Abstract
Background
Acute kidney injury (AKI) can affect hospitalized patients with coronavirus disease 2019 (COVID-19), with estimates ranging between 0.5% and 40%. We performed a systematic review and meta-analysis of studies reporting incidence, mortality and risk factors for AKI in hospitalized COVID-19 patients.
Methods
We systematically searched 11 electronic databases until 29 May 2020 for studies in English reporting original data on AKI and kidney replacement therapy (KRT) in hospitalized COVID-19 patients. Incidences of AKI and KRT and risk ratios for mortality associated with AKI were pooled using generalized linear mixed and random-effects models. Potential risk factors for AKI were assessed using meta-regression. Incidences were stratified by geographic location and disease severity.
Results
A total of 3042 articles were identified, of which 142 studies were included, with 49 048 hospitalized COVID-19 patients including 5152 AKI events. The risk of bias of included studies was generally low. The pooled incidence of AKI was 28.6% [95% confidence interval (CI) 19.8–39.5] among hospitalized COVID-19 patients from the USA and Europe (20 studies) and 5.5% (95% CI 4.1–7.4) among patients from China (62 studies), whereas the pooled incidence of KRT was 7.7% (95% CI 5.1–11.4; 18 studies) and 2.2% (95% CI 1.5–3.3; 52 studies), respectively. Among patients admitted to the intensive care unit, the incidence of KRT was 20.6% (95% CI 15.7–26.7; 38 studies). Meta-regression analyses showed that age, male sex, cardiovascular disease, diabetes mellitus, hypertension and chronic kidney disease were associated with the occurrence of AKI; in itself, AKI was associated with an increased risk of mortality, with a pooled risk ratio of 4.6 (95% CI 3.3–6.5).
Conclusions
AKI and KRT are common events in hospitalized COVID-19 patients, with estimates varying across geographic locations. Additional studies are needed to better understand the underlying mechanisms and optimal treatment of AKI in these patients.
Keywords: acute kidney injury, COVID-19, kidney replacement therapy, meta-analysis, SARS-CoV-2
INTRODUCTION
Although coronavirus disease 2019 (COVID-19) primarily manifests as an acute pulmonary infection, multiple organs can be affected, including the kidney [1, 2]. Preliminary data suggest that acute kidney injury (AKI) and kidney abnormalities such as proteinuria and haematuria may be common among patients with COVID-19 [3–5]. This also became apparent in certain epicentres such as New York that faced a critical shortage of dialysis equipment due to the high incidence and severity of AKI caused by COVID-19 [6]. Furthermore, recent autopsy studies show direct pathological evidence of invasion of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into kidney tissue as well as significant acute tubular injury and endothelial damage [7–9].
The estimated incidence of AKI and the need for kidney replacement therapy (KRT) among hospitalized COVID-19 patients varies between studies, ranging from 0.5% to as high as 40% [10, 11]. Two of the largest studies exemplify this wide variation: Guan et al. [10] reported an AKI incidence of only 0.5% in an analysis of 1099 hospitalized patients across China, while a recent analysis from New York reported an AKI incidence of 26.9% among 5700 hospitalized COVID-19 patients [12].
It is critical to provide an accurate estimation of the incidence of AKI and KRT in COVID-19 patients as well as exploration of differences in these estimations to improve treatment strategies, facilitate healthcare planning and gain pathophysiological insight into this novel disease. We therefore performed a systematic review and meta-analysis of studies reporting incidence, outcomes and risk factors for AKI in hospitalized COVID-19 patients. In addition, we determined the incidence of KRT in this population.
MATERIALS AND METHODS
This systematic review and meta-analysis was reported following the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines [13]. The protocol was not registered in any database of systematic reviews.
Literature search
According to the predetermined protocol, a systematic literature search of 11 databases (PubMed, PMC PubMed Central, WHO COVID-19 database, MEDLINE, Embase, Web of Science, Cochrane Library, Emcare, Academic Search Premier, ScienceDirect and Google Scholar) was performed from 1 December 2019 to 29 May 2020 in cooperation with a trained librarian. Keywords such as ‘AKI’, ‘KRT’ and ‘COVID-19’ were used. As AKI and KRT are often secondary outcome measures and may not always be mentioned in the title or abstract, we additionally included keywords such as ‘endpoint’, ‘outcome’ and ‘clinical characteristics’. The full search string and details of the search strategy are detailed in the Supplementary Material.
Study selection
Studies written in English with original data for at least 10 hospitalized patients with COVID-19 were eligible for inclusion. To be included, studies had to report data on the incidence of AKI or KRT and the majority of patients (>80%) had to be ≥18 years of age. Eligible study designs were cohort studies, case–control studies, case series and clinical trials. Cross-sectional studies, case reports, reviews without original data, preprints and reports for which no full text could be found were excluded. Two reviewers (E.L.F. and R.J.J.) independently screened titles and abstracts of all identified articles. Full-text screening of potential articles was thereafter performed independently by pairs of reviewers (composed from E.L.F., R.J.J., Y.d.J., V.v.d.E., J.M., E.v.d.W. and E.d.R.). Manual cross-referencing of included studies was performed. Discrepancies between the investigators were resolved through mutual discussion or, if necessary, by referring to the senior author (M.v.D.).
Data extraction and quality assessment
The same seven reviewers independently extracted relevant data using a custom-made, predesigned data extraction form. Bibliographic details, eligibility criteria, details about the study population, study design, risk of bias and results were extracted. If necessary, authors of included studies were contacted for additional information. We assessed the risk of bias by focusing on study elements that could potentially bias the estimation of the incidence of AKI or KRT using a set of predefined questions [14]. A detailed explanation for these questions and the assessment of risk of bias is given in the Supplementary Methods.
Statistical analysis
The primary outcome of the meta-analysis was the incidence of AKI and KRT. As we anticipated clinical heterogeneity, incidences are presented stratified by geographic location with similar admission policies (China or South Korea versus the USA or Europe) [15–17]. Weighted incidences are also reported separately for hospitalized patients and patients admitted to the intensive care unit (ICU). Estimates for the ICU were not stratified by geographic location, as we hypothesized that patients admitted to the ICU would have similar disease severity. A generalized linear mixed model (random intercept logistic regression model) was used to pool incidences of AKI and KRT and the maximum likelihood was used to estimate the between-study variance [18]. This method has been recommended over the double arcsine transformation but does not provide individual study weights [19, 20]. A continuity correction was applied for studies with zero events. We used random-effects models since heterogeneity between included studies was expected even after stratification for geographic location and types of patients. Heterogeneity was visually assessed with forest plots and quantified by I2. Potential small study bias was assessed using funnel plots and more formally with Egger’s test when sufficient studies were available (n = 10) [21]. It should be kept in mind that AKI was not the main outcome parameter of most included studies, which makes it unlikely that the incidence of AKI per se is a reason for not publishing the study [22].
For the association between AKI and subsequent mortality, we summarized relative risk estimates [(risk ratios or hazard ratios (HRs)] using random-effects models. Between-study variance was estimated using restricted maximum likelihood and we used a continuity correction for cells with zero counts. When studies did not report these measures but provided relevant numbers, we calculated crude risk ratios and their 95% confidence intervals (CIs). A continuity correction was applied for studies with a zero cell count, adding 0.5 to all cells.
In exploratory analyses, we investigated possible risk factors for AKI using random-effects meta-regression. Incidences were logit transformed, restricted maximum likelihood was used for calculating between-study variance and results were pooled using inverse variance weighting. Risk factors were prespecified and included age, sex, history of cardiovascular disease, diabetes mellitus, chronic kidney disease (CKD) and hypertension.
We performed a sensitivity analysis for the pooled incidence to test the robustness of our result, using a random-effects model with the inverse variance method and logit transformation instead of generalized linear mixed models. Second, we repeated our analyses but excluded studies for which there was suspected overlap of patients. This was predominantly the case for studies from China; reasons for exclusion are listed in the Supplementary data, Table S1. All data were analysed using R version 3.6.2 using the packages meta and metafor (R Foundation, Vienna, Austria) [23, 24].
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
RESULTS
Search results
The literature search yielded 3042 potentially eligible publications after deduplication. Of these, 2442 were excluded after screening titles and abstracts. Full-text assessment was performed of the remaining 600 publications. Through cross-referencing, 24 additional studies were identified, of which 4 were included. A total of 142 studies [3, 4, 10–12, 25–163] involving 49 048 patients were included (Supplementary data, Figure S1). We did not include the study by Richardson et al. [12] since the same data were used in another study specifically focusing on AKI [105].
Study characteristics
A detailed overview of study characteristics is presented in the Supplementary data, Table S2. Most studies (84 studies) reported data on COVID-19 patients from China [3, 4, 10, 11, 28–38, 42–44, 46–53, 55, 56, 58–77, 85, 87, 88, 90, 107–109, 112, 118–123, 132–135, 137–140, 142, 143, 146–148, 150–160], 27 studies were conducted in the USA [11, 12, 25, 27, 40, 57, 78, 81, 84, 94, 98–101, 103, 105, 111, 114, 116, 124, 129–131, 136, 141, 145, 149, 163], 23 studies reported data from Europe [26, 39, 45, 54, 80, 82, 83, 86, 89, 91–93, 95–97, 104, 110, 115, 125, 128, 144, 161, 162], 3 studies from South Korea [106, 113, 117], 1 study from Qatar [79], 1 study from Canada [126] and 3 studies reported data from multiple countries [41, 102, 127]. Sample size ranged from 10 to 7337 patients. Sample size was <300 patients in 117 studies and 7 studies (4 from China, 2 from the USA and 1 from South Korea) contained a sample size >1000 patients [10, 12, 34, 73, 105, 113, 131, 159]. The majority of studies (85 studies) reported data on general hospitalized patients [3, 4, 10, 12, 28, 30–38, 40–42, 44, 46–50, 52, 53, 55, 56, 58–63, 65–67, 69–75, 77, 78, 80, 84, 85, 87, 88, 90–92, 97, 99–103, 105–107, 109, 110, 112, 113, 118, 119, 121, 123, 128, 129, 131, 132, 134–136, 138–140, 143, 147, 148, 153, 155, 157, 159], 32 studies solely included ICU patients [27, 39, 45, 54, 64, 68, 79, 81–83, 86, 94–96, 98, 104, 111, 115, 117, 120, 122, 124, 126, 130, 141, 149, 154, 158, 160–163] and 25 studies were on populations with specific characteristics, including 5 studies on kidney transplant patients and 3 studies on dialysis patients [11, 25, 26, 29, 43, 51, 57, 76, 89, 93, 108, 114, 116, 125, 127, 133, 137, 142, 144–146, 150–152, 156].
The reported mean or median age ranged from 30 to 85 years and 0–100% of participants were male (one study included pregnant women only and one study included only male heart failure patients). The median prevalence of prior cardiovascular disease was 12.7% (range 0–69), 25.8% for hypertension (range 6–100), 17.9% for diabetes (range 0–100) and 4% for CKD (range 0–100). The mean age and proportion of patients with hypertension and diabetes tended to be higher in studies from the USA and Europe compared with those from Asia (Supplementary data, Figure S2).
Laboratory characteristics at hospital admission are reported in the Supplementary data, Table S3. Thirteen studies reported eGFR [3, 26, 63, 64, 66, 92, 102, 105, 107, 112, 137, 138, 140]. Reported mean serum creatinine varied from 0.54 to 11.8 (haemodialysis population) mg/dL. Seven studies reported data about haematuria and proteinuria [3, 4, 35, 47, 94, 105, 140]. The prevalence of proteinuria ranged from 31.2 to 87% and the prevalence of haematuria between 26.7 and 51%.
Incidence of AKI and KRT
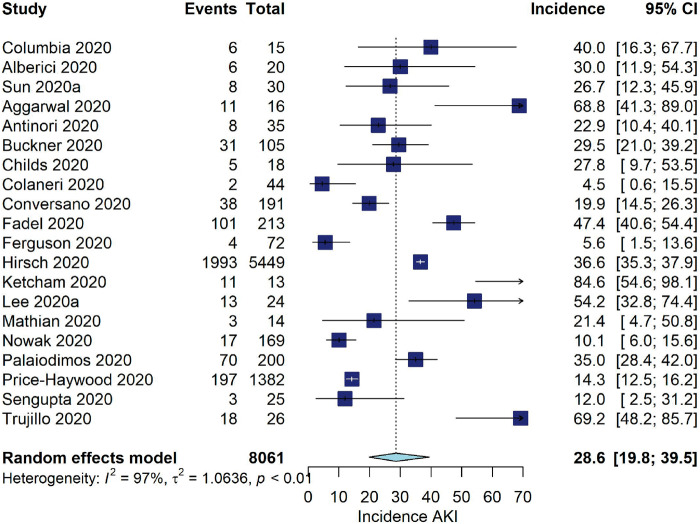
Among studies from Asia, the pooled incidence of AKI was 5.5% (95% CI 4.1–7.4, I2 = 94%, 62 studies, N = 19 378, 884 AKI events; Figure 1). For studies from the USA and Europe, the pooled incidence of AKI was 28.6% (95% CI 19.8–39.5, I2 = 97%, 20 studies, N = 8061, 2545 AKI events).
FIGURE 1:
Incidence of AKI (%) among hospitalized COVID-19 patients stratified by geographic location.
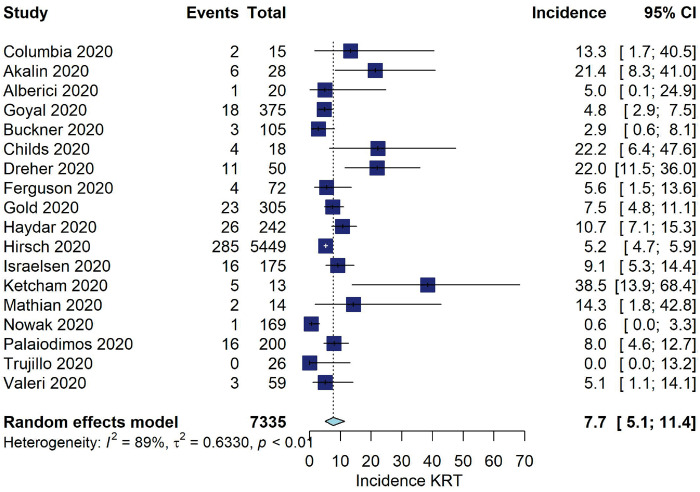
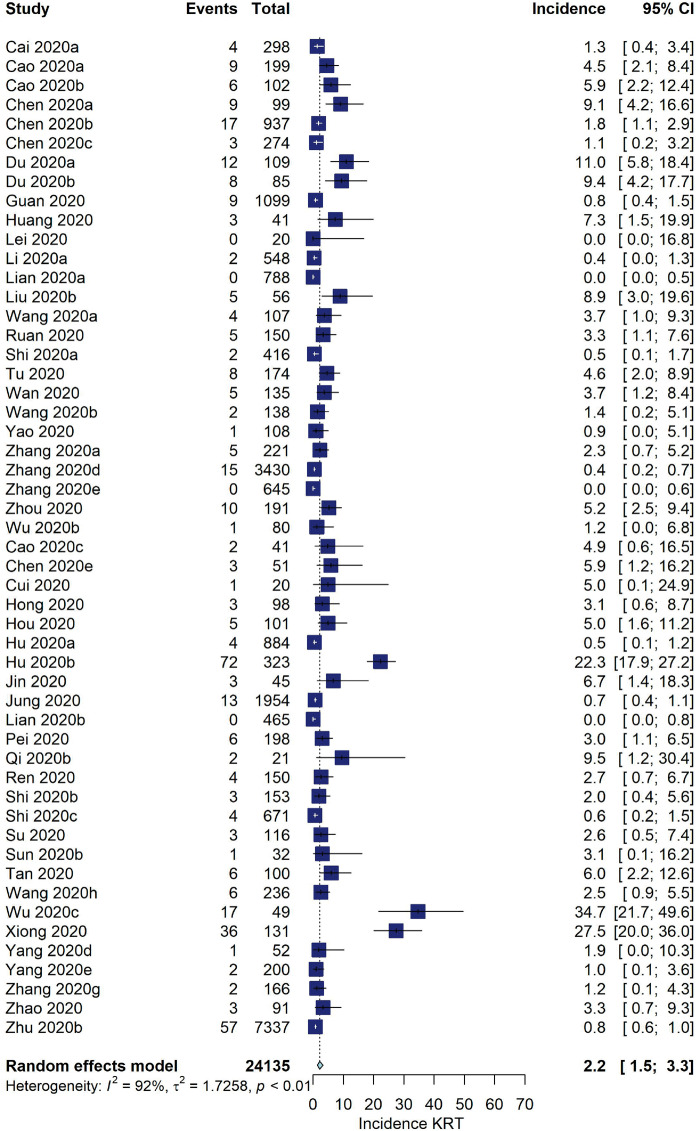
Among studies from China, the pooled incidence of KRT was 2.2% (95% CI 1.5–3.3, I2 = 92%, 52 studies, N = 24 135, 404 KRT events; Figure 2). For the USA and Europe, the pooled incidence was 7.7% (95% CI 5.1–11.4, I2 = 80%, 18 studies, N = 7335, 426 KRT events).
FIGURE 2:
Incidence of KRT (%) among hospitalized COVID-19 patients stratified by geographic location.
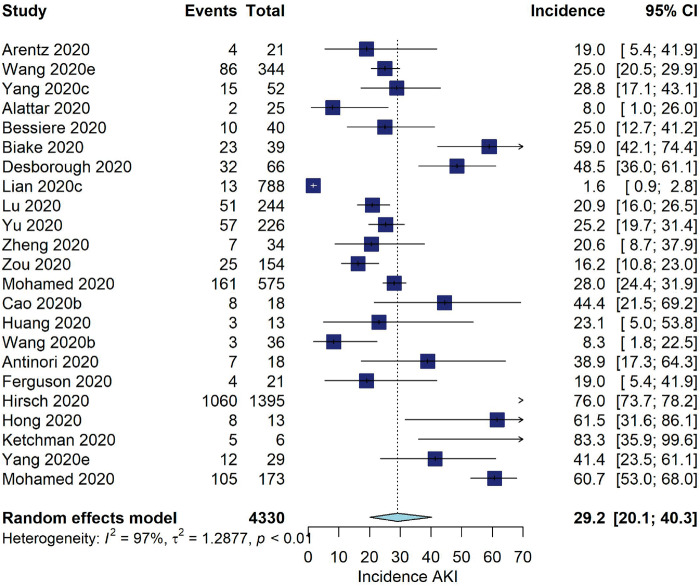
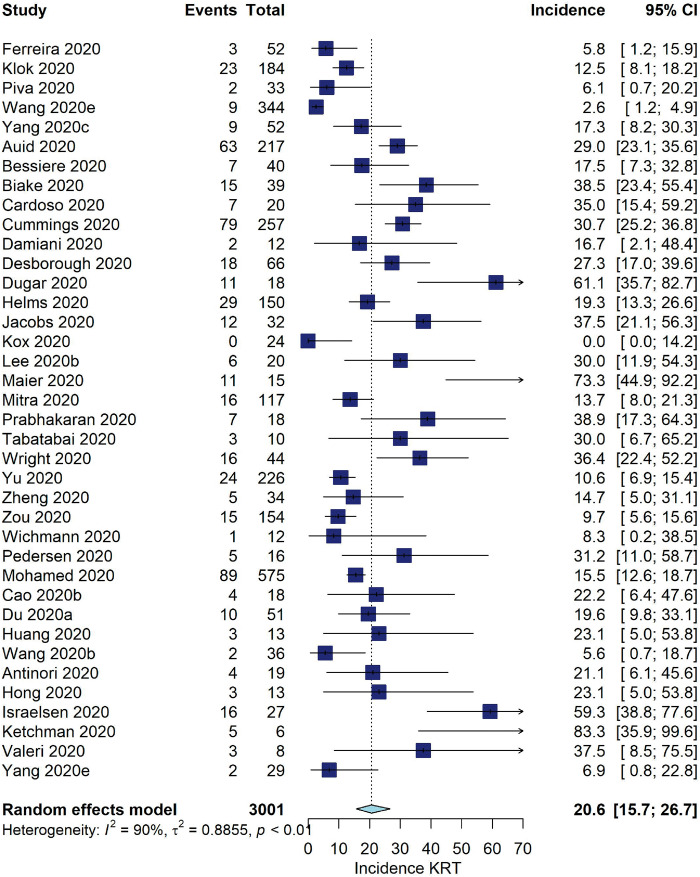
Five studies reported data from kidney transplant patients [11, 25, 26, 76, 144], reporting an incidence of AKI between 30 and 69% and an incidence of KRT between 0 and 21%. Among patients admitted to the ICU, the pooled incidence of AKI was 29.2% (95% CI 20.1–40.3, I2 = 97%, 23 studies, N = 4330, 1701 AKI events; Figure 3). The pooled incidence of KRT was 20.6% (95% CI 15.7–26.7, I2 = 90%, 38 studies, N = 3001, 539 KRT events; Figure 4).
FIGURE 3:
Incidence of AKI (%) among COVID-19 patients admitted to the ICU.
FIGURE 4:
Incidence of KRT (%) among COVID-19 patients admitted to the ICU.
Risk factors for AKI
Meta-regression demonstrated that studies including subjects with higher age, a higher percentage of males, cardiovascular disease, diabetes or hypertension tended to have a greater incidence of AKI (Supplementary data, Figure S3, Table 1). The associated odds ratios (ORs) for AKI incidence were 2.15 (95% CI 1.54–3.00) per mean/median 10-year increase in age, 1.36 (95% CI 1.07–1.73) per 10% increase in male sex proportion, 1.53 (95% CI 1.13–2.08) per 10% increase in cardiovascular disease, 1.48 (95% CI 1.24–1.77) per 10% increase in diabetes, 1.64 (95% CI 1.40–1.93) per 10% increase in CKD and 1.50 (95% CI 1.33–1.69) per 10% increase in hypertension.
Table 1.
ORs for the association between risk factors and AKI incidence in meta-regression analyses
| Study level variables | Studies included, n | OR (95% CI) per 10 years or 10% increase | P-value | I 2 (%) | R 2 (%) |
|---|---|---|---|---|---|
| Age (years) | 83 | 2.15 (1.54–3.00) | <0.001 | 96.16 | 22.71 |
| Male (%) | 83 | 1.36 (1.07–1.73) | 0.01 | 96.48 | 7.04 |
| Cardiovascular disease (%) | 64 | 1.53 (1.13–2.08) | 0.01 | 97.14 | 11.48 |
| Diabetes (%) | 72 | 1.48 (1.24–1.77) | <0.001 | 95.85 | 22.87 |
| CKD (%) | 46 | 1.64 (1.40–1.93) | <0.001 | 92.85 | 49.21 |
| Hypertension (%) | 72 | 1.50 (1.33–1.69) | <0.001 | 94.49 | 43.64 |
ORs are interpreted as the OR associated with a 10-year or 10% increase in the prevalence of the risk factor. I2 is the amount of heterogeneity present. R2 is interpreted as the amount of heterogeneity explained by the risk factor.
Association between AKI and mortality
We identified 21 studies that reported mortality for AKI for which crude risk ratios could be calculated [4, 11, 31, 35, 36, 53, 55, 59, 64, 68, 69, 71, 75, 96, 105, 123, 128, 133, 137, 146, 160]. In addition, two studies reported adjusted effect estimates for the association between AKI and mortality. Cheng et al. [3] found that, compared with no AKI, patients with Stage 1 AKI had an HR of 1.9 (95% CI 0.8–4.8), those with Stage 2 AKI had an HR of 3.5 (95% CI 1.5–8.3) and those with Stage 3 AKI had an HR of 4.7 (95% CI 2.6–8.8), with a pooled HR of AKI versus no AKI of 3.5 (95% CI 2.3–5.5; own calculations). Wang et al. [62] reported outcomes for 339 patients with COVID-19 admitted to hospital in Wuhan. After adjustment for age the HR for AKI was 1.2 (95% CI 0.6–2.4). The pooled risk ratio for mortality associated with AKI was 4.6 (95% CI 3.3–6.5, I2 = 90%, 23 studies) (Supplementary data, Figure S4).
Sensitivity analyses
Inverse variance weighting with a logit transformation gave slightly higher estimates (∼0.5%) of AKI and KRT incidence compared with generalized linear mixed models (Supplementary data, Figure S5). When studies with potential overlap in patients were excluded, a similar incidence of AKI and KRT to the main analysis was observed (Supplementary data, Figure S6).
Risk of bias
The risk of bias assessment is presented in the Supplementary data, Tables S4–S5 and Supplementary data, Figure S7. Most studies (82%) included solely laboratory-confirmed SARS-CoV-2 patients who were diagnosed with positive reverse transcription polymerase chain reaction nasal or oropharyngeal swab. The majority of studies had a loss to follow-up <20% (96%) and sampled consecutive hospitalized patients (75%). Fifty-four percent of studies used the Kidney Disease: Improving Global Outcomes (KDIGO) definition for assessment of AKI. A definitive outcome (death or hospital discharge) in >80% of patients occurred in 39% of studies, as most initial studies on COVID-19 only had a short follow-up. In addition, 15% of studies presented results only for the subsample with a definitive outcome and excluded patients that were still in hospital. Overall, 81% of studies reporting AKI (for which all risk of bias questions could be answered) scored ≥5 points and were judged to be at low risk of bias. Of the studies reporting only KRT (for which five of the seven questions could be answered), 80% scored ≥3.5 points.
Publication bias
Funnel plots and Egger’s test for the incidence of AKI provided some evidence that in smaller studies the incidence estimates were slightly lower (Supplementary data, Figure S8). No firm evidence for small sample bias was present for KRT incidence and the association between AKI and mortality.
DISCUSSION
To our knowledge, this is the most comprehensive systematic review and meta-analysis of studies conducted to date focusing on kidney involvement in patients infected with SARS-CoV-2. We found that the incidence of AKI and KRT was high among hospitalized patients with COVID-19 and varied considerably according to geographic location. In addition, mortality risk was higher in COVID-19 patients with AKI and exploratory analyses suggested that higher age, the presence of diabetes and hypertension may increase the risk of AKI.
An important observation from our systematic review is that the incidence of AKI varies considerably according to geographic location, a finding that was noted earlier [164]. Among studies from Asia, we found a pooled incidence of AKI of 5.5% compared with 28.6% among studies from the USA and Europe. This may be caused by differences in guideline recommendations regarding hospital admission and hence differing patient populations [15–17]. For example, Chinese guidelines by the National Health Commission state that all ‘suspected and confirmed cases should be isolated and treated in designated hospitals with effective isolation and protection conditions’ [16]. Other countries, such as the USA and The Netherlands, only admit symptomatic patients with moderate to severe illness [15, 17]. Indeed, patients admitted for COVID-19 from the USA and Europe tended to be older and have more comorbidities compared with patients from China. We similarly observed differences in KRT incidence according to geographic location among hospitalized patients. The incidence of KRT was ~20.6% among patients admitted to the ICU, which is somewhat higher than the 17.4% incidence reported by the Intensive Care National Audit & Research Centre among 8250 critically ill patients across ICUs in England, Wales and Northern Ireland [165]. In addition, we included five studies on kidney transplant patients [11, 25, 26] that reported AKI incidences between 30 and 69%, indicating that certain groups may be especially vulnerable for adverse kidney outcomes.
To date, studies specifically investigating risk factors and outcomes associated with AKI are limited. We found that patients who developed AKI had a strongly increased risk for mortality, with a pooled risk ratio of 4.6. However, these findings should be regarded as hypothesis-generating since most studies only provided crude numbers and our estimates were not adjusted for confounding. The recent study by Hirsch et al. [164], which studied 5449 individuals admitted to hospitals across New York and was included in our meta-analysis, found that 35% of patients who developed AKI died. A recent preprint shows an adjusted OR for mortality of 9.6 [5]. These findings underline that AKI portends a poor prognosis. In meta-regression analyses, we found that age, male sex and history of cardiovascular disease, diabetes, hypertension and CKD were significantly associated with a higher incidence of AKI. These findings are consistent with studies investigating risk factors for COVID-19-related mortality [35, 75, 166]. Future studies should confirm our observations.
Studies included in our review [3, 35] indicate that up to 60% of patients present with haematuria and proteinuria. A recent study showed that 75% of patients admitted to hospital had kidney abnormalities, with either haematuria (42%), proteinuria (66%) or AKI (5%) [4]. There are several plausible mechanisms for the kidney involvement in COVID-19 [2]. Recent autopsy reports showed evidence of SARS-CoV-2 nucleocapsid proteins in kidney tubules, including significant acute tubular injury, endothelial damage, pigment casts related to rhabdomyolysis and inflammation [7–9]. The limited studies to date suggest acute tubular necrosis as the major cause of AKI, although cytokine storm, a prothrombotic state, organ crosstalk between lungs and kidneys and rhabdomyolysis may also contribute to the development of AKI [2, 4, 163].
To our knowledge, this is the most comprehensive overview of AKI and KRT among hospitalized COVID-19 patients to date. The strengths of this systematic review include the large number of studies included that allowed for precise estimation of incidences and the rich diversity of the populations included. Our results can be used to support decision-making and workforce planning. In addition, the systematic overview of all published studies allowed the discernment of patterns of geographic variability in AKI incidence that were previously not well understood. Our study has some limitations. First, most studies had only a short follow-up and in less than half of studies >80% of patients were discharged or had died. This could potentially underestimate the incidence of AKI since patients in-hospital can still develop AKI. Second, our estimates for risk factors and outcomes associated with AKI were not adjusted for confounders. These findings should therefore be considered as hypothesis-generating. Third, we did not include non-English publications. Fourth, the definition of AKI was unclear in 31% of studies. When contacting the authors of studies that did not report the definition of AKI, the majority acknowledged that KDIGO criteria were used. For the remaining studies, only the most severe cases could have been reported, leading to an underestimate of AKI incidence. We therefore urge researchers to report definitions according to KDIGO criteria. Lastly, it is possible that some studies from China included overlapping patients. However, our sensitivity analysis excluding all studies with suspicion of potential overlap showed very similar estimates to our main analyses.
In conclusion, AKI and KRT are common among individuals hospitalized for COVID-19 and are associated with a poor prognosis. Future studies need to further elucidate the long-term outcomes of COVID-19-associated AKI.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Jan Schoones, biomedical information specialist of the Walaeus Library, Leiden University Medical Center, for his assistance with the systematic literature search.
AUTHORS’ CONTRIBUTIONS
E.L.F., O.M.D., J.I.R. and M.v.D. contributed to the research idea and study design. E.L.F., R.J.J., Y.d.J., V.v.d.E., J.M., E.v.d.W. and E.d.R. contributed to data acquisition. E.L.F., O.M.D., J.I.R. and M.v.D. contributed to data analysis/interpretation. E.L.F. performed statistical analysis. M.v.D. provided supervision and mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest related to this work.
REFERENCES
- 1. Sise ME, Baggett MV, Shepard JO. et al. Case 17-2020: a 68-year-old man with Covid-19 and acute kidney injury. N Engl J Med 2020; 382: 2147–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Batlle D, Soler M J, Sparks M A. et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol 2020; 31: 1380–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng Y, Luo R, Wang K. et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020; 97: 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pei G, Zhang Z, Peng J. et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 2020; 31: 1157–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan L, Chaudhary K, Saha A. et al. Acute kidney injury in hospitalized patients with COVID-19. medRxiv 2020; doi: 10.1101/2020.05.04.20090944 [Google Scholar]
- 6. Mahase E. Covid-19: increasing demand for dialysis sparks fears of supply shortage. BMJ 2020; 369: m1588. [DOI] [PubMed] [Google Scholar]
- 7. Farkash EA, Wilson AM, Jentzen JM.. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol 2020; doi: 10.1681/ASN.2020040432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Su H, Yang M, Wan C. et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020; 98: 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Puelles VG, Lutgehetmann M, Lindenmeyer MT. et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 2020; doi: 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guan WJ, Ni ZY, Hu Y. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Columbia University Kidney Transplant Program. Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol 2020; 31: 1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richardson S, Hirsch JS, Narasimhan M. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323: 2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shamseer L, Moher D, Clarke M. et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015; 349: g7647. [DOI] [PubMed] [Google Scholar]
- 14. Dekkers OM, Vandenbroucke JP, Cevallos M. et al. COSMOS-E: guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med 2019; 16: e1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health. COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/overview/management-of-covid-19/ (20 May 2020, date last accessed) [PubMed]
- 16.National Health Commission and State Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). https://www.chinadaily.com.cn/pdf/2020/1.Clinical.Protocols.for.the.Diagnosis.and.Treatment.of.COVID-19.V7.pdf (20 May 2020, date last accessed)
- 17.Federatie Medisch Specialisten. Guidance for admitting patients with suspected COVID-19 to hospital. https://www.demedischspecialist.nl/sites/default/files/Leidraad%20voor%20opname%20van%20patiënten%20met%20verdenking%20covid%20in%20het%20ziekenhuis.pdf (20 May 2020, date last accessed)
- 18. Stijnen T, Hamza TH, Ozdemir P.. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med 2010; 29: 3046–3067 [DOI] [PubMed] [Google Scholar]
- 19. Schwarzer G, Chemaitelly H, Abu-Raddad LJ. et al. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Syn Meth 2019; 10: 476–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Warton DI, Hui FK.. The arcsine is asinine: the analysis of proportions in ecology. Ecology 2011; 92: 3–10 [DOI] [PubMed] [Google Scholar]
- 21. Egger M, Smith GD, Schneider M. et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dekkers OM. Meta-analysis: key features, potentials and misunderstandings. Res Pract Thromb Haemost 2018; 2: 658–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balduzzi S, Rucker G, Schwarzer G.. How to perform a meta-analysis with R: a practical tutorial. Evid Based Mental Health 2019; 22: 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36: 1–48 [Google Scholar]
- 25. Akalin E, Azzi Y, Bartash R. et al. Covid-19 and kidney transplantation. N Engl J Med 2020; 382: 2475–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alberici F, Delbarba E, Manenti C. et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020; 97: 1083–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arentz M, Yim E, Klaff L. et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020; 323: 1612–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cai Q, Huang D, Ou P. et al. COVID‐19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy 2020; 75: 1742–1752 [DOI] [PubMed] [Google Scholar]
- 29. Cai Q, Huang D, Yu H. et al. COVID-19: abnormal liver function tests. J Hepatol 2020; doi: 10.1016/j.jhep.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao B, Wang Y, Wen D. et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; 382: 1787–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cao J, Hu X, Cheng W. et al. Clinical features and short-term outcomes of 18 patients with corona virus disease 2019 in intensive care unit. Intensive Care Med 2020; 46: 1298–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cao J, Tu WJ, Cheng W. et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen N, Zhou M, Dong X. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen R, Liang W, Jiang M. et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest 2020; 158: 97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen T, Wu D, Chen H. et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 368: m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deng Y, Liu W, Liu K. et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J (Engl) 2020; 133: 1261–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Du RH, Liu LM, Yin W. et al. Hospitalization and critical care of 109 decedents with COVID-19 pneumonia in Wuhan, China. Ann Am Thorac Soc 2020; 17: 839–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Du Y, Tu L, Zhu P. et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med 2020; 201: 1372–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferreira M, Blin T, Collercandy N. et al. Critically ill SARS-CoV-2-infected patients are not stratified as sepsis by the qSOFA. Ann Intensive Care 2020; 10: 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goyal P, Choi JJ, Pinheiro LC. et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med 2020; 382: 2372–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grein J, Ohmagari N, Shin D. et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med 2020; 382: 2327–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guo T, Fan Y, Chen M. et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; 5: 811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. He W, Chen L, Chen L. et al. COVID-19 in persons with haematological cancers. Leukemia 2020; 34: 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Klok F, Kruip M, van der Meer N. et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020; 191: 145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lei Z, Cao H, Jie Y. et al. A cross-sectional comparison of epidemiological and clinical features of patients with coronavirus disease (COVID-19) in Wuhan and outside Wuhan, China. Travel Med Infect Dis 2020; 35: 101664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li X, Xu S, Yu M. et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 2020; 146: 110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lian J, Jin X, Hao S. et al. Analysis of epidemiological and clinical features in older patients with corona virus disease 2019 (COVID-19) out of Wuhan. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu F, Xu A, Zhang Y. et al. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis 2020; 95: 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu K, Chen Y, Lin R, Han K.. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect 2020; 80: e14–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu Y, Chen H, Tang K, Guo Y.. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect 2020; doi: 10.1016/j.jinf.2020.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu Y, Yang Y, Zhang C. et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020; 63: 364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang D, Yin Y, Hu C et al Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care 2020; 24: 188 [DOI] [PMC free article] [PubMed]
- 54. Piva S, Filippini M, Turla F. et al. Clinical presentation and initial management critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Brescia, Italy. J Crit Care 2020; 58: 29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ruan Q, Yang K, Wang W. et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46: 1294–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shi S, Qin M, Shen B. et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020; 5: 802–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sun H, Lee J, Meyer BJ. et al. Characteristics and palliative care needs of COVID-19 patients receiving comfort directed care. J Am Geriatr Soc 2020; 68: 1162–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tang X, Du R, Wang R. et al. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest 2020; 158: 195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tu WJ, Cao J, Yu L. et al. Clinicolaboratory study of 25 fatal cases of COVID-19 in Wuhan. Intensive Care Med2020; 46: 1117–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wan S, Xiang Y, Fang W. et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol 2020; 92: 797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang D, Hu B, Hu C. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang L, He W, Yu X. et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect 2020; 80: 639–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang L, Li X, Chen H. et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol 2020; 51: 343– 346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang Y, Lu X, Chen H. et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med 2020; 201: 1430–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wu C, Chen X, Cai Y. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180: 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang F, Shi S, Zhu J. et al. Analysis of 92 deceased patients with COVID-19. J Med Virol 2020; doi: 10.1002/jmv.25891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yang W, Cao Q, Qin L. et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect 2020; 80: 388–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yang X, Yu Y, Xu J. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yao Q, Wang P, Wang X. et al. Retrospective study of risk factors for severe SARS-Cov-2 infections in hospitalized adult patients. Pol Arch Intern Med 2020; 130: 390–399 [DOI] [PubMed] [Google Scholar]
- 70. Zhang G, Hu C, Luo L. et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol 2020; 127: 104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang J, Wang X, Jia X. et al. Risk factors for disease severity, unimprovement, and mortality of COVID-19 patients in Wuhan, China. Clin Microbiol Infect 2020; 26: 767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang JJ, Cao YY, Dong X. et al. Distinct characteristics of COVID-19 patients with initial rRT-PCR positive and negative results for SARS-CoV-2. Allergy 2020; 75: 1809–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang P, Zhu L, Cai J. et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin ii receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020; 126: 1671–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang X, Cai H, Hu J. et al. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int J Infect Dis 2020; 94: 81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhou F, Yu T, Du R. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhu L, Gong N, Liu B. et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol 2020; 77: 748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wu J, Liu J, Zhao X. et al. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Aggarwal S, Garcia-Telles N, Aggarwal G. et al. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis (Berl) 2020; 7: 91–96 [DOI] [PubMed] [Google Scholar]
- 79. Alattar R, Ibrahim TBH, Shaar SH. et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol 2020; doi: 10.1002/jmv.25964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Antinori S, Cossu MV, Ridolfo AL. et al. Compassionate remdesivir treatment of severe Covid-19 pneumonia in intensive care unit (ICU) and Non-ICU patients: clinical outcome and differences in post-treatment hospitalisation status. Pharmacol Res 2020; 158: 104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Auld SC, Caridi-Scheible M, Blum JM. et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med 2020; doi: 10.1097/CCM.0000000000004457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bessière F, Roccia H, Delinière A. et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol 2020; doi: 10.1001/jamacardio.2020.1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Blake A, Collins D, O'Connor E. et al. Clinical and biochemical characteristics of patients admitted to ICU with SARS-CoV-2. Med Intensiva 2020; doi: 10.1016/j.medin.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Buckner FS, McCulloch DJ, Atluri V. et al. Clinical features and outcomes of 105 hospitalized patients with COVID-19 in Seattle, Washington. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cao Y, Wei J, Zou L. et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol 2020; 146: 137–146.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cardoso FS, Pereira R, Germano N.. Liver injury in critically ill patients with COVID-19: a case series. Crit Care 2020; 24: 190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chen G, Wu D, Guo W. et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130: 2620–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chen L, Zhang B, Ti MN. et al. Clinical course of severe and critically ill patients with coronavirus disease 2019 (COVID-19): a comparative study. J Infect 2020; 81: e82–e84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Childs K, Post FA, Norcross C. et al. Hospitalized patients with COVID-19 and HIV: a case series. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chu Y, Li T, Fang Q, Wang X.. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect 2020; 81: 147–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Colaneri M, Sacchi P, Zuccaro V. et al. Clinical characteristics of coronavirus disease (COVID-19) early findings from a teaching hospital in Pavia, North Italy, 21 to 28 February 2020. Euro Surveill 2020; 25:2000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Conversano A, Melillo F, Napolano A. et al. RAAs inhibitors and outcome in patients with SARS-CoV-2 pneumonia. A case series study. Hypertension 2020; 76: e10–e12 [DOI] [PubMed] [Google Scholar]
- 93. Cui C, Yao Q, Zhang D. et al. Approaching otolaryngology patients during the COVID-19 pandemic. Otolaryngol Head Neck Surg 2020; 163: 121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cummings MJ, Baldwin MR, Abrams D. et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020; 395: 1763–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Damiani E, Carsetti A, Casarotta E. et al. Microvascular alterations in patients with SARS-COV-2 severe pneumonia. Ann Intensive Care 2020; 10:60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Desborough MJR, Doyle AJ, Griffiths A. et al. Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom. Thromb Res 2020; 193: 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dreher M, Kersten A, Bickenbach J. et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch Arztebl Int 2020; 117: 271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dugar S, Duggal A, Bassel A. et al. Spontaneous echo contrast in venous ultrasound of severe COVID-19 patients. Intensive Care Med 2020; 46: 1637–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fadel R, Morrison AR, Vahia A. et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ferguson J, Rosser JI, Quintero O. et al. Characteristics and outcomes of coronavirus disease patients under nonsurge conditions, Northern California, USA, March-April 2020. Emerg Infect Dis 2020; 26: 1679–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gold JAW, Wong KK, Szablewski CM. et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 - Georgia, March 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 545–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Goldman JD, Lye DCB, Hui DS. et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med 2020; doi: 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Haydar A, Lo KB, Goyal A. et al. Palliative care utilization among patients with COVID-19 in an underserved population: a single-center retrospective study. J Pain Symptom Manage 2020; 60: e18–e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Helms J, Tacquard C, Severac F. et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020; 46: 1089–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hirsch JS, Ng JH, Ross DW. et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020; 98: 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hong KS, Lee KH, Chung JH. et al. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: a brief descriptive study. Yonsei Med J 2020; 61: 431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hou W, Zhang W, Jin R. et al. Risk factors for disease progression in hospitalized patients with COVID-19: a retrospective cohort study. Infect Dis (Lond) 2020; 52: 498–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hu J, Zhang X, Zhang X. et al. COVID-19 patients with hypertension have more severity condition, and ACEI/ARB treatment have no influence on the clinical severity and outcome. J Infect 2020; doi: 10.1016/j.jinf.2020.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hu L, Chen S, Fu Y. et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Israelsen SB, Kristiansen KT, Hindsberger B. et al. Characteristics of patients with COVID-19 pneumonia at Hvidovre Hospital, March–April 2020. Dan Med J 2020; 67: A04200232 [PubMed] [Google Scholar]
- 111. Jacobs JP, Stammers AH, St. Louis J. et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in coronavirus disease 2019: experience with 32 patients. ASAIO J 2020; 66: 722–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Jin A, Yan B, Hua W. et al. Clinical characteristics of patients diagnosed with COVID-19 in Beijing. Biosaf Health 2020; 2: 104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jung SY, Choi JC, You SH. et al. Association of renin-angiotensin-aldosterone system inhibitors with COVID-19-related outcomes in Korea: a nationwide population-based cohort study. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ketcham SW, Adie SK, Malliett A. et al. Coronavirus disease-2019 in heart transplant recipients in southeastern Michigan: a case series. J Card Fail 2020; 26: 457–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kox M, Frenzel T, Schouten J. et al. COVID-19 patients exhibit less pronounced immune suppression compared with bacterial septic shock patients. Crit Care 2020; 24:263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lee BT, Perumalswami PV, Im GY. et al. COVID-19 in liver transplant recipients: an initial experience from the U.S. epicenter. Gastroenterology 2020; 10.1053/j.gastro.2020.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lee J, Lee YH, Chang HH. et al. Comparison of short-term mortality between mechanically ventilated patients with COVID-19 and influenza in a setting of sustainable healthcare system. J Infect 2020; 81: e76–e78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Li D, Chen Y, Liu H. et al. Immune dysfunction leads to mortality and organ injury in patients with COVID-19 in China: insights from ERS-COVID-19 study. Signal Transduct Target Ther 2020; 5:62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lian J, Jin X, Hao S. et al. Epidemiological, clinical, and virological characteristics of 465 hospitalized cases of coronavirus disease 2019 (COVID-19) from Zhejiang province in China. Influenza Other Respir Viruses 2020; doi: 10.1111/irv.12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lian JS, Cai H, Hao SR. et al. Comparison of epidemiological and clinical characteristics of COVID-19 patients with and without Wuhan exposure history in Zhejiang Province, China. J Zhejiang Univ Sci B 2020; 21: 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Liao J, Fan S, Chen J. et al. Epidemiological and clinical characteristics of COVID-19 in adolescents and young adults. Innovation 2020; 1: 100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lu X, Chen T, Wang Y. et al. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit Care 2020; 24:241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ma S, Lai X, Chen Z. et al. Clinical characteristics of critically ill patients co-infected with SARS-CoV-2 and the influenza virus in Wuhan, China. Int J Infect Dis 2020; 96: 683–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Maier CL, Truong AD, Auld SC. et al. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet 2020; 395: 1758–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mathian A, Mahevas M, Rohmer J. et al. Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis 2020; 79: 837–839 [DOI] [PubMed] [Google Scholar]
- 126. Mitra AR, Fergusson NA, Lloyd-Smith E. et al. Baseline characteristics and outcomes of patients with COVID-19 admitted to intensive care units in Vancouver, Canada: a case series. CMAJ 2020; 192: E694–E701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Moon AM, Webb GJ, Aloman C. et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol 2020; doi: 10.1016/j.jhep.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nowak B, Szymański P, Pańkowski I. et al. Clinical characteristics and short-term outcomes of coronavirus disease 2019: retrospective, single-center experience of designated hospital in Poland. Pol Arch Intern Med 2020; 130: 407–411 [DOI] [PubMed] [Google Scholar]
- 129. Palaiodimos L, Kokkinidis DG, Li W. et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism 2020; 108: 154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Prabhakaran K, Malcom R, Choi J. et al. Open tracheostomy for covid19 positive patients: a method to minimize aerosolization and reduce risk of exposure. J Trauma Acute Care Surg 2020; doi: 10.1097/TA.0000000000002780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Price-Haywood EG, Burton J, Fort D. et al. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med 2020; 382: 2534–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Qi L, Yang Y, Jiang D. et al. Factors associated with duration of viral shedding in adults with COVID-19 outside of Wuhan, China: a retrospective cohort study. Int J Infect Dis 2020; 96: 531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Qi X, Liu Y, Wang J. et al. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut 2020; doi: 10.1136/gutjnl-2020-321666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Qiu C, Deng Z, Xiao Q. et al. Transmission and clinical characteristics of coronavirus disease 2019 in 104 outside-Wuhan patients, China. J Med Virol 2020; 10.1101/2020.03.04.20026005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ren D, Ren C, Yao RQ. et al. Clinical features and development of sepsis in patients infected with SARS-CoV-2: a retrospective analysis of 150 cases outside Wuhan, China. Intensive Care Med 2020; 46: 1630–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sengupta V, Sengupta S, Lazo A Jr. et al. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev 2020; 29: 747–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Shi Q, Zhang X, Jiang F. et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care 2020; 43: 1382–1391 [DOI] [PubMed] [Google Scholar]
- 138. Shi S, Qin M, Cai Y. et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J 2020; 41: 2070–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Su Y, Tu G, Ju M. et al. Comparison of CRB-65 and quick sepsis-related organ failure assessment for predicting the need for intensive respiratory or vasopressor support in patients with COVID-19. J Infect 2020; doi: 10.1016/j.jinf.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sun DQ, Wang TY, Zheng KI. et al. Subclinical acute kidney injury in COVID-19 patients: a retrospective cohort study. Nephron 2020; 144: 347–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Tabatabai A, Rabin J, Menaker J. et al. Factor VIII and functional protein c activity in critically ill patients with coronavirus disease 2019: a case series. A A Pract 2020; 14: e01236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tan N-D, Qiu Y, Xing X-B. et al. Associations between angiotensin converting enzyme inhibitors and angiotensin ii receptor blocker use, gastrointestinal symptoms, and mortality among patients with COVID-19. Gastroenterology 2020; 10.1053/j.gastro.2020.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Tang W, Cao Z, Han M. et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 2020; 369: m1849 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Trujillo H, Caravaca-Fontán F, Sevillano Á. et al. SARS-CoV-2 infection in hospitalized patients with kidney disease. Kidney Int Rep 2020; doi: 10.1016/j.ekir.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Valeri AM, Robbins-Juarez SY, Stevens JS. et al. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol 2020; 31: 1409–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wang D, Yin Y, Hu C. et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan. Crit Care 2020; 24: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wang Y, Liu S, Liu H. et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol 2020; doi: 10.1016/j.jhep.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wang Y, Zhang D, Du G. et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395: 1569–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wright FL, Vogler TO, Moore EE. et al. Fibrinolysis shutdown correlates to thromboembolic events in severe COVID-19 infection. J Am Coll Surg 2020; 231: 193–203.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wu J, Li J, Zhu G. et al. Clinical features of maintenance hemodialysis patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Clin J Am Soc Nephrol 2020; doi: 10.2215/CJN.04160320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Xiong F, Tang H, Liu L. et al. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol 2020; 31: 1387–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Yang F, Shi S, Zhu J. et al. Clinical characteristics and outcomes of cancer patients with COVID-19. J Med Virol 2020; doi: 10.1002/jmv.25972 [DOI] [PubMed] [Google Scholar]
- 153. Yang L, Liu J, Zhang R. et al. Epidemiological and clinical features of 200 hospitalized patients with corona virus disease 2019 outside Wuhan, China: a descriptive study. J Clin Virol 2020; 129: 104475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Yu Y, Xu D, Fu S. et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care 2020; 24: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zhang L, Feng X, Zhang D. et al. Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation 2020; 142: 114–128 [DOI] [PubMed] [Google Scholar]
- 156. Zhang Y, Li H, Zhang J. et al. The clinical characteristics and outcomes of diabetes mellitus and secondary hyperglycaemia patients with coronavirus disease 2019: a single-center, retrospective, observational study in Wuhan. Diabetes Obes Metab 2020; 22: 1443–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zhao XY, Xu XX, Yin HS, Hu QM. et al. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis 2020; 20: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zheng Y, Sun LJ, Xu M. et al. Clinical characteristics of 34 COVID-19 patients admitted to intensive care unit in Hangzhou, China. J Zhejiang Univ Sci B 2020; 21: 378–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zhu L, She ZG, Cheng X. et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab 2020; 31: 1068–1077.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zou X, Li S, Fang M. et al. Acute physiology and chronic health evaluation ii score as a predictor of hospital mortality in patients of coronavirus disease 2019. Crit Care Med 2020; 48: e657–e665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wichmann D, Sperhake JP, Lütgehetmann M. et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med 2020; doi: 10.7326/M20-2003 [DOI] [PubMed] [Google Scholar]
- 162. Pedersen HP, Hildebrandt T, Poulsen A. et al. Initial experiences from patients with COVID-19 on ventilatory support in Denmark. Dan Med J 2020; 67: A04200232 [PubMed] [Google Scholar]
- 163. Mohamed MM, Lukitsch I, Torres-Ortiz AE. et al. Acute kidney injury associated with coronavirus disease 2019 in Urban New Orleans. Kidney360 2020; doi: 10.34067/KID.0002652020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Hirsch JS, Ng JH, Ross DW. et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020; 98: 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.ICNARC report on COVID-19 in critical care 15 May 2020. https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports (20 May 2020, date last accessed)
- 166. Williamson E, Walker AJ, Bhaskaran KJ. et al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature 2020; 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.