Abstract
Rationale: Chronic bronchitis (CB) is characterized by productive cough with excessive mucus production, resulting in quality-of-life impairment and increased exacerbation risk. Bronchial rheoplasty uses an endobronchial catheter to apply nonthermal pulsed electrical fields to the airways. Preclinical studies have demonstrated epithelial ablation followed by regeneration of normalized epithelium.
Objectives: To evaluate the feasibility, safety, and initial outcomes of bronchial rheoplasty in patients with CB.
Methods: Pooled analysis of two separate studies enrolling 30 patients undergoing bilateral bronchial rheoplasty was conducted. Follow-up through 6 months (primary outcome) and 12 months included assessment of adverse events, airway histology, and changes in symptoms using the Chronic Obstructive Pulmonary Disease (COPD) Assessment Test and St. George’s Respiratory Questionnaire (SGRQ).
Measurements and Main Results: Bronchial rheoplasty was performed in all 30 patients (63% male; mean [SD] age, 67 [7.4]; mean [SD] postbronchodilator FEV1, 65% [21%]; mean [SD] COPD Assessment Test score 25.6 [7.1]; mean [SD] SGRQ score, 59.6 [15.3]). There were no device-related and four procedure-related serious adverse events through 6 months, and there were none thereafter through 12 months. The most frequent nonserious, device- and/or procedure-related event through 6 months was mild hemoptysis in 47% (14 of 30) patients. Histologically, the mean goblet cell hyperplasia score was reduced by a statistically significant amount (P < 0.001). Significant changes from baseline to 6 months in COPD Assessment Test (mean, −7.9; median, −8.0; P = 0.0002) and SGRQ (mean, −14.6; median, −7.2; P = 0.0002) scores were observed, with similar observations through 12 months.
Conclusions: This study provides the first clinical evidence of the feasibility, safety, and initial outcomes of bronchial rheoplasty in symptomatic patients with CB.
Clinical trial registered with www.anzctr.org.au (ACTRN 12617000330347) and clinicaltrials.gov (NCT 03107494).
Keywords: chronic obstructive pulmonary disease; obstructive, lung diseases, respiratory tract diseases, bronchial diseases, pulsed electric field
At a Glance Commentary
Scientific Knowledge on the Subject
In chronic bronchitis (CB), chronic inflammation of the respiratory tract leads to an increased number and hyperplasia of bronchial mucus–producing cells and a mucus hypersecretion phenotype, which in turn contributes to the chronic cough and sputum production characteristic of the disease. There is an unmet clinical need for the treatment of CB. Locally acting, mucosal-ablative interventions may be useful in treating CB.
What This Study Adds to the Field
This study provides proof of concept for the safety and technical feasibility of bronchial rheoplasty, an endoscopic technique using nonthermal pulsed electrical fields to ablate airway mucosa and reduce airway mucus production in the treatment of CB. Reductions in goblet cell hyperplasia and changes in patient-reported quality-of-life measures were observed after the procedure.
Chronic obstructive pulmonary disease (COPD) is a major public health problem that is projected to rank fifth worldwide in terms of disease burden and third in terms of mortality (1). The predominant subtypes of COPD include emphysema and chronic bronchitis (CB). CB is defined as chronic cough and sputum production for 3 mo/yr for 2 consecutive years. The pathophysiology of CB is chronic inflammation of the respiratory tract, leading to overproduction and hypersecretion of mucus together with impaired mucociliary clearance (2–4). As a result, patients with CB report symptoms such as persistent cough, production of phlegm, and/or shortness of breath. The diagnosis of CB is associated with more frequent and severe exacerbations, quality-of-life impairments, and increased morbidity and mortality (5–11). The prevalence of CB in the adult population ranges from 3.4% to 22.0%, depending on the definition used, and affects smokers with and without COPD (12, 13). In fact, previous studies suggest that symptoms of CB may predict the development of COPD (13–16).
Despite the impact of CB on morbidity and mortality, little progress has been made in developing effective therapies. Common therapies in managing COPD, including inhaled β2-agonists, anticholinergics, and glucocorticoids, have either not been studied specifically in the CB subtype or have yielded conflicting results (12). Roflumilast (a phosphodiesterase-4 inhibitor) has been shown to be effective in treating CB, but side effects limit its use in clinical practice (17, 18). Despite guideline-directed therapy, many patients with CB remain significantly symptomatic.
Bronchial rheoplasty is a procedure that uses nonthermal pulsed electrical fields with the intention of ablating the abnormal mucus-producing cells of the airway epithelium, thereby allowing normal, healthy epithelial regeneration to occur. The current study evaluates the technical feasibility, safety, and initial outcomes of this therapy in patients with CB. Some of the results of this study have been previously reported in the form of abstracts (19–21).
Methods
Study Design
Two prospective, multicenter, single-arm clinical studies under two nearly identical protocols, differing mainly by local specifications and requirements (Australia: ACTRN 12617000330347; Austria and Chile: NCT 03107494), were conducted in patients with diagnosed CB. The study informed-consent forms were approved by the local ethics committee at each study center. All patients provided written informed consent before screening.
Patients were recruited at five tertiary academic centers in Austria, Australia, and Chile between February 2017 and October 2018. The study population included adult patients who were at least 40 years of age and had a smoking history of at least 10 pack-years, with CB defined as a productive cough for 3 months in each of 2 successive years, and in whom other causes of productive cough had been excluded. The initial protocol required patients to have a post-bronchodilator FEV1 of ≥30% and ≤80% of predicted volume within 3 months of enrollment, together with a FEV1/FVC ratio < 0.70. Early during the enrollment period, however, it was noted that there was a significant number of patients suffering from significant CB symptoms who had relatively normal lung function (FEV1/FVC ratio ≥ 0.7 or FEV1 > 80%) but otherwise appeared to be good candidates for bronchial rheoplasty. The protocol was amended to remove the FEV1/FVC ratio criterion and to allow enrollment of patients with FEV1 > 80% if they had a COPD Assessment Test (CAT) total score ≥ 10 and the first two items on their CAT (“I never cough” vs. “I cough all the time” and “I have no phlegm in my chest at all” vs. “My chest is full of phlegm”) summed to a score ≥7 points.
Exclusion criteria included oral steroid–dependent conditions (>10 mg/d), active respiratory infection, COPD exacerbation within 6 weeks before treatment, abnormal cardiac rhythm at the time of the first procedure, history of arrhythmia within the past 2 years, presence of implantable cardiac devices, prior lung surgery, history of asthma before age 30, and current smoking (within 6 mo of treatment). See the complete listing of entry criteria in the online supplement.
Patients were to be maintained on stable pharmacologic treatment regimens throughout the study follow-up.
Bronchial Rheoplasty System Description
The RheOx System (Gala Therapeutics) consists of an electrosurgical generator and a single-use catheter (see Figure E1 in the online supplement). The system is designed to deliver pulsed electrical fields (high-frequency, short-duration, nonthermal electrical fields) to the airway epithelium and mucosa. This targeted energy delivery is intended to cause cell death by disrupting cellular homeostasis, leading to processes such as osmotic swelling and apoptosis (19–21). This cell death does not destroy the architectural function of the tissue, permitting subsequent regeneration of normalized epithelium and a reduction in airway mucus production. Additional detail on the RheOx System is provided in the online supplement.
Procedure Description
The procedure is performed under general anesthesia through a bronchoscope with at least a 2.8-mm working channel. Once the bronchoscope is in place, the endobronchial catheter is inserted and delivered to the target location in the airway, and the electrode is expanded to circumferentially contact the airway wall using the catheter-handle mechanism. The generator is then activated to deliver energy to the airway over approximately 5 seconds. The catheter is then collapsed and redeployed at the next target location adjacent to the prior activation, and, once again, energy is delivered. This sequence is repeated until all of the accessible airway mucosa has been treated. The operator treats from the subsegmental airways to the main carina. All accessible lobar segments and subsegments are treated. A minimum number of activations per procedure was not prespecified in the study protocol. It was assumed that the number of activations would depend on the accessibility and dimensions of the airways. Additional procedural details are provided in the online supplement.
During the first bronchoscopic session, endobronchial cryobiopsy samples (Erbe Elektromedizin GmbH, Tübingen, Germany) of the airway mucosa were acquired from multiple standardized regions (see online supplement for description of sampling techniques) of the right bronchial airways, followed by treatment of the right lung. The second bronchoscopic session, including (baseline) endobronchial cryobiopsy and treatment of the left lung, was scheduled at approximately 1 month after the initial treatment to allow for patients to recover from the initial procedure. A third bronchoscopy was performed, for bilateral-airway biopsy-sample collection only, 3 months after the second treatment; treatment was not administered at this visit. Patients were observed and discharged per the standard of care of the treating institution. Periprocedural corticosteroids and/or antibiotics (intravenous or oral) were permitted if deemed medically necessary by the treating physician. Follow-up visits were scheduled at 1 week (phone call) and 1 month after the first treatment and at 1 week (phone call) and 3, 6, and 12 months after the second treatment. A comprehensive schedule of study events is provided in Table E1.
Histological samples from the right bronchus intermedius and the left lower lobe were formalin fixed, paraffin embedded, and sectioned per standard histology protocols. Hematoxylin and eosin– and periodic acid–Schiff–stained slides were reviewed by a board-certified anatomic pathologist with subspecialty expertise in pulmonary pathology. The pathologist graded the degree of epithelial goblet cell hyperplasia using a prespecified, semiquantitative methodology consisting of a categorical scale, described in detail in Figure E2. The pathologist was blinded to the patient identification and to the time point of the sample collection.
Outcomes
The primary outcome of the study was safety, as assessed by the incidence and evaluation of serious adverse events (SAEs) associated with the device through 6 months. Safety was also assessed by evaluating the type, frequency, and severity of nonserious adverse events (AEs), their potential relationship to the study device or procedure, and their timing in relation to the treatment. Events were defined as occurring during the treatment recovery period if they occurred within 30 days of either treatment. Events thereafter were defined as occurring during the 3-, 6-, or 12-month periods after the second treatment, excluding the treatment recovery period. Spirometry testing (FEV1 and FVC) was incorporated as an additional safety measure.
Further outcomes included evaluation of the effects of bronchial rheoplasty on goblet cell hyperplasia score and disease-related symptoms and quality of life using CAT and St. George’s Respiratory Questionnaire (SGRQ) total scores. Goblet cell hyperplasia was assessed at 3 months after the second procedure. CAT and SGRQ scores were collected at baseline and at 3, 6 (primary assessment), and 12 months after the second procedure visit, and responder rates were calculated using 2-point and 4-point thresholds for CAT (22) and SGRQ (23), respectively.
Statistical Methods
Because this was a safety and feasibility study, no formal sample-size calculation was performed. Descriptive statistics and graphical representations were used to summarize the data. For categorical variables, counts and percentages were calculated. For continuous variables, means, medians, quartiles, SDs, and, when appropriate, 95% confidence intervals for the mean, assuming a normal distribution, were calculated. All calculations were based on available data; no imputations or extrapolations were used to replace missing values. P values for longitudinal secondary outcome measures (CAT and SGRQ) at baseline and 3, 6, and 12 months were computed using a nonparametric test appropriate for repeated measures (Friedman’s test) (24), followed by pairwise Wilcoxon signed rank tests of changes from baseline, with a Hochberg adjustment for multiplicity. Data analyses were performed using SAS version 9.4 (SAS Institute).
Results
Forty-two patients were screened, and 30 patients were enrolled (Table E2). Follow-up to 6 and 12 months after the second study treatment was available for 30 and 29 out of 30 patients, respectively (Figure 1).
Figure 1.
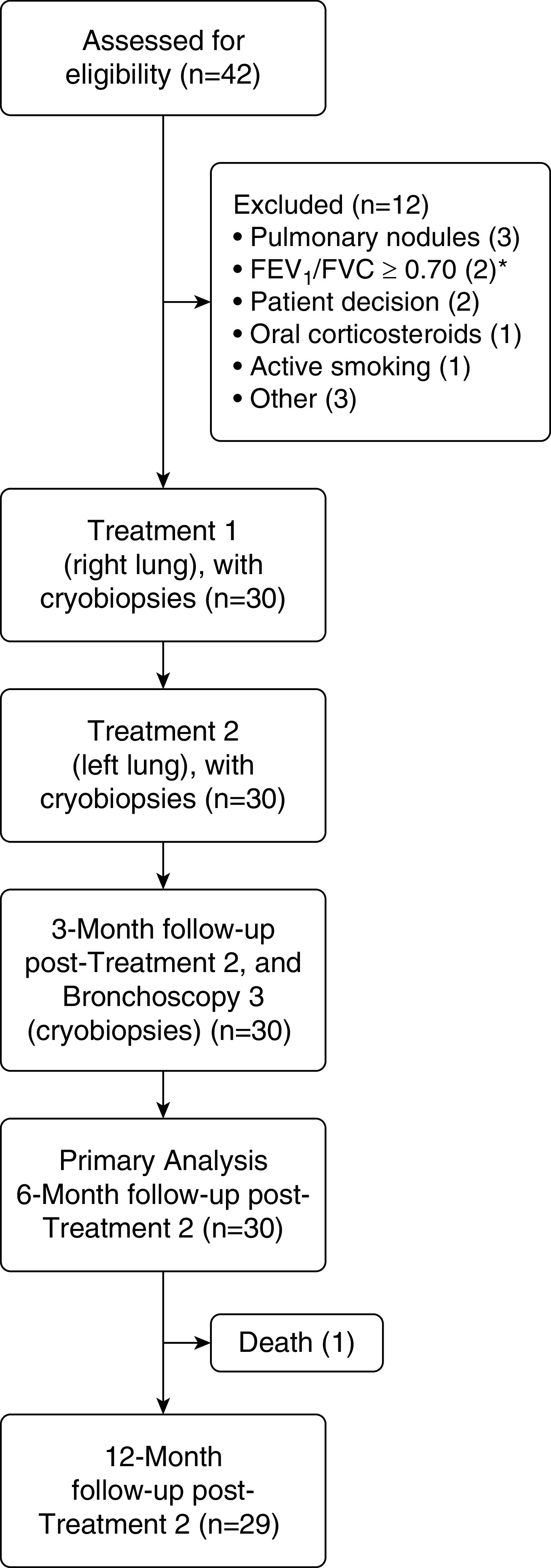
Disposition of study patients (CONSORT [Consolidated Standards of Reporting Trials] diagram). *The original version of the protocol required an FEV1/FVC ratio < 0.70. This requirement was removed in a later protocol amendment.
Enrolled patients had a mean (SD) FEV1% predicted of 65% (21%), CAT score of 25.6 (7.1) points, and SGRQ score of 59.6 (15.3) points, indicating a high symptom burden, despite most patients being on inhaled long-acting bronchodilator treatment and nearly half of patients being on inhaled corticosteroid therapy (Table 1). The medication regimen at study entry was maintained through the 12-month follow-up in 27 of the 30 patients. Two patients transitioned from short-acting bronchodilator inhaler therapy to long-acting bronchodilator treatment with inhaled corticosteroid therapy per the treating physician’s recommendation, and one patient had his long-acting antimuscarinic antagonist bronchodilator inhaler therapy withdrawn at the 3-month visit.
Table 1.
Patient Demographics, Baseline Clinical Characteristics, and Medications
| Characteristic | Value (N = 30 Patients) |
|---|---|
| Age, yr | 67 (7.4) |
| Sex, M, n (%) | 19 (63.3) |
| BMI, kg/m2 | 27.5 (4.7) |
| Smoking history, pack-years | 40.7 (26.5) |
| FEV1% predicted* | 65.0 (21.2) |
| FEV1/FVC ratio* | 0.53 (0.14) |
| Airflow obstruction, n (%) | |
| CB w/o airflow obstruction | 4 (13.3) |
| GOLD I | 4 (13.3) |
| GOLD II | 13 (43.3) |
| GOLD III | 9 (30.0) |
| TLC% predicted* | 111.6 (14.2) |
| RV% predicted* | 142.2 (39.8) |
| RV/TLC* | 48.7 (10.1) |
| Emphysema, % (−950 HU) | 8.0 (9.2) |
| 6MWT, m† | 443.2 (92.4) |
| CAT total score | 25.6 (7.1) |
| CAT phlegm score | 4.1 (0.8) |
| CAT cough score | 3.6 (0.9) |
| SGRQ total score | 59.6 (15.3) |
| SGRQ symptoms score | 76.1 (13.4) |
| Inhaled pharmacologic treatment, n (%) | |
| Short-acting only | 2 (6.7) |
| LABA/LAMA/ICS, LABA/LAMA, LABA only, LAMA only | 27 (90.0) |
| ICS only | 1 (3.3) |
| Oral roflumilast, n (%) | 3 (10) |
Definition of abbreviations: 6MWT = 6-minute-walk test; BMI = body mass index; CAT = Chronic Obstructive Pulmonary Disease Assessment Test; CB = chronic bronchitis; GOLD = Global Initiative for Chronic Obstructive Lung Disease; HU = Hounsfield units; ICS = inhaled corticosteroids; LABA = long-acting β-agonist bronchodilator; LAMA = long-acting muscarinic antagonist bronchodilator; RV = residual volume; SGRQ = St. George’s Respiratory Questionnaire; w/o = without.
Data are mean (SD) unless otherwise noted. Lung-function parameters are after bronchodilator treatment.
One patient did not have post-bronchodilator pulmonary-function testing done (protocol deviation).
Three patients did not perform the 6MWT.
Procedural Results and Feasibility
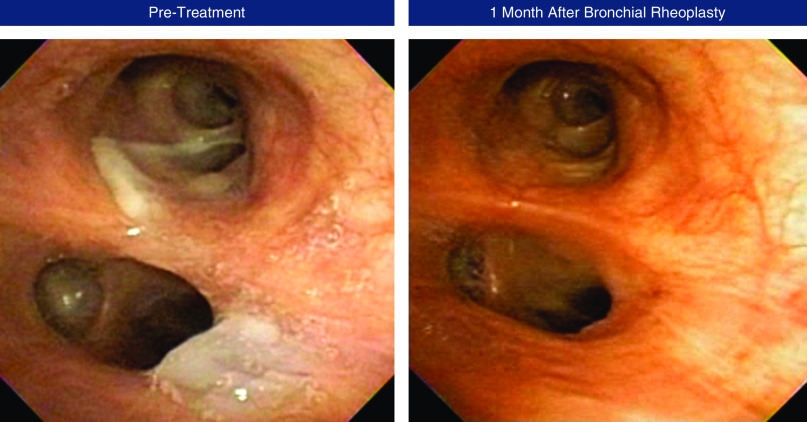
Bilateral bronchial rheoplasty was completed in all 30 enrolled patients (60 procedures), with successful catheter deployment and energy delivery to the target sites in all procedures. A mean (SD) of 43 (21) activations were applied per lung. The median post-procedure hospital stay was 1 day (range, 0–4). In 92% of procedures, patients were discharged within 48 hours of the procedure. Figure 2 shows bronchoscopic images taken before and after bronchial rheoplasty in a representative patient.
Figure 2.
Pre- and post-treatment bronchoscopic images from a study patient. Note the thick mucus before treatment, with resolution after treatment. The images show the endoscopic view of the left upper lobe carina with both upper and lower lobe airways visible.
Primary Study Outcome and Additional Safety Assessments
No device-related SAEs and four procedure-related SAEs were reported through 6 months, with no additional device- or procedure-related SAEs in the 6- to 12-month period. Three of the four procedure-related SAEs occurred during the treatment recovery period, including one case of pneumonia 2 days after the procedure; one case of mucosal scarring observed at the second bronchoscopy, which was determined to have been related to cryobiopsy sampling during the first bronchoscopy procedure; and one case of COPD exacerbation, which started the same day as the second procedure. The fourth procedure-related SAE was a COPD exacerbation that occurred the day of the third bronchoscopy, which was for research only.
Other than the 4 procedure-related SAEs described above, 12 SAEs unrelated to either device or procedure were reported in nine patients through 12 months (Table 2). The most frequently reported SAE was COPD exacerbation (13.3%, 4 of 30 patients). There was one case of atrial fibrillation 1 week after the procedure, judged by the investigator to be unrelated to the procedure or device but instead related to an unreported preexisting condition exacerbated by alcohol consumption. This event resolved without sequelae 1 day later after prescription of an intravenous β-blocker. No acute cardiac rhythm abnormalities occurred during the procedure in any patient. One patient died of end-stage COPD, unrelated to either the device or the procedure, 381 days after the second procedure.
Table 2.
Serious Adverse Events
| Event Type | Treatment Recovery Period* (n Events) | 3 mo† (n Events) | 6 mo‡ (n Events) | 12 mo§ (n Events) |
|---|---|---|---|---|
| Device-related‖ | 0 | 0 | 0 | 0 |
| Procedure-related‖ | ||||
| COPD exacerbation | 1 | 1 | 0 | 0 |
| Mucosal scarring | 1 | 0 | 0 | 0 |
| Pneumonia | 1 | 0 | 0 | 0 |
| Unrelated to device or procedure | ||||
| Atrial fibrillation | 1 | 0 | 0 | 0 |
| COPD exacerbation | 0 | 1 | 1 | 3 |
| Erysipelas | 0 | 0 | 1 | 0 |
| Femoral artery stenosis | 0 | 0 | 1 | 1 |
| Lung nodule | 0 | 0 | 0 | 1 |
| Musculoskeletal injury | 0 | 0 | 0 | 1 |
| Pyelonephritis | 0 | 0 | 0 | 1 |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
There were a total of 16 events in 11 patients. One patient experienced 2 COPD exacerbations in separate time intervals.
Thirty days after either bronchial rheoplasty procedure.
Follow-up period through 3 months after treatment 2, excluding either treatment recovery period.
Follow-up period between 3 months and 6 months after treatment 2.
Follow-up period between 6 months and 12 months after treatment 2.
Events are considered device-related or procedure-related if judged by the treating investigator to be possibly, probably, or definitely related to the device or procedure, respectively.
Nonserious device-related and procedure-related AEs through 12 months are presented in Tables E3 and E4, respectively. The majority (85%) of these events occurred during the treatment recovery period. The most frequent nonserious AEs that were device and/or procedure related were COPD exacerbations (30%, 9 of 30 patients), which were generally moderate in severity and successfully treated with antibiotic and/or systemic steroid therapy; mild hemoptysis (47%, 14 of 30 patients); and sore throat (33%, 10 of 30 patients). All hemoptysis events resolved spontaneously without any intervention or follow-up required. No unexpected AEs related to the device or procedure were reported during the study.
Moderate and severe COPD-exacerbation event rates during the study, defined as the number of events per patient-year of follow-up, were 1.20 (SD, 1.30) and 0.21 (SD, 0.60), respectively.
There were no statistically significant changes in lung-function parameters (FEV1, FVC) at 3, 6, or 12 months after the procedure compared with baseline.
Histological Assessments
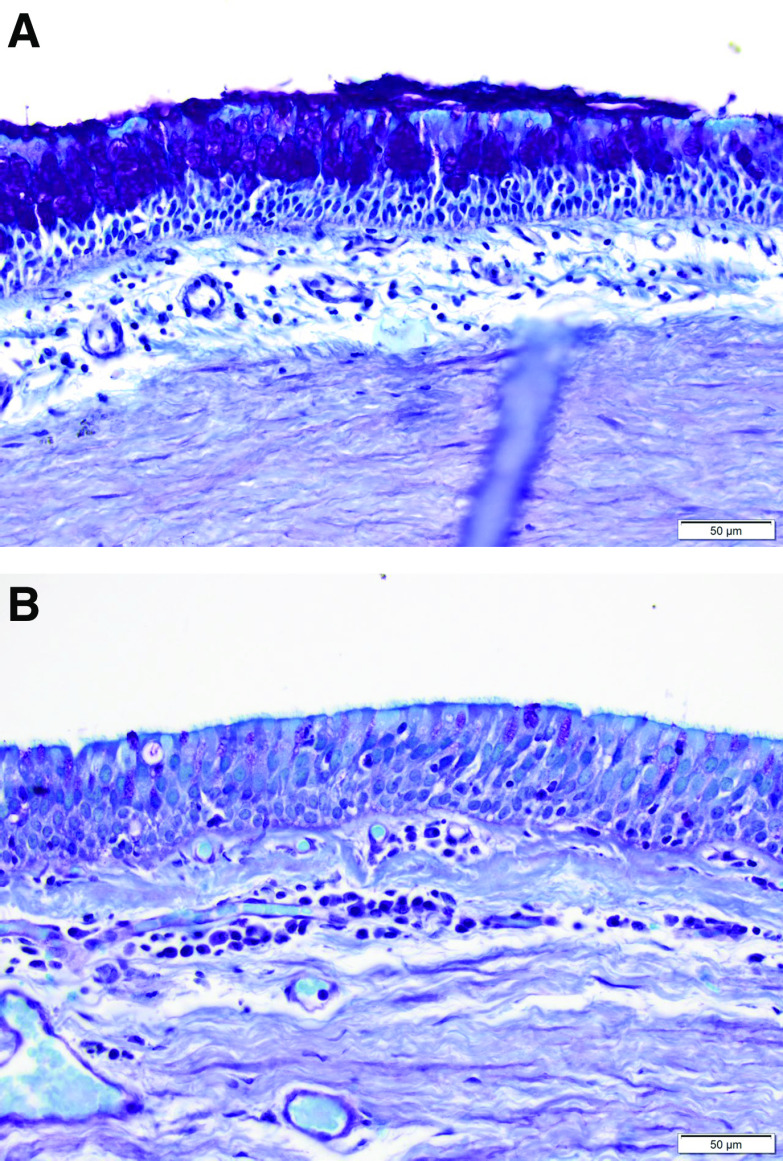
A representative example of the histological changes between the pre- and post-treatment biopsies shows marked goblet cell hyperplasia of the right bronchus intermedius at baseline (Figure 3A, score = 2), followed by a 1-point reduction (i.e., improvement) in goblet cell hyperplasia from the same location at the 3-month follow-up bronchoscopy (Figure 3B, score = 1).
Figure 3.
Histological findings from the right bronchus intermedius of a study patient. The goblet cells, with magenta-colored cytoplasmic mucin highlighted by periodic acid–Schiff staining, are seen in the superficial bronchial epithelium. (A) On Day 0 immediately before therapy, significant goblet cell hyperplasia can be seen (score of 2). (B) Right bronchus intermedius 120 days after the initial treatment, demonstrating complete regeneration of the pseudostratified columnar epithelium with a reduction of goblet cell numbers (semiquantitative assessment score of 1).
Overall, 54 matched sets (baseline and follow-up) of histological samples were available for analysis. In six lungs, one or more of the samples demonstrated insufficient material for further analysis. The mean (SD) goblet cell hyperplasia score was reduced from 1.48 (0.91) at baseline to 0.91 (0.81) after treatment, a relative reduction of 39% (P < 0.001; Table 3). Of the 25 matched lung biopsies with baseline goblet cell hyperplasia scores of 2 or 3 (indicating a moderately or severely increased ratio of goblet cells to ciliated bronchial epithelial cells, respectively), 21 (84%) were reduced by at least 1 point after treatment (Table 4).
Table 3.
Histopathology Results: Goblet Cell Hyperplasia Scores
| Statistics | Baseline | Follow-up | Change from Baseline |
|---|---|---|---|
| N (lungs biopsied) | 54 | 54 | |
| Mean score (SD) | 1.48 (0.91) | 0.91 (0.81) | −0.57* |
| 95% CI | 1.23 to 1.73 | 0.69 to 1.13 | −0.83 to −0.32 |
Definition of abbreviation: CI = confidence interval.
Table presents changes from baseline to 90 days and 120 days after the procedure for the left and right lungs, respectively. One biopsy sample is assessed for each lung at each of the two time points (baseline and follow-up).
P < 0.001.
Table 4.
Goblet Cell Hyperplasia Score: Change by Baseline Score
| Baseline Goblet Cell Hyperplasia Score* (N = 54 Airway Biopsies) | Improved | No Change | Worsened |
|---|---|---|---|
| 0 | 0 | 5 | 2 |
| 1 | 6 | 13 | 3 |
| 2 | 14 | 2 | 1 |
| 3 | 7 | 1 | 0 |
Table presents changes from baseline to 90 days and 120 days after the procedure for the left and right lungs, respectively. One biopsy sample is assessed for each lung at each of the two time points (baseline and follow-up).
Graded on a 4-point scale in which 0 = normal ratio of goblet cells to ciliated bronchial epithelial cells (1 goblet cell per 10 or more bronchial epithelial cells); 1 = mild goblet cell hyperplasia (1 goblet cell per approximately 3–10 ciliated bronchial epithelial cells); 2 = moderate goblet cell hyperplasia (approaching 1 goblet cell per ciliated bronchial epithelial cell); and 3 = severe goblet cell hyperplasia (>1 goblet cell per ciliated bronchial epithelial cell).
Symptoms and Quality-of-Life Assessments
Statistically significant changes in health-related quality-of-life outcomes were observed at 3, 6, and 12 months after the second treatment (Figure 4 and Table E5). At the 6-month primary assessment, the median (interquartile range) changes from baseline in CAT and SGRQ total scores were −8.0 (−14.0 to −2.0) points (P = 0.0002) and −7.2 (−19.8 to −3.1) points (P = 0.0002), respectively, and mean (SD) changes from baseline were −7.9 (8.3) points and −14.6 (19.4) points, respectively. Responder rates for the CAT and SGRQ at 6 months were 76.7% and 70.0%, respectively. At the 12-month follow-up, median (interquartile range) changes from baseline in CAT and SGRQ were −8.0 (−14.0 to 1.0) points and −14.7 (−27.8 to −2.0) points, respectively, and mean (SD) changes from baseline were −7.0 (8.9) points and −15.2 (20.4) points, respectively. Responder rates for the CAT and SGRQ at 12 months were 69% (20 of 29) and 72% (21 of 29), respectively.
Figure 4.
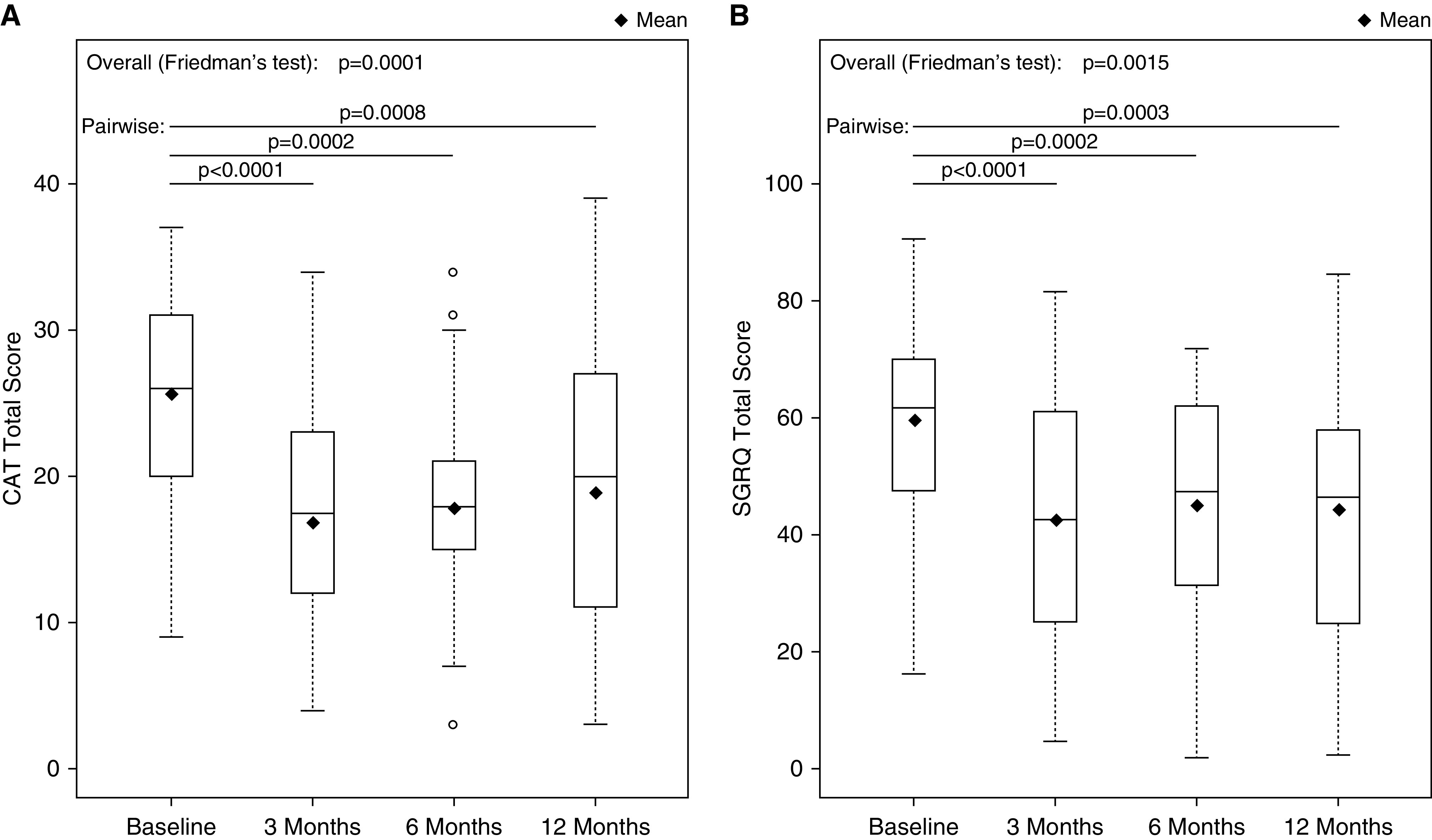
Quality-of-life outcomes. The 3-, 6-, and 12-month time points are in relation to the second treatment procedure. Box plots indicate minimum, first-quartile, median (solid horizontal line), third-quartile, and maximum values. Diamonds indicate mean values. (A) Total CAT score. (B) Total SGRQ score. CAT = Chronic Obstructive Pulmonary Disease Assessment Test; SGRQ = St. George’s Respiratory Questionnaire.
Post hoc analyses of 6-month component scores from the CAT and SGRQ questionnaires showed reductions in CAT cough and phlegm scores (questions 1 and 2), and in all three SGRQ domains (Table E5). Individual patient changes from baseline to 6 months in CAT and SGRQ scores are also provided in Figure E3.
Discussion
Bronchial rheoplasty is an endoscopic technique that uses nonthermal pulsed electrical fields to ablate the airway mucosa and mucus-producing cells of the airway epithelium. On the basis of preclinical research in animals, the local extracellular matrix is left intact after the procedure (19–21), which is believed to promote healthier regeneration of the epithelium.
This initial study of bronchial rheoplasty demonstrated that the procedure is technically feasible, with an acceptable safety profile. Pulsed electrical fields were delivered from the main bronchus to the subsegmental airways, the airways most likely to contain goblet cells. The histological findings appear to confirm the proposed mechanism of action, demonstrating a statistically significant reduction in the number of epithelial goblet cells, particularly in those patients with pretreatment evidence of moderate-to-severe goblet cell hyperplasia. These observations were accompanied by statistically significant reductions in CAT and SGRQ scores through 12 months.
The above findings were observed in the presence of an acceptable safety profile. No patients experienced a device-related SAE, and four patients experienced a procedure-related SAE, each of which resolved without sequelae. No device- or procedure-related SAEs were observed beyond 3 months. Given that the RheOx System delivers high-voltage energy to the airways in close proximity to the heart, monitoring for cardiac arrythmias was of particular interest in characterizing safety. No acute cardiac rhythm abnormalities occurred during the procedure in any patient. One patient developed atrial fibrillation 1 week after the procedure, which was, however, considered to be unrelated to the device or procedure. This patient failed to report a history of prior atrial fibrillation, which may have been exacerbated by general anesthesia, the procedure, and/or the patient’s behavior (i.e., significant alcohol consumption).
There was a 30% rate of nonserious, moderate COPD exacerbations during the treatment recovery period. The overall rates of moderate and severe COPD exacerbations in the present study were 1.20 and 0.21 events per patient-year, respectively. These rates appear to be similar to those of a cross-sectional analysis of 112 French patients with COPD and a CB phenotype, in which reported moderate and severe COPD exacerbation rates were 1.80 and 0.43 events per patient-year, respectively (25). Nevertheless, we acknowledge that the occurrence of COPD exacerbations may in part have been influenced by the bronchoscopic interventions, which included multiple cryobiopsies. To put this rate into context, an analysis of the SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) trial demonstrated a 27% rate of respiratory complications after research bronchoscopy performed in patients with COPD (26).
Spirometry was performed primarily for safety reasons and was unaffected by the procedure. Given the absence of a relationship between goblet cell density and classic markers of lung function (FEV1 and FVC) in a previous report (27), we did not expect to observe substantive changes in these measures. Improvements in CB symptoms may better correlate with small airway function and are better measured with impulse oscillometry, body plethysmography, and/or computed tomography–derived parameters, such as airway count and/or airway volumes (28–30).
Few other interventional device systems have been developed to ablate the abnormal airway epithelium and mucosa in patients with obstructive airway disease. In contrast to bronchial rheoplasty, these systems deliver thermal energy to achieve changes in airway pathology. Bronchial thermoplasty is a U.S. Food and Drug Administration–approved procedure that uses radiofrequency energy to reduce airway smooth muscle mass through bronchoscopy to all visible airways. Clinical benefits accompanied by histological changes underlying the mechanism of action have been demonstrated in patients with refractory asthma, in the presence of significant procedure- and device-related side effects after treatment (31). Bronchial thermoplasty, however, has not yet been studied in COPD.
More recently, metered cryospray therapy has been introduced to ablate airway mucosa in patients with CB. Five out of 11 patients scheduled to undergo surgery for lung cancer underwent this endoscopic approach, followed by lung resection and assessments of airway histology at 2 weeks (32). Similar to findings in the present study, reepithelialization at the treatment site was observed in that report. However, because of the lack of published safety, histology, and outcome data beyond 2 weeks, it is not possible to draw further conclusions or otherwise compare these current technologies.
With any new interventional technique, however, careful patient selection is one of the most important considerations. Patients were selected for this study using a high CB symptom threshold, as determined by the first two items of the CAT instrument (cough and phlegm or mucus). Although there is no agreed-on method for staging patients with CB, several definitions have been used (27, 33), and higher scores on the first two CAT items have been correlated with worse quality-of-life scores, lower FEV1 and FVC, worse computed tomography–derived airway parameters, and more frequent COPD-related hospitalizations (33). In our study, the final protocol versions did not require a COPD diagnosis based on pulmonary function tests. Patients with preserved lung function were allowed if they met other study criteria, including the CB symptom thresholds. During the enrollment phase, we identified a significant number of patients suffering from significant CB symptoms who had relatively normal lung function (FEV1/FVC ratio ≥ 0.7) and were thus deemed to be good candidates for bronchial rheoplasty. This is consistent with recent studies that have shown that smokers and former smokers with chronic respiratory symptoms of chronic mucus hypersecretion, cough, and dyspnea, but relatively normal spirometry, constitute a significant proportion of clinical consultations (13, 34) and that these individuals may have early stages of COPD not yet evidenced by airflow limitation (35), representing a significant unmet clinical need. Çolak and colleagues (13) also showed that chronic respiratory symptoms are associated with respiratory hospitalizations and death in individuals with normal spirometry.
As an initial safety and feasibility study, this study had limitations that included a small sample size and lack of a control group. Although the observed changes in airway histology and the observed findings in CAT and SGRQ scores suggest a treatment effect warranting further investigation, we cannot rule out the potential influence of an intervention or placebo effect on patient-reported outcomes. In a randomized controlled trial of targeted lung denervation in patients with moderate-to-severe COPD, mean changes in SGRQ of −3.8 and −2.5 points at 6 and 12 months were observed, respectively, in the group of patients who underwent sham bronchoscopy (36). Similar results have been reported in other blinded studies evaluating endoscopic interventions in COPD, with changes in CAT and SGRQ data with bronchoscopy alone in the range between 0 and −3.7 points (37, 38). Additional limitations of the current study are that the goblet cell hyperplasia scoring was performed by a single pathologist and that the semiquantitative scale has not been validated. Furthermore, given the sample size of the current study, we were not able to calculate an intrarater variability of the histological reads. A randomly selected subset of 20 of the 54 samples were, however, regraded by the pathologist in a blinded fashion, resulting in a similarly statistically significant reduction of the goblet cell hyperplasia score (P = 0.0013, data not shown). That said, and though no standardized scoring system has been developed for assessing changes in airway biopsy samples in CB, a similar 4-point scale was employed by Gordon and colleagues (39) when assessing goblet cell hyperplasia in biopsy samples collected from asthma patients treated with bronchial thermoplasty. Moreover, previous reports have shown that goblet cell density—a comparable measure to the one used in our study—in endobronchial biopsy samples correlated well with smoking history (3, 27) and was better at discriminating mucosal pathology than mucin-volume density (27). Other established markers of submucosal gland hypertrophy, such as the Reid Index, failed to show any differences between those with CB and those without (40, 41) and were therefore not used. Finally, it is not known whether a bilateral treatment during a single procedure would potentially decrease the total risk of procedure-related events by reducing the number of airway interventions.
In summary, this study provides the first clinical evidence of the feasibility, safety, and initial outcomes of bronchial rheoplasty in symptomatic patients with CB.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Gala Therapeutics for providing support for this study, including data analysis and administrative and logistical support in coordinating the study across the study sites. They thank Paul VanderLaan, M.D., Ph.D., (Boston, Massachusetts) for performing pathological evaluation and grading of the airway biopsy samples.
Footnotes
Supported by Gala Therapeutics.
Author Contributions: Substantial contributions to the conception or design of the work: A.V., W.S.K., and J.W. Substantial contribution to the acquisition of data for the work: A.V., S.F.-B., A.J.I., D.P.S., G.I.S., J.P.W., T.S., L.B.I., and E.J.D. Substantial contribution to the analysis or interpretation of data for the work: A.V., W.S.K., and J.W. Drafting the work or revising it critically for important intellectual content and final approval of the version to be published: all authors. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: A.V.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201908-1546OC on May 14, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49:1700214. doi: 10.1183/13993003.00214-2017. [DOI] [PubMed] [Google Scholar]
- 2.Verra F, Escudier E, Lebargy F, Bernaudin JF, De Crémoux H, Bignon J. Ciliary abnormalities in bronchial epithelium of smokers, ex-smokers, and nonsmokers. Am J Respir Crit Care Med. 1995;151:630–634. doi: 10.1164/ajrccm/151.3_Pt_1.630. [DOI] [PubMed] [Google Scholar]
- 3.Saetta M, Turato G, Baraldo S, Zanin A, Braccioni F, Mapp CE, et al. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med. 2000;161:1016–1021. doi: 10.1164/ajrccm.161.3.9907080. [DOI] [PubMed] [Google Scholar]
- 4.Innes AL, Woodruff PG, Ferrando RE, Donnelly S, Dolganov GM, Lazarus SC, et al. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest. 2006;130:1102–1108. doi: 10.1378/chest.130.4.1102. [DOI] [PubMed] [Google Scholar]
- 5.Vestbo J, Prescott E, Lange P Copenhagen City Heart Study Group. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Am J Respir Crit Care Med. 1996;153:1530–1535. doi: 10.1164/ajrccm.153.5.8630597. [DOI] [PubMed] [Google Scholar]
- 6.Pelkonen M, Notkola IL, Nissinen A, Tukiainen H, Koskela H. Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural men. Chest. 2006;130:1129–1137. doi: 10.1378/chest.130.4.1129. [DOI] [PubMed] [Google Scholar]
- 7.Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax. 2009;64:894–900. doi: 10.1136/thx.2008.110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgel PR. Chronic cough and sputum production: a clinical COPD phenotype? Eur Respir J. 2012;40:4–6. doi: 10.1183/09031936.00022412. [DOI] [PubMed] [Google Scholar]
- 9.Kim V, Davey A, Comellas AP, Han MK, Washko G, Martinez CH, et al. COPDGene Investigators. Clinical and computed tomographic predictors of chronic bronchitis in COPD: a cross sectional analysis of the COPDGene study. Respir Res. 2014;15:52. doi: 10.1186/1465-9921-15-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim V, Zhao H, Boriek AM, Anzueto A, Soler X, Bhatt SP, et al. COPDGene Investigators. Persistent and newly developed chronic bronchitis are associated with worse outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13:1016–1025. doi: 10.1513/AnnalsATS.201512-800OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lahousse L, Seys LJM, Joos GF, Franco OH, Stricker BH, Brusselle GG. Epidemiology and impact of chronic bronchitis in chronic obstructive pulmonary disease. Eur Respir J. 2017;50:1602470. doi: 10.1183/13993003.02470-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:228–237. doi: 10.1164/rccm.201210-1843CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Çolak Y, Nordestgaard BG, Vestbo J, Lange P, Afzal S. Prognostic significance of chronic respiratory symptoms in individuals with normal spirometry. Eur Respir J. 2019;54:1900734. doi: 10.1183/13993003.00734-2019. [DOI] [PubMed] [Google Scholar]
- 14.Lindberg A, Eriksson B, Larsson LG, Rönmark E, Sandström T, Lundbäck B. Seven-year cumulative incidence of COPD in an age-stratified general population sample. Chest. 2006;129:879–885. doi: 10.1378/chest.129.4.879. [DOI] [PubMed] [Google Scholar]
- 15.de Marco R, Accordini S, Cerveri I, Corsico A, Antó JM, Künzli N, et al. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med. 2007;175:32–39. doi: 10.1164/rccm.200603-381OC. [DOI] [PubMed] [Google Scholar]
- 16.Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180:3–10. doi: 10.1164/rccm.200901-0047OC. [DOI] [PubMed] [Google Scholar]
- 17.Baye J. Roflumilast (daliresp): a novel phosphodiesterase-4 inhibitor for the treatment of severe chronic obstructive pulmonary disease. P&T. 2012;37:149–161. [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez FJ, Rabe KF, Sethi S, Pizzichini E, McIvor A, Anzueto A, et al. Effect of roflumilast and inhaled corticosteroid/long-acting β2-agonist on chronic obstructive pulmonary disease exacerbations (RE(2)SPOND): a randomized clinical trial. Am J Respir Crit Care Med. 2016;194:559–567. doi: 10.1164/rccm.201607-1349OC. [DOI] [PubMed] [Google Scholar]
- 19.Valipour A, Ing A, Williamson J, Saghaie T, Steinfort D, Irving L, et al. Late breaking abstract - first-in-human results of bronchial rheoplasty: an endobronchial treatment for chronic bronchitis (CB) [abstract] Eur Respir J. 2018;52:OA2162. [Google Scholar]
- 20.Valipour A, Ing A, Williamson JP, Saghaie T, Irving L, Steinfort D, et al. First-in-human results of bronchial rheoplasty: an endobronchial treatment for chronic bronchitis (CB) [abstract] Am J Respir Crit Care Med. 2019;199:A7037. doi: 10.1164/rccm.201908-1546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valipour A, Ing A, Williamson J, Saghaie T, Steinfort D, Irving L, et al. Late breaking abstract - bronchial rheoplasty for treatment of chronic bronchitis: 6 month results from a prospective multi-center study [abstract] Eur Respir J. 2019;54:RCT448. [Google Scholar]
- 22.Kon SS, Canavan JL, Jones SE, Nolan CM, Clark AL, Dickson MJ, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med. 2014;2:195–203. doi: 10.1016/S2213-2600(14)70001-3. [DOI] [PubMed] [Google Scholar]
- 23.Jones PW. St. George’s respiratory questionnaire: MCID. COPD. 2005;2:75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 24.Hollander M, Wolfe DA. Nonparametric statistical methods. 2nd ed. New York: John Wiley & Sons; 1999. [Google Scholar]
- 25.Burgel PR, Nesme-Meyer P, Chanez P, Caillaud D, Carré P, Perez T, et al. Initiatives Bronchopneumopathie Chronique Obstructive (BPCO) Scientific Committee. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009;135:975–982. doi: 10.1378/chest.08-2062. [DOI] [PubMed] [Google Scholar]
- 26.Wells JM, Arenberg DA, Barjaktarevic I, Bhatt SP, Bowler RP, Christenson SA, et al. Safety and tolerability of comprehensive research bronchoscopy in chronic obstructive pulmonary disease: results from the SPIROMICS bronchoscopy substudy. Ann Am Thorac Soc. 2019;16:439–446. doi: 10.1513/AnnalsATS.201807-441OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim V, Oros M, Durra H, Kelsen S, Aksoy M, Cornwell WD, et al. Chronic bronchitis and current smoking are associated with more goblet cells in moderate to severe COPD and smokers without airflow obstruction. PLoS One. 2015;10:e0116108. doi: 10.1371/journal.pone.0116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones PW. Health status and the spiral of decline. COPD. 2009;6:59–63. doi: 10.1080/15412550802587943. [DOI] [PubMed] [Google Scholar]
- 29.Coxson HO, Leipsic J, Parraga G, Sin DD. Using pulmonary imaging to move chronic obstructive pulmonary disease beyond FEV1. Am J Respir Crit Care Med. 2014;190:135–144. doi: 10.1164/rccm.201402-0256PP. [DOI] [PubMed] [Google Scholar]
- 30.Jetmalani K, Thamrin C, Farah CS, Bertolin A, Chapman DG, Berend N, et al. Peripheral airway dysfunction and relationship with symptoms in smokers with preserved spirometry. Respirology. 2018;23:512–518. doi: 10.1111/resp.13215. [DOI] [PubMed] [Google Scholar]
- 31.D’Anci KE, Lynch MP, Leas BF, Apter AJ, Bryant-Stephens T, Kaczmarek JL, et al. ECRI Institute. Effectiveness and safety of bronchial thermoplasty in management of asthma. Rockville, MD: Agency for Healthcare Research and Quality; 2017. AHRQ Publication No. 18-EHC003-EF. [PubMed] [Google Scholar]
- 32.Slebos DJ, Breen D, Coad J, Klooster K, Hartman J, Browning R, et al. Safety and histological effect of liquid nitrogen metered spray cryotherapy in the lung. Am J Respir Crit Care Med. 2017;196:1351–1352. doi: 10.1164/rccm.201611-2220LE. [DOI] [PubMed] [Google Scholar]
- 33.Lim JU, Lee JH, Kim TH, Lee JS, Lee SD, Oh YM, et al. Alternative definitions of chronic bronchitis and their correlation with CT parameters. Int J Chron Obstruct Pulmon Dis. 2018;13:1893–1899. doi: 10.2147/COPD.S164055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. SPIROMICS Research Group. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Celli BR, Decramer M, Wedzicha JA, Wilson KC, Agustí A, Criner GJ, et al. ATS/ERS Task Force for COPD Research. An official American Thoracic Society/European Respiratory Society statement: research questions in COPD. Eur Respir J. 2015;45:879–905. doi: 10.1183/09031936.00009015. [DOI] [PubMed] [Google Scholar]
- 36.Slebos DJ, Shah PL, Herth FJF, Pison C, Schumann C, Hübner RH, et al. AIRFLOW-2 Study Group. Safety and adverse events after targeted lung denervation for symptomatic moderate to severe chronic obstructive pulmonary disease (AIRFLOW): a multicenter randomized controlled clinical trial. Am J Respir Crit Care Med. 2019;200:1477–1486. doi: 10.1164/rccm.201903-0624OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davey C, Zoumot Z, Jordan S, McNulty WH, Carr DH, Hind MD, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet. 2015;386:1066–1073. doi: 10.1016/S0140-6736(15)60001-0. [DOI] [PubMed] [Google Scholar]
- 38.Shah PL, Slebos DJ, Cardoso PF, Cetti E, Voelker K, Levine B, et al. EASE trial study group. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trial. Lancet. 2011;378:997–1005. doi: 10.1016/S0140-6736(11)61050-7. [DOI] [PubMed] [Google Scholar]
- 39.Gordon IO, Husain AN, Charbeneau J, Krishnan JA, Hogarth DK. Endobronchial biopsy: a guide for asthma therapy selection in the era of bronchial thermoplasty. J Asthma. 2013;50:634–641. doi: 10.3109/02770903.2013.794239. [DOI] [PubMed] [Google Scholar]
- 40.Mullen JB, Wright JL, Wiggs BR, Pare PD, Hogg JC. Reassessment of inflammation of airways in chronic bronchitis. Br Med J (Clin Res Ed) 1985;291:1235–1239. doi: 10.1136/bmj.291.6504.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagai A, West WW, Thurlbeck WM. The National Institutes of Health Intermittent Positive-Pressure Breathing trial: pathology studies. II. Correlation between morphologic findings, clinical findings, and evidence of expiratory air-flow obstruction. Am Rev Respir Dis. 1985;132:946–953. doi: 10.1164/arrd.1985.132.5.946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.