Abstract
Pathologies affecting the small intestine contribute significantly to the disease burden of both the developing and the developed world, which has motivated investigation into the disease mechanisms through in vitro models. Although existing in vitro models recapitulate selected features of the intestine, various important aspects have often been isolated or omitted due to the anatomical and physiological complexity. The small intestine's intricate microanatomy, heterogeneous cell populations, steep oxygen gradients, microbiota, and intestinal wall contractions are often not included in in vitro experimental models of the small intestine, despite their importance in both intestinal biology and pathology. Known and unknown interdependencies between various physiological aspects necessitate more complex in vitro models. Microfluidic technology has made it possible to mimic the dynamic mechanical environment, signaling gradients, and other important aspects of small intestinal biology. This review presents an overview of the complexity of small intestinal anatomy and bioengineered models that recapitulate some of these physiological aspects.
Impact statement
There is a need for improved cell culture models of the small intestine. To develop such models, it is necessary to give engineers a better understanding of intestinal biology, and conversely, to give biologists an idea of what is possible with microfluidic devices and biomaterial platforms. This work provides a detailed overview of aspects of intestinal biology that are important for tissue engineers to understand when designing models of the small intestine and provides a summary of current approaches. This review highlights both unique and common features of various strategies for modeling the intestinal cell environment, not only using microfluidic chip-based systems but also several elegant designs that use a materials-based static culture approach.
Keywords: gut-on-a-chip, microfluidic models of intestine, small intestine model, tissue engineered intestine
Introduction
The small intestine is the site of numerous pathologies that contribute significantly to the disease burden of both the developing and the developed world. Investigation into the pathological mechanisms contributing to small intestinal disease is impeded by the complexity and dynamism of the organ's physiology. The small intestinal mucosa has an intricate microanatomy with fine topographical features that organize its epithelium into distinct proliferative and differentiated compartments.1 A steep oxygen gradient extends from the intestinal wall into the lumen and supports a complex microbial community growing in a generally anaerobic environment in close proximity to the aerobic cells of the mucosa.2 Finally, the contractions of the intestinal wall and the movement of luminal chyme create a dynamic mechanical and chemical environment that contributes to normal intestinal homeostasis and influences the crosstalk between the mucosal surface and the luminal microbiota.3,4
Despite their importance in both intestinal biology and pathology, these aforementioned features and other aspects of intestinal anatomy are often not included in in vitro experimental models of the small intestine. This review covers an overview of the complexity of small intestinal anatomy and the bioengineered models that recapitulate some of these physiological aspects.
Anatomy
Structure
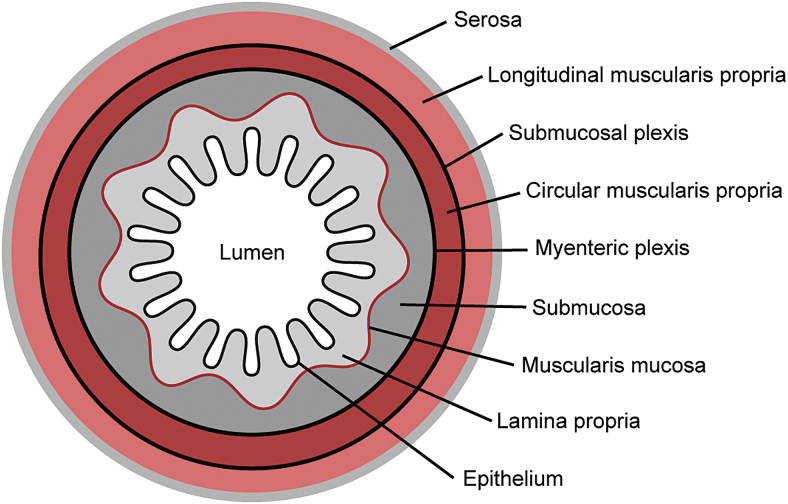
The main function of the small intestine is to digest and absorb nutrients from ingested food.1 It begins at the distal end of the stomach (the pyloric sphincter) and ends at the proximal opening of the large intestine (the ileocecal valve).1 Starting from the stomach, the sections of the small intestine are the duodenum, jejunum, and ileum.1 Anatomically, the intestine is a tubular organ composed of several concentric layers, each with distinct compositions and functions (Fig. 1).
FIG. 1.
Cross section of the small intestine. Color images are available online.
The outermost layer is the serosa, which is composed of a single layer of mesothelial cells that rest on a thin layer of connective tissue containing scattered fibroblasts, macrophages, mast cells, and other immune cells.1 The serosa is continuous with the mesentery and functions as a conduit for blood vessels, lymphatics, and nerves that connect to the small intestine.1 The serosa surrounds two layers of smooth muscle known as the muscularis externa.1 These layers are oriented in different directions: the outer one is oriented longitudinally and the inner one is oriented circumferentially.1 Their coordinated actions cause peristalsis, a series of radially symmetric contractions that move in an aboral manner and are responsible for mixing and homogenizing the digestate.4
Inside of the muscularis is the submucosa, a layer of highly vascularized connective tissue composed of fibrillar collagens and hydrated glycosaminoglycans, which is populated by fibroblasts and a variety of immune cells.5,6 The submucosa is bounded internally by the mucosa, itself consisting of three more layers: the muscularis mucosae, the lamina propria, and the epithelium.1 Like the muscularis externa, the fibers within the muscularis mucosae are oriented longitudinally near the submucosa and circumferentially near the lamina propria; however, this muscularis layer is significantly thinner than the muscularis externa.1
The two regions of muscularis are innervated by the enteric nervous system (ENS), which is composed of two main ganglionic plexi.1 The myenteric plexus is located in the muscularis externa between the circumferential and longitudinal layers and is responsible for coordinating peristalsis, while the submucosal plexus is located within the submucosa and regulates intestinal secretion and the motions of the muscularis mucosae.7
Immediately on top of the muscularis mucosae is a layer of loose connective tissue known as the lamina propria.1 The lamina propria forms an array of pocket-like invaginations and finger-like protrusions, called crypts and villi, respectively.1Bundles of muscle fibers extend from the muscularis mucosae through the core of each villus, enabling villus contractility.8
The lamina propria is highly vascularized and contains a wide variety of mesenchymal and immune cells that support and nourish the overlying epithelium.1 The intestinal epithelium is covered by a layer of mucus, which physically separates the epithelium from the bacteria that reside in the lumen of the small intestine.9
Cell types
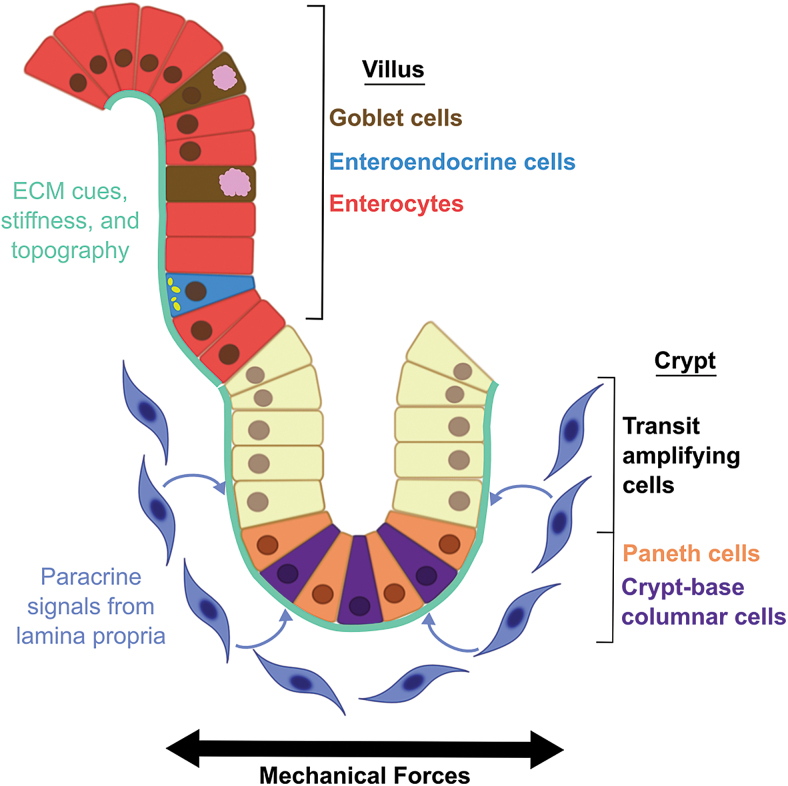
The intestinal epithelium is anchored to the underlying lamina propria by the intestinal basement membrane.1 The epithelium is composed of five main cell types, which are organized into a proliferative compartment located in the intestinal crypt and a differentiated compartment located on the intestinal villus.1 This organization is known as the crypt-villus axis (Fig. 2).
FIG. 2.
The crypt-villus axis. Color images are available online.
The intestinal stem cells (ISCs), also called crypt-base columnar cells, are found at the base of the crypts and are marked by leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5), which is involved in the Wnt-signaling pathway.10 The Paneth cells are also found at the crypt base, interspersed with the ISCs.11 They help maintain the stem cell population by providing Wnt, epidermal growth factor, and Notch signals.11 They also produce antimicrobial peptides, such as lysozyme and α-defensins, that regulate the intestinal microbial population and protect the ISCs from infection.11 The higher regions of the crypt are filled with transit-amplifying cells, which have committed to a differentiated epithelial lineage but are still proliferating.12
In the differentiated villus region of the intestinal epithelium, three main types of differentiated intestinal epithelial cells (IECs) are found: enterocytes, goblet cells, and enteroendocrine cells.1
Enterocytes are the most common cell in the intestinal epithelium and are responsible for the digestion and absorption of nutrients from the lumen. They have a well-developed array of apical microvilli studded with a large amount of digestive enzymes and transport proteins.1 This structure is known as the brush border, and it serves to increase the surface area available to each cell for nutrient processing and absorption.
Goblet cells are the next most common cell type and are characterized by an apical mass of mucin-containing secretory granules.1 Mucus secreted from these cells maintains a continuous layer of mucus over the intestinal epithelium, which provides a protective barrier and helps lubricate the inner wall of the intestine.9
The last most common cell type in the villus intestinal epithelium is the enteroendocrine cell.1 They are the least prevalent and are characterized by prominent basal secretory granules filled with peptide and monoamine hormones. When enteroendocrine cells are activated, they release their granules into the lamina propria; these granules then exert local paracrine effects or are absorbed into the vasculature and perform endocrine functions.1 Enteroendocrine cells produce a wide variety of hormones and regulate a number of different functions, such as stimulating gastrointestinal (GI) motility, inducing insulin secretion, and regulating gastric emptying.13
Other cell types in the villus intestinal epithelium include tuft cells, microfold cells, and cup cells. Tuft cells are important in regulating the immune response to parasites.14 Microfold cells, also called M cells, play a role in immune response to other pathogens and antigen tolerance.15 Cup cells are a distinct cell type, but their specific function is undetermined.
Variation along the length of the small intestine represents additional challenges for in vitro modeling. The duodenum experiences lower pH than the rest of the intestine and is the site of mixing for chyme from the stomach, enzymes from the pancreas, and bile from the liver.16 The jejunum is responsible for most nutrient absorption. Vitamin B12 and bile salts are absorbed by the ileum, along with nutrients not absorbed by the jejunum.16
In addition to having differences arising from their distinct functions, the sections of the small intestine are affected differently by diseases and pathogens. For example, the characteristic villous atrophy of celiac disease is most often present in the duodenum and proximal jejunum, but mostly or completely absent in the ileum.17 Crohn's disease most frequently effects the distal end of the ileum.18 The distal ileum is also likely the initial site of invasion for pathogenic bacteria such as Salmonella and Shigella.19 These are just a few examples of the many differences between longitudinal sections in the small intestine. These differences necessitate the informed selection of a particular section or the inclusion of cells or tissues from multiple sections in studies investigating diseases or phenomenon not known to be strongly associated with a particular section.
Barrier function
One of the main functions of the intestinal epithelium is to form a selective barrier between the lamina propria and the intestinal lumen that allows absorption of nutrients, water, and ions, but excludes toxins and infectious microbes.1 Tight junction proteins expressed by every IEC are key to this barrier function.20 Tight junctions are composed of occludins and claudins, which are transmembrane proteins anchored to the actin cytoskeleton by adaptor molecules, such as zonula occludens-1 or ZO-1 (also known as tight junction protein 1 or TJP1).20 The extracellular domains of occludins and claudins interact to form semipermeable barriers that allow paracellular ion transport, but restrict large molecules from passing between cells.21 Tight junctions form belt-like barriers around every IEC, and define the border between apical and basolateral regions.21
Adherens junctions and spot desmosomes are located basal to the tight junctions and mediate cell–cell attachment.21 Like tight junctions, both of these complexes are composed of transmembrane proteins that interface with the cytoskeleton through adaptor proteins: for adherens junctions, cadherins connect to the actin cytoskeleton via a cadherin/catenin complex, while for spot desmosomes, desmoglein and desmocollin connect to intermediate filaments via a distinct catenin complex that contains desmoplakin.1
The layer of mucus that lies on top of the intestinal epithelium is also important for the barrier function of the intestine.9 It helps protect the small intestinal epithelium from the shear flow of materials in the lumen,9 and it creates a barrier between the epithelium and the intestinal microbiota.9 Intestinal mucus is primarily composed of mucin-2, a gel-forming mucin, although it also contains many other mucins.22 These mucins form a dense barrier that is difficult for microbes to penetrate, although some pathogenic microbes, notably Salmonella, have evolved the ability to traverse the mucus layer.23 Furthermore, secretory immunoglobulin A (IgA) produced by B cells in the lamina propria and extruded into the lumen via transcytosis, and antimicrobial peptides, secreted by Paneth cells, reside in the mucus layer and present additional obstacles to luminal microbes.24
Homeostasis
To carry out its nutrient absorption and barrier functions appropriately, the entire intestinal epithelium must renew itself about every 5 days, making it one of the most proliferative tissues in the human body.1 The regeneration process starts at the base of the intestinal crypt, where the Lgr5+ ISCs cycle daily, generating two symmetric daughter cells. These daughter cells compete for space at the base of the crypt: those that remain in the crypt base maintain their stem cell phenotype, while those that are forced out of the stem cell niche become transit-amplifying cells.25
Transit-amplifying cells continue to proliferate as they migrate up the crypt and eventually terminally differentiate into enterocytes, goblet cells, or enteroendocrine cells once they reach the villus.25 Paneth cells, in contrast to the other differentiated IECs, migrate back down after differentiation to assume their position at the crypt base.11 Differentiated IECs are shed from the tip of the villus through anoikis, a type of apoptosis caused by loss of cell anchorage.26
Cellular signaling networks
The homeostasis of the intestinal epithelium is regulated by the interplay of several gene networks that include Wnt, bone morphogenetic protein (BMP), Hedgehog (Hh), and Notch signaling pathways.27,28 Along the crypt-villus axis, Wnt and BMP have an antagonistic relationship. Wnt promotes a proliferative, undifferentiated epithelial phenotype, while BMP drives epithelial differentiation.27 In the crypts, Wnt is provided by the Paneth cells and the sheath of stromal cells that surrounds each intestinal crypt.28 These stromal cells also produce BMP antagonists to further strengthen the Wnt signal in the crypt.28
In the villi, differentiated IECs produce Hh that diffuses to the adjacent mesenchyme and induces BMP expression, which then diffuses back to the IECs and promotes further differentiation.28 Mesenchymal production of Wnt antagonists also drives epithelial differentiation in the villus.29
The Notch signaling pathway is important for lineage specification of differentiated IECs. In the canonical Notch pathway, signals are dependent on cell-to-cell contact and are sent and received by adjacent cells.30 IECs that receive large amounts of Notch signal become enterocytes, while IECs that receive low amounts are driven toward a secretory lineage (i.e., goblet, Paneth, or enteroendocrine cells).30 Atoh-1, which is expressed in ISCs, appears to have a role in directing Notch signaling to the ISCs to maintain these stem cell populations.31 Notch signaling is also necessary to maintain the Notch Delta-like ligands, Dll1 and Dll4, that are expressed by Paneth cells, enteroendocrine cells, and goblet cells.30,32,33
Mechanobiology
The homeostasis of the intestinal epithelium is also impacted by mechanical forces. During fasting, peristaltic muscle contractions travel down the length of the intestines, every 90–120 min.3 After eating, more vigorous patterns of peristaltic waves move down the intestine to mix the contents of the lumen and to propel them toward the end of the GI tract.4 These waves consist of contractions of the longitudinal and circumferential muscle layers of the muscularis externa that are coordinated by the ENS and gap junctions between individual smooth muscle cells.7 Exogenous factors other than food can also influence contraction. For example, murine organoids that were cultured at an air-liquid interface demonstrated an increased contraction rate, due to enhanced oxygen transport.34
In addition to the movement of the intestinal walls, the individual villi also undergo complicated motility patterns.8 There are two main types of villus contractility: piston-like shortening and pendular swaying.8 Both movements are independent of the movement of the intestinal walls, and are caused by bundles of smooth muscle that run from the base to the tip of the villi.8 These bundles surround the villus lacteal and are thought to aid the movement of lymph from the villus tip to larger ducts in the lamina propria.8 Villus contraction is coordinated by ENS neurons from the submucosal plexus.35
Both peristalsis and villus motility expose the intestinal mucosa to a variety of mechanical forces, as summarized in Table 1. These physical inputs are sensed by the cytoskeletons of the IECs and become converted to biochemical signals by a complex and poorly understood process known as mechanotransduction.36 Although the identity of these biochemical signals and their mechanism of action remain mostly unknown, it is clear that mechanical forces alter both epithelial differentiation and proliferation and have a significant impact on intestinal homeostasis.37
Table 1.
Mucosal Deformations Caused by Intestinal Motility
| Action | Force experienced by mucosa |
|---|---|
| Contraction and relaxation of muscularis externa | Longitudinal uniaxial stretching |
| Contraction and relaxation of circular muscularis externa | Compression |
| Villus motility | Radial uniaxial stretching |
| Movement of luminal contents | Shear stress |
Although it is not possible to model simultaneously all of the forces experienced by the intestinal epithelium in vitro, it is possible to recreate each force independently. Uniaxial stretch is the most commonly modeled force and has been shown to affect IEC barrier function by altering tight junction integrity38 and to promote IEC differentiation39,40 and proliferation.41–43 Compressive forces and shear stress have been modeled less frequently, but are important for IEC adhesion44 and proliferation,45 respectively.
Enteric nervous system
The ENS is responsible for regulating intestinal motility, local blood flow, mucosal secretion, endocrine function, immune activity, and transport of fluid and ions in the small intestine and the rest of the digestive system.46 The ENS is capable of both receiving input from the central nervous system (CNS) and functioning autonomously, receiving input from chemical and mechanical stimuli and responding dynamically to food and pathogens.46 The ENS is involved in triggering diarrhea in response to pathogens, such as cholera-toxin and rotavirus.47 Sometimes referred to as the “second brain,” the ENS secretes many of the same neurotransmitters as the CNS.48
Diseases that affect the CNS or peripheral nerves, such as Parkinson's syndrome and diabetes, can also affect the ENS and cause intestinal dysfunction.48 Irritable bowel syndrome and other disorders of GI function are thought to be related to altered serotonin production by the ENS.49 The ENS also communicates with the CNS, playing a significant role in regulating mood and appetite.50
Cell–extracellular matrix interactions
The intestinal basement membrane forms a mesh-like network that physically separates the epithelium from the underlying lamina propria.51 It is composed of a variety of extracellular matrix (ECM) proteins, namely laminins, collagen IV, perlecan, nidogen, and other assorted glycoproteins.51Cells attach to it through both focal adhesions (β1 integrins connected to actinomyosin cytoskeleton) and hemidesmosomes (β4 integrins connected to intermediate filaments).51
Interestingly, both the composition of the ECM and the expression of integrin isoforms varies along the crypt-villus axis. In the crypt, laminin 111 and 211 predominate, while on the villus laminin 322 and 511 are highly expressed.52,53
Similarly, α1β1 and α1β2 integrins, which have a high affinity for the laminin 111 and 211, are highly expressed in human intestinal crypts, while α3β1 and α7β1 integrins, which bind to laminin 322 and 511, are present in the villi.53 The functional significance of these observations has not been confirmed, but it has been demonstrated in vitro that ECM proteins can alter intestinal epithelial differentiation and proliferation.54,55
Furthermore, conditional ablation of laminin 511 alters the murine small intestinal epithelial architecture by blunting and fusing villi.56 Although changes in Wnt and phosphoinositide 3-kinase (PI3K) signaling are seen in these mice,57 the reasons for this conversion are not well understood. Nonetheless, it is likely that the basement membrane sends important signals that help to direct intestinal homeostasis.
Immune activity
Up to 70% of the body's immunocytes reside in gut-associated lymphoid tissue, which partially consists of lymphoid follicles and larger organized lymphoid nodules called Peyer's patches (PP).15 M or microfold cells, which are specialized epithelial cells that overlie PP, have the ability to transport antigens and bacteria from the lumen of the intestine.15 Although PP are critical to mucosal immunity, they can be exploited as a route of entry by viruses, bacteria, and prions.15
In addition to protecting the host from pathogens, mucosal immunity plays a role in inducing antigen tolerance and maintaining the symbiotic relationship with commensal gut bacteria.58 Dendritic cells monitor antigens in the blood and activate mucosal T cells.59 The distinct anatomic regions of the intestine have different distributions of innate and adaptive immune cells; PP, for example, are located in the jejunum and ileum.60,61 Certain inflammatory intestinal diseases affect particular segments of the intestines,17,18 which could be attributed to regional differences in immune cells. In addition to its roles in autoimmune disorders, allergies, and infectious diseases, immune activity in the small intestine also maintains homeostasis in the gut microbiome.62,63
Gut microbiome
The gut microbiome is estimated to consist of more than 1000 types of bacteria.64 These bacteria occupy the loose, superficial mucus layer and the lumen of the intestine.2 The oxygen gradient between the epithelium and the center of the intestinal lumen is steep.2 In fact, some bacteria in the intestine are strict anaerobes; oxygen is toxic to them.2 Some bacterial populations produce antimicrobial compounds or are dependent on nutrient processing by others, forming a complex web of interdependencies.65
The intestinal cells and the gut microbiome communicate bidirectionally.62,64 Signals from the host, notably immune signaling molecules and stress hormones, can regulate bacterial proliferation and gene expression of individual populations.62,63 Bacteria are also influenced by the mechanical and chemical properties of the mucin layer, and thus their populations vary along the length of the intestine.66 The gut microbiome is affected by diet, disease history, age, antibiotics, breastfeeding, and genetics.67–70 In turn, the composition of the gut microbiome can influence host susceptibility to various pathogens and autoimmune diseases.71 The importance of the gut microbiome is increasingly recognized as connections have been found between the microbiome and autism, mental disorders, transplant integration, obesity, allergies, and cancer.72–77
In summary, intestinal homeostasis is a complex phenomenon that depends on many factors: intercellular signaling within the epithelium, paracrine communication between the epithelium and mesenchyme, mechanical stimuli, and ECM cues. Moreover, it is incredibly important in both intestinal physiology and pathology.
Cell Types for Experimental Models
The small intestine is the location of a diverse array of pathologies that include Crohn's disease, infectious diarrhea, and cancer, and all of these pathologies display alterations in intestinal homeostasis.78–80 Despite significant research effort, our knowledge of these diseases is still incomplete, and our ability to treat these conditions is limited. One reason for this is the lack of good experimental models. Many types of infectious diarrhea cannot be modeled in animals, since the causative organisms only affect humans.
While animal models of Crohn's disease and cancer have significantly advanced our understanding of these illnesses, no animal model faithfully recapitulates all features of the disease, making interpretation of experiments challenging.81,82 Furthermore, many treatments that have been identified and developed using these models have not been successful when translated to humans, likely because of interspecies biological differences.81
In vitro models using human cells are an alternative to animal models and have been used for decades. These models have the potential to recapitulate human biology better than animal cells or models do; however, this potential is often countered by the nonphysiological culture conditions in which the cells are grown.
Traditionally, in vitro studies involving human cells have consisted of homogenous monolayers of transformed cells growing on tissue culture polystyrene, where they are separated from the biophysical and biochemical cues that instruct their behavior in the human body.83 However, recent efforts in bioengineering have introduced physiological substrate stiffness, ECM ligands, mechanical forces, and cell–cell communication into in vitro models to make them more representative of the human body.84 Known as “organotypic” culture systems, these technologies have started to address the shortcomings of traditional in vitro cultures, and have already attracted considerable interest as models of human disease and drug toxicity.84–89
Explants
Intestinal explants are sections of intestinal tissue that have been removed from a human or animal to be cultured and manipulated ex vivo.90,91 Since these samples come directly from a living organism, the proportions of cell types, ECM composition, and tissue structure are all physiological, making explants an attractive platform to study complex aspects of intestinal biology, such as inflammatory intestinal diseases, drug toxicity and interactions, and the transport of nutrients, bile-acids, and drugs.91–93 It can be generally challenging to obtain human samples and more or less impossible to control factors that influence intestinal biology, such as diet. Another drawback to using explants is the limited survival time, which is usually around 48 h.94 The Ussing chamber is a popular apparatus for measuring barrier function in explants.95
Other useful systems have been developed to allow coculture of intestinal explants with other cell types, such as hepatocytes, or to introduce luminal flow.96,97 Although the complexity of intestinal explants makes them the most physiological of in vitro models, the inclusion of all of the cell types can make interpretation of experimental results challenging. If a study would benefit from a focus on the epithelium or longer culture time, it can be advantageous to use cells instead of explants.
Transformed cell types
Transformed intestinal cell lines are commercially available and straightforward to culture and are therefore widely used despite their limited physiological relevance. Two popular transformed IECs, Caco-2 and HT-29 cells, are derived from colon cancer lines, but display properties of enterocytes and goblet cells, respectively, when cultured under the correct conditions.98 Although the use of these cell lines is well-established, they differ significantly from the small intestinal epithelium in gene expression, villi formation, and permeability to numerous drugs.99,100 Since most absorption occurs in the small intestine, the relevance of permeability studies that use colon cell lines is debatable. For these reasons, the use of transformed cell lines in models of the small intestinal epithelium has been questioned.
Other cell lines
One popular nontransformed cell line is HIEC-6 (human crypt IECs). Although these crypt-like cells have some of the fat absorbative capabilities of normally differentiated enterocytes,101 they do not differentiate into other types of epithelial cells, limiting their usefulness for studying pathologies that involve multiple epithelial cell types. Although cell lines are often convenient and well characterized, intestinal organoids or enteroids can be better models for studying the diverse cell types that compose the small intestinal epithelium.
Human intestinal organoids
Recently, significant advances in stem cell biology have led to the derivation of intestinal tissue from pluripotent stem cells (PSCs) in vitro. Known as human intestinal organoids (HIOs), these spheroids consist of an epithelial layer that is surrounded by a mesenchymal layer containing fibroblasts and smooth muscle cells. Although the epithelium contains both differentiated and undifferentiated regions, it more closely resembles the epithelium of the fetal intestine.102
When implanted in a murine kidney capsule, HIOs become more differentiated and gain distinct crypt and villus architecture, as well as muscularis externae.103 However, this process is lengthy and inconvenient, and their three-dimensional (3D) spherical architecture makes it quite difficult to perform common in vitro procedures, such as measuring apical-basal transport and epithelial permeability, or adding commensal and pathogenic microorganisms to the apical epithelium. Moreover, HIOs in vitro do not contain some of the normal components of the intestinal lamina propria, including vascular cells.104
Human intestinal enteroids
The discovery of Lgr5 as a marker of the ISC,10 as well as the elucidation of the various niche factors required to maintain them, allowed cultivation of polarized, functional units of human intestinal epithelium that contain every major cell type along the crypt-villus axis.105 Small intestinal crypts are isolated from intestinal tissues left over from surgeries and endoscopic biopsies by washing with EDTA, and these crypts are then grown in Matrigel, a poorly defined ECM material derived from mouse sarcoma cells, where they form budding cystic structures.106 These structures are referred to as adult stem cell-derived HIOs or “enteroids” to distinguish them from PSC-derived HIOs, which include mesenchyme.
Unlike the transformed cell lines, human intestinal enteroids (HIEs) recapitulate the natural cell differentiation process and physiology of the small intestine.107,108 When grown in the presence of Wnt and other growth factors that are found in the ISC niche in vivo, HIEs are predominantly composed of high numbers of proliferating cells and can be considered to be “crypt-like.” When these growth factors are withdrawn, the crypt cells within HIEs differentiate, producing epithelial cultures with abundant enterocytes, goblet cells, and enteroendocrine cells and can be considered to be “villus-like.”108 These two types of HIEs can be used to model the physiology of the two compartments (i.e., crypt and villus) of the intestinal epithelium.
Unlike HIOs, HIEs lack any cell types native to the lamina propria.109 However, HIEs have the distinct advantage of being relatively easy to manipulate, as they are only composed of a single layer of epithelium.109 Techniques have been developed to coculture HIEs with other cell types, such as T cells, macrophages, dendritic cells, and subepithelial myofibroblasts,110–114 and to create HIE monolayers from enzymatically digested 3D cultures.31 These abilities have facilitated the incorporation of HIEs into bioengineered, organotypic intestinal models, which are better able to model the intestinal epithelium.
Bioengineered models of HIEs
Normal HIE culture requires the exogenous administration of numerous growth factors that are normally supplied by the intestinal mesenchyme.106 Attempts to coculture HIEs with primary stromal cells to administer those growth factors endogenously have met with mixed success. Mesenchymal tissue from adult humans and mice is unable to sustain HIE culture, but tissue from human infants permits HIE culture for at least 56 weeks.111
RNA sequencing from supportive and nonsupportive mesenchymal cells demonstrated significant differences in expression levels of secreted proteins in the Wnt pathway.112 However, the human mesenchymal cells used in this study demonstrated low levels of α-smooth muscle actin (α-SMA) expression,111 suggesting that the mesenchymal cells used in this study may differ from the intestinal subepithelial myofibroblasts that express high levels of α-SMA and surround the crypt base in vivo.115
Expanding on work performed with Caco-2 cells, HIEs have been grown on topographically patterned surfaces. In one study, colonic HIEs (known as colonoids) were suspended in Matrigel in arrays of polydimethylsiloxane microwells.116 As the colonoids grew, they formed an organized architecture reminiscent of the structure of the colonic epithelium, with proliferative crypt domains located in the recessed microwells and nonproliferative domains found on the flat regions between and above the microwells.116 In a separate study, Caco-2 and HT29-MTX cells were grown on porous poly(lactic-co-glycolic acid) villus scaffolds and displayed differentiation along the villi and migration of cells up from the crypts.117 Although this result is promising, it did not demonstrate the full crypt-villus axis, including enterocytes and enteroendocrine cells.
Bioengineered In Vitro Models
Coculture models
Early bioengineering efforts at constructing organotypic models of the intestinal mucosa consisted primarily of coculturing immune or mesenchymal cells with transformed IECs on collagen gels on top of porous Transwell membranes.114,118–120 These models demonstrated that IEC monolayers on top of hydrogels could engage in paracrine communication with cells embedded within the hydrogel bulk. Primary IECs have also been cocultured with microvascular cells on porous membranes within microfluidic chips and on Transwell membranes.100 In coculture with microvascular cells, intestinal epithelial monolayers show decreased permeability and increased mucin production. These models have been used to model drug absorption and a variety of GI pathologies.114,120
Bioreactors
There is an unmet need for bioreactors to scale up intestinal cell culture. HIEs are currently grown in 3D in Matrigel, a culture method that is labor intensive and expensive. If HIEs, HIOs, or other intestinal cells are ever to be used for cellular therapy or tissue engineering applications, there needs to be a method to grow them in large numbers and without the undefined animal factors found in Matrigel and other natural scaffolds. Plurioptent stem cell-derived intestinal organoids have been successfully cultured using alginate, poly(ethylene glycol) conjugated to an RGD peptide (PEG-RGD), or four-arm poly(ethylene glycol) macromer (PEG-4MAL) as the 3D scaffold,121–123 but HIEs do not grow in alginate.
Besides being able to expand intestinal cell numbers, the use of larger bioreactors has also been explored to expose intestinal cell cultures to mechanical stimulation. In addition to studies involving cyclic stretch, complex physical forces have been applied to INT-407 cells in rotating wall vessels. In these devices, cells cultured on porous microbeads coated with collagen type I are added to a sterile cylinder, which is then filled with media and axially rotated, causing the beads to tumble against the other microbeads and the cylinder walls.87 Although the nature of the physical forces experienced by the cells is difficult to characterize, it likely involves a combination of compression and shear stress.
IECs cultured in these bioreactors exhibit increased polarization and junctional complex expression and increased expression of surface markers that support the infection and replication of pathogenic bacteria. The results from infections with cells in these bioreactors and microfluidic devices suggest that physical forces affect host–pathogen interactions, and that static culture conditions might not accurately recapitulate the in vivo relationship between the intestinal epithelium and the intestinal microbiome.87 These studies clearly provided a significant foundation for applying bioengineering technology to in vitro intestinal models, even though their use of transformed IECs, such as Caco-2 and HT-29 cells, limits the physiological relevance of those models in particular.
A different type of bioreactor was used to house full thickness intestinal biopsies and perfuse media over the luminal and serosal sides, maintaining viability for up to 72 h.124 Although this type of ex vivo device may aid in studying certain diseases, the complexity of full thickness intestinal tissue complicates the analysis of experimental results and makes certain mechanisms difficult to identify.
Continuous-flow bioreactors show promise for culturing the complete gut microbiome. In these bioreactors, populations of bacteria are maintained by introducing nutrients and removing waste continuously. A continuous-flow anaerobic bioreactor has enabled the in vitro culture of a large part of the diverse population of gut microbiota.125 The populations in these bioreactors often shift over time and change significantly in response to the nutrient composition of the media. Since communication between the IECs and the gut microbiome helps regulate bacterial populations, incorporating IECs into bioreactors may help maintain physiologically balanced bacterial populations. Additional innovations in the way samples are collected, stored, and cultured would help preserve the entirety of both the aerobic and strict anaerobic bacterial populations.
Microfabrication
More recent attempts to model the IEC microenvironment have applied microfabrication techniques, such as photolithography, to generate arrays of pillars and microwells to recapitulate crypt-villus architecture (Fig. 3). When cultured on these topographical surfaces, Caco-2 monolayers demonstrated increased metabolic activity, decreased expression of brush border enzymes, and lower transepithelial resistance (TER) values compared to those grown on flat surfaces.126,127
FIG. 3.
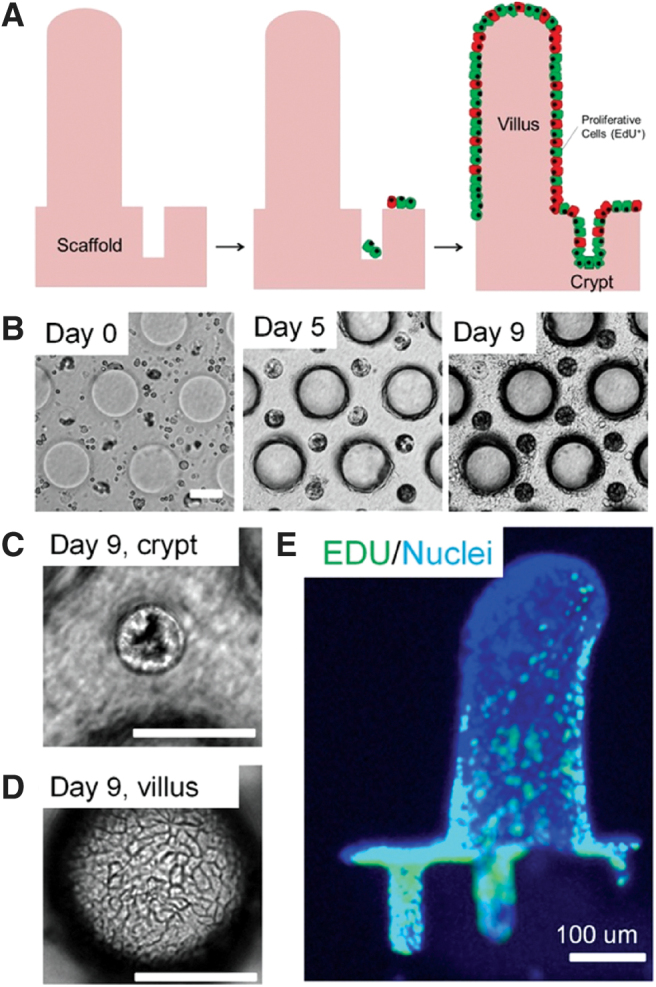
(A–E) Small intestinal cells growing on microfabricated crypt-villus structures. Reproduced with permission from Wang et al.128 Copyright 2017 Elsevier Ltd. Color images are available online.
Other groups have generated villus-like structures by laser-ablating 500 μm depressions into a plastic block and using a series of molding steps to generate an array of conical pillars.128 To preserve the delicate villus structures, alginate has been used as a dissolvable mold.129 These arrays of villi have been made out of several materials, including collagen gels, poly(ethylene glycol) diacrylate (PEG-DA), and porous poly(lactide-co-glycolide) membranes.116,128–130 IECs seeded on these scaffolds demonstrated gradients of differentiation along the height of the villus, with poorly differentiated, unpolarized cells located in the intervillus regions and differentiated polarized cells located on the villi. TER values of monolayers cultured on these 3D villi were shown to be lower than those from monolayers cultured on flat surfaces, and they exhibit increased permeability to drugs.
These villus-like structures have also been incorporated into well-plate inserts for transport studies131 and into microfluidic devices.131–133 However, micropatterned villus structures have not been incorporated into devices that direct flow over the epithelium or cocultured with vascular, lymphatic, or neural cells under flow.
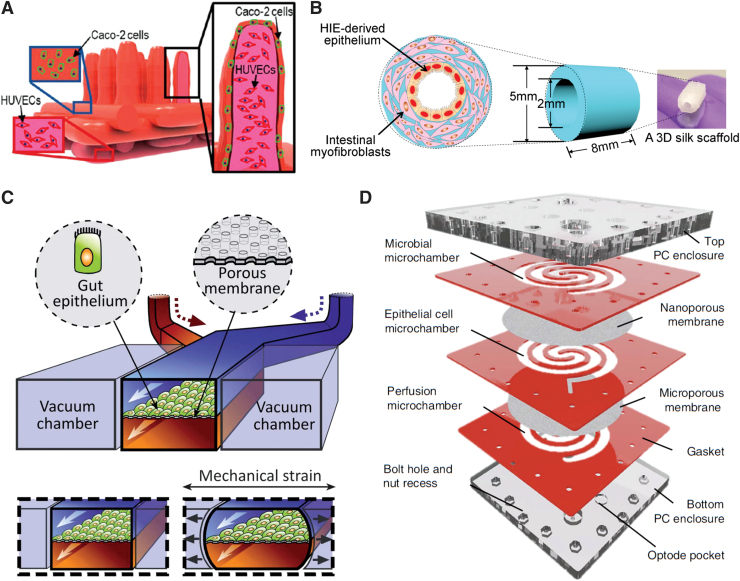
In addition to these indirect fabrication methods, villi also have been directly fabricated with 3D bioprinting (Fig. 4A).134 By coaxially extruding two collagen-based bioinks containing a suspension of human umbilical vein endothelial cells (HUVECs) and Caco-2 cells, the Kim group was able to fabricate exceptionally tall and narrow villi, matching physiological aspect ratios more closely than any of the molding attempts. The HUVECs formed capillaries within the villi and encouraged higher cell growth, glucose uptake, and mucin expression.
FIG. 4.
(A) Three-dimensional printed villi in collagen using Caco-2 cells with HUVECs in the mesenchyme. Adapted and reprinted with permission from Kim and Kim.134 Copyright 2018 American Chemical Society. (B) Tube-shaped silk scaffold model containing intestinal myofibroblasts in the bulk gel and HIE-derived epithelial monolayer on the inner surface. Adapted and reproduced with permission from Chen et al.135 Copyright 2017 PLoS One. (C) Gut-on-a-chip device featuring two perfusable channels separated an epithelial monolayer on a permeable membrane that can be stretched by pressurizing adjacent chambers. Adapted and reproduced with permission from Kim and Ingber.138 Copyright 2013 Royal Society of Chemistry. (D) Human-microbial crosstalk (HuMiX) system for culturing aerobic and anaerobic bacteria in close proximity to epithelial monolayers and studying human–microbe interactions. Adapted and reproduced with permission from Shah et al.142 Copyright 2018 Nature. HIE, human intestinal enteroids; HUVEC, human umbilical vein endothelial cells. Color images are available online.
Cylindrical fabrication
The aforementioned modeling approaches have endeavored to recapitulate 3D intestinal crypt-villus microarchitecture, but the resulting structures were nonetheless planar at the mesoscale. This planar setup greatly simplifies handling, fabrication, and imaging. However, one group created a tubular model of the small intestine, more representative of physiological geometry (Fig. 4B). They seeded patient-derived intestinal cells within 2 mm cylindrical channels in a biocompatible silk matrix with intestinal myofibroblasts in the bulk.135,136 Their innovative approach also incorporated ridged channels, which contributed to patterned cell migration. Due to high cell density, this model developed a proximal to distal oxygen gradient. The cells grown in these tubes produced more mucus than those grown on Transwell membranes.136
The utility of this model was limited by the high opacity of the silk matrix, which makes it impossible to perform live cell imaging, although the cells could be imaged by fixing and cutting the scaffold to lay the cell layer flat. From a tissue engineering perspective, however, the use of the durable and easily sutured silk material brings us closer to generating segments of intestine for transplant. Culturing HIE monolayers within tubes also addresses a limitation that has been largely neglected by other investigators: after about 2 weeks in planar culture, IEC monolayers begin to curl up and detach from the substrate, precluding longer studies.137 Monolayers within tubes remain attached for more than 8 weeks, making longer term studies possible.135
Microfluidic devices
Other bioengineering efforts have led to the development of microfluidic “gut-on-a-chip” platforms. The most common design for these microfluidic devices involves two chambers separated by a porous membrane, which supports a monolayer of IECs. As with Transwell, this design provides access to the apical and basolateral sides of the monolayer, allowing epithelial barrier function and transport to be measured.
There are several variations on this central design. One such variation includes empty chambers on either side of the membrane, allowing stretch to be administered to the cells by applying vacuum to the empty chambers (Fig. 4C).138 Both chambers of this design are perfusable, which allows shear stress to be applied to the apical and basolateral sides of the membrane and permeability to be measured over time. Commensal bacteria can be added to the apical channel to simulate the effects of the gut microbiome.139
Villi-like structures have formed spontaneously in these devices. Although these structures were only as large as 300 μm and appear to be structured more like ridges than true villi, even this level of self-assembly highlights the importance of signaling gradients in the self-organization of the intestinal epithelium. This model of intestinal epithelium was also cocultured with a monolayer of endothelial cells on the opposite side of the porous membrane.140 The intestinal vasculature is a critical part of the gut-blood barrier and therefore especially important to include in studies of transport and pathology. Using this platform to study radiation damage, the presence of endothelial cells was found to exacerbate damage to the intestinal epithelium.140
Other similar microfluidic designs do not include stretch or allow simultaneous flow over both sides of the membrane, but have made significant innovations to other design aspects.132,133,141 One model was designed to be integrated into a body-on-a-chip device. The intestine and liver components were connected and used to assess hepatotoxicity of ingested nanoparticles.132 There is a need for more multiorgan in vitro studies involving the intestine and other organs, especially the liver. Two other models have made significant improvements to the membrane itself. One design used a collagen membrane141 and the other patterned villi in collagen atop the polyester (PET) membrane.133 In the future, these innovations in micropatterning and the membrane material could be combined with stretch and flow elements from other devices.
One especially innovative device was designed specifically to study the crosstalk between the gut microbiome and the intestinal epithelium (Fig. 4D).142 This system featured three chambers separated by permeable membranes with the long channels arranged in a swirled pattern for convenience of handling. This design allowed physiological oxygen gradients to be established and supported simultaneous culture of aerobic and obligate anaerobic bacterial.
Other devices have also established coculture of anaerobic bacteria and epithelial monolayers, including a microfluidic device143 and a system that establishes an oxygen gradient across each Transwell in an array.144 These devices share this design element of two chambers separated by a porous membrane, because most intestinal device development has been focused on transport of nutrients, drugs, and infectious agents between the apical and basolateral sides of the intestinal epithelium. Further design innovations to recapitulate physiological microstructure, signaling gradients, and mechanical stimuli could advance research in other areas such as the gut microbiome, wound healing, tissue engineering, and chronic inflammatory conditions.
Applying existing practical improvements to the microfluidic devices that have been designed for intestinal research will substantially improve their ease of use and broader translation. To tackle leaks and clogs, it would be worthwhile to incorporate bubble traps, on-chip pumps, valves, and built-in sensors; these are just a few examples of improvements that could be applied to microfluidics for intestinal research.145–150
The small size of microfluidic devices can also exacerbate difficulties in handling. For applications where neither the cells nor the reagents are especially expensive or rare, larger devices could be used. Larger devices also make it easier to co-culture intestinal cells with other cell types and bacteria and to establish oxygen gradients.144 Moreover, in cases where the component of interest is produced in small quantities, having a larger device that contains more cells could be a useful strategy.
An ideal platform for studying a broad range of intestinal pathologies would recapitulate the physiological aspects most relevant to intestinal barrier function. A device that provides access to the basolateral and apical sides of a human, polarized epithelial monolayer fulfils the basic requirements. Crypt-villus structure, signaling gradients, and mechanical stimulation would make the model more physiological. An ideal platform would also facilitate coculture with other cell types relevant to disease, including vascular, nerve, and immune cells as well as bacteria. Mimicking the physiological oxygen gradient and the anaerobic environment of the lumen are crucial to incorporating the gut microbiome.
A practical feature of an ideal device would be large size for easy handling and to provide plenty of biological material for analysis. A device that is easy to image and able to be deconstructed for sectioning would enable morphological studies and localization of pathogens. Other convenient features would be embedded sensors to measure transepithelial electrical resistance, mechanical actuators, and on-chip pumps.
Conclusion
The challenges in engineering models of the small intestine arise from the complexity of this organ and our lack of knowledge about the potential interdependencies between numerous aspects of intestinal physiology. As a rule, in vitro models of human tissues attempt to strike a balance between physiological complexity and experimental simplicity. Innovations in primary intestinal cell culture, biomaterials, chip-based microfluidic devices, microfabrication, and 3D printing have contributed substantially to the development of more mechanically faithful intestinal cell culture models. Given the intricate nature of this organ, future models will need to move even further toward physiological complexity to generate more accurate results.
Acknowledgments
The authors thank Dr. Jennifer Connell for help with editing this review.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was funded by National Institutes of Health (U19 AI116497).
References
- 1. Shroyer N.F., and Kocoshis S.A.. Anatomy and physiology of the small and large intestines. In: Mahajan, L.A., Kaplan, B., Mahajan, L.A., and Kaplan, B., eds. Pediatr Gastrointest Liver Dis. Philadelphia, PA: Saunders Elsevier, 2011, pp. 324–336.e2 [Google Scholar]
- 2. Zheng L., Kelly C.J., and Colgan S.P.. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol 309, C350, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takahashi T. Mechanism of interdigestive migrating motor complex. J Neurogastroenterol Motil 18, 246, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Otterson M.F., and Sarr M.G.. Normal physiology of small intestinal motility. Surg Clin North Am 73, 1173, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Hodde J.P., Badylak S.F., Brightman A.O., and Voytik-Harbin S.L.. Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng 2, 209, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Komuro T., and Hashimoto Y.. Three-dimensional structure of the rat intestinal wall (mucosa and submucosa). Arch Histol Cytol 53, 1, 1990 [DOI] [PubMed] [Google Scholar]
- 7. Furness J.B. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9, 286, 2012 [DOI] [PubMed] [Google Scholar]
- 8. Womack W.A., Kvietys P.R., and Granger N. Villous motility. Compr Physiol 975, 2011 [Google Scholar]
- 9. Nordgård C.T., and Draget K.I.. Dynamic responses in small intestinal mucus: relevance for the maintenance of an intact barrier. Eur J Pharm Biopharm 95(Pt A), 144, 2015 [DOI] [PubMed] [Google Scholar]
- 10. Barker N., van Es J.H., Kuipers J., et al. . Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Clevers H.C., and Bevins C.L.. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol 75, 289, 2013 [DOI] [PubMed] [Google Scholar]
- 12. Shaker A., and Rubin D.C.. Intestinal stem cells and epithelial-mesenchymal interactions in the crypt and stem cell niche. Transl Res 156, 180, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Furness J.B., Rivera L.R., Cho H.-J., Bravo D.M., and Callaghan B.. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol 10, 729, 2013 [DOI] [PubMed] [Google Scholar]
- 14. Howitt M.R., Lavoie S., Michaud M., et al. . Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351, 1329, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jung C., Hugot J.-P., and Barreau F.. Peyer's patches: the immune sensors of the intestine. Int J Inflamm 2010, 823710, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Collins J.T., and Badireddy M. Anatomy, abdomen and pelvis, small intestine. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing, 2019. Available at http://www.ncbi.nlm.nih.gov/books/NBK459366/ (accessed January21, 2020) [PubMed]
- 17. Freeman H.J. Pearls and pitfalls in the diagnosis of adult celiac disease. Can J Gastroenterol 22, 273, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caprilli R. Why does Crohn's disease usually occur in terminal ileum? J Crohns Colitis 2, 352, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Rhodes J.M. The role of Escherichia coli in inflammatory bowel disease. Gut 56, 610, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fromm M., and Schulzke J.D.. Molecular structure and function of the tight junction. Ann N Y Acad Sci 1165, 1, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol 2, 14, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim Y.S., and Ho S.B.. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 12, 319, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hansson G.C. Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol 15, 57, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dharmani P., Srivastava V., Kissoon-Singh V., and Chadee K.. Role of intestinal mucins in innate host defense mechanisms against pathogens. J Innate Immun 1, 123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van der Flier L.G., and Clevers H.C.. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71, 241, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Günther C., Neumann H., Neurath M.F., and Becker C.. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut 62, 1062, 2013 [DOI] [PubMed] [Google Scholar]
- 27. Noah T.K., Donahue B., and Shroyer N.F.. Intestinal development and differentiation. Exp Cell Res 317, 2702, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Powell D.W., Pinchuk V.I., Saada J.I., Chen X., and Mifflin R.C.. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol 73, 213, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gracz A.D., and Magness S.T.. Defining hierarchies of stemness in the intestine: evidence from biomarkers and regulatory pathways. Am J Physiol Gastrointest Liver Physiol 307, G260, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Noah T.K., and Shroyer N.F.. Notch in the intestine: regulation of homeostasis and pathogenesis. Annu Rev Physiol 75, 263, 2013 [DOI] [PubMed] [Google Scholar]
- 31. VanDussen K.L., Marinshaw J.M., Shaikh N., et al. . Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 64, 911, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pellegrinet L., Rodilla V., Liu Z., et al. . Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 140, 1230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stamataki D., Holder M., Hodgetts C., et al. . Delta1 expression, cell cycle exit, and commitment to a specific secretory fate coincide within a few hours in the mouse intestinal stem cell system. PLoS One 6, e24484, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. DiMarco R.L., Su J., Yan K.S., Dewi R., Kuo C.J., and Heilshorn S.C.. Engineering of three-dimensional microenvironments to promote contractile behavior in primary intestinal organoids. Integr Biol 6, 127, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. See N.A., Greenwood B., and Bass P.. Submucosal plexus alone integrates motor activity and epithelial transport in rat jejunum. Am J Physiol 259, G593, 1990 [DOI] [PubMed] [Google Scholar]
- 36. Eyckmans J., Boudou T., Yu X., and Chen C.S.. A hitchhiker's guide to mechanobiology. Dev Cell 21, 35, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gayer C.P., and Basson M.D.. The effects of mechanical forces on intestinal physiology and pathology. Cell Signal 21, 1237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Samak G., Gangwar R., Crosby L.M., et al. . Cyclic stretch disrupts apical junctional complexes in Caco-2 cell monolayers by a JNK-2-, c-Src-, and MLCK-dependent mechanism. AJP Gastrointest Liver Physiol 306, G947, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Basson M.D., Li G.D., Hong F., Han O., and Sumpio B.E.. Amplitude-dependent modulation of brush border enzymes and proliferation by cyclic strain in human intestinal Caco-2 monolayers. J Cell Physiol 168, 476, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Basson M.D. Effects of repetitive deformation on intestinal epithelial cells. Inflammopharmacology 15, 109, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Gayer C.P., Craig D.H., Flanigan T.L., Reed T.D., Cress D.E., and Basson M.D.. ERK regulates strain-induced migration and proliferation from different subcellular locations. J Cell Biochem 109, 711, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Li W., Duzgun A, Sumpio B.E., and Basson M.D.. Integrin and FAK-mediated MAPK activation is required for cyclic strain mitogenic effects in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol 280, G75, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Gayer C.P., Chaturvedi L.S., Wang S., Craig D.H., Flanigan T.L., and Basson M.D.. Strain-induced proliferation requires the phosphatidylinositol 3-kinase/AKT/glycogen synthase kinase pathway. J Biol Chem 284, 2001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thamilselvan V., and Basson M.D.. Pressure activates colon cancer cell adhesion by inside-out focal adhesion complex and actin cytoskeletal signaling. Gastroenterology 126, 8, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Balcells M., Fernández Suárez M., Vázquez M., and Edelman E.R.. Cells in fluidic environments are sensitive to flow frequency. J Cell Physiol 204, 329, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Costa M., Brookes S.J.H., and Hennig G.W.. Anatomy and physiology of the enteric nervous system. Gut 47, iv15, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lundgren O., and Svensson L.. I, 3. The enteric nervous system and infectious diarrhea. Perspect Med Virol 9, 51, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nezami B.G., and Srinivasan S.. Enteric nervous system in the small intestine: pathophysiology and clinical implications. Curr Gastroenterol Rep 12, 358, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Crowell M.D. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol 141, 1285, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mayer E.A. Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci 12, 453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Simon-Assmann P., Spenlé C., Lefebvre O., and Kedinger M.. The role of the basement membrane as a modulator of intestinal epithelial-mesenchymal interactions. Prog Mol Biol Transl Sci 96, 175, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Teller I.C., Auclair J., Herring E., Gauthier R., Ménard D., and Beaulieu J.-F.. Laminins in the developing and adult human small intestine: relation with the functional absorptive unit. Dev Dyn Off Publ Am Assoc Anat 236, 1980, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Beaulieu. J.F. Extracellular matrix components and integrins in relationship to human intestinal epithelial cell differentiation. Prog Histochem Cytochem 31, III–76, 1997 [DOI] [PubMed] [Google Scholar]
- 54. Hahn U., Stallmach A., Hahn E.G., and Riecken E.O.. Basement membrane components are potent promoters of rat intestinal epithelial cell differentiation in vitro. Gastroenterology 98, 322, 1990 [DOI] [PubMed] [Google Scholar]
- 55. Basson M.D., Turowski G., and Emenaker N.J.. Regulation of human (Caco-2) intestinal epithelial cell differentiation by extracellular matrix proteins. Exp Cell Res 225, 301, 1996 [DOI] [PubMed] [Google Scholar]
- 56. Mahoney Z.X., Stappenbeck T.S., and Miner J.H.. Laminin alpha 5 influences the architecture of the mouse small intestine mucosa. J Cell Sci 121, 2493, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ritié L., Spenlé C., Lacroute J., et al. . Abnormal Wnt and PI3Kinase signaling in the malformed intestine of lama5 deficient mice. PLoS One 7, e37710, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yap Y.A., and Mariño E.. An insight into the intestinal web of mucosal immunity, microbiota, and diet in inflammation. Front Immunol 9, 2617, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chang S.-Y., Song J.-H., Guleng B., et al. . Circulatory antigen processing by mucosal dendritic cells controls CD8+ T cell activation. Immunity 38, 153, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mowat A.M., and Agace W.W.. Regional specialization within the intestinal immune system. Nat Rev Immunol 14, 667, 2014 [DOI] [PubMed] [Google Scholar]
- 61. Mutwiri G., Watts T., Lew L., et al. . Ileal and jejunal Peyer's patches play distinct roles in mucosal immunity of sheep. Immunology 97, 455, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Freestone P. Communication between bacteria and their hosts. Scientifica 2013, 361073, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mahdavi J., Royer P.-J., Sjölinder H.S., et al. . Pro-inflammatory cytokines can act as intracellular modulators of commensal bacterial virulence. Open Biol 3, 130048, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Keely S.J. Decoding host–microbiota communication in the gut—now we're flying! J Physiol 595, 417, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Foster J.A., Krone S.M., and Forney L.J.. Application of ecological network theory to the human microbiome. Interdiscip Perspect Infect Dis 2008, 839501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Eckburg P.B., Bik E.M., Bernstein C.N., et al. . Diversity of the human intestinal microbial flora. Science 308, 1635, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nistal E., Caminero A., Herrán A.R., et al. . Differences of small intestinal bacteria populations in adults and children with/without celiac disease: effect of age, gluten diet, and disease. Inflamm Bowel Dis 18, 649, 2012 [DOI] [PubMed] [Google Scholar]
- 68. Goodrich J.K., Waters J.L., Poole A.C., et al. . Human genetics shape the gut microbiome. Cell 159, 789, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. O'Sullivan A., Farver M., and Smilowitz J.T.. The influence of early infant-feeding practices on the intestinal microbiome and body composition in infants. Nutr Metab Insights 8, 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dudek-Wicher R.K., Junka A., and Bartoszewicz M.. The influence of antibiotics and dietary components on gut microbiota. Gastroenterol Rev 13, 85, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Round J.L., and Mazmanian S.K.. The gut microbiome shapes intestinal immune responses during health and disease. Nat Rev Immunol 9, 313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Parekh P.J., Balart L.A., and Johnson D.A.. The influence of the gut microbiome on obesity, metabolic syndrome and gastrointestinal disease. Clin Transl Gastroenterol 6, e91, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gopalakrishnan V., Helmink B.A., Spencer C.N., Reuben A., and Wargo J.A.. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell 33, 570, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Riiser A. The human microbiome, asthma, and allergy. Allergy Asthma Clin Immunol 11, 35, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li Q., Han Y., Dy A.B.C., and Hagerman R.J.. The gut microbiota and autism spectrum disorders. Front Cell Neurosci 11, 120, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mathewson N.D., Jenq R., Mathew A.V., et al. . Corrigendum: gut microbiome–derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol 17, 1235, 2016 [DOI] [PubMed] [Google Scholar]
- 77. Lach G., Schellekens H., Dinan T.G., and Cryan J.F.. Anxiety, depression, and the microbiome: a role for gut peptides. Neurother J Am Soc Exp Neurother 15, 36, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Foulke-Abel J., In J., Kovbasnjuk O., et al. . Human enteroids as an ex-vivo model of host-pathogen interactions in the gastrointestinal tract. Exp Biol Med 239, 1124, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Coskun M. Intestinal epithelium in inflammatory bowel disease. Front Med 1, 1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vermeulen L., and Snippert H.J.. Stem cell dynamics in homeostasis and cancer of the intestine. Nat Rev Cancer 14, 468, 2014 [DOI] [PubMed] [Google Scholar]
- 81. Goyal N., Rana A., Ahlawat A., Bijjem V.K.R., and Kumar P.. Animal models of inflammatory bowel disease: a review. Inflammopharmacology 22, 219, 2014 [DOI] [PubMed] [Google Scholar]
- 82. Johnson R.L., and Fleet J.C.. Animal models of colorectal cancer. Cancer Metastasis Rev 32, 39, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wikswo J.P. The relevance and potential roles of microphysiological systems in biology and medicine. Exp Biol Med 239, 1061, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shamir E.R., and Ewald A.J.. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol 15, 647, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yu J., Carrier R.L., March J.C., and Griffith L.G.. Three dimensional human small intestine models for ADME-Tox studies. Drug Discov Today 19, 1587, 2014 [DOI] [PubMed] [Google Scholar]
- 86. Hartman K.G., Bortner J.D., Falk G.W., et al. . Modeling inflammation and oxidative stress in gastrointestinal disease development using novel organotypic culture systems. Stem Cell Res Ther 4 Suppl 1, S5, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Barrila J., Radtke A.L., Crabbé A., et al. . Organotypic 3D cell culture models: using the rotating wall vessel to study host-pathogen interactions. Nat Rev Microbiol 8, 791, 2010 [DOI] [PubMed] [Google Scholar]
- 88. Gjorevski N., Ranga A., and Lutolf M.P.. Bioengineering approaches to guide stem cell-based organogenesis. Development 141, 1794, 2014 [DOI] [PubMed] [Google Scholar]
- 89. Benam K.H., Dauth S., Hassell B., et al. . Engineered in vitro disease models. Annu Rev Pathol Mech Dis 10, 195, 2015 [DOI] [PubMed] [Google Scholar]
- 90. Kothari A., and Rajagopalan P.. Isolating rat intestinal explants for in vitro cultures. Curr Protoc Toxicol 80, e79, 2019 [DOI] [PubMed] [Google Scholar]
- 91. Randall K.J., Turton J., and Foster J.R.. Explant culture of gastrointestinal tissue: a review of methods and applications. Cell Biol Toxicol 27, 267, 2011 [DOI] [PubMed] [Google Scholar]
- 92. Li M., de Graaf I.A.M., van de Steeg E., de Jager M.H., and Groothuis G.M.M.. The consequence of regional gradients of P-gp and CYP3A4 for drug-drug interactions by P-gp inhibitors and the P-gp/CYP3A4 interplay in the human intestine ex vivo. Toxicol In Vitro 40, 26, 2017 [DOI] [PubMed] [Google Scholar]
- 93. Li M., Vokral I., Evers B., de Graaf I.A.M., de Jager M.H., and Groothuis G.M.M.. Human and rat precision-cut intestinal slices as ex vivo models to study bile acid uptake by the apical sodium-dependent bile acid transporter. Eur J Pharm Sci 121, 65, 2018 [DOI] [PubMed] [Google Scholar]
- 94. Maresca M., Pinton P., Ajandouz E.H., Menard S., Ferrier L., and Oswald I.P.. Overview and comparison of intestinal organotypic models, intestinal cells, and intestinal explants used for toxicity studies. Curr Top Microbiol Immunol 2018. [Epub ahead of print]; DOI: 10.1007/82_2018_142 [DOI] [PubMed]
- 95. Thomson A., Smart K., Somerville M.S., et al. . The Ussing chamber system for measuring intestinal permeability in health and disease. BMC Gastroenterol 19, 98, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kothari A., and Rajagopalan P.. The assembly of integrated rat intestinal-hepatocyte cultures. Bioeng Transl Med 5, e10146, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yissachar N., Zhou Y., Ung L., et al. . An intestinal organ culture system uncovers a role for the nervous system in microbe-immune crosstalk. Cell 168, 1135, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Simon-Assmann P., Turck N., Sidhoum-Jenny M., Gradwohl G., and Kedinger M.. In vitro models of intestinal epithelial cell differentiation. Cell Biol Toxicol 23, 241, 2007 [DOI] [PubMed] [Google Scholar]
- 99. Sun D., Lennernas H., Welage L.S., et al. . Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequences tags and correlation with permeability of 26 drugs. Pharm Res 19, 1400, 2002 [DOI] [PubMed] [Google Scholar]
- 100. Kasendra M., Tovaglieri A., Sontheimer-Phelps A., et al. . Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci Rep 8, 2871, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Beauséjour M., Noël D., Thibodeau S., et al. . Integrin/Fak/Src-mediated regulation of cell survival and anoikis in human intestinal epithelial crypt cells: selective engagement and roles of PI3-K isoform complexes. Apoptosis 17, 566, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Spence J.R., Mayhew C.N., Rankin S.A, et al. . Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Watson C.L., Mahe M.M., Múnera J., et al. . An in vivo model of human small intestine using pluripotent stem cells. Nat Med 20, 1310, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Brugmann S.A., and Wells J.M.. Building additional complexity to in vitro-derived intestinal tissues. Stem Cell Res Ther 4 Suppl 1, S1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sato T., Vries R.G.J., Snippert H.J., et al. . Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262, 2009 [DOI] [PubMed] [Google Scholar]
- 106. Sato T., Stange D.E., Ferrante M., et al. . Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141, 1762, 2011 [DOI] [PubMed] [Google Scholar]
- 107. Kovbasnjuk O., Zachos N.C., In J., et al. . Human enteroids: preclinical models of non-inflammatory diarrhea. Stem Cell Res Ther 4, S3, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sato T., and Clevers H.C.. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340, 1190, 2013 [DOI] [PubMed] [Google Scholar]
- 109. Mahe M.M., Sundaram N., Watson C.L., Shroyer N.F., and Helmrath M.A.. Establishment of human epithelial enteroids and colonoids from whole tissue and biopsy. J Vis Exp 2015; DOI: 10.3791/52483 [DOI] [PMC free article] [PubMed]
- 110. Rogoz A., Reis B.S., Karssemeijer R.A., and Mucida D.. A 3-D enteroid-based model to study T-cell and epithelial cell interaction. J Immunol Methods 421, 89, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lahar N., Lei N.Y., Wang J., et al. . Intestinal subepithelial myofibroblasts support in vitro and in vivo growth of human small intestinal epithelium. PLoS One 6, e26898, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lei N.Y., Jabaji Z., Wang J., et al. . Intestinal subepithelial myofibroblasts support the growth of intestinal epithelial stem cells. PLoS One 9, e84651, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Noel G., Baetz N.W., Staab J.F., et al. . A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci Rep 7, 45270, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Leonard F., Collnot E.-M., and Lehr C.-M.. A 3-dimensional co-culture of enterocytes, macrophages and dendritic cells to model the inflamed intestinal mucosa in vitro. Mol Pharm 7, 2103, 2010 [DOI] [PubMed] [Google Scholar]
- 115. Pinchuk V.I., Mifflin R.C., Saada J.I., and Powell D.W.. Intestinal mesenchymal cells. Curr Gastroenterol Rep 12, 310, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang Y., Ahmad A.A., Sims C.E., Magness S.T., and Allbritton N.L.. In vitro generation of colonic epithelium from primary cells guided by microstructures. Lab Chip 14, 1622, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Costello C.M., Hongpeng J., Shaffiey S., et al. . Synthetic small intestinal scaffolds for improved studies of intestinal differentiation. Biotechnol Bioeng 111, 1222, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Li N., Wang D., Sui Z., et al. . Development of an improved three-dimensional in vitro intestinal mucosa model for drug absorption evaluation. Tissue Eng Part C Methods 19, 708, 2013 [DOI] [PubMed] [Google Scholar]
- 119. Pereira C., Araújo F., Barrias C.C., Granja P.L., and Sarmento B.. Dissecting stromal-epithelial interactions in a 3D in vitro cellularized intestinal model for permeability studies. Biomaterials 56, 36, 2015 [DOI] [PubMed] [Google Scholar]
- 120. Araújo F., and Sarmento B.. Towards the characterization of an in vitro triple co-culture intestine cell model for permeability studies. Int J Pharm 458, 128, 2013 [DOI] [PubMed] [Google Scholar]
- 121. Capeling M.M., Czerwinski M., Huang S., et al. . Nonadhesive alginate hydrogels support growth of pluripotent stem cell-derived intestinal organoids. Stem Cell Reports 12, 381, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cruz-Acuna R., Quiros M., Farkas A., et al. . PEG-4MAL hydrogels for in vitro culture of human organoids and in vivo delivery to sites of injury. Protoc Exch 13, 2012, 2018 [Google Scholar]
- 123. Gjorevski N., Sachs N., Manfrin A., et al. . Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560, 2016 [DOI] [PubMed] [Google Scholar]
- 124. Dawson A., Dyer C., Macfie J., et al. . A microfluidic chip based model for the study of full thickness human intestinal tissue using dual flow. Biomicrofluidics 10, 064101, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Auchtung J.M., Robinson C.D., and Britton R.A.. Cultivation of stable, reproducible microbial communities from different fecal donors using minibioreactor arrays (MBRAs). Microbiome 3, 42, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wang L., Murthy S.K., Barabino G.A., and Carrier R.L.. Synergic effects of crypt-like topography and ECM proteins on intestinal cell behavior in collagen based membranes. Biomaterials 31, 7586, 2010 [DOI] [PubMed] [Google Scholar]
- 127. Wang L., Murthy S.K., Fowle W.H., Barabino G.A., and Carrier R.L.. Influence of micro-well biomimetic topography on intestinal epithelial Caco-2 cell phenotype. Biomaterials 30, 6825, 2009 [DOI] [PubMed] [Google Scholar]
- 128. Wang Y., Gunasekara D.B., Reed M.I., et al. . A microengineered collagen scaffold for generating a polarized crypt-villus architecture of human small intestinal epithelium. Biomaterials 128, 44, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sung J.H., Yu J., Luo D., Shuler M.L., and March J.C.. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip 11, 389, 2011 [DOI] [PubMed] [Google Scholar]
- 130. Costello C.M., Sorna R.M., Goh Y., Cengic I., Jain N.K., and March J.C.. 3-D intestinal scaffolds for evaluating the therapeutic potential of probiotics. Mol Pharm 11, 2030, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kim S.H., Lee J.W., Choi I., Kim Y.-C., Lee J.B., and Sung J.H.. A microfluidic device with 3-D hydrogel villi scaffold to simulate intestinal absorption. J Nanosci Nanotechnol 13, 7220, 2013 [DOI] [PubMed] [Google Scholar]
- 132. Esch M.B., Sung J.H., Yang J., et al. . On chip porous polymer membranes for integration of gastrointestinal tract epithelium with microfluidic “body-on-a-chip” devices. Biomed Microdevices 14, 895, 2012 [DOI] [PubMed] [Google Scholar]
- 133. Shim K.-Y., Lee D., Han J., Nguyen N.-T., Park S., and Sung J.H.. Microfluidic gut-on-a-chip with three-dimensional villi structure. Biomed Microdevices 19, 37, 2017 [DOI] [PubMed] [Google Scholar]
- 134. Kim W., and Kim G.. Intestinal villi model with blood capillaries fabricated using collagen-based bioink and dual-cell-printing process. ACS Appl Mater Interfaces 10, 41185, 2018 [DOI] [PubMed] [Google Scholar]
- 135. Chen Y., Zhou W., Roh T., Estes M.K., and Kaplan D.L.. In vitro enteroid-derived three-dimensional tissue model of human small intestinal epithelium with innate immune responses. PLoS One 12, e0187880, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Chen Y., Lin Y., Davis K.M., et al. . Robust bioengineered 3D functional human intestinal epithelium. Sci Rep 5, 13708, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kauffman A.L., Gyurdieva A.V., Mabus J.R., Ferguson C., Yan Z., and Hornby P.J.. Alternative functional in vitro models of human intestinal epithelia. Front Pharmacol 4, 79, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kim H.J., and Ingber D.E.. Gut-on-a-chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr Biol 5, 1130, 2013 [DOI] [PubMed] [Google Scholar]
- 139. Kim H.J., Huh D., Hamilton G.A., and Ingber D.E.. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12, 2165, 2012 [DOI] [PubMed] [Google Scholar]
- 140. Jalili-Firoozinezhad S., Prantil-Baun R., Jiang A., et al. . Modeling radiation injury-induced cell death and countermeasure drug responses in a human gut-on-a-chip. Cell Death Dis 9, 223, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wang C., Tanataweethum N., Karnik S., and Bhushan A.. Novel microfluidic colon with an extracellular matrix membrane. ACS Biomater Sci Eng 4, 1377, 2018 [DOI] [PubMed] [Google Scholar]
- 142. Shah P., Fritz J.V., Glaab E., et al. . A microfluidics-based in vitro model of the gastrointestinal human–microbe interface. Nat Commun 7, 11535, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Jalili-Firoozinezhad S., Gazzaniga F.S., Calamari E.L., et al. . Complex human gut microbiome cultured in anaerobic human intestine chips. bioRxiv 2018 [Google Scholar]
- 144. Fofanova T.Y., Stewart C., Auchtung J.M., et al. A novel human enteroid-anaerobe co-culture system to study microbial-host interaction under physiological hypoxia. bioRxiv 2019 [Google Scholar]
- 145. Maoz B.M., Herland A., Henry O.Y.F., et al. Organs-on-chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab Chip 17, 2294, 2017 [DOI] [PubMed] [Google Scholar]
- 146. Zhang X., Chen Z., and Huang Y.. A valve-less microfluidic peristaltic pumping method. Biomicrofluidics 9, 014118, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chen A., and Pan T.. Manually operatable on-chip bistable pneumatic microstructures for microfluidic manipulations. Lab Chip 14, 3401, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rhee M., and Burns M.A.. Microfluidic pneumatic logic circuits and digital pneumatic microprocessors for integrated microfluidic systems. Lab Chip 9, 3131, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Stucki J.D., and Guenat O.T.. A microfluidic bubble trap and oscillator. Lab Chip 15, 4393, 2015 [DOI] [PubMed] [Google Scholar]
- 150. Skelley A.M., and Voldman J.. An active bubble trap and debubbler for microfluidic systems. Lab Chip 8, 1733, 2008 [DOI] [PubMed] [Google Scholar]