Abstract
Human‐severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) interaction can have an array of various outcomes—it could be mortal, morbid or merely carrying minor health consequences. The very rapid global spread has raised the issue whether there are further multi‐dimensional consequences of SARS‐CoV‐2 infection on human behavior, the key of its transmission. During the coronavirus crisis, odd, abnormal, and irresponsible behavior has been reported in coronavirus disease 2019 (COVID‐19) individuals, particularly in super‐spreaders, that is, persons with a high viral load, thus constituting also super‐emitters. Indeed, cases of infected persons ignoring self‐confinement orders, intentionally disregarding physical distancing and multiplying social interactions, or even deliberately sneezing, spitting or coughing were reported. While it is known that some other viruses, such as rabies and even influenza do change human behavior, this remains unclear for SARS‐CoV‐2. In this perspective, we highlight the possibility that COVID‐19 is facilitated by altered human social behavior that benefits SARS‐CoV‐2 transmission, through showcasing similar virus‐induced changed behavior by other pathogens and relating this to reports from the gray literature.
Keywords: abnormal behavior, brain‐immune axis, CNS‐changes, COVID‐19, obligate parasites, social interactions
Highlights
Viruses such as rabies and even influenza were reported to result in altered demeanor.
SARS‐CoV‐2 behavioral manipulation may concern only a fraction of infected persons, especially super‐spreaders.
SARS‐CoV‐2 may influence behavior, likely in the asymptomatic phase, favoring its prompt spread.
This may explain odd behavior, e.g. disregarding confinement and deliberate viral‐spreading.
This warrants further investigation, including implications for brain‐immune and brain‐gut axis.
1. INTRODUCTION
The devastating numbers of coronavirus disease 2019 (COVID‐19) cases (over 23 million cases worldwide as of 24 August 2020) raised the question as to whether severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) employs a more sophisticated strategy to ensure its rapid spread over the globe, by modulating human behavior, the key of pathogenic spread. Understanding any such strategy is paramount to keep viral spread in check. Beyond its basic strategy to hijack our cell machinery, does SARS‐CoV‐2 influence our behavior to make it more likely to spread?
It has been debated that the COVID‐19 spread depends on clusters, in which super‐spreaders are playing a key role in the impressive rapid transmission of the novel coronavirus. 1 In Wuhan, it was estimated that undiagnosed asymptomatic individuals or those with only mild‐symptoms were responsible for up to 79% of viral infections. 2 It is remarkable that most of the infected people did not spread the virus at all, 1 though it has been postulated that super‐spreaders may carry rather high viral loads. 3 , 4 Exhaled virus‐containing aerosols (and also droplets >5‐10 µm) from super‐emitters, during breathing, speaking, singing, vocalizing, coughing, and sneezing, constitute the key factor for the spread, 2 , 3 even if other means of transmission may exist, such as via hand contact. However, this is the first time that behavioral changes, especially in super‐spreaders, are presented as a plausible key to the successful global spread of the new coronavirus. Indeed, behavioral aspects can be considered as the first line of defense, resulting in either avoidance or exposure to the virus. This hypothesis is perhaps less far‐stretched as it may appear on a first glance. It should be kept in mind that a variety of intracellular obligate parasites, including viruses, have been reported to exactly do this, as exemplified in the following. Furthermore, gray literature has been increasingly adding to the body of evidence of strange abnormal behavioral patterns during COVID‐19, in line with virus‐induced altered mannerism. In the following, we briefly emphasize the possibility of COVID‐19 instigated behavior, contributing to the rapid spread of the virus we observe.
2. DISCUSSION
Parasite‐induced behavioral manipulation is a multi‐dimensional process impacting host behavioral decisions in a manner that benefits pathogen transmission. 5 , 6 This is corroborated by the gray literature around COVID‐19. During the coronavirus crisis, the international media have highlighted, in several parts of the world, peculiar cases of men and women intentionally ignoring physical distancing, deliberately sneezing or coughing in public places or on money, or even spitting on door handles, elevator buttons, shopping carts, credit cards, into people's faces, etc. 7 , 8 Defying coronavirus health warnings by licking and kissing holy shrines in Iranian mausoleums was also observed. Disregarding social distancing, benefitting SARS‐CoV‐2 transmission, was reported during anti‐lockdown movements in both US and Germany, among other countries. Cell phone data suggested that anti‐lockdown participants may have widely spread the virus in the US, as some of them did move hundreds of miles across states. Patients with COVID‐19 escaping from hospitals were reported in several countries including Spain, where several cases leaving hospitals before they were formally discharged were recorded, resulting in being tracked down, as well as their close contacts, by the police. Cases of infected individuals deliberately behaving to spread the novel coronavirus, for example, in bars were also reported. Certain governments consider deliberate transmission of COVID‐19 as an offense under the general criminal laws. Indeed, individuals intentionally spreading the novel coronavirus could be charged as terrorists in the US, 9 and risk imprisonment for life, the ultimate penalty, in Australia. 8 In this context, deliberate human immunodeficiency virus transmission for reasons of personal vengeance or frustration, was already reported and this type of behavior was criminalised by several governments.
Stunningly, vaccinating humans with an influenza vaccine 10 significantly increased the number of interactions post‐immunization compared with before, that is, in the 48 hours presymptomatic period before the expected onset of symptoms or sickness. In case of a real infection, this would contribute to a rapid spread of the virus. Unlike the precedent SARS‐CoV, where its transmission originated from patients with more severe symptoms, 11 the SARS‐CoV‐2 is further transmissible by asymptomatic individuals or those with only mild‐symptoms. 2 , 3 , 11 It is believed that a large portion of the spread of COVID‐19 has been occurring through airborne transmission by asymptomatic individuals, particularly in indoor conditions, 2 , 3 where aerosols can remain airborne for hours, accumulate over time and follow air flows over distances further than 2 m2. Moreover, the swine flu virus (H1N1) has been demonstrated to cause a variety of neuropsychiatric disorders, 12 including mood disorder and schizophrenia. Thus, the possibility of host‐pathogen interactions toward a more active phenotype following infection must be considered. Indeed, host‐parasite interactions constitute a well‐known dynamic concept leading to abnormal behavioral patterns in the host, which are advantageous either to the host—such as employing defensive behavior—or to the parasite, as a manipulative behavior. 5 , 6 However, from a co‐evolutionary point of view, it is unclear how such behavioral changes can be produced by viruses which have been only interacting with humans for a short time, but they appear to occur, at least for individuals with influenza, and also certain patients with acquired immunodeficiency syndrome.
Such irresponsible behavior has been reported to result in an impressive number of cases of contamination in a single night—over 170—by one individual who visited several clubs (termed “the nightclub's patient zero”), with thousands of potential contacts. 1 As a consequence, South Korean authorities warned of a pandemic's second wave. Interestingly, the first pandemic wave in South Korea was attributed to a single super‐spreader known as patient 31, the 31st confirmed COVID‐19 case. Until then, SARS‐CoV‐2 was under control and the number of patients was stable, with 30 confirmed cases. Instead of self‐quarantine, the patient 31 (a female), had rather multiple social interactions, with thousands of contacts, a demeanor that turned South Korea's virus situation into an epidemic crisis. Indeed, on 18 March, at least 60% of all cases in South Korea could be attributed to patient 31. 13 Undoubtedly, traveling has been a major determining factor for the international spread of the novel coronavirus. In India, a super‐spreader found asymptomatic at Delhi airport after a trip on 17 March had ignored orders for self‐quarantine, infecting around 100 people in Jaipur. 14 Worse still, a religious Sikh, who had returned from a trip to Europe's virus epicenter at the time—Italy—and Germany, and had ignored self‐confinement orders, was suspected to have infected several hundreds of people, causing mass quarantining in India, as 40,000 persons from 20 villages in the Punjab state underwent strict home quarantine. 15 Despite knowing he had contracted COVID‐19, a super‐spreader teacher continued to come to work to a Jerusalem high school, infecting around 200 students and staff members, forcing more than 1400 individuals to enter quarantine. 16 Ms. “S” was identified as the COVID‐19 super‐spreader in Ningbo, China, in mid‐January. 4 Despite having flu‐like symptoms, she joined a blessing ceremony in a temple without mask. On 12 February, a total of 77 confirmed infected cases were reported, and an accumulative total of 1257 contacts were isolated; a spread in Ningbo was stopped only due to the aggressive contact‐tracing and testing adopted by the Chinese government. 4 In the Alpine ski resort of Ischgl, Austria, hundreds of tourists were believed to have contracted the coronavirus and taken it to their homeplace, mostly to Northern Europe and Germany. Thus, Ischgl, which attracts at least 500,000 tourist each winter, became one of the principal epicenters of the COVID‐19 spread in Europe. A German bartender, believed to be Ischgl's “patient‐zero,” fell sick with flu‐like symptoms on 5 February. However, the novel coronavirus lingered around Ischgl undetected for more than a month, spreading from customer to customer, infecting 611 Austrians—and many more international guests. Indeed, the bartender was tested positive for coronavirus only on 7 March and the bar was finally closed on 9 March. 17
Gray literature and social media have reported numerous such stories of infection and super‐spreading events that we cannot detail here. It should be highlighted that if such behavioral manipulation by SARS‐CoV‐2 takes place, this still concerns only a small fraction of infected persons, as the majority of infected individuals appeared to well‐respect self‐confinement orders, followed social distancing and employed other defensive behavior, such as hand washing. Interestingly, it was also estimated that 10% of the cases are super‐spreaders, resulting in 80% of viral spread, meaning that the majority of SARS‐CoV‐2 carriers do not appear to unaccountably transmit the virus. Indeed, most patients with COVID‐19 are believed not to transmit the disease. 1 It seems that the mode of the transmission of COVID‐19 is highly heterogeneous, since the dispersion factor (k), which describes how much a disease clusters, is very low (k = 0.1), indicating that transmissions origin from only a small number of people. Most of the discussions around the transmission of SARS‐CoV‐2 have focused on the average number of new infections caused by each patient. Without social distancing, the reproduction number (R) is about three. But “The consistent pattern is that the most common number is zero,” that is, most people do not transmit. 1 Our hypothesis stipulates that super‐spreaders, who are already known as heavily infected regarding viral load and also super‐emitters, are further able to infect large numbers of individuals, following a pattern of odd and irresponsible behavior triggered by the novel coronavirus, most likely during the asymptomatic phase. Research should focus urgently on the psychological dimension of individuals to address whether their personality traits, including personality disorders with aggressive impulses, are more predisposed to SARS‐CoV‐2 manipulation. This should also address the question whether there exists a close relationship between higher viral load and the manipulative ability of the virus. Ethically, it should be stressed that even if behavioral manipulation is present, this does not acquit the individual from acting responsible.
Of course, it is possible or even likely that other aspects influencing personal behavior of super‐spreaders, including cultural and religious aspects, do also play important roles. Given that behavior is being determined by several factors including biological, psychological, and social determinants, as well as situational factors including environmental challenges, it is likewise possible that this behavior, reflecting the human complexity, is explainable by the stressful situation—perceived or real—in which persons find themselves.” For instance, the behavior of ignoring social distancing and public safety rules could also be explainable by anger and resentment triggered by fear for loss of rights, labor, and income, among others. It could be interesting to study whether during the COVID‐19 crisis, more such odd behavior is truly found—and reported—compared with other stressful, comparable incidences or diseases, or to compare the behavior in diseased versus non‐diseased subjects or in the preinfection versus postinfection state, especially in super‐spreaders—but such data has at present not been at our disposal and would require carefully controlled studies.
However, similar virus‐induced modified behavior was observed in the deadly rabies virus, typically hosted in dogs and foxes, which can inhibit nicotinic acetylcholine receptors in the central nervous system (CNS), 18 the area required for rational behavior and decision‐making. This causes structural changes of the CNS, induced by a snake‐like glycoprotein, resulting in hyperactivity, increased bite‐frequency (and elevated saliva‐flow) and longer social contacts, a behavioral pattern fundamental to the persistence of rabies in dog populations for millennia. 19 As another viral example, irritability episodes were reported in children recently affected by the Herpes simplex virus, and infected mice were hyperactive. Many more examples of parasitical behavior changes have been reported. For instance, Toxoplasma gondii, a protozoa, manipulates rodent behavior, converting host innate aversion to cat odors into rather an attraction, enhancing the chance of cat predating, thus ensuring its transmission efficiency to complete its life‐cycle within the cat's intestine. Toxoplasma gondii is also influencing personality and behavior of infected humans, for example, by tampering with psychomotor performance. 20
3. CONCLUSION
In this perspective, we highlighted the possibility that COVID‐19 is facilitated by altered human social behavior that benefits SARS‐CoV‐2 transmission (Figure 1). Regrettably, direct scientific evidence for behavioral changes from COVID‐19 infected individuals are not (yet) available, and carefully controlled studies in this domain are warranted. However, a loss of chemosensory function has already been reported for COVID‐19, influencing taste and smell, which may be related to CNS changes. Brain alteration and a myriad of neuropsychological symptoms including cerebrovascular complications and encephalopathies were reported as a consequence of SARS‐CoV‐2 infection. Evidence has shown that SARS‐CoV‐2 may affect directly CNS. CNS disturbances could also result from the immune response to SARS‐CoV‐2 or as the consequence of the virus' effects on the gastrointestinal tract (Figure 1), thus both the brain‐immune and the gut‐brain axis could be involved. Therefore, while we surely should monitor our behavior in handling the COVID‐19 crisis, we must also consider that we may be somewhat handled by the virus, emphasizing that we should be double‐cautious regarding our behavior.
Figure 1.
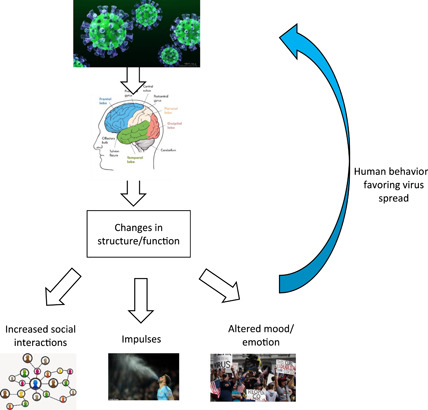
Possible close relationship between the spread of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and altered behavior. The scheme highlights the potential manipulative strategy of the novel coronavirus, resulting in viral spread, following an altered behavioral pattern in some patients with coronavirus disease 2019 (COVID‐19), as a consequence of a direct impact on brain structure/function, owing to viral infiltration into the central nervous system (CNS), and/or via perturbation of the brain‐immune axis or the gut‐brain axis
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Both JB and TB wrote the article and revised it.
Bouayed J, Bohn T. Behavioral manipulation—key to the successful global spread of the new coronavirus SARS‐CoV‐2? J Med Virol. 2021;93:1748–1751. 10.1002/jmv.26446
Contributor Information
Jaouad Bouayed, Email: jaouad.bouayed@univ-lorraine.fr.
Torsten Bohn, Email: torsten.bohn@lih.lu.
REFERENCES
- 1. Kupferschmidt K. Case clustering emerges as key pandemic puzzle. Science. 2020;368:808‐809. [DOI] [PubMed] [Google Scholar]
- 2. Asadi S, Bouvier N, Wexler AS, Ristenpart WD. The coronavirus pandemic and aerosols: does COVID‐19 transmit via expiratory particles? Aerosol Sci Technol. 2020;54:635‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prather KA, Wang CC, Schooley RT. Reducing transmission of SARS‐CoV‐2. Science. 2020;368:1422‐1424. 10.1126/science.abc6197 [DOI] [PubMed] [Google Scholar]
- 4. Lin J, Yan K, Zhang J, Cai T, Zheng J. A super‐spreader of COVID‐19 in Ningbo city in China. J Infect Public Health. 2020;13:935‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poulin R. Parasite manipulation of host personality and behavioural syndromes. J Experim Biol. 2013;216:18‐26. [DOI] [PubMed] [Google Scholar]
- 6. Moore J. An overview of parasite‐induced behavioral alterations—and some lessons from bats. J Exp Biol. 2013;216:11‐17. [DOI] [PubMed] [Google Scholar]
- 7. Farrell J. Coronavirus: ticket collector dies with COVID‐19 after man claiming to have virus spat on her. Sky. 2020. https://news.sky.com/story/coronavirus-mother-dies-with-covid-19-after-man-claiming-to-have-virus-spits-on-her-11986808
- 8. Vrajlal A. 'Imprisonment for life': COVID‐19 deliberate transmission maximum penalty announced. Huffington post. 2020. https://www.huffingtonpost.com.au/entry/coronavirus-deliberate-transmission_au_5e8d41cac5b6e1d10a6ba789
- 9. LeBlanc P. People intentionally spreading coronavirus could be charged with terrorism, DOJ says. CNN. 2020. https://edition.cnn.com/2020/03/25/politics/coronavirus-terrorism-justice-department/index.html
- 10. Reiber C, Shattuck EC, Fiore S, Alperin P, Davis V, Moore J. Change in human social behavior in response to a common vaccine. Ann Epidemiol. 2010;20(10):729‐733. [DOI] [PubMed] [Google Scholar]
- 11. Colvin K. The people versus COVID‐19. EMJ. 2020;5(1):20‐23. [Google Scholar]
- 12. Manjunatha N, Math SB, Kulkarni GB, Chaturvedi SK. The neuropsychiatric aspects of influenza/swine flu: a selective review. Industr Psychiatry J. 2011;20:83‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hernandez M, Scarr S, Manas S. The Korean clusters. Reuters. 2020. https://graphics.reuters.com/CHINA-HEALTH-SOUTHKOREA-CLUSTERS/0100B5G33SB/index.html
- 14. Bohra S. Super spreader' infects nearly 100 people in Jaipur, Rajasthan gets second COVID‐19 hotspot. The Print. 2020. https://theprint.in/india/super-spreader-infects-nearly-100-people-in-jaipur-rajasthan-gets-second-covid-19-hotspot/398537/
- 15. BBC staff member . Coronavirus: India 'super spreader' quarantines 40,000 people BBC News. 2020. https://www.bbc.com/news/world-asia-india-52061915
- 16. YWN Israel Desk–Jerusalem . Teacher at J‐m school at center of outbreak Knew He Was Ill but came to work anyway. 2020. https://www.theyeshivaworld.com/news/headlines-breaking-stories/1866010/incriminating-report-teacher-at-j-m-school-at-center-of-outbreak-knew-he-was-ill-but-came-to-work-anyway.html
- 17. Tavernini M. Vector spread from Austrian ski town was toxic mix of business and politics. TRT. World. 2020;6. https://www.trtworld.com/magazine/vector-spread-from-austrian-ski-town-was-toxic-mix-of-business-and-politics-35161 [Google Scholar]
- 18. Hueffer K, Khatri S, Rideout S, et al. Rabies virus modifies host behaviour through a snake‐toxin like region of its glycoprotein that inhibits neurotransmitter receptors in the CNS. Sci Rep. 2017;7:12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brookes VJ, Dürr S, Ward MP. Rabies‐induced behavioural changes are key to rabies persistence in dog populations: investigation using a network‐based model. PLOS Negl Trop Dis. 2019;13(9):e0007739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flegr J. Effects of toxoplasma on human behavior. Schizophr Bull. 2007;33:757‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]


