Abstract
Mitochondrial function is essential for ATP-supply, especially in response to different cellular stressors. Increased mitochondrial biogenesis resulting from caloric restriction (CR) has been reported. Resveratrol (RSV) is believed to mimic the physiological effects of CR mainly via a sirtuin (SIRT) 1-dependent pathway. The effect of RSV on the physiological function of mitochondrial respiratory complexes was evaluated using a Seahorse XF96. Myoblasts of five patients harboring the m.3243A>G mutation and five controls were analyzed. The relative expression of several genes involved in mitochondrial biogenesis was evaluated for a better understanding of the coherent mechanisms. Additionally, media-dependent effects of nutritional compounds and hormonal restrictions (R) on myoblasts from patients and controls in the presence or absence of RSV were investigated. Culturing of myoblasts under these conditions led to an upregulation of almost all the investigated genes compared to normal nutrition. Under normal conditions, there was no positive effect of RSV on mitochondrial respiration in patients and controls. However, under restricted conditions, the respiratory factors measured by Seahorse were improved in the presence of RSV. Further studies are necessary to clarify the involved mechanisms and elucidate the controversial effects of resveratrol on SIRT1 and SIRT3 expression.
Keywords: resveratrol, m.3243A>G mutation, SIRT1, SIRT3, OXPHOS
1. Introduction
Mitochondria are the cells’ main energy sources, converting nutrients into usable energy [1]. The mitochondrial DNA (mtDNA) is a double-stranded 16.5 kb circle molecule, encoding for 13 essential subunits of the mitochondrial respiratory chain unit, two ribosomal mt-RNAs (rRNAs) and 22 mitochondrial transfer RNA (mt-tRNA)s [2,3]. Mitochondrial diseases can either be caused by mutations in the mtDNA itself or by mutations of nuclear origin and are associated with a wide range of different clinical phenotypes, from mild to severe [4]. The coexistence of mutant and wild-type mtDNA molecules within the same cell is defined as heteroplasmy [5]. It is already known that patients with higher heteroplasmy levels tend to have more severe disease burden and progression rate; however, disease burden and progression vary greatly between individuals and tissue [3]. Recently, it has been shown that heteroplasmy levels did not differ between clinically affected and unaffected m.3243 patients [3]. The m.3243A>G point mutation in the MT-TL1 gene (encoding mt-tRNALeu(UUR)) can be found in approximately 80% of patients with MELAS (mitochondrial encephalopathy, lactate acidosis, and stroke-like episodes)-syndrome [3,6,7,8].
Resveratrol (3,4,5-trihydroxystilbene, RSV) is a small phenolic compound and found in grapes, nuts, berries, and various other plants [9]. During the years 2008–2010, the effect of RSV for the treatment of patients with MELAS-syndrome has been evaluated in a clinical study [10]. For this purpose, the Resveratrol analog SRT501 (Sirtris Pharmaceuticals, Cambridge, MA, USA) was used. RSV has been referred to as the caloric restriction “mimetic” compound [11].
The dual control of mitochondrial biogenesis by sirtuin (SIRT) 1 and SIRT3 is widely believed [12]. SIRT1 activates the peroxisome proliferator-activated receptor Gamma coactivator 1 α (PGC-1α)-mediated transcription of nuclear and mitochondrial genes. PGC-1α is known to be a central inducer of mitochondrial biogenesis [13], and co-activates the transcription of Nuclear Respiratory Factor (NRF) 1, which regulates the transcription of Tfam. Mitochondrial transcription factor A (TFAM) stimulates mitochondrial DNA replication and mitochondrial gene expression in the mitochondrial matrix. The regulatory effect of SIRT1 on PGC-1α activity and its role in mitochondrial biogenesis is controversially discussed [14]. Some groups reported the induction of genes for oxidative phosphorylation and mitochondrial biogenesis and an increase of PGC-1α activity by SIRT1 [15]. On the other hand, others opposed the obligatory regulatory role of SIRT1 for the PGC-1α- mediated mitochondrial biogenesis in muscle. They showed the downregulation of PGC-1α and Tfam resulting from the overexpression of SIRT1 in muscle and the downregulated levels of SIRT1 by upregulation of PGC-1α in this tissue [16]. SIRT3 directly activates important proteins for oxidative phosphorylation, tricarboxylic acid (TCA) cycle, and fatty-acid oxidation, and indirectly affects PGC-1α and AMP-activated protein kinase (AMPK) [12].
Nevertheless, the activating effect of RSV on SIRT1 and SIRT3 is a matter of debate. Many studies reported on SIRT1 and SIRT3 activation by RSV and their structurally related compounds [17,18]. Others, however, denied RSV and its analogs as direct SIRT1-activators [19]. In a zebrafish-model, RSV did not affect the mRNA level of SIRT1 and PGC-1α and even decreased the expression of SIRT3 and SIRT4 genes [20].
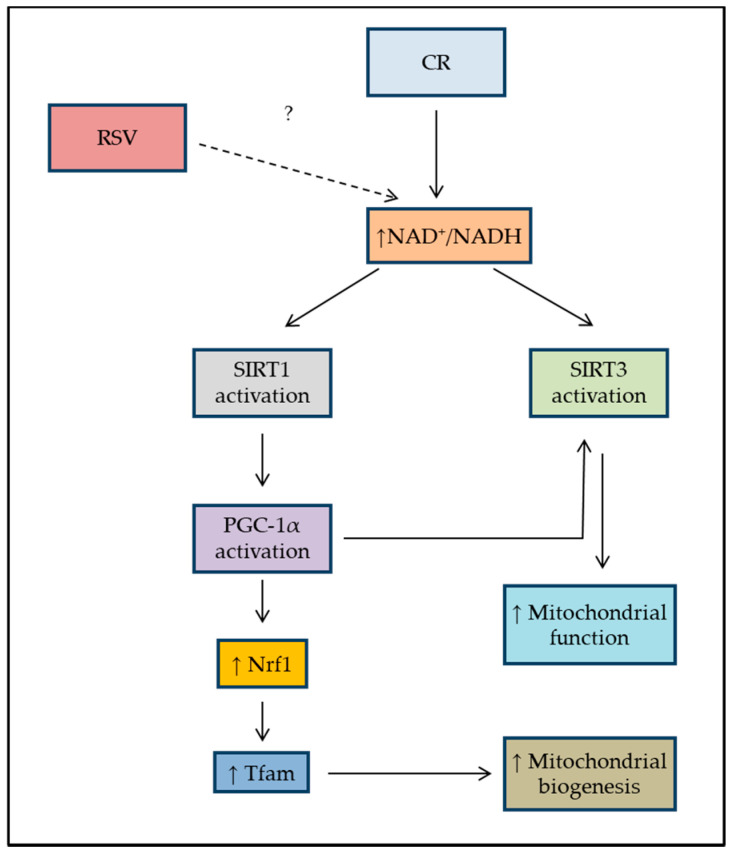
The aim of this study was to assess the effect of RSV on oxidative phosphorylation in patients harboring the m.3243A>G mutation and in controls. The controversially discussed caloric restriction (CR) stimulating effect of RSV on mitochondrial respiratory activity and mitochondrial biogenesis was evaluated in patients and in controls under normal and restricted conditions. The investigated pathway is schematically shown in Figure 1. The potential protective effects of RSV were only investigated under restricted cultural conditions to comply with the basic cellular needs, as well.
Figure 1.
Schematic diagram showing the investigated pathways in the present study using RSV in patients and controls, adopted accordingly [21]. Caloric restriction (CR) activates the SIRT1 levels or NAD+ levels leading to the activation of PGC-1α in the nucleus, which then activates the transcription of genes that are necessary for mitochondrial function and biogenesis. CR also leads to activation of AMPK and, therefore, the activation of PGC-1α in skeletal muscle. RSV: Resveratrol; NAD: Nicotinamide adenine dinucleotide; SIRT1: sirtuin 1; SIRT3: sirtuin 3; PGC-1α: peroxisome proliferator-activated receptor gamma co-activator 1α; Nrf1: Nuclear Respiratory Factor 1; Tfam: Mitochondrial Transcription Factor A.
2. Materials and Methods
2.1. Human Myoblasts
Muscle primary cells from five patients harboring the genetically confirmed m.3243A>G mutation and controls were provided by the Muscle Tissue Culture Collection (MTCC) from the University of Munich. The presence of a mutation was confirmed in myoblasts of all patients. Further details are given in Table 1. Five patients served as controls (two males, three females), who had muscle biopsy for the diagnosis of a suspected neuromuscular disorder. They were deemed to be ‘normal controls’ if they were ultimately found to have no muscle disease by combined clinical and histologic criteria. The age of the controls ranged from 35 to 53 years.
Table 1.
Sex, age, and location of muscle biopsy of five patients with the genetically confirmed m.3243A>G mutation and five healthy controls, F: female, M: male.
| Gender | Age at Biopsy | Location of Muscle Biopsy | |
|---|---|---|---|
| Patients | |||
| P 1 | M | 43 | biceps brachii muscle |
| P 2 | M | 42 | biceps brachii muscle |
| P 3 | F | 70 | quadriceps muscle |
| P 4 | M | 34 | deltoideus muscle |
| P 5 | F | 40 | biceps brachii muscle |
| Controls | |||
| C1 | F | 50 | biceps brachii muscle |
| C 2 | M | 53 | quadriceps muscle |
| C 3 | F | 40 | quadriceps muscle |
| C 4 | M | 35 | biceps brachii muscle |
| C 5 | F | 49 | biceps brachii muscle |
Myoblast Culture Conditions
The experiments were divided into two main groups depending on the culture conditions of the myoblasts: (I) normal (N)- or, (II) substrate restricted (R)-conditions, both, either without or with 10 µM or 20 µM of RSV. At first, all cells were grown in skeletal muscle cell growth medium (Promocell, Heidelberg, Germany) supplemented with 10% fetal bovine serum (FBS), GlutaMAX-1 (Gibco, Life Technologies, Grand Island, NY, USA), and Supplement mix (Fetuin (bovine, 50 ng/mL), human epidermal growth factor (hEGF, 10 pg/mL), human basic fibroblast growth factor (hbFGF, 1 pg/mL), Dexamethasone (0.4 pg/mL), and human recombinant insulin (10 ng/mL), Promocell, Heidelberg, Germany). After the first 24 h, the medium was changed and the cells were cultured for another 48 h. In the R group, the medium was replaced by a substrate-limited medium (DMEM with 0.5 mM glucose, 1.0 mM glutamine, and 1% FBS) with or without 10 µM or 20 µM RSV. Promocell skeletal muscle cell growth medium was used either without or with 10 µM or 20 µM RSV in the normal group. Resveratrol (>99% purity) was obtained from Sigma-Aldrich (St. Louis, MO, USA). All cells were maintained in 5% CO2 at 37 °C.
2.2. The Seahorse XF96 Analysis of Metabolic Function
To evaluate the mitochondrial function and the effect of RSV in patients and controls, the Mito Stress test was performed using a Seahorse XF96 Cell Analyzer (Seahorse Bioscience, Billerica, MA, USA), either under N or restricted R conditions according to manufacturer’s recommendations. Briefly, myoblasts from patients and controls were seeded to Seahorse XF96 cell culture microplates (2.5 × 104 cells per well) in skeletal muscle cell growth medium supplemented with 10% FBS. After a 24-hour incubation at 37 °C, the medium was replaced depending on six different experimental conditions, as described in Section 2.1 (R or N cultural conditions without or either with 10 µM or 20 µM RSV).
Forty-eight h later the cells were washed twice with the pre-warmed assay medium (XF base medium supplemented with 10 mM glucose, 2 mM glutamine, and 1 mM sodium pyruvate; pH 7.4).
Oxygen consumption rate (OCR) values were measured following sequential injections of oligomycin (2 µM), carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP, 2 µM), and rotenone (0.5 µM) + antimycin A (0.5 µM), with three OCR measurements after each injection following an injection of cell-permeable Hoechst 33342 (2 µg/mL) dye. The key parameters of mitochondrial function such as basal respiration (BR), ATP-linked respiration, maximal respiration (MR), and spare respiratory (SRC) capacity were analyzed using the above-described measurements. The ATP linked respiration (ATP production rate, ATP-R) was derived from the difference between the OCR at baseline and respiration following oligomycin addition. Maximal OCR was determined by subtracting the OCR after antimycin A addition from the OCR induced by FCCP. The SRC was calculated by the difference between maximal and basal respiration. The data were normalized to cell numbers by measurement of Hoechst dye staining of nuclei with excitation and emission wavelengths 355 nm and 465 nm, accordingly, using a Tecan InfiniteTM M1000 (Tecan, Groedig, Austria) and plotted as OCR (pmol/min/cell ± SD).
2.3. Gene Expression by Quantitative Real-Time (qRT)-PCR
The cells from the six different groups, as described in Section 2.1 (N or R with or without 10 µM or 20 µM RSV), were harvested, shock frozen in liquid nitrogen, and stored at −80 °C until RNA extraction. RNA was extracted using a NucleoSpin RNA kit (Macherey and Nagel, Duren, Germany), according to the manufacturer’s instructions. cDNA was next synthesized using the reverse transcription of 1 µg of RNA with RevertAid H Minus First Strand cDNA Synthesis kit (Thermo Scientific, Vilnius, Lithuania), according to the manufacturer’s instructions. cDNAs were kept at −20 °C until analysis.
Quantitative Real-Time (qRT)-PCR was carried out using PowerUp™ SYBR™ Green Master Mix (Thermo Scientific, Vilnius, Lithuania) using a QuantStudio 3 real-time PCR machine (Applied Biosystems, Thermo Fisher, Foster City, CA, USA). Each 10 µL-reaction contained 5 µL (2×) SYBR Green master mix, 500 nM forward and reverse primer, 0.5 µL cDNA, and nuclease-free water. The used primer pairs are listed in Table 2. The following thermal program was applied: a single cycle of DNA polymerase activation for 15 min at 95 °C followed by 40 amplification cycles of 15 s at 95 °C (denaturation) and 1 min at 60 °C (annealing and extension). Subsequently, a melting temperature analysis of the amplification products was performed by gradually increasing the temperature from 60 to 95 °C in 15 min. The fluorescent reporter signal was normalized against the internal reference dye (ROX) signal. The relative gene expression (ΔΔCT) was calculated first by correcting each gene cycle threshold (CT) by the average CT value for the housekeeping genes HPRT1 and β-Actin, that were stable across groups (calculation of relative expression—reported as 2−ΔΔCt and CT representing the cycle threshold). Three technical replicates were measured for each sample in three independent experiments.
Table 2.
Primers used for quantitative RT-PCR.
| Target Gene | Forward Primer | Reverse Primer |
|---|---|---|
| SIRT1 | AGAAGAACCCATGGAGGATG | TCATCTCCATCAGTCCCAAA |
| SIRT3 | CAGCAGTACGATCTCCCGTA | GAAGCAGCCGGAGAAAGTAG |
| PGC-1α | GTCCAGGCAGGAGCTTTTAGA | AGCTTTGATTTGCTCAAGCCAT |
| Nrf1 | AGGAACACGGAGTGACCCAA | TATGCTCGGTGTAAGTAGCCA |
| Tfam | ATGGCGTTTCTCCGAAGCAT | TCCGCCCTATAAGCATCTTGA |
| HPRT1 | ACCAGTCAACAGGGGACATAA | CTTCGTGGGGTCCTTTTCACC |
| β-Actin | GCGCCGTTCCGAAAGTTG | CGCGCCGCTGGGTTTTATAG |
2.4. Statistical Analysis
Statistical analysis, calculation, and visualization were performed using Prism 8 (GraphPad, San Diego, CA, USA). An analysis of correlation was carried out using a two-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. The level of significance was set to p = 0.05. The statistical tests chosen were predetermined by the size of the study group and the numerical range of values.
2.5. Ethical Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local Ethics Committee of the University Halle-Wittenberg (Project identification codes 215/20.01.10/3 and 2020-019). A written informed consent was received from all patients.
3. Results
For better readability of the results, the experiments using 48 h cultures were divided between the ones conducted either under normal (N) or restricted (R) conditions.
3.1. The Seahorse XF96 Analysis of Metabolic Function
3.1.1. The Effect of Restricted Conditions
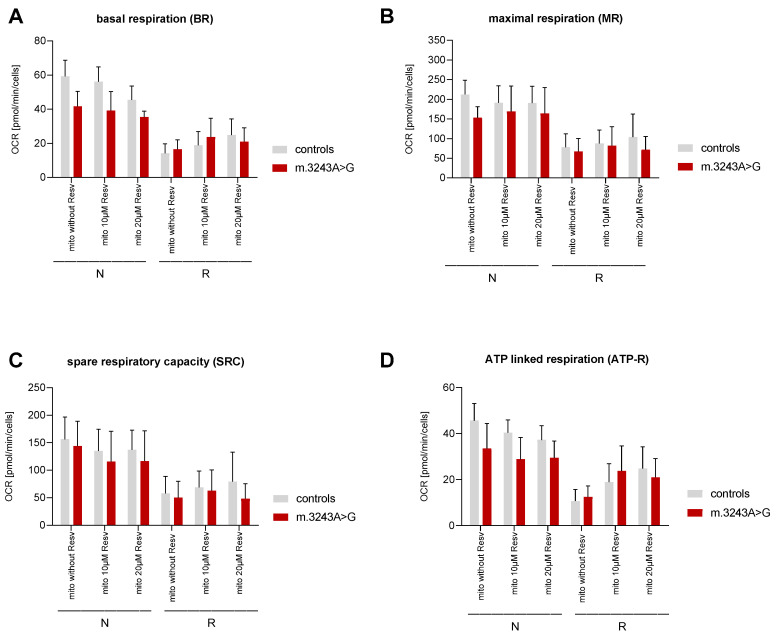
Independent of RSV-absence or presence, restriction in the culture medium led to a decrease of oxidative phosphorylation (OXPHOS) factors (Figure 2). This decrease was significant, except in one case—the decrease of ATP production in the presence of 10 µM RSV was only significant in controls.
Figure 2.
Evaluation of mitochondrial function using a Seahorse XF96 Cell Analyzer in myoblasts from patients (n = 5) and controls (n = 5). The key parameters of mitochondrial function such as basal respiration (BR), ATP production (ATP-R) and spare respiratory capacity (SRC) were analyzed as previously described. (A) Basal respiration (BR), (B) maximal respiration (MR), (C) spare respiratory capacity (SRC) and (D) ATP-linked respiration (ATP-R) after 48 h under normal (N) and restricted (R) conditions. The significant differences are shown in Table 3 and Table 4, and Table S1.
The mean values are presented in Supplementary Table S1 (p values are only shown in case of significance).
3.1.2. Differences between Patients Harboring the m.3243A>G Mutation and Controls
Mito Stress Test in the Absence of Resveratrol
Under normal (N) conditions, BR, MR, and ATP-R were all significantly higher in controls compared to patients without the addition of RSV (Figure 2 and Table 3). However, under R conditions, no significant differences in the above-mentioned factors were detected between patients and controls.
Table 3.
Comparison of the mean values of the key parameters for mitochondrial function (basal, MR, ATP production and SRC) using a Seahorse XF96 Cell Analyzer in myoblasts between patients (n = 5) and controls (n = 5) under normal (N) or restricted (R) conditions. p values are only shown in the case of significant differences between patients and controls. −RSV = without RSV.
| N conditions | |||||||||
| − RSV | 10 µM RSV | 20 µM RSV | |||||||
| Controls (mean) | Patients (mean) | p value | Controls (mean) | Patients (mean) | p value | Controls (mean) | Patients (mean) | p value | |
| Basal | 59.24 | 41.71 | 0.0005 | 56.11 | 39.19 | <0.0001 | 45.44 | 35.44 | 0.05 |
| MR | 212.3 | 153.3 | 0.05 | 191.3 | 169.2 | 190.4 | 164 | ||
| SRC | 156.4 | 144.1 | 135.2 | 115.8 | 137.3 | 116.8 | |||
| ATP | 45.64 | 33.51 | 0.01 | 40.39 | 28.82 | 0.001 | 37.24 | 29.4 | 0.03 |
| R conditions | |||||||||
| −RSV | 10 µM RSV | 20 µM RSV | |||||||
| Controls (mean) | Patients (mean) | p value | Controls (mean) | Patients (mean) | p value | Controls (mean) | Patients (mean) | p value | |
| Basal | 14.19 | 16.52 | 18.91 | 23.8 | 24.84 | 21 | |||
| MR | 78.04 | 66.87 | 87.21 | 81.92 | 103.8 | 71.5 | |||
| SRC | 57.88 | 50.34 | 68.79 | 62.84 | 79.22 | 48.38 | |||
| ATP | 10.67 | 12.45 | 18.9 | 23.8 | 24.84 | 21 | |||
Mito Stress Test in RSV-treated Groups
In experiments under N conditions, BR and ATP production were significantly higher in controls than in patients in the presence of RSV. MR and SRC were similar in patients and controls (Figure 2 and Table 3).
In all R groups, all the above-mentioned values were comparable between patients and controls (Figure 2 and Table 3).
3.1.3. The Effect of RSV on OXPHOS Factors
The Effect of RSV on OXPHOS Factors under Normal Conditions
Upon treatment of myoblasts with 10 or 20 µM RSV under N condition, there was no significant difference between values resulting from either 10 or 20 µM RSV in all of the main OXPHOS factors (BR, MR, SRC, and ATP-R) with only one exception. As the only exception, BR was significantly lower in controls in the presence of 20 µM RSV (Figure 2 and Table 4).
Table 4.
Comparison of the effect of 10 or 20 µM RSV on basal, MR, ATP production, and SRC measured under normal (N) or restricted (R) conditions in patients (n = 5) and controls (n = 5). p values are only shown in the case of a significant difference between the two conditions.
| Controls | ||||||||||
| N | R | |||||||||
| −RSV | 10 | p (10) | 20 | p (20) | −RSV | 10 | p (10) | 20 | p (20) | |
| Basal | 59.24 | 56.11 | 45.44 | 0.02 | 14.19 | 18.91 | 24.84 | 0.008 | ||
| MR | 212.3 | 191.3 | 190.4 | 78.04 | 87.21 | 103.8 | ||||
| SRC | 156.4 | 135.2 | 137.3 | 57.88 | 68.79 | 79.22 | ||||
| ATP | 45.64 | 40.39 | 37.24 | 10.67 | 18.9 | 0.03 | 24.84 | <0.0001 | ||
| Patients | ||||||||||
| N | R | |||||||||
| −RSV | 10 | p (10) | 20 | p (20) | −RSV | 10 | p (10) | 20 | p (20) | |
| Basal | 41.71 | 39.19 | 35.44 | 16.52 | 23.8 | 0.05 | 21 | |||
| MR | 153.3 | 169.2 | 164 | 66.87 | 81.92 | 71.5 | ||||
| SRC | 144.1 | 115.8 | 116.8 | 50.34 | 62.84 | 48.38 | ||||
| ATP | 33.51 | 28.82 | 29.4 | 12.45 | 23.8 | <0.0001 | 21 | 0.005 | ||
The Effect of RSV on OXPHOS Factors under Restricted Conditions
The addition of 10 or 20 µM RSV under the R condition led to an improvement of ATP-R in controls and patients (Figure 2 and Table 4).
3.2. Gene Expression by qRT-PCR
3.2.1. The Effect of Restrictions
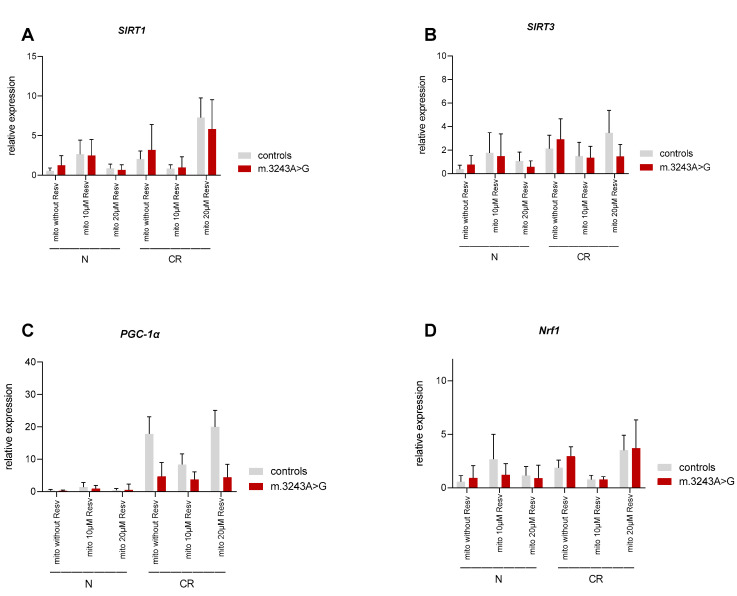
Under R conditions, the expression of SIRT1, SIRT3, PGC-1α, Nrf1, and Tfam tended to be increased in the absence of RSV or in the presence of 20 µM RSV compared to normal cultural conditions in both, controls and patients. The expression rates in the presence of 10 µM RSV did not follow any specific pattern (Table 5 and Figure 3).
Table 5.
Comparison of the relative expression rate of the genes SIRT1, SIRT3, PGC-1α, Nrf1, and Tfam measured under N or R conditions in patients (n = 5) and controls (n = 5). p values are only shown in the case of significant differences between patients and controls. −RSV = without RSV.
| Controls | |||||||||
| −RSV | 10 µM RSV | 20 µM RSV | |||||||
| N (mean) | R (mean) | p value | N (mean) | R (mean) | p value | N (mean) | R(mean) | p value | |
| SIRT1 | 0.56 | 2.04 | 2.65 | 0.82 | 0.84 | 7.1 | <0.0001 | ||
| SIRT3 | 0.4 | 2.1 | 0.02 | 1.77 | 1.47 | 1.07 | 3.46 | 0.0003 | |
| PGC-1α | 0.3 | 17.8 | <0.0001 | 1.41 | 8.3 | <0.0001 | 0.45 | 19.97 | <0.0001 |
| Nrf1 | 0.57 | 1.87 | 2.68 | 0.77 | 0.007 | 1.16 | 3.51 | 0.0004 | |
| Tfam | 2.99 | 4.45 | 12.44 | 2.67 | <0.0001 | 4.96 | 7.55 | ||
| Patients | |||||||||
| −RSV | 10 µM RSV | 20 µM RSV | |||||||
| N (mean) | R (mean) | p value | N (mean) | R (mean) | p value | N (mean) | R (mean) | p value | |
| SIRT1 | 1.26 | 3.19 | 2.463 | 0.95 | 0.67 | 5.82 | <0.0001 | ||
| SIRT3 | 0.77 | 2.9 | 0.0009 | 1.49 | 1.36 | 0.57 | 1.45 | ||
| PGC-1α | 0.27 | 4.7 | 0.006 | 0.94 | 3.78 | 0.54 | 4.43 | 0.02 | |
| Nrf1 | 0.93 | 2.97 | 0.003 | 1.21 | 0.79 | 0.9 | 3.7 | <0.0001 | |
| Tfam | 2.71 | 4.4 | 8.34 | 2.27 | 0.0003 | 1.53 | 2.7 | ||
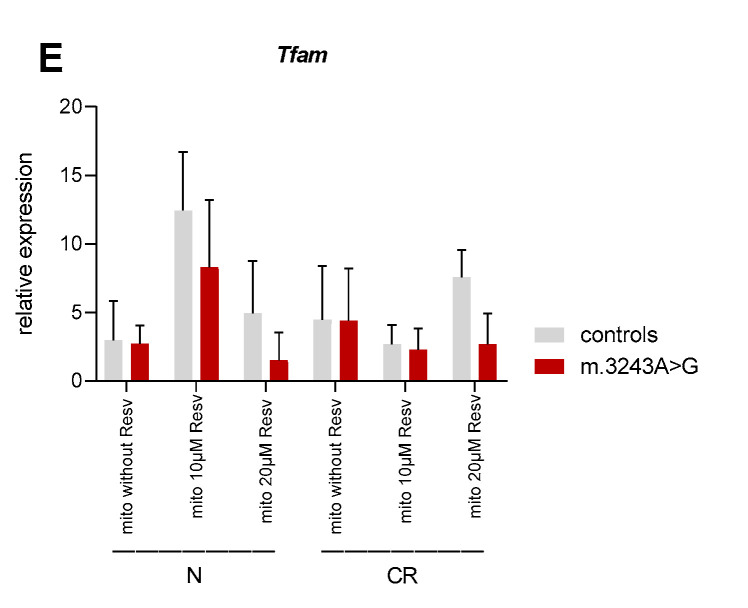
Figure 3.
Evaluation of gene expression in myoblasts from patients (n = 5) and controls (n = 5), analyzed as previously described. (A) SIRT1, (B) SIRT3, (C) PGC-1 α, (D) Nrf1, and (E) Tfam after 48 h, N vs. R conditions. The significant differences are shown in Table 5 and Table 6, and Table S2.
3.2.2. The Difference between Patients and Controls
Without RSV, there was no significant difference in expression of SIRT1, SIRT3, Nrf1, and Tfam between patients and controls under normal and restricted conditions. The expression of PGC-1 α was significantly lower in patients compared to controls only in restricted conditions in the absence or presence of RSV (Figure 3). In the majority of cases, the difference in the expression of other genes in the presence of RSV was not significant. The exceptions are shown in Supplementary Table S2.
3.2.3. The Effect of RSV
Generally, the addition of RSV under N or R conditions did not lead to a significant difference in the expressions of SIRT1, SIRT3, PGC-1α, Nrf1, and Tfam in both patients and controls (Table 6 and Figure 3).
Table 6.
The effect of 10 or 20 µM RSV on the relative expression rate of the genes SIRT1, SIRT3, PGC-1α, Nrf1, and Tfam measured under normal (N) or restricted (R) conditions in patients (n = 5) and controls (n = 5). p values are only shown in the case of significant differences between the two conditions.
| Controls | ||||||||||
| N | R | |||||||||
| −RSV | 10 | p (10) | 20 | p (20) | −RSV | 10 | p (10) | 20 | p (20) | |
| SIRT1 | 0.56 | 2.65 | 0.84 | 2.04 | 0.82 | 7.1 | <0.001 | |||
| SIRT3 | 0.4 | 1.77 | 1.07 | 2.1 | 1.47 | 3.46 | ||||
| PGC-1α | 0.3 | 1.41 | 0.45 | 17.8 | 8.3 | <0.0001 | 19.97 | |||
| Nrf1 | 0.57 | 2.68 | 0.002 | 1.16 | 1.87 | 0.77 | 3.51 | 0.03 | ||
| Tfam | 2.99 | 12.44 | <0.0001 | 4.96 | 4.45 | 2.67 | 7.55 | |||
| Patients | ||||||||||
| N | R | |||||||||
| −RSV | 10 | p (10) | 20 | p (20) | −RSV | 10 | p (10) | 20 | p (20) | |
| SIRT1 | 1.26 | 2.463 | 0.67 | 3.19 | 0.95 | 5.82 | 0.02 | |||
| SIRT3 | 0.77 | 1.49 | 0.57 | 2.9 | 1.36 | 1.45 | ||||
| PGC-1α | 0.27 | 0.94 | 0.54 | 4.7 | 3.78 | 4.43 | ||||
| Nrf1 | 0.93 | 1.21 | 0.9 | 2.97 | 0.79 | 0.004 | 3.7 | |||
| Tfam | 2.71 | 8.34 | 0.004 | 1.53 | 4.4 | 2.27 | 2.7 | |||
4. Discussion
Resveratrol is believed to mimic the physiological effects of CR in a mainly SIRT1- or SIRT3-dependent manner [22,23]. Functional mitochondria have been reported to be important for the effects of RSV [24]. Thus, in the present study, this potential effect of RSV was evaluated in oxidative phosphorylation capacities and transcription factors involved in mitochondrial biogenesis in myoblasts of five patients harboring the m.3243A>G point mutation and five controls. Furthermore, it was assessed whether mitochondrial dysfunction based on an mtDNA defect in patients could trigger cellular signals provoking compensatory adaptations.
Analyzing the mitochondrial activity was performed using a Seahorse XF96 Cell Analyzer. In patients, there was no effect resulting from the addition of RSV under the N condition. Under this condition (glucose as substrate), independent of RSV-absence or presence, the important respiratory factors BR and ATP-R were higher in the controls than in the patients. This is consistent with another study reporting reduced ATP-linked respiration, MR, and overall, a decrease in mitochondrial function in fibroblasts of MELAS patients. [25]. In the R group, the medium was only used in concentrations that are necessary to fulfil the cellular basic needs, including CR, as well as lacking of several other factors, including insulin. These restrictions generally led to a decrease of respiratory values in both patients and controls compared to normal conditions. The presented findings are partly in contrast to data from previous studies, which reported an increase of the mitochondrial ATP synthesis efficiency and oxidative metabolism resulting from CR conditions [26,27,28]. The different experimental conditions or different species might be the reason for the contradicting results. In a study using C2C12 myoblasts, the measurements were performed in three groups; assaying oöconditions identical to culture conditions, with 1 g/L or without glucose. Considering the results upon assaying with 1 g/l glucose, they report a slightly higher basal mitochondrial respiration and ATP turnover-driven respiration in groups with glucose in culture medium compared to those with glucose depletion [26]. Other studies performed the experiments in mice and evaluated the CR effect by subsequent measurements in tissues. Their results should be considered as a reaction of several organs involved [27,28]. Moreover, in the present study, the effects seen in the R groups resulted from a reduction, not only of glucose but of other supplements as well, compared to the normal medium.
The acquired data under normal conditions did not confirm the reported positive effect of RSV on OXPHOS values [15,29]. Low doses of RSV have been reported to ameliorate the mitochondrial respiratory dysfunction in fibroblasts of patients carrying homoplasmic mtDNA mutations [30]. However, there are other studies reporting either no effect or a detrimental effect of RSV on ATP production in fibroblasts of controls or patients with mitochondrial disorders [31]. It has been suggested that the therapeutic effects of RSV for the treatment of mitochondrial disorders might depend on many factors, including the severity of the underlying defect and the administered dose. RSV might be beneficial to some patients as a supportive therapeutic supplement and as part of a multi-component therapy [32]. Under restricted conditions, OXPHOS values improved in both patients and controls in the presence of RSV (especially 10µM). In this situation, the above-mentioned respiratory factors were in general similar in patients and controls (Figure 2 and Table 4).
The lacking positive effect of RSV under normal conditions is consistent with a study on C2C12 cells. The addition of RSV for 24 h at a concentration of 1 µM to 10 µM did not affect the ATP production but led to a 50% decrease in ATP concentration in the 20 µM RSV group [33].
Other studies showed an inhibitory effect of RSV on mitochondrial F0F1-ATPase activity in a concentration-dependent manner in rat brain and liver mitochondria, suggesting that RSV can also impair mitochondrial metabolic pathways [34,35]. In the present study, RSV was used at 10 µM and 20 µM because even higher concentrations of RSV have been known to be lethal to cells [33].
For the evaluation of the cellular response resulting from impaired OXPHOS in patients, the relative expression of the key genes related to energy metabolism and mitochondrial function SIRT1, SIRT3, PGC-1α, Nrf1, and Tfam were investigated. While one study showed comparable expression of Nrf1 and Tfam and upregulation of PGC-1α and SIRT3 in MELAS patients compared to controls [25], another one reported similar expression rates of PGC-1α and upregulation of Tfam in patients compared with that of controls [36]. In the present study, the expression of the above-mentioned genes was similar in patients and controls; however, the PGC-1α values were only higher under R conditions in controls compared to patients.
There are some tissue-specific metabolic pathways to maintain energy and nutrient homeostasis in mammals, acting as a response to environmental and nutritional conditions. Fasting induces PGC-1α deacetylation by SIRT1 in skeletal muscle [37]. An increase in SIRT3 and SIRT1 protein level and expression in skeletal muscle of mice has been reported by fasting, correlated with an induction of PGC-1α as well. Resveratrol, in contrast, induced the SIRT1 expression in mice skeletal muscle but did not affect the SIRT3 level. The inability of resveratrol to induce SIRT3 has been interpreted as an ineffectiveness of resveratrol to mimic CR-mediated health benefits [23,38]. In the present study, the restricted condition led to upregulation of SIRT3, PGC-1α, and Nrf1 in both patients and controls. PGC-1α’s upregulation was particularly pronounced in controls under R conditions (about 60× higher than under N condition). However, Tfam-expression was not affected upon restricted conditions. Notably, the addition of 10 µM RSV, under restricted conditions, led to downregulation of Tfam-expression in both patients and controls.
The restricted condition led to an increased expression of the investigated genes, which might indicate stimulation of mitochondrial biogenesis. On the other hand, respiratory key parameters were decreased under R conditions. The lower maximal capacity might either result from decreased substrate availability or a comprised mitochondrial mass/integrity or a mitophagic turnover under stressful situations to prevent accumulation of additional damage [39,40]. The studied genes showed slight upregulation in the presence of 10 µM RSV under N conditions, and upregulation in the presence of 20 µM RSV under R conditions compared to the conditions without RSV; however, not always significant (Table 6 and Figure 3).
Limitations
Due to lacking several factors in the culturing of the restricted group, the obtained results cannot be seen as a pure effect of glucose depletion, and a direct comparison of N and R groups is not easy to establish. Further studies are necessary to investigate alternative pathways and individual factors that are influenced by RSV in stressed models. Moreover, assay conditions (N or R) were similar for cells from both conditions. An adjustment to specific experimental conditions might be considered in future works. It should be noticed that mRNA quantification does not always represent the expressed amount of protein or changed modification of these proteins as deacetylation, phosphorylation, or methylation. These factors could play a role in protein translation and, subsequently the number of active proteins.
5. Conclusions
The data in the present study confirmed the reduced mitochondrial respiration in patients harboring the m.3243A>G mutation. The fasting stimulating effect of RSV in myoblasts under normal conditions was not demonstrated. Interestingly, under restricted conditions, there was an improvement in ATP-linked respiration, resulting from RSV in both patients and controls. It might show that benefits of RSV occur only in stressed models. The positive effect of RSV was not always concomitant with an increase in the expression of the investigated transcription factors involved in mitochondrial biogenesis in this study.
Acknowledgments
We thank the Muscle Tissue Culture Collection MTCC at the University hospital Munich Muenchen for providing the samples and Julia Emmerich for excellent technical assistance. The Muscle Tissue Culture Collection is part of the German network on muscular dystrophies (MD-NET) and the German network for mitochondrial disorders (mito-NET, 01GM1113A) funded by the German ministry of education and research (BMBF, Bonn, Germany). The Muscle Tissue Culture Collection is a partner of Eurobiobank (www.eurobiobank.org) and TREAT-NMD (www.treat-nmd.eu). L.M.S., D.L.U. and S.Z. are members of the German mitoNET funded by the German Ministry of Education and Research. We thank the center of basic medical research (ZMG) of the medical school at the University of Halle-Wittenberg for providing the Seahorse device. We acknowledge the financial support within the funding program Open Access Publishing by the German Research Foundation (DFG).
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-273X/10/8/1103/s1, Table S1: Comparison of the mean values of basal, maximal respiration (MR), ATP production, and spare respiratory capacity (SRC) measured under normal (N) or restricted (R) conditions in patients (n = 5) and controls (n = 5), Table S2: Relative expression rates of the genes SIRT1, SIRT3, PGC-1α, Nrf1, and Tfam in myoblasts between patients (n = 5) and controls (n = 5) under normal (N) or restricted (R) conditions.
Author Contributions
Conceptualization, L.M.S., H.S., F.D., S.Z.; methodology, L.M.S., H.S., S.A.-R., A.T.; software, L.M.S., D.L.U., H.S., S.A.-R.; data curation, L.M.S., D.L.U., H.S., S.A.-R.; writing—original draft preparation, L.M.S., D.L.U., A.T.; writing—review and editing, L.M.S., D.L.U., F.D.; project administration, L.M.S., S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
D.L.U. is funded by the Hertha-Nathorff-Programm (HNP) of the Medical University of Ulm, Germany.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Wallace D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schon E.A., DiMauro S., Hirano M. Human mitochondrial DNA: Roles of inherited and somatic mutations. Nat. Rev. Genet. 2012;13:878–890. doi: 10.1038/nrg3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scholle L.M., Zierz S., Mawrin C., Wickenhauser C., Urban D.L. Heteroplasmy and Copy Number in the Common m.3243A>G Mutation-A Post-Mortem Genotype-Phenotype Analysis. Genes. 2020;11:212. doi: 10.3390/genes11020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehmann D., Schubert K., Joshi P.R., Baty K., Blakely E.L., Zierz S., Taylor R.W., Deschauer M. A novel m.7539C>T point mutation in the mt-tRNA(Asp) gene associated with multisystemic mitochondrial disease. Neuromuscul. Disord. 2015;25:81–84. doi: 10.1016/j.nmd.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majamaa K., Moilanen J.S., Uimonen S., Remes A.M., Salmela P.I., Karppa M., Majamaa-Voltti K.A., Rusanen H., Sorri M., Peuhkurinen K.J., et al. Epidemiology of A3243G, the mutation for mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes: Prevalence of the mutation in an adult population. Am. J. Hum. Genet. 1998;63:447–454. doi: 10.1086/301959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman S., Poulton J., Marchington D., Suomalainen A. Decrease of 3243 A-->G mtDNA mutation from blood in MELAS syndrome: A longitudinal study. Am. J. Hum. Genet. 2001;68:238–240. doi: 10.1086/316930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi Y., Ichihashi K., Ohta S., Nihei K., Kagawa Y., Yanagisawa M., Momoi M.Y. The mutant mitochondrial genes in mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS) were selectively amplified through generations. J. Inherit. Metab. Dis. 1992;15:803–808. doi: 10.1007/BF01800025. [DOI] [PubMed] [Google Scholar]
- 8.Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348:651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- 9.Wahab A., Gao K., Jia C., Zhang F., Tian G., Murtaza G., Chen J. Significance of Resveratrol in Clinical Management of Chronic Diseases. Molecules. 2017;22:1329. doi: 10.3390/molecules22081329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fong D., Chan M.M. Mitochondria as Targets for Phytochemicals in Cancer Prevention and Therapy. Springer; Berlin/Heidelberg, Germany: 2013. Dietary phytochemicals target cancer stem cells for cancer chemoprevention; pp. 85–125. [Google Scholar]
- 11.Civitarese A.E., Carling S., Heilbronn L.K., Hulver M.H., Ukropcova B., Deutsch W.A., Smith S.R., Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenmoehl J., Hoeflich A. Dual control of mitochondrial biogenesis by sirtuin 1 and sirtuin 3. Mitochondrion. 2013;13:755–761. doi: 10.1016/j.mito.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Austin S., St-Pierre J. PGC1alpha and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 2012;125:4963–4971. doi: 10.1242/jcs.113662. [DOI] [PubMed] [Google Scholar]
- 14.Tang B.L. Sirt1 and the Mitochondria. Mol. Cells. 2016;39:87–95. doi: 10.14348/molcells.2016.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Gurd B.J., Yoshida Y., Lally J., Holloway G.P., Bonen A. The deacetylase enzyme SIRT1 is not associated with oxidative capacity in rat heart and skeletal muscle and its overexpression reduces mitochondrial biogenesis. J. Physiol. 2009;587:1817–1828. doi: 10.1113/jphysiol.2008.168096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J., Wang N., Li J., Zhang J., Feng P. Effects of resveratrol on NO secretion stimulated by insulin and its dependence on SIRT1 in high glucose cultured endothelial cells. Endocrine. 2010;37:365–372. doi: 10.1007/s12020-010-9314-8. [DOI] [PubMed] [Google Scholar]
- 18.Price N.L., Gomes A.P., Ling A.J., Duarte F.V., Martin-Montalvo A., North B.J., Agarwal B., Ye L., Ramadori G., Teodoro J.S. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pacholec M., Bleasdale J.E., Chrunyk B., Cunningham D., Flynn D., Garofalo R.S., Griffith D., Griffor M., Loulakis P., Pabst B. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. Biol. Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schirmer H., Pereira T.C.B., Rico E.P., Rosemberg D.B., Bonan C.D., Bogo M.R., Souto A.A. Modulatory effect of resveratrol on SIRT1, SIRT3, SIRT4, PGC1α and NAMPT gene expression profiles in wild-type adult zebrafish liver. Mol. Biol. Rep. 2012;39:3281–3289. doi: 10.1007/s11033-011-1096-4. [DOI] [PubMed] [Google Scholar]
- 21.Milton-Laskibar I., Aguirre L., Etxeberria U., Milagro F.I., Martínez J.A., Portillo M.P. Do the effects of resveratrol on thermogenic and oxidative capacities in IBAT and skeletal muscle depend on feeding conditions? Nutrients. 2018;10:1446. doi: 10.3390/nu10101446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baur J.A. Resveratrol, sirtuins, and the promise of a DR mimetic. Mech. Ageing Dev. 2010;131:261–269. doi: 10.1016/j.mad.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palacios O.M., Carmona J.J., Michan S., Chen K.Y., Manabe Y., Ward III J.L., Goodyear L.J., Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1α in skeletal muscle. Aging. 2009;1:771. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Widlund A.L., Baral K., Dalgaard L.T., Vang O. Functional Mitochondria Are Important for the Effect of Resveratrol. Molecules. 2017;22:847. doi: 10.3390/molecules22050847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin D.-S., Kao S.-H., Ho C.-S., Wei Y.-H., Hung P.-L., Hsu M.-H., Wu T.-Y., Wang T.-J., Jian Y.-R., Lee T.-H. Inflexibility of AMPK-mediated metabolic reprogramming in mitochondrial disease. Oncotarget. 2017;8:73627. doi: 10.18632/oncotarget.20617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elkalaf M., Anděl M., Trnka J. Low glucose but not galactose enhances oxidative mitochondrial metabolism in C2C12 myoblasts and myotubes. PLoS ONE. 2013;8:e70772. doi: 10.1371/journal.pone.0070772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barazzoni R., Zanetti M., Bosutti A., Biolo G., Vitali-Serdoz L., Stebel M., Guarnieri G. Moderate caloric restriction, but not physiological hyperleptinemia per se, enhances mitochondrial oxidative capacity in rat liver and skeletal muscle—tissue-specific impact on tissue triglyceride content and AKT activation. Endocrinology. 2005;146:2098–2106. doi: 10.1210/en.2004-1396. [DOI] [PubMed] [Google Scholar]
- 28.Cerletti M., Jang Y.C., Finley L.W., Haigis M.C., Wagers A.J. Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell. 2012;10:515–519. doi: 10.1016/j.stem.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes Costa A., Le Bachelier C., Mathieu L., Rotig A., Boneh A., De Lonlay P., Tarnopolsky M.A., Thorburn D.R., Bastin J., Djouadi F. Beneficial effects of resveratrol on respiratory chain defects in patients’ fibroblasts involve estrogen receptor and estrogen-related receptor alpha signaling. Hum. Mol. Genet. 2014;23:2106–2119. doi: 10.1093/hmg/ddt603. [DOI] [PubMed] [Google Scholar]
- 30.Mizuguchi Y., Hatakeyama H., Sueoka K., Tanaka M., Goto Y.-i. Low dose resveratrol ameliorates mitochondrial respiratory dysfunction and enhances cellular reprogramming. Mitochondrion. 2017;34:43–48. doi: 10.1016/j.mito.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Douiev L., Soiferman D., Alban C., Saada A. The effects of ascorbate, N-acetylcysteine, and resveratrol on fibroblasts from patients with mitochondrial disorders. J. Clin. Med. 2017;6:1. doi: 10.3390/jcm6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Paepe B., Van Coster R. A critical assessment of the therapeutic potential of resveratrol supplements for treating mitochondrial disorders. Nutrients. 2017;9:1017. doi: 10.3390/nu9091017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higashida K., Kim S.H., Jung S.R., Asaka M., Holloszy J.O., Han D.-H. Effects of resveratrol and SIRT1 on PGC-1α activity and mitochondrial biogenesis: A reevaluation. PLoS Biol. 2013;11:e1001603. doi: 10.1371/journal.pbio.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng J., Ramirez V.D. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br. J. Pharmacol. 2000;130:1115–1123. doi: 10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zini R., Morin C., Bertelli A., Bertelli A.A., Tillement J. Effects of resveratrol on the rat brain respiratory chain. Drugs Exp. Clin. Res. 1999;25:87–97. [PubMed] [Google Scholar]
- 36.Joseph A.-M., Rungi A.A., Robinson B.H., Hood D.A. Compensatory responses of protein import and transcription factor expression in mitochondrial DNA defects. Am. J. Physiol. Cell Physiol. 2004;286:C867–C875. doi: 10.1152/ajpcell.00191.2003. [DOI] [PubMed] [Google Scholar]
- 37.Gerhart-Hines Z., Rodgers J.T., Bare O., Lerin C., Kim S.H., Mostoslavsky R., Alt F.W., Wu Z., Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tauriainen E., Luostarinen M., Martonen E., Finckenberg P., Kovalainen M., Huotari A., Herzig K.-H., Lecklin A., Mervaala E. Distinct effects of calorie restriction and resveratrol on diet-induced obesity and fatty liver formation. J. Nutr. Metab. 2011;2011:525094. doi: 10.1155/2011/525094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill B.G., Benavides G.A., Lancaster J.R., Jr., Ballinger S., Dell’Italia L., Jianhua Z., Darley-Usmar V.M. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abeliovich H., Dengjel J. Mitophagy as a stress response in mammalian cells and in respiring S. cerevisiae. Biochem. Soc. Trans. 2016;44:541–545. doi: 10.1042/BST20150278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.