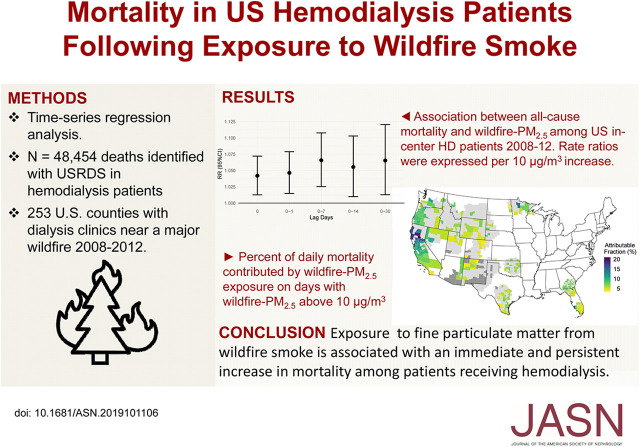
Significance Statement
Wildfires generate high concentrations of fine particulate matter (PM2.5), which are linked to increased morbidity and mortality. When inhaled, PM2.5 can travel into the respiratory tract and trigger oxidative stress and systemic inflammation. Because of their frailty, patients with ESKD might be especially susceptible to this environmental stressor, but little is known about the effects of air pollution exposures in this population. In a retrospective cohort analysis, the authors found a 4% increase in daily mortality per 10-μg/m3 increase in wildfire PM2.5 and a 7% increase in mortality over 30 days after exposure. On days with wildfire PM2.5 >10 μg/m3, fires accounted for 8.4% of daily mortality. The findings highlight an immediate and persistent effect of wildfire smoke on mortality among individuals receiving hemodialysis.
Keywords: environmental exposure, wildfire, hemodialysis patients, mortality
Visual Abstract
Abstract
Background
Wildfires are increasingly a significant source of fine particulate matter (PM2.5), which has been linked to adverse health effects and increased mortality. ESKD patients are potentially susceptible to this environmental stressor.
Methods
We conducted a retrospective time-series analysis of the association between daily exposure to wildfire PM2.5 and mortality in 253 counties near a major wildfire between 2008 and 2012. Using quasi-Poisson regression models, we estimated rate ratios (RRs) for all-cause mortality on the day of exposure and up to 30 days following exposure, adjusted for background PM2.5, day of week, seasonality, and heat. We stratified the analysis by causes of death (cardiac, vascular, infectious, or other) and place of death (clinical or nonclinical setting) for differential PM2.5 exposure and outcome classification.
Results
We found 48,454 deaths matched to the 253 counties. A 10-μg/m3 increase in wildfire PM2.5 associated with a 4% increase in all-cause mortality on the same day (RR, 1.04; 95% confidence interval [95% CI], 1.01 to 1.07) and 7% increase cumulatively over 30 days following exposure (RR, 1.07; 95% CI, 1.01 to 1.12). Risk was elevated following exposure for deaths occurring in nonclinical settings (RR, 1.07; 95% CI, 1.02 to 1.12), suggesting modification of exposure by place of death. “Other” deaths (those not attributed to cardiac, vascular, or infectious causes) accounted for the largest portion of deaths and had a strong same-day effect (RR, 1.08; 95% CI, 1.03 to 1.12) and cumulative effect over the 30-day period. On days with a wildfire PM2.5 contribution >10 μg/m3, exposure accounted for 8.4% of mortality.
Conclusions
Wildfire smoke exposure was positively associated with all-cause mortality among patients receiving in-center hemodialysis.
The frequency of large wildfires has increased worldwide.1,2 In the United States, over the past 40 years, the incidence has doubled in the western part of the country, and the wildfire season is now year round.3–5 Smoke generated during fires contains high concentrations of particulate matter, carbon monoxide, polycyclic aromatic compounds, nitrogen dioxide, and other trace gases, all of which have previously been linked to adverse health outcomes.6–9 Among the components of smoke, fine particulate matter (particles with an aero-diameter<2.5 μm; PM2.5) presents a growing public health concern due to the widespread exposure and its demonstrated biologic effects.10–12
Inhaled PM2.5 can travel deep into the respiratory tract, triggering oxidative stress and systemic inflammation which, in turn, can cause acute exacerbations of respiratory and cardiovascular disease, subacute infections, and death. Most PM2.5 is a by-product of combustion and has been determined to be causally related to cardiovascular mortality and morbidity, and is one of the six pollutants regulated under the Clean Air Act by the US Environmental Protection Agency.13 Importantly, epidemiologic studies show that exposure to PM2.5 emitted during wildfires increases the risk of cardiorespiratory morbidity and all-cause mortality in the general population.13–17
Identifying populations that are susceptible to the effects of wildfire smoke and PM2.5 is a critical first step in reducing the public health burden, often disproportionately borne by individuals with comorbidities.18,19 Patients with ESKD requiring dialysis therapy are a fragile population.16,20,21 The majority of these patients have multiple chronic conditions that may affect their overall burden of disease and a high annual risk of mortality in the United States of 16%.22–27 This population could be sensitive to the effects of wildfire smoke exposure, yet, to our knowledge, no studies have examined the effects of wildfire smoke among patients with ESKD.
We assessed the association between short-term wildfire-PM2.5 and mortality among patients receiving in-center hemodialysis (HD) in areas affected by wildfire smoke across the United States. We assessed the effect of daily exposure to wildfire-PM2.5 on all-cause and cause-specific mortality on the day of the exposure (same-day effect) and up to 30 days after exposure (cumulative lagged effect). Because of the potential for differential exposure to wildfire-PM2.5 during the extended hospital stays before death, we examined the effects stratified by place of death, clinical versus nonclinical settings.
Methods
Study Setting
An open cohort of individuals receiving in-center HD between 2008 and 2012 was constructed using data from the US Renal Data System (USRDS). The USRDS is a national registry for patients with both chronic kidney disease and ESKD, and includes records on almost all United States patients with ESKD receiving in-center HD. To study the association between daily mortality and wildfire-PM2.5 we restricted the analysis to the 253 counties within 200 km of at least one large wildland fire (>50,000 acres) and with an in-center dialysis clinic during the study period (Supplemental Table 1).17 Study inclusion criteria were: (1) Medicare as a primary payer; (2) time on dialysis exceeded 3 months; and (3) received in-center HD treatment in a clinic located within the study area before death (Supplemental Figure 1). We excluded person-time and events during the first 3 months of dialysis because of the insurance coverage transferring issues and the known exceptionally high mortality rates during this period.
Outcome Assessment
The outcome variables included daily county-level all-cause and cause-specific death counts, by place of death. Date, cause, and place of death, together with the last visited dialysis clinic before death, were extracted from the ESKD Death Notification Form (Centers for Medicare & Medicaid Services [CMS] 2746) and other relevant USRDS files. CMS 2746 cause-of-death categories were used to classify mortality into cardiac, vascular, infection related, and with nonaccidental “other” causes (listed as other, unknown, unlisted other, and missing) on the basis of the primary cause of death (Supplemental Table 2). The form indicated four additional categories of death (liver disease, gastro-intestinal, metabolic, and endocrine causes) that were not examined in this study due to lack of biologic plausibility documented in the literature. Place of death information was used to classify mortality into clinical (hospitals and dialysis clinics) and nonclinical settings (home, nursing home, other, and missing). Place of death was treated as a confounder because (1) hospital visits of patients receiving HD average 7 days, altering individuals’ exposure in non-negligible ways, and (2) distribution of the diagnostic codes varies by the place of death. As such, “place of death” had a direct effect on both the exposure and outcome of interest, justifying stratification on the basis of place of death. Each death was assigned to the county where patients received their last in-center HD. Death counts were then aggregated for each county and day.
Exposure Assessment
We utilized the Community Multiscale Air Quality (CMAQ) modeling system, an emissions-based chemical transport atmospheric model, to assess daily exposure to PM2.5 attributable to wildfire smoke.28 Briefly, CMAQ incorporates pollutant precursor emissions, gridded meteorologic fields, and boundary conditions data to model chemistry and transport of ambient air pollutant concentrations hourly on a 12-km grid. Ambient PM2.5 concentrations were simulated using CMAQ models with and without wildland fire emissions, and the difference was taken to represent the contribution of wildland fire emissions.5,28 The CMAQ with-fire model included emissions from all wild and prescribed fires. From hourly concentrations, we calculated daily county-level averages of PM2.5 concentrations which were then used as a primary exposure variable in the analysis. In the United States, there are 70,000–80,000 fires annually, whose contribution to ambient concentrations of PM2.5 is modeled by CMAQ; however, many areas of the country have a negligible contribution of smoke. Therefore, for this analysis, we restricted study population to the counties with dialysis clinics within 200 km of large fires. Patient deaths were matched to county-level PM2.5 estimates on the basis of the location of the dialysis clinic last visited.
Statistical Analyses
Mortality data were aggregated as daily death counts among patients receiving in-center HD on the basis of the county of the dialysis clinic last visited. Quasi-Poisson regression models were used to model daily mortality and estimate the association between mortality and wildfire-PM2.5
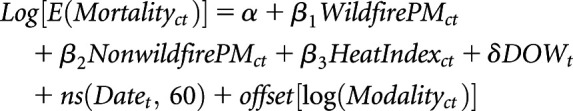 |
Equation (1) |
where c and t indexed counties and days, respectively. Time-series regressions have been widely used in environmental epidemiology, notably in investigating the short-term effects of exposures such as air pollution or weather variables on health outcomes such as mortality or health service utilizations.30,31 We adjusted for daily levels of background PM2.5 (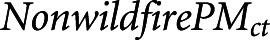 ), day of week (
), day of week ( ), daily continuous measure of heat index (
), daily continuous measure of heat index (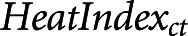 ), seasonal and long-term time trends (natural spline of continuous dates, 60 degrees of freedom), and population size (offset of logged modality count). We used the continuous measure of heat index, which measures what temperature feels like to the body, accounting for relative humidity, to control for the confounding effect of heat.
), seasonal and long-term time trends (natural spline of continuous dates, 60 degrees of freedom), and population size (offset of logged modality count). We used the continuous measure of heat index, which measures what temperature feels like to the body, accounting for relative humidity, to control for the confounding effect of heat.
All patients receiving HD enrolled in Medicare undergo one of the two treatment regimens: Mon/Wed/Fri or Tues/Thu/Sat. An extra day without treatment at the end of each week is known to elevate the risk of death; therefore, we controlled for the day of week.32 As part of the exploratory analysis, we observed both a monthly and an annual trend of mortality rate among patients on dialysis; thus, we used a degree of freedom for every month in the study period (12×5=60). Modality, which is the count of monthly in-center HD sessions aggregated to the county level, was included as an offset to be the denominator for rate estimation and to control for population size at risk differences across counties and over time. Finally, all-cause and cause-specific analyses were stratified by “place of death” to control for potential confounding.
The reported rate ratios (RRs) were expressed per 10-μg/m3 increase in wildfire-PM2.5, together with corresponding 95% confidence intervals (95% CIs). The same-day effect was defined as the effect of a 10-μg/m3 increase in daily wildfire-PM2.5 on the rate of death on the day of the exposure. To assess the persistent effect of wildfire-PM2.5 on mortality across multiple days (lag periods) after exposure, we used a distributed lag model.33 Here, the RR is interpreted as effect per 10-μg/m3 increase in wildfire-PM2.5 distributed up to 1, 7, 14, and 30 days after exposure.
A lag period was defined as the period starting with the lag day until the index day (including the index day). The index day, referred to as lag day 0, was the day when the event of death took place. Lag day n is the nth day before the index day. The cumulative lagged effects of exposure were assessed over four different lag periods: 0–1, 0–7, 0–14, and 0–30 lag days. Cumulative RR estimated the effect of a 10-μg/m3 increase in wildfire-PM2.5 on mortality rate distributed between the index day and the lag day. Detailed specification of this model is described in the Supplemental Material for all-cause mortality.
We also calculated the proportion of daily mortality attributed to wildfire-PM2.5 exposure relative to the daily mortality with no wildfire-PM2.5 exposure, or the exposed attributable fraction (EAF).34 First, for all county-days, using the estimated risk coefficient and no wildfire-PM2.5 exposure as the exposure minimum, the average RR was evaluated at the value of the daily wildfire-PM2.5 concentration as 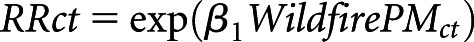 . Then, we calculated average RR
. Then, we calculated average RR 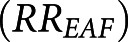 by county across all days and for days when wildfire-PM2.5 was >10 μg/m3 (Supplemental Material). The EAF was evaluated as
by county across all days and for days when wildfire-PM2.5 was >10 μg/m3 (Supplemental Material). The EAF was evaluated as 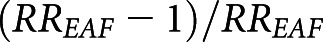 .
.
A sensitivity analysis was performed where all causes of death reported (up to five per death, including the primary cause of death) were used to classify deaths into categories. Because there was little difference in the effect estimates for deaths categorized by this alternative classification, we report results here using the primary cause of death only.
Data management and statistical analyses were conducted using the R Project for Statistical Computing Version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria). Distributed lag model parameters were estimated using the “dlnm” R package.35 Mortality data extraction from USRDS for patients receiving in-center HD was performed with SAS software, Version 9.4, of the SAS System for Windows (SAS Institute Inc., Cary, NC). The study was exempted of Institutional Review Board approval by the Office of Human Research Ethics at the University of North Carolina at Chapel Hill on May 15, 2018 (Study no.: 18–0886).
Results
Among the 268,399 deaths of United States patients receiving in-center HD in the 5-year period of 2008–2012, 48,454 deaths (Supplemental Figure 1) were matched to the 253 counties affected by a large wildfire (Figure 1, Supplemental Table 1).36 The mean age at dialysis initiation was 64.9 (±14.5) years and the mean duration of time between the first day of dialysis treatment and the day of death was 4.1 (±3.6) years (Table 1). Approximately 45% of the study population were female, 73% were white, and the mean body mass index at dialysis initiation was 28.0 (±7.4) kg/m2 (Table 1). At dialysis initiation, 4.6% and 1.2% of the study population reported tobacco use and alcohol dependence, respectively. On the basis of the CMS 2728 form collected at the initiation of dialysis, diabetes was listed as the most predominant primary cause of renal failure, accounting for 37.8% of the study population. When comparing the baseline characteristics for deaths that occurred inside or outside of clinical settings or inside or outside of study counties, we did not observe major differences, other than expected racial differences between the western and the eastern parts of the United States.
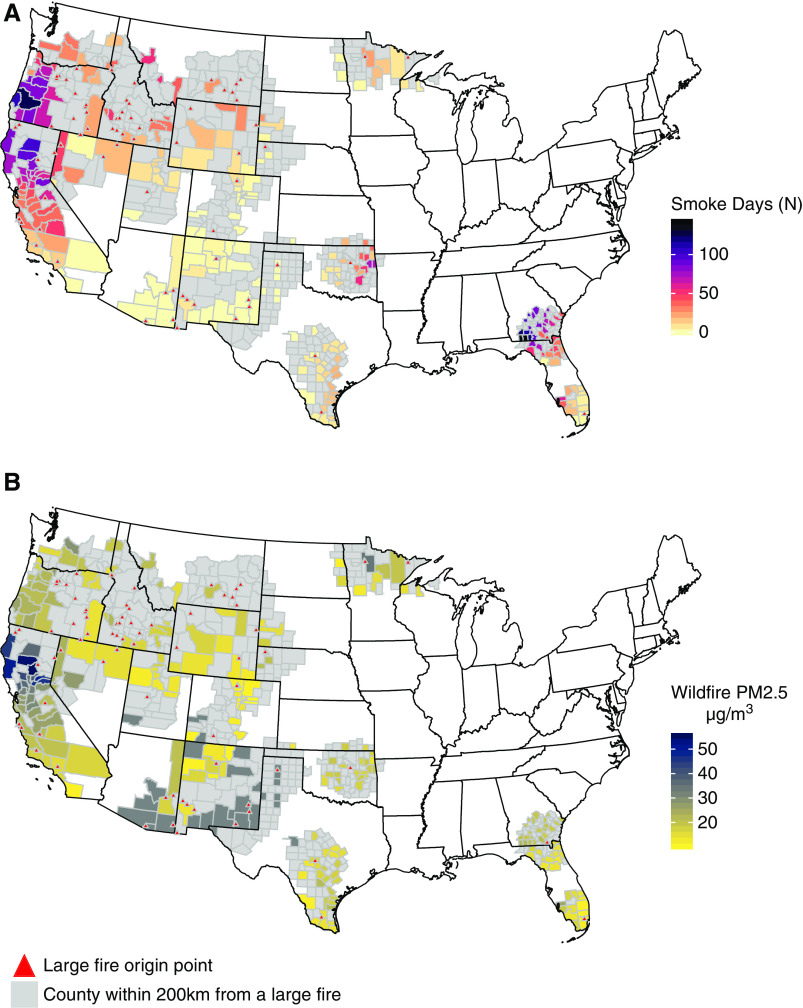
Figure 1.
Map of the study area and distribution of wildfire-related fine particulate matter (wildfire-PM2.5) by county. The locations of 63 large fires are marked with triangles. (A) The number of days with wildfire-PM2.5 contribution >10 μg/m3 for each of the 253 counties with in-center HD clinics near fire. (B) Average wildfire PM2.5 concentration on days with wildfire-PM2.5 contribution >10 μg/m3. Only counties with dialysis clinic(s) are labeled for smoke days and PM2.5. Counties in gray were within 200 km of the fire origin but did not have an in-center HD clinic or wildfire-PM2.5 >10 μg/m3.
Table 1.
Study population characteristics on the basis of CMS 2728 form, collected at the time of dialysis initiation
| Characteristicsa | Within Study Area | Out of Study Area Total N (%) | ||
|---|---|---|---|---|
| Place of Deathb | Total N (%) | |||
| Clinical N (%) | Nonclinical N (%) | |||
| n | 28,139 | 20,315 | 48,454 | 217,601 |
| Incident age, mean (SD) | 63.78 (14.6) | 66.52 (14.3) | 64.9 (14.5) | 65.08 (14.4) |
| Time since start of dialysis, mean (SD) | 4.24 (3.67) | 3.92 (3.41) | 4.1 (3.6) | 3.98 (3.6) |
| BMI, mean (SD) | 28.20 (7.5) | 27.81 (7.3) | 28.0 (7.4) | 28.79 (7.9) |
| Female | 12,763 (45.4) | 8785 (43.2) | 21,548 (44.6) | 97,197 (44.7) |
| Race | ||||
| White | 19,813 (70.4) | 15,413 (75.9) | 35,226 (72.7) | 136,931 (62.9) |
| Black | 5057 (18.0) | 2846 (14.0) | 7903 (16.3) | 70,866 (32.6) |
| Other | 3269 (11.6) | 2056 (10.1) | 5325 (11.0) | 9804 (4.5) |
| Access type | ||||
| AVF | 2099 (11.5) | 1804 (12.9) | 3903 (12.1) | 20,696 (13.8) |
| Graft | 688 (3.8) | 529 (3.8) | 1217 (3.8) | 6309 (4.2) |
| Catheter | 15,306 (83.9) | 11,562 (82.7) | 26,868 (83.4) | 121,241 (80.9) |
| Other | 147 (0.8) | 85 (0.6) | 232 (0.7) | 1679 (1.1) |
| Primary cause of ESKD | ||||
| Diabetes | 10,686 (38.0) | 7616 (37.5) | 18,302 (37.8) | 76,663 (35.2) |
| GN | 741 (2.6) | 614 (3.0) | 1355 (2.8) | 6687 (3.1) |
| Hypertension | 5270 (18.7) | 4204 (20.7) | 9474 (19.6) | 48,517 (22.3) |
| Other | 11,442 (40.7) | 7881 (38.8) | 19,323 (39.9) | 85,734 (39.4) |
| Tobacco use | 1215 (4.3) | 1000 (4.9) | 2215 (4.6) | 14,526 (6.7) |
| Alcohol dependence | 330 (1.2) | 241 (1.2) | 571 (1.2) | 3010 (1.4) |
| Comorbidities | ||||
| COPD | 1974 (7.0) | 1665 (8.2) | 3639 (7.5) | 23,335 (10.7) |
| Cancer | 1411 (5.0) | 1434 (7.1) | 2845 (5.9) | 16,890 (7.8) |
| CHF | 9629 (34.2) | 6743 (33.2) | 16,372 (33.8) | 81,145 (37.3) |
| ASHD | 4151 (14.8) | 3360 (16.5) | 7511 (15.5) | 42,865 (19.7) |
| Diabetes | 13,526 (48.1) | 9732 (47.9) | 23,258 (48.0) | 104,182 (47.9) |
BMI, body mass index; AVF, arteriovenous fistula; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; ASHD, arteriosclerotic heart disease.
Baseline characteristics were obtained from CMS 2728 form, collected at the time of dialysis initiation.
Place of death definitions: clinical: hospital, dialysis clinic; nonclinical: home, other, nursing home, missing.
The distribution of daily wildfire-PM2.5 varied across counties and was skewed, with a smaller number of days accounting for the largest exposure. Among 444,894 county-days, 16.8% had a contribution of wildfire-PM2.5 between 1 and 10 μg/m3 and 1.7% had >10 μg/m3. A small fraction of county-days (0.2%, n=990) had wildfire-PM2.5 concentrations that exceeded 35 μg/m3, the level equivalent to the daily standard (Table 3). The highest daily concentration of wildfire-PM2.5 was estimated at 277.45 μg/m3, whereas the highest background was 63.48 μg/m3. The geographic distribution of wildfire-PM2.5 is provided in Figure 1.
Table 3.
Summary of county-level PM2.5 concentrations (μg/m3)
| Exposure Metrics | Mean | Maximum | County-Day N (%) | |||
|---|---|---|---|---|---|---|
| 1 to <10 µg/m3 | 10 to <35 µg/m3 | 35 µg/m3 and above | Total | |||
| Wildfire-PM2.5 | 1.01 | 277.45 | 74,888 (16.8) | 6542 (1.5) | 990 (0.2) | 444,894 |
| Background-PM2.5 | 4.52 | 63.48 | 401,155 (90.2) | 27,649 (6.2) | 79 (0.02) | 444,894 |
| Total PM2.5 | 5.54 | 280.64 | 379,800 (85.4) | 50,001 (11.2) | 1641 (0.4) | 444,894 |
Among deaths included in this study, 58% occurred in clinical settings and 42% occurred in nonclinical settings. Deaths with primary diagnoses of cardiac, vascular, infection, and other accounted for 40.3%, 4.9%, 10.6%, and 41.4% of total deaths, respectively. Within the “other” cause-of-death category, 10%, 36%, and 13% of deaths were coded other (98), unknown (99), or missing causes of death, respectively, which accounts for one-fourth of the total deaths included. There was about 30% higher proportion of “other” deaths in nonclinical settings in comparison with the clinical settings. The distributions of deaths by cause and place of death are present in Supplemental Table 3.
All-Cause Mortality
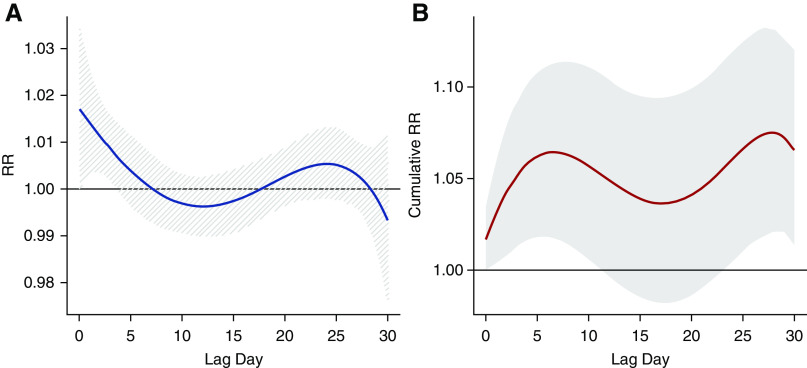
Exposure to wildfire-PM2.5 was associated with an increase in all-cause mortality (Figure 2). The RR associated with a 10-μg/m3 increase in same-day wildfire-PM2.5 exposure was 1.04 (95% CI, 1.01 to 1.07). The cumulative lagged effects distributed across 0–1-, 7-, 14-, and 30-day lag periods were 1.05 (95% CI, 1.01 to 1.08), 1.07 (95% CI, 1.03 to 1.11), 1.05 (95% CI, 1.01 to 1.10), and 1.07 (95% CI, 1.01 to 1.12), respectively (Figure 2, Table 2), for every 10-μg/m3 increase in wildfire-PM2.5. When examining the RR over 0–30 days, we observed that the risk was highest at lag 0 and declined over the subsequent 2 weeks (Figure 3). After approximately 3 weeks, we observed another small increase in risk leading to a statistically significant change in cumulative effect, although with larger uncertainty.
Figure 2.
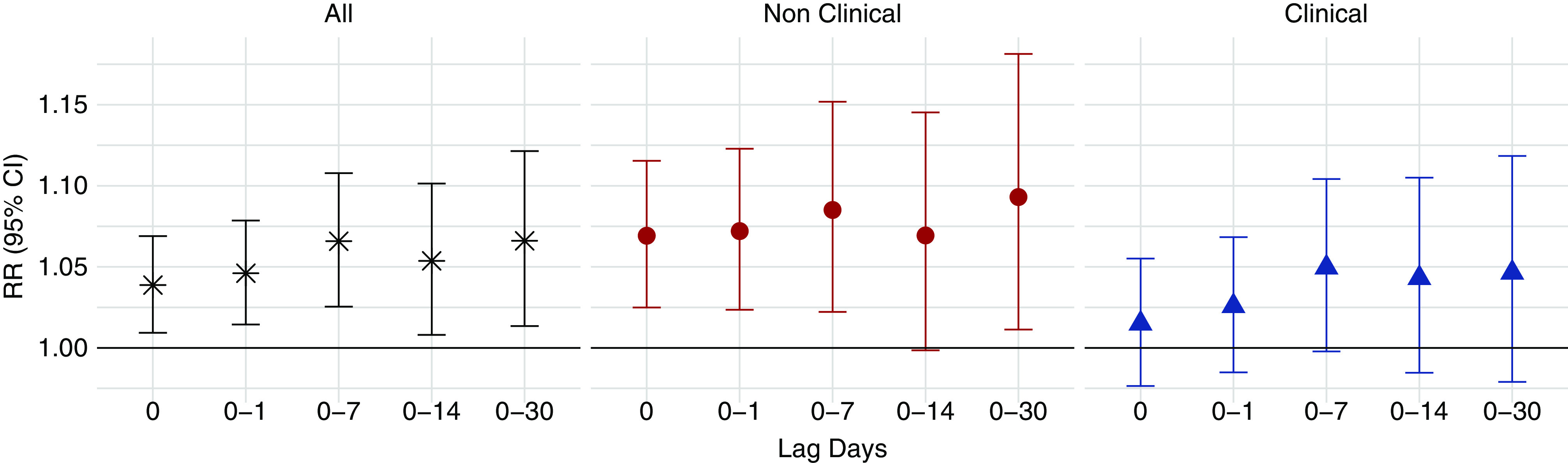
Association between all-cause mortality and wildfire-PM2.5 among United States patients receiving in-center HD during 2008–2012. RRs and 95% CIs were expressed per 10-μg/m3 increase in wildfire-PM2.5. Numeric values are provided in the Supplemental Material.
Table 2.
Associations between wildfire-PM2.5 and all-cause and cause-specific mortalities, overall and by place of death, among the United States in-center HD population 2008–2012 per 10-μg/m3 increase in smoke PM2.5
| Cause | Lag Period | Overall RR (95% CI) | Place of Death | |
|---|---|---|---|---|
| Nonclinical RR (95% CI) | Clinical RR (95% CI) | |||
| All cause | n=48,454 (100.0%) | n=20,315 (41.9%) | n=28,139 (58.1%) | |
| 0a | 1.04 (1.01 to 1.07) | 1.07 (1.02 to 1.12) | 1.02 (0.98 to 1.06) | |
| 0–1b | 1.05 (1.01 to 1.08) | 1.07 (1.02 to 1.12) | 1.03 (0.98 to 1.07) | |
| 0–7b | 1.07 (1.03 to 1.11) | 1.09 (1.02 to 1.15) | 1.05 (1.00 to 1.10) | |
| 0–14b | 1.05 (1.01 to 1.10) | 1.07 (1.00 to 1.15) | 1.04 (0.98 to 1.11) | |
| 0–30b | 1.07 (1.01 to 1.12) | 1.09 (1.01 to 1.18) | 1.05 (0.98 to 1.12) | |
| Cardiac | n=19,530 (40.3%) | n=6579 (32.4%) | n=12,951 (46.0%) | |
| 0a | 1.02 (0.98 to 1.07) | 1.03 (0.95 to 1.11) | 1.02 (0.97 to 1.08) | |
| 0–1b | 1.03 (0.99 to 1.08) | 1.03 (0.95 to 1.12) | 1.03 (0.98 to 1.10) | |
| 0–7b | 1.02 (0.96 to 1.09) | 0.99 (0.88 to 1.12) | 1.03 (0.96 to 1.11) | |
| 0–14b | 0.99 (0.91 to 1.06) | 0.98 (0.86 to 1.11) | 0.99 (0.90 to 1.08) | |
| 0–30b | 0.99 (0.91 to 1.08) | 1.00 (0.87 to 1.16) | 0.98 (0.88 to 1.10) | |
| Vascular | n=2360 (4.9%) | n=452 (2.2%) | n=1908 (6.8%) | |
| 0a | 0.96 (0.80 to 1.15) | 1.10 (0.87 to 1.38) | 0.90 (0.71 to 1.14) | |
| 0–1b | 0.97 (0.81 to 1.17) | 1.13 (0.90 to 1.41) | 0.90 (0.71 to 1.15) | |
| 0–7b | 0.93 (0.73 to 1.18) | 1.12 (0.80 to 1.56) | 0.84 (0.62 to 1.15) | |
| 0–14b | 0.92 (0.71 to 1.19) | 1.10 (0.75 to 1.61) | 0.84 (0.60 to 1.17) | |
| 0–30b | 0.93 (0.70 to 1.23) | 1.25 (0.87 to 1.80) | 0.78 (0.54 to 1.14) | |
| Infection | n=5116 (10.6%) | n=692 (3.4%) | n=4424 (15.7%) | |
| 0a | 0.93 (0.83 to 1.05) | 0.95 (0.67 to 1.34) | 0.93 (0.82 to 1.05) | |
| 0–1b | 0.93 (0.82 to 1.05) | 0.97 (0.69 to 1.37) | 0.92 (0.81 to 1.05) | |
| 0–7b | 0.95 (0.82 to 1.09) | 0.76 (0.44 to 1.32) | 0.95 (0.82 to 1.11) | |
| 0–14b | 0.96 (0.82 to 1.12) | 0.83 (0.49 to 1.40) | 0.96 (0.82 to 1.13) | |
| 0–30b | 0.88 (0.73 to 1.05) | 0.83 (0.48 to 1.41) | 0.85 (0.69 to 1.05) | |
| Other | n=20,068 (41.4%) | n=12,155 (59.8%) | n=7913 (28.1%) | |
| 0a | 1.08 (1.03 to 1.12) | 1.09 (1.03 to 1.15) | 1.06 (1.00 to 1.13) | |
| 0–1b | 1.08 (1.04 to 1.13) | 1.09 (1.02 to 1.16) | 1.07 (1.01 to 1.14) | |
| 0–7b | 1.13 (1.07 to 1.19) | 1.12 (1.04 to 1.21) | 1.13 (1.04 to 1.21) | |
| 0–14b | 1.13 (1.06 to 1.20) | 1.11 (1.02 to 1.21) | 1.15 (1.06 to 1.24) | |
| 0–30b | 1.17 (1.09 to 1.25) | 1.13 (1.03 to 1.25) | 1.20 (1.10 to 1.31) | |
Lag 0 represents the same-day effect estimates from the model adjusting for background PM2.5, heat index, day of the week, and continuous dates with an offset of modality.
Lag 0–1, 0–7, 0–14, or 0–30 represents the cumulative lagged effect of exposure 0–1, 0–7, 0–14, or 0–30 d before death estimates with the distributed lag model, adjusting for background PM2.5, heat index, day of the week, and continuous dates with an offset of modality.
Figure 3.
Association between all-cause mortality and 10-μg/m3 increase in wildfire-PM2.5 on the basis of 0–30-day distributed lag model. (A) RR by lag day. (B) Cumulative effect of wildfire-PM2.5 on all-cause mortality.
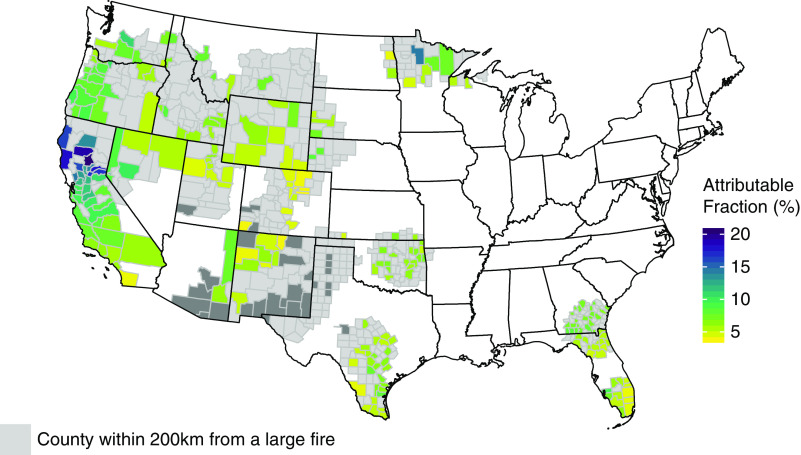
The attributable fraction among the exposed was calculated for the all-cause mortality using the estimated effect for lag day 0. Overall, wildfire-PM2.5 attributed 0.29% of deaths during the period between 2008 and 2012. However, on days with wildfire-PM2.5 concentration >10 μg/m3, on average, 8.4% of deaths were attributed to the exposure. The EAF varied between 3% and 20.5%, with the largest EAF (20.5%) observed in Northern Californian counties where exposure was the most significant (Figure 4).
Figure 4.
Attributable fraction of daily mortality on days with wildfire-PM2.5 contribution >10 μg/m3. Attributable fraction is calculated for the counties with dialysis clinic.
All-Cause Mortality by Place of Death
The mortality rate in clinical settings was elevated after exposure, but not statistically significant. The RR for the same-day effect for deaths occurring in clinical settings was 1.02 (95% CI, 0.98 to 1.06), and the RRs of cumulative lagged effect across 0–1-, 7-, 14-, and 30-day lag periods were 1.03 (95% CI, 0.98 to 1.07), 1.05 (95% CI,1.00 to 1.10), 1.04 (95% CI, 0.98 to 1.11), and 1.05 (95% CI, 0.98 to 1.12), respectively (Figure 2, Table 2). For deaths occurring outside of clinical settings, the RR of same-day effect was 1.07 (95% CI, 1.02 to 1.12), and the RRs of cumulative lagged effect estimated with distributed lag models across 0–1-, 7-, 14-, and 30-day lag period were 1.07 (95% CI, 1.02 to 1.12), 1.09 (95% CI, 1.02 to 1.15), 1.07 (95% CI, 1.00 to 1.15), and 1.09 (95% CI, 1.01 to 1.18), respectively (Figure 2, Table 2). Individual lag day effects were also estimated for all-cause mortality, as summarized in Supplemental Table 4.
Cause-Specific Mortality
The RRs for cardiac mortality associated with wildfire-PM2.5 exposure on the same day and across 0–1-day and 0–7-day lag periods (Figure 2, Table 2) were 1.02 (95% CI, 0.98 to 1/07), 1.03 (95% CI, 0.99 to 1.08), and 1.02 (95% CI, 0.96 to 1.09), respectively; there was no apparent association between wildfire PM2.5 and vascular or infection-related deaths for any lag period. However, there were significant and strong associations for wildfire-PM2.5 and deaths classified with “other” causes (Table 2). The highest effect was observed for the cumulative lagged effect over 0–30 days for death with “other” causes, with RR of 1.17 (95% CI, 1.09 to 1.25).
Discussion
This study of wildfire-PM2.5 across the United States over a 5-year period demonstrated a consistent association with all-cause mortality among patients with ESKD receiving chronic HD. We found a 4% increase in all-cause mortality for every 10-μg/m3 increase in wildfire-PM2.5 on the same day and the effect persisted up to a month after exposure. The cumulative lagged effects of wildfire-PM2.5 exposure demonstrated a bimodal distribution, peaking at 7 and 30 days after exposure (Figure 3, Supplemental Figure 2). When examining the association by place of death, the strength of association with mortality was stronger in nonclinical settings. Regarding cause-specific mortality, we did not find a significant association with cardiac, vascular, or infection-related mortality, but did observe a strong association with deaths related to “other” causes.
Same-day exposure to 10 μg/m3 of wildfire-PM2.5 accounted for 3.8% of daily mortality. On days when wildfire-PM2.5 concentration exceeded 10 μg/m3, the attributable fraction rose to 8.4%, but varied across counties depending on the exposure level (3.0%–20.5%). We also examined for nonlinear exposure-response relationship and cumulative exposure but did not find supporting evidence. However, we note that the analysis may have not been well suited to examining nonlinear effects due to small daily counts.
Health risks of PM2.5 exposure in the HD population have not been extensively evaluated. In the absence of prior reports and considering the high complexity of health risks in this population, we grouped causes of death on the basis of CMS form 2746 classification, and we considered “place of death” as a confounder. We hypothesized that the significantly longer average hospital stays in this population (7 days) could result in non-negligible differences in personal exposure to wildfire-PM2.5. Hospitals are usually equipped with high-efficiency air purifiers that keep the in-hospital patients from being exposed to high concentrations of PM2.5 from outside. Furthermore, the distribution of death causes differed by “place of death.” As such, “place of death” had a direct effect on characterization of exposure and outcome and was considered a confounder that motivated stratification analysis. The risk of wildfire-PM2.5-related all-cause and vascular-related mortality was lower in the clinical setting but not significantly different from the nonclinical setting. The risk of cardiac, infection, and other causes was not differentiated by place of death.
Previous research efforts related to health effects due to smoke exposure in wider populations have reported effects delayed by days and weeks.17,21,41,49 We examined the effects up to 30 days after exposure, hypothesizing that there is an increased chance of developing complications related to existing conditions or worsening of new conditions such as pneumonia or congestive heart failure. These effects are expected to be delayed days and weeks from the initial exposure, eventually contributing to all-cause mortality at longer lag periods. A bimodal distribution of association across lag days 0 through 30 with peaks immediately after and approximately 3 weeks after exposure, particularly among “other” causes, suggests plausibility of our hypothesis. Further investigations are needed to test the exact pathologic mechanisms for this hypothesis.
Although there are currently no published studies examining the effects of wildfire-PM2.5 on mortality among patients on dialysis, the effects have been examined in a variety of other populations.37–47 Linares and colleagues reported a mortality RR of 1.02 (95% CI, 1.01 to 1.03) for each 10-μg/m3 increase in PM2.5 (all sourced including wildfire emission) with 1-day lag among the inhabitants of Madrid, Spain, 2004–2009.40 Several studies also observed positive associations with coarse particulate matter (PM10), which in wildfire smoke is dominated by smaller-sized particles found in PM2.5, and yielded RRs ranging from 1.00 to 1.01 among general populations in Europe and Australia.38,41,44 For cause-specific effect, a number of studies reported primarily positive and consistent associations with respiratory effects,29,48–51 whereas evidence of cardiovascular effects, primarily in terms of cardiovascular related hospitalizations, has been less consistent.10,14,21,49,50 This study also expands on recent investigations of the potential effects of air pollution among patients on dialysis and individuals with and at risk for CKD.52–55
The magnitude of the wildfire-PM2.5 effect appeared to be greater in the dialysis population compared with the effects reported in other populations,38–44 which highlights the need to further examine the differential frailty of this population to PM2.5 and reinforces the importance of the focus on populations that may be particularly susceptible to environmental exposures.13,56 This pronounced effect observed among patients on HD could be explained by the following factors. First, almost all patients with ESKD receiving HD have at least one other chronic condition, including hypertension, coronary heart disease, diabetes, peripheral vascular disease, stroke, and others, which are known factors of increased vulnerability to PM2.5. Second, patients on dialysis are likely to be resistant to relocating, which could lead to a prolonged exposure to wildfire smoke. Logistically, it is difficult to change dialysis clinics within a short period of time in the event of a wildfire or other natural disaster.57 In the exploratory analysis, with a model using the modality as the response variable and wildfire-PM2.5 as the explanatory variable, controlling for other factors, we observed that the number of dialysis sessions did not change during smoke periods—suggesting that patients on dialysis were not likely to move in response to wildfire-PM2.5.
Among the cause-specific mortalities, the strongest association of PM2.5 was found for deaths with “other” primary causes, which accounted for >40% of deaths. Other causes of death were more likely to be given to deaths occurring outside of the hospitals or dialysis clinics. Furthermore, the proportion of deaths with an unidentifiable primary cause was high, which may have reflected the uncertainty in determining the primary cause of death in the ESKD Death Notification Form.27,58,59 Therefore, we suspect that cause-specific mortality analysis in the population may be prone to a high degree of inaccuracy in cause-of-death designation, and the primary cause of death recorded may not reflect changes in the status of the patient’s multiple chronic conditions in response to wildfire smoke exposure.
This study has several strengths. The USRDS dataset provides an opportunity to assess the exposure’s effects on patients receiving dialysis on a national level and to apply strong analytic methods. Near-complete representativeness and characterization of the population at risk at each time point allows us to control for the potential confounding of temporally and spatially varying factors (varying population size). The information on the last dialysis clinic visited before death extracted from the USRDS claims enables us to map the exposure to each death accurately. Previous studies have shown that United States patients on HD reside close to their dialysis clinics with average distances between dialysis clinic and residential address ranging from 6.1 to 7.9 miles, which indicates that the county-level exposure assigned on the basis of clinic address is a valid surrogate.60,61 Time-series regression enables us to control for time-invariant factors such as age, sex, social economic status, and race by design, with the individuals in the risk set acting as their own control. Similarly, time-series regression models have been used extensively in the field of air pollution epidemiology.31 Additionally, at-risk population size can vary across time and places substantially, which would lead to biased effect estimates when ignored. The monthly count of HD sessions in USRDS data makes it possible to enumerate the population at risk within each county and to control for temporal as well as spatial variation in the time-series context.
Despite the strengths, these findings should be considered in the context of the following limitations. First, exposure misclassification is always a concern in studies of environmental exposures as the individual level of exposure is not known. Instead, the assumption is made that the average exposure is similar across patients living in the same county and that the degree of exposure misclassification does not vary simultaneously with daily variation in average exposure and mortality. In addition, misclassification due to movement in response to wildfires was also possible. However, the results of the secondary analysis suggest that patients on dialysis were not likely to move in response to wildfires. Thus, the chance of exposure misclassification due to movement is assumed low in our study. Second, previous examination of effect modification in a cohort of Medicare recipients using the same exposure did not result in substantial differences in risk on the basis of geographic region.49 Exposure misclassification and composition of smoke could vary geographically, and further examination of this effect may be beneficial for characterizing risk in this population.
Third, we must consider possible misclassifications that occur due to misspecification of physical and chemical properties in the CMAQ modeling framework. Such misspecifications can lead to errors in estimation of exposure and therefore an attenuation of the effect, but are unlikely to confound or lead to spurious associations with daily mortality. In previously published studies, we found a good agreement among associations estimated on the basis of CMAQ and those on the basis of measured PM2.5 concentrations at the monitoring sites.17 Alternative models have been used as well, including monitoring data, satellite images,21,29,49 dispersion models,15,62 or data fusion models.63 Finally, we also acknowledge the limitations in classification of outcomes by Death Notification Form in this population. (1) Our analysis excluded one of the most clinically vulnerable periods in this population. Namely, deaths within 90 days of HD initiation were excluded due to uncertainty in reporting medical coverage. All patients with ESKD are eligible for medical coverage under Medicare; however, the transition from a private to single-payer system may take a few weeks to months, which would result in reporting errors. By including only Medicare patients, the results of the analysis may not be generalizable to the patient cohort with private insurance. (2) We did not investigate code-specific deaths such as sudden death, cardiac arrest, etc., which have previously been linked to air pollution exposures. A cohort analysis where each individual is followed through time could provide further information on the risk of wildfire-PM2.5 in these patients. However, the number of these omitted cases is relatively small and is unlikely to systematically affect reported risk estimates. Our analysis also excluded patients on peritoneal dialysis for two reasons: because their disease status is likely to be substantially different, and because their personal exposure may be substantially different from the exposure at the location of the dialysis clinic. Finally, as in all observational studies, although we accounted for known confounders such as heat index and slow-varying trends, we cannot discount the possibility of unmeasured confounding. In the presence of unmeasured confounding, our estimates could not be interpreted as causal.
In conclusion, in a multi-year, large, epidemiologic analysis, we found evidence of an immediate and persistent effect of wildfire smoke exposure on mortality among patients receiving chronic in-center HD. Although smoke accounted for small fraction of daily mortality (0.29%) over the study period, that fraction was substantial (8.4%) on days where wildfire PM2.5 smoke concentration exceeded 10 μg/m3. The findings support the need for more research to develop and implement interventions to manage exposure to particulate matter during wildfire smoke episodes in this and other populations with a high prevalence of frailty.
Disclosures
All authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
We thank Dr. Wang Lily for performing data extraction from the United States Renal Data System (USRDS) claims. The authors would like to thank the reviewers for their helpful comments.
The data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the United States government. The views expressed in this manuscript do not necessarily reflect the views or policies of the US Environmental Protection Agency.
Dr. Abhijit V. Kshirsagar reports grants from the National Institutes of Health/National Institute of Nursing Research (NIH/NINR), grants from NIH/National Institute of Diabetes and Digestive and Kidney Diseases, and other from Up To Date, outside the submitted work. All authors contributed to study design. Dr. Yuzhi Xi and Dr. Ana G. Rappold carried out the analysis. Dr. Yuzhi Xi made the figures and drafted the paper. All authors revised and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019101066/-/DCSupplemental.
Supplemental Table 1. Major fire events that occurred 2008–2012 in the United States*.
Supplemental Table 2. Classification of cause-specific mortality on the basis of death cause codes and categorization from CMS 2746 form.
Supplemental Table 3. Mortality count by cause of death and by place of death.
Supplemental Figure 1. Study outcome extraction protocol.
Supplemental Texts 1. Specification of distributed lag model.
Supplemental Texts 2. Exposed attributable fraction calculation.
Supplemental Table 4. Individual lag day estimates for all-cause mortality.
References
- 1.Portier CJ, Thigpen Tart K, Carter SR, et al.: A Human Health Perspective on Climate Change: A Report Outlining the Research Needs on the Human Health Effects of Climate Change, Research Triangle Park, NC, Environmental Health Perspectives and the National Institute of Environmental Health Sciences, 2013, p 621 [Google Scholar]
- 2.Kenward A, Adams-Smith D, Raja U: Wildfires and Air Pollution: The Hidden Health Hazards of Climate Change, Princeton, NJ, Climate Central, 2013 [Google Scholar]
- 3.Westerling AL, Hidalgo HG, Cayan DR, Swetnam TW: Warming and earlier spring increase western U.S. forest wildfire activity. Science 313: 940–943, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Spracklen DV, Mickley LJ, Logan JA, et al.: Impacts of climate change from 2000 to 2050 on wildfire activity and carbonaceous aerosol concentrations in the western United States. J Geophys Res 114: D20301, 2009 [Google Scholar]
- 5.Fann N, Alman B, Broome RA, Morgan GG, Johnston FH, Pouliot G, et al.: The health impacts and economic value of wildland fire episodes in the U.S.: 2008-2012. Sci Total Environ 610–611: 802–809, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gan RW, Ford B, Lassman W, Pfister G, Vaidyanathan A, Fischer E, et al.: Comparison of wildfire smoke estimation methods and associations with cardiopulmonary-related hospital admissions. Geohealth 1: 122–136, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennekamp M, Abramson MJ: The effects of bushfire smoke on respiratory health. Respirology 16: 198–209, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Doerr SH, Santín C: Global trends in wildfire and its impacts: Perceptions versus realities in a changing world. Philos Trans R Soc Lond B Biol Sci 371: 20150345, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu JC, Mickley LJ, Sulprizio MP, Dominici F, Yue X, Ebisu K, et al.: Particulate air pollution from wildfires in the Western US under climate change. Clim Change 138: 655–666, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennekamp M, Straney LD, Erbas B, Abramson MJ, Keywood M, Smith K, et al.: Forest fire smoke exposures and out-of-hospital cardiac arrests in Melbourne, Australia: A case-crossover study. Environ Health Perspect 123: 959–964, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reisen F, Meyer CP, McCaw L, et al.: Impact of smoke from biomass burning on air quality in rural communities in southern Australia. Atmos Environ 45: 3944–3953, 2011 [Google Scholar]
- 12.Phuleria HC, Fine PM, Zhu YF, Sioutas C: Air quality impacts of the October 2003 Southern California wildfires. J Geophys Res Atmos 110: 11, 2005 [Google Scholar]
- 13.US EPA : Integrated Science Assessment (ISA) for Particulate Matter, Washington, DC, US EPA, 2009 [Google Scholar]
- 14.Liu JC, Pereira G, Uhl SA, Bravo MA, Bell ML: A systematic review of the physical health impacts from non-occupational exposure to wildfire smoke. Environ Res 136: 120–132, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rappold AG, Fann NL, Crooks J, Huang J, Cascio WE, Devlin RB, et al.: Forecast-based interventions can reduce the health and economic burden of wildfires. Environ Sci Technol 48: 10571–10579, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Reid CE, Brauer M, Johnston FH, Jerrett M, Balmes JR, Elliott CT: Critical review of health impacts of wildfire smoke exposure. Environ Health Perspect 124: 1334–1343, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFlorio-Barker S, Crooks J, Reyes J, Rappold AG: Cardiopulmonary effects of fine particulate matter exposure among older adults, during wildfire and non-wildfire periods, in the United States 2008-2010. Environ Health Perspect 127: 37006, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell ML, Zanobetti A, Dominici F: Evidence on vulnerability and susceptibility to health risks associated with short-term exposure to particulate matter: A systematic review and meta-analysis. Am J Epidemiol 178: 865–876, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacks JD, Stanek LW, Luben TJ, Johns DO, Buckley BJ, Brown JS, et al.: Particulate matter-induced health effects: Who is susceptible? Environ Health Perspect 119: 446–454, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanderslice R, Poole K, Sloan K, Krueger N, Bessler A: Wildfire Smoke: A Guide for Public Health Officials, Washington, DC, USEPA, 2016 [Google Scholar]
- 21.Wettstein ZS, Hoshiko S, Fahimi J, Harrison RJ, Cascio WE, Rappold AG: Cardiovascular and cerebrovascular emergency department visits associated with wildfire smoke exposure in California in 2015. J Am Heart Assoc 7: e007492, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fadili W, Al Adlouni A, Louhab N, Habib Allah M, Kissani N, Laouad I: Prevalence and risk factors of cognitive dysfunction in chronic hemodialysis patients. Aging Ment Health 18: 207–211, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Freburger JK, Ng LJ, Bradbury BD, Kshirsagar AV, Brookhart MA: Changing patterns of anemia management in US hemodialysis patients. Am J Med 125: 906–914.e9, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Rigler SK, Wetmore JB, Mahnken JD, Dong L, Ellerbeck EF, Shireman TI: Impact of a modified data capture period on Liu comorbidity index scores in Medicare enrollees initiating chronic dialysis. BMC Nephrol 14: 51, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santoro D, Satta E, Messina S, Costantino G, Savica V, Bellinghieri G: Pain in end-stage renal disease: A frequent and neglected clinical problem. Clin Nephrol 79[Suppl 1]: S2–S11, 2013. [PubMed] [Google Scholar]
- 26.USRD System : 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, 2016 [Google Scholar]
- 27.USRD System : 2018 USRDS Annual Data Report: End-Stage Renal Disease (ESRD) in the United States, Bethesda, MD, National Institutes of Health, 2018 [Google Scholar]
- 28.Rappold AG, Reyes J, Pouliot G, Cascio WE, Diaz-Sanchez D: Community Vulnerability to Health Impacts of Wildland Fire Smoke Exposure. Environ Sci Technol 51: 6674–6682, 2017. 10.1021/acs.est.6b06200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rappold AG, Stone SL, Cascio WE, Neas LM, Kilaru VJ, Carraway MS, et al.: Peat bog wildfire smoke exposure in rural North Carolina is associated with cardiopulmonary emergency department visits assessed through syndromic surveillance. Environ Health Perspect 119: 1415–1420, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkinson RW, Kang S, Anderson HR, Mills IC, Walton HA: Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: A systematic review and meta-analysis. Thorax 69: 660–665, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhaskaran K, Gasparrini A, Hajat S, Smeeth L, Armstrong B: Time series regression studies in environmental epidemiology. Int J Epidemiol 42: 1187–1195, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foley RN, Gilbertson DT, Murray T, Collins AJ: Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 365: 1099–1107, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Gasparrini A: Modeling exposure-lag-response associations with distributed lag non-linear models. Stat Med 33: 881–899, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poole C: A history of the population attributable fraction and related measures. Ann Epidemiol 25: 147–154, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Gasparrini A: Distributed lag linear and non-linear models in R: The package dlnm. J Stat Softw 43: 1–20, 2011. [PMC free article] [PubMed] [Google Scholar]
- 36.Short KC: Spatial Wildfire Occurrence Data for the United States, 1992-2015, 4th Ed., Fort Collins, CO, Forest Service Research Data Archive, 2017 [Google Scholar]
- 37.Haikerwal A, Akram M, Del Monaco A, Smith K, Sim MR, Meyer M, et al.: Impact of fine particulate matter (PM2.5) exposure during wildfires on cardiovascular health outcomes. J Am Heart Assoc 4: e001653, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faustini A, Alessandrini ER, Pey J, Perez N, Samoli E, Querol X, et al.; MED-PARTICLES Study Group: Short-term effects of particulate matter on mortality during forest fires in Southern Europe: Results of the MED-PARTICLES Project. Occup Environ Med 72: 323–329, 2015. [DOI] [PubMed] [Google Scholar]
- 39.Johnston F, Hanigan I, Henderson S, Morgan G, Bowman D: Extreme air pollution events from bushfires and dust storms and their association with mortality in Sydney, Australia 1994-2007. Environ Res 111: 811–816, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Linares C, Carmona R, Tobías A, Mirón IJ, Díaz J: Influence of advections of particulate matter from biomass combustion on specific-cause mortality in Madrid in the period 2004-2009. Environ Sci Pollut Res Int 22: 7012–7019, 2015. [DOI] [PubMed] [Google Scholar]
- 41.Morgan G, Sheppeard V, Khalaj B, Ayyar A, Lincoln D, Jalaludin B, et al.: Effects of bushfire smoke on daily mortality and hospital admissions in Sydney, Australia. Epidemiology 21: 47–55, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Robinson BM, Zhang J, Morgenstern H, Bradbury BD, Ng LJ, McCullough KP, et al.: Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int 85: 158–165, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sastry N: Forest fires, air pollution, and mortality in southeast Asia. Demography 39: 1–23, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Shaposhnikov D, Revich B, Bellander T, Bedada GB, Bottai M, Kharkova T, et al.: Mortality related to air pollution with the moscow heat wave and wildfire of 2010. Epidemiology 25: 359–364, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al.: Air pollution and mortality in the Medicare population. N Engl J Med 376: 2513–2522, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Q, Dai L, Wang Y, Zanobetti A, Choirat C, Schwartz JD, et al.: Association of short-term exposure to air pollution with mortality in older adults. JAMA 318: 2446–2456, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Guo Y, Tong S, et al.: Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med 381: 705–715, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu JC, Wilson A, Mickley LJ, Dominici F, Ebisu K, Wang Y, et al.: Wildfire-specific fine particulate matter and risk of hospital admissions in urban and rural counties. Epidemiology 28: 77–85, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delfino RJ, Brummel S, Wu J, Stern H, Ostro B, Lipsett M, et al.: The relationship of respiratory and cardiovascular hospital admissions to the southern California wildfires of 2003. Occup Environ Med 66: 189–197, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore D, Copes R, Fisk R, Joy R, Chan K, Brauer M: Population health effects of air quality changes due to forest fires in British Columbia in 2003: Estimates from physician-visit billing data. Can J Public Health 97: 105–108, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mott JA, Mannino DM, Alverson CJ, Kiyu A, Hashim J, Lee T, et al.: Cardiorespiratory hospitalizations associated with smoke exposure during the 1997, Southeast Asian forest fires. Int J Hyg Environ Health 208: 75–85, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z: Particulate matter air pollution and the risk of incident CKD and progression to ESRD. J Am Soc Nephrol 29: 218–230, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z: Associations of ambient coarse particulate matter, nitrogen dioxide, and carbon monoxide with the risk of kidney disease: A cohort study. Lancet Planet Health 1: e267–e276, 2017. [DOI] [PubMed] [Google Scholar]
- 54.Huang WH, Yen TH, Chan MJ, Su YJ: Impact of environmental particulate matter and peritoneal dialysis-related infection in patients undergoing peritoneal dialysis. Medicine (Baltimore) 93: e149, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weng CH, Hu CC, Yen TH, Huang WH: Association between environmental particulate matter and arterial stiffness in patients undergoing hemodialysis. BMC Cardiovasc Disord 15: 115, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu JC, Wilson A, Mickley LJ, Ebisu K, Sulprizio MP, Wang Y, et al.: Who among the elderly is most vulnerable to exposure to and health risks of fine particulate matter from wildfire smoke? Am J Epidemiol 186: 730–735, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson AH, Cohen AJ, Kutner NG, Kopp JB, Kimmel PL, Muntner P: Missed dialysis sessions and hospitalization in hemodialysis patients after Hurricane Katrina. Kidney Int 75: 1202–1208, 2009. [DOI] [PubMed] [Google Scholar]
- 58.Lovell N, Jones C, Baynes D, Dinning S, Vinen K, Murtagh FE: Understanding patterns and factors associated with place of death in patients with end-stage kidney disease: A retrospective cohort study. Palliat Med 31: 283–288, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pun PH, Herzog CA, Middleton JP: Improving ascertainment of sudden cardiac death in patients with end stage renal disease. Clin J Am Soc Nephrol 7: 116–122, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prakash S, Coffin R, Schold J, et al.: Travel distance and home dialysis rates in the United States. Perit Dial Int 34: 24–32, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stephens JM, Brotherton S, Dunning SC, et al.: Geographic disparities in patient travel for dialysis in the United States. J Rural Health 29: 339–348, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Henderson SB, Brauer M, Macnab YC, Kennedy SM: Three measures of forest fire smoke exposure and their associations with respiratory and cardiovascular health outcomes in a population-based cohort. Environ Health Perspect 119: 1266–1271, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watson GL, Telesca D, Reid CE, Pfister GG, Jerrett M: Machine learning models accurately predict ozone exposure during wildfire events. Environ Pollut 254: 112792, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.