Dear Editor,
Acral lesions represent 63% of cutaneous manifestations of COVID‐19. Most lesions resemble chilblains affecting hands and feet with asymmetric distribution. 1 , 2 Acro‐ischaemic lesions have been reported in critical patients due to the disseminated intravascular coagulation that can occur in these patients. 3 However, cases of acute acro‐ischaemic lesions have also been reported in mild or asymptomatic forms of COVID‐19. 4
The most typical finding in coagulation tests on patients with COVID‐19 is an increased D‐dimer concentration. Inflammation of endothelial cells could lead to the massive release of plasminogen activators, which could explain the high concentrations of D‐dimer and fibrin degradation products in patients with COVID‐19. 5
A 10‐year‐old boy presented with a dry cough and a fever for 12 days and had acral lesions similar to acro‐ischaemia on the toes of both feet during April 2020 in Spain, which coincides with spring and a warm climate (Fig. 1). The patient had no relevant medical history and had not taken any drugs. Blood tests, which included biochemical, haemogram, coagulation, hepatic, and renal profiles, immunoglobulins and an urinalysis, did not show any alterations apart from an elevation in D‐dimer (4231 µg/L) and IgE immunoglobulin (1570 U.L/mL). An autoimmunity study was performed that included antinuclear antibodies, complement, autoantibodies against extractable nuclear antigens, anti‐dsDNA, anti‐histone anti‐PM/Scl‐100, anti‐centromere protein‐A/B, anti‐ku, anti‐ribosomal, anti‐thyroid peroxidase antibodies, anti‐thyroglobulin antibodies, cold agglutinins, circulating immunocomplexes, rheumatoid factor and a profile of myositis antibodies, which were all negative. Acute‐phase reactants, such as C‐reactive protein (0.037 mg/dL), erythrocyte sedimentation rate in the first hour (8 mm and serum ferritin levels (39 ng/mL) did not show elevations. Serologies for parvovirus B19, Epstein–Barr virus, cytomegalovirus, Mycoplasma pneumoniae, herpes simplex virus, hepatitis B and C, and RT‐PCR for enterovirus were all negative. A nasopharyngeal swab for RT‐PCR and a SARS‐CoV‐2 IgM/IgG rapid antibody test were performed with negative results. After 10 days, new serologies were carried out adding serologies for SARS‐CoV‐2 (IgA + IgM and IgG antibodies), which were negative.
Figure 1.

(a) Erythematous–purpuric lesions can be seen on the toes with the presence of superficial crusts. (b) Purpuric lesions on the pads of the toes.
A skin biopsy showed both superficial and deep perivascular lymphocytic infiltrates with vacuolar degeneration in the basal epidermal layer. The blood vessels showed swollen endothelium, oedema, fibrin and extravasation of red blood cells in the papillary dermis (Fig. 2). There was complete resolution of the lesions and normalisation of the D‐dimer levels after 4 weeks.
Figure 2.
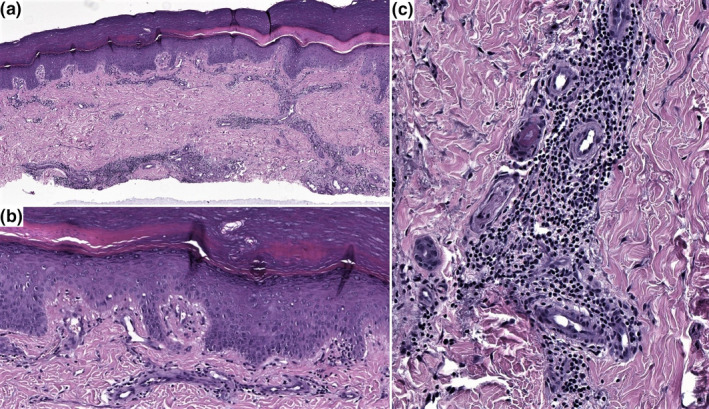
(a) Skin biopsy shows superficial and deep perivascular lymphoid infiltrate. (HE, x2). (b) Basal vacuolar changes are present. (HE, x10). (c) The endothelium is enlarged, and fibrin is present focally. (HE, x20).
Reports of acral lesions in children and young adults coinciding with the COVID‐19 pandemic have become more frequent. Despite this, most studies have not been able to show evidence of active or past SARS‐CoV‐2 infection in diagnostic tests, including serologies, presenting positive diagnostic tests in only 7% of cases. 1
The clinical and histopathological findings of the reported case are similar to those of other studies. 1 , 2 However, extremely high levels of D‐dimer have not been reported in children or young adults with these lesions. This is probably due to a lack of analytical and coagulation studies carried out when patients present with only mild or asymptomatic forms of COVID‐19. In our case, the endothelial damage present in the histopathological findings may explain the elevation of D‐dimer levels, as occurs in seriously ill patients with COVID‐19.
Significant elevations in D‐dimer levels coinciding with acro‐ischaemic lesions and the subsequent normalisation along with the exclusion of other cases of D‐dimer elevation suggest that the skin lesions are related to COVID‐19. These findings reinforce the hypothesis that SARS‐CoV‐2 infection may cause the lesions, which can be explained by various hypotheses. One of them is that acro‐ischaemic lesions are evidence of endothelial damage induced by SARS‐CoV‐2, 2 which in turn produces a hypercoagulable state that raises D‐dimer levels. Another explanation would be that the thromboembolic phenomena that usually occur in COVID‐19 patients contributed to endothelial damage and subsequent appearance of acro‐ischaemic lesions. However, it could also be explained as a combination of the previous aetiopathogenic hypotheses.
We propose, supported by other studies, that this type of skin lesion be added to the testing criteria for COVID‐19 and the consideration for performing PCR tests and serologies. 1
Acknowledgements
We gratefully acknowledge the multidisciplinary team that contributed to the patient’s medical care.
Conflict of interest: The authors declare that they have no conflict of interest.
Funding statement: The authors received no financial support for the research, authorship and/or publication of this article.
References
- 1. Freeman EE, McMahon DE, Lipoff JB et al. Pernio‐like skin lesions associated with COVID‐19: a case series of 318 patients from 8 countries. J. Am. Acad. Dermatol. 2020; 83: 486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colmenero I, Santonja C, Alonso‐Riaño M et al. SARS‐CoV‐2 endothelial infection causes COVID‐19 chilblains: histopathological, immunohistochemical and ultraestructural study of 7 paediatric cases [published online ahead of print, 2020 Jun 20]. Br. J. Dermatol. 2020. 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Y, Cao W, Xiao M et al. Clinical and coagulation characteristics of 7 patients with critical COVID‐2019 pneumonia and acro‐ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020; 41: E006. [DOI] [PubMed] [Google Scholar]
- 4. Mazzotta F, Trocoli T. Acute acro‐ischemia in the child at the time of COVID‐19. [Online ahead of print, 2020]. Eur. J. Pediat. Dermatol. 2020. [Google Scholar]
- 5. Levi M, Thachil J, Iba T et al. Coagulation abnormalities and thrombosis in patients with COVID‐19. Lancet Haematol. 2020; 7(6): e438–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]


