Abstract
A lack of knowledge on metal speciation in the microenvironment surrounding phytoplankton cells (i.e., the phycosphere) represents an impediment to accurately predicting metal bioavailability. Phycosphere pH and O2 concentrations from a diversity of algae species were compiled. For marine algae in the light, the average increases were 0.32 pH units and 0.17 mM O2 in the phycosphere, whereas in the dark the average decreases were 0.10 pH units and 0.03 mM O2, in comparison to bulk seawater. In freshwater algae, the phycosphere pH increased by 1.28 units, whereas O2 increased by 0.38 mM in the light. Equilibrium modeling showed that the pH alteration influenced the chemical species distribution (i.e., free ion, inorganic complexes, and organic complexes) of Al, Cd, Co, Cu, Fe, Hg, Mn, Ni, Pb, Sc, Sm, and Zn in the phycosphere, and the O2 fluctuation increased oxidation rates of Cu(I), Fe(II) and Mn(II) from 2 to 938-fold. The pH/O2-induced changes in phycosphere metal chemistry were larger for freshwater algae than for marine species. Reanalyses of algal metal uptake data in the literature showed that uptake of the trivalent metals (Sc, Sm and Fe), in addition to divalent metals, can be better predicted after considering the phycosphere chemistry.
Introduction
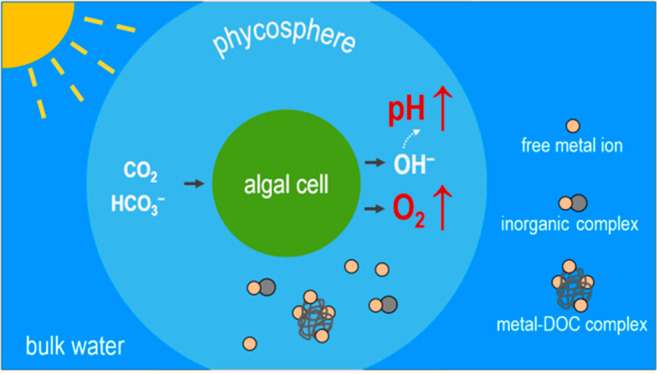
Microscopic plant-like organisms called phytoplankton carry out more than 50% of global CO2 fixation,1 and their growth is influenced by the availability of micronutrient metals such as Fe, Cu, Zn, Co, and Mn in many aquatic ecosystems, especially the oceans.2 Alternatively, these photosynthetic microorganisms also “accidentally” take up nonessential metals, such as Al, Cd, Hg, Pb, and rare earth metals,3 and they are an important link in the food chain propagating contaminants into human food sources.4,5 Numerous studies have suggested that metal bioavailability to phytoplankton is largely controlled by ambient metal speciation, such as the free metal ion concentration, in ambient bulk water.6 The current “algal metal uptake” paradigm assumes that chemical conditions and metal speciation in the microenvironment surrounding phytoplankton cells (i.e., the phycosphere) are similar to those in bulk waters. The phycosphere is the unstirred boundary layer in the immediate vicinity of an algal cell, where the effects of algal exudates and other associated microorganisms are significant,7 but given current technical limitations metal speciation in this tiny space has been little investigated.
Interestingly, several studies consistently show that key chemical conditions such as pH and O2 concentrations in the phycosphere significantly differed from those in bulk waters.8−10 For example, when a marine diatom was exposed to light, the phycosphere pH increased up to 0.9 units and the phycosphere O2 concentration doubled.9 In contrast, both pH and the O2 in the phycosphere decreased in the dark.11 The phycosphere pH and O2 concentrations tended to covary with ambient light intensity.8
These local changes in pH and O2 concentration likely alter metal speciation in the phycosphere and hence influence metal bioavailability to phytoplankton. Our recent experimental studies on the uptake of Cd and Pb by freshwater algae and marine diatoms are well explained by the postulated metal speciation change in the phycosphere.12−15 Similarly, with natural Phaeocystis colonies, it was shown that Mn accumulation in the algae was significantly influenced by the pH and O2 concentrations in the mucilaginous matrix in which the cells were imbedded.16 In contrast, Eichner et al.17 reported that the utilization of Fe minerals by Trichodesmium colonies was little influenced by changes in pH and O2 in and around the colonies. Moreover, two modeling studies showed that speciation of Si18 and Cd19 in the phycosphere differed from that in the bulk media. Here, we hypothesize that metal bioavailability can be better predicted by taking phycosphere metal speciation into account, rather than that in bulk waters. Quantification of metal speciation in this tiny microspace is technically very challenging, and no data are yet available on phycosphere metal speciation.
In the present study, we first compiled data on pH and O2 concentrations in the phycosphere of both marine and freshwater phytoplankton in the literature. Upon the basis of these data, we then calculated pH-induced alterations in the speciation of 12 metals (Al, Cd, Co, Cu, Fe, Hg, Mn, Ni, Pb, Sc, Sm, and Zn) of ecological importance and/or environmental concern. We also evaluated how changes in the local O2 concentrations would affect the oxidation kinetics of redox sensitive metals (i.e., Cu(I), Fe(II), and Mn(II)). We were particularly interested in any differences in metal speciation/oxidation kinetics between the phycosphere and bulk waters, and how other environmental variables, such as luminosity (light vs dark), dissolved organic carbon (DOC), and water types (freshwater vs seawater), influence the local metal speciation. Finally, we examined whether or not the “phycosphere metal speciation” hypothesis can explain published “unexpected” metal uptake data that could not be easily related to metal speciation in bulk solutions.
Data Sources and Methods
Data Sources for pH and O2 Concentrations in the Phycosphere
Only a few studies have determined the pH and O2 concentrations in the micrometre-scale phycosphere,8,9,11,20 largely focusing on algal colonies/aggregates and giant diatoms, given technical limitations. We have compiled the available data for marine and freshwater species in Table S1 of the Supporting Information (SI), and the differences in pH and O2 concentrations between the phycosphere and ambient bulk waters are shown in Figure 1.
Figure 1.
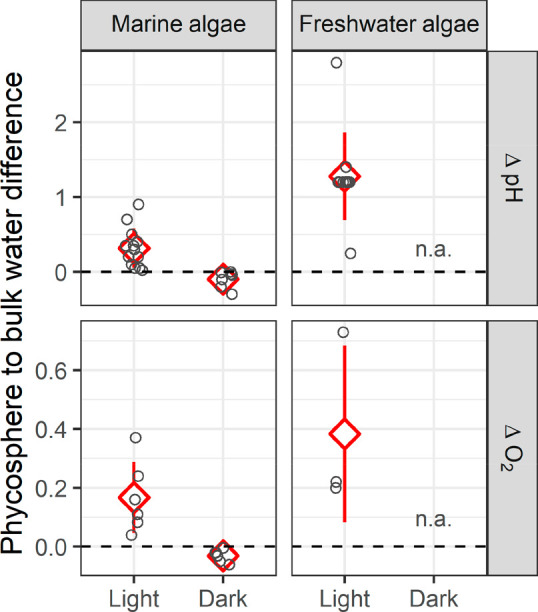
Differences in pH and O2 concentrations (mM) between the phycosphere and bulk water. The data are from in situ measurements in the phycosphere of a diversity of marine and freshwater algae species (see Table S1 of the SI for details). The dashed line indicates no difference between the phycosphere and bulk waters, and n.a. indicates that data are not available.
The data sources include a diversity of taxonomic groups including green algae, diatoms, cyanobacteria, chromista algae, and yellow-green algae from freshwater or marine environments, and the size of these species ranges from a few micrometers (e.g., Thalassiosira weissflogii)20 to hundreds of micrometers (e.g., Coscinodiscus wailesii(9,10) and Odontella sinensis(8)). The studied samples include individual cells8,9,20 and cell aggregates/colonies.11,21 Most of the measurements were carried out with pH and O2 microelectrodes.8,21 Some studies employed fluorescence pH dyes20 or monitored pH sensitive extracellular reactions,22 and one recent work used a novel pH nanoprobe.10 Concentration gradients of both pH and O2 in the phycosphere were reported for cell aggregates/colonies in some studies, but the data are rare for microalgae simply due to technical limitations in spatial resolution. The data we compiled in Figure 1 were those measured within the phycosphere, but the exact distances of the measurement points from the cell membrane are unknown. The light intensity is also shown in Table S1, since it influenced phycosphere pH and O2 concentrations.
Calculation of the Influence of Phycosphere pH on Local Metal Speciation
To calculate metal speciation in the phycosphere, the chemical composition of this microspace, including concentrations of major cations/anions, trace metals, and DOC, is required, in addition to the local pH. In the present study, we assumed that all of these parameters except for pH were the same as those in the bulk waters, given the current lack of data on their changes in the phycosphere. Here, the focus was on the trend and magnitude of any change in phycosphere metal speciation, and we did the speciation modeling in the presence of low to high concentrations of DOC23,24 (i.e., 0.3 to 32 mg/L in freshwater and 0.14 to 16 mg/L in seawater).
The chemical compositions of the two studied water types (i.e., freshwater and seawater, Table S2) were taken from other studies that focused on metal speciation in bulk waters.25−28 The potential influence of other chemical, physical, or biological factors on metal behavior in the phycosphere is discussed in the SI (Note S1).
Calculation of metal speciation in the presence of DOC was carried out with Visual MINTEQ 3.1 software, which includes the Stockholm Humic Model (SHM).29 The performance of the model is similar to that of other software including the Windermere Humic Aqueous Model (WHAM) and the nonideal competitive adsorption (NICA)-Donnan model.30,31 The default database imbedded in the Visual MINTEQ 3.1 software was used. In the Discussion we compare the present modeling results with those obtained with other models (e.g., WHAM and NICA-Donnan model) under comparable chemical conditions.
Note, we calculated the influence of pH on metal speciation with the assumption of thermodynamic equilibrium being reached in the phycosphere, because complexation of most metals by natural ligands including exudates and DOC is relatively fast.32,33 An example of the calculation of Cu complexation kinetic by DOC in the phycosphere is given in the SI (Note S3), and the results suggested that Cu equilibrated with DOC in this thin layer. The phycosphere thickness was simply assumed to be equivalent to one cell radius;19,34 the actual thickness might be altered by factors such as algal shape/size and cell movement relative to water.35
Three groups of metal species (i.e., free metal ions, inorganic metal complexes, and organic metal complexes) were calculated within the observed phycosphere pH range, and speciation in bulk waters was also calculated for comparison. Among these three metal groups, the free metal ion is considered as the best predictor of metal bioavailability,6 although inorganic complexes of some metals such as Fe have been shown to contribute to metal uptake by some phytoplankton species.36,37 Alternatively, metals complexed by DOC generally cannot be directly assimilated by algal cells.38
Calculation of the Influence of Phycosphere O2 Concentrations on Local Oxidation Kinetics
In addition to pH, we were also interested in how changes in the phycosphere O2 concentrations might influence the redox status of redox-sensitive metals, such as Fe, Cu, and Mn. Even in oxic surface waters, there are significant amounts of Fe(II),39 Cu(I),40 and Mn(II),41 partly due to photochemical reactions. The lifetime of the reduced species can be longer than minutes,42 so potential oxidation of Fe(II), Cu(I), and Mn(II) in the O2-enriched phycosphere can be relevant to metal uptake by phytoplankton. Moreover, algal metal uptake is sensitive to the redox status of the metals.43,44
Specifically, we calculated oxidation rates of Cu(I), Fe(II), and Mn(II) in two phycosphere O2 scenarios (i.e., for algae exposed to light and for algae in the dark), and the rates were compared to those calculated for the bulk waters. The calculations are given in the SI (Note S2), and differences in the oxidation rate between the phycosphere and bulk water are shown in Figure S1. The corresponding phycosphere pH condition was taken into account for the calculation of oxidation rates, since metal oxidation is known to be sensitive to pH change.42,45
Case Verification—“Phycosphere Free Metal Ion Concentration” versus “Bulk Free Metal Ion Concentration” in Predicting Metal Uptake
In the literature, a number of “unexpected” results of metal uptake (i.e., significant derivation from the uptake rates predicted on the basis of the free metal ion concentration in the bulk solution) have been reported, even in chemically well-defined media. These puzzling data include both divalent metals (e.g., Cd, Zn, Cu, and Pb)12,14,15,46−49 and trivalent ones (e.g., rare earth metals and Fe),50−55 in the presence of either synthetic or natural ligands. Here, the focus was on cases involving trivalent metals, because we have already shown that the “unexpected” uptake of several divalent metals including Cd and Pb (see Figure S2 for an example) can be well explained by the phycosphere effect.13−15
Studies of short-term (e.g., minutes to hours) metal uptake by algae at low cell densities in a chemically defined medium are suitable for the validation of the “phycosphere effect” hypothesis. Both algal physiology and medium chemistry, except within the phycosphere, undergo relatively little change under such experimental conditions. Experiments under known light intensity are particularly useful for this validation, because chemical changes in the phycosphere, including pH, are closely associated with ambient light intensity. Several uptake tests on rare earth metals satisfied these requirements.50−54
Few data are available to examine the phycosphere effect on Fe bioavailability, because almost all the short-term Fe uptake experiments44 were carried out in the dark to avoid photolysis of Fe–ligand complexes (e.g., Fe-EDTA); the minor changes in the phycosphere that occur in the absence of light are unlikely to alter local Fe speciation. However, the results of Fe uptake experiments under a red light55 and in the presence of photostable ligands and light (e.g., the siderophore desferrioxamine B)56 are particularly useful to examine the phycosphere effect.
To calculate metal speciation in the phycosphere of the algae in the selected studies, we used a series of pH levels (within the range of the compiled data) and exactly the same chemical composition as that of original exposure medium. Fixed pH values were used, although there would probably be a pH gradient (and hence a gradient of free metal ions) in the phycosphere. Our calculated phycosphere free metal ion concentration was thus a rough estimation of the average concentration of gradient-distributed metal ions in this microspace. Finally, the quantitative relationship between our calculated phycosphere free metal ion concentration and the original measured metal uptake was compared with that between the bulk free metal ion concentration and the metal uptake.
Statistical Analyses
The SPSS 16.0 software package (SPSS Inc.) was used to analyze data. The differences in pH and O2 concentrations between the phycosphere and bulk water were compared using a t-test (two-tailed), and the significant level α = 0.05 was used. The figures were processed using the “ggplot2” package of R software (V 3.6.1). A Michaelis–Menten equation was fitted to each set of metal uptake data using the “nls” function in R. The goodness of fit was assessed in terms of the Root Mean Square Logarithmic Error (RMSLE) (Note S4).
Results and Discussion
There were two key objectives in the present work. One was to estimate metal speciation in the phycosphere based on average observed differences between bulk and local pH and O2 concentrations. The other objective was to examine whether or not “unexpected” metal uptake data in the literature can be explained by the “phycosphere effect” hypothesis.
Phycosphere pH and O2 in Marine and Freshwater Phytoplankton
Consistently, in both marine and freshwater algae, the phycosphere pH was higher in the light than in the dark (Figure 1), and it increased with ambient light intensity (Table S1). The phycosphere pH of marine algae in the light was 0.32 ± 0.26 units (mean ± standard deviation, SD) higher on average (N = 13) than that of the bulk seawater (p < 0.01), and the local pH change was in the range of 0.02 to 0.90 units. For the experiments run in the dark, the phycosphere pH was 0.10 ± 0.11 units on average lower than bulk seawater (N = 7, ranging from 0 to 0.30), but the difference was not statistically significant (p = 0.06).
For freshwater algae, the pH differences between the phycosphere and the bulk solution were greater than those for marine algae (Figure 1). The average increase in phycosphere pH for freshwater algae in the light was 1.28 ± 0.59 units (N = 11, ranging from 0.25 to 2.80, p < 0.01); no data were available for the phycosphere pH in the dark. The larger fluctuation in the freshwater environment might be partly due to its relatively poor pH-buffering capacity; seawater has much higher concentrations of dissolved inorganic carbon to resist pH change.
The phycosphere pH enhancement in the light probably results from uptake of inorganic carbon and nitrogen nutrients, as reflected by the following photosynthesis stoichiometry (bulk water pH 7 to 9):
Theoretically, when ambient nutrients (i.e., HCO3–, NO3–, or NH4+) are sufficient, the photosynthetically driven uptake of dissolved inorganic carbon (DIC) is the primary factor influencing the phycosphere pH, with nitrogen sources playing a secondary role. This scenario is consistent with the frequent observation of pH changes in ambient bulk water during algal blooms.57 However, when DIC is more limiting than inorganic nitrogen, we found that nitrogen sources can be the dominant factor in influencing extracellular pH (Figure S3). Similarly, the recent modeling data by Lavoie et al.19 on freshwater chrysophytes suggested that nitrogen uptake influenced phycosphere pH more than DIC uptake. At present, the precise molecular pathways involved in modulating phycosphere pH remain speculative. One recent study8 with marine diatoms reported that the extracellular transformation of bicarbonate by external carbonic anhydrase largely contributed to the phycosphere pH change.
One might expect that an algal colony would induce higher local pH fluctuations than a single cell, since the group of cells have greater photosynthetic/respiratory capacity and thus more easily induce local chemical changes. Interestingly, the pH close to an individual Coscinodiscus wailesii cell can increase by up to 0.90 units at 170 μmol photons m–2 s–1, whereas for the Trichodesmium colonies the pH only increased by 0.30 units at 1000 μmol photons m–2 s–1. The intriguing phenomena might be associated with photosynthetic capacity, algae species, solution buffering conditions or technical resolution.
Similar to the case for phycosphere pH, the local O2 concentration in the light was consistently higher than in the dark, and it covaried with light intensity (Table S1). The phycosphere O2 concentration for marine algae in the light was 0.17 ± 0.12 mM higher on average (N = 6, p = 0.02) than in the bulk seawater, and the local increase was in the range of 0.04 to 0.37 mM. For these same algae in the dark, the phycosphere O2 decreased by 0.03 ± 0.02 mM on average (N = 7, in the range 0 to 0.06 mM, p < 0.01) (Figure 1). In the experiments with freshwater algae in the light, the increase in local O2 was 0.38 ± 0.30 mM (ranging from 0.20 to 0.73 mM) higher than in the bulk waters, and the average increase did not differ significantly from zero, partly due to the limited number of cases (N = 3, p = 0.16). Also, the average O2 increase in the phycosphere of these freshwater algae (i.e., 0.38 mM) did not significantly differ from that in marine algae (i.e., 0.17 mM, p = 0.15). No data were available for freshwater algae in the dark.
Influence of Local pH on Metal Speciation in the Phycosphere
Marine Phytoplankton
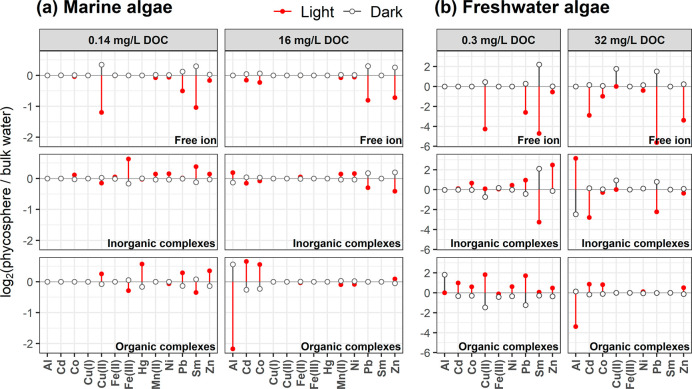
We used the average phycosphere pH changes (i.e., a 0.3-unit increase in the light and a 0.1 decrease in the dark for marine algae) to calculate changes in the relative proportions of the free metal ion, total inorganic metal complexes and total organic metal complexes in the microspace, and we compared phycosphere metal speciation to that in the bulk seawater at pH 8.0. The differences in metal speciation between the phycosphere and the bulk solution were compiled for both low and high concentrations of DOC; the data are shown in Figure 2a and Table S3.
Figure 2.
Fold changes in the calculated concentrations of the free-metal ion, total inorganic metal complexes and total organic metal complexes in the phycosphere of marine algae (a) and freshwater algae (b), in comparison to bulk waters. Data are shown on a binary logarithmic scale. For marine algae, the average phycosphere pH changes (i.e., a 0.3-unit increase in the light, and a 0.1-unit decrease in the dark) were used to calculate the speciation changes, and the pH of bulk seawater was 8.0. For freshwater algae, the average phycosphere pH changes (i.e., a 1.3-unit increase in the light, and an assumed 0.5-unit decrease in the dark) were used to calculate the speciation change, and the pH of bulk freshwater was 7.0.
For marine phytoplankton, the current modeling data showed that the speciation of Al, Cd, Co, Cu(II), Fe(III), Hg, Pb, Sm, and Zn in the phycosphere differed notably from that in the ambient bulk seawater, and the magnitude of the local speciation changes was influenced by the ambient DOC concentration. For instance, when the phycosphere pH increased by 0.3 units, the concentration of free Cu2+ ion was halved (4.4% to 1.9%) in the microenvironment at an ambient DOC concentration of 0.14 mg/L. This decrease was due to pH-induced changes in Cu complexation by DOC (41.2% to 49.1%), carbonates (45.7% to 41.5%) or hydroxides (6.2% to 6.3%). Consistent with our modeling data, Millero et al.58 have suggested that the concentration of free Cu2+ ion in seawater (activity corrected using an ion interaction approach) was lower at a higher pH when inorganic complexes dominated Cu species. Using the NICA-Donnan model, Avendaño et al.59 similarly showed that the concentration of the free Cu2+ ion was lower at a higher seawater pH, even when Cu was predominantly bound to DOC (71–95%). Moreover, using WHAM and Humic Ion Binding Model VII, Stockdale et al.60 predicted a lower concentration of free Cu2+ ion at a higher solution pH as well.
For the other divalent metals (e.g., Pb, Co, Zn, and Cd), notable differences in speciation between the bulk water and the phycosphere were also observed, especially at 16 mg/L DOC (Figure 2a). For instance, when the phycosphere pH increased by 0.3 units, the free ion concentrations of Pb, Co, Zn, and Cd decreased from 0.12% to 0.07%, 59.1% to 50.4%, 8.2% to 5.0%, and 3.0% to 2.7%, respectively (Table S3). For the same metals, the contributions of their complexes with strong acid anions, such as Cl– and SO42–, decreased as well. Consequently, the total concentrations of inorganic Pb, Co, Zn, and Cd complexes decreased as the pH increased, despite relatively small increases in the concentrations of their complexes with OH– and CO32–.
The speciation of the trivalent metals Al, Fe, and Sm in the phycosphere was strongly influenced by the local pH change (Figure 2a, Table S3). When the pH rose by 0.3 units, total organic Al complexes in the phycosphere decreased 4.5-fold at 16 mg/L DOC, total inorganic Fe species increased 1.5-fold at 0.14 mg/L DOC, and the free Sm3+ ion concentration was halved at the low DOC concentration. Similarly, Stockdale et al.60 showed that organic Al complexes decreased 9-fold and inorganic Fe species increased 3.4-fold, when seawater pH increased by 0.35 units at 1 mg/L DOC. Also, Millero et al.58 observed that the free Sm3+ ion concentration was halved when seawater pH increased by 0.3 in the absence of DOC. These consistent trends are expected for trivalent metals, because they form strong complexes with hydroxide (OH–) or carbonate (CO32–) anions and thus undergo dramatic changes in speciation as the phycosphere pH changes. Indeed, we found that >93% of total inorganic complexes of the three metals were hydrolyzed Al (i.e., Al(OH)x(3–x)+), hydrolyzed Fe (i.e., Fe(OH)x(3–x)+), and Sm–carbonate complexes. The free ions of Al, Fe, and Sm were much lower in the higher pH phycosphere than in ambient bulk seawater, although they were a very small fraction of the total metal concentrations (i.e., < 0.01%).
In the dark, speciation changes for all metals in the phycosphere were relatively small, simply due to the relatively small pH change under these conditions. Note, however, that free ion concentrations of Cu and Sm at 0.14 mg/L DOC and of Pb and Zn at 16 mg/L DOC were clearly higher in the phycosphere than those in bulk seawater. More precisely, when the phycosphere pH decreased by 0.1 unit, the free ion concentrations of Cu, Sm, Pb, and Zn increased 1.3-, 1.2-, 1.2-, and 1.2-fold, respectively.
Freshwater Phytoplankton
For freshwater algae, we similarly calculated the pH-induced changes in metal speciation in their phycosphere at an ambient bulk pH of 7 and two DOC concentrations, using a 1.3 pH-unit increase in the light and assuming a 0.5-unit decrease in the dark. The data for freshwater algae are presented in Figure 2b and Table S4.
More marked changes in phycosphere metal speciation were observed in the freshwater system in comparison to seawater. For example, when the phycosphere pH increased by 1.3 units in the light, free ion concentrations of Cd, Co, Pb, and Zn at 32 mg/L DOC decreased 10-, 2-, 49-, and 10-fold, respectively. For the simulations at the low 0.3 mg/L DOC, the free ion concentrations of Cu, Pb, Sm, and Zn decreased 19-, 6-, 26-, and 1.5-fold, respectively. The decreases in the free ions of Cd, Pb, and Sm were not related to any possible increases in the complexes associated with OH– and CO32–; on the contrary, they were mainly due to the enhanced DOC complexation.
Influence of the Local O2 Concentration on Oxidation Rates of Cu(I), Fe(II) and Mn(II) in the Phycosphere
The calculated oxidation rates of Cu(I), Fe(II), and Mn(II) were very sensitive to phycosphere O2 fluctuation; oxidation of the three metals in the phycosphere of algae in the light was faster than that in bulk solutions, whereas it was slower in the dark (Figure S1). Taking marine phytoplankton as an example, when the phycosphere O2 increased by 0.17 mM, the oxidation rates of Fe, Cu, and Mn in the microspace were enhanced 7.6-, 2.0-, and 7.6-fold, respectively; when the local O2 decreased by 0.03 mM, the oxidation rates in the phycosphere decreased by 45%, 18%, and 45%, respectively. However, the changes of metal oxidation rates in the phycosphere of freshwater algae were more dramatic; the local oxidation rates of Fe, Cu and Mn in the light were enhanced 938-, 5.9-, and 938-fold, respectively.
In addition to considering changes in O2 concentrations, we simultaneously considered the effect of a concurrent pH change in the phycosphere on metal oxidation (Note S2), since an increase in pH can accelerate metal oxidation.42 In other words, the light-driven high pH and O2 in the phycosphere might work together to facilitate oxidation of Cu(I), Fe(II) and Mn(II) to the oxidized species Cu(II), Fe(III), and Mn(III, IV, etc.). Consequently, redox-active metal precipitation might be favored near the algal cell surface in the light. However, the local oxidation might be hindered by possible organic exudates from algae.61
The effect of the chemical conditions in the phycosphere on metal oxidation rates may be less significant than the calculated fold changes mentioned above. The degree of oxidation will depend on the residence time of the metals in this microspace before being taken up by algae. In theory, at a moderate metal oxidation rate, when metal uptake rate by algae is very fast, and the amount of the metal in the phycosphere is very low, all metals would quickly pass through the phycosphere and hence few metals would be oxidized. However, taking Cu as an example (Note S3), a simple calculation suggests that the phycosphere residence time of Cu (>230 s) would be sufficiently long to allow Cu(I) to be oxidized, and 24%–98% of the Cu(I) in the phycosphere of the marine diatoms Thalassiosira pseudonana and Thalassiosira oceanica would be oxidized. Note, any oxidized Cu in the phycosphere will be probably reduced to Cu(I) again by membrane-bound Cu(II) reductases to being internalized by algal cells,43 since membrane transporters for Cu(II) only play a role at high concentrations of ambient Cu.48
Predictions of Metal Bioavailability That Consider Phycosphere Metal Speciation
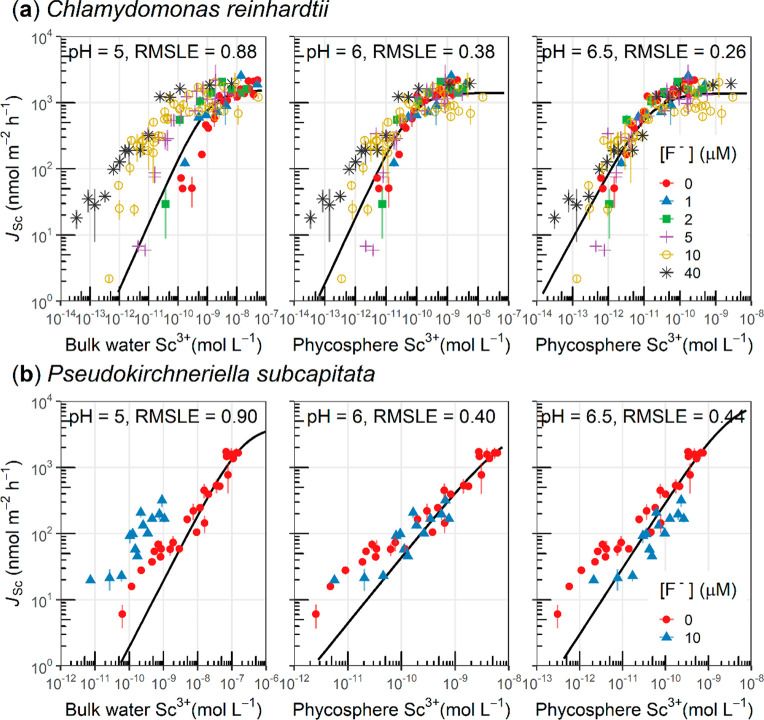
Our second key objective was to test the “phycosphere effect” hypothesis with published metal uptake data. The first case was for Sc uptake in the presence of an inorganic ligand, fluoride (F–), by the freshwater alga Chlamydomonas reinhardtii (Figure 3a), for which the uptake data cannot be completely explained by the concentration of free Sc3+ ion in the bulk exposure solution.51 Here, we assumed that the phycosphere pH was higher than in the bulk medium, because the algae were exposed to 100 μmol photons m–2 s–1 during the uptake experiments. The hypothetical phycosphere pH levels (i.e., 6.0 and 6.5) were used to calculate the concentration of free Sc3+ ion in the phycosphere. At both of the tested phycosphere pH levels, the fittings between the phycosphere free Sc3+ concentration and the Sc uptake rate (RMSLE = 0.26 and 0.38, respectively) were consistently better than that using the bulk free Sc3+ concentration (RMSLE = 0.88). Few techniques are available for direct measurement of local pH near a single cell of 6 μm in diameter,10 but we have shown that under an illumination of 100 μmol photons m–2 s–1 this strain can enhance extracellular pH.15
Figure 3.
Relationships between the measured Sc uptake rate (JSc) and the calculated free Sc3+ concentration in the bulk water and in the phycosphere of freshwater algae under constant light (i.e., 100 μmol photons m–2 s–1). (a) The freshwater alga Chlamydomonas reinhardtii CPCC11 and (b) the freshwater alga Pseudokirchneriella subcapitata. The bulk medium pH51 was 5, and the phycosphere Sc3+ concentration was calculated by assuming a phycosphere pH 6 or 6.5. Both algae species were supplied with nitrate and ammonium, whereas the nitrate reductase-deficient C. reinhardtii CPCC11 cannot utilize nitrate. The solid lines correspond to the fittings by the Michaelis–Menten equation.
The second case was also based on Sc uptake, but in another algal species, Pseudokirchneriella subcapitata exposed to 100 μmol photons m–2 s–1 (Figure 3b). Similarly, we found that the calculated phycosphere free Sc3+ concentration at the assumed phycosphere pH levels (i.e., again based on phycosphere pHs of 6.0 and 6.5) better predicted the Sc uptake rate than did the bulk free Sc3+ concentration. The best fitting was found with the assumption that the phycosphere pH was 1.0 unit higher than the bulk medium; a 1.0 pH unit enhancement is very close to the average pH increase (i.e., 1.3 pH units) of several other freshwater species (Figure 1 and Table S1). Although the Sc uptake was better predicted after considering the phycosphere pH effect, uptake in the lower range of phycosphere Sc3+ concentrations was still systematically underestimated, which may suggest the existence of two uptake sites for Sc in P. subcapitata.
The third case was for the uptake of Sm by the freshwater alga C. reinhardtii (Figure S4).52 The uptake test was carried out under a low light condition (<20 μmol photons m–2 s–1) with algal cells that had been precultured with NH4+ as the nitrogen source. Because the low light intensity could only slightly increase phycosphere pH (Table S1), the supply of NH4+ would dominate the extracellular pH change (i.e., result in an overall lower phycosphere pH than in the bulk water) (Figure S5). Under such a condition, we assumed that the phycosphere pH was lower than that of the bulk medium (pH = 6). Two lower phycosphere pH levels, 5.5 and 5.0, were used to calculate the free Sm3+ concentration near the cell surface, and again the fittings between the phycosphere free Sm3+ concentration and Sm uptake (RMSLE = 0.42 or 0.53) were better than that with the bulk free Sm3+ concentration (RMSLE = 0.61). Note, the uptake data in the presence of an organic ligand (diglycolate) still depart from the fitting curve, and this is likely due to little change in the concentration of free diglycolate ligand in the tested pH range (i.e., pKa = 3.01 and 4.36 < solution pH 5 to 6).
The last example was for another trivalent metal–Fe (Figure 4), and we only found two studies suitable for validation of the “phycosphere effect” hypothesis. Specifically, Kranzler et al.55 observed that the short-term Fe uptake rate under a red light by the nonsiderophore producing cyanobacterium Synechocystis sp. was 3-fold higher than that in the dark in the presence of ferrozine. In this experiment, Fe(III) would have dominated Fe uptake since any uptake of Fe(II) was inhibited by the Fe(II)-complexing ligand ferrozine. Interestingly, our calculation suggests that the concentration of inorganic Fe(III) complexes (dominated by Fe(OH)x(3–x)+) in the phycosphere was 3-fold higher under a red light than in the dark, if it was assumed that the phycosphere pH in the red light was 0.3 units higher than in the dark. Note that Kranzler et al.55 concluded that this potential effect of red light on bulk Fe speciation and Fe uptake physiology was negligible, given the minor effect of red light on abiotic/biotic Fe reduction and the algal Fe uptake determined in the absence of ferrozine. Since neither bulk Fe speciation nor algal Fe physiology contributed to the enhanced Fe uptake in the red light, the phycosphere pH induced change in Fe(III) speciation might be the key factor.
Figure 4.
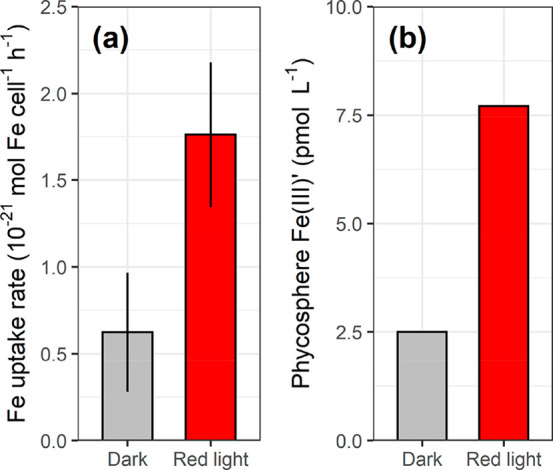
(a) Measured short-term Fe uptake in the presence of Fe(II)-specific ligand, ferrozine, in Fe-limited nonsiderophore producing planktonic cyanobacteria Synechocystis sp. PCC6803 in the dark and red light. (b) The calculated total concentration of inorganic Fe(III)′ complexes (i.e., Fe(OH)x(3–x)+) in the phycosphere in the dark and red light, and it was assumed the phycosphere pH in the red light was 0.3 units higher than in the dark. The cells were cultured with NO3– as the nitrogen source, and the uptake of Fe(II) was inhibited by ferrozine. The original study showed that the effect of red light on Fe reduction was negligible.55
Also, Fe(III) uptake data obtained in the presence of photostable ligands (i.e., siderophore desferrioxamine B)56 can be alternatively explained by the phycosphere pH effect. Briefly, Strzepek et al. observed that the Fe uptake rate by axenic single Phaeocystis cells was 2-fold higher in the light than in the dark. The higher uptake might be due to a higher efficiency in Fe reduction/transport at a higher phycosphere pH in the light. In this connection, it has been shown that the Fe uptake rate in the presence of desferrioxamine B by other marine diatoms was higher at a higher pH.62 Note, in addition to free-living single cells, the Phaeocystis could form colonies, and the colonial cells synthesize extracellular polymeric substances,63 which might alter phycosphere metal behavior (Note S1).
Acknowledgments
We acknowledge the helpful comments received from the reviewers and thank Yeala Shaked (Hebrew University of Jerusalem) for providing Fe uptake data. F.L. was supported by Newton International Fellowship, Royal Society of the United Kingdom (grant number NF170808). A.C. is supported by the Natural Sciences and Engineering Research Council of Canada (discovery grant number RGPIN-2019-04400), and C.F. is supported by the Canada Research Chair program (grant number 950-231107).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.9b07773.
Tables showing the summary of phycosphere pH and O2 concentrations in marine and freshwater phytoplankton, chemical composition of freshwater and seawater used for the calculation of metal speciation, equilibrium modeled relative distributions of free ion, total inorganic complexes, and total organic complexes of metals in the phycosphere; figures showing calculated oxidation rates of Fe(II), Mn(II), and Cu(I) in the phycosphere, algal uptake of Pb or Sm being better predicted by the phycosphere free metal ion concentration, and the bulk pH changes of growth media with nitrate or ammonium-fed algae; discussion of potential influences of several physical/chemical/biological factors, other than pH and O2, on metal behavior in the phycosphere; and details for calculation of oxidation rates for Fe(II), Cu(I), and Mn(II) in the phycosphere, as well as the calculation of phycosphere Cu oxidation and complexation kinetics (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Falkowski P. Ocean Science: The power of plankton. Nature 2012, 483, S17. 10.1038/483S17a. [DOI] [PubMed] [Google Scholar]
- Morel F. M. M.; Price N. M. The biogeochemical cycles of trace metals in the oceans. Science 2003, 300 (5621), 944–947. 10.1126/science.1083545. [DOI] [PubMed] [Google Scholar]
- Campbell P. G. C.; Errécalde O.; Fortin C.; Hiriart-Baer V. P.; Vigneault B. Metal Bioavailability to Phytoplankton-Applicability of the Biotic Ligand Model. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2002, 133, 189–206. 10.1016/S1532-0456(02)00104-7. [DOI] [PubMed] [Google Scholar]
- Luoma S. N.; Rainbow P. S.. Metal Contamination in Aquatic Environments: Science and Lateral Management; Cambridge University Press: Cambridge, UK, 2008; p 573. [Google Scholar]
- Wang W.-X. Interactions of trace metals and different marine food chains. Mar. Ecol.: Prog. Ser. 2002, 243, 295–309. 10.3354/meps243295. [DOI] [Google Scholar]
- Slaveykova V. I.; Wilkinson K. J. Predicting the bioavailability of metals and metal complexes: critical review of the biotic ligand model. Environ. Chem. 2005, 2 (1), 9–24. 10.1071/EN04076. [DOI] [Google Scholar]
- Azam F.; Malfatti F. Microbial structuring of marine ecosystems. Nat. Rev. Microbiol. 2007, 5 (10), 782–791. 10.1038/nrmicro1747. [DOI] [PubMed] [Google Scholar]
- Chrachri A.; Hopkinson B. M.; Flynn K.; Brownlee C.; Wheeler G. L. Dynamic changes in carbonate chemistry in the microenvironment around single marine phytoplankton cells. Nat. Commun. 2018, 9 (1), 74. 10.1038/s41467-017-02426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S. F.; Raven J. A. Photosynthetic oscillation in individual cells of the marine diatom Coscinodiscus wailesii (Bacillariophyceae) revealed by microsensor measurements. Photosynth. Res. 2007, 95 (1), 37–44. 10.1007/s11120-007-9221-x. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Takahashi Y.; Hong S. P.; Liu F.; Bednarska J.; Goff P. S.; Novak P.; Shevchuk A.; Gopal S.; Barozzi I.; et al. High-resolution label-free 3D mapping of extracellular pH of single living cells. Nat. Commun. 2019, 10 (1), 5610. 10.1038/s41467-019-13535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner M. J.; Klawonn I.; Wilson S. T.; Littmann S.; Whitehouse M. J.; Church M. J.; Kuypers M. M. M.; Karl D. M.; Ploug H. Chemical microenvironments and single-cell carbon and nitrogen uptake in field-collected colonies of Trichodesmium under different pCO2. ISME J. 2017, 11, 1305. 10.1038/ismej.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F.; Fortin C.; Campbell P. G. C. Can freshwater phytoplankton access cadmium bound to low-molecular-weight thiols?. Limnol. Oceanogr. 2017, 62 (6), 2604–2615. 10.1002/lno.10593. [DOI] [Google Scholar]
- Liu F.; Fortin C.; Campbell P. G. C. Chemical conditions in the boundary layer surrounding phytoplankton cells modify cadmium bioavailability. Environ. Sci. Technol. 2018, 52 (14), 7988–7995. 10.1021/acs.est.8b01408. [DOI] [PubMed] [Google Scholar]
- Liu F.; Tan Q.-G.; Fortin C.; Campbell P. G. C. Why does cysteine enhance metal uptake by phytoplankton in seawater but not in freshwater?. Environ. Sci. Technol. 2019, 53 (11), 6511–6519. 10.1021/acs.est.9b00571. [DOI] [PubMed] [Google Scholar]
- Sánchez-Marín P.; Liu F.; Chen Z.; Fortin C.; Campbell P. G. C. Microalgal-driven pH changes in the boundary layer lead to apparent increases in Pb internalization by a unicellular alga in the presence of citrate. Limnol. Oceanogr. 2018, 63 (3), 1328–1339. 10.1002/lno.10774. [DOI] [Google Scholar]
- Schoemann V.; Wollast R.; Chou L.; Lancelot C. Effects of photosynthesis on the accumulation of Mn and Fe by Phaeocystis colonies. Limnol. Oceanogr. 2001, 46 (5), 1065–1076. 10.4319/lo.2001.46.5.1065. [DOI] [Google Scholar]
- Eichner M.; Basu S.; Wang S.; Beer D.; Shaked Y. Mineral iron dissolution in Trichodesmium colonies: The role of O2 and pH microenvironments. Limnol. Oceanogr. 2020, 65 (6), 1149. 10.1002/lno.11377. [DOI] [Google Scholar]
- Wischmeyer A. G.; Del Amo Y.; Brzezinski M.; Wolf-Gladrow D. A. Theoretical constraints on the uptake of silicic acid species by marine diatoms. Mar. Chem. 2003, 82 (1), 13–29. 10.1016/S0304-4203(03)00033-1. [DOI] [Google Scholar]
- Lavoie M.; Duval J. F. L.; Raven J. A.; Maps F.; Béjaoui B.; Kieber D. J.; Vincent W. F. Carbonate disequilibrium in the external boundary layer of freshwater chrysophytes: Implications for contaminant uptake. Environ. Sci. Technol. 2018, 52 (16), 9403–9411. 10.1021/acs.est.8b00843. [DOI] [PubMed] [Google Scholar]
- Milligan A. J.; Mioni C. E.; Morel F. M. M. Response of cell surface pH to pCO2 and iron limitation in the marine diatom Thalassiosira weissflogii. Mar. Chem. 2009, 114 (1–2), 31–36. 10.1016/j.marchem.2009.03.003. [DOI] [Google Scholar]
- Ploug H. Cyanobacterial surface blooms formed by Aphanizomenon sp. and Nodularia spumigena in the Baltic Sea: Small-scale fluxes, pH, and oxygen microenvironments. Limnol. Oceanogr. 2008, 53 (3), 914–921. 10.4319/lo.2008.53.3.0914. [DOI] [Google Scholar]
- Richardson L. L.; Stolzenbach K. D. Phytoplankton cell size and the development of microenvironments. FEMS Microbiol. Ecol. 1995, 16 (3), 185–191. 10.1111/j.1574-6941.1995.tb00282.x. [DOI] [Google Scholar]
- Pastor J.; Solin J.; Bridgham S. D.; Updegraff K.; Harth C.; Weishampel P.; Dewey B. Global warming and the export of dissolved organic carbon from boreal peatlands. Oikos 2003, 100 (2), 380–386. 10.1034/j.1600-0706.2003.11774.x. [DOI] [Google Scholar]
- Thurman E. M.Amount of organic carbon in natural waters. In Organic Geochemistry of Natural Waters; Springer: Dordrecht, 1985; Vol. 2. [Google Scholar]
- Tipping E.; Lofts S.; Stockdale A. Metal speciation from stream to open ocean: modelling v. measurement. Environ. Chem. 2016, 13 (3), 464–477. 10.1071/EN15111. [DOI] [Google Scholar]
- Lofts S.; Tipping E. Assessing WHAM/Model VII against field measurements of free metal ion concentrations: model performance and the role of uncertainty in parameters and inputs. Environ. Chem. 2011, 8 (5), 501–516. 10.1071/EN11049. [DOI] [Google Scholar]
- Stockdale A.; Tipping E.; Hamilton-Taylor J.; Lofts S. Trace metals in the open oceans: speciation modelling based on humic-type ligands. Environ. Chem. 2011, 8 (3), 304–319. 10.1071/EN11004. [DOI] [Google Scholar]
- Stockdale A.; Tipping E.; Lofts S. Dissolved trace metal speciation in estuarine and coastal waters: Comparison of WHAM/Model VII predictions with analytical results. Environ. Toxicol. Chem. 2015, 34 (1), 53–63. 10.1002/etc.2789. [DOI] [PubMed] [Google Scholar]
- Gustafsson J. P. Modeling the acid–base properties and metal complexation of humic substances with the Stockholm Humic Model. J. Colloid Interface Sci. 2001, 244 (1), 102–112. 10.1006/jcis.2001.7871. [DOI] [Google Scholar]
- Kinniburgh D. G.; Milne C. J.; Benedetti M. F.; Pinheiro J. P.; Filius J.; Koopal L. K.; Van Riemsdijk W. H. Metal ion binding by humic acid: Application of the NICA-Donnan model. Environ. Sci. Technol. 1996, 30 (5), 1687–1698. 10.1021/es950695h. [DOI] [Google Scholar]
- Tipping E. WHAMC—A chemical equilibrium model and computer code for waters, sediments, and soils incorporating a discrete site/electrostatic model of ion-binding by humic substances. Comput. Geosci. 1994, 20 (6), 973–1023. 10.1016/0098-3004(94)90038-8. [DOI] [Google Scholar]
- Buffle J.; Zhang Z.; Startchev K. Metal flux and dynamic speciation at (Bio)interfaces. part 1: Critical evaluation and compilation of physicochemical parameters for complexes with simple Ligands and Fulvic/Humic substances. Environ. Sci. Technol. 2007, 41 (22), 7609–7620. 10.1021/es070702p. [DOI] [PubMed] [Google Scholar]
- Louis Y.; Garnier C.; Lenoble V.; Mounier S.; Cukrov N.; Omanović D.; Pižeta I. Kinetic and equilibrium studies of copper-dissolved organic matter complexation in water column of the stratified Krka River estuary (Croatia). Mar. Chem. 2009, 114 (3), 110–119. 10.1016/j.marchem.2009.04.006. [DOI] [Google Scholar]
- Wolf-Gladrow D.; Riebesell U. Diffusion and reactions in the vicinity of plankton: A refined model for inorganic carbon transport. Mar. Chem. 1997, 59 (1), 17–34. 10.1016/S0304-4203(97)00069-8. [DOI] [Google Scholar]
- Whitfield M.; Turner D. R.. Critical assessment of the relationship between biological thermodynamic and electrochemical availability. In Chemical Modeling in Aqueous Systems; American Chemical Society: 1979; Vol. 93, pp 657–680. [Google Scholar]
- Hudson R. J. M.; Morel F. M. M. lron transport in marine phytoplankton: Kinetics of cellular and medium coordination reactions. Limnol. Oceanogr. 1990, 35 (5), 1002–1020. 10.4319/lo.1990.35.5.1002. [DOI] [Google Scholar]
- Sunda W. Feedback interactions between trace metal nutrients and phytoplankton in the ocean. Front. Microbiol. 2012, 3, 1–22. 10.3389/fmicb.2012.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneault B.; Campbell P. G. C. Uptake of cadmium by freshwater green algae: effects of pH and aquatic humic substances. J. Phycol. 2005, 41, 55–61. 10.1111/j.1529-8817.2005.04068.x. [DOI] [Google Scholar]
- Hopwood M. J.; Santana-González C.; Gallego-Urrea J.; Sanchez N.; Achterberg E. P.; Ardelan M. V.; Gledhill M.; González-Dávila M.; Hoffmann L.; Leiknes Ø.; Santana-Casiano J. M.; Tsagaraki T. M.; Turner D. Fe(II) stability in seawater. Biogeosci. Discuss. 2018, 1–29. [Google Scholar]
- Moffett J. W.; Zika R. G. Measurement of copper(I) in surface waters of the subtropical Atlantic and Gulf of Mexico. Geochim. Cosmochim. Acta 1988, 52 (7), 1849–1857. 10.1016/0016-7037(88)90008-7. [DOI] [Google Scholar]
- Johnson K. S.; Coale K. H.; Berelson W. M.; Michael Gordon R. On the formation of the manganese maximum in the oxygen minimum. Geochim. Cosmochim. Acta 1996, 60 (8), 1291–1299. 10.1016/0016-7037(96)00005-1. [DOI] [Google Scholar]
- Yuan X.; Pham A. N.; Xing G.; Rose A. L.; Waite T. D. Effects of pH, chloride, and bicarbonate on Cu(I) oxidation kinetics at circumneutral pH. Environ. Sci. Technol. 2012, 46 (3), 1527–1535. 10.1021/es203394k. [DOI] [PubMed] [Google Scholar]
- Kong L.; Price N. M. A reduction-dependent copper uptake pathway in an oceanic diatom. Limnol. Oceanogr. 2020, 65, 601–611. 10.1002/lno.11329. [DOI] [Google Scholar]
- Shaked Y.; Lis H. Disassembling iron availability to phytoplankton. Front. Microbiol. 2012, 3 (123), 1–26. 10.3389/fmicb.2012.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. J. Kinetics of reaction between O2 and Mn(II) species in aqueous solutions. Geochim. Cosmochim. Acta 2005, 69 (1), 35–48. 10.1016/j.gca.2004.06.013. [DOI] [Google Scholar]
- Aristilde L.; Xu Y.; Morel F. M. M. Weak organic ligands enhance zinc uptake in marine phytoplankton. Environ. Sci. Technol. 2012, 46 (10), 5438–5445. 10.1021/es300335u. [DOI] [PubMed] [Google Scholar]
- Semeniuk D. M.; Bundy R. M.; Payne C. D.; Barbeau K. A.; Maldonado M. T. Acquisition of organically complexed copper by marine phytoplankton and bacteria in the northeast subarctic Pacific Ocean. Mar. Chem. 2015, 173, 222–233. 10.1016/j.marchem.2015.01.005. [DOI] [Google Scholar]
- Walsh M. J.; Goodnow S. D.; Vezeau G. E.; Richter L. V.; Ahner B. A. Cysteine enhances bioavailability of copper to marine phytoplankton. Environ. Sci. Technol. 2015, 49 (20), 12145–12152. 10.1021/acs.est.5b02112. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Shi D. L.; Aristilde L.; Morel F. M. M. The effect of pH on the uptake of zinc and cadmium in marine phytoplankton: Possible role of weak complexes. Limnol. Oceanogr. 2012, 57 (1), 293–304. 10.4319/lo.2012.57.1.0293. [DOI] [Google Scholar]
- Crémazy A.; Campbell P. G. C.; Fortin C. The biotic ligand model can successfully predict the uptake of a trivalent ion by a unicellular alga below pH 6.50 but not above: Possible role of hydroxo-species. Environ. Sci. Technol. 2013, 47 (5), 2408–2415. 10.1021/es3038388. [DOI] [PubMed] [Google Scholar]
- Crémazy A.; Campbell P. G. C.; Fortin C. In the presence of fluoride, free Sc3+ is not a good predictor of Sc bioaccumulation by two unicellular algae: Possible role of fluoro-complexes. Environ. Sci. Technol. 2014, 48 (16), 9754–9761. 10.1021/es5016247. [DOI] [PubMed] [Google Scholar]
- Tan Q.-G.; Yang G.; Wilkinson K. J. Biotic ligand model explains the effects of competition but not complexation for Sm biouptake by Chlamydomonas reinhardtii. Chemosphere 2017, 168, 426–434. 10.1016/j.chemosphere.2016.10.051. [DOI] [PubMed] [Google Scholar]
- Yang G.; Wilkinson K. J. Biouptake of a rare earth metal (Nd) by Chlamydomonas reinhardtii – Bioavailability of small organic complexes and role of hardness ions. Environ. Pollut. 2018, 243, 263–269. 10.1016/j.envpol.2018.08.066. [DOI] [PubMed] [Google Scholar]
- Zhao C.-M.; Wilkinson K. J. Biotic ligand model does not predict the bioavailability of rare earth elements in the presence of organic ligands. Environ. Sci. Technol. 2015, 49 (4), 2207–2214. 10.1021/es505443s. [DOI] [PubMed] [Google Scholar]
- Kranzler C.; Lis H.; Shaked Y.; Keren N. The role of reduction in iron uptake processes in a unicellular, planktonic cyanobacterium. Environ. Microbiol. 2011, 13 (11), 2990–2999. 10.1111/j.1462-2920.2011.02572.x. [DOI] [PubMed] [Google Scholar]
- Strzepek R. F.; Maldonado M. T.; Hunter K. A.; Frew R. D.; Boyd P. W. Adaptive strategies by Southern Ocean phytoplankton to lessen iron limitation: Uptake of organically complexed iron and reduced cellular iron requirements. Limnol. Oceanogr. 2011, 56 (6), 1983–2002. 10.4319/lo.2011.56.6.1983. [DOI] [Google Scholar]
- Buapet P.; Gullström M.; Björk M. Photosynthetic activity of seagrasses and macroalgae in temperate shallow waters can alter seawater pH and total inorganic carbon content at the scale of a coastal embayment. Mar. Freshwater Res. 2013, 64 (11), 1040–1048. 10.1071/MF12124. [DOI] [Google Scholar]
- Millero F. J.; Woosley R.; Ditrolio B.; Waters J. Effect of ocean acidification on the speciation of metals in seawater. Oceanography 2009, 22 (4), 72–85. 10.5670/oceanog.2009.98. [DOI] [Google Scholar]
- Avendaño L.; Gledhill M.; Achterberg E. P.; Rérolle V. M. C.; Schlosser C. Influence of ocean acidification on the organic complexation of iron and copper in northwest European shelf seas; a combined observational and model study. Front. Mar. Sci. 2016, 3 (58), 1–19. 10.3389/fmars.2016.00058. [DOI] [Google Scholar]
- Stockdale A.; Tipping E.; Lofts S.; Mortimer R. J. G. Effect of ocean acidification on organic and inorganic speciation of trace metals. Environ. Sci. Technol. 2016, 50 (4), 1906–1913. 10.1021/acs.est.5b05624. [DOI] [PubMed] [Google Scholar]
- González A. G.; Santana-Casiano J. M.; González-Dávila M.; Pérez-Almeida N.; Suárez de Tangil M. Effect of Dunaliella tertiolecta organic exudates on the Fe(II) oxidation kinetics in seawater. Environ. Sci. Technol. 2014, 48 (14), 7933–7941. 10.1021/es5013092. [DOI] [PubMed] [Google Scholar]
- Shi D.; Xu Y.; Hopkinson B. M.; Morel F. M. M. Effect of ocean acidification on iron availability to marine phytoplankton. Science 2010, 327 (5966), 676–679. 10.1126/science.1183517. [DOI] [PubMed] [Google Scholar]
- Janse I.; van Rijssel M.; Gottschal J. C.; Lancelot C.; Gieskes W. W. C. Carbohydrates in the North Sea during spring blooms of Phaeocystis: a specific fingerprint. Aquat. Microb. Ecol. 1996, 10 (1), 97–103. 10.3354/ame010097. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.