Abstract
A nonmedical switch policy is currently being considered in Alberta, which would force patients on originator biologics to biosimilar alternatives with the hypothetical aim of reducing costs to the health care system. The evidence to support the safety of nonmedical switching in patients with inflammatory bowel disease (IBD) is of low to very low quality; in fact, existing data suggest a potential risk of harm. In a pooled analysis of randomized controlled trials, one patient would lose response to infliximab for every 11 patients undergoing nonmedical switching. Switching to a biosimilar has important logistical and ethical implications including potential forced treatment changes without appropriate patient consent and unfairly penalizing patients living in rural areas and those without private drug insurance. Even in the best-case scenario, assuming perfectly executed switching without logistical delays, we predict switching 2,000 patients with Remicade will lead to over 60 avoidable surgeries in Alberta. Furthermore, nonmedical switching has not been adequately studied in vulnerable populations such as children, pregnant women, and elderly patients. While the crux of the argument for nonmedical switching is cost savings, biosimilar switching may not be cost effective: Particularly when originator therapies are being offered at the same price as biosimilars. Canadian patients with IBD have been surveyed, and their response is clear: They are not in support of nonmedical switching. Policies that directly influence patient health need to consider patient perspectives. Solutions to improve cost efficiency in health care exist but open, transparent collaboration between all involved stakeholders is required.
Keywords: Biologic, Biosimilar, Infliximab, Inflammatroy bowel disease
Key Messages.
The evidence evaluating the safety of nonmedical switching to a biosimilar is of very low quality and suggests potential harm to patients with IBD.
A meta-analysis of randomized controlled trials demonstrated that one patient is expected to lose response to their infliximab for every 11 patients who are switched to a biosimilar.
A nonmedical switch policy is expected to cause over 60 avoidable surgeries in Alberta.
Nonmedical switching has not been adequately studied in highly vulnerable populations including children, pregnant women and elderly patients.
Nonmedical switching has important logistical and ethical implications.
Biosimilar switching may not be cost effective, particularly when originator therapies are offered at the same price as biosimilars.
Canadian patients with IBD have significant anxiety and concern over switching.
Introduction
In 2019, the prevalence of the inflammatory bowel disease (IBD) in Canada was 0.7% with an estimated 270,000 Canadians, and nearly 33,000 Albertans, living with IBD (1,2). IBD is a potentially debilitating disease associated with impaired quality of life, need for hospitalizations, and surgeries in patients who do not respond to medical therapy (3). Medical management was revolutionized with the introduction of biologics—the first being the tumour necrosis factor (TNF) antagonist, infliximab (Remicade), with approval for Crohn’s disease in 2000. Since 2000, rates of hospitalizations and surgeries for Crohn’s disease and ulcerative colitis have steadily decreased across Canada (4). For example, a population-based study in the Calgary Zone demonstrated that from 2002 to 2010, surgical resections fell significantly by 3.5% per year, which was driven by a substantial 10.1% annual decrease in emergency operations for patients with Crohn’s disease, much of which is attributable to the introduction of TNF antagonists (5).
The introduction of biologics has been associated with an increase in the direct medical costs of managing IBD, which is estimated at over $1 billion per annum in Canada (6). Estimating the precise cost of IBD management is complex, but the major driving forces are rising drug costs with the advent of new biologics juxtaposed against decreasing costs of fewer hospitalizations and surgeries resulting from better disease control (6). One approach to reducing drug costs of IBD is the introduction of biosimilars to infliximab (Inflectra or CT-P13 infliximab-dyyb in 2016 and Renflexis infliximab-abda in 2018). A biosimilar (also known as a subsequent entry biologic) is a biologic product that is highly similar to a reference product. However, the production of biologics requires replication in living cells, resulting in variability of these complex proteins and rendering the products bio-similar but not bio-identical. Regulatory authorities state that biosimilars must show a ‘high degree of similarity’ to the original product and have ‘no clinically meaningful differences in safety, purity, and potency’, although these terms are not clearly defined (7–9).
Biosimilars of infliximab are associated with an approximately 30 to 40% decrease in the listed price compared to Remicade. As stewards of the health care system, gastroenterologists are committed to supporting policies aimed at reducing the cost of care that do not substantially influence effectiveness or safety. In 2016, gastroenterologists in Alberta supported a policy that mandated starting biosimilar Inflectra in patients naive to infliximab instead of the originator (Remicade) (10). Mandating new starts of biosimilar infliximab has saved Alberta’s health care system significant dollars while maintaining a collaborative and informed decision process between physician and patient by allowing patient choice in their care, which may include Inflectra or other indicated biologics.
In May 2019, the Government of British Columbia launched the Biosimilars Initiative through their PharmaCare program, with the goal of switching patients using originator biologics, including Remicade, to a biosimilar version by March 2020 (11). A portion of the economic savings were re-invested into the IBD community, including funding models for nurses and expanded fee codes for doctors. However, a provincial postswitch surveillance program evaluating the impact associated with switching from Remicade to a biosimilar was not instituted. Moreover, the biosimilar switch policy was enacted prior to the Joint Canadian Association of Gastroenterology (CAG) and Crohn’s and Colitis Canada (CCC) Position Statement on Biosimilars for the Treatment of Inflammatory Bowel Disease (8). This recent publication recommended against nonmedical switching from infliximab to a biosimilar after evidence was reviewed and graded (12).
Currently, the Government of Alberta is considering a similar policy to switch patients on originator infliximab to biosimilar and has engaged some stakeholders in discussions after execution of a Non-Disclosure Agreement. This Perspective is written with the purpose of providing the rationale for our decision to not support a nonmedical switch; to estimate the potential harm; to address understudied vulnerable and high-risk populations; and to explore alternative approaches for saving dollars.
Evidence for Nonmedical Biosimilar Switching
The current state of evidence evaluating the efficacy and safety of nonmedical switching between originator biologics and subsequent entry biosimilars is summarized in the Joint CAG/CCC Position Statement. The authors reviewed the literature to evaluate efficacy, safety, and patient acceptance of using biosimilar versus originator biologics in both patients naïve to anti-TNF therapy as well as patients undergoing a nonmedical switch. In contrast to other position statements, the CAG/CCC recommendations were based on a systematic review of the literature with formal evaluation of evidence quality using GRADE criteria (12). Overall, the quality of evidence was low to very low.
There are several important considerations when evaluating the evidence for and against nonmedical switching. First, there is a paucity of high-quality randomized controlled trials evaluating this question: Existing studies are underpowered to determine noninferiority in IBD patients, much less equivalence. Second, existing studies have primarily been randomized transition studies, where patients are randomized to switch to the biosimilar or continue the originator biologic; the requisite crossover and interchangeability studies necessary to answer critical questions regarding the risk of immunogenicity postswitching have not yet been performed. Third, while several jurisdictions have implemented nonmedical switch policies, appropriate postswitch pharmacovigilance and surveillance studies have not been rigorously conducted and often fail to include adequate follow-up time or evaluation of meaningful endpoints. Determining the degree of attributable excessive risk in uncontrolled studies is difficult given that the annual risk of loss of response to anti-TNF therapy is as high as 10 to 20% per patient-year, which may be underestimated when other biologic therapies are not available (13), and there may be a nocebo effect in patients switching to biosimilar therapy (14). Finally, extrapolating results from biosimilar switch experiences for rheumatologic or dermatologic indications to patients with IBD may not be valid. There are differential effects of biologic treatments across disease states and the consequences of loss of response in IBD are arguably more significant related to the lack of advanced therapeutic options and the reality that surgical intervention carries a high risk of morbidity and unacceptable mortality rate (15,16).
With these considerations in mind, what does the existing randomized controlled trial evidence tell us about the risks and benefits associated with biosimilar switching? The NOR-SWITCH study was a randomized, double-blind, noninferiority (assuming a 15% noninferiority margin), phase IV transition trial including adult patients with Crohn’s disease, ulcerative colitis, rheumatoid arthritis, spondyloarthritis, psoriatic arthritis and psoriasis (17). Patients on stable reference infliximab for at least 6 months were randomized 1:1 to continue reference infliximab or switch to biosimilar CT-P13 for 52 weeks with no change in dosing regimen. A total of 482 Norwegian patients were randomized, including 155 patients with Crohn’s disease and 93 patients with ulcerative colitis. While there was overall noninferiority with switching to biosimilar CT-P13 for disease worsening (adjusted treatment difference −4.4% [95% CI: −12.7%, 3.9%]) and occurrence of adverse events, the data in Crohn’s disease patients was far less reassuring with a treatment difference of −14.3% [95% CI: −29.3%, 0.7%], favouring continuation of infliximab originator. A second, double-blind, prospective randomized controlled trial in 200 IBD patients from a single center in Munich, Germany has also been described (18). In this trial, 111 patients were switched to CT-P13 and 89 patients were continued on infliximab originator. While this study was also underpowered to detect either noninferiority or equivalence, there was an 11% nominal difference (62.2% versus 73.0%, P = 0.104) in patients achieving the primary endpoint (clinical remission and continuation of study drug at 52 weeks), again, favouring originator infliximab. When results from these two randomized control trials were pooled, the relative risk of loss of response/worsening disease was 0.64 (95% CI: 0.44, 0.94; P = 0.02), favouring continuation of originator infliximab resulting in a number needed to harm (NNH) of 11 (95% CI: 6, 50).
The CAG/CCC position statement also identified two uncontrolled cohort studies that compared patients continuing originator infliximab versus switching to biosimilar CT-P13 (19,20). The primary limitations of both of these studies relate to a lack of power necessary to detect a clinically relevant difference and risk of bias in open-label designs where the decision to switch is made by the patient and clinician. However, statistically nonsignificant signals for worsening disease activity were detected: In a cohort study of 219 adults with IBD, patients who continued on infliximab originator had lower risks of disease worsening (relative risk [RR]: 0.66 [95% CI: 0.28, 1.57]), and biologic dose increase/treatment discontinuation (RR: 0.69 [95% CI: 0.38, 1.25]), mirroring observations from randomized controlled trials. Furthermore, a larger cohort of 1,388 patients continuing on infliximab originator therapy across all indications compared to 136 patients switching to CT-P13 demonstrated a greater than fivefold increased hazard of discontinuing treatment at 12 months (hazard ratio: 5.53 [95% CI: 4.01, 7.63]) (21).
Importantly, we acknowledge that there were no significant differences observed in either randomized control trials or observational studies with respect to clinical remission or adverse events in patients continuing originator infliximab or biosimilar CT-P13. However, we caution readers that these data points should be interpreted carefully. First, the overall proportion of patients who are in remission at 1 year is biased if dropout of nonresponders is not adjusted for. Second, worsening of IBD is the most relevant adverse event in biologic trials, which has typically been considered separately in biosimilar noninferiority studies (22). Third, the existing data in IBD has predominantly evaluated switching to biosimilar CT-P13 (Inflectra) but there are no data specifically evaluating outcomes after switching to Renflexis in this population, which is particularly relevant in Alberta because switching to either formulation may be implemented. Given the overall data suggesting that nonmedical biosimilar switching leads to an increased risk of disease worsening, dose escalation, and/or switching to an alternative therapy, the CAG/CCC Joint Position statement provides caution against nonmedical switching from originator infliximab to biosimilar in patients with stable disease on Remicade (23).
Logistical and Ethical Implications of Biosimilar Switching
Murdoch and Caulfield from the University of Alberta wrote the most comprehensive review on the legal and ethical implications of instituting a provincial biosimilar switch initiative (24). The authors state: “At a minimum, the controversy surrounding the switch will necessitate, as part of the consent process, a robust and thorough disclosure of relevant risks, benefits and reasonable alternatives.” Consequently, gastroenterologists have an ethical duty to explain to their patients the rationale and implications of abiding by a government mandated switch program. In Alberta, approximately 2,000 Albertans with IBD are prescribed Remicade (23). The logistical challenges associated with attempting to switch this large cohort of patients from Remicade to biosimilar infliximab are tremendous. Due to logistical challenges in transitioning to biosimilars, including geographic barriers unique to a large province like Alberta, biosimilar switch data from centres in Europe (25–29) are unlikely to approximate the Canadian experience. Further, we identify several areas where potential harm may come to patients as a direct result of an uncoordinated switching policy.
First, gastroenterologists are obligated to properly consent patients to a treatment switch. These clinic visits are necessary to address the fear and anxiety that many patients feel about switching their biologic (30). Assuming a 20-minute follow-up appointment, a minimum of 650 additional clinic hours will need to be scheduled by gastroenterologists within the timeframe allowed by the government to complete the switch program. In British Columbia, the timeframe was 6 months; though, due to the 2-month infusion interval of infliximab, transition to a biosimilar needs to be organized within 4 months. This clinic time will undeniably add to already burdened digestive disease ambulatory clinics in Alberta. For example, the Calgary Zone’s Central Triage and Access system receives over 1,100 referrals to gastroenterologists with wait times for routine triaged referrals exceeding 18 months (31). Gastroenterologists in Alberta are already struggling to provide timely access to care, and a biosimilar switch program will needlessly add to long wait times.
Second, because infliximab is administered as a scheduled outpatient intravenous infusion, substantial logistical and human resources are dedicated to maintaining an infusion network that provides equitable care across the province. These principles may be threatened by a biosimilar switch policy: Once a patient on Remicade visits the gastroenterologist, a switch necessitates a new prescription, change in a biologic coordinator and patient support program, coordinating transfer of insurance coverage, transition to an outpatient infusion clinic that supports biosimilars, and surveillance monitoring including fecal calprotectin and drug levels. Whereas the BioAdvance outpatient infusion clinic network for Remicade has been operational in Alberta for nearly two decades and has broad geographic distribution, the efficacy and efficiency of biosimilar transition will be dependent on the capacity of the two biosimilar companies to handle the transition of the 2,000 Albertans on Remicade.
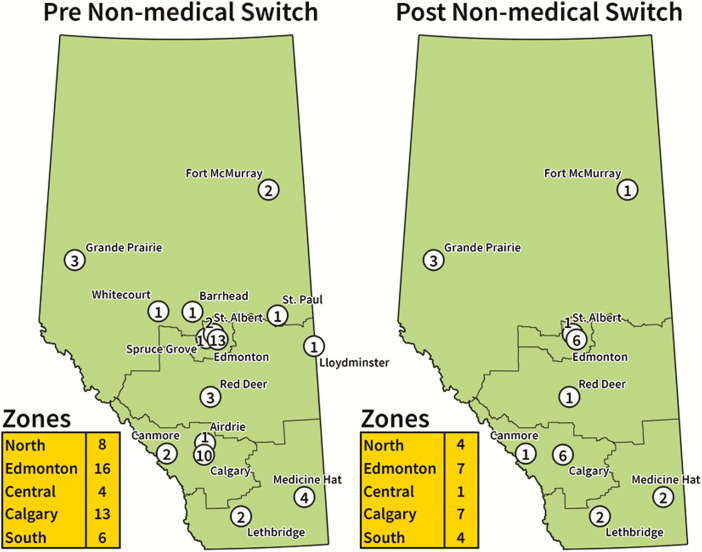
In November 2019, 47 independent outpatient infusion clinics distributed throughout Alberta were operational for providing originator and biosimilar infliximab (Figure 1). These outpatient infusion clinics are not funded by the Alberta government. The large distribution of outpatient infusion clinics supports patients with IBD living in rural regions and some operate in evenings and weekends to reduce loss of work productivity. Following a biosimilar switch program, 24 BioAdvance outpatient infusion clinics that are under contractual obligation to only infuse Remicade will cease to infuse patients who are switched to biosimilars. Figure 1 displays the geographic location of outpatient infusion clinics available to provide infliximab biosimilars: Based on the distribution of infusion clinics in November 2019, the number of outpatient infusion centers may drop in the Calgary Zone (13 to 7), Edmonton Zone (16 to 7), Central Zone (4 to 1), North Zone (8 to 4) and South Zone (6 to 4). This will necessitate some patients to travel several hours to receive services and potentially impact them further with time off work. The alternative would be for Alberta Health Services to assume the burden of infusions, leading to greater utilization of hospital-based infusion clinics, which would cut into the proposed cost savings. Although new biosimilar outpatient infusion sites will be introduced following the announcement of a biosimilar switch policy, centers with a long-track record of infusing patients will be most prepared to seamlessly enact a switch process. Logistical challenges in transferring infusion clinics have a high probability to induce delays in the dosing interval of infliximab, whose maintenance dose ranges from every 4 to 8 weeks.
Figure 1.
Alberta infliximab outpatient infusion network before and after nonmedical biosimilar infliximab switch, based on distribution of sites in November 2019.
Patients treated with infliximab have a high propensity for immunogenicity: Antibodies form in the environment of low drug levels (32). Consequently, logistical delays in transitioning from originator to biosimilar may lead to decreasing levels and increase the likelihood of antibody formation. Downstream effects include subsequent need to increase doses and, more importantly, clinically meaningful loss of response. This increased cost has been shown in other autoimmune conditions (33–35). This pathway of harm will not only drive costs up but also put patients at risk as they will need to transition to second-line biologic therapy, which is not associated with any appreciable cost savings and has reduced efficacy when used second line. Nonresponders experience impaired quality of life, need for otherwise avoidable hospitalizations and surgeries, and in a subset, postoperative mortality. Patients residing outside of metropolitan cities will be disproportionately negatively affected by the switch policy as they lack access to gastroenterologists and have less availability of infusion centres. Moreover, access to infusion centres for children will be restricted, as infusion of children requires nurses trained in Pediatric Advanced Life Support and paediatric life-saving equipment (36).
Finally, patients with private, third-party insurance may continue on originator infliximab. This raises important ethical implications for nonmedical switching. Effectively, this policy will create two tiers of health care access: Patients without private drug insurance will be disproportionately affected. Importantly, an individual patient in remission on originator biologic stands to gain no benefit from nonmedical switching but rather only adopts the risk of harm from loss of response. Hence, to place that burden of potential harm solely on the shoulders of patients without the means to afford private insurance coverage stands in stark contrast to the principles of universality that Canadians have proudly associated with our health care system.
Biosimilar Switching in Vulnerable Populations
Special populations with IBD may be more vulnerable to an adverse outcome related to a biosimilar switch including children, pregnant women, and the elderly where data on switching is limited (37). The European Society for Pediatric Gastroenterology, Hepatology, and Nutrition released an updated position statement in January 2019, stating that a biosimilar switch may be considered in children with IBD in clinical remission, following at least three induction infusions (38). However, these paediatric guidelines were based on an expert opinion from evaluation of the literature without using GRADE methodology (12). Further, special cohorts including individuals who are actively in the process of transferring to adult care, pregnant women, and the elderly need to be taken into consideration (31,39,40). Given the lack of published data, it may be prudent to delay switching in individuals who are in the process of transferring from paediatric to adult care (41), as many other changes are already occurring, including change in physician, hospital, clinic and insurance. Minimizing additional changes during this crucial time may improve the success of paediatric to adult transition. Similarly, although there is no published data on the effect of biosimilar switch during pregnancy on materno-fetal outcomes, pregnancy constitutes a defined time period and delaying a mandated switch to the postpartum period may result in less mental and physical stress. Finally, data on switching in seniors with IBD are lacking. In the elderly population, age and increased comorbidities reduce the physiological reserve available to recover from the consequences of an IBD flare.
High-risk patients with IBD who are stabilize on Remicade are at significant risk for surgery if they develop secondary loss of response. Examples of high risk patients with IBD include those patients with acute severe disease at presentation, hospitalization, are in the midst of induction therapy, have perianal disease or extra-intestinal manifestations, are obese, or are active smokers (42). When these patients flare, options for medical rescue are limited. Stratified analyses documenting safety of switching in these populations are exceedingly limited. Consequently, exemptions for switching should be offered to vulnerable and high-risk populations with IBD. An exemption process that permits certain patients to continue on the originator biologic therapy based on medical justification from a physician may mitigate the risks of nonmedical switching in high-risk patients, although there is no current guidance on who may qualify (43).
Predicting Outcomes of Nonmedical Switching in Alberta
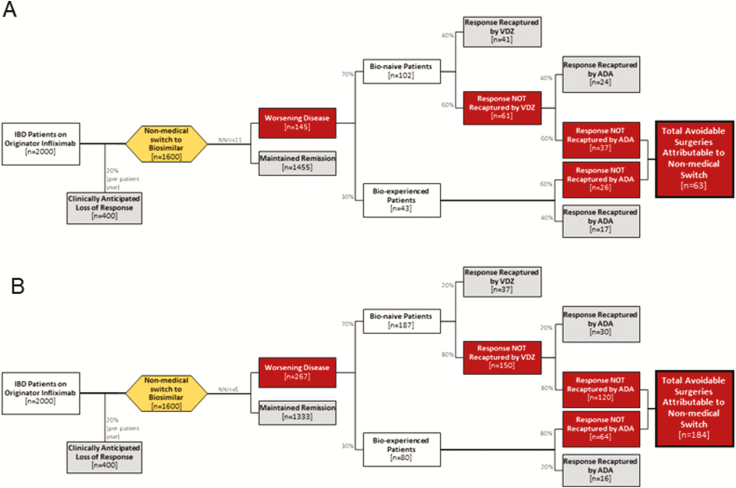
Applying the available data to an Alberta IBD context, we provide two scenarios for the likely patient impact of nonmedical switching. Both projections are based on the existing literature, and are summarized in Figure 2. The primary output of these predictions is the estimated number of patients undergoing avoidable surgical intervention directly attributable to a nonmedical biosimilar switch policy. The risk of harm was estimated from the CAG/CCC position statement (NNH = 11). All other assumptions used in these projections were based on highly conservative estimates favouring nonmedical biosimilar switching, such that all projections of potential patient harm reflect optimistic scenarios. In fact, it is plausible that the potential realized patient harm may be higher than our current projections because of the possible logistical challenges (e.g., change in infusion centers) in a province-wide switch program.
Figure 2.
Anticipated patient health outcomes after nonmedical biosimilar infliximab switch assuming best (A) and worst (B) case scenarios.
We estimated that there are currently 2,000 patients with IBD on originator infliximab in Alberta who would be affected by a nonmedical biosimilar switch (23). We accounted for an estimated 20% loss of response over 1 year (13), irrespective of switching policy: The subsequent 1,600 IBD patients in remission on originator infliximab were carried forward to a biosimilar switch. We created projections for two scenarios: a best-case and worst-case scenario. For both scenarios, we assumed a high proportion (70%) of these patients were receiving infliximab as their first biologic to maximize the number of postflare medical treatment options favouring biosimilar switching. In the best-case scenario, we assumed the NNH for switch was 11, and that bio-naive patients could be treated with either adalimumab (with 40% treatment response after infliximab failure (44)), or vedolizumab (with 40% treatment response after anti-TNF failure (45)). For bio-experienced patients who have already failed vedolizumab, we carried forward the assumption that 40% would still respond to adalimumab prior to requiring surgery. In the worst-case scenario, we assumed an NNH = 6 and 20% remission recaptured with either vedolizumab or adalimumab. We did not include ustekinumab as a treatment option in patients losing response after biosimilar switch because this medication is currently not covered by Alberta Blue Cross and compassionate access to ustekinumab may be revoked after a biosimilar switch policy.
In the best-case scenario, 63 avoidable surgical procedures would occur, directly attributable to a biosimilar switch policy. In the worst-case scenario, 184 avoidable surgical procedures would occur. Patients undergoing an avoidable surgery are at risk for surgery-related mortality: A meta-analysis of population-based studies has demonstrated a postoperative mortality of 3.6% (Crohn’s disease) and 5.3% (ulcerative colitis) for emergency surgery, and 0.6% (Crohn’s disease) and 0.7% (ulcerative colitis) in elective settings (16).
Patient Perspective
A substantial gap exists between patients with IBD and policy makers who are mandating a nonmedical biosimilar switch. CCC recently conducted a survey across Canada of nearly 800 patients with IBD or their caregivers to understand their perspective regarding a nonmedical biosimilar switch policy. The survey revealed that over three quarters of patients or their caregivers disapproved of a nonmedical switch. Open comments from the survey revealed that opposition for the policy was driven by substantial fear and anxiety (41).
Mental anguish of patients with IBD who are stable on originator infliximab and forced to switch to a biosimilar has far-reaching consequences. A nocebo effect has been proposed in this setting whereby anxiety over a medical decision leads to patient harm that was not caused directly by the intervention (14,22,46,47). Studies have documented that between 20% and 30% of patients with IBD suffer from mental illness, such as depression and anxiety (48). Consequently, patients with IBD are highly susceptible to a nocebo effect. Moreover, studies indicate that depression can affect intestinal inflammation such that the mental anguish of switching could directly worsen disease activity irrespective of the efficacy of the new medication (49). These concerns are heightened among high-risk patients who have previously experienced debilitating symptoms, hospitalizations, and surgeries that preceded remission on anti-TNF therapy.
Not surprisingly, over 11,000 patients with IBD or their caregivers have contacted the Alberta government to voice their opinion against a mandated biosimilar switch policy. Patients have advocated that highly individualized medical decisions should be left between themselves and their physicians.
Cost of Biosimilar Switching
In most jurisdictions that implemented nonmedical switching, the rationale is to achieve cost savings. Collaborative efforts exist in which part of the cost savings were re-invested into health care resources that directly benefit the patients affected by the switch (29). In contrast, the Alberta government has failed to communicate transparently with key stakeholders (including physicians and patients) to consider a gain-share agreement. Furthermore, although the list prices of biosimilars are lower than the listed price of originator infliximab, this is unlikely to result in any realized cost savings for the province for several reasons.
First, both the manufacturer of Remicade and the government have publicly acknowledged that the price of the originator was offered at the same price as the biosimilar, essentially negating any cost savings as a rationale for considering a nonmedical switch policy. Second, economic modeling must consider the costs associated with managing the logistical challenges of switching patients on infliximab, including costs associated with establishing new infusion networks and changing patient support programs. Third, there are multiple sources of opportunity cost to switching to a biosimilar, including unnecessary physician and time resources expended on counselling and educating patients. Fourth, both the direct and indirect costs of managing disease flares, including lost work productivity, poor quality of life, increased ambulatory visits for medical treatment, and, ultimately, hospitalization and surgery must to be considered. Prior economic evaluations in Europe that were favourable towards switching did not account for these factors (29). Moreover, in one European cohort study, transitioning to biosimilar was associated with 20% higher costs than remaining on originator biologic (50). Further, a systematic review of nonmedical switching of all types of drugs did not support cost savings while nearly 70% of studies reported cost savings with originator drugs (51).
One must then question the ethics of nonmedical switching when there are no anticipated cost savings to be achieved and real patient harms that are anticipated. Stakeholders should work collaboratively to preserve the health and wellness of patients. Indeed, in the world of multiple biologic options, alternatives to forced nonmedical switching that achieve the goals of patients, physicians, and policy makers do exist. This includes, for example, maximal allowed cost strategies or lowest cost alternatives where government would allow for a fair market pricing while allowing for patient and physician choice and minimizing the cost to the system. However, to have meaningful discussions around patient-oriented, cost-effective strategies, these conversations need to be conducted transparently and with input from patients at the ground level, not just post-hoc after policies have already been unilaterally crafted for political expediency.
Conclusion
It is important for all stakeholders to be accountable for the costs within the health care system. Physicians need to be stewards of the system but their first priority should be to the health and wellness of their individual patients. This first dictum of the Hippocratic Oath remains ‘First do no Harm’. We argue that even in the best-case scenario, a nonmedical switch policy will harm at least some patients with IBD; notwithstanding the logistical challenges and ethical implications of such a policy that comes with no clearly measurable cost savings.
Conflict of Interests: G.G.K. has received honoraria for speaking or consultancy from Abbvie, Janssen, Pfizer, and Takeda. He has received research support from Ferring, Janssen, Abbvie, GlaxoSmith Kline, Merck and Shire. He shares ownership of a patent: TREATMENT OF INFLAMMATORY DISORDERS, AUTOIMMUNE DISEASE, AND PBC. UTI Limited Partnership, assignee. Patent WO2019046959A1. PCT/CA2018/051098. September 7, 2018. C.M. has received honoraria for speaking or consultancy from Janssen, AbbVie, Takeda, Pfizer and Robarts Clinical Trials Inc. C.H.S. has received honoraria for speaking or consultancy from Janssen, Abbvie, Takeda, Ferring, Shire and Pfizer. She has received research support from Janssen. K.K. has received honoraria for speaking or consultancy from AbbVie, Janssen, Takeda and Pfizer. R.P. has received honoraria for speaking or consultancy from AbbVie, Allergan, Amgen, Biogen, BMS, Celgene, Eisai, Elan, Eli Lily, Ferring, Gilead, Glaxo-Smith Kline, Janssen, Merck, Pfizer, Robarts, Samsung, Sublimity, Sandoz, Shire Takeda, UCB, Protagonist, Arena Pharma, SatisfaiHealth and Roche.
References
- 1. Kaplan GG, Bernstein CN, Coward S, et al. . The impact of inflammatory bowel disease in Canada 2018: Epidemiology. J Can Assoc Gastroenterol 2019;2(Suppl 1):S6–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coward S, Clement F, Benchimol EI, et al. . Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology 2019;156(5):1345–1353.e4. [DOI] [PubMed] [Google Scholar]
- 3. Benchimol EI, Bernstein CN, Bitton A, et al. . The impact of inflammatory bowel disease in Canada 2018: A scientific report from the Canadian gastro-intestinal epidemiology consortium to Crohn’s and colitis Canada. J Can Assoc Gastroenterol 2019;2(Suppl 1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. King JA, Underwood FE, Panaccione N, et al. . Trends in hospitalisation rates for inflammatory bowel disease in western versus newly industrialised countries: A population-based study of countries in the Organisation for Economic Co-operation and Development. Lancet Gastroenterol Hepatol 2019;4(4):287–95. [DOI] [PubMed] [Google Scholar]
- 5. Ma C, Moran GW, Benchimol EI, et al. . Surgical rates for Crohn’s disease are decreasing: A population-based time trend analysis and validation study. Am J Gastroenterol 2017;112(12):1840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuenzig ME, Benchimol EI, Lee L, et al. . The impact of inflammatory bowel disease in Canada 2018: Direct costs and health services utilization. J Can Assoc Gastroenterol 2019;2(Suppl 1):17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Radin M, Sciascia S, Roccatello D, et al. . Infliximab biosimilars in the treatment of inflammatory bowel diseases: A systematic review. BioDrugs 2017;31(1):37–49. [DOI] [PubMed] [Google Scholar]
- 8. Moayyedi P, Benchimol EI, Armstrong D, Yuan C, Fernandes A, Leontiadis GI. Joint Canadian association of gastroenterology and crohn’s and colitis Canada position statement on biosimilars for the treatment of inflammatory bowel disease. J Can Assoc Gastroenterol. 2020;3(1):e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. U.S. Food and Drug Administration. Biosimilar Development, Review, and Approval. 2017. https://www.fda.gov/drugs/biosimilars/biosimilar-development-review-and-approval.
- 10. CADTH Canadian Drug Expert Committee. Infliximab: Notice of final recommendation. Common Drug Review. 2016. https://www.cadth.ca/sites/default/files/cdr/complete/SE0483_IBD_Inflectra-Oct-28-16.pdf. [Google Scholar]
- 11. Biosimilars Initiative. Biosimilars Initiative for Prescribers. 2019. https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/pharmacare/prescribers/biosimilars-initiative-prescribers.
- 12. Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ben-Horin S, Chowers Y. Review article: Loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther 2011;33(9):987–95. [DOI] [PubMed] [Google Scholar]
- 14. Odinet JS, Day CE, Cruz JL, et al. . The biosimilar nocebo effect? a systematic review of double-blinded versus open-label studies. J Manag Care Spec Pharm 2018;24(10):952–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Silva S, Ma C, Proulx MC, et al. . Postoperative complications and mortality following colectomy for ulcerative colitis. Clin Gastroenterol Hepatol 2011;9(11):972–80. [DOI] [PubMed] [Google Scholar]
- 16. Singh S, Al-Darmaki A, Frolkis AD, et al. . Postoperative mortality among patients with inflammatory bowel diseases: A systematic review and meta-analysis of population-based studies. Gastroenterology 2015;149(4):928–37. [DOI] [PubMed] [Google Scholar]
- 17. Jørgensen KK, Olsen IC, Goll GL, et al. ; NOR-SWITCH study group Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): A 52-week, randomised, double-blind, non-inferiority trial. Lancet 2017;389(10086):2304–16. [DOI] [PubMed] [Google Scholar]
- 18. Roder H, Schnitzler F, Borchardt J, Janelidze S, Ochsenkuen T. Switch of iinfiximab originator to biosimilar CT-P13 in patients with Crohn’s disease and ulcerative colitis in a large German IBD center. A one year, randomized and prospective trial. United European Gastroenterol J 2018. https://www.ueg.eu/education/document/switch-of-infliximab-originator-to-biosimilar-ct-p13-in-patients-with-crohn-s-disease-and-ulcerative-colitis-in-a-large-german-ibd-center-a-one-year-randomized-and-prospective-trial/181336/ [Google Scholar]
- 19. Kang B, Lee Y, Lee K, et al. . Long-term outcomes after switching to CT-P13 in pediatric-onset inflammatory bowel disease: A single-center prospective observational study. Inflamm Bowel Dis 2018;24(3):607–16. [DOI] [PubMed] [Google Scholar]
- 20. Haifer C, Srinivasan A, Menon S, An Y, Picardo S. Switching Australian patients with moderate to severe inflammatory bowel disease from originator infliximab to biosimilar Inflectra: Interim results from a multicenter parallel cohort study. J Gastroenterol Hepatol. 2018;34:155–156. [Google Scholar]
- 21. Phillips K, Juday T, Zhang Q, Keshishian A. Economic outcomes, treatment patterns, and adverse events and reactions for patients prescribed infliximab or CT-P13 in the Turkish population. Ann Rheum Dis. 2017;76:835. [Google Scholar]
- 22. van Hoeve K, Dreesen E, Hoffman I, et al. . Efficacy, Pharmacokinetics, and immunogenicity is not affected by switching from infliximab originator to a biosimilar in pediatric patients with inflammatory bowel disease. Ther Drug Monit 2019;41(3):317–24. [DOI] [PubMed] [Google Scholar]
- 23. Coward S. Forecasting the Future: A Trek through the Changing Landscape of Inflammatory Bowel Disease. Calgary, Alberta, Canada: Community Health Sciences, University of Calgary; 2019. [Google Scholar]
- 24. Murdoch B, Caulfield T. The Law and Ethics of Switching from Biologic to Biosimilar in Canada.2019. https://crohnsandcolitis.ca/Crohns_and_Colitis/images/events/2019-September-CCC-Biosimilars-Biologics-Murdoch-Caulfield-NEW-EN.pdf.
- 25. Bronswijk M, Moens A, Lenfant M, et al. . Evaluating efficacy, safety, and pharmacokinetics after switching from infliximab originator to biosimilar CT-P13: Experience from a large tertiary referral center. Inflamm Bowel Dis. 2020;26(1):161. [DOI] [PubMed] [Google Scholar]
- 26. Guerra Veloz MF, Argüelles-Arias F, Castro Laria L, et al. . Loss of efficacy and safety of the switch from infliximab original to infliximab biosimilar (CT-P13) in patients with inflammatory bowel disease. World J Gastroenterol 2018;24(46):5288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Plevris N, Jones GR, Jenkinson PW, et al. . Implementation of CT-P13 via a managed switch programme in Crohn’s disease: 12-Month real-world outcomes. Dig Dis Sci 2019;64(6):1660–7. [DOI] [PubMed] [Google Scholar]
- 28. Martelli L, Peyrin-Biroulet L. Efficacy, safety and immunogenicity of biosimilars in inflammatory bowel diseases: A systematic review. Curr Med Chem 2019;26(2):270–9. [DOI] [PubMed] [Google Scholar]
- 29. Razanskaite V, Bettey M, Downey L, et al. . Biosimilar infliximab in inflammatory bowel disease: Outcomes of a managed switching programme. J Crohns Colitis 2017;11(6):690–6. [DOI] [PubMed] [Google Scholar]
- 30. Crohn’s and Colitis Canada. http://www.crohnsandcolitis.ca/Crohns_and_Colitis/documents/2019-September-CCC-Patient-HCP-Survey-Input-Full-Report.pdf. 2019.
- 31. Nguyen GC, Targownik LE, Singh H, et al. . The impact of inflammatory bowel disease in Canada 2018: IBD in seniors. J Can Assoc Gastroenterol 2019;2(Suppl 1):68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vermeire S, Gils A, Accossato P, et al. . Immunogenicity of biologics in inflammatory bowel disease. Therap Adv Gastroenterol 2018;11:1756283X17750355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gibofsky A, Skup M, Mittal M, et al. . Effects of non-medical switching on outcomes among patients prescribed tumor necrosis factor inhibitors. Curr Med Res Opin 2017;33(11):1945–53. [DOI] [PubMed] [Google Scholar]
- 34. Gibofsky A, Skup M, Yang M, et al. . Short-term costs associated with non-medical switching in autoimmune conditions. Clin Exp Rheumatol 2019;37(1):97–105. [PubMed] [Google Scholar]
- 35. Liu Y, Yang M, Garg V, et al. . Economic impact of non-medical switching from originator biologics to biosimilars: A systematic literature review. Adv Ther 2019;36(8):1851–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barfield E, Sockolow R, Hoffenberg E, et al. . Assuring quality for non-hospital-based biologic infusions in pediatric inflammatory bowel disease: A clinical report from the North American society for pediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr 2018;66(4):680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singh S, Picardo S, Seow CH. Management of inflammatory bowel diseases in special populations: Obese, old or obstetric. Clin Gastroenterol Hepatol. 2019. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Ridder L, Assa A, Bronsky J, et al. ; Paediatric IBD Porto group of ESPGHAN Use of biosimilars in pediatric inflammatory bowel disease: An updated position statement of the pediatric IBD porto group of ESPGHAN. J Pediatr Gastroenterol Nutr 2019;68(1):144–53. [DOI] [PubMed] [Google Scholar]
- 39. Carroll MW, Kuenzig ME, Mack DR, et al. . The impact of inflammatory bowel disease in Canada 2018: Children and adolescents with IBD. J Can Assoc Gastroenterol 2019;2(Suppl 1):49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nguyen GC, Seow CH, Maxwell C, et al. ; IBD in Pregnancy Consensus Group; Canadian Association of Gastroenterology The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology 2016;150(3):734–57.e1. [DOI] [PubMed] [Google Scholar]
- 41. Shapiro JM, El-Serag HB, Gandle C, et al. . Recommendations for successful transition of adolescents with inflammatory bowel diseases to adult care. Clin Gastroenterol Hepatol 2019. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 42. Lichtenstein GR, Loftus EV, Isaacs KL, et al. . ACG clinical guideline: Management of Crohn’s disease in adults. Am J Gastroenterol 2018;113(4):481–517. [DOI] [PubMed] [Google Scholar]
- 43. Pfizer. Understanding Biosimilars.2019. https://www.pfizer.ca/understanding-biosimilars.
- 44. Sandborn WJ, Rutgeerts P, Enns R, et al. . Adalimumab induction therapy for Crohn disease previously treated with infliximab: A randomized trial. Ann Intern Med 2007;146(12):829–38. [DOI] [PubMed] [Google Scholar]
- 45. Sands BE, Feagan BG, Rutgeerts P, et al. . Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014;147(3):618–27.e3. [DOI] [PubMed] [Google Scholar]
- 46. Pouillon L, Socha M, Demore B, et al. . The nocebo effect: A clinical challenge in the era of biosimilars. Expert Rev Clin Immunol 2018;14(9):739–49. [DOI] [PubMed] [Google Scholar]
- 47. Spanou I, Mavridis T, Mitsikostas DD. Nocebo in biosimilars and generics in neurology: A systematic review. Front Pharmacol 2019;10:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Frolkis AD, Vallerand IA, Shaheen AA, et al. . Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut 2019;68(9):1606–12. [DOI] [PubMed] [Google Scholar]
- 49. Mawdsley JE, Rampton DS. Psychological stress in IBD: New insights into pathogenic and therapeutic implications. Gut 2005;54(10):1481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Phillips K, Juday T, Zhang Q, Keshishian A. SAT0172 Economic outcomes, treatment patterns, and adverse events and reactions for patients prescribed infliximab or ct-p13 in the Turkish population. Ann Rheum Dis. 2017;76(Suppl 2):835. [Google Scholar]
- 51. Nguyen E, Weeda ER, Sobieraj DM, et al. . Impact of non-medical switching on clinical and economic outcomes, resource utilization and medication-taking behavior: A systematic literature review. Curr Med Res Opin 2016;32(7):1281–90. [DOI] [PubMed] [Google Scholar]