Abstract
Purpose
To examine the foveal avascular zone (FAZ) in patients with congenital achromatopsia (ACHM).
Methods
Forty-two patients with genetically confirmed ACHM were imaged either with Optovue's AngioVue system or Zeiss's Plex Elite 9000, and the presence or absence of a FAZ was determined. For images where a FAZ was present and could be confidently segmented, FAZ area, circularity index, and roundness were measured and compared with previously published normative values. Structural optical coherence tomography images were acquired to assess the degree of foveal hypoplasia (number and thickness of inner retinal layers present at the fovea).
Results
A FAZ was present in 31 of 42 patients imaged (74%), although no determination could be made for 11 patients due to poor image quality (26%). The mean ± SD FAZ area for the ACHM retina was 0.281 ± 0.112 mm2, which was not significantly different from the previously published normative values (P = 0.94). However, their FAZs had decreased circularity (P < 0.0001) and decreased roundness (P < 0.0001) compared to the normative cohort. In the patients with ACHM examined here, the FAZ area decreased as the number and thickness of the retained inner retinal layers increased.
Conclusions
Our data demonstrate that despite the presence of foveal hypoplasia, patients with ACHM can have a FAZ. This is distinct from other conditions associated with foveal hypoplasia, which generally show an absence of the FAZ. In ACHM, FAZ formation does not appear to be sufficient for complete pit formation, contrary to some models of foveal development.
Keywords: foveal avascular zone, achromatopsia, retinal development, optical coherence tomography angiography
The human fovea is a highly specialized region of the retina that underpins our high-acuity vision and color discrimination.1,2 The foveal region is characterized by an avascular zone (aka, the foveal avascular zone, or FAZ) and an absence of the inner retinal layers. While the fovea occupies only about 0.02% of the retinal surface area, some 40% of primary visual cortex (V1) receives input from the fovea.3 This is made possible by the high density of cone photoreceptors,4 the specialized “private line” circuitry between cones and midget ganglion cells,5,6 and increased cortical sampling of foveal inputs in V1.7,8 Given that foveal structure and function is affected in a wide range of retinal diseases that lead to severe visual impairment,9–13 there is significant interest in understanding how the fovea develops, especially the relationship between different foveal specializations.
Histologic studies have shown that a FAZ is defined before the pit begins to form.14 Additionally, in vivo studies using optical coherence tomography (OCT) have demonstrated a positive correlation between the size of the FAZ and the size of the pit in control populations.15–18 Moreover, patients with albinism lack both a FAZ and complete formation of the pit (i.e., foveal hypoplasia).11,19 Taken together, these data support current models of foveal development that propose the FAZ as a prerequisite for the formation of the pit.1,20 However, recent data have shown the presence of foveal microvessels (absence of a normal FAZ) in individuals with a normal-appearing pit,21–27 rather than overt foveal hypoplasia as might be expected. As foveal hypoplasia is present in a wide range of diseases (and even in normally sighted individuals),11,28–34 studying the FAZ in these patients may provide further insight into how these foveal specializations are related. Such data would be valuable in advancing our understanding of foveal development.
Congenital achromatopsia (ACHM) is an autosomal recessive disease characterized by almost complete loss of cone photoreceptor function.35 Symptoms of this disorder include nystagmus, low-acuity vision, reduced or absent color discrimination, and photophobia. These patients have a varying amount of inner retina present at the fovea, ranging from severe foveal hypoplasia to normal (complete) displacement of inner retinal layers at the fovea,36 although there have been no studies of the FAZ in these patients. Here we report the discovery of normal-sized FAZs in patients with ACHM, most of whom have persistent inner retinal layers at the fovea. Our data demonstrate that the formation of a FAZ is not sufficient for normal pit formation in ACHM.
Materials and Methods
Patients
The study followed the tenets of the Declaration of Helsinki and was approved by the institutional review boards at the Medical College of Wisconsin and Moorfields Eye Hospital. Informed consent was obtained from all patients after the nature and possible consequences of the study were explained. Forty-two patients with genetically confirmed ACHM were enrolled for imaging (Supplementary Table S1). The patients with ACHM imaged at the Medical College of Wisconsin (n = 27) were screened for a history of premature birth. In addition, a 26-year-old woman with no known vision pathology and no history of premature birth was recruited for this study for illustrative purposes. Axial length measurements were obtained from all individuals using an IOLMaster (Carl Zeiss Meditec, Dublin, CA, USA). When necessary, one drop each of tropicamide (1%) and of phenylephrine hydrochloride (2.5%) were instilled into the eye for pupillary dilation and cycloplegia.
Spectral Domain OCT (SD-OCT) Imaging
High-resolution structural images of the macula were acquired using a Bioptigen Envisu R2200 SD-OCT system (Leica Microsystems, Wetzlar, Germany) or Spectralis SD-OCT system (Heidelberg Engineering, Heidelberg, Germany). For patients imaged using the Bioptigen (n = 35), high-density line scans (1000 A-scans/B-scans, 80 repeated B-scans) and nominal 7-mm volume scans (750 A-scans/B-scans, 250 B-scans) were acquired at the fovea. Line scans were registered based on the presence of the foveal reflex and averaged to reduce speckle noise in the image.37 For patients imaged using the Spectralis (n = 7), 20˚ or 30˚ OCT volume or line scans were acquired. To ensure the scans used for analysis were at the foveal center, volume scans were manually inspected by one observer (R.E.L.) to confirm the location of the fovea. One observer (K.M.L.) graded all foveal line scans for disruption of the ellipsoid zone (i.e., photoreceptor band) using a previously established grading system.36,38 Three graders (R.E.L., E.N.W., and J.C.) reviewed all the foveal line scans to determine if there were retained inner retinal layers and (if present) which layers were retained. Graders were masked as to whether a given subject had a FAZ, and a consensus approach was used in cases of disagreement. Additionally, using logarithmic grayscale images and custom software (OCT Reflectivity Analytics, Milwaukee, WI, USA), longitudinal reflectance profiles were used to measure the thickness of the inner retinal layers and the outer nuclear layer at the fovea for each subject.39 Two repeated thickness measurements were made for each layer and the averaged values were used for subsequent analyses.
OCT Angiography (OCT-A) Imaging and Analysis
Patients were imaged either using the AngioVue system (Optovue, Inc., Fremont, CA, USA) at the Medical College of Wisconsin or the Plex Elite 9000 (Carl Zeiss; Meditec, Dublin, CA, USA) at Moorfields Eye Hospital. When possible, both eyes were imaged, and the eye with the better image quality was chosen for analysis. The remaining patients had only one eye dilated for other imaging, so that eye was used for this study. For the AngioVue system, two volumes consisting of 304 B-scans at 304 A-scans/B-scans were acquired, centered on the fovea, with a nominal size of 3 × 3 mm. One volume had a horizontal fast scanning axis, while the other volume had a vertical fast scanning axis. The two volumes were registered (AngioVue software version: 2017.1.0.151) to create a single volume with reduced motion artifact and increased signal-to-noise ratio (SNR). From each volume, the full-thickness angiogram was extracted by integrating motion contrast data from the inner limiting membrane (ILM) to 9 µm above the outer plexiform layer (OPL). Three to seven volumes were obtained per patient based image quality. Where possible, three to five volumes were then averaged together as previously described40,41 to further increase image SNR. The averaged image was used for FAZ analyses.
For the Plex Elite 9000, one volume consisting of 1024 B-scans and 350 A-scans/B-scans was acquired with a horizontal fast scanning axis. The nominal size was also 3 × 3 mm. The superficial layer, measuring from the ILM to the outer boundary of the inner plexiform layer (IPL), was extracted and single frames were used for FAZ analyses. The real scale of each image (µm/pixel) was calculated by multiplying the nominal image scale by the ratio of the patient's axial length to the axial length assumed by the OCT-A device (Zeiss = 24.46 mm, Optovue = 23.95 mm).
Determination of whether a FAZ was present was done by a single observer (R.E.L.) with three different possibilities: FAZ present, FAZ not present, and FAZ presence not determined due to poor image quality (Fig. 1). For the images in which a FAZ was present, a single observer (R.E.L.) subjectively determined if the image was of sufficient quality for segmentation (i.e., minimal motion artifacts around the FAZ, good vessel contrast, no motion artifacts, etc.). If the image quality was deemed sufficient, the FAZ for each patient was segmented by the same observer using ImageJ software's multipoint tool (National Institutes of Health, Bethesda, MD, USA).42 The segmentation coordinates were entered into a custom MATLAB (MathWorks, Inc., Natick, MA, USA) script based on previous work in our lab.43,44 The area of the FAZ (in pixels) was computed using the poly2area function. The perimeter of the FAZ (in pixels) was calculating by simply summing the distances between each pair of neighboring segmentation coordinates. The area and perimeter were both converted into retinal units (millimeters) by multiplying by the real scale of that image (described above). Circularity is a unitless metric that is defined by the following equation:
Figure 1.
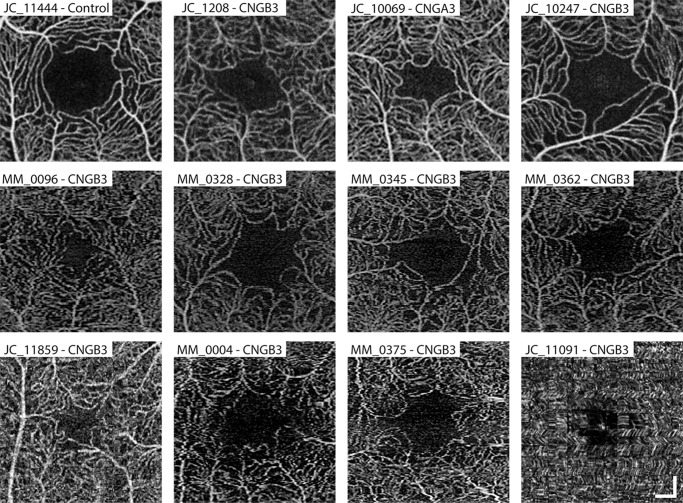
Representative OCT-A images used to subjectively decide if a FAZ is present and, if present, analyzable. An OCT-A scan from the right eye of a 26-year-old female subject with normal vision is shown for reference (top left). This normal-appearing FAZ has an area of 0.389 mm2, a circularity index of 0.593, and a roundness of 0.852. The remaining panels in the top row are examples of patients imaged using the Optovue AngioVue system with multiple frames averaged together. The second row includes single-frame images from patients imaged using the Zeiss Plex Elite 9000. All seven patients in the top two rows were determined to have a present and analyzable FAZ. The first three patients in the bottom row were all graded as having a FAZ, but distortions at the fovea make segmentation and measurement of the FAZ unreliable. The subject in the bottom right had poor image quality that made it not possible to determine whether a FAZ was present. Scale bars: 200 µm.
Additionally, using the MATLAB function poly2mask, a mask was produced from the segmentation coordinates and regionprops was used to derive an ellipse with the same normalized second central moment (variance) as the mask. The normalized second central moment is calculated as follows:
where the centroid of the FAZ mask (or ellipse) is xc,yc and xi,yi represents every point within the FAZ mask (or ellipse). The major axis (longest diameter, pixels) and the minor axis (shortest diameter, pixels) of the resulting ellipse were extracted. These values were again converted into retinal units (millimeters) by multiplying by the real scale of the image. Roundness is a unitless metric that is defined by the following equation:
All statistical analyses were conducted using Prism v8.2.1 (GraphPad Software, La Jolla, CA, USA). Specific statistical tests are reported alongside the individual results. The choice of parametric versus nonparametric test was based on an analysis of normality for each group using the Shapiro-Wilk test (using P > 0.05 as the criterion).
Results
The demographics for the 42 patients with ACHM recruited for this study are given in Supplementary Table S1, 25 of whom have been previously reported in other studies (Supplementary Table S2).36,38,45–49 There were 19 men and 23 women, with a mean ± SD age of 29.2 ± 13.4 years. None of the 27 patients with ACHM imaged on the AngioVue system reported a history of premature birth. A FAZ was present in 31 of 42 individuals imaged (74%), and no determination could be made for 11 patients due to poor image quality (26%). Example OCT-A images, including those for which no determination could be made, are shown in Figure 1. Of the 31 patients who were judged to have a FAZ, 24 (77%) had minimal motion artifacts around the FAZ, which allowed for confident segmentation and derivation of quantitative FAZ metrics. No significant difference in FAZ area, circularity, and roundness was observed between the devices used here (Table 1; P = 0.91, P = 0.93, and P = 0.72, respectively). As such, we treated the patients with ACHM as a single group for subsequent analyses.
Table 1.
FAZ Metrics Summary of the Subject Population
| FAZ Metrics*,† | ||||
|---|---|---|---|---|
| OCTA Device | Number of Eyes | Area (mm2) | Circularity Index | Roundness |
| AngioVue | 16 | 0.279 ± 0.120 | 0.502 ± 0.098 | 0.756 ± 0.073 |
| Plex Elite 9000 | 8 | 0.285 ± 0.100 | 0.498 ± 0.088 | 0.766 ± 0.061 |
| P = 0.91‡ | P = 0.93‡ | P = 0.72‡ | ||
All data are listed as mean ± standard deviation.
All data passed Shapiro-Wilk normality test (P > 0.05).
Unpaired t-test.
In order to evaluate whether the FAZ morphometry observed in our patients with ACHM differed from normal, we compared their data to a group of 175 previously published patients with no vision pathology.50 The mean ± SD FAZ area for the control group was 0.280 ± 0.101 mm2, which was not significantly different from the patients with ACHM (0.281 ± 0.112 mm2; P = 0.94, Mann-Whitney U test). In contrast, the circularity index of the FAZ in the control group (mean ± SD = 0.682 ± 0.106) was significantly greater than that for the patients with ACHM (0.501 ± 0.093; P < 0.0001, unpaired t-test). Likewise, the roundness of the FAZ in the control group (mean ± SD = 0.860 ± 0.068) was significantly greater than that for the patients with ACHM (0.759 ± 0.068; P < 0.0001, Mann-Whitney U test). Additional normative FAZ morphometry data from other published studies are provided in Table 2 for reference, which are consistent with the FAZ in ACHM being normal in area but more irregular in overall contour.
Table 2.
Summary of Previous OCT-A Studies Assessing FAZ Area, Circularity Index, and Roundness in Patients Without Disease
| FAZ Metrics* | |||||
|---|---|---|---|---|---|
| Study | OCTA Device | Number of Eyes | Area (mm2)† | Circularity Index | Roundness |
| Yasin Alibhai et al65 | AngioVue | 40 | 0.33 ± 0.15 | 0.73 ± 0.19‡ | ND |
| Arya et al66 | Angioplex | 8 | 0.333 ± 0.063 | ND | ND |
| AngioVue | 0.312 ± 0.073 | ND | ND | ||
| Plex Elite 9000 | 0.305 ± 0.071 | ND | ND | ||
| Borrelli et al67 | Angiovue | 77 | 0.261 ± 0.149 | ND | ND |
| Chen et al68 | Angiovue | 50 | 0.233 ± 0.108§ | ND | ND |
| Choi et al69 | Angioplex | 52 | 0.35 ± 0.11 | 0.81 ± 0.07 | ND |
| Corvi et al70 | Angioplex | 36 | 0.232 ± 0.004 | ND | ND |
| AngioVue | 0.2221 ± 0.1002 | ND | ND | ||
| Plex Elite 9000 | 0.2250 ± 0.1004 | ND | ND | ||
| Durbin et al71 | Angioplex | 50 | 0.25 ± 0.10 | 0.82 ± 0.06 | ND |
| Hsieh et al72 | AngioVue | 22 | 0.35 ± 0.08 | 0.74 ± 0.11‡ | ND |
| Inanc et al73 | AngioVue | 57 | 0.27 ± 0.13 | 0.94 ± 0.13‡ | ND |
| Ishii et al74 | Plex Elite 9000 | 40 | 0.244 ± 0.81 | ND | ND |
| Lin et al75 | Angioplex | 35 | 0.255 ± 0.112 | 0.64 ± 0.14 | ND |
| Linderman et al50∥ | AngioVue | 175 | 0.280 ± 0.101§ | 0.682 ± 0.106 | 0.860 ± 0.068 |
| Sacconi et al76 | Plex Elite 9000 | 32 | 0.199 ± 0.100 | ND | ND |
| Shiihara et al77 | AngioVue | 70 | 0.329 ± 0.115 | 0.769 ± 0.064 | 0.878 ± 0.071 |
ND, not determined.
All data are listed as mean ± standard deviation.
Significant digits are listed as reported in the original study.
Study originally reported circularity as acircularity. Acircularity was converted to circularity using the following equation: Circularity = 1/Acircularity2.
§Study corrected FAZ area for ocular magnification.
The right eye of the original data was reprocessed with the same code used in the current study.
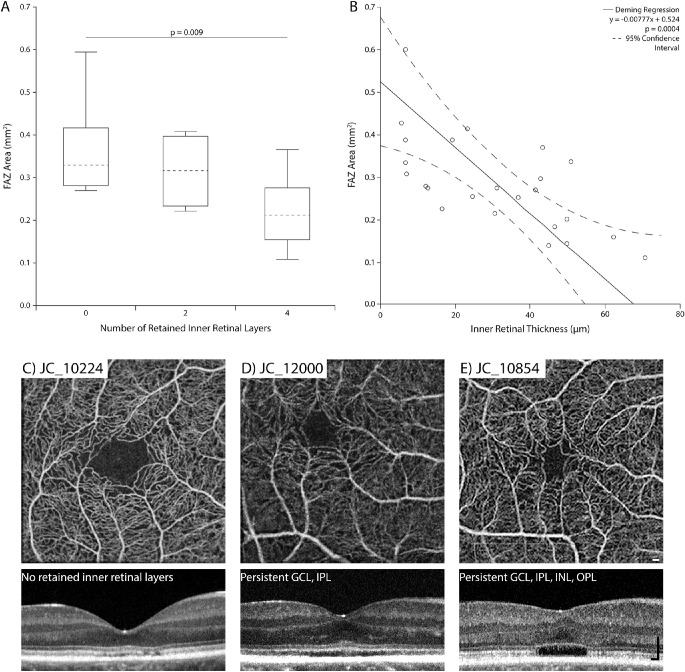
We sought to determine if there was a relationship between the size of the FAZ and the foveal retinal layers. Of the 31 patients in whom a FAZ was determined to be present, 22 (71%) had retained inner retinal layers at the fovea. Of these patients, 17 (77%) had retention of all inner retinal layers (ganglion cell layer (GCL), IPL, INL, and OPL) and 5 (23%) had retention of GCL and IPL, but no inner nuclear layer (INL) and OPL. The remaining 9 patients (29%) appeared to have complete displacement of the inner retinal layers at the fovea. As shown in Figure 2A, FAZ area decreased as the number of retained inner retinal layers increased (one-way ANOVA, P = 0.01), although there was significant overlap between groups. FAZ area was significantly smaller in patients with four retained inner retinal layers compared to those with no observable retained inner retinal layers (Tukey's multiple comparison test, P = 0.009). However, there was no significant difference in FAZ area between patients with no observable retained inner retinal layers and those with two retained inner retinal layers (Tukey's multiple comparison test, P = 0.64) or between patients with two retained inner retinal layers and those with four retained inner retinal layers (Tukey's multiple comparison test, P = 0.22). Additionally, as the thickness of the retained inner retinal layers increased, the FAZ area decreased (Deming regression, P = 0.0004, Fig. 2B). Images from patients demonstrating this trend are shown in Figures 2C–2E. In contrast to FAZ area, there was no significant relationship between inner retinal layer thickness and either FAZ circularity index (Deming regression, P = 0.27) or FAZ roundness (Deming regression, P = 0.44), as shown in Supplementary Figure S1, although there was small but significant relationship between outer nuclear layer thickness and FAZ area (Supplementary Figure S2; Deming regression, P = 0.05). Finally, there was no association between FAZ area and the severity of the ellipsoid zone disruption (Kruskal-Wallis test, P = 0.14).
Figure 2.
Relationship between retained inner retinal layers at the fovea and FAZ morphometry in patients with ACHM. (A) As shown in the box-and-whiskers plot (dashed line: median; box limits: 25th–75th percentiles; whiskers: minimum and maximum), there was a significant difference in FAZ area between patients with different numbers of retained inner retinal layers (one-way ANOVA, P = 0.01). Further analysis showed the only significant difference between groups occurred between patients with no retained inner retinal layers (0) and those with all four layers retained (Tukey's multiple comparison test, P = 0.009). (B) There was a significant negative relationship between the inner retinal layer thickness and FAZ area (Deming regression, y = –0.00777x + 0.524, P = 0.004). The 95% confidence intervals (dashed lines) were calculated using the jackknife method. (C) This subject has normal inner retinal layer appearance at the fovea (zero layers retained). Inner retinal thickness = 7.17 µm, FAZ area = 0.384 mm2, circularity index = 0.524, and roundness = 0.731. (D) This subject has two layers retained (GCL and IPL). Inner retinal thickness = 16.73 µm, FAZ area = 0.220 mm2, circularity index = 0.560, and roundness = 0.786. (E) This subject has four layers retained (GCL, IPL, INL, and OPL). Inner retinal thickness = 62.74 µm, FAZ area = 0.155 mm2, circularity index = 0.383, and roundness = 0.597. Note that this subject has a disrupted ellipsoid zone (aka, a hyporeflective zone) at the fovea, which is commonly seen in patients with ACHM. Scale bars: 100 µm.
Since we could not determine the presence of the FAZ in all patients, we wanted to examine whether a selection bias may be influencing our results relating FAZ area with foveal inner retinal lamination. There was no difference in inner retinal thickness (unpaired t-test, P = 0.71) or age (Mann-Whitney U test, P = 0.74) between patients with a FAZ and patients for whom the presence of a FAZ could not be determined. Likewise, we saw no difference in the distribution of mutation (Fisher’ exact test, P = 0.64), sex (Fisher's exact test, P = 0.73), ellipsoid zone disruption (chi-square test, P = 0.66), or the number of persistent inner retinal layers at the fovea (chi-square test, P = 0.29). These findings suggest that our observed relationship between FAZ area and inner retinal layer thickness likely holds for all patients with CNGA3- or CNGB3-associated ACHM.
Discussion
As OCT and OCT-A imaging have become standard for both clinical care in ophthalmology and vision research, the relationship between the fovea and the FAZ has been scrutinized in an attempt to understand how the fovea develops. Despite a number of studies,11,16,17,20 the relationship between these specializations remains unclear. Studies suggest that the FAZ is delineated prior to any pit formation; however, the mechanism for how the pit is formed as well as the potential role of the FAZ in this process remains uncertain. One theory suggests that the pit is passively created due to an increased elasticity of the incipient fovea (due to a lack of vasculature in the region), an increase in interocular pressure, and normal eye growth.1 Another theory suggests the metabolic demands of the foveal region causes a hypoxic environment, which in turn causes the displacement of the inner retinal layers.2 Here we show, for the first time, FAZ areas within the normal anatomical range in patients with foveal hypoplasia. It is important to consider that 9 of the 31 patients with ACHM for whom we measured the FAZ had no inner retinal layers present at their fovea. In these patients, the presence of a FAZ is not at all surprising. Even if we remove these 9 patients from the analysis, the average FAZ area (0.245 mm2, n = 22) was not significantly different from the controls (Mann-Whitney U test, P = 0.157). However, we do see a negative relationship between the size of the FAZ present and the residual inner retina at the fovea, which supports a facilitatory role of the FAZ in the process of pit development as previously predicted from finite element modeling.51 Taken together, our data clearly demonstrate that a normal-sized FAZ alone is not sufficient to effect complete pit formation in ACHM.
Further insight on foveal development comes from comparing our findings in ACHM to findings in albinism. Patients with albinism have a similar persistence of inner retinal layers across the fovea, but the sparse data available to date have shown that they lack a FAZ (Phillips E, et al. IOVS 2016;57:ARVO E-Abstract 5461).19,52,53 While patients with albinism have reduced peak foveal cone densities compared to control populations,11 these cones are nonetheless functional. In contrast, patients with ACHM have severely diminished to no cone function.54 This, in turn, may lead to reduced activity of the midget ganglion cells in the foveal region in patients with ACHM, which could reduce the overall metabolic demands of this region. If true, this would support a role for cone pathway activity in the process of foveal development and offers a possible area for further investigation in nonhuman primate models.
Our findings in ACHM (normally sized FAZs in the presence of foveal hypoplasia) are in stark contrast to those in other conditions associated with foveal hypoplasia.12,13,34,55–57 For example, Yanni et al.57 observed a FAZ in preterm children, although again about half the area of that seen in full-term children. They used an en face OCT method that examined the deep capillary plexus, so they would have missed any persistent capillaries in the superficial plexus. Falavarjani et al.12 found that a distinct FAZ was absent in 42.8% of eyes in preterm children. When a FAZ was present, it was accompanied by an absence of inner retinal layers at the fovea and was considerably smaller than normal and appeared as a fragmented structure opposed to a single avascular area. This is similar to that reported by Miki et al.,56 who observed small (average = 0.14 mm2), fragmented FAZ-like structures in patients with a history of laser treatment for retinopathy of prematurity. Another study of children with a history of treatment for retinopathy of prematurity (laser or intravitreal ranibizumab) also reported very small and fragmented FAZ-like structures, often accompanied by an absence of persistent inner retinal layers at the fovea.55 Not only are the FAZs reported in our study not significantly different from normal in area, but there was only one subject with ACHM who had a fragmented FAZ, assessed using our recently described objective and automated method.58 It would be interesting to reexamine OCT-A images from patients with foveal hypoplasia and exclude those with a fragmented FAZ before quantitative analyses.
Our study has a number of important limitations. Most notably, only individuals with CNGA3 or CNGB3 genotypes were included. While there are reported differences in cone structure in the GNAT2,59,60 ATF6,61 and PDE6C forms of ACHM,62 it is unknown whether a FAZ is present in individuals with ACHM caused by these genotypes. Additionally, there are reports of residual cone function in patients with ACHM,63 and patients with GNAT2-associated ACHM have been reported to have cone-mediated visual function at high radiances (although only at low temporal frequencies).64 While we did not assess cone function in the patients imaged in the present study, it would be valuable to examine the FAZ in patients with ACHM who have clearly demonstrable cone function as this would advance our understanding of how metabolic demands may impact FAZ and pit formation. Another limitation is that we did not employ an age-, race-, or sex-matched control group. These variables have been shown to affect the FAZ morphometry, although the numerous studies listed in Table 2 provide confidence that the FAZ area observed in our ACHM cohort is well within published normative ranges. A final limitation relates to the interpretation of our key observation (presence of a normal-sized FAZ in ACHM). While our youngest subject was 10 years old, the FAZ normally forms prenatally.1 Thus, we cannot rule out that there was no FAZ at birth in any of our patients and that vascular remodeling (such as that occurs in diabetic retinopathy9) occurred postnatally to effectively create a FAZ. The normal area of the FAZ in ACHM would argue against this, although the decreased circularity/roundness of the FAZ in ACHM could be considered consistent with this alternate interpretation. However, the nystagmus present in patients with ACHM can result in distortions in the OCTA image (even in images with high SNR), which would affect the measurements of the circularity/roundness of the FAZ. Thus, we believe the simplest explanation is that the FAZ developed normally but the pit did not fully form in all patients.
In conclusion, patients with ACHM-associated foveal hypoplasia have a FAZ that suggests that the FAZ is necessary but not sufficient for normal pit formation for these patients. These data should help in further constraining comprehensive models of foveal development as well as shed additional light on the complex relationship between the different anatomic specializations of the human fovea.
Supplementary Material
Acknowledgments
The authors thank Erin Curran, Brian Higgins, Kaitlin McKenney, Phyllis Summerfelt, and Vesper Williams for their contributions to this work.
Supported by the National Eye Institute, the National Institute of Aging, the National Institute of General Medical Sciences, the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award numbers R01EY024969, R01EY017607, F32EY029148, T35AG029793, T32GM080202, TL1TR001437, and UL1TR001436; the National Institute for Health Research Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology; Onassis Foundation; Leventis Foundation; Moorfields Eye Hospital Special Trustees; Moorfields Eye Charity; Retina UK; and the Foundation Fighting Blindness (USA). This investigation was conducted in a facility constructed with support from the Research Facilities Improvement Program, grant C06RR016511, from the National Center for Research Resources, NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional support given from the Thomas M. Aaberg, Sr. Retina Research Fund and AchromaCorp.
Disclosure: R.E. Linderman, Optovue (C); M. Georgiou, MeiraGTx (C); E.N. Woertz, None; J.A. Cava, None; K.M. Litts, None; S. Tarima, None; R. Rajendram, Carl Zeiss AG (F, R); J.M. Provis, None; M. Michaelides, MeiraGTx (C); J. Carroll, AGTC (F), MeiraGTx (F, C), Optovue (F), Translational Imaging Innovations (I), US Patent 9,427,147 (P)
References
- 1. Provis JM, Dubis AM, Maddess T, Carroll J. Adaptation of the central retina for high acuity vision: cones, the fovea, and the avascular zone. Prog Retin Eye Res. 2013; 35: 63–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bringmann A, Syrbe S, Görner K, et al.. The primate fovea: structure, function and development. Prog Retin Eye Res. 2018; 66: 49–84. [DOI] [PubMed] [Google Scholar]
- 3. Hendrickson A. Organization of the adult primate fovea. In: Penfold PL, Provis JM, eds. Macular Degeneration. Heidelberg, Germany: Springer-Verlag; 2005: 1–20. [Google Scholar]
- 4. Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990; 292: 497–523. [DOI] [PubMed] [Google Scholar]
- 5. Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990; 300: 5–25. [DOI] [PubMed] [Google Scholar]
- 6. Dacey DM. The mosaic of midget ganglion cells in the human retina. J Neurosci. 1993; 13: 5334–5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Azzopardi P, Cowey A. Preferential representation of the fovea in the primary visual cortex. Nature. 1993; 361: 719–721. [DOI] [PubMed] [Google Scholar]
- 8. Rossi EA, Achtman RL, Guidon A, et al.. Visual function and cortical organization in carriers of blue cone monochromacy. PLoS One. 2013; 8: e57956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lynch G, Andrade Romo JS, Linderman R, et al.. Within-subject assessment of foveal avascular zone enlargement in different stages of diabetic retinopathy using en face OCT reflectance and OCT angiography. Biomed Opt Express. 2018; 9: 5982–5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sanders RJ, Brown GC, Rosenstein RB, Magargal L. Foveal avascular zone diameter and sickle cell disease. Arch Ophthalmol. 1991; 109: 812–815. [DOI] [PubMed] [Google Scholar]
- 11. Wilk MA, McAllister JT, Cooper RF, et al.. Relationship between foveal cone specialization and pit morphology in albinism. Invest Ophthalmol Vis Sci. 2014; 55: 4186–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Falavarjani KG, Iafe NA, Velez FG, et al.. Optical coherence tomography angiography of the fovea in children born preterm. Retina. 2017; 37: 2289–2294. [DOI] [PubMed] [Google Scholar]
- 13. Mintz-Hittner HA, Knight-Nanan DM, Satriano DR, Kretzer FL. A small foveal avascular zone may be an historic mark of prematurity. Ophthalmology. 1999; 106: 1409–1413. [DOI] [PubMed] [Google Scholar]
- 14. Provis JM, Sandercoe T, Hendrickson AE. Astrocytes and blood vessels define the foveal rim during primate retinal development. Invest Ophthalmol Vis Sci. 2000; 41: 2827–2836. [PubMed] [Google Scholar]
- 15. Samara WA, Say EA, Khoo CT, et al.. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina. 2015; 35: 2188–2195. [DOI] [PubMed] [Google Scholar]
- 16. Dubis AM, Hansen BR, Cooper RF, Beringer J, Dubra A, Carroll J. Relationship between the foveal avascular zone and foveal pit morphology. Invest Ophthalmol Vis Sci. 2012; 53: 1628–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chui TYP, Zhong Z, Song H, Burns SA. Foveal avascular zone and its relationship to foveal pit shape. Optom Vis Sci. 2012; 89: 602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chui TYP, VanNasdale DA, Elsner AE, Burns SA. The association between the foveal avascular zone and retinal thickness. Invest Ophthalmol Vis Sci. 2014; 55: 6870–6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marmor MF, Choi SS, Zawadzki RJ, Werner JS. Visual insignificance of the foveal pit: reassessment of foveal hypoplasia as fovea plana. Arch Ophthalmol. 2008; 126: 907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tick S, Rossant F, Ghorbel I, et al.. Foveal shape and structure in a normal population. Invest Ophthalmol Vis Sci. 2011; 52: 5105–5110. [DOI] [PubMed] [Google Scholar]
- 21. Yokoyama T, Maruko I, Koizumi H, Ishikawa Y, Iida T. Unmeasurable small size of foveal avascular zone without visual impairment in optical coherence tomography angiography. Eye (Lond). 2018; 32: 1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carpineto P, Mastropasqua R, Marchini G, Toto L, Di Nicola M, Di Antonio L. Reproducibility and repeatability of foveal avascular zone measurements in healthy subjects by optical coherence tomography angiography. Br J Ophthalmol. 2016; 100: 671–676. [DOI] [PubMed] [Google Scholar]
- 23. Sousa DC, Leal I, Abegão Pinto L. Vessels crossing the fovea: a familial normal variant? Ophthalmol Retina. 2017; 1: 67. [DOI] [PubMed] [Google Scholar]
- 24. Takagi M, Maruko I, Yamaguchi A, Kakehashi M, Hasegawa T, Iida T. Foveal abnormalities determined by optical coherence tomography angiography in children with history of retinopathy of prematurity. Eye (Lond). 2019; 33: 1890–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeung J, Crock G, Cairns J, Heinze J, Troski S, Billson F. Macular-foveal capillaries in human retina. Aust J Ophthalmol. 1973; 1: 17–23. [DOI] [PubMed] [Google Scholar]
- 26. Cicinelli MV, Carnevali A, Rabiolo A, et al.. Clinical spectrum of macular-foveal capillaries evaluated with optical coherence tomography angiography. Retina. 2017; 37: 436–443. [DOI] [PubMed] [Google Scholar]
- 27. Pilotto E, Leonardi F, Deganello D, Convento E, Midena E, Frizziero L. Morphofunctional evaluation of macular-foveal capillaries: a comparative optical coherence tomography angiography and microperimetry study. Retina. 2020; 40: 1279–1285. [DOI] [PubMed] [Google Scholar]
- 28. Thomas MG, Kumar A, Kohl S, Proudlock FA, Gottlob I. High-resolution in vivo imaging in achromatopsia. Ophthalmology. 2011; 118: 882–887. [DOI] [PubMed] [Google Scholar]
- 29. Thomas MG, Kumar A, Mohammad S, et al.. Structural grading of foveal hypoplasia using spectral-domain optical coherence tomography: a predictor of visual acuity? Ophthalmology. 2011; 118: 1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han J, Lee T, Lee JB, Han SH. Retinal microstructures are altered in patients with idiopathic infantile nystagmus. Graefes Arch Clin Exp Ophthalmol. 2017; 255: 1661–1668. [DOI] [PubMed] [Google Scholar]
- 31. Ecsedy M, Szamosi A, Karko C, et al.. A comparison of macular structure imaged by optical coherence tomography in preterm and full-term children. Invest Ophthalmol Vis Sci. 2007; 48: 5207–5211. [DOI] [PubMed] [Google Scholar]
- 32. Saffra N, Agarwal S, Chiang JP, Masini R, Bertolucci A. Spectral-domain optical coherence tomographic characteristics of autosomal recessive isolated foveal hypoplasia. Arch Ophthalmol. 2012; 130: 1324–1327. [DOI] [PubMed] [Google Scholar]
- 33. Pedersen HR, Baraas RC, Landsend ECS, et al.. PAX6 genotypic and retinal phenotypic characterization in congenital aniridia. Invest Ophthalmol Vis Sci. 2020; 61: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsushita I, Nagata T, Hayashi T, et al.. Foveal hypoplasia in patients with Stickler syndrome. Ophthalmology. 2017; 124: 896–902. [DOI] [PubMed] [Google Scholar]
- 35. Hirji N, Aboshiha J, Georgiou M, Bainbridge J, Michaelides M. Achromatopsia: clinical features, molecular genetics, animal models and therapeutic options. Ophthalmic Genet. 2018; 39: 149–157. [DOI] [PubMed] [Google Scholar]
- 36. Sundaram V, Wilde C, Aboshiha J, et al.. Retinal structure and function in achromatopsia: implications for gene therapy. Ophthalmology. 2014; 121: 234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tanna H, Dubis AM, Ayub N, et al.. Retinal imaging using commercial broadband optical coherence tomography. Br J Ophthalmol. 2010; 94: 372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Langlo CS, Patterson EJ, Higgins BP, et al.. Residual foveal cone structure in CNGB3-associated achromatopsia. Invest Ophthalmol Vis Sci. 2016; 57: 3984–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilk MA, Wilk BM, Langlo CS, Cooper RF, Carroll J. Evaluating outer segment length as a surrogate measure of peak foveal cone density. Vision Res. 2017; 130: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mo S, Phillips E, Krawitz BD, et al.. Visualization of radial peripapillary capillaries using optical coherence tomography angiography: the effect of image averaging. PLoS One. 2017; 12: e0169385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Uji A, Balasubramanian S, Lei J, et al.. Multiple enface image averaging for enhanced optical coherence tomography angiography imaging. Acta Ophthalmologica. 2018; 96: e820–e827. [DOI] [PubMed] [Google Scholar]
- 42. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Linderman R, Salmon AE, Strampe M, Russillo M, Khan J, Carroll J. Assessing the accuracy of foveal avascular zone measurements using optical coherence tomography angiography: segmentation and scaling. Transl Vis Sci Technol. 2017; 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilk MA, Dubis AM, Cooper RF, Summerfelt P, Dubra A, Carroll J. Assessing the spatial relationship between fixation and foveal specializations. Vision Res. 2017; 132: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Langlo CS, Erker LR, Parker M, et al.. Repeatability and longitudinal assessment of foveal cone structure in CNGB3-associated achromatopsia. Retina. 2017; 37: 1956–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hirji N, Georgiou M, Kalitzeos A, et al.. Longitudinal assessment of retinal structure in achromatopsia patients with long-term follow-up. Invest Ophthalmol Vis Sci. 2018; 59: 5735–5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aboshiha J, Dubis AM, Cowing J, et al.. A prospective longitudinal study of retinal structure and function in achromatopsia. Invest Ophthalmol Vis Sci. 2014; 55: 5733–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Georgiou M, Litts KM, Kalitzeos A, et al.. Adaptive optics retinal imaging in CNGA3-associated achromatopsia: retinal characterization, interocular symmetry, and intrafamilial variability. Invest Ophthalmol Vis Sci. 2019; 60: 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mastey RR, Gaffney M, Litts KM, et al.. Assessing the interocular symmetry of foveal outer nuclear layer thickness in achromatopsia. Transl Vis Sci Technol. 2019; 8: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Linderman RE, Muthiah MN, Omoba SB, et al.. Variability of foveal avascular zone metrics derived from optical coherence tomography angiography images. Transl Vis Sci Technol. 2018; 7: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Springer AD, Hendrickson AE.. Development of the primate area of high acuity. 1. Use of finite element analysis models to identify mechanical variables affecting pit formation. Vis Neurosci. 2004; 21: 53–62. [DOI] [PubMed] [Google Scholar]
- 52. Andrade Romo JS, Linderman RE, Pinhas A, Carroll J, Rosen RB, Chui TYP. Novel development of parafoveal capillary density deviation mapping using an age-group and eccentricity matched normative OCT angiography database. Transl Vis Sci Technol. 2019; 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pakzad-Vaezi K, Keane PA, Cardoso JN, Egan C, Tufail A. Optical coherence tomography angiography of foveal hypoplasia. Br J Ophthalmol. 2017; 101: 985–988. [DOI] [PubMed] [Google Scholar]
- 54. Aboshiha J, Dubis AM, Carroll J, Hardcastle AJ, Michaelides M. The cone dysfunction syndromes. Br J Ophthalmol. 2016; 100: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhao J, Wu Z, Lam W, et al.. Comparison of OCT angiography in children with a history of intravitreal injection of ranibizumab versus laser photocoagulation for retinopathy of prematurity [published online ahead of print February 12, 2020]. Br J Ophthalmol, doi: 10.1136/bjophthalmol-2019-315520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miki A, Yamada Y, Nakamura M. The size of the foveal avascular zone is associated with foveal thickness and structure in premature children. J Ophthalmol. 2019; 2019: 8340729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yanni SE, Wang J, Chan M, et al.. Foveal avascular zone and foveal pit formation after preterm birth. Br J Ophthalmol. 2012; 96: 961–966. [DOI] [PubMed] [Google Scholar]
- 58. Linderman RE, Cava JA, Salmon AE, et al.. Visual acuity and foveal structure in eyes with fragmented foveal avascular zones. Ophthalmol Retina. 2019; 4: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dubis AM, Cooper RF, Aboshiha J, et al.. Genotype-dependent variability in residual cone structure in achromatopsia: towards developing metrics for assessing cone health. Invest Ophthalmol Vis Sci. 2014; 55: 7303–7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Georgiou M, Singh N, Kane T, et al.. Photoreceptor structure in GNAT2-associated achromatopsia. Invest Ophthalmol Vis Sci. 2020; 61: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mastey RR, Georgiou M, Langlo CS, et al.. Characterization of retinal structure in ATF6-associated achromatopsia. Invest Ophthalmol Vis Sci. 2019; 60: 2631–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Georgiou M, Robson AG, Singh N, et al.. Deep phenotyping of PDE6C-associated achromatopsia. Invest Ophthalmol Vis Sci. 2019; 60: 5112–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nishiguchi KM, Sandberg MA, Gorji N, Berson EL, Dryja TP. Cone cGMP-gated channel mutations and clinical findings in patients with achromatopsia, macular degeneration, and other hereditary cone diseases. Hum Mutat. 2005; 25: 248–258. [DOI] [PubMed] [Google Scholar]
- 64. Stockman A, Smithson HE, Michaelides M, Moore AT, Webster AR, Sharpe LT. Residual cone vision without α-transducin. J Vis. 2007; 7: 8. [DOI] [PubMed] [Google Scholar]
- 65. Yasin Alibhai A, Moult EM, Shahzad R, et al.. Quantifying microvasculature changes using OCT angiography in diabetic eyes without clinical evidence of retinopathy. Ophthalmol Retina. 2018; 2: 418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Arya M, Rebhun CB, Yasin Alibhai A, et al.. Parafoveal retinal vessel density assessment by optical coherence tomography angiography in healthy eyes. Ophthalmic Surg Lasers Imaging Retina. 2018; 49: S5–S17. [DOI] [PubMed] [Google Scholar]
- 67. Borrelli E, Lonngi M, Balasubramanian S, et al.. Macular microvascular networks in healthy pediatric subjects. Retina. 2019; 39: 1216–1224. [DOI] [PubMed] [Google Scholar]
- 68. Chen FK, Menghini M, Hansen A, Mackey DA, Constable IJ, Sampson DM. Intrasession repeatability and interocular symmetry of foveal avascular zone and retinal vessel density in OCT angiography. Transl Vis Sci Technol. 2018; 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Choi J, Kwon J, Shin JW, Lee J, Lee S, Kook MS. Quantitative optical coherence tomography angiography of macular vascular structure and foveal avascular zone in glaucoma. PLoS One. 2017; 12: e0184948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Corvi F, Pellegrini M, Erba S, Cozzi M, Staurenghi G, Giani A. Reproducibility of vessel density, fractal dimension, and foveal avascular zone using 7 different optical coherence tomography angiography devices. Am J Ophthalmol. 2018; 186: 25–31. [DOI] [PubMed] [Google Scholar]
- 71. Durbin MK, An L, Shemonski ND, et al.. Quantification of retinal microvascular density in optical coherence tomography angiography images in diabetic retinopathy. JAMA Ophthalmol. 2017; 35: 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hsieh YT, Alam MN, Le D, et al.. OCT angiography biomarkeres for predicting visual outcomes after ranibizumab treatment for diabetic macular edema. Ophthalmol Retina. 2019; 3: 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Inanc M, Tekin K, Kiziltoprak H, Ozalkak S, Doguizi S, Aycan Z. Changes in retinal microcirculation precede the clinical onset of diabetic retinopathy in children with type 1 diabetes mellitus. Am J Ophthalmol. 2019; 207: 37–44. [DOI] [PubMed] [Google Scholar]
- 74. Ishii H, Shoji T, Yoshikawa Y, Kanno J, Ibuki H, Shinoda K. Automated measurement of the foveal avascular zone in swept source optical coherence tomography angiography images. Transl Vis Sci Technol. 2019; 8: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lin A, Fang D, Li C, Cheung CY, Chen H. Reliability of foveal avascular zone metrics automatically measured by cirrus optical coherence tomography angiography in healthy subjects. Int Ophthalmol. 2020; 40: 763–773. [DOI] [PubMed] [Google Scholar]
- 76. Sacconi R, Casaluci M, Borrelli E, et al.. Multimodal imaging assessment of vascular and neurodegenerative retinal alterations in type 1 diabetic patients without fundoscopic signs of diabetic retinopathy. J Clin Med. 2019; 8: 1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shiihara H, Terasaki H, Sonoda S, et al.. Objective evaluation of size and shape of superficial foveal avascular zone in normal subjects by optical coherence tomography angiography. Sci Rep. 2018; 8: 10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.