 (2.00) | 32.06 (1.80) | 31.83 (2.32) |
| Granger Score (score > 0 on any of the 3 days) | 15 (9.7%) | 12 (18.2%) | 6 (10.5%) |
| Median Highest Parental Education | Master’s Degree | Master’s Degree | Master’s Degree |
| Median Household Income | 100,001 to 150,000 | 100,001 to 150,000 | 100,001 to 150,000 |
| Months at adoption** | – | 10.95 (2.65) | 29.38 (12.30) |
| Months in home** | – | 133 (25) | 99 (28) |
| % Time institutional care | – | 0.96 (0.09) | 0.94 (0.10) |
| Country of origin2 | |||
| China | – | 13 (19.7%) | 8 (14.0%) |
| Colombia | – | 2 (3.0%) | 3 (5.3%) |
| Ecuador | – | 1 (1.5%) | 0 (0%) |
| Ethiopia | – | 3 (4.5%) | 1 (1.8%) |
| Guatemala | – | 7 (10.6%) | 1 (1.8%) |
| Haiti | – | 0 (0%) | 2 (3.5%) |
| India | – | 7 (10.6%) | 9 (15.8%) |
| Kazakhstan | – | 3 (4.5%) | 0 (0%) |
| Nepal | – | 0 (0%) | 1 (1.8%) |
| Philippines | – | 1 (1.5%) | 0 (0%) |
| Russia | – | 28 (42.4%) | 23 (40.4%) |
| Slovakia | – | 0 (0%) | 2 (3.5%) |
| Ukraine | – | 0 (0%) | 5 (8.8%) |
| Vietnam | – | 1 (1.5%) | 2 (3.5%) |
Note. Superscripts denote group differences within continuous variables. Oneway ANOVA was run to assess group differences on continuous variables across three groups (t-test was run for two group comparisons). Chi Square test was run to assess group differences on categorical variables.
NA group has a significantly greater proportion of White participants compared to both adopted groups. EA and LA did not differ on a chi square test of race.
Group differences were not analyzed for country of origin.
p < .001.
p < .05.
2.2. Recruitment and inclusion/exclusion criteria
Children in the NA group were recruited from a registry of families potentially interested in participating in research. Most of these families had been recruited to the registry through a letter received soon after the child’s birth in Minnesota. PI children were recruited from a registry of families that responded to a survey sent out after adopting internationally through Minnesota agencies and through on-going recruitment via major local international adoption agencies. Inclusion criteria for the PI children were adoption from an institution between 6 and 60 months of age and having spent at least 50% of their preadoption life in institutional care. Exclusion criteria for both PI and NA children were: endocrine disorders and/or use of systemic glucocorticoid medication. Children intermittently using glucocorticoid-based nasal sprays were retained, but this was incorporated into a score of cortisol-impacting drugs and included as a covariate (Granger et al., 2009). PI children were further assessed and excluded for evidence of Fetal Alcohol Syndrome. Only one child per household was included.
2.3. Procedures
These data are from year one of an accelerated longitudinal (cross-sequential) study with data collected from cohorts initially aged 7–8, 9–10, 11–12, and 13–14 years. Because girls progress through puberty earlier than boys, girls were recruited towards the lower end of each age group and boys towards the higher end. Accordingly, girls were somewhat, but not significantly, younger (M = 11.05, SD = 2.28) than boys (M = 11.55, SD = 2.32; t (df = 241) = −1.78, p = 0.076). Participants were assessed at three time-points, each one year apart. At each assessment, participants came to the research laboratory twice, within about a 2-week period. At one of the visits, a nurse exam was performed to determine pubertal stage; parents and children also completed a puberty questionnaire. At the other visit, other tests were performed that are not described here. Between visits, saliva was collected to assess the CAR. Concern for participant burden and compliance led us to only request wakeup and 30 min saliva samples to assess the CAR.
The research was approved by the University of Minnesota Institutional Review Board. Informed consent was obtained from all participants before beginning data collection.
2.4. Measures
2.4.1. Background questionnaires
All parents completed a questionnaire providing information on child race, medications, parental education, and family income in 25 thousand-dollar increments. Parents of the adopted children completed a questionnaire providing information on the child’s age at adoption, amount of pre-adoption time in an institution, pre-adoption care quality, and birth country.
2.4.2. Pubertal stage
Nurses trained to Marshall and Tanner (1969, 1970) criteria by a pediatric endocrinologist collaborator (BM) conducted a physical exam to measure pubertal stage. To assess interrater reliability, 33 boys and 50 girls were scored simultaneously by the three nurse assessors. Cohen’s k for these scores was .87 (p < .001, using Landis and Koch criteria of > .81; Landis and Koch, 1977). Of the 277 participants in this study, 258 underwent the nurse’s exam. The remaining 19 (7%) refused the exam and self-reported pubertal stage. All parents and children completed the Petersen Pubertal Development Scale (PDS; Petersen et al., 1988). Responses ranged from 1 (not yet started) to 4 (seems complete). Menstruation was coded (1 = not begun; 4 = had begun) and self-report of age in months at menarche was provided. A summary mean score ranged from 1 (puberty has not begun) to 4 (puberty is complete). These scores were used to estimate pubertal stage for the seven percent who refused the nurse exam (see Reid et al., 2017 for details).
2.4.3. Post-waking cortisol
Participants were asked to provide saliva samples for three days. Participants were strongly encouraged to sample on weekdays, not necessarily consecutively, but on days with regular activity to increase the consistency of the wake-sleep cycle and sampling times. Samples were not to be taken on days in which the participant was sick with a fever. The instructions were to take samples immediately upon waking (“wake”) and 30 min after waking (“30 m”). Bedtime samples were also taken but were not analyzed for this manuscript as interest was just in the awaking response. Directions included refraining from eating, drinking, using tobacco, or brushing teeth in between the two morning samples, especially relating to substances containing dairy or caffeine. The participants were shown a video using child actors and humor to help them and their parents understand the instructions. Participants were also provided with a diary sheet to record wake time, sample time, sleep hours and quality, day of the week, month of the year, daily medications, sick or not, and daily events.
Participants collected saliva by mouthing an oral sponge (Sarstedt Salivettes for Cortisol Testing) until it was wet. This was then placed in a provided vial, which was stored in the freezer until being brought to the next visit. Sponges were provided in a MEMS® 5 TrackCaps that recorded time of opening to help check compliance. Samples returned to the lab were stored at −20 °C until being shipped to Trier, Germany for assay processing. Samples were assayed in duplicate using a time-resolved fluorescence immunoassay (DELFIA) and values were then averaged. The intra-assay coefficient of variation was between 4.0% and 6.7%, and the corresponding inter-assay coefficients of variation were between 7.1% and 9.0%.
2.4.4. Medications
A Granger score was calculated for each day in order to assess the likelihood of medication impact on cortisol levels. This was based on a sum of scores for each medication reported to be taken on that day, indicating plausibility of influencing cortisol levels (0 = no plausible effects on the HPA axis, 1 = possible effects, 2 = very plausible effects; Granger et al., 2009).
2.5. Data processing and missing values
Prior to analyses, the home data for participants were reviewed to determine adherence to protocol. Initially there were 321 participants; of these 29 provided no home samples and another 15 were completely eliminated (4 for overall poor compliance to protocol, 1 for use of steroids, 1 for having a sibling in the study, 1 for not fitting study criteria, 1 for implausible cortisol values, 5 for FAS concern discovered after enrollment, 2 for likely sample evaporation). Participant information described in this manuscript represents the 277 participants retained in the analysis after these eliminations. An additional 16 samples from 10 participants were missing due to insufficient saliva volumes. In addition, individual samples were eliminated due to failure to adhere to protocol, using the following rules derived from Stalder et al. (2016): (1) sample time differed from the track-cap time by more than 10 min (nsamples = 39), (2) wake samples were more than 15 min after reported wake time, or (3) 30 min. samples were either less than 15 min from wake sample or more than 40 min from wake time (nsamples = 101) (Stalder et al., 2016). Seven samples were eliminated for biological implausibility. In total, 163 (5%) samples were eliminated. For the CAR analysis, complete data were 6 samples; two samples per day across three days. Seventy-two percent of participants had complete data while the remainder averaged 3.9 of the 6 samples. Missing samples did not differ by adoption group or age. There were also no differences between adoption groups (EA, LA, NA) on wake time or time between waking and sampling (details reported in Table 1).
2.6. Data analysis
A mixed effects model (Pinheiro and Bates, 2006) was used to model each individual’s CAR. The model was fit in R (R Core Team, 2017) using the lme function in the nlme package (Pinheiro et al., 2017). This mixed effects model has been used by other developmental scientists to model the CAR (e.g., Adam et al., 2015). Using this approach allowed a disentangling of the within- and between-subjects variation in waking cortisol and the CAR across the multiple days (Eq. (1) details this model and is provided in the Supplemental materials). Statistical analyses were appropriate given the missing data mechanism is assumed to be missing at random. Cortisol values in μg/dL were examined for normality and natural log transformed to correct for positively skewed distributions.
Preliminary analyses were conducted on a host of potential confounding variables that had been identified in the literature (Stalder et al., 2016; see Supplemental materials). None of these were found to be associated with the cortisol measures, and thus were not included in subsequent analyses. For the final model, we regressed cortisol onto the time since wake while including primary predictors and covariates in the model (see below). Cortisol values at 6 time-points were the outcome variables, examined as a function of time since wakening. Variables that applied by person included the primary predictors of adoption status (NA, EA, or LA) and participant age and wake time for each day, Granger score for each day, sex, and day of sampling (1st, 2nd, or 3rd). Time was centered as minutes since waking, so that the intercept corresponded to waking cortisol. To explicitly model the post-waking rise in cortisol (slope), a dummy variable was created for the 30 min post-wake sample (0 = wake and 1 = 30 min post-wake). If this dummy variable was significant, this would indicate a significant change in cortisol from wake to 30-min post-wake, controlling for variation in wake time and time of sampling along with the other variables mentioned above. In the model, there was a random intercept, reflecting subject-level variation in cortisol values, and a random slope, reflecting subject-level variation in the post-waking rise in cortisol.
Initial analyses were done as described above, using age, because age was a continuous variable and had a high degree of correlation with pubertal status (polychoric correlation of 0.853). A group by age interaction was investigated in order to see if there were differences in the post-waking rise in cortisol for group members of younger and older ages. If a significant age by group effect was obtained, the analysis plan was then to follow this up to see where in pubertal stage the effect was observed.
Residual and normal Q-Q plots were examined to assess modeling assumptions and to look for the presence of outlier or influential observations. The residual plot was acceptable (i.e. no pattern or heteroscedasticity) with the exception that two observations for two different participants had extremely large and negative standardized residuals. Upon examination of these observations, cortisol values were extremely small (0.002 and 0.016 μg/dL), but were not biologically implausible. Because these observations were so aberrant for these participants, these observations were removed from the analysis. The assumption of normality was assessed by creating normal Q-Q plots for the within-group errors (eijk), the random intercepts (b1i), and the random slopes (b2i). These plots indicated that the normality assumptions were plausible and appropriate to make.
We investigated other potential covariates that were supported by the literature as impacting cortisol (e.g. season, weekend versus weekday sampling), but as they had non-significant effects they were removed to simplify the model (see Supplemental material for descriptive statistics on these variables).
3. Results
Full results from the mixed effects model are presented in Table 2. The analysis conducted with Tanner stage instead of age is provided in the Supplemental materials. Fig. 1 displays results by adoption group. Fig. 2 displays results by age.
Table 2.
Parameter estimates from mixed effect model.
| Estimate | SE | df | t-value | p-value | |
|---|---|---|---|---|---|
| Model for waking cortisol, β1i | |||||
| Intercept, β1 | −0.907 | 0.138 | 1126 | −6.580 | <.001 |
| LA, β3 | 0.086 | 0.069 | 261 | 1.266 | 0.207 |
| EA, β4 | −0.054 | 0.067 | 261 | −0.809 | 0.419 |
| Age (mean-centered), β5 | −0.044 | 0.012 | 261 | −3.733 | <.001 |
| Time Since Wake, β6 | 0.021 | 0.007 | 1126 | 3.022 | 0.003 |
| Wake Time (mean-centered), β7 | 0.000 | 0.000 | 1126 | −0.450 | 0.653 |
| Granger Score, β8 | 0.001 | 0.028 | 1126 | 0.049 | 0.961 |
| Sex, β9 | −0.028 | 0.054 | 261 | −0.518 | 0.605 |
| Day 2, β10 | −0.053 | 0.036 | 1126 | −1.474 | 0.141 |
| Day 3, β11 | −0.030 | 0.037 | 1126 | −0.811 | 0.418 |
| Model for 30 m cortisol slope, β2i | |||||
| Intercept, β2 | 0.411 | 0.231 | 1126 | 1.777 | 0.076 |
| LA, β12 | −0.163 | 0.063 | 1126 | −2.592 | 0.010 |
| EA, β13 | 0.019 | 0.062 | 1126 | 0.304 | 0.761 |
| Age (mean-centered), β14 | 0.042 | 0.011 | 1126 | 3.863 | <.001 |
| Time Since Wake, β15 | −0.030 | 0.008 | 1126 | −3.502 | <.001 |
| Wake Time (mean-centered), β16 | 0.000 | 0.000 | 1126 | −2.263 | 0.024 |
| Granger Score, β17 | 0.027 | 0.028 | 1126 | 0.980 | 0.328 |
| Sex, β17 | −0.082 | 0.050 | 1126 | −1.639 | 0.102 |
| Day 2, β18 | −0.028 | 0.051 | 1126 | −0.551 | 0.582 |
| Day 3, β19 | −0.059 | 0.052 | 1126 | −1.133 | 0.257 |
| Residual and random effects Estimate (95% confidence interval) | |
|---|---|
| sd(eijk) | 0.389 (0.371, 0.407) |
| sd(b1i) | 0.351 (0.309, 0.398) |
| sd(b2i) | 0.192 (0.132, 0.278) |
| cor(b1i, b2i) | −0.649 (−0.802, −0.415) |
Fig. 1.
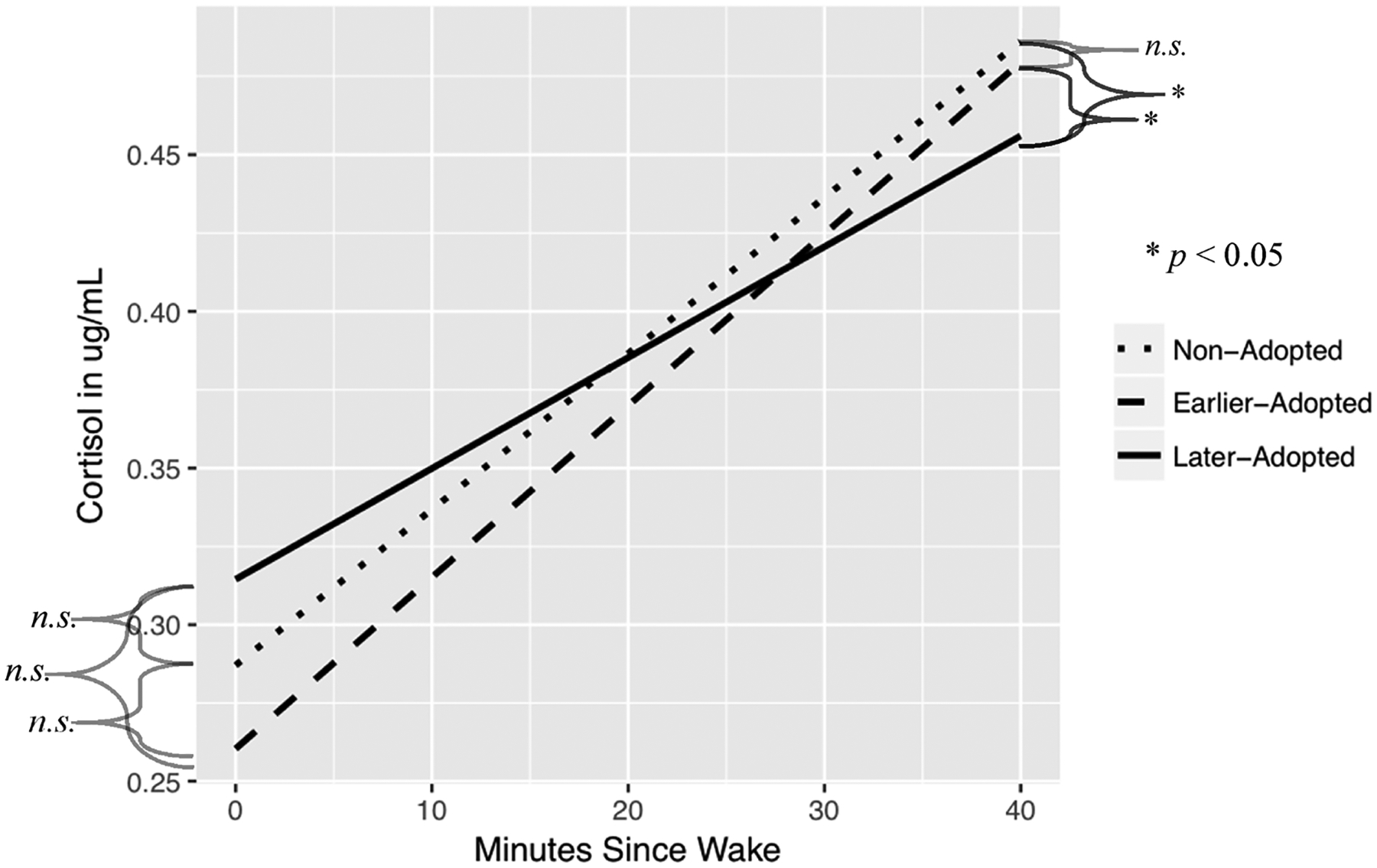
Change in cortisol from wake sample to 30 m sample, by group.
Fig. 2.
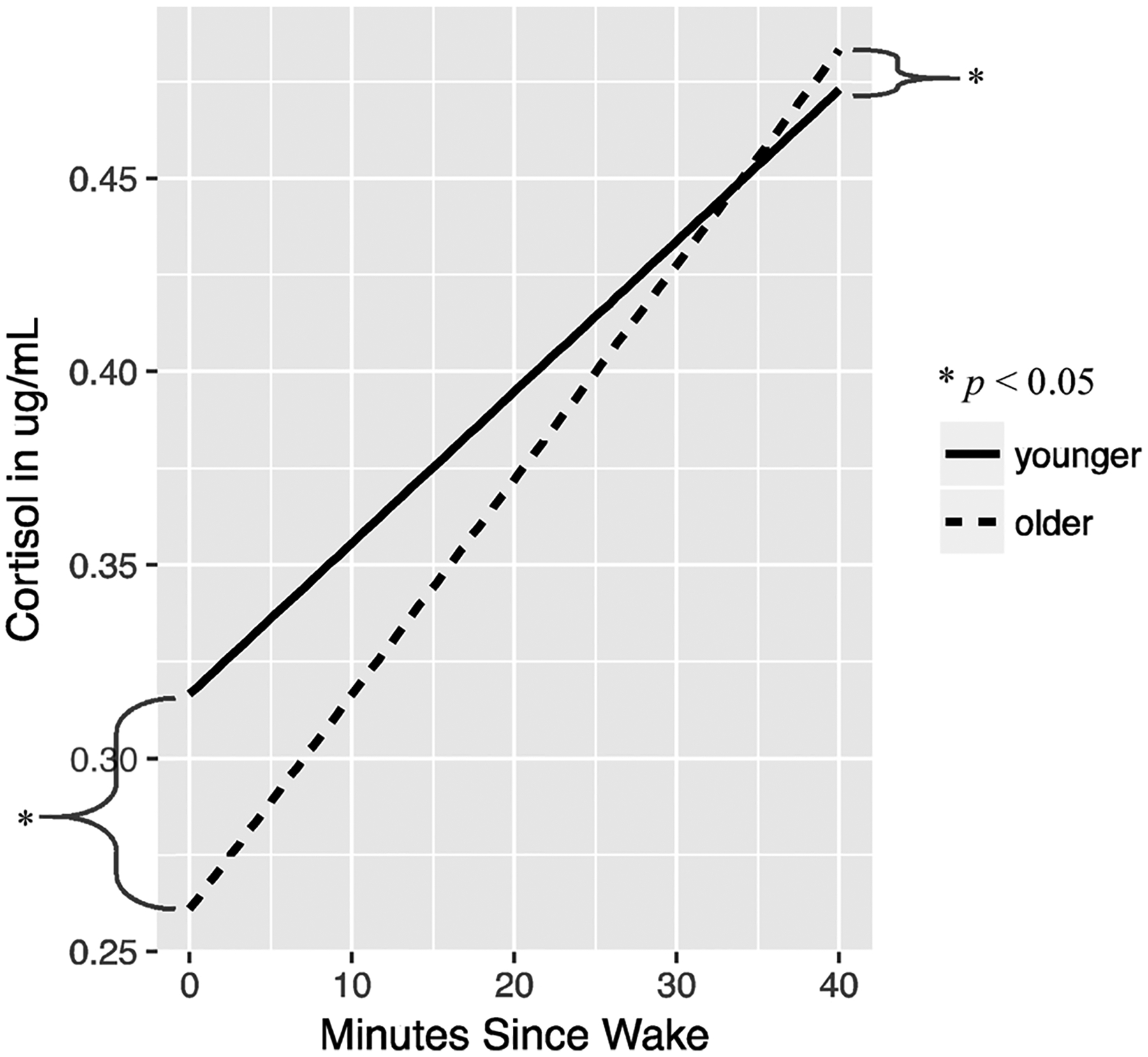
Graphical representation of relationship between cortisol and age. Grouped based on median split at 11-years-old for demonstration purposes.
3.1. Descriptive statistics
Descriptive statistics are presented in Table 1, by group. Groups differed on age (F(2, 274) = 5.53, p = .009), sex (χ2(2) = 6.13, p = .047), and Tanner stage (χ2 (8) = 27.34, p < 0.001). Groups did not differ on mean wake time (F(2,265) = 2.24, ns), average time between wake and sampling (wake F(2,207) = 1.73, ns; 30 m F (2,188) = .759, ns), or presence of meds (Granger score above 0 on any of the days; χ2 (16) = 19.51, ns).
3.2. Waking cortisol levels
There were no significant effects of sex for waking cortisol level (t (261) = −0.50, p = 0.62). There were no group differences between EA, LA, and NA on cortisol level upon awakening (p > .05). Older children demonstrated lower levels of cortisol upon awakening (t(261) = −3.70, p < 0.001). All interactions were non-significant and removed to simplify the model.
3.3. Post awakening rise in cortisol
There were no significant effects of sex for the (t(1126) = −1.64, p = 0.26). However, there were significant group differences. When compared to NA children, LA children had a significantly blunted rise in cortisol from waking to 30 min later (t(1126) = −2.59, p < 0.01). LA children were also blunted compared to EA children (t(1126) = 2.39, p = 0.017). NA and EA children did not differ from one another in the post-waking cortisol slope (p > .05, contrast presented in Table 3). There was a significant age effect, with older children exhibiting a steeper post-waking rise in cortisol. For a one year increase in age, the slope in log cortisol levels from waking to 30 min later increased by 0.04 (t(1126) = 3.88, p < 0.001). There were no significant interaction effects, including the age by group interaction. Therefore, interactions were removed to simplify the model. [To be thorough, the analysis was also conducted using Tanner stage instead of age with similar results, as shown in the Supplemental materials.]
Table 3.
Parameter estimates from mixed effect model. LA as reference group, to look at EA-LA comparison.
| Estimate | SE | df | t-value | p-value | |
|---|---|---|---|---|---|
| Model for waking cortisol, β1i | |||||
| EA (compared to LA) | −0.141 | 0.082 | 261 | −1.725 | 0.086 |
| Model for 30 m cortisol slope, β2i | |||||
| EA (compared to LA) | 0.182 | 0.076 | 1126 | 2.396 | 0.017 |
As a sensitivity analysis, we included the two excluded observations mentioned in Section 2.6 and found that our results were generally robust to their inclusion with the exception that the difference in cortisol level upon awakening between EA and LA became significant (t (261) = −2.13, p = 0.03). Further examination showed that one of these observations was driving the significance of this relationship.
4. Discussion
This study contributes to the developing literature on how early life adversity relates to stress response system functioning in children and young adolescents. We observed a significantly blunted slope of the post-waking rise in cortisol from awakening to 30-min for the post-institutionalized children who had been adopted at 16 months or later, but not for those adopted earlier in infancy. This provides a partial picture of the cortisol awakening response (CAR), though a more complete understanding would come from the collection of a 45-, and if possible a 60-min post-wake sample. Future studies should strive to include these data. The mechanisms of this blunting cannot be determined from these data, but the fact that it was found in children years after removal from deprived conditions suggests that early experiences produced significant alterations in axis regulation. The fact that this was not observed in the children adopted earlier hints at a possible sensitive period effect. Either deprivation of supportive, individualized care affects regulatory processes that only emerge later in infancy or the system becomes less plastic into the second year of life and is thus more capable of recovering if removed from deprived conditions earlier. Given that differences in cortisol levels upon waking between EA and LA emerged when two extreme outliers were included, it will be fruitful to reevaluate this finding with more data or in future studies. However, there was no strong evidence of this difference here.
There are several alternative explanations for these findings that can be ruled out. First, it is possible that age differences among the groups explained the slope differences, as LA youth were slightly, but statistically significantly, younger than EA youth. However, as LAs and NAs did not differ in age, but did in the slope, this limits the utility of that alternate explanation. Additionally, age was included in the model; therefore, any effect of group would have been over and above the effect of age. However, since the LA group was younger than the EA group and were also adopted later in life, this equates to less time in an advantaged environment. If this is the explanation, then as we follow these youth, differences in the post-waking cortisol slope should decrease with more time in the home. Notably, however, all of the PI participants had been with their adoptive families for a number of years. As noted, in preliminary analyses (see Supplemental materials) we also ruled out a host of potential confounding variables as not differing by group or being associated with cortisol levels.
Previous studies had observed an increase in the CAR to levels similar to non-adopted youth at higher stages of pubertal development (Quevedo et al., 2011; King et al., 2016). In the present study, as shown in Table 1, 72% of the youth were in the lower three stages of puberty, with only 28% being in later stages previously associated with recalibration of the CAR. Given this, it is perhaps not surprising that we observed no significant age by group or puberty by group interactions. Nonetheless, the percentage of participants scoring at later stages of puberty in the present study and in earlier studies was roughly equivalent, so this may not provide a sufficient explanation for the null interaction results. Additionally, while in this and others studies (King et al., 2016; Mangold et al., 2010; Quevedo et al., 2011) significant effects of early adversity were observed in the first 30 min post awakening, as noted above, a more complete picture would emerge if the full CAR were to be assessed (see (Stalder et al., 2016). Another potential explanation could be that institutionalization early in life has an effect of pubertal acceleration, meaning that PI children are more highly represented in the later stages of puberty, making observing an interaction challenging. However, this explanation is ruled out by previous work from our group assessing puberty concurrent with the measures reported here and demonstrating that PI children were not farther along in puberty than their non-adopted peers (Reid et al., 2017). In addition, in the present study we used age in our primary analyses, while previous studies used self-reported pubertal stage (Quevedo et al., 2011; King et al., 2016). While it would seem likely that pubertal stage is more relevant to recalibration, we also examined nurse exam measures of pubertal stage and found no evidence of recalibration (see Supplemental materials).
Thus, despite two previous studies suggesting a pubertal recalibration of the CAR, other explanations may be needed for those findings. The CAR has been associated with changes in expectations for the day, with an increased CAR indicating increased anticipation and allocation of metabolic resources. With this interpretation, a blunted CAR could reflect a reduced ability to mount morning metabolic preparations. We observed that as children got older, the slope of the first 30 min of the CAR got steeper and cortisol levels were lower upon waking (perhaps enabling a steeper rise by the 30 m sample). When the CAR appears to reorganize with puberty or among young adolescents, as in previous studies, it may in fact be that greater demands are being placed on youth as they move from childhood to adolescence. Our LA youth either may not be in situations that place these demands, may be more scaffolded by parents, or may simply not perceive their days as being that challenging. Unfortunately, we do not have the data to test these hypotheses. We did ask whether there were any exceptionally arousing events expected on the day of sampling, letting the youth and their parents define those events, and there were no differences in these reports among NA, EA and LA groups. Regardless of the mechanisms accounting for the blunted CAR in LA youth and the reasons why we may not have observed a recalibration with puberty, while others have, the question remains, is this blunting an indication of HPA axis dysregulation? If so, what are its implications for the physical and mental health of these children? If the blunted CAR reflects dysregulation, then it might indicate that LA youth are not mounting a sufficient preparatory response for the day ahead.
Finally, the pubertal recalibration hypothesis argues that the HPA axis will normalize if the level of adversity is low in late childhood/early adolescence. While the conditions of these children had certainly improved in terms of physical resources and attentive parents, some were likely experiencing significant stressors in their lives. Parents have described PI children as being likely to be bullied and some struggle with being competent in peer relations (Pitula et al., 2017). Thus, it may be that current life stress needs to be examined in order to differentiate youth who are able to recalibrate to more typical, low risk functioning with puberty, and those that do not follow this trajectory.
Some limitations accompanied these data. Strict guidelines for CAR assessment have been outlined (Stalder et al., 2016); however, as this study began before the guidelines were published, some but not all of the recommendations were followed. Actigraphs were not used, due to expense. However, participants were asked to report on their time of awakening each morning and were given clear instructions regarding the importance of accurate reporting and sampling immediately after awakening. Videos were also shown to reinforce this message. As mentioned, only the wake and 30-min post-wake sample were collected. Stalder et al. (2016) point out that a 45-minute post-wake sample is needed for accurate CAR measurement of post-pubertal girls. To reduce participant burden and increase compliance, we chose to only ask for two samples, 0 (wake) and 30 min post-wake. Because we examined slope rather than peak value of the CAR, and slope has not been shown to vary with pubertal stage (Oskis et al., 2009), we believe that our results are still interpretable and contribute to an understanding of HPA axis functioning under conditions of early life stress. We are further confident in our findings as other groups assessing early adversity and trauma on the CAR have also found significant effects in the first 30 min, but not on the later slope of the CAR (Mangold et al., 2010; Quevedo et al., 2011). Nonetheless, because we did not collect a 45-minute sample, in the post-menarchial girls we likely did not capture the peak of the CAR response for these participants and are thus limited in our ability to interpret post-pubertal group differences for girls.
We were also limited in examining sex differences in PI youth because in international adoption, there is an over-representation of girls. Thus, while we included sex in the model as a covariate for both waking cortisol values and CAR, it is the case that to more effectively assess CAR changes across puberty a more equal distribution boys and girls across the 5 stages would be needed in both the PI and NA groups.
Future studies of the CAR should make every effort to collect 4 post-waking samples, at 0-, 30-, 45-, and 60-min post-waking, across at least two days. One approach to collecting this quantity of samples while also reducing participant burden, which was our main concern, is to sample only on weekends when there is more time and flexibility in the hour after waking. Accompanying downsides to weekend sampling include irregularity of sleep quantity, bed time, and wake time. Furthermore, while we did not find differences in cortisol between weekdays and weekend days, other studies have shown differences here (Law et al., 2013).
In conclusion, this study provides further information about whether early adversity is associated with altered HPA axis activity around the time of awakening. It provides support for previous work showing that children adopted later show continued alteration in HPA axis functioning compared to those adopted earlier and children without adverse early life care (Gunnar et al., 2009; Mclaughlin et al., 2015). As yet, we found no evidence for a pubertal recalibration of the start of the CAR, as measured in the first 30 min post-waking; however, most of the youth were still early in pubertal development. Subsequent years of this study will provide more definitive data with which to test whether the post-waking rise in cortisol recalibrates with puberty for youth experiencing low levels of stressors during the pubertal transition.
Acknowledgments
This study was funded by grant number R01 HD075349 (to MG) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) at the National Institute of Health (NIH). The authors wish to thank the many families and youth who participated in this study. We also thank the International Adoption Project. We thank Tori Simenec, Bao Moua, and Lea Neumann for their assistance with the study, as well as our nurses Janet Goodwalt, Terri Jones, and Melissa Stoll. Additionally, we thank Lorah Dorn for assistance in initial Tanner staging training. Finally, this study was conducted, in part, at the Center for Neurobehavioral Development at the University of Minnesota.
Footnotes
Conflicts of interest
None.
References
- Adam EK, Heissel JA, Zeiders KH, Richeson JA, Ross EC, Ehrlich KB, et al. , 2015. Developmental histories of perceived racial discrimination and diurnal cortisol profiles in adulthood: a 20-year prospective study. Psychoneuroendocrinology 62, 279–291. 10.1016/j.psyneuen.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumler D, Kirschbaum C, Kliegel M, Alexander N, Stalder T, 2013. The cortisol awakening response in toddlers and young children. Psychoneuroendocrinology 38 (11), 2485–2492. 10.1016/j.psyneuen.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S, 2009. Morning cortisol levels in preschool-aged foster children: differential effects of maltreatment type. Dev. Psychobiol 51, 14–23. 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler K, Klaus K, Edwards L, Pennington K, 2017. Elevated cortisol awakening response associated with early life stress and impaired executive function in healthy adult males. Horm. Behav 95, 13–21. 10.1016/j.yhbeh.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N, 2005. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta). Dev. Psychobiol 46, 318–330. 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A, 2009. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol. Psychol 80, 265–278. 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F, 2004. The awakening cortisol response: methodological issues and significance. Stress 7 (1), 29–37. 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Doom JR, Gunnar MR, 2013. Stress physiology and developmental psychopathology: past, present and future. Dev. Psychopathol 25 (4). 10.1002/ana.22528.Toll-like. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH, 2009. Medication effects on salivary cortisol: tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology 34, 1437–1448. 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ, 2009. Moderate versus severe early life stress: associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology 34 (1), 62–75. 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel AS, Hunt RH, Cowell RA, Van Den Heuvel SE, Gunnar MR, Thomas KM, 2015. Duration of early adversity and structural brain development in post-institutionalized adolescents. NeuroImage 105, 112–119. 10.1016/j.neuroimage.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ, 2008. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience 1132–1142. 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Colich NL, LeMoult J, Humphreys KL, Ordaz SJ, Price AN, Gotlib IH, 2016. The impact of the severity of early life stress on diurnal cortisol: the role of puberty. Psychoneuroendocrinology 77, 68–74. 10.1016/j.psyneuen.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss KJ, Mliner SB, Donzella B, Gunnar MR, 2016. Early adversity, hypocortisolism, and behavior problems at school entry: a study of internationally adopted children. Psychoneuroendocrinology 66, 31–38. 10.1016/j.psyneuen.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumsta R, Schlotz W, Golm D, Moser D, Kennedy M, Knights N, et al. , 2017. HPA axis dysregulation in adult adoptees twenty years after severe institutional deprivation in childhood. Psychoneuroendocrinology 86, 196–202. 10.1016/j.psyneuen.2017.09.021. [DOI] [PubMed] [Google Scholar]
- Landis R, Koch GG, 1977. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 33 (2), 363–374. [PubMed] [Google Scholar]
- Law R, Hucklebridge F, Thorn L, Evans P, Clow A, 2013. State variation in the cortisol awakening response. Stress 16 (5), 483–492. 10.3109/10253890.2013.817552. [DOI] [PubMed] [Google Scholar]
- Levine S, 2005. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology 30 (10), 939–946. 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Loman MM, Gunnar MR, 2010. Early experience and the development of stress reactivity and regulation in children. Neurosci. Biobehav. Rev 34 (6), 867–876. 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold D, Wand G, Javors M, Mintz J, 2010. Acculturation, childhood trauma and the cortisol awakening response in Mexican-American adults. Horm. Behav 58 (4), 637–646. 10.1016/j.yhbeh.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM, 1969. Variations in pattern of pubertal changes in girls. Arch. Dis. Child 22, 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM, 1970. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child 45, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, Nelson CA, 2015. Causal effects of the early caregiving environment on development of stress response systems in children. PNAS 112 (18). 10.1073/pnas.1423363112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskis A, Loveday C, Hucklebridge F, Thorn L, Clow A, 2009. Diurnal patterns of salivary cortisol across the adolescent period in healthy females. Psychoneuroendocrinology 34 (3), 307–316. 10.1016/j.psyneuen.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A, 1988. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc 17 (2), 117–133. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, 2006. Mixed-effects models in S and S-PLUS Springer Science & Business Media. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, CR Core Team R, 2017. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–131 URL: https://CRAN.R-project.org/package=nlme.
- Pitula CE, Depasquale CE, Mliner SB, Gunnar MR, 2017. Peer problems among postinstitutionalized, internationally adopted children: relations to hypocortisolism, parenting quality, and ADHD symptoms. Child Dev 0 (0), 1–17. 10.1111/cdev.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, Von Auer K, Jobst S, et al. , 1997. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci 61 (26), 2539–2549. 10.1016/S0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Quevedo K, Johnson AE, Loman ML, LaFavor TL, Gunnar M, 2011. The confluence of adverse early experience and puberty on the cortisol awakening response. Int. J. Behav. Dev 36 (1), 19–28. 10.1177/0165025411406860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid BM, Miller BS, Dorn LD, Desjardins C, Donzella B, Gunnar M, 2017. Early-growth faltering in post-institutionalized youth and later anthropometric and pubertal development. Pediatr. Res 82 (2), 278–284. 10.1016/j.biopsych.2013.07.017.Anatomical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, Patel R, Pham L, So VM, 2016. Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neurosc. Biobehav. Rev 70, 206–216. 10.1016/j.neubiorev.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM, 2001. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev. Psychopathol 13, 419–449. 10.1017/S0954579401003029. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wüst S, et al. , 2016. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology 63, 414–432. 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH, Palacios J, Sonuga-Barke EJS, Gunnar MR, Vorria P, McCall RB, et al. , 2011. I. Children in institutional care: delayed development and resilience. Monogr. Soc. Res. Child Dev 76 (4), 8–30. 10.1111/j.1540-5834.2011.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


