Abstract
Aim:
To address the unmet needs of patients interested in regenerative medicine, Mayo Clinic created a Regenerative Medicine Consult Service (RMCS). We describe the service and patient satisfaction.
Materials & methods:
We analyzed RMCS databases through retrospective chart analysis and performed qualitative interviews with patients.
Results:
The average patient was older to elderly and seeking information about regenerative options for their condition. Patients reported various conditions with osteoarthritis being most common. Over a third of consults included discussions about unproven interventions. About a third of patients received a clinical or research referral. Patients reported the RMCS as useful and the consultant as knowledgeable.
Conclusion:
An institutional RMCS can meet patients’ informational needs and support the responsible translation of regenerative medicine.
Keywords: : consultation service, ethical, evidence-based medicine, information for patients, patient education, patient navigation, regenerative medicine education, responsible translation, unproven stem cell therapies
Patients, clinicians, scientists and the public are enthusiastic about the potential of regenerative therapies [1–3] to address unmet medical needs of patients [4,5]. As with many emerging biotechnologies, the immediate therapeutic potential of regenerative medicine has been exaggerated and there is considerable misinformation on the internet and elsewhere concerning the clinical readiness of regenerative medicine products [6,7]. ‘Stem cell hype’ has contributed to inflating public expectations and skewing public understanding of the state of development of stem cell therapies and other regenerative medicine products [8]. With patients increasingly seeking health information online [9–11], the internet is playing an important role in distributing and amplifying inaccurate scientific and therapeutic claims. False, misleading and incomplete health information is not exclusive to regenerative medicine; an estimated 11% of 2805 health-related websites contain medical misinformation [12,13]. Exposure to medical misinformation can influence patients’ decisions to undergo costly unproven interventions that may carry undisclosed risks. In particular, stem cell interventions have garnered considerable public interest even though only a handful of evidence-based stem cell therapies having received US Food and Drug Administration (FDA) premarketing or marketing authorization [14]. Many among the public believe there already exist safe and effective stem cell treatments capable of healing a wide variety of medical conditions. Marketing representations emphasizing the purported safety and efficacy of such products have resulted in increased demand and the creation of a direct-to-consumer market where clinics around the world advertise unproven regenerative and stem cell interventions that lack regulatory approval [15–18] and often are promoted with little or no supporting evidence. The US direct-to-consumer market for unlicensed cell-based products is perhaps the largest with estimates of approximately one thousand clinics offering unproven regenerative interventions [19–21]. Much has been written about the features and concerns surrounding the marketplace, summarized in Table 1.
Table 1. . Summary of the features and risks of unproven stem cell and regenerative medicine intervention industry.
| Risks to patients and society | Ref. |
|---|---|
| Aggressive marketing by clinics directly to consumers using company websites and blogs, social media accounts and patient seminars. | [22–31] |
| Advertisements about unproven stem cell and regenerative medicine interventions marketed as low risk, high benefits, safe and effective, cutting-edge, innovative and natural. | [22,23,25–30,32,33] |
| Some commercial products do not contain stem cells but are marketed under the broader label of ‘stem cell therapy.’ | |
| Marketing strategies include the use of patient and celebrity testimonials, clinical trial registration, technical language, obtaining accreditation, collaborating with scientific establishments, incorrect referencing of scientific information, and claiming commercial activities are supported by published preclinical and clinical studies to showcase scientific legitimacy. | [6,22,23,26–30,34–37] |
| Clinics may also actively promote distrust of regulators and conventional medical establishments. | [31,34,35,38–40] |
| Unqualified physicians and clinicians providing stem cell and regenerative interventions to patients. | [41,42] |
| Physical, emotional and economic harms including blindness, tumors, lesions, death, emotional distress and financial loss. | [43,44] |
| Concerns about informed consent and patient confusion. | [45,46] |
| Confusion in the general public about the state of stem cell science (hype). | |
| Possible decreased trust in legitimate scientific translation of regenerative medicine products and services. | [14] |
Patients confronted with conflicting information about unproven regenerative options may not know what claims are credible or which sources are trustworthy. Difficulty distinguishing between sources of information from misinformation can impact patients’ capacity to make informed decisions about their healthcare needs. Developing constructive and trustworthy resources to engage patients seeking regenerative interventions is critical to ensuring that individuals can make informed decisions about their healthcare, especially in the context of medical misinformation. Informing patients about evidence-based regenerative interventions also serves as one of the pillars to responsible clinical translation [47].
Many scientific and medical institutions put significant effort into informing patients about potential harms associated with unproven stem cell and regenerative interventions. The International Society for Stem Cell Research, International Society for Cellular and Gene Therapy, the FDA, Stem Cells Australia, the Mayo Clinic and other organizations have all supported patient education and public engagement in this space [45,48–55]. Despite such noteworthy efforts, it is unclear whether such messaging reaches patients and whether patients consider these sources reputable and trustworthy [56,57].
Determining how best to meet patients’ informational needs, especially given fast-moving developments in regenerative medicine, is a significant challenge. Although proposed countermeasures target individual patients and the public [58–60], communication strategies that maintain or enhance trust are likely to be most effective at dispelling misinformation [61]. A stem cell counseling service was proposed to help patients make well-informed decisions about their medical care [62]. Similar approaches are being used to promote vaccination [63]. Taking into consideration the speed with which static resources can become outdated, a dynamic and evolving information exchange system may be more likely to remain up-to-date with scientific advances in regenerative medicine and permits dialogue with patients. Toward this end, Mayo Clinic developed the first Regenerative Medicine Consult Service (RMCS) in the USA in 2011. This service arose organically in response to patient requests for information about possible regenerative therapies [3,47]. The goal of the RMCS is to inform patients about evidence-based regenerative interventions and navigate patients to appropriate clinical and research interventions. Other similar services have been or are being developed. Funded by the California Institute of Regenerative Medicine, many of the Alpha Clinics in California including at the University of California San Diego, University of California San Francisco, and the City of Hope have developed similar patient consultation services [64]. At Stem Cells Australia and EuroStemCell, many patients initiate contact to find out more about stem cell interventions related to their conditions and experts within the organization strive to correct misperceptions and offer fact-based information [65].
While the concept of counselling patients in regenerative and stem cell medicine is being implemented at several institutions, no study has yet to characterize the nature of such a consult service at an academic medical center and assess the extent to which patients find such a resource helpful. In this study, we analyzed the nature of the Mayo Clinic RMCS at two campuses: Rochester, Minnesota and Jacksonville, Florida. We performed a retrospective chart analysis through our electronic health record database and characterized several features about patients and the consult service. We also conducted a pilot analysis through interviews with patients who recently engaged the consult service to explore their satisfaction with the service.
Materials & methods
This project uses multiple methods to capture information about patients calling into the RMCS at Mayo Clinic including retrospective chart analysis and qualitative interviews.
Mayo Clinic RMCS
Mayo Clinic has three major US campuses. They are located in Rochester, Minnesota, Jacksonville, Florida and Phoenix and Scottsdale, Arizona. The RMCS was created to address patient queries about the availability of stem cell and regenerative interventions in response to clinical trials conducted at Mayo Clinic [66]. The RMCS was first established in Rochester, Minnesota, where patients could call a toll-free number readily found on the internet [67]. Utilizing the free consultation service begins with a conversation with a scheduling specialist who records demographic and basic clinical information about the patient and triages the call to the appropriate consultant or clinical group best suited to addressing the patient's questions. Skilled patient navigators, registered nurses, and physicians serve as consultants.
Calls related to the use of stem cells to treat cancers using bone or hematopoietic stem cell transplantation were forwarded to Oncology and Hematology departments. In 2016, the Department of Orthopedics in Florida established a consultation service as part of the Regenerative Therapeutics Program [47]. The Mayo Clinic campus in Arizona does not have a consultation service. In 2017, calls related to certain orthopedics conditions were also separated in Rochester, Minnesota and forwarded to Physical Medicine and Rehabilitation or Sports Medicine in Minnesota. Calls for all other conditions were directed to members of the RMCS team in Minnesota. For our analysis, we focused on analyzing database information and electronic health records (EHR) of the RMCS in both Minnesota and Florida as these both had systematically recorded patient information related to the service.
Chart & database – data collection
Demographic and appointment information of patients was recorded in password-protected Microsoft Excel databases behind a firewall at both Minnesota and Florida. For Minnesota, a written description of the consultation discussion along with patient health information was recorded in the EHR platform ‘Epic’. In this study, we examined the Excel databases of both Minnesota and Florida in addition to the EHR consultation notes only available in Minnesota. A qualitative content analysis was performed for individual EHR notes where condition information and the nature of the discussion was obtained.
Under Minnesota Statute 144.335, researchers are permitted to use health records generated on or after 1 January 1997 unless the patient objects to authorizing the use of their records for research [68,69]. We excluded patients whose clinical records were not eligible for research due to this law. In addition, we excluded duplicate records, those with inactive or unidentifiable Mayo Clinic patient numbers, and patients who did not have an associated EHR clinical note in Minnesota or the clinical note did not correspond to the consultation service. We included all patients in the RMCS Florida database. We analyzed consultations conducted between 2011 and 2017 from Minnesota and 2016 and 2017 in Florida.
Chart & database – data analysis
Ordinal data on several variables were captured in Excel databases; some of it was also recorded in the EHRs of patients calling into the RMCS. For ordinal data, we performed descriptive statistics on several variables including whether patients or other parties contacted the RMCS, basis for calling the consult service, year of the consult, referral source and patient’s recorded gender, age, state of residence, and condition. Referral source and patient’s gender was not available for Florida patients. We also collected all clinical conditions for which patients called in as captured in the databases and when possible, verified through the EHR for Minnesota patients.
Codebook development for analyzing electronic health records
To qualitatively analyze EHR information written as free text, we developed a comprehensive codebook. We first developed a codebook deductively by analyzing empirical studies of clinic web-based marketing practices [7,15–17,21,23,25,27,29,70,71] and interviews with patients traveling to clinics to receive unproven stem cell interventions [22,34,35,72,73]. This permitted us to consider information about patients including their general medical areas and specific conditions of concern. We were also able to include the location of clinics, sources of information about clinics and the reasons patients may consider undertaking (or have undertaken) an unproven regenerative intervention. We then purposely selected 30 RMCS notes recorded in the ‘Epic’ EHR based on a diversity of years (2012–2017) and four consultants with the highest number of consultations to inductively modify the codebook and add additional codes. Several of the codes written in patient EHRs were used to corroborate data from Excel databases (patients’ age, sex, year of consultation and state of residence). In cases where there was a discrepancy between information contained within the Excel database versus the ‘Epic’ EHR, information in the EHR was chosen since it represented the nature of the discussion and was written by the consultant who interacted directly with the patient. Additional codes in the codebook included the following categories: patient medical history, clinical problem, purpose of consultation, nature of the discussion and outcomes of the consultation (please see final Codebook in Supplementary Material 1).
We performed intercoder analysis between two coders (CS and JDH) to determine independence of data based on the Cohen’s kappa statistic [74]. A random 20% of entries was chosen and analyzed to find excellent intercoder reliability (, range 84–100% and average k = 0.82, range 0.23–1, respectively; Supplementary Table 1). In two cases, poor intercoder scores were obtained due to an initial difference in interpreting the codes. After discussion and consensus coding, additional clarification to the codebook was written to ensure a common understanding between both coders. Subsequent coding used the revised definitions and the remaining consultation notes were divided and coded by two coders (CS and JDH). We used descriptive statistics to represent the data.
Only nonorthopedic consultation notes from the Minnesota database were included for analysis. Specific notes regarding orthopedic conditions were excluded from the analysis because in many cases those notes did not contain any information related to the variables examined as part of the consultation or the education that may have been offered. These notes reflected referrals to other physicians or the Florida service for more specific consultation and we did not want to double up our counts. We analyzed all telephone consultations in Florida where several demographic variables of interest were maintained in an Excel database. In-person Florida consults were maintained in the EHR which was not analyzed because the information reflected a different population of patients undergoing physical exam and testing and did not contain the variables of interest in the study.
Length of time of consultations
The length of the consultation conversation was not routinely recorded in either Minnesota or Florida record systems. From November 2019 to January 2020, we had consultants record the time of patient conversations for 25 consultations in both Minnesota and Florida totaling 50 consultations.
Patient interviews & qualitative analysis
We conducted 25 pilot telephone interviews with individuals who had called into the RMCS Minnesota service between August and November 2018. We purposively sampled for patients representing a range of clinical conditions and attempted to balance for gender. Potential participants were contacted if the EHR indicated a conversation with the consultant about unproven regenerative options. We contacted potential participants by phone no more than three-times on separate days and left voicemail messages. To explain the study, we asked if individuals had approximately 5 min to answer several questions about their experience with the Mayo Clinic RMCS. We then outlined the study procedures and risks and benefits to participation. We did not elicit health information from patients.
After obtaining oral informed consent, we asked three brief questions: did you find the consultation experience helpful? Did the consultant provide you with knowledge about stem cell therapies? Did the consultant change your opinion concerning the decision to undergo unapproved stem cell therapies? Interviews were recorded with an encrypted recorder. Interviews averaged about 6 min. Detailed notes were taken on the content of each interview and a simple descriptive analysis was performed. Given the length of the conversations and the type of information we were aiming to collect about patients’ experiences, we did not undertake an in-depth qualitative analysis.
Ethics review
Our study received an IRB waiver of consent from Mayo Clinic for the chart review portion of the study. Patient interviews were granted approval by the Mayo Clinic IRB #18-006931.
Results
We included a total of 1319 consultations between Minnesota and Florida campuses (Figure 1). Between 2011 and 2017, a total of 2534 consultations were conducted in Minnesota and between 2016 and 2017, 374 consultations were conducted in Florida. Based on the exclusion criteria described above for Minnesota, a total of 1598 consultations were excluded, mostly due to removal of 1390 records of patients who did not provide authorization under the Minnesota Statute. Of the 945 consultations notes available for analysis, 442 were excluded mostly due to the removal of orthopedic cases as explained above. These exclusions left a final count of 503 individual patient consultation notes from the Minnesota EHR for analysis.
Figure 1. . Selection of electronic health records from Regenerative Medicine Consult Services.
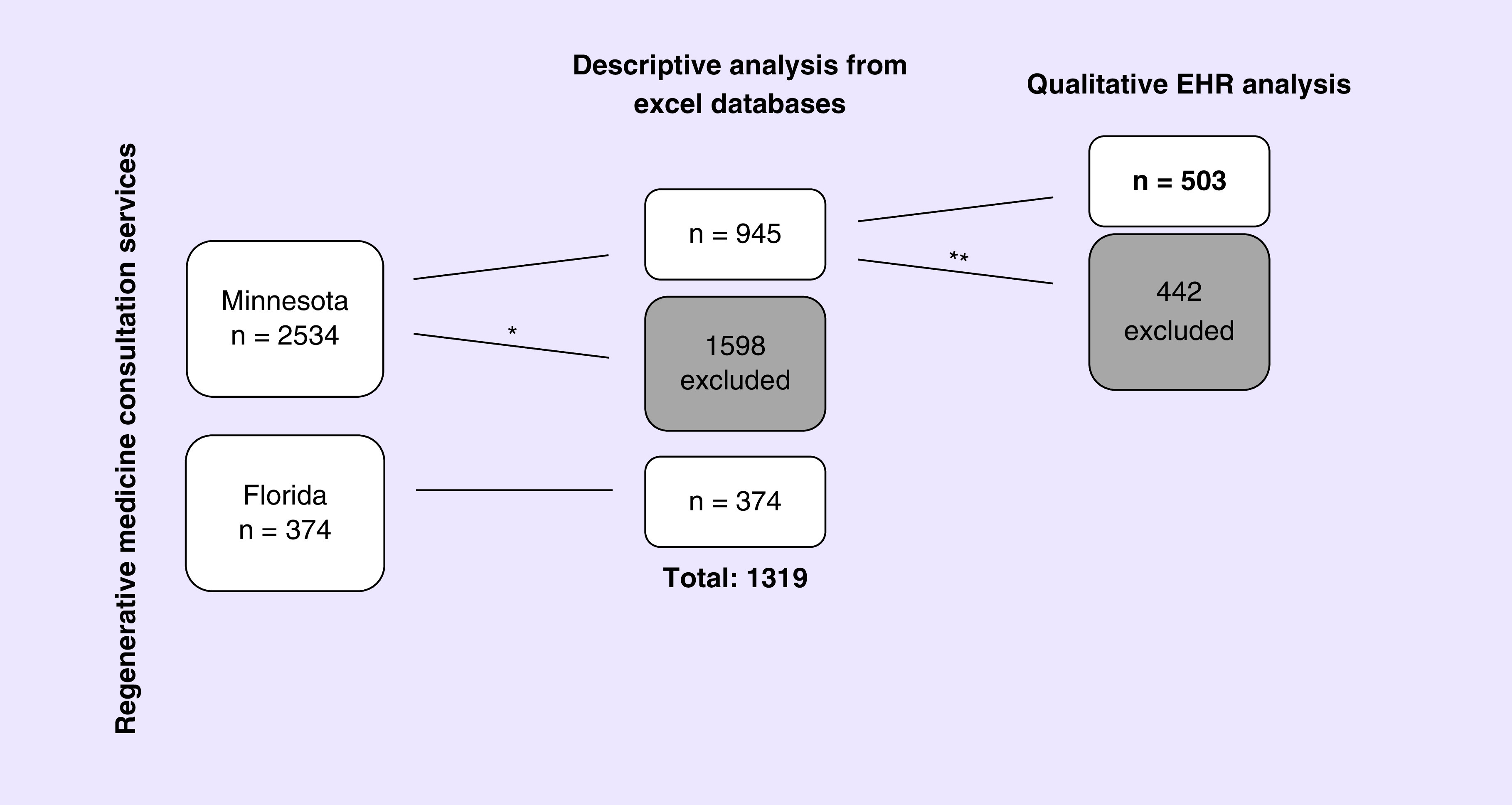
*Exclusion criteria 1: Removal of patient records based on the Minnesota Statute (n = 1390), did not have a Mayo Clinic number, or duplicates.
**Exclusion criteria 2: Removal of all orthopedic consultation notes and duplicate notes from the EHR.
EHR: Electronic health records.
Patient information, demographics & clinical conditions
Most regenerative medicine consultations were conducted by telephone (91.1%; n = 861 Minnesota, 95.5%; n = 357 Florida), although some consultations were made in person (n = 38) or by video (n = 26). We found roughly equal number of males (56.3%, n = 530) and females who engaged the RMCS in Minnesota. The majority of callers in the RMCS in Minnesota were new Mayo Clinic patients (63.2%; n = 1,602) with 931 previous patients (36.7%). For Florida, slightly less than half of callers had prior engagement with the Mayo System (47.1%; n = 132), with 148 (52.9%) being new Mayo Clinic patients. Most often patients themselves called into the RMCS (75.8% n = 709 in Minnesota; 93.3%, n = 349 in Florida) which was followed by spouses (8.3%, n = 78 in Minnesota; 4.8% n = 18, in Florida), parents (7.2%, n = 67 in Minnesota; 0.8%, n = 3 in Florida), children (3.6%, n = 34 in Minnesota; 0.5%, n = 2 in Florida) and medical providers (2.6%; n = 24 in Minnesota) who called to ask questions on behalf of patients. Consultations averaged about 32 min in length (27 min in Minnesota and 36 min in Florida) with an overall range of 12–52 min.
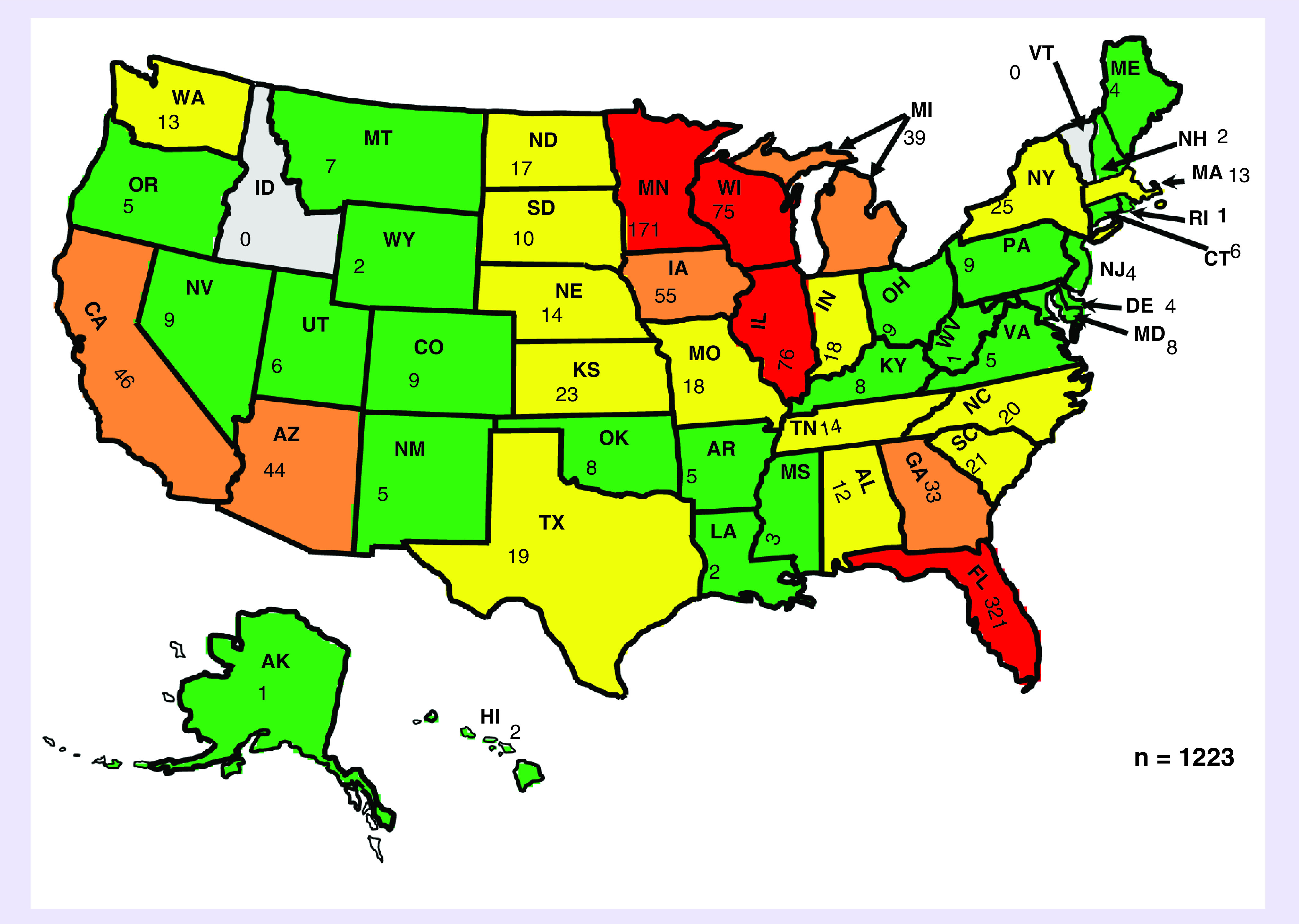
The mean age of patients was 60.7 years of those contacting the service in Minnesota and 64.6 years for Florida. The youngest patient was less than 1 year old and the oldest patient was 97 years of age (Figure 2). The majority of patients had their home residence in the USA (97.8%, n = 919 from the Minnesota consultation service, 98.7%, n = 304 in the Florida consultation service). Patients engaged the Minnesota service from across 48 US states and eight other countries, while patients engaged the Florida consultation service across 33 US states and four other countries (Figure 3). Patient residences were mostly clustered in the upper Midwest and southeast USA; the southwest region of the country was the next most common region. Mayo Clinic’s reputation (54.1%; n = 387) and the internet (31.8%; n = 228) combined represented over 85% of the referral sources for patients engaging the Minnesota RMCS. This information was not captured for Florida. Physicians (7.3%; n = 52) represented the next most common referral source followed by family members or friends (2.8%; n = 20), social media (1.0%; n = 7) and academic journal articles (0.7%; n = 5).
Figure 2. . Age of patients seeking Regenerative Medicine Consult Service consultation.
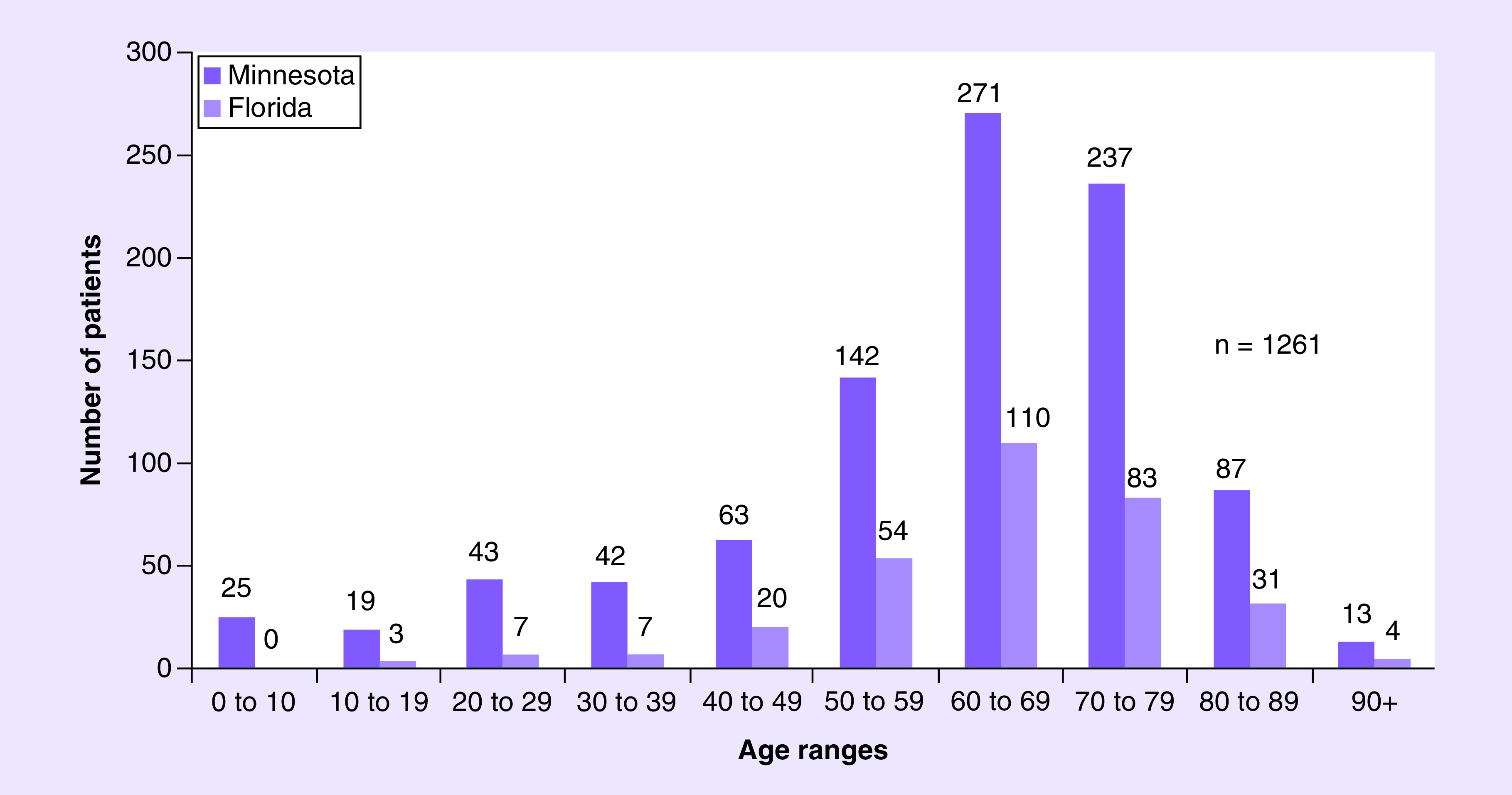
Figure 3. . Frequency of consultations by Minnesota and Florida Regenerative Medicine Consult Services.
Patients engaging the RMCS in Minnesota had questions about 152 unique clinical conditions or complaints in 17 clinical areas of interest whereas those engaging the Florida service had 32 unique conditions or complaints in eight clinical areas of interest (please see Supplementary Table 2 for all clinical conditions represented). Orthopedic issues represented the most common area of interest for which patients contacted the RMCS in Minnesota (43.1%, n = 379), followed by cardiology, neurology and pulmonology (Table 2). In Florida, patients almost exclusively called the consult service for orthopedic conditions (93%, n = 346). The twelve most common clinical conditions patients contacted the RMCS in Minnesota were osteoarthritis (30.2%; n = 266), heart failure (7.3%; n = 64), chronic obstructive pulmonary disease (COPD) (6.4%; n = 56), cardiomyopathy (4.0%; n = 35), hypoplastic left heart syndrome (3.6%; n = 32), degenerative disc disease (3.0%; n = 26), Parkinson’s disease (2.4%; n = 21), avascular necrosis (1.6%; n = 14), stroke (1.5%; n = 13), multiple sclerosis (1.4%; n = 12), spinal cord injury (1.4%; n = 12) and hip pain (1.4%; n = 12). Osteoarthritis represented 81.7% (n = 304) of all calls at the Florida service.
Table 2. . Patients’ clinical areas of interest among Minnesota and Florida consultations.
| Clinical area of interest | Consultations | |
|---|---|---|
| Minnesota n (%) | Florida n (%) | |
| Orthopedics | 379 (43.1%) | 346 (93.0%) |
| Cardiology | 170 (19.3%) | |
| Neurology | 133 (15.1%) | 6 (1.6%) |
| Pulmonology | 92 (10.5%) | 6 (1.6%) |
| Nephrology | 23 (2.6%) | 2 (0.5%) |
| Hepatology | 17 (1.9%) | |
| Ophthalmology | 15 (1.7%) | |
| Endocrinology | 12 (1.4%) | |
| Rheumatology | 8 (0.9%) | 5 (1.3%) |
| Oncology | 8 (0.9%) | 2 (0.5%) |
| Gastrointestinal | 7 (0.8%) | |
| Ear, nose, throat | 4 (0.5%) | |
| Sexual Health | 4 (0.5%) | |
| Dermatology | 4 (1.1%) | |
| Vascular | 3 (0.3%) | 1 (0.3%) |
| Plastic Surgery | 3 (0.3%) | |
| Infectious Disease | 1 (0.1%) | |
| Urology | 1 (0.1%) | |
| Total | 880 | 372 |
Examining patient queries about unproven regenerative therapies & consultants’ responses
An analysis of 503 consultations recorded by consultants in patient EHRs in Minnesota revealed that nearly all of the patients engaging the service (99.2%; n = 499) wanted to know about stem cell or regenerative interventions related to their condition. The EHR notes documented the consultant explaining the state of stem cell and regenerative research for the patient’s condition in 93.4% (n = 470) of cases. More than a third of the notes (36.6%; n = 184) captured the consultant discussing the for-profit, direct-to-consumer industry and some of the risks involved with unproven, out-of-pocket interventions. A small number of consults documented patients conducting research on a specific stem cell clinic (16%; n = 82), with about half of those (n = 40) reporting that they contacted the clinic. Of the 43 specific locations where patients reported undertaking research and/or contacting a stem cell clinic, 69.8% (n = 30) were located in the USA with clinics in California (n = 5), Arizona (n = 5) and Florida (n = 3) being most frequently mentioned. Mexico was the most frequently mentioned international destination for unproven regenerative interventions (n = 7). A few consult notes recorded patients (2.6%; n = 13) undergoing an unproven stem cell intervention in the past of which seven reported mostly a positive experience receiving the intervention and the remainder had either mixed (n = 3) or mostly negative (n = 3) experiences.
Consultants also offered medical and clinical research referrals as appropriate. Nearly a third of the patients (29%; n = 146) received medical referrals for their condition with the majority of these being at Mayo Clinic (87.0%; n = 127). Slightly more than a third of the patients (37.0%, n = 186) received clinical research referrals with 71.4% (n = 132) being referred to research at Mayo Clinic. In addition to medical and research referrals, 13.1% (n = 66) of notes recorded that consultants provided additional educational materials to patients. Examples of these materials include the International Society for Stem Cell Research’s website [52] and patient handbook [53] or Clinicaltrials.gov [75].
Overall, 12.9% (n = 65) of EHRs documented patients actively considering an unproven stem cell or regenerative intervention. Among those, 32.3% (n = 21) of the consultation notes explained that patients were still considering a stem cell or regenerative intervention, 16.9% (n = 11) explained that patients had decided not to pursue an unproven intervention, and 50.8% (n = 33) of the notes did not address whether the patient would pursue an unproven intervention after the discussion.
Patients’ satisfaction with a regenerative medicine consultation
To perform a preliminary assessment of patient satisfaction with the RMCS, we conducted individual interviews with 25 patients who engaged the RMCS between August and November 2018. Out of 25 patients, 84% (n = 21) found the consultation service informative and helpful. Almost all callers (n = 22) reported that the consultant was knowledgeable about the state of stem cell and regenerative medicine research, although multiple participants reported not learning about any additional therapeutic options for their specific medical conditions. Patients described the service as ‘thorough’, ‘excellent’, ‘professional’, ‘convenient’, ‘informative’ and remarked that “all possible information was given.” Many expressed gratitude for the service and the information provided by consultants.
Some patients explained that the consultation confirmed their suspicions about unproven stem cell interventions. Two patients who were actively planning to pursue such procedures decided not to undergo such interventions following their consultation, one citing the exorbitant cost for the procedure coupled with the family not realizing the intervention was currently unapproved by regulators and the second due to the lack of scientific evidence for the intervention. Several patients reported being disappointed in the information they received from a consultant since they did not previously understand how few stem cell interventions were currently available as evidence-based and FDA-approved treatments. One patient explained that they decided to go through with an unproven stem cell intervention even after the consultation, but appreciated learning ‘the Mayo view’ and recognized that no treatment was perfect. The participant had conducted research on various options for over half a year and felt that he had enough information to make an informed decision.
Discussion
Our data serves to characterize a unique group of patients with unmet informational needs surrounding regenerative therapies. Members of this group consist of older to elderly persons with degenerative conditions, reside throughout the USA and are avid information seekers considering an unproven stem cell or regenerative intervention. The role of the RMCS is to provide information and to help patients navigate their clinical options based on their medical conditions, preferences and goals, and the availability of evidence-based regenerative interventions or clinical investigations. The informational needs of patients and families are not, however, restricted to the USA as a recent study shows that patients have contacted EuroStemCell and Stem Cells Australia who similarly address written patient queries about stem cell interventions [65].
Our analysis indicates that Mayo’s RMCS appears to satisfy the informational needs of patients seeking regenerative medicine interventions. Patients and families are being exposed to fact-based information about regenerative medicine through conversations about the state of the science and the availability of evidence-based options for their medical conditions. Pilot data from patient interviews suggests that patients are mostly satisfied with the information provided by the consultant and that the consultant was knowledgeable. A discussion of the science also may serve to help correct some patients’ misunderstandings about unproven interventions. Correcting misinformation and helping patients make informed decisions is an especially important function of such a service. Given the number of patient queries related to unproven stem cell interventions, RMCS consultants have revised their standard operating procedures to now include discussion of unproven interventions even if the patient does not specifically ask about this subject. This is an important attribute to convey since patients are interested in knowing more about unproven interventions, including where they should go to receive treatment and whether trusted organizations recommend undertaking them [65].
To help patients distinguish credible claims from misinformation, consultants can convey evidence-based information to patients about the unproven regenerative industry while also being attentive to each individual patient's unique circumstances. While we recommend basing these conversations around individual patients’ needs and interests, a generalized approach can consist of four parts. First, consultants should listen to the patient’s particular story and understand what information they are seeking, what sorts of treatments they have previously tried and the patient’s understanding of regenerative medicine. Next, consultants should convey evidence-based information at the level of patient understanding, addressing the risks and benefits associated with whatever interventions they are considering. This information should be communicated in an accessible manner and teach-back methods can be used to determine what the patient is taking from the conversation. Throughout such conversations, consultants should attempt to address potential questions a patient might ask. If they cannot address the question directly they should provide patients with useful information resources. Finally, consultants should attempt to counter misinformation and misperceptions in a professional manner that is nonjudgmental, patient centered, and respectful. Consultants providing information to patients should consider both the cultural and regulatory contexts in different jurisdictions.
Results from our study show that interpersonal communication with consultants (patient navigators, nurses and physicians) not only serves as a vehicle for delivering information about regenerative medicine, but may also influence health behavior in the context of direct-to-consumer marketing. Our analysis of EHRs revealed that nearly 17% of 65 documented cases of patients actively considering an unproven stem cell or regenerative intervention resulted in patient statements that they would instead not undergo the intervention after speaking with a consultant. However, it remains unclear whether other patients considering a regenerative intervention also changed their opinions since this conversation may not have been documented by the consultant. This result was corroborated in several interviews conducted with patients. Together, these results suggest that a RMCS may be effective at influencing health behavior of patients considering an unproven regenerative intervention, although there were also 21 patients who maintained interest in an unproven stem cell intervention after the consultation. Yet whether a RMCS ought to consider health behavior change as one of its goals needs deeper reflection and consideration. While we do not aim to comprehensively address the ethical plausibility of health behavior change in the context of a RMCS, the literature on interpersonal relations between provider and patient, health coaching and health behavior offers insights on effective communication pertinent to conveying information and communicating effectively with patients and families through a RMCS.
The ecological model of health behavior posits that health behavior is influenced at multiple levels, including interpersonal communication where relationships between people are crucial to identity and behavior [76]. Interpersonal communication recognizes the roles and interactions between a patient’s friends, family and social networks, including interactions with health providers as being central to formulating beliefs, norms and guiding health decisions. Healthcare decisions are not made in isolation based on biomedical information, but instead involve a complex interplay between patients' illness experiences, knowledge, values and emotions. Healthcare decisions also involve negotiations between a range of different actors and the healthcare system. While family members, close friends and patient advocacy groups that include patients with similar experiences play a significant role in shaping health behavior, nurses and physicians continue to be one of the most trusted professions for honesty and ethics [77,78] and are likely to play an important role in shaping patients’ healthcare decisions. Communicating with patients by addressing their emotional state is one of the recommendations made by Zarzeczny et al. who found that many patients and families contacting Stem Cells Australia and EuroStemCell expressed distress related to their or a loved one's clinical condition and their willingness to try an unproven intervention [65].
In addition to conversationally-based education during the consultation, written informational sources may serve as important complements to consultation services [65]. Most patient booklets designed about regenerative medicine [50–53], the new informed consent standards put forth by the International Society for Stem Cell Research [45,54] and academic literature in this space [18,22,23,25–30,32] are helpful resources for providing evidence-based accounts of the current state of regenerative medicine, anticipating questions and dispelling myths surrounding cell-based interventions. One recent study indicated that web-based education may have an initial impact in influencing the health behavior of stroke patients seeking unproven stem cell interventions [79]. Providing additional reputable sources that patients can use when considering an unproven intervention may help patients retain this information, especially given that it is unlikely that everything will be remembered from a single conversation. Written information may also help patients understand the distinctions between proven therapies, interventions provided on an experimental basis in clinical trials and unproven interventions that should not be offered clinically because of a lack of adequate safety and efficacy data [57]. Moreover, providing additional resources, especially from multiple sources, may suggest to patients that the information given is important and can be trusted since it comes from different reputable scientific organizations. Although our data suggests that only 13% of patients received additional educational materials, this is likely under-reported due to the limitation that not everything discussed verbally is recorded in an EHR. Anecdotally, RMCS consultants report that they almost always provide additional educational resources to patients.
Limitations
Five limitations must be noted. First, our study analyzed patients who sought to engage the RMCS through internet searches and knowledge of Mayo Clinic and thus does not capture the experiences, preferences and information-seeking behavior of all patients and families considering regenerative interventions. Second, given the organic nature of how the RMCS evolved, undocumented splintering of services and private consultations with individual physicians would not be captured in our analysis. We are in the process of refining our consultation service to ensure a single institutional door of entry for all patients. Third, application of the Minnesota Statute on research authorization partially limits our reporting of a complete dataset of Minnesota consultations through the service, although there remains an accurate portrayal of the trends reported in the manuscript. Fourth, because consultants may not record everything discussed in the consultation within the EHR, our analysis cannot capture the full nature of the dialogue between consultant, patient and family members. Therefore, some data may not accurately reflect what information was collected or reported to patients. Fifth, because we excluded the analysis of orthopedics consults for the qualitative EHR note analysis, our findings may be more relevant for patients seeking out information for nonorthopedic conditions. Further exploration into the methods of consultation that can be best used to engage patients interested in orthopedic interventions is needed.
Conclusion
The responsible and compassionate translation of regenerative medicine involves addressing the informational needs of patients. Considering the ecological model of health framework and the relevant public health context, we recommend that the scientific community takes seriously the idea of patient-centered approaches when developing communication tools intended to help individuals make informed healthcare decisions. Patients report higher levels of trust when they perceive providers to be informative, respectful, nonjudgmental, sensitive and emotionally supportive [80,81]. Similarly, a good interpersonal interaction between a patient and physician is likely to facilitate information exchange and shared decision-making [82]. A patient perceiving someone as caring and showing empathy can improve the emotional health of patients by reducing anxiety, increasing self-confidence and improving satisfaction and trust [83–85]. It is important for consultants to listen and build a rapport with patients and families since those considering unproven regenerative interventions often express anxiety about living with chronic illness and sometimes report medical teams as dismissive to their concerns, thus causing frustration and fueling distrust in conventional medicine [34,35,39,72]. Strategies from health coaching on empathic communication where patients are empowered and participate in goal setting and healthcare decision-making could enhance information exchange and add to the repertoire of communication strategies supported by health consultation services [86,87].
Interpersonal communication is at the core of this service and patients should be given the opportunity to express their worries, concerns and questions about regenerative medicine amidst confusion and misinformation replete in this health area. This will require active listening by consultants to meet patients’ needs. Having trained healthcare professionals available to listen and empathize while providing an explanation of risks and providing insight into the direct-to-consumer marketplace for unproven stem cell and regenerative interventions may help patients critically evaluate and possibly reconsider unproven and potentially harmful therapies. Whether academic and medical institutions should engage in promoting services that result in behavior change require deeper reflection, moral consideration and public engagement to better understand whether this should be an aim of such services. Additional important and practical issues about the service to consider include economic costs and returns on investment for the institution to sustain such programs, institutional reputation and public perception and social responsibilities to patients and the public who support the advancement of regenerative medicine. Despite the potential of stem cell and regenerative medicine research to provide new treatments, for many patients today, there are no evidence-based options. Having an opportunity to discuss medical concerns with a caring individual may be of modest benefit in and of itself, lending additional value to regenerative consultative services.
Summary points.
Unproven regenerative therapies and direct-to-consumer health advertising necessitate new ways of engaging with patients. Unproven stem cell interventions are often marketed with misinformation and this can impact patient autonomy and the ability for patients to make informed health decisions. This obligates healthcare professionals to facilitate patient access to evidence-based information.
Mayo Clinic in Minnesota created a Regenerative Medicine Consult Service (RMCS) in 2011 to provide patients with reliable information and navigate them to evidence-based clinical and research opportunities. In 2016, Mayo Clinic in Florida started a consult service focused mainly on orthopedic conditions.
Using mixed methods, we characterized the nature of patient consults in the RMCS. Specifically, we captured various patient data from 1319 patients among two Excel databases, undertook a qualitative analysis of 503 consultation notes in patient electronic health records and conducted short interviews with 25 patients.
We identified a unique group of information seekers who were older to elderly patients, clustered in the upper midwest, southeast and southwest regions of the USA and interested in learning more about regenerative options for their medical conditions.
Callers were provided with information about the state of stem cell research for a patient’s medical condition and discussed the unproven stem cell therapy industry in over a third of the consults. About a third of patients received either a clinical or research referral.
An analysis of the consult notes showed that among patients who were actively pursuing an unproven stem cell intervention (12.9%; n = 65), about a third (32.3%; n = 21) documented that patients were still considering an unproven intervention toward the end of the consult. However, 16.9% (n = 11) notes reported that patients said they would not pursue an unproven intervention at the end of the consult, and the remaining notes did not describe the patient's stated intentions.
Patients who were interviewed reported that the consultant was knowledgeable and that the service was well received.
Given the challenges associated with providing patients with up-to-date and evidence-based information in the digital age, our results suggest that provider–patient consultations may be helpful for engaging patients struggling to navigate health information about regenerative medicine. The RMCS was able to deliver evidence-based information, navigate patients to research and clinical services and potentially influence health behavior.
Supplementary Material
Acknowledgments
We would like to thank BS Edwards in the Transplant Center of Mayo Clinic in Minnesota and KP Krucker for their leadership in spearheading the RMCS. We would also like to thank the anonymous reviewers for their feedback which served to strengthen the manuscript.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/rme-2020-0018
Financial & competing interests disclosure
Z Master would like to acknowledge research support from the Mayo Clinic CTSA Small Grant Program from UL1 TR002377, NIH/NCATS. Several of the authors are employees of Mayo Clinic. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The study received an institutional review board (IRB) waiver of consent from Mayo Clinic for the chart review portion of the study. Patient interviews were granted approval by the Mayo Clinic IRB #18-006931.
References
- 1.Ronfard V, Vertes AA, May MH, Dupraz A, van Dyke ME, Bayon Y. Evaluating the past, present and future of regenerative medicine: a global view. Tissue Eng. Part B Rev. 23(2), 199–210 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Mao AS, Mooney DJ. Regenerative medicine: current therapies and future directions. PNAS 112(47), 14452–14459 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terzic A, Harper CM, Jr, Gores GJ, Pfenning MA. Regenerative medicine blueprint. Stem Cells Dev. 22(Suppl. 1), 20–24 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Hirsch T, Rothoeft T, Teig N. et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 551(7680), 327–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siprashvili Z, Nguyen NT, Gorell ES. et al. Safety and wound outcomes following genetically corrected autologous epidermal grafts in patients with recessive dystrophic epidermolysis bullosa. JAMA 316(17), 1808–1817 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Sipp D, Caulfield T, Kaye J. et al. Marketing of unproven stem cell-based interventions: a call to action. Sci. Transl. Med. 9(397), (2017). [DOI] [PubMed] [Google Scholar]
- 7.Kamenova K, Caulfield T. Stem cell hype: media portrayal of therapy translation. Sci. Transl. Med. 7(278), 278ps274 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Cuchiara ML, Olive JK, Matthews K. Regulating the therapeutic translation of regenerative medicine. Expert Opin. Biol. Ther. 15(10), 1387–1390 (2015). [DOI] [PubMed] [Google Scholar]
- 9.UnitedHealthcare. UnitedHealthcare consumer sentiment survey executive summary. 1–17 (2019). https://newsroom.uhc.com/content/dam/newsroom/2019%20UHC%20Consumer%20Sentiment%20Survey%2009.23.19%20-%20FINAL.pdf
- 10.Hesse BW, Nelson DE, Kreps GL. et al. Trust and sources of health information: the impact of the Internet and its implications for health care providers: findings from the first Health Information National Trends Survey. Arch. Intern. Med. 165(22), 2618–2624 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Fox S. The social life of health information, 2011 (2011). www.pewresearch.org/internet/2011/05/12/the-social-life-of-health-information-2011/ [Google Scholar]
- 12.Gregory J. Health websites are notoriously misleading. So we rated their reliability (2019). www.statnews.com/2019/07/26/health-websites-are-notoriously-misleading-so-we-rated-their-reliability/ [Google Scholar]
- 13.NewsGuard. Inside NewsGuard's first year fighting misinformation (2019). www.newsguardtech.com/press/newsguards-first-year/
- 14.Cossu G, Birchall M, Brown T. et al. Lancet commission: stem cells and regenerative medicine. Lancet 391(10123), 883–910 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Ogbogu U, Du J, Koukio Y. The involvement of Canadian physicians in promoting and providing unproven and unapproved stem cell interventions. BMC Med. Ethics 19(1), 32 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner L, Knoepfler P. Selling stem cells in the USA: assessing the direct-to-consumer industry. Cell Stem Cell 19(2), 154–157 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Berger I, Ahmad A, Bansal A, Kapoor T, Sipp D, Rasko JEJ. Global distribution of businesses marketing stem cell-based interventions. Cell Stem Cell 19(2), 158–162 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Munsie M, Hyun I. A question of ethics: selling autologous stem cell therapies flaunts professional standards. Stem Cell Res. 13(3 Pt B), 647–653 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Knoepfler PS. Rapid change of a cohort of 570 unproven stem cell clinics in the USA over 3 years. Regen. Med. 14(8), 735–740 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Marso A. Peddling unproven treatments, stem cell industry ‘fleeces,’ sometimes harms patients (2019). www.kansascity.com/news/business/health-care/article233635472.html [Google Scholar]
- 21.Turner L. The US direct-to-consumer marketplace for autologous stem cell interventions. Perspect. Biol. Med. 61(1), 7–24 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Gottweis H. Stem cell treatments in China: rethinking the patient role in the global bio-economy. Bioethics 27(4), 194–207 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Lau D, Ogbogu U, Taylor B, Stafinski T, Menon D, Caulfield T. Stem cell clinics online: the direct-to-consumer portrayal of stem cell medicine. Cell Stem Cell 3(6), 591–594 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Marcon AR, Murdoch B, Caulfield T. Fake news portrayals of stem cells and stem cell research. Regen. Med. 12(7), 765–775 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Murdoch B, Zarzeczny A, Caulfield T. Exploiting science? A systematic analysis of complementary and alternative medicine clinic websites' marketing of stem cell therapies. BMJ Open 8(2), e019414 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regenberg AC, Hutchinson LA, Schanker B, Mathews DJ. Medicine on the fringe: stem cell-based interventions in advance of evidence. Stem Cells 27(9), 2312–2319 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Ryan KA, Sanders AN, Wang DD, Levine AD. Tracking the rise of stem cell tourism. Regen. Med. 5(1), 27–33 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Hawke B, Przybylo AR, Paciulli D, Caulfield T, Zarzeczny A, Master Z. How to peddle hope: an analysis of YouTube patient testimonials of unproven stem cell treatments. Stem Cell Reports 12(6), 1186–1189 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamenova K, Reshef A, Caulfield T. Representations of stem cell clinics on Twitter. Stem Cell Rev. Rep. 10(6), 753–760 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Robillard JM, Cabral E, Hennessey C, Kwon BK, Illes J. Fueling hope: stem cells in social media. Stem Cell Rev. Rep. 11(4), 540–546 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Knoepfler PS. The stem cell hard sell: report from a clinic's patient recruitment seminar. Stem Cells Transl. Med. 6(1), 14–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarzeczny A, Rachul C, Nisbet M, Caulfield T. Stem cell clinics in the news. Nature Biotechnol. 28(12), 1243–1246 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Kingery MT, Schoof L, Strauss EJ, Bosco JA, Halbrecht J. Online direct-to-consumer advertising of stem cell therapy for musculoskeletal injury and disease: misinformation and violation of ethical and legal advertising parameters. J. Bone Joint Surg. Am. 102(1), 2–9 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Rachul C. “What have i got to lose?”: an analysis of stem cell therapy patients' blogs. Health Law Review 20, 5–12 (2011). [Google Scholar]
- 35.Song P. Biotech pilgrims and the transnational quest for stem cell cures. Med. Anthropol. 29(4), 384–402 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Turner L. ClinicalTrials.gov, stem cells and ‘pay-to-participate’ clinical studies. Regen. Med. 12(6), 705–719 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Richley A, Frow EK. The role of scientific papers cited by direct-to-consumer stem cell businesses in the Southwest US. : International Society for Stem Cell Research. International Society for Stem Cell Research, Abstract 4050 CA, USA: (2019). [Google Scholar]
- 38.Caulfield T, Marcon AR, Murdoch B. et al. Health misinformation and the power of narrative messaging in the public sphere. Can. J. Bioethics 2(2), 52–60 (2019). [Google Scholar]
- 39.Levine AD, Wolf LE. The roles and responsibilities of physicians in patients' decisions about unproven stem cell therapies. J. Law Med. Ethics 40(1), 122–134 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Petersen A, Tanner C, Munsie M. Between hope and evidence: how community advisors demarcate the boundary between legitimate and illegitimate stem cell treatments. Health 19(2), 188–206 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Frow EK, Brafman DA, Muldoon A. et al. Characterizing direct-to-consumer stem cell businesses in the southwest United States. Stem Cell Reports 13(2), 247–253 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu W, Smith C, Turner L, Fojtik J, Pacyna JE, Master Z. Characteristics and scope of training of clinicians participating in the US direct-to-consumer marketplace for unproven stem cell interventions. JAMA 321(24), 2463–2464 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bauer G, Elsallab M, Abou-El-Enein M. Concise review: a comprehensive analysis of reported adverse events in patients receiving unproven stem cell-based interventions. Stem Cells Transl. Med. 7(9), 676–685 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horner C, Tenenbaum E, Sipp D, Master Z. Can civil lawsuits stem the tide of direct-to-consumer marketing of unproven stem cell interventions. NPJ Regen. Med. 3(5), 1–5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugarman J, Barker RA, Charo RA. A professional standard for informed consent for stem cell therapies. JAMA 322(17), 1651–1652 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Master Z, Smith C, Tilburt JC. Informed consent for stem cell-based interventions. JAMA 323(9), 893 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Shapiro SA, Smith CG, Arthurs JR, Master Z. Preparing regenerative therapies for clinical application: proposals for responsible translation. Regen. Med. 14(2), 77–84 (2019). [DOI] [PubMed] [Google Scholar]
- 48.US Food and Drug Administration. Statement on stem cell clinic permanent injunction and FDA's ongoing efforts to protect patients from risks of unapproved products (2019). www.fda.gov/news-events/press-announcements/statement-stem-cell-clinic-permanent-injunction-and-fdas-ongoing-efforts-protect-patients-risks
- 49.US Food and Drug Administration. FDA warns about stem cell therapies (2019). www.fda.gov/consumers/consumer-updates/fda-warns-about-stem-cell-therapies
- 50.Stem Cells Australia. The Australian Stem cell handbook (2015). www.stemcellsaustralia.edu.au/AboutUs/Document-Library/The-Australian-Stem-Cell-Handbook.aspx
- 51.Master Z, Caulfield T. Patient booklet: what you need to know about stem cell therapies (2014). www.amc.edu/academic/bioethics/stem_cell_patient_booklet.cfm
- 52.International Society for Stem Cell Research. A closer look at stem cell therapies (2020). www.closerlookatstemcells.org
- 53.International Society for Stem Cell Research. Patient handbook on stem cell therapies (2008). www.isscr.org/docs/default-source/patient-handbook/isscrpatienthandbook.pdf
- 54.International Society for Stem Cell Research. Informed consent standard for stem cell-based interventions offered outside of formal clinical trials (2019). www.isscr.org/docs/default-source/policy-documents/isscr-informed-consent-standards-for-stem-cell-based-interventions.pdf
- 55.Mayo Clinic Center for Regenerative Medicine. Regenerative medicine education for the general public. www.mayo.edu/research/centers-programs/center-regenerative-medicine/education/regenerative-medicine-education-for-the-general-public
- 56.Master Z, Robertson K, Frederick D, Rachul C, Caulfield T. Stem cell tourism and public education: the missing elements. Cell Stem Cell 15(3), 267–270 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Master Z, Zarzeczny A, Rachul C, Caulfield T. What's missing? discussing stem cell translational research in educational information on stem cell “tourism”. J. Law Med. Ethics 41(1), 254–268 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Armstrong PW, Naylor CD. Counteracting health misinformation: a role for medical journals? JAMA 321(19), 1863–1864 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Merchant RM, Asch DA. Protecting the value of medical science in the age of social media and “fake news”. JAMA 320(23), 2415–2416 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Levy N. Nudges in a post-truth world. J. Med. Ethics 43(8), 495–500 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hooker C, Capon A, Leask J. Communicating about risk: strategies for situations where public concern is high but the risk is low. Public Health Res. Pr. 27(1), e2711709 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Scott CT. The case for stem cell counselors. Stem Cell Reports 4(1), 1–6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boodman E. The vaccine whisperers: counselors gently engage new parents before their doubts harden into certainty. (2019). www.statnews.com/2019/08/05/the-vaccine-whisperers-counselors-gently-engage-new-parents-before-their-doubts-harden-into-certainty/ [Google Scholar]
- 64.Lomax GP, Adams J, Jamieson C, Zaia J, Milian MT. Proceedings: accelerating stem cell treatments for patients: the value of networks and collaboration. Stem Cell Transl. Med. 4(7), 692–694 (2015). [Google Scholar]
- 65.Zarzeczny A, Tanner C, Barfoot J, Couturier A, Blackburn C, Munsie M. ‘Contact us for more information’: an analysis of public enquiries about stem cells. Regen. Med. 14(12), 1137–1150 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Terzic A, Nelson TJ. Regenerative medicine primer. Mayo Clin. Proc. 88(7), 766–775 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Mayo Clinic Center for Regenerative Medicine. Regenerative medicine consult service (2020). www.mayo.edu/research/centers-programs/center-regenerative-medicine/patient-care/regenerative-medicine-consult-service
- 68.Citizens' Council on Healthcare. What consent? - MN's medical & genetic research law. : CCHC Policy Brief. Citizen's Council on Health Care, MN, USA: (2005). [Google Scholar]
- 69.Woodward B, Hammerschmidt D. Requiring consent vs. waiving consent for medical records research: a Minnesota law vs. the U.S. (HIPAA) privacy rule. Health Care Anal. 11(3), 207–218 (2003). [DOI] [PubMed] [Google Scholar]
- 70.Ogbogu U, Rachul C, Caulfield T. Reassessing direct-to-consumer portrayals of unproven stem cell therapies: is it getting better? Regen. Med. 8(3), 361–369 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Munsie M, Lysaght T, Hendl T, Tan HYL, Kerridge I, Stewart C. Open for business: a comparative study of websites selling autologous stem cells in Australia and Japan. Regen. Med. 12(7), 777–790 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Peterson A, Tanner C, Munsie M. Therapeutic journeys: the hopeful travails of stem cell tourists. Sociol. Health Illness 36(5), 670–685 (2014). [DOI] [PubMed] [Google Scholar]
- 73.Prasad A. Ambivalent journeys of hope: embryonic stem cell therapy in a clinic in India. Health 19(2), 137–153 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Neuendorf K. The Content Analysis Guidebook. SAGE Publications, CA, USA, 149–151 (2002). [Google Scholar]
- 75.US National Library of Medicine. ClinicalTrials.gov [DOI] [PubMed]
- 76.Sallis JF, Owen N. Chapter 3: ecological models of health behavior. : Health Behavior: Theory, Research and Practice. Glanz K, Rimer BK, Viswanath K. (). Wiley, CA, USA: (2015). [Google Scholar]
- 77.Brenan M. Nurses again outpace other professions for honesty, ethics (2018). https://news.gallup.com/poll/245597/nurses-again-outpace-professions-honesty-ethics.aspx
- 78.Gallup. Honesty/ethics in professions. https://news.gallup.com/poll/1654/honesty-ethics-professions.aspx
- 79.Unsworth DJ, Mathias JL, Dorstyn DS, Koblar SA. Are patient educational resources effective at deterring stroke survivors from considering experimental stem cell treatments? A randomized controlled trial. Patient Educ. Couns. 10.1016/j.pec.2020.02.012 (2020) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 80.Deledda G, Moretti F, Rimondini M, Zimmermann C. How patients want their doctor to communicate. A literature review on primary care patients' perspective. Patient Educ. Couns. 90(3), 297–306 (2013). [DOI] [PubMed] [Google Scholar]
- 81.Gordon HS, Street RL, Sharf BF, Kelly PA, Souchek J. Racial differences in trust and lung cancer patients' perceptions of physician communication. J. Clin. Oncol. 24(6), 904–909 (2006). [DOI] [PubMed] [Google Scholar]
- 82.Arora NK. Interacting with cancer patients: the significance of physicians' communication behavior. Soc. Sci. Med. 57(5), 791–806 (2003). [DOI] [PubMed] [Google Scholar]
- 83.Fuertes JN, Mislowack A, Bennett J. et al. The physician-patient working alliance. Patient Educ. Couns. 66(1), 29–36 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Quirk M, Mazor K, Haley HL. et al. How patients perceive a doctor's caring attitude. Patient Educ. Couns. 72(3), 359–366 (2008). [DOI] [PubMed] [Google Scholar]
- 85.Uhas AA, Camacho FT, Feldman SR, Balkrishnan R. The relationship between physician friendliness and caring and patient satisfaction findings from an internet-based survey. Patient 1(2), 91–96 (2008). [DOI] [PubMed] [Google Scholar]
- 86.Howick J, Moscrop A, Mebius A. et al. Effects of empathic and positive communication in healthcare consultations: a systematic review and meta-analysis. J. Roy. Soc. Med. 111(7), 240–252 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thom DH, Wolf J, Gardner H. et al. A qualitative study of how health coaches support patients in making health-related decisions and behavioral changes. Ann. Fam. Med. 14(6), 509–516 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


