Abstract
Interventional oncology (IO) has proven to be highly efficient in the local therapy of numerous malignant tumors in addition to surgery, chemotherapy, and radiotherapy. Due to the advent of immune-oncology with the possibility of tumor control at the molecular and cellular levels, a system change is currently emerging. This will significantly rule oncology in the coming decades. Therefore, one cannot think about IO in the 21st century without considering immunology. For IO, this means paying much more attention to the immunomodulatory effects of the interventional techniques, which have so far been neglected, and to explore the synergistic possibilities with immuno-oncology. It can be expected that the combined use of IO and immuno-oncology will help to overcome the limitations of the latter, such as limited local effects and a high rate of side-effects. To do this, however, sectoral boundaries must be removed and interdisciplinary research efforts must be strengthened. In case of success, IO will face an exciting future.
Introduction
Until now, the primary goal of cancer treatment was to reduce a malignant tumor to its absolute minimum. Though, the challenge is essentially twofold; on one hand fighting a malignant disease, which in turn most likely limits the life expectancy and quality, but on the other hand also dealing with a chronic disease. This might necessitate various treatment components as surgery, chemotherapy, and radiotherapy.
Radiological interventional oncology (IO) matured from diagnostic and interventional vascular radiology over the last decades. The diverse assortment of IO techniques resembles the underlying concepts of “classic” treatments in a minimal-invasive fashion. The decisive criteria can be quantified by the focal or locoregional application/effects of IO techniques accompanied by fewer side-effects and superior patient tolerance. For many clinical situations, IO outcomes are comparable to surgery and chemotherapy or even superior as in early and intermediate hepatocellular carcinoma (HCC), oligometastatic disease of the liver and lung, or in small renal cell cancers. Consequently, IO can be seen as the fourth pillar of oncological therapy in various malignant diseases and often as the problem-solver in multimodality treatment concepts.
Although the classical and minimal-invasive concepts for tumor therapy have evolved and improved over the decades, tumor relapse has been a recurring issue, when considering the treatment effectiveness. The macroscopic epiphenomena of malignant disease – typically depicted by radiological imaging and/or by laboratory chemistry – may then be treated again. Yet, it is still possible, that the fundamental cellular pathology remains untouched.
Nevertheless, continuous research over the last decades leads to a deepened understanding of tumor biology and of the manifold, often quite sophisticated, cross-linked immunological processes stimulating or suppressing the growth of malignant cells.
This rise of immune-oncology is going to change the face of oncology as well as current and future therapy concepts – the utilization of IO included. Essentially, the “classic” oncology model in terms of treating a defined disease by a standard protocol is evolving towards precision medicine customizing all components of health care to an individual patient.
Rather than elaborating on the development and specific outcomes of each ablative technique for its respective tumor, this article gives an insight on the evolution of oncology to immune-oncology and its possible impact on other fields, like IO. Additionally, it sketches the last and future developments in ablative radiological oncological techniques but also drafts some scenarios on how and where interventional oncology might head to.
Developments in medical oncology
Over the last century, oncology meant therapeutically chasing a disease. At the same time, the findings and developments of biomedical research provided more and more insights into the molecular machinery of cancer. Lately, numerous tools were developed to identify oncological control mechanisms and to utilize/modify them for potential therapeutical purposes.
The ambitious vision is that we may enter an era of pre-onset identification of malignant diseases together with treatment options before a significant tumor manifestation. Pinpointing and understanding mutations, as well as identifying predictive and prognostic biomarkers, is equally as important as learning how an insufficient mutation control can be influenced by using and modifying cellular signaling pathways and finally, enhancing the immunological control of malignant cells, in our ambitious endeavor of treating and curing cancer, no matter its stage.
After (and in addition to) “classical” chemotherapies and metabolic inhibitor therapies, immune-oncology is considered to be the cutting edge of medical oncology. Since the hallmarks of cancer had been proposed first in 2000 by Hanahan and Weinberg,1 they have been revised over the years,2–4 leading to seven up-to-date hallmarks required for carcinogenesis: “selective growth and proliferative advantage, altered stress response favoring overall survival, vascularization, invasion and metastasis, metabolic rewiring, an abetting microenvironment, and immune modulation.”5 Most of these are very likely to be linked to immunological process, which leads to the conclusion, that they could be altered immunologically.6,7 This widely prevalent hope (and hype) in biological therapies is supported by enormous research efforts. A very recent “Nature Reviews” analysis revealed an increase of 91% only in immune drug development between 2017 and 2019 with currently almost 5.200 active trials worldwide (clinicaltrials.gov database).8
A complete breakdown of this seemingly endless topic of immune mechanisms and their respective therapies in consideration of oncology is simply not possible within one article. Nevertheless, the main types which might interact with IO will be presented briefly9 :
At present, there are five main categories of immunotherapy with several subcategories (Table 1):
Table 1.
Categories of immune therapy
| Categories | Type | Substance (generic) | Target | TU types | |
|---|---|---|---|---|---|
| Passive | Targeted antibodies | Monoclonal antibodies | Bevacizumab, Cetuximab, Denosumab, Panitumumab, Rituximab, Trastuzumab … | CD20/33/52, VEGFA, EGFR, Her2, RANK | Leukemia, lymphoma, BRC, NSCLC, CRC, RCC, glioblastoma … |
| ADC | Ado-trastuzumab, brentuximab … |
HER2+ CD30 |
BRC, leukemia, lymphoma | ||
| Bispecific antibodies | Blinatumomab | Leukemia | |||
| Immunomodulators | Cytokines | Interferon Interleukin |
|||
| Adoptive cell transfer | TILs CIK cells CAPRI cells TCR cells CAR cells NK cells LAK |
EBR2 MART-1 CD19 |
Bile duct CA, lymphoma, melanoma | ||
| Active | Checkpoint inhibitors | Ipilimumab Nivolumab Atezolizumab |
CTLA-4 PD-1 PD-L1 |
Melanoma, NSCLC,RCC, lymphoma … | |
| Vaccines | Peptide vaccines | Oncophage | RCC | RCC | |
| DC vaccines | Sipuleucel-T | GM-CSF | PRC | ||
| Allogenic whole-cell vaccines | CD4, CD8 | Melanoma, PaC, BRC … | |||
| Oncolytic viruses | T-vec | Melanoma | |||
ADC, Antibody-drug conjugates;BRC, Breast cancer; CD, Cluster of differentiation; CRC, Colorectal cancer; CTLA, cytotoxic T-lymphocyte-associated Protein; EGFR, Epidermal Growth Factor Receptor; GM-CSF, Granulocyte-macrophage colony-stimulating factor; HER2, human epidermal growth factor receptor 2; MART, melanoma antigen recognized by T cells; NSCLC, Not small cell lung cancer; PD, programmed death; PD-L, programmed death-ligand; PRC, Prostate cancer; PaC, Pancreas cancer; RCC, Renal cell cancer;VEGFA, Vascular endothelial growth factor A.
Monoclonal antibodies (mAB)
Laboratory engineered AB made for a specific target (a protein on the surface of the cancer cell), increasing the immune response, killing the cancer cell, or stopping it from growth.
Immune modulation
Conjugated mABs tocarry a drug or radioactivity to a specific cell.
Bispecific mABs made of 2 AB components binding to two proteins at the same time.
Specific mABs (checkpoint inhibitors) blocking so-called checkpoint proteins (e.g. PD-1) which may be hindered to make tumour cells “invisible” for the immune system and re-releasing immune processes
-
Tumour-agnostic therapies
mABs targeting mutations caused by cancer cells but not the specific tumor cells which mean that any cancer containing these mutations can be addressed.
-
Non-specific immunotherapies
Cytokines as interferons and interleukins, stimulating the immune system without targeting cancer cells specifically, often applied together with chemotherapy (CTx) or radiotherapy (RT).
-
Oncolytic virus therapy
Genetically modified viruses to target and kill cancer cells with a secondary release of antigens stimulating an immune response.
-
Adoptive T-cell therapy
Isolation and ex-vivo expansion of cancer-specific T-cells to amplify T cells in-vivo
Genetically engineered chimeric antigen receptor (CAR) T-cells to produce an artificial T-cell receptor combining antigen-binding and T-cell activation.
Ex-vivo modified tumour-infiltrating lymphocytes (TILs) to amplify cancer lysis in-vivo.
Cancer vaccines
Preventive vaccines to prevent cancer cells from developing
Therapeutic vaccines to stimulate the immune system to attack tumor cells.
As known from numerous trials, most of these therapies produce a limited response, sometimes only in-vitro. Therefore, it is expected that there will be more and more complex personalized regimes by combining several modalities customized for the specific patient (“personalized therapy”). This will be based on selective genetic and immunological analysis identifying eligible patients and eligible therapy components. In order to further advance these therapies, a larger sampling of cells and tissues will be required. The general use of liquid biopsies (i.e. sampling of non-solid probes from body liquids as e.g. blood and analyzing them for e.g. circulating tumor cells, DNA and RNA components, or various other biomarkers) in particular will also be promoted in the field of oncology. Even though it is not certain if these biopsies yield sufficient results for targeted therapies.10
Moreover, current experience, e.g. in checkpoint inhibitor-based therapies shows that the tumor microenvironment is of ample importance for an effective treatment. The suitable tumor/patient can be identified by DNA, RNA, and germline DNA sequencing11 consequently, image-guided biopsies of target tissue(s) will get even more critical.12
Admittedly, all of this may sound too good to be true, suggesting that almost every problem and complication could be solved by understanding biological mechanisms – similar to how nuclear physics was perceived 100 years ago. Nonetheless, our increasing comprehension seemingly matches our increasing knowledge of the complexity of biological mechanisms and their interrelations, but of course, leads to the discovery of even more complex mechanisms and potential side-effects.
As of now, only limited data are available on the capabilities and risks of these constantly evolving molecular therapies. Even though only a small subset of patients qualifies for and benefits from these costly immune therapies, it is still our utmost priority to find the right therapies (or even combinations thereof) for the right patient and the right time. Particularly in this context, big data analysis and AI might help to identify appropriate targets, optimize the selection of the right immunological tools, and to predict a therapeutical outcome.13
Where will medical oncology be heading to?
In the first place, the noble ambition of oncology in the 21st century is still about curing cancer patients.
This goal becomes more and more achievable, due to immune-oncology and its allied sciences continuously deciphering, understanding, and modifying the biological mechanisms of carcinogenesis on a molecular basis.
A cure might (still) not be possible in many cases, nevertheless, immune-oncology alone or in combination with other systemic and/or local therapies which may also present some immune-modulatory effects, all tailored to the patient’s specific condition, may significantly contribute to controlling a malignant disease. “Healing” in terms of best achievable life quality then could be interpreted as the “cancer host” not experiencing significant impairment, even though tumor cells are still present in the body, which are controlled to the extent of being harmless.
Interventional oncology – the past and presence (The first 30–40 years)
Continuous developments regarding underlying techniques and clinical applications made IO a highly effective component in the therapy of primary and secondary malignant tumors of, e.g. liver, lung, kidney, or bone. IO is characterized by the absence or only mild presentation of systemic side effects, but moreover by the ability to combine it with “classical” surgery, medical and radiation oncology.
Applying the basic principles of established treatment concepts as surgery, chemotherapy, or radiotherapy IO techniques are acting mainly in a local or locoregional fashion, so far representing already some kind of individualized therapy.
According to the route of access and treatment delivery IO techniques are grouped into transvascular and percutaneous techniques (Table 2).
Table 2.
Interventional oncology techniques according to the route of access, treatment delivery, and specific mode of action
| Route of access/delivery | Technique of ablation | “Mode of action” | Target |
|---|---|---|---|
| Transarterial | Chemo- | HAI TACE (classic,+bland beads,+drug-eluting beads) Chemosaturation |
Liver: local, locoregional (e.g. super-selective, selective lobar or segmental whole liver |
| Radio- | TARE | Liver: local, loco-regional (e.g. super-selective, selective lobar or segmental, so-called radioresection) | |
| Transcutaneous | Thermo- | RFA MWA Cryoablation LITT |
Liver, lung, kidney, lymph node, bone, soft-tissue |
| Electromechanical- | IRE HiFU | Limited indications under investigation |
HAI, hepatic arterial perfusion; HiFU, high-focused ultrasound ; IRE, irreversible electroporation; LITT, laser-induced thermotherapy; MWA, Microwave ablation; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; TARE, transarterial radioembolization.
When the first IO technique transarterial chemoembolization (TACE) for unresectable hepatocellular carcinoma was introduced in 1977 by Yamada et al14 it was considered the state-of-the-art treatment for HCC worldwide. In recent years, various refinements and modifications of the original, lipiodol-based technique were developed. Current advancements head for a more selective and super-selective application with an increased awareness of intra- and peritumoral flow dynamics which translates into more elaborated embolization techniques.15
Still far away from standardization, current meta-analyses of TACE revealed 5 year survival rates of around 15–30% for intermediate and even some cases of advanced HCC, whereas over the last years a tendency to better outcomes is seen by drug-eluting beads (DEB)-TACE.16,17
Also, in hepatic metastatic disease – with pronounced evidence for hepatic metastatic colorectal cancer (mCRC) – transarterial perfusion techniques as hepatic arterial infusion (HAI) with 5-fluorodeoxyuridine (FUDR), 5-fluorouracil (5FU), and oxaliplatin gained significant interest among oncologists. In primarily unresectable liver metastases, the combination of HAI and systemic chemotherapy enables 5 year median survival rates of 70–85% in comparison to 40–50% of systemic chemotherapy alone.18–20
A particular “version” of HAI is percutaneous chemosaturation, a technique for isolated liver perfusion adopted from a primarily surgically developed perfusion technique. For this technique which is still performed sporadically only in selected centers, the only approved chemotherapy as of now is melphalan, a drug specifically for advanced ovarian cancer, myeloma, and melanoma, not very well suited for metastases of the vast majority of malignancies.21,22 Therefore, the future will show if this technique will gain a major clinical impact.
Transarterial radioembolization (TARE) with yttrium-90 as a “brachytherapy variant” of TACE has been causing controversy over the last years. Recent, large, multicentric RCTs comparing TARE, TARE combined with systemic therapies, and systemic therapies alone as 1. line therapy for HCC and mCRC provided mainly equivalence of the compared therapies –in contrary to expectations. Not surprisingly, a thorough work-up of these RCTs revealed the crucial impact of study design and patient selection and could explain the results.23–25 Nevertheless, numerous studies could establish the versatility of yttrium-90 in primary and secondary hepatic tumors. This proved its value for downstaging in curative intention, which furthermore could be used in radio-segmentectomy or -lobectomy. By subsequent induction of lobar hypertrophy, the usability in liver-transplantations or palliative treatments in post 1.- and 2.- line regimens just add to the excellent safety profiles regarding the procedure.26–29
The recently introduced compound Holmium-166 may have some practical features in terms of radiation characteristics in comparison to yttrium-90, however, its effectiveness has not been ascertained clinically yet.30
Among the different percutaneous thermal ablation techniques, radiofrequency ablation (RFA) was performed the first in 1988.31,32 Technical evolution and new developments such as cooled systems, microwave ablation, pulsed energy delivery, etc. made thermal ablation a very robust technique with well-established indications and excellent documented outcomes. The majority of recent studies and large registries including several thousand patients were able to confirm an effective tumor control by thermal ablation comparable to surgery for HCC,33–36 hepatic mCRC,37–40 lung tumors,41–44 and renal tumors.45–48 The major predictive factor for this success and the common ground in all studies is the tumor size ≤3 cm. Delineating this as the optimal maximum tumor diameter for thermal ablation, given it is adequately operated, leads to considerable outcomes: The available ablations systems are creating reliable and reproducible ablation volumes, additionally having an adequate safety margin, without any relevant side-effects or impaired quality of life. Larger volumes may be achievable in particular cases, e.g. by combining TACE and thermal ablation in larger HCCs or by complex 3D probe re-positioning, nevertheless, thermodynamics (i.e. depth of heat penetration, heat dissipation, heat sink effects) and pathophysiology (peritumoral cell spread, larger intra- and peritumoral vessels, invasion of lymphatics) are making an unimpeded increase in ablation volume not appear sensible.
Other ablative techniques as cryoablation, irreversible electroporation (IRE), and high-intensity focused ultrasound (HiFU) have found a niche or are still searching for it. First reports on percutaneous cryoablation (Cryo) of the prostate are dating back to the early 1990s. After some technical refinements as downsizing of cryoprobes, there was a growing interest for cryoablation also in other organs. At present, cryoablation is mainly used for renal cell cancer and bone tumors. Despite some benefits as less pain during ablation and MRI compatibility, there is no proven superiority of cryoablation over one of the other techniques.49–52
IRE and HiFU, both non-thermal, electromechanical ablation methods created some hype-expectations once they were introduced. Concerning the currently available data, both are not well-suited for the usual clinical needs. Besides the somewhat cumbersome practicability, local tumor control and complication rate IRE is inferior in comparison to the other minimal-invasive techniques in liver, lung, and renal tumors,53–56 whereas no relevant data are available yet for HiFU. However, IRE might get some meaning in the treatment of non-resectable pancreatic cancer wherein first studies report median survival rates of up to 27 months after IRE.57
Technical developments and future perspectives in IO
Ablation techniques
In general, the physicotechnical characteristics of current interventional material meets the clinical needs. From a practical point, further minimization/miniaturization of catheters, percutaneous probes, or embolic agents makes not too much sense since manual handling, visibility, and carrying capacities (e.g. embolic particles) must be warranted. This might not mean that there is no further development anymore, however, the usual minor improvements (e.g. controllable catheter tips, expandable MW probes, RFA/MWA/IRE/Cryo generator technology, etc.) can be expected, might improve practicability somewhat but will probably not change utilization significantly.
Considering that immune oncological development will represent the major impact on oncology during the next years and decades, it will be more relevant how the interaction of IO techniques with immunological treatment concepts might interact and which synergistic effects will enhance therapeutical outcomes.
For instance, recent developments in microcatheter technology for pressure-enabled drug delivery as microballoon occlusion or antireflux valves are addressing the intra- and peritumorous flow and pressure environment. E.g. Hardaway et al. could show that transarterial pressure-enabled delivery of CAR-T cells increased the CAR-T presence within liver metastases from pancreatic cancer significantly in comparison to conventional microcatheter hepatic artery infusion.58 The assumed pathophysiological effects are yet not coherent and the clinical impact and potential advantages of such a technique still need to be affirmed.59,60
There was also a substantial development in embolizing agents for oncological procedures from irregularly formed crumbs as polyvenylalcohol (PVA) towards high-tech beads calibrated for various sizes (40–1300 µm) allowing for precise and definite embolization at different caliber levels within a vascular environment. Some of these newer particles can load specific chemodrugs based on their electrical charge (e.g. doxorubicin, irinotecan). However, many current chemo drugs (e.g. oxaliplatin) as well as immunological active molecules cannot bind to such beads.Therefore, there could be a need for particles/carriers as liposomes or nanoparticles loadable with various types of chemodrugs and molecules as antibodies.61,62
Since TACE and TARE are carrier techniques for “classical” chemodrugs and radioactivity future device research has to deal more with the question of what might be useful to be transported and promoted via these techniques and less with developing new transvascular modalities.
All ablative techniques are interacting and interfering with the immune system, addressing T-cells, T helper cells and cytokines (TH1, TH2), cytotoxic T cells (CD8+), memory T cells (e.g. CD4+, CD8+), regulatory T cells (Tregs), and innate-like natural killer T cells, etc.and may even cause abscopal effects (Table 3). Moreover, immunological signatures generated by the ablated tissue in situ may also stimulate immune suppressive effects as myeloid-derived suppressor cells (MDSCs), cytokines, etc which are “protecting” tumour cells (Figure 1).
Table 3.
Immune effects of ablative therapies (modified from Greten et al63)
| Modality | Activation |
|---|---|
| TACE | IL12p70, IFN-c, IL-17A, IL-2, IL-10, IL-9, IL-22, IL-6, IL-13, IL-4, IL-5, IL-1b, and TNF-a |
| TARE | TNF-a, IL-6, Il-8, oxidative stress markers |
| RFA | Th1 (IL-2, TNF-a, IFN-c), Th2 (IL-4, IL-6, IL-10) |
| Cryo | T cells |
| myeloid-derived suppressor cells | |
| Antigen-presenting cells | |
| TU-associated antigen-derived peptides | |
| Glypican-3-specific CTLs | |
| Immune potentiating antigens in the serum, Ficolin-3 HSP HMGB1 |
|
| MWA | T cell, IL-12 |
| CD3, CD4, CD4+,CD8, CD16, CD25+, CD56, NK, TH17, Tregs | |
| IRE, HifU | T cells |
HMGB1, High-Mobility-Group-Protein B1; HSP, heat shock proteins;MWA, Microwave ablation; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; TARE, transarterial radioembolization.
Figure 1.
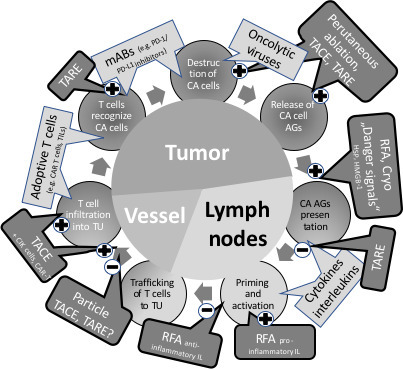
Modified and simplified “cancer cycle”,63–67 illustrating some immunological effectors (light gray boxes) and potential stimulating (+) or supressing (-) interactions by IO techniques (gray boxes). IO, interventional oncology; MWA, Microwave ablation
For instance, TARE increases TNF-α and antigen-presenting cells resulting in an abscopal effect, but may also induce lymphopenia and hinder anti inflammatory processes by diminishing lymphocyte proliferation and cytokine production followed by unwanted tumor progression.64,65
Nevertheless, over the last years, a comparatively small but constantly growing number of studies are exploring immune oncological and IO combination therapies with promising results.63,66–68 Mizukoshi et al. showed that RFA in HCC amplifies tumor-associated antigen-specific T cell response resulting in improved recurrence-free survival.69 Initial data on TACE plus cytokine-induced killer cells (CIK) in HCC patients could show a significantly prolonged survival in comparison to TACE alone.70 Similar synergistic effects could be seen also for TARE in combination with checkpoint inhibitors71,72 (Figure 2).
Figure 2.
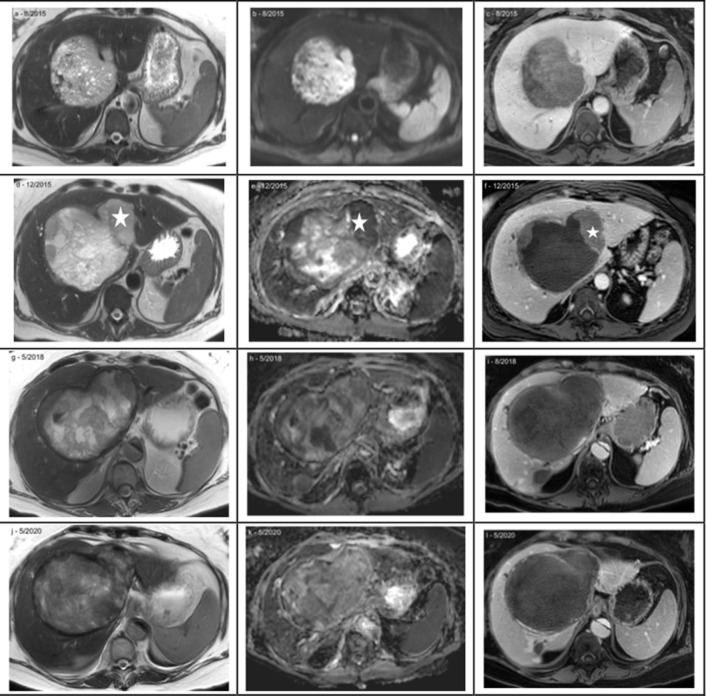
Incidentally found, large solitary tumour in a 55-y-o female. Histology confirmed a G3 sarcomatous hepatocellular carcinoma, baseline MRI (a – c). Initial treatment with two superselective TACE (40 µm particles loaded with 100 mg doxorubicin) resulted in substantial necrosis of the tumor with still some growth in the periphery; asterix (d- f). Sytemic therapy with a proteinkinase inhibitor (sorfenib) was not tolerated and switched to a checkpoint-inhibitor (PD-1 inhibitor, nivolumab). Nivolumab could maintain (g – i) and promote the tumor necrosis, last control study in 5/2020 (j – l). 5 years after initial diagnosis, the patient is still alive without compromized life quality. The asymptomatic, incidentally in 2017 detected Standford type B aortic dissection was not treated yet since the patient refused to any other treatments beside the HCC therapy. HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization. (Left column: T2W; middle: DWI ADC map; right: T1W 20 min post Gd-EOB DTPA)
How an immune answer is promoted or suppressed by ablative therapies is not yet well understood. So far, the pre-existing tumor-specific and humoral immune situation, as well as the type of local action (the type of cell destruction, residual cellular components after destruction, periablation vascular maintenance, etc), may interact in a complex fashion which will determine the impact on an eligible concomitant immune therapy, the timing of this therapy, and biomarkers for response assessment.73
In consequence, future research must identify the molecular-biological patterns created by the ablative footprint, assess when it will be useful to deliver an immune therapy locally, evaluate if a combination with local/locoregional technique is useful, and decode effects which might act synergistically or antagonistically with minimal-invasive immunological promoters.
Supporting technologies
The enormous progress of information technology (artificial intelligence (AI), big data, machine and deep learning by smart algorithms, and robotics) is not only affecting daily life but will significantly change how medicine is practiced in the future. Especially in oncology, the analytical crosslinking of medical databases and individual patient data as, e.g. genomic information from blood or tumor samples will support diagnostic and therapeutic decisions.
Before taking over the interventionalists work, AI and all the related technologies will rather support disease and treatment management. Comprehensive image processing as Radiomic will assist more and more in image-guided procedures and directing drug delivery, in pre- and post-procedural monitoring and follow-up.74–76 Advanced imaging technologies as fusion imaging based on ultrasound, MRI, and probably very new techniques as dielectric or optical imaging, AI,and even robotic-assisted imaging/procedures including augmented and virtual reality will allow highly accurate IO procedures together with minimized radiation exposure to the patient and operator.77
Capturing all technical developments that are entering new dimensions of accuracy in imaging and guidance, and some of which are still in their infancy goes far beyond the scope of this article. However, one could argue about how much precision is finally needed in the era of precision medicine, where the bullet is much more precise than the rifle scope.
Look into the 21st century with the crystal ball
If this article would have been written 100 years ago addressing the question of how medicine and particularly radiology might develop in the 20th century, ultrasound, CT, MRI, catheter-based procedures would have been far from everyone’s imagination. In 1842, Christian Doppler detected the later called Doppler effect, in 1906 the first naval sonar was presented by Lewis Nixon, to medical ultrasonography it took another 50 years. Fourier transformation was described in 1805, the X-rays were detected in 1895, almost 80 later the first CT – not possible without Fourier transformation – was presented. Wolfgang Pauli postulated in 1925 the electron spin, another 50 years lasted till the first MRI scanner. And lastly, the first right heart catheterization was performed by Werner Forssmann in a self-experiment in 1924, getting forgotten and reinvented and modified with the first balloon angioplasty in 1977 by Andreas Gruentzig.
For the next decades, we should consider an even faster-turning wheel of knowledge and assume that the fundamentals for many striking, future developments are already laid.
Unquestionably, all oncological therapies progressed enormously over the last decades and may progress even more in this 21st century of biology. Moreover, the next decades will provide specific new challenges in the service for oncological patients. These challenges might be mainly driven by demographic and economic developments throughout the world. In many very well developed countries of the eastern and western world the aging populations will provide more and more medically well “maintained“ people experiencing probably several malignancies during their life time and at higher ages (https://ourworldindata.org/cancer).78 Therefore, treatment concepts considering elderly cancer patients with various comorbidities and the affordability within the different healthcare systems will be needed. In poorer, less developed countries, often with still rapidly growing populations the main challenge will be to provide adequate health service at all. The proportion of oncological diseases versus, e.g. infectious diseases might be lower (albeit high in absolute numbers) than in richer countries, whereas the stages of malignant diseases could be more advanced.78–80
Medical knowledge will change again, current treatments and procedures will vanish as already in the past, and new ones will arise. By all means, due to growing numbers in every respect, it is not hard to predict that the medical world will be getting even more and more complex due to
growing populations producing more and older patients necessitating individualized therapies due to comorbidities,
growing health-economical and health-structural pressure (e.g. as seen during the Covid-19 pandemia),
increased requirement for “patient comfort” emphasizing on short inpatient stay or outpatient therapy concepts,
more drugs, mainly immune-based,
more medical data processed by smart algorithms producing new evidence and most likely unexpected cross-connections,
cybertechnologies for self-optimization and autodiagnostics by implantable devices (“nano-machines”) allowing for earlier detection of disease, etc.
Also, according to many current recommendations in various national and international cancer treatment guidelines (ESMO, ECCO, EASL, NCCN), the mindset of therapeutical concepts seems to change towards personalized therapy approaches. Complete tumor eradication might be still the goal, however, transforming a primarily dismal diagnosis into a chronic disease controlled by a chronic (recurrent) treatment could be a sufficient therapeutical goal maintaining a patient’s life quality at a preferably high level and the respective (controlled) tumor load at a preferably low level. In scenarios of limited resources and economic capabilities, these concepts could be reasonable, acceptable, and affordable.
Furthermore, we are dealing with a moving target from various perspectives. Many if not all tumors may change their genomic, micro- and macrobiological behavior over time while the tumor hosting patient is also changing in terms of age, comorbidities, as well as physiological tolerance to and psychological acceptance of a given therapy.
Precision medicine is addressing many of these issues. Nevertheless, at present genome adjusted precision medicine is suitable only for a minority of patients, is still very costly, and is often accompanied by severe side-effects limiting patient’s tolerance.
In all likelihood, in clinically manifest malignancies with significant tumour load immune-oncology will significantly impact how treatments will be performed. But as long as immune-oncology – or maybe better immune-system control – is not eradicating any evolving tumor in its preclinical phase, there will be still a strong demand for surgery, radiation therapy, chemotherapy, and IO as the minimal-invasive pillar of oncology.
What will be the challenge for IO?
Currently, with the hype of immune, targeted, hormone, and precision medicine IO is not in the main focus of awareness of most oncologists and oncological societies. For example, on the website of the NIH national cancer institute (https://www.cancer.gov/about-cancer/treatment/types) IO is not even mentioned among the listed eight types of cancer treatment (i.e. surgery, radiation therapy, chemotherapy, immunotherapy, targeted therapy, hormone therapy, stem cell transplant, and precision medicine) – well-intentioned, one might assume that IO is included within “targeted and precision medicine.”
Forecasting the future of IO for more than a few years might be like reading from the crystal ball.
Though, some recognizable trends and lessons from the past might help to get an idea where IO could head to and which obstacles must be overcome.
Considering that immune-oncology with all its facets and sidearms is still at the beginning of its era and may run through the common hype cycle of emerging technologies,81 IO might already have reached a stable “plateau of productivity”(Figure 3). Over the last decades, interventional radiology had created a comprehensive base of evidence regarding effectiveness for various IO techniques. In a nutshell, a limited tumor extent suitable for the capabilities of IO techniques can be effectively treated similar to surgery or radiation therapy in several types of cancer in many organs. Even if IO cannot treat a systemic tumor spread, some immune-modulatory effects of IO procedures might act systemically what is the link to synergistic effects for immune-oncology as discussed in the paragraphs above.
Figure 3.
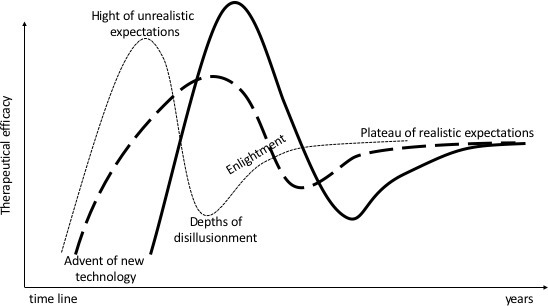
Hype cycle. Borrowing from the term coined by the Gartner consultant Jackie Fenn for evaluation in the introduction of new technologies: dashed line IO techniques, solid
line immuno-oncology.81 IO, interventional oncology.
Besides the already mentioned synergistic effects, IO with its imaging, guidance, and treatment capabilities can offer manifold added value to all kinds of oncological and immune-oncological therapies. This added value is ranging from usually technical easy, highly precise tissue sampling procedures for intrinsic tumor characterization based on complex imaging analysis to percutaneous or transvascular treatments with utmost precision. Especially precise local drug/molecule delivery may overcome the limits of systemic applications in terms of focal improved distribution and concentration together with less systemic toxicities.82
Concerning image guidance and guided therapy, IO will further significantly benefit from AI-driven developments in imaging and imaging-based data processing. This is supported by cheap, permanently increasing, and available computing power at all scales. Moreover, the generation of digital natives is capable, willing, and competent to incorporate technologies as machine and deep learning by AI, robotics, and cyber technologies not only in daily life but also in medicine.83 This could also mean that IO procedures can be easier performed and are getting increasingly available not only to trained IR/IOspecialists.
One could see these developmentsas disruptive, but future developments are often feared as disruptive, but could rather be understood as natural evolution. This means that we will provide medicine differently in the next 20, 50, and 100 years, which will be true also for radiology and IO. Advanced medicobiological knowledge will produce new treatments, technical developments will allow new procedures, and both will create new needs and opportunities for IO practice. It is equivocal if this practice will be performed in categories still assigned to distinct medical specialties as today, where interdisciplinarity is still celebrated as a great achievement.
The more the next generations of medical professionals, engineers, and administrators break away from traditional medical categories and distinct specialties, new structures of medical services may evolve which may unite disease system relevant specialties and skills. With the presumably blurring boundaries between the various specialties, there will be an increasing demand for substantial cross-over knowledge (not only about a specific therapy but also about the overall understanding of a disease and treatment concepts, side-effects, and concomitant therapies), paralleled by new concepts in specialist training.
To take part in this, IO must leave the bubble of a subspecialty by creating increased visibility and acceptance as a valuable therapy provider. This might be achieved by being consequently involved in interdisciplinary studies and registries, conducting more trials on health service research, getting involved in interdisciplinary counseling and guideline boards, being actively present in oncological societies and meetings.
Conclusion
Immunological therapies are the current hype and may dominate oncology in the 21st century most likely. So far, they have shown impressive success, unfortunately only in vivo with a certain number of patients and often at the high price of serious side-effects.
On the other hand, the IO methods have already expressly proven their effectiveness in terms of local tumor control in various tumor entities and low side-effects, but their previously known immune-modulating effects are usually too low to achieve significant system-therapeutic effects.
The combination of IO and immuno-oncology opens the possibility of overcoming these limitations through synergy and symbiosis.
However, this requires leaving sectoral thinking behind and focusing on the goal – finding the best treatment for the patient.
A crucial step here is to strengthen the interdisciplinary presence of IO to promote the mutual exchange of knowledge between the competing specialties and to bundle the research efforts as comprehensively as possible.
Therefore, the future of IO which holds a wide-range assortment of great tools and concepts might be bright, and if the homework is properly done IO will be IO2.
Footnotes
Acknowledgements: I thank my son cand. med. Thomas Maximilian Helmberger for his writing assistance, language editing, and proofreading.
REFERENCES
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. doi: 10.1016/s0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–74. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 3.Sonnenschein C, Soto AM. The aging of the 2000 and 2011 hallmarks of cancer reviews: a critique. J Biosci 2013; 38: 651–63. doi: 10.1007/s12038-013-9335-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sukari A, Nagasaka M, Al-Hadidi A, Lum LG. Cancer immunology and immunotherapy. Anticancer Res 2016; 36: 5593–606. doi: 10.21873/anticanres.11144 [DOI] [PubMed] [Google Scholar]
- 5.Fouad YA, Aanei C. Revisiting the hallmarks of cancer. Am J Cancer Res 2017; 7: 1016–36. [PMC free article] [PubMed] [Google Scholar]
- 6.Velcheti V, Schalper K. Basic overview of current immunotherapy approaches in cancer. Am Soc Clin Oncol Educ Book 2016; 35: 298–308. doi: 10.1200/EDBK_156572 [DOI] [PubMed] [Google Scholar]
- 7.Nicolini A, Ferrari P, Rossi G, Carpi A. Tumour growth and immune evasion as targets for a new strategy in advanced cancer. Endocr Relat Cancer 2018; 25: R577–604. doi: 10.1530/ERC-18-0142 [DOI] [PubMed] [Google Scholar]
- 8.Xin Yu J, Hubbard-Lucey VM, Tang J. Immuno-oncology drug development goes global. Nat Rev Drug Discov 2019; 18: 899–900. doi: 10.1038/d41573-019-00167-9 [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Chen J. Current status and future directions of cancer immunotherapy. J Cancer 2018; 9: 1773–81. doi: 10.7150/jca.24577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arneth B. Update on the types and usage of liquid biopsies in the clinical setting: a systematic review. BMC Cancer 2018; 18: 527. doi: 10.1186/s12885-018-4433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 2019; 19: 133–50. doi: 10.1038/s41568-019-0116-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoenberg SO, Attenberger UI, Solomon SB, Weissleder R. Developing a roadmap for interventional oncology. Oncologist 2018; 23: 1162–70. doi: 10.1634/theoncologist.2017-0654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khorrami M, Prasanna P, Gupta A, Patil P, Velu PD, Thawani R, et al. Changes in CT radiomic features associated with lymphocyte distribution predict overall survival and response to immunotherapy in non-small cell lung cancer. Cancer Immunol Res 2020; 8: 108-119. doi: 10.1158/2326-6066.CIR-19-0476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada R, Nakatsuka H, Nakamura K, Sato M, Tamaoka K, Takemoto K, et al. Super-Selective arterial embolization in unresectable hepatomas (author's transl. Nihon Igaku Hoshasen Gakkai Zasshi 1979; 39: 540–3. [PubMed] [Google Scholar]
- 15.Horikawa M, Miyayama S, Irie T, Kaji T, Arai Y. Development of conventional transarterial chemoembolization for hepatocellular carcinomas in Japan: historical, strategic, and technical review. AJR Am J Roentgenol 2015; 205: 764–73. doi: 10.2214/AJR.15.14825 [DOI] [PubMed] [Google Scholar]
- 16.Raoul J-L, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev 2019; 72: 28–36. doi: 10.1016/j.ctrv.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 17.Han T, Yang X, Zhang Y, Li G, Liu L, Chen T, et al. The clinical safety and efficacy of conventional transcatheter arterial chemoembolization and drug-eluting beads-transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a meta-analysis. Biosci Trends 2019; 13: 374–81. doi: 10.5582/bst.2019.01153 [DOI] [PubMed] [Google Scholar]
- 18.Ranieri G, Laforgia M, Nardulli P, Ferraiuolo S, Molinari P, Marech I, et al. Oxaliplatin-Based intra-arterial chemotherapy in colo-rectal cancer liver metastases: a review from pharmacology to clinical application. Cancers 2019; 11: E14124 01 2019. doi: 10.3390/cancers11020141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapelle N, Matysiak-Budnik T, Douane F, Metairie S, Rougier P, Touchefeu Y. Hepatic arterial infusion in the management of colorectal cancer liver metastasis: current and future perspectives. Dig Liver Dis 2018; 50: 220–5. doi: 10.1016/j.dld.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 20.Zervoudakis A, Boucher T, Kemeny NE. Treatment options in colorectal liver metastases: hepatic arterial infusion. Visc Med 2017; 33: 47–53. doi: 10.1159/000454693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel A, Gupta S, Zeile M, von Haken R, Brüning R, Lotz G, et al. Chemosaturation percutaneous hepatic perfusion: a systematic review. Adv Ther 2017; 33: 2122–38. doi: 10.1007/s12325-016-0424-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marquardt S, Kirstein MM, Brüning R, Zeile M, Ferrucci PF, Prevoo W, et al. Percutaneous hepatic perfusion (chemosaturation) with melphalan in patients with intrahepatic cholangiocarcinoma: European multicentre study on safety, short-term effects and survival. Eur Radiol 2019; 29: 1882–92. doi: 10.1007/s00330-018-5729-z [DOI] [PubMed] [Google Scholar]
- 23.Gibbs P, Heinemann V, Sharma NK, Taieb J, Ricke J, Peeters M, et al. Effect of primary tumor side on survival outcomes in untreated patients with metastatic colorectal cancer when selective internal radiation therapy is added to chemotherapy: combined analysis of two randomized controlled studies. Clin Colorectal Cancer 2018; 17: e617–29. doi: 10.1016/j.clcc.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 24.van Hazel GA, Heinemann V, Sharma NK, Findlay MPN, Ricke J, Peeters M, et al. SIRFLOX: randomized phase III trial comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus mFOLFOX6 (plus or minus bevacizumab) plus selective internal radiation therapy in patients with metastatic colorectal cancer. J Clin Oncol 2016; 34: 1723–31. doi: 10.1200/JCO.2015.66.1181 [DOI] [PubMed] [Google Scholar]
- 25.Ricke J, Klümpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol 2019; 71: 1164–74. doi: 10.1016/j.jhep.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 26.Wehrenberg-Klee E, Gandhi RT, Ganguli S. Patient selection and clinical outcomes of Y90 in hepatocellular carcinoma. Tech Vasc Interv Radiol 2019; 22: 70–3. doi: 10.1053/j.tvir.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 27.Türk G, Eldem G, Kılıçkap S, Bozkurt FM, Salancı BV, Çil BE, et al. Outcomes of radioembolization in patients with chemorefractory colorectal cancer liver metastasis: a single-center experience. J Gastrointest Cancer 2019; 50: 236–43. doi: 10.1007/s12029-018-0053-z [DOI] [PubMed] [Google Scholar]
- 28.Saini A, Wallace A, Alzubaidi S, Knuttinen MG, Naidu S, Sheth R, et al. History and evolution of yttrium-90 radioembolization for hepatocellular carcinoma. J Clin Med 2019; 8: E5507 01 2019. doi: 10.3390/jcm8010055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salem R, Hassan S, Lewandowski RJ, Grace K, Martin RCG, Sichlau MJ, et al. Quality of life after radioembolization for hepatocellular carcinoma using a digital patient-reported outcome tool. J Vasc Interv Radiol 2020; 31: 311-314.e1. doi: 10.1016/j.jvir.2019.06.018 [DOI] [PubMed] [Google Scholar]
- 30.Reinders MTM, Smits MLJ, van Roekel C, Braat AJAT. Holmium-166 microsphere radioembolization of hepatic malignancies. Semin Nucl Med 2019; 49: 237–43. doi: 10.1053/j.semnuclmed.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 31.McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol 1990; 25: 267–70. doi: 10.1097/00004424-199003000-00011 [DOI] [PubMed] [Google Scholar]
- 32.Rossi S, Fornari F, Pathies C, Buscarini L. Thermal lesions induced by 480 kHz localized current field in guinea pig and pig liver. Tumori 1990; 76: 54–7. [DOI] [PubMed] [Google Scholar]
- 33.Xia Y, Li J, Liu G, Wang K, Qian G, Lu Z, et al. Long-Term effects of repeat hepatectomy vs percutaneous radiofrequency ablation among patients with recurrent hepatocellular carcinoma: a randomized clinical trial. JAMA Oncol 2019;27 Nov 2019. doi: 10.1001/jamaoncol.2019.4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uhlig J, Sellers CM, Stein SM, Kim HS. Radiofrequency ablation versus surgical resection of hepatocellular carcinoma: contemporary treatment trends and outcomes from the United States National cancer database. Eur Radiol 2019; 29: 2679–89. doi: 10.1007/s00330-018-5902-4 [DOI] [PubMed] [Google Scholar]
- 35.Yin Z, Jin H, Ma T, Zhou Y, Yu M, Jian Z. A meta-analysis of long-term survival outcomes between surgical resection and radiofrequency ablation in patients with single hepatocellular carcinoma ≤ 2 cm (BCLC very early stage. Int J Surg 2018; 56: 61–7. doi: 10.1016/j.ijsu.2018.04.048 [DOI] [PubMed] [Google Scholar]
- 36.Lee S, Kang TW, Cha DI, Song KD, Lee MW, Rhim H, et al. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: propensity score analyses of long-term outcomes. J Hepatol 2018; 69: 70–8. doi: 10.1016/j.jhep.2018.02.026 [DOI] [PubMed] [Google Scholar]
- 37.Lee BC, Lee HG, Park IJ, Kim SY, Kim K-H, Lee JH, et al. The role of radiofrequency ablation for treatment of metachronous isolated hepatic metastasis from colorectal cancer. Medicine 2016; 95: e4999. doi: 10.1097/MD.0000000000004999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sag AA, Selcukbiricik F, Mandel NM. Evidence-Based medical oncology and interventional radiology paradigms for liver-dominant colorectal cancer metastases. World J Gastroenterol 2016; 22: 3127–49. doi: 10.3748/wjg.v22.i11.3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shady W, Petre EN, Do KG, Gonen M, Yarmohammadi H, Brown KT, et al. Percutaneous Microwave versus Radiofrequency Ablation of Colorectal Liver Metastases: Ablation with Clear Margins (A0) Provides the Best Local Tumor Control. J Vasc Interv Radiol 2018; 29: 268–75. doi: 10.1016/j.jvir.2017.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hof J, Joosten HJ, Havenga K, de Jong KP. Radiofrequency ablation is beneficial in simultaneous treatment of synchronous liver metastases and primary colorectal cancer. PLoS One 2018; 13: e0193385. doi: 10.1371/journal.pone.0193385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Baère T, Aupérin A, Deschamps F, Chevallier P, Gaubert Y, Boige V, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol 2015; 26: 987–91. doi: 10.1093/annonc/mdv037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li G, Xue M, Chen W, Yi S. Efficacy and safety of radiofrequency ablation for lung cancers: a systematic review and meta-analysis. Eur J Radiol 2018; 100: 92–8. doi: 10.1016/j.ejrad.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 43.Prud'homme C, Deschamps F, Moulin B, Hakime A, Al-Ahmar M, Moalla S, et al. Image-Guided lung metastasis ablation: a literature review. Int J Hyperthermia 2019; 36: 37–45. doi: 10.1080/02656736.2019.1647358 [DOI] [PubMed] [Google Scholar]
- 44.Uhlig J, Ludwig JM, Goldberg SB, Chiang A, Blasberg JD, Kim HS. Survival rates after thermal ablation versus stereotactic radiation therapy for stage 1 non-small cell lung cancer: a national cancer database study. Radiology 2018; 289: 862–70. doi: 10.1148/radiol.2018180979 [DOI] [PubMed] [Google Scholar]
- 45.Johnson B, Sorokin I, Cadeddu JA. Ten-Year outcomes of renal tumor radiofrequency ablation. J Urol 2018;. [DOI] [PubMed] [Google Scholar]
- 46.Wendler JJ, Liehr BU, Damm R, Powerski M, Brunner T, Schostak M, et al. Small renal carcinoma: the "when" and "how" of operation, active surveillance, and ablation. Pol J Radiol 2018; 83: e561–8. doi: 10.5114/pjr.2018.81282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pietropaolo A, Jones P, Aboumarzouk OM, Rai BP, Lockyer CRW, Hayes MC, et al. Trends in surgical and ablative treatment of localised renal cell carcinoma: a review of publication trends over 16 years (2000-2015. Arab J Urol 2019; 17: 120–4. doi: 10.1080/2090598X.2019.1590516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uhlig J, Strauss A, Rucker G. Seif Amir Hosseini a, Lotz J, Trojan L, et al. Partial nephrectomy versus ablative techniques for small renal masses: a systematic review and network meta-analysis. Eur Radiol 2019; 29: 1293–307. [DOI] [PubMed] [Google Scholar]
- 49.Wu S, Hou J, Ding Y, Wu F, Hu Y, Jiang Q, et al. Cryoablation versus radiofrequency ablation for hepatic malignancies: a systematic review and Literature-Based analysis. Medicine 2015; 94: e2252. doi: 10.1097/MD.0000000000002252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan F, Ho AM, Jamal JE, Gershbaum MD, Katz AE, Hoffmann JC. Long-Term outcomes after percutaneous renal cryoablation performed with adjunctive techniques. Clin Imaging 2018; 50: 62–7. doi: 10.1016/j.clinimag.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 51.Aboumarzouk OM, Ismail M, Breen DJ, Van Strijen M, Garnon J, Lagerveld B, et al. Laparoscopic vs percutaneous cryotherapy for renal tumors: a systematic review and meta-analysis. J Endourol 2018; 32: 177–83. doi: 10.1089/end.2017.0791 [DOI] [PubMed] [Google Scholar]
- 52.Zondervan PJ, Buijs M, De Bruin DM, van Delden OM, Van Lienden KP. Available ablation energies to treat cT1 renal cell cancer: emerging technologies. World J Urol 2019; 37: 445–55. doi: 10.1007/s00345-018-2546-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ricke J, Jürgens JHW, Deschamps F, Tselikas L, Uhde K, Kosiek O, et al. Irreversible electroporation (IRE) fails to demonstrate efficacy in a prospective multicenter phase II trial on lung malignancies: the Alice trial. Cardiovasc Intervent Radiol 2015; 38: 401–8. doi: 10.1007/s00270-014-1049-0 [DOI] [PubMed] [Google Scholar]
- 54.Niessen C, Beyer LP, Pregler B, Dollinger M, Trabold B, Schlitt HJ, et al. Percutaneous ablation of hepatic tumors using irreversible electroporation: a prospective safety and midterm efficacy study in 34 patients. J Vasc Interv Radiol 2016; 27: 480–6. doi: 10.1016/j.jvir.2015.12.025 [DOI] [PubMed] [Google Scholar]
- 55.Distelmaier M, Barabasch A, Heil P, Kraemer NA, Isfort P, Keil S, et al. Midterm safety and efficacy of irreversible electroporation of malignant liver tumors located close to major portal or hepatic veins. Radiology 2017; 285: 1023–31. doi: 10.1148/radiol.2017161561 [DOI] [PubMed] [Google Scholar]
- 56.Langan RC, Goldman DA, D'Angelica MI, DeMatteo RP, Allen PJ, Balachandran VP, et al. Recurrence patterns following irreversible electroporation for hepatic malignancies. J Surg Oncol 2017; 115: 704–10. doi: 10.1002/jso.24570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Narayanan G, Hosein PJ, Beulaygue IC, Froud T, Scheffer HJ, Venkat SR, et al. Percutaneous image-guided irreversible electroporation for the treatment of unresectable, locally advanced pancreatic adenocarcinoma. J Vasc Interv Radiol 2017; 28: 342–8. doi: 10.1016/j.jvir.2016.10.023 [DOI] [PubMed] [Google Scholar]
- 58.Hardaway JC, Prince E, Arepally A, Katz SC. Regional infusion of chimeric antigen receptor T cells to overcome barriers for solid tumor immunotherapy. J Vasc Interv Radiol 2018; 29: 1017–21. doi: 10.1016/j.jvir.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 59.Xu Z, Jernigan S, Kleinstreuer C, Buckner GD. Solid tumor Embolotherapy in hepatic arteries with an anti-reflux catheter system. Ann Biomed Eng 2016; 44: 1036–46. doi: 10.1007/s10439-015-1411-7 [DOI] [PubMed] [Google Scholar]
- 60.Rose SC, Narsinh KH, Isaacson AJ, Fischman AM, Golzarian J. The beauty and bane of Pressure-Directed Embolotherapy: hemodynamic principles and preliminary clinical evidence. AJR Am J Roentgenol 2019; 212: 686–95. doi: 10.2214/AJR.18.19975 [DOI] [PubMed] [Google Scholar]
- 61.Zhou Q, Wang K, Dou J, Cao F, Liu F, Yuan H, et al. Theranostic liposomes as nanodelivered chemotherapeutics enhanced the microwave ablation of hepatocellular carcinoma. Nanomedicine 2019; 14: 2151–67. doi: 10.2217/nnm-2018-0424 [DOI] [PubMed] [Google Scholar]
- 62.Saeed BA, Lim V, Yusof NA, Khor KZ, Rahman HS, Abdul Samad N. Antiangiogenic properties of nanoparticles: a systematic review. Int J Nanomedicine 2019; 14: 5135–46. doi: 10.2147/IJN.S199974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greten TF, Mauda-Havakuk M, Heinrich B, Korangy F, Wood BJ. Combined locoregional-immunotherapy for liver cancer. J Hepatol 2019; 70: 999–1007. doi: 10.1016/j.jhep.2019.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernandez-Ros N, Iñarrairaegui M, Paramo JA, Berasain C, Avila MA, Chopitea A, et al. Radioembolization of hepatocellular carcinoma activates liver regeneration, induces inflammation and endothelial stress and activates coagulation. Liver Int 2015; 35: 1590–6. doi: 10.1111/liv.12592 [DOI] [PubMed] [Google Scholar]
- 65.Seidensticker M, Powerski M, Seidensticker R, Damm R, Mohnike K, Garlipp B, et al. Cytokines and 90Y-Radioembolization: Relation to Liver Function and Overall Survival. Cardiovasc Intervent Radiol 2017; 40: 1185–95. doi: 10.1007/s00270-017-1622-4 [DOI] [PubMed] [Google Scholar]
- 66.Kudo M. Targeted and immune therapies for hepatocellular carcinoma: predictions for 2019 and beyond. World J Gastroenterol 2019; 25: 789–807. doi: 10.3748/wjg.v25.i7.789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slovak R, Ludwig JM, Gettinger SN, Herbst RS, Kim HS. Immuno-thermal ablations - boosting the anticancer immune response. J Immunother Cancer 2017; 5: 78. doi: 10.1186/s40425-017-0284-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takaki H, Cornelis F, Kako Y, Kobayashi K, Kamikonya N, Yamakado K. Thermal ablation and immunomodulation: from preclinical experiments to clinical trials. Diagn Interv Imaging 2017; 98: 651–9. doi: 10.1016/j.diii.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 69.Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 2013; 57: 1448–57. doi: 10.1002/hep.26153 [DOI] [PubMed] [Google Scholar]
- 70.Jia C-C, Chen Y-H, Cai X-R, Li Y, Zheng X-F, Yao Z-C, et al. Efficacy of cytokine-induced killer cell-based immunotherapy for hepatocellular carcinoma. Am J Cancer Res 2019; 9: 1254–65. [PMC free article] [PubMed] [Google Scholar]
- 71.Wehrenberg-Klee E, Goyal L, Dugan M, Zhu AX, Ganguli S. Y-90 radioembolization combined with a PD-1 inhibitor for advanced hepatocellular carcinoma. Cardiovasc Intervent Radiol 2018; 41: 1799–802. doi: 10.1007/s00270-018-1993-1 [DOI] [PubMed] [Google Scholar]
- 72.Choi C, Yoo GS, Cho WK, Park HC. Optimizing radiotherapy with immune checkpoint blockade in hepatocellular carcinoma. World J Gastroenterol 2019; 25: 2416–29. doi: 10.3748/wjg.v25.i20.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Erinjeri JP, Fine GC, Adema GJ, Ahmed M, Chapiro J, den Brok M, et al. Immunotherapy and the interventional oncologist: challenges and Opportunities-A Society of interventional oncology white paper. Radiology 2019; 292: 25–34. doi: 10.1148/radiol.2019182326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Z, Wang S, Dong D, Wei J, Fang C, Zhou X, et al. The applications of Radiomics in precision diagnosis and treatment of oncology: opportunities and challenges. Theranostics 2019; 9: 1303–22. doi: 10.7150/thno.30309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shan Q-Y, Hu H-T, Feng S-T, Peng Z-P, Chen S-L, Zhou Q, et al. Ct-Based peritumoral radiomics signatures to predict early recurrence in hepatocellular carcinoma after curative tumor resection or ablation. Cancer Imaging 2019; 19: 11. doi: 10.1186/s40644-019-0197-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mokrane F-Z, Lu L, Vavasseur A, Otal P, Peron J-M, Luk L, et al. Radiomics machine-learning signature for diagnosis of hepatocellular carcinoma in cirrhotic patients with indeterminate liver nodules. Eur Radiol 2020; 30: 558–70. doi: 10.1007/s00330-019-06347-w [DOI] [PubMed] [Google Scholar]
- 77.Solbiati M, Passera KM, Rotilio A, Oliva F, Marre I, Goldberg SN, et al. Augmented reality for interventional oncology: proof-of-concept study of a novel high-end guidance system platform. Eur Radiol Exp 2018; 2: 18. doi: 10.1186/s41747-018-0054-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Global burden of disease cancer C, Fitzmaurice C, Akinyemiju TF, al Lami FH, Alam T, Alizadeh-Navaei R, et al. global, regional, and National cancer incidence, mortality, years of life lost, years lived with disability, and Disability-Adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol 2018; 4: 1553–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farid M, Toh HC, Day WC. - Don't Stop Thinking About Tomorrow. Ann Acad Med Singapore 2019;. ; 48: 42–42019. [PubMed] [Google Scholar]
- 80.Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 2018; 103: 356–87. doi: 10.1016/j.ejca.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 81.Bortfeld T, Marks LB. Hype cycle in radiation oncology. Int J Radiat Oncol Biol Phys 2013; 86: 819–21. doi: 10.1016/j.ijrobp.2013.03.027 [DOI] [PubMed] [Google Scholar]
- 82.de Baère T, Tselikas L, Deschamps F, Soria JC, Marabelle A. Immuno-Oncology in cancer care is a fantastic opportunity for interventional oncology: IO4IO (interventional oncology for Immuno-Oncology) initiative. Cardiovasc Intervent Radiol 2018; 41: 825–7. doi: 10.1007/s00270-018-1935-y [DOI] [PubMed] [Google Scholar]
- 83.Rice SL, Bale R, Breen DJ, de Baere T, Denys A, Guiu B, et al. The management of colorectal cancer liver metastases: the interventional radiology viewpoint. Int J Radiat Oncol Biol Phys 2019; 103: 537–9. doi: 10.1016/j.ijrobp.2018.08.069 [DOI] [PubMed] [Google Scholar]