Abstract
Taxonomic complexities, like environmental plasticity and homoplasy, make precise identification challenging in Calamus, the genus of spiny climbing palms of the subfamily Calamoideae (Arecaceae). In the present study, the species discriminatory power of twelve potential DNA barcode regions (rbcL, matK, psbA-trnH, rpoC, rpoB, psbK-psbI, atpF-atpH, psbZ-trnfM, ITS1, ITS2, PRK, and RPB2) were evaluated in 21 species of Calamus from the Western Ghats region of India, using distance, tree, and similarity based statistical methods. Except for the low copy nuclear region, RPB2, none of the tested plastid loci or nuclear loci ITS, either singly or in combinations, could discriminate all the species of Calamus due to low substitution rate of plastid regions and multiple copies of ITS respectively. The RPB2 locus showed highest species resolution with 96% accuracy in similarity based analysis, indicating its potential and efficiency as a barcode locus for the genus. The putative “Calamus gamblei complex” based on overlapping morphology was successfully resolved as six distinct, though closely related, species. The analysis also indicates that C. delessertianus is a morphological variant of C. dransfieldii. In spite of being a low copy nuclear gene region, RPB2 provided an efficient barcode to delineate Calamus species and has the potential to further extend its use as a prospective barcode to other Palm genera.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00864-5) contains supplementary material, which is available to authorized users.
Keywords: Calamus, Species complex, Rattans, DNA barcoding, Low copy nuclear region, RPB2
Introduction
DNA barcoding, a species identification method using standardized short sequences of DNA, called DNA barcode (Hebert et al. 2003) is widely used in various fields of research, such as conservation biology (Stoeckle 2003), timber forensics and tracking (Asif and Cannon 2005; Fuji 2007), identification of adulterants (Baker et al. 2012; Dev et al. 2014), phylogeography (Kuppu et al. 2019), and in biosystematics (Gao et al. 2010; Pang et al. 2010) due to its high species discriminatory ability. Mitochondrial DNA barcodes for animals using cox1/CO1 (Hebert et al. 2004) turned out to be successful having the necessary universality and variability, unlike in plants where very low substitution rates impede its utility as a barcode (Wolfe et al. 1987). Therefore, a number of DNA regions, mostly from the plastid genome, have been tested for their discriminatory power in plants (Kress et al. 2005; Newmaster et al. 2006; Kress and Erickson 2007; Hollingsworth et al. 2009). In 2009, the Plant Working Group of the Consortium for Barcode of Life (CBOL) recommended the use of the combination of rbcL and matK as the core barcode and psbA-trnH, rpoB, rpoC, psbK-psbI, and atpF-atpH as the supplementary barcode regions, based on reproducibility and universality of these regions (CBOL 2009). Subsequently, the nuclear Internal Transcribed Spacer (ITS) region has also been reported as the most divergent barcode region in plants (Chase et al. 2005; Kress et al. 2005; China Plant BOL Group et al. 2011). However, universal barcode region in plants is still to be a reality and therefore major studies have focussed on finding appropriate barcode loci, good enough to discriminate species of their specific group of interest (Valentini et al. 2009; Ballardini et al. 2013; Techen et al. 2014; Joly et al. 2014; Paracchini et al. 2017). The major problems with plant species, while locating a barcode region are the slow evolutionary rates, widespread hybridization, introgression, or incomplete lineage sorting (Hollingsworth et al. 2011), which ultimately hinders species discrimination. Therefore, identification of a perfect barcode region, specific to the study group, is essential to address the taxonomical complexities.
Rattans are spiny climbing palms and are heavily exploited for commercial purposes in handicrafts, furniture industries, basketry, etc. (Dransfield and Manokaran 1994). Calamus, the largest rattan genus, comes under the subfamily Calamoideae of the family Arecaceae (Palmae). The genus has a worldwide distribution from the Indian subcontinent to the southern part of China, east towards Malaysia, Indonesia, Fiji, and tropical and subtropical regions of eastern Australia and Africa (Uhl and Dransfield 1987; Dransfield 1992). In India, Calamus is represented by 46 species, distributed in three phytogeographical regions, viz. Peninsular India, Eastern Himalayas, and Andaman and Nicobar Islands (Renuka 2001). Identification of rattan species is mainly based on the morphology of leaf, stem, fruit, and inflorescence which is extremely challenging due to the unavailability of flowers round the year in this genus (Uhl et al. 1995). Several taxonomic complexities like homoplasy, environmental plasticity, and species complexities that exist within this group further complicate species identification using morphological characters (Boer 1968; Sreekumar and Henderson 2014; Atria et al. 2017). Moreover, the species' in the “Calamus gamblei sp. complex”, comprising of C. gamblei, C. lacciferus, C. neelagiricus, C. prasinus, C. dransfieldii, C. shendurunii, and C. renukae, are quite difficult to discriminate owing to their overlapping morphological characters (Sreekumar and Henderson 2014). Species delimitation in this complex is based on morphological characters, like size, colour and spininess of sheaths and pinnae, the shape and colour of the fruits, but these characters vary within as well as amongst species (Renuka 1986; Lakshmana and Renuka 1990; Renuka et al. 1997; Jacob et al. 2008). This species complex also shares several morphological characters, such as the presence of long, parallel, apical pinnae joined at their bases, pinnae with long spines on the veins adaxially, recurved rachillae with pistillate dyads arranged in alternate, non-opposite rows so that one side of the rachillae is without flowers, pistillate dyads, borne on a distinct pedicel and yellow fruits with raised scale. Since the taxonomic positioning of these species is still a controversy, the present study also attempted to unravel the complexities in this group using the DNA barcode tool.
DNA barcoding has the potential to accurately identify and classify species’ (Hebert et al. 2004; Kress et al. 2005; Kress and Erickson 2007; Hollingsworth et al. 2009; Jeanson et al. 2011). Since chloroplast regions have slow rates of evolution in palms (Wilson et al. 1990; Gaut et al. 1992), the use of the CBOL recommended chloroplast barcode regions is inadequate for accurate species identification in Arecaceae. However, nuclear ribosomal ITS2 has proven its utility for species identification in some species of palms (Jeanson et al. 2011), yet the presence of multiple copies of ITS regions have led to erroneous species identification in Calamoideae (Baker et al. 2000). psbA-trnH was further recommended by Yang et al. (2012) as a barcode for 15 Chinese Calamus species but the region showed only 58 per cent of species discrimination ability, indicating its inefficiency. Later, the chloroplast region, psbZ-trnfM was recommended as the potential DNA barcode region for species identification within the genus Phoenix (Arecaceae) (Ballardini et al. 2013). The species delimitation studies on Daemonorops, using the nuclear regions, reported the inability of a single gene or marker alone to delineate the species with precision (Umapathy et al. 2015).
With this background, the present study is aimed to identify an ideal DNA barcode region to address the prevailing taxonomic complexities and to delineate species in the genus Calamus. The study proposed to analyse the species discrimination ability of eight plastid barcode gene regions viz., rbcL, matK, psbA-trnH, rpoC, rpoB, psbK-psbI, atpF-atpH, psbZ-trnfM and four nuclear regions ITS1, ITS2, PRK and RPB2.
Materials and methods
Taxon sampling
Multiple accessions of 21 species of Calamus (Calamus brandisii, C. hookerianus, C. delessertianus, C. dransfieldii, C. shendurunii, C. neelagiricus, C. viminalis, C. rotang, C. metzianus, C. wightii, C. pseudotenuis, C. nagbettai, C. karnatakensis, C. lakshmanae, C. prasinus, C. stoloniferus, C. travancoricus, C. vattayila, C. thwaitesii, C. lacciferus and C. gamblei) (Renuka et al. 2010) were collected from their natural distribution zones in the Western Ghats of India. Taking account of the effect of the geographical scale of sampling and intraspecific variability on DNA barcoding, multiple accessions were included. List of samples collected for the study and their location are provided as supplementary table S1. Voucher specimens are deposited in the Kerala Forest Research Institute Herbarium (KFRI) (www.kfriherbarium.org). In order to test the efficiency of the recommended barcode locus in other palm genera, sequences of palm species were downloaded from GenBank (http://www.ncbi.nlm.nih.gov/genbank/). For this, multiple accessions of 27 species (13 genera) belonging to 5 subfamilies of Arecaceae were downloaded (Supplementary table S2).
DNA extraction, PCR amplification and Sequencing
Total genomic DNA was extracted from fresh and silica gel dried leaf materials using a modified Cetyl trimethyl ammonium bromide (CTAB) method (Doyle and Doyle 1990) as well as DNeasy Plant Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. Eight barcode loci of the plastid genome (four coding regions viz., matK, rbcL, rpoB, rpoC and four intergenic spacers viz., psbA-trnH, psbK-psbI, atpF-atpH, psbZ-trnfM), two high copy nuclear regions (ITS1 and ITS2) and two low copy nuclear gene regions (PRK and RPB2) were evaluated.
Polymerase chain reaction (PCR) amplification was performed using 50 μL reaction containing 2X Taq buffer with 1.5 mM MgCl2, 200 μM dNTPs, 10 pm of each primer, and 2U Taq DNA polymerase (Genei, Bangalore) and 50–100 ng template DNA. The PCR reaction conditions included an initial denaturation at 94 °C for 5 min, 30 cycles of denaturation at 94 °C for 30 s, primer annealing at specified temperatures (Table 1) and an extension at 72 °C for 1 min followed by a final extension at 72 °C for 10 min. The primer information and optimal annealing temperatures are provided in Table 1. The amplified products were checked on a 2% agarose gel and purified using a Nucleospin Gel and PCR Clean-up kit (Macherey–Nagel, USA) before sequencing. Sanger dideoxy sequencing was performed in both forward and reverse directions (Chromous, Bangalore).
Table 1.
Barcoding primers used and annealing temperature–time
| Barcode region | Primer | Primer sequence 5′–3′ | References | Annealing temperature |
|---|---|---|---|---|
| rbcL |
1F 724R |
ATGTCACCACAAACAGAAAC TCGCATGTACCTGCAGTAGC |
Kress et al. (2005) | 60 °C–40 s |
| matK |
472F 1248R |
CCCRTYCATCTGGAAATCTTGGTT GCTRTRATAATGAGAAAGATTTCTGC |
Yu et al. (2011) | 60 °C–40 s |
| trnH- psbA |
trnH psbA |
GTWATGCAYGAACGTAATGCTC CGCGCATGGTGGATTCACAATCC |
Kress et al. (2005) | 59 °C–50 s |
| rpoC |
rpoC rpoC |
GGCAAAGAGGGAAGATTTCG CCATAAGCATATCTTGAGTTGG |
Sass et al. (2007) | 58 °C–50 s |
| rpoB |
rpoB F rpoB R |
AAGTGCATTGTTGGAACTGG GATCCCAGCATCACAATTCC |
Ford et al. (2009) | 58 °C–40 s |
| psbK-psbI |
psbK psbI |
TTAGCCTTTGTTTGGCAAG AGAGTTTGAGAGTAAGCAT |
Lee et al. (2007) | 55 °C–50 s |
| atpF-atpH |
AtpF atpH |
ACTCGCACACACTCCCTTTCC GCTTTTATGGAAGCTTTAACCAAT |
Hollingsworth et al. (2009) | 60 °C–50 s |
| psbZ-trnfM |
psbZ trnfM |
GGTACMTCATTATGGATTGG GCGGAGTAGAGCAGTTTGGT |
Scarcelli et al. (2011) | 50–65 °C–1 min |
| ITS1 |
ITSF ITSR |
TCCGTAGGTGAACCTGCGG GCTGCGTTCATCGATGC |
White et al. (1990) | 60 °C–40 s |
| ITS2 |
ITS3F IT4R |
GCATCGATGAAGAACGCAGC TCCTCCGCTTATTGATATGC |
White et al. (1990) | 60 °C–40 s |
| PRK |
PRKF PRKR |
GTGATATGGAAGAACGTGG ATTCCAGGGTATGAGCAGC |
Lewis and Doyle (2002) | 50–60 °C–1 min |
| RPB2 |
RPB2F RPB2R |
CAACTTATTGAGTGCATCATGG CCACGCATCTGATATCCAC |
Roncal et al. (2005) | 58 °C–40 s |
Sequence alignment
The raw sequences were edited manually using BioEdit (Hall 1999) and aligned using the default alignment parameters in CLUSTAL X (Jeanmougin et al. 1998). The refined sequences were confirmed via BLASTn (http://blast.ncbi.nlm.nih.gov/Blast.cgi) against the nucleotide database and deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/).
Data analysis
Distance-based analysis
For pairwise genetic distance (PWG-distance) method, the genetic distance was determined using MEGA v.6.0 adopting Kimura 2-Parameter (K2P) model (Kimura 1980) with complete deletion option (Tamura et al. 2012). The average interspecific distance, theta prime, and minimum interspecific distance were used to represent interspecific divergence. The average intraspecific distance, theta, and maximum intraspecific distance (coalescent depth) were calculated to evaluate the intraspecific variation, adopting K2P model (Meyer and Paulay 2005; Chen et al. 2010) for all the barcode regions. DNA barcoding gaps were calculated as the differences between average intraspecific and interspecific distances (Erickson et al. 2008). The significance of barcoding gap was assessed using Wilcoxon matched pairs signed rank test in SPSS v 17.0 (SPSS Inc 2007) for the selected barcoding loci, which showed higher barcoding gap (RPB2, matK + psbA-trnH, psbA-trnH and matK).
Similarity based analysis
Two similarity based methods, Best Match (BM) and Best Close Match (BCM) were employed for the species identification success in Calamus species using TaxonDNA/Species Identifier 1.7.7 based on uncorrected p-distances (Meier et al. 2006) in order to define how similar a barcode match. The criteria for successful, ambiguous and incorrect identifications as well as no match were set according to previous studies (Meier et al. 2006).
Tree based analysis
Phylogenetic trees were constructed for individual data matrix and concatenated sequence data adopting Bayesian Inference (BI) using Mr Bayes 3.2.2 (Ronquist and Huelsenbeck 2003) (http://www.phylo.org/). jModelTest 2.1.4 (Posada and Buckley 2004) was used to select the best fit model of nucleotide substitution under Akaike Information Criterion (AIC) prior to Bayesian analysis. Bayesian Markov Chain Monte Carlo (MCMC) algorithm was run 5,00,00,000 generations with one cold chain and three heated chains, starting from random trees and sampling trees every 100 generations, until getting standard deviation value below 0.01. The 50% majority-rule consensus trees were constructed after the first 25% of sampled trees were removed during the burn in period. The posterior probability (PP) of each topological bipartition was calculated across the remaining trees in Bayesian Inference. A neighbor-joining tree (NJ) (Saitou and Nei 1987) was also constructed for each dataset of the studied barcode regions using MEGA v.6.0 adopting the K2P model (Kimura 1980; Tamura et al. 2013). A phylogenetic tree was also constructed with sequences from Calamus species’ of other geographical regions and related genera of palms to confirm the efficiency of the recommended barcoding locus, using Mr Bayes 3.2.2.
Results
Amplification and sequence analysis
Basic sequence information of the selected DNA barcode regions, viz., rbcL, matK, psbA-trnH, rpoC, rpoB, psbK-psbI, atpF-atpH and RPB2 and their combinations were compared (Table 2). ITS1 and ITS2 gave multiple amplicons and failed to produce good quality chromatogram peaks. psbZ-trnfM and PRK also failed to yield good amplification (Supplementary Fig. 1). Other barcoding regions exhibited good PCR amplification and sequencing rates. Combinations of loci, which showed higher barcoding gap were also taken for the analysis (RPB2 + psbA- trnH and matK + psbA-trnH). Among the nine regions analysed, RPB2 (11.6%) showed the highest per cent of parsimony informative sites as a single barcode region followed by RPB2 + psbA-trnH (9.09%), psbA-trnH (6.74%), matK + psbA-trnH (6.2%) and matK (4.51%). Parsimony informative sites in rbcL, rpoB, rpoC, psbK-psbI and atpF-atpH were negligible as compared to other barcode regions. RPB2 had the greatest percentage of nucleotide variation (12.3%) based on number of variable sites and showed species specific differences (Supplementary Fig. 2). Indels were more prevalent in psbA-trnH and aligned sequence lengths ranged from 500 to 800 bp. psbA-trnH sequences in C. hookerianus and C. pseudotenuis showed a deletion of 300 bp (Supplementary Fig. 3). All rpoC sequences were similar without much variation, indicating the conserved nature of this region. A same transversion was observed in the RPB2 sequences of species’ in the C. gamblei species complex (Supplementary Fig. 4). All the generated nucleotide sequences are deposited in NCBI (supplementary Table S1).
Table 2.
Sequence characteristics of eight DNA regions and their combinations
| Comparison | rbcL | matK | psbA-trnH | rpoB | rpoC | psbK-psbI | atpF-atpH | matK + psbA-trnH | RPB2 + psbA- trnH | RPB2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sequence length (bp) | 674 | 709 | 786 | 463 | 456 | 271 | 637 | 1495 | 1518 | 732 |
| Conserved regions | 666 | 659 | 675 | 456 | 453 | 271 | 620 | 1330 | 1319 | 642 |
| Variable regions | 7 | 50 | 87 | 7 | 3 | 4 | 16 | 142 | 175 | 90 |
| Parsimony informative sites | 4 | 32 | 53 | 4 | 2 | 4 | 11 | 93 | 138 | 85 |
| Singleton sites | 3 | 18 | 33 | 3 | 1 | 0 | 5 | 47 | 36 | 5 |
Distance-based analysis
Genetic divergence was estimated using six parameters in MEGA 6.0 (Table 3). Maximum average interspecific distance was observed in RPB2 (0.24), followed by RPB2 + psbA-trnH (0.238), psbA-trnH (0.23), matK + psbA-trnH (0.19), matK (0.17), atpF-atpH (0.004), rpoB (0.036), psbK-psbI (0.03), rbcL (0.028), and rpoC (0.02) regions. The combination of matK + psbA-trnH exhibited the highest average intra specific distance (0.012), while rpoC, rpoB, rbcL and psbK-psbI had the lowest average intra specific distances (0.0004).
Table 3.
Parameters of interspecific divergence and intraspecific variation of eight barcode regions and their combinations
| Parameters | rbcL | matK | psbA- trnH | rpoB | rpoC | psbK-psbI | atpF-atpH | matK + psbA- trnH | RPB2 + psbA- trnH | RPB2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Average inter specific | 0.028 ± 0.019 | 0.170 ± 0.04 | 0.23 ± 0.077 | 0.036 ± 0.026 | 0.02 ± 0.016 | 0.03 ± 0.028 | 0.07 ± 0.036 | 0.191 ± 0.040 | 0.238 ± 0.057 | 0.246 ± 0.074 |
| Average theta prime | 0.0019 ± 0.0014 | 0.009 ± 0.003 | 0.017 ± 0.005 | 0.002 ± 0.001 | 0.0009 ± 0.0009 | 0.002 ± 0.001 | 0.004 ± 0.002 | 0.012 ± 0.003 | 0.017 ± 0.003 | 0.013 ± 0.004 |
| Interspecific distance | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Average intraspecific distance | 0.0004 ± 0.0004 | 0.009 ± 0.002 | 0.0076 ± 0.002 | 0.0004 ± 0.0003 | 0.0004 ± 0.0003 | 0.0004 ± 0.0004 | 0.0011 ± 0.0003 | 0.0119 ± 0.002 | 0.007 ± 0.021 | 0.0008 ± 0.0008 |
| Maximum intra specific (coalescent depth) | 0.002 ± 0.002 | 0.023 ± 0.007 | 0.027 ± 0.007 | 0.004 ± 0.003 | 0.003 ±+0.002 | 0.002 ± 0.002 | 0.015 ± 0.002 | 0.088 ± 0.001 | 0.087 ± 0.002 | 0.010 ± 0.003 |
| Average theta | 0.0004 ± 0.0004 | 0.0008 ± 0.0005 | 0.009 ± 0.0027 | 0.0004 ± 0.0004 | 0.0004 ± 0.0003 | 0.0004 ± 0.0004 | 0.0009 ± 0.0003 | 0.0116 ± 0.004 | 0.004 ± 0.001 | 0.0009 ± 0.0005 |
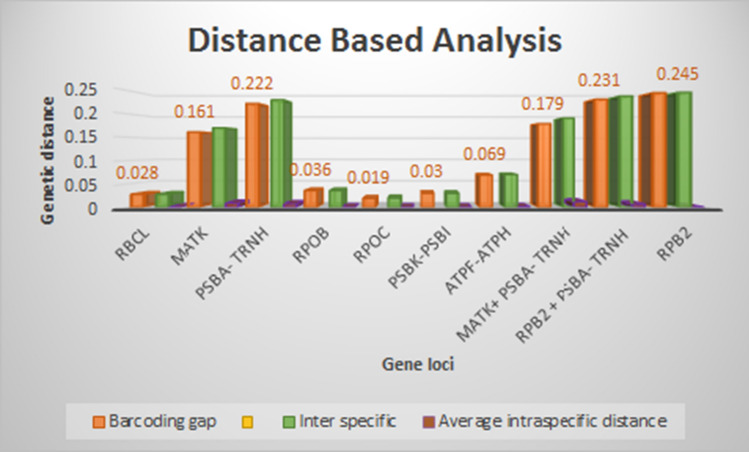
| Barcoding gap | 0.0276 | 0.161 | 0.2224 | 0.0356 | 0.0196 | 0.0296 | 0.0689 | 0.1791 | 0.231 | 0.2452 |
Based on barcoding gap analysis, RPB2 with the highest barcoding gap of 0.2452, can be recommended as a promising barcode locus in the selected species of Calamus (Fig. 1). Four more barcode regions viz., RPB2 + psbA-trnH, psbA-trnH, matK and matK + psbA-trnH also displayed distinct barcode gaps (0.231, 0.222, 0.161 and 0.1791 respectively). Barcoding gap was negligible in the remaining loci viz., rpoC, rpoB, rbcL, atpF-atpH and psbK-psbI with 0.0196, 0.0356, 0.0276, 0.0689, and 0.0296, respectively. The interspecific divergence measures displayed by RPB2 were significantly higher than that of other regions (matK + psbA-trnH, psbA-trnH, matK) as evident by the Wilcoxon’s signed rank test (Table 4).
Fig. 1.
DNA barcoding gap of barcoding loci based on the differences between average intraspecific and average interspecific distances
Table 4.
Test of significance of interspecific divergence among loci in Wilcoxon-signed rank test
| W+ | W− | Relative ranks | n | P > | Result | |
|---|---|---|---|---|---|---|
| W+ | W− | |||||
| RPB2 | matK + trnH | 7398 | 4229 | 153 | 0.001 | RPB2 > matK + trnH |
| RPB2 | psbA-trnH | 9763 | 4942 | 171 | 0.001 | RPB2 > psbA- trnH |
| RPB2 | matK | 10,266 | 4268 | 171 | 0.001 | RPB2 > matK |
| matK + psbA- trnH | psbA- trnH | 8767 | 3014 | 153 | 0.001 | matK + trnH > psbA-trnH |
| matK + psbA- trnH | matK | 7922 | 3705 | 153 | 0.001 | matK + trnH > matK |
| psbA- trnH | matK | 12,480 | 2225 | 171 | 0.001 | psbA- trnH> matK |
Similarity based analysis
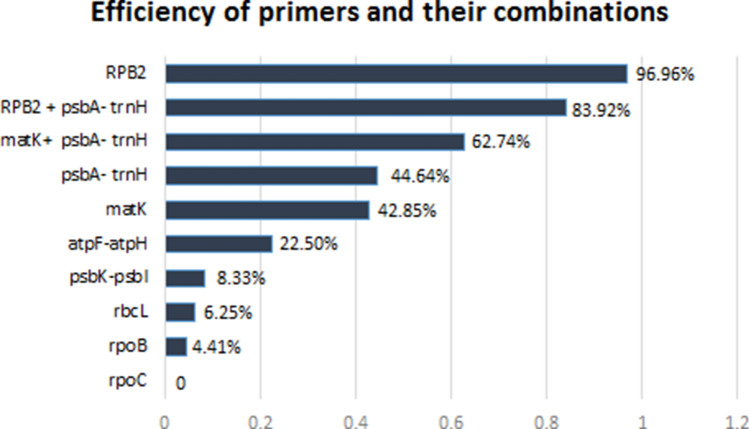
The results of similarity-based method performed employing BM and BCM parameters in TaxonDNA are shown in Table 5. Among the candidate loci, RPB2 had the highest successful identification rate (96.96%), while rpoC (0%) failed to show any successful identification under the ‘BM’ method. The combinations of RPB2 + psbA-trnH, matK + psbA-trnH, psbA-trnH and matK showed 83.9%, 62.7%, 44.6% and 42.8% species discrimination efficiencies respectively and had higher identification efficiencies than other regions (Fig. 2). The results of the ‘BCM’ method were similar to ‘BM’ method in all regions.
Table 5.
Identification success rates of eight barcode regions singly and in their combination using TaxonDNA under ‘Best Match’ and ‘Best Close Match’ criteria
| Parameters | rbcL (%) | matK (%) | psbA-trnH (%) | rpoB (%) | rpoC | psbK-psbI (%) | atpF-atpH (%) | matK + psbA-trnH (%) | RPB2 + psbA-trnH | RPB2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Correct identifications (BM) | 6.25 | 42.85 | 44.64 | 4.41 | 0 | 8.33 | 22.5 | 62.74 | 83.92% | 96.96 |
| Ambiguous (BM) | 93.75 | 34.28 | 32.14 | 95.58 | 96.61% | 90.0 | 70.0 | 3.92 | 0 | 1.51 |
| Incorrect identifications (BM) | 0.0 | 22.85 | 23.21 | 0.0 | 3.38% | 1.66 | 7.5 | 33.33 | 16.07% | 1.51 |
| Correct identifications (BCM) | 6.25 | 42.85 | 44.64 | 4.41 | 0 | 8.33 | 22.5 | 62.74 | 83.92% | 96.96 |
| Ambiguous (BCM) | 93.75 | 34.28 | 32.14 | 95.58 | 96.61% | 90.0 | 70.0 | 3.92 | 0 | 1.51 |
| Incorrect identifications (BCM) | 0.0 | 22.85 | 23.21 | 0.0 | 3.38% | 1.66 | 7.5 | 33.33 | 16.07% | 1.51 |
Fig. 2.
Efficiency of eight DNA barcode regions singly and in their combinations based on similarity based method
Tree based analysis
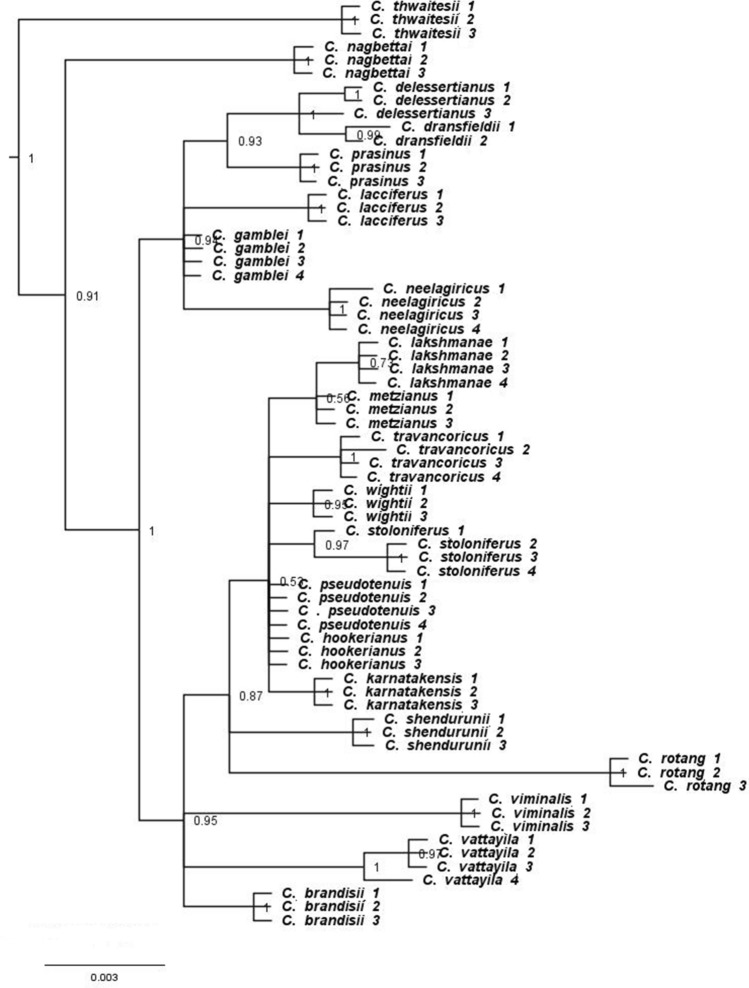
The discriminatory success of single or combined barcode regions, was also determined by evaluating the percentage of each species being monophyletic using a phylogenetic tree. The phylogenetic trees constructed based on Bayesian Inference and Neighbor-joining showed similar topologies. RPB2 gave a robust phylogenetic tree with high number of monophyletic clades in the genus Calamus using Bayesian Inference (Fig. 3). Even though some of the remaining barcode loci (psbA-trnH, matK, atpF-atpH, RPB2 + psbA-trnH and matK + psbA-trnH) showed species-specific monophyletic clades, they failed to show species resolution in less than half of the analysed species. The analysed barcode regions, rpoC, rpoB, rbcL and psbK-psbI failed to resolve species in the phylogenetic analysis. RPB2 based phylogenetic tree showed well resolved topology wherein species having multiple accessions formed well defined clusters with higher bootstrap support and good posterior probability values (1.00). Additionally, BI and NJ trees also formed a well resolved tree with the RPB2 gene region, in which members of the genus Calamus formed a single clade with a majority of species clustered into different subclades (Fig. 3). Out of the 21 species analysed, 15 species (C. brandisii, C. shendurunii, C. neelagiricus, C. viminalis, C. rotang, C. wightii, C. nagbettai, C. karnatakensis, C. lakshmanae, C. prasinus, C. stoloniferus, C. travancoricus, C. vattayila, C. thwaitesii, and C. lacciferus) displayed well supported distinct monophyletic clades in the phylogenetic tree. C. metzianus, C. gamblei and C. hookerianus showed polytomy in the phylogenetic analysis. C. pseudotenuis clustered with C. hookerianus, C. dransfieldii and C. delessertianus, whereas C. thwaitesii was a standalone species with strongly supported bootstrap value (100%) in all the phylogenetic trees. Neighbor-joining tree is given in Supplementary Fig. 5.
Fig. 3.
Phylogenetic tree with posterior probability using low copy nuclear region RPB2, based on GTR + G model and AIC in MrBayes v.3.2.2
The phylogenetic trees constructed, in order to check the efficiency of RPB2 region in other Calamus species from India as well as South East Asia and palm species showed a well resolved tree with high number of monophyletic clades (Supplementary Figs. 6 & 7). The species delineation was seen noticeably in these trees.
Discussion
Identification of plants may be challenging, especially if they are beset with taxonomic complexities and thereby identification purely based on morphological characters alone may not be feasible. Recent efforts with DNA barcoding have proved it to be a reliable molecular tool for species delineation in many plant taxa (Gogoi and Bhau 2018; Duan et al. 2019; Moura et al. 2019; Dev et al. 2020). However, in Calamus and other taxa such as Aspalathus (Edwards et al. 2008), wild potatoes (Spooner 2009), Indian berberis (Roy et al. 2010), Picea (Ran et al. 2010), Bromeliaceae (Maia et al. 2012), willows (Percy et al. 2014), Alooideae (Daru et al. 2017), these reported barcode regions were not efficient enough. Consequently, there is intense debate and confusion in the plant barcoding community regarding universal barcodes and many suggestions put forth in terms of combination of the recommended regions and introduction of new regions (Valentini et al. 2009; Ballardini et al. 2013; Techen et al. 2014; Joly et al. 2014; Paracchini et al. 2017).
Twelve barcoding loci, including both plastid and nuclear regions viz., rbcL, matK, psbA-trnH, rpoC, rpoB, psbK-psbI, atpF-atpH, psbZ-trnfM, ITS1, ITS2, PRK and RPB2 and their combinations, based on the prevalence in literature, were analysed in the present study to demonstrate their efficiency in species discrimination in Calamus. The plastid barcode regions recommended as universal barcode failed to discriminate the rattan species’, supporting the emerging consensus that plastid sequences often fail due to slow rate of evolution in palms (Wilson et al. 1990). In palms, substitution rate estimates from restriction site variation in chloroplast DNA are 5 to 13-fold slower than the estimates for grasses (Wilson et al. 1990). The slow evolutionary rate of palm DNA further hinders the use of plastid genes in palm molecular studies (Gaut et al. 1992, 1996; Wilson et al. 1990). Calamoideae has the lowest substitution rate (1.3 × 10−10) in chloroplast region when compared to other palms (Wilson et al. 1990), clearly indicating the inefficiency of plastid primers to discriminate species. Low copy nuclear regions such as PRK (phosphoribulokinase), RPB2 (RNA polymerase II) and MS (malate synthase) were, on the other hand, successfully used in the phylogenetic studies of palms (Lewis and Martinez 2000; Lewis and Doyle 2001, 2002; Bayton 2005; Roncal et al. 2005; Thomas et al. 2006; Loo et al. 2006; Norup 2006; Zona et al. 2011), revealing their utility in the palm molecular studies.
Even though psbA-trnH (Al-Qurainy et al. 2011; Yang et al. 2012), psbK-psbI (Enan and Ahmed 2016) and the ribosomal locus ITS2 (Jeanson et al. 2011) have proven their utility as barcodes in other palms, they failed to succeed in the genus Calamus. rbcL and matK have been recommended for discriminating palm genera (Naeem et al. 2014; Elansary et al. 2017), but accurate species identification in palms requires more gene regions in addition to core barcodes (Ahmad et al. 2019). The nuclear regions, ITS1 and ITS2 failed to produce good PCR amplicons in our study, probably due to the presence of multiple copies as reported by Baker et al. (2000) in Calamoideae. Moreover, the ITS region recommended for barcoding (China Plant BOL Group et al. 2011), has been reported to have incomplete concerted evolution and consequently intragenomic heterogeneity among copies in palms (Baker et al. 2000). In this study, psbA-trnH exhibited dramatic differences in sequence lengths due to the insertion/deletions among the analysed congeneric species, which led to difficulties in sequence alignment, thus making it unsuitable for species identification (Kress et al. 2005). Further, the different combinations tried in our study (matK + psbA-trnH as well as RPB2 + psbA-trnH), exhibited sufficient DNA barcoding gap, but showed large intraspecific differences thereby making it inefficient for barcoding. The low species discrimination ability of rbcL, rpoB, rpoC, atpF-atpH and psbK-psbI, reported previously (CBOL 2009; Han et al. 2016; Feng et al. 2013) was also confirmed by our study.
Low-copy nuclear regions have been successfully used instead of plastid regions in resolving phylogenetic relationships of palms, especially at lower taxonomic levels, regardless of the difficulties like paralogy, concerted evolution and intragenic polymorphism (Lewis and Doyle 2001, 2002; Gunn 2004; Roncal et al. 2005; Bayton 2005; Thomas et al. 2006; Loo et al. 2006; Norup 2006). Even though the utility of low copy nuclear regions as a barcode is restricted (Kress et al. 2005), 100 per cent PCR amplification success with maximum discriminatory ability of RPB2 was evident in this study. This region showed the highest successful species identification rate (96.96%, Fig. 2) in Calamus, based on the similarity based method. Furthermore, RPB2 also showed significant barcoding gap and gave a robust phylogenetic tree. Therefore, considering all the three analyses viz. distance, similarity and tree based, the RPB2 nuclear barcode region is proposed as an ideal DNA barcode for Calamus species identification. Earlier, RPB2 has also been successfully used in angiosperm phylogenetics (Denton et al. 1998; Oxelman and Bremer 2000; Oxelman et al. 2004; Pfeil et al. 2004). The efficiency of other low copy nuclear regions as potential barcode was also successfully demonstrated in Clermontia (Campanulaceae) and Cyrtandra (Gesneriaceae), in which plastid genes had shown slow evolutionary rates (Pillon et al. 2013). Furthermore, these regions that can evolve up to five times faster than the plastid genome and can also resolve even recently and rapidly diversifying lineages (Sang 2002; Small et al. 2004; Norup 2006), make a successful candidate for DNA barcoding in slow evolving plant groups.
Taxonomic recommendations
This study also addressed the potential of the RPB2 barcode region in resolving the ‘C. gamblei species complex’. RPB2 showed the same transversion event (T → G) in all species of this complex, indicating close similarity of the species (Supplementary Fig. 4). In spite of a similar mutational event, the RPB2 barcode region was able to precisely discriminate all of them except C. delessertianus and C. dransfieldii as distinct species. C. shendurunii, previously reported (Sreekumar and Henderson 2014) as a member of the complex formed a distant clade, thereby separating itself from the complex. The formation of individual distinct clusters by the members of the complex showed a clear delimitation between the species, thus resolving the complex.
C. dransfieldii (identified from Dhoni Hills, Palakkad described by Renuka, 1986) and C. delessertianus (reported by Beccari, 1908 and later described by Renuka, 1999) were morphologically similar in many characteristics. C. delessertianus has dark green coloured leaf sheath and leaflets while it is pale green for C. dransfieldii. Both the species’ were clustered together in the phylogram (Fig. 3) with no significant nucleotide differences in the analysed barcoding regions (rbcL, matK, psbA-trnH, RPB2). Further, morphological differences were also insignificant to consider it as two different species. Hence, we recommend that the specimens hitherto considered as C. delessertianus (Renuka 1999) are merely morphological variants of C. dransfieldii.
Specimen examination and field observations revealed overlapping morphological character states of the two species, C. hookerianus and C. pseudotenuis. The present study recommends them as closely related species based on similarity of RPB2 sequences and particularly the presence of a shared 300 bp insertion in psbA-trnH spacer region besides the similarities in morphology. Further analysis of more specimens from Sri Lanka and Karnataka may be required before considering the merging of the species’. Likewise, the species, C. wightii, C. pseudotenuis, C. karnatakensis, C. stoloniferus, C. travancoricus and C. hookerianus had a 2 base pair (CT) deletion in common in RPB2 region, indicating that they are closely related.
In this study, C. thwaitesii was found to be a standalone species in the phylogenetic tree of the Calamus species of south India (Fig. 3). Similarly in Beccari’s classification (1908), C. thwaitesii was grouped apart from the south Indian species’. These results were also supported by recent molecular phylogenetic studies (Senthilkumar 2015; Kurian 2018) indicating C. thwaitesii has more similarities with rattans from Andaman Nicobar islands and northeast India. In the molecular and morphology based phylogenetic studies of rattans of India, C. thwaitesii was grouped with flagelliferous, clustering, large diameter rattans from the Andamans and northeast India (C. longisetus and C. flagellum) (Sreekumar 2005; Kurian 2018).
Considering the vast diversity of species in the genus spread across wide geographical area, sampling of Calamus from other countries is essential to further prove the potentiality of the recommended barcode. Hence, the proposed barcoding region, RPB2 was also evaluated for efficacy with Calamus species from other parts of India and south East Asia. This barcoding locus succeeded in demarking the species boundaries in all the species tested (15 species including C. andamanicus, C. baratangensis, C. caesius, C. dilaceratus, C. flagellum, C. floribundus, C. gracilis, C. latifolius, C. longisetus, C. nambariensis, C. palustris, C. pseudorivalis, C. tenuis, C. tetradactylus and C. unifarius) signifying the use of this region as a potential DNA barcode in the genus Calamus (Supplementary Fig. 6). Additionally, the efficacy of the RPB2 as a barcoding region in other palm genera was also tested in 13 genera (27 species) of 5 subfamilies of Arecaceae and this region succeeded in delimiting species boundaries in all the subfamilies of palms showing their utility as a promising DNA barcode in the palm family (Supplementary Fig. 7). Therefore, the use of RPB2 as a potential barcode region to discriminate species in other groups of plants, which show slow rate of evolution, is another recommendation from this study.
Conclusion
The inefficiency of the recommended plastid regions was a hurdle to the development of suitable DNA barcodes for accurate species identification in the taxonomically complex genus Calamus. The low copy nuclear region, RPB2 showed highest species resolution and was successful in unravelling the species complexities in the genus. Species in the “C. gamblei species complex” with overlapping morphological characteristics could be resolved as distinct species using the RPB2 barcode locus. Based on similarities in the barcode sequence data and morphological characteristics, the study could recommend C. delessertianus to be only a morphological variant of C. dransfieldii. Molecular data indicates C. hookerianus and C. pseudotenuis to be closely related to each other. The RPB2 nuclear region therefore emerges as a strong candidate barcode for the genus Calamus. The barcode potential of this region may also be exploited in resolving taxonomic problems in other problematic palm genera as well.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig.1. PCR amplified products of the analysed DNA barcode loci (TIFF 202 kb)
Supplementary Fig.2. Multiple Sequence Alignment showing species specific differences in the Calamus species using RPB2 (TIFF 2057 kb)
Supplementary Fig.3. Multiple Sequence Alignment showing 300bp deletion in the C. hookerianus and C. pseudotenuis using psbA-trnH. (TIFF 580 kb)
Supplementary Fig.4. Multiple Sequence Alignment showing transversions in the Calamus gamblei species complex (TIFF 1553 kb)
Supplementary Fig.5. Neighbor-joining tree (NJ) of the genus Calamus using RPB2 based on p-distance using MEGA v.6.0 with bootstrap percentage value shown below (TIFF 53 kb)
Supplementary Fig.6. Phylogenetic tree with posterior probability using RPB2 of the genus Calamus (TIFF 553 kb)
Supplementary Fig.7. Phylogenetic tree with posterior probability using RPB2 of the family Arecaceae (TIFF 114 kb)
Supplementary Table S1. List of samples collected for DNA Barcoding, their location and GenBank Accession numbers (DOC 118 kb)
Supplementary Table S2. List of sequences downloaded from GenBank and their Accession numbers. (DOC 29 kb)
Supplementary File 1. Multiple Sequence Alignment of RPB2 (MAS 50 kb)
Acknowledgements
We thank Kerala Forest Department, Govt. of Kerala and Karnataka Forest Department, Govt. of Karnataka for their permission to collect samples. The financial support received from Kerala State Council for Science Technology and Environment (KSCSTE) is also acknowledged.
Author contributions
AK: contributed towards conducting wet lab experiments, sample collections from the field, writing of the paper and analysing the data, SAD: involved in writing of the paper and designing the project, SVB: sample collections from the field site, identification and writing of the paper, MEM: involved in coordination of project and writing of the paper
Funding
The financial support for this study was received from Kerala State Council for Science, Technology and Environment (KSCSTE) (Grant No. KFRI RP 617/2011).
Data Availability Statement
All the nucleotide sequence data generated in the study was submitted to NCBI and the list of accession numbers are provided as supplementary table S1.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmad I, Khan S, Naeem M, Hayat M, Azmi UR, Ahmed S, Murtaza G, Irfan M. Molecular identification of ten palm species using DNA Fingerprinting. Int J Pure App Biosci. 2019;7(1):46–51. [Google Scholar]
- Al-Qurainy F, Khan S, Al-Hemaid FM, Ajmal Ali A, Tarroum M, Ashraf M. Assessing molecular signature for some potential date (Phoenix dactylifera L.) cultivars from Saudi Arabia, based on chloroplast DNA sequences rpoB and psbA trnH. Int J Mol Sci. 2011;12:6871–6880. doi: 10.3390/ijms12106871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif MJ, Cannon CH. DNA extraction from processed wood: a case study for the identification of an endangered timber species (Gonystylus bancanus) Plant Mol Biol Rep. 2005;23:185–192. [Google Scholar]
- Atria M, Mil H, Baker WJ, Dransfield J, Welzen PC. Morphometric analysis of rattan Calamus javensis complex (Arecaceae:Calamoideae) Syst Bot. 2017;42:494–506. [Google Scholar]
- Baker WJ, Hedderson TA, Dransfield J. Molecular phylogenetics of subfamily Calamoideae (Palmae) based on nrDNA ITS and cpDNA rps16 intron sequence data. Mol Phylogenet Evol. 2000;14:195–217. doi: 10.1006/mpev.1999.0696. [DOI] [PubMed] [Google Scholar]
- Baker DA, Stevenson DW, Little DP. DNA barcode identification of black cohosh herbal dietary supplements. J AOAC Int. 2012;95(4):1023–1034. doi: 10.5740/jaoacint.11-261. [DOI] [PubMed] [Google Scholar]
- Ballardini M, Mercuri A, Littardi C, et al. The chloroplast DNA locus psbZ-trnfM as a potential barcode marker in Phoenix L. (Arecaceae) ZooKeys. 2013;365:71–82. doi: 10.3897/zookeys.365.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayton RP (2005) Borassus L. and the Borassoid palms: systematics and evolution. Ph.D. Thesis, University of Reading, UK
- Beccari O. Asiatic palms—Lepidocaryeae. Part II. The species of Calamus. Ann R Bot Gard. 1908;11:1–518. [Google Scholar]
- Boer WJ. The Genomoid palms. Verh Kon Ned Akad Wetensch Afd Natuurk Tweede Sect Ser. 1968;58:1–202. [Google Scholar]
- CBOL Plant Working Group A DNA barcode for land plants. Proc Natl Acad Sci USA. 2009;106:12794–12797. doi: 10.1073/pnas.0905845106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase MW, Salamin N, Wilkinson M, et al. Land plants and DNA barcodes: short-term and long-term goals. Philos Trans R Soc Lond B Biol Sci. 2005;360:1889–1895. doi: 10.1098/rstb.2005.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Yao H, Han J, et al. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE. 2010;5:e8613. doi: 10.1371/journal.pone.0008613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China Plant BOL Group. Li DZ, Gao LM, Li HT, et al. Comparative analysis of a large dataset indicates that internal transcribed spacer (ITS) should be incorporated into the core barcode for seed plants. Proc Natl Acad Sci USA. 2011;108:19641–19646. doi: 10.1073/pnas.1104551108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daru BH, van der Bank M, Bello A, Yessoufou K. Testing the reliability of standard and complementary DNA barcodes for the monocot subfamily Alooideae from South Africa. Genome. 2017;60:337–347. doi: 10.1139/gen-2015-0183. [DOI] [PubMed] [Google Scholar]
- Denton AL, Mc Conaughy BL, Hall BD. Usefulness of RNA polymerase II coding sequences for estimation of green plant phylogeny. Mol Biol Evol. 1998;15:1082–1085. doi: 10.1093/oxfordjournals.molbev.a026007. [DOI] [PubMed] [Google Scholar]
- Dev SA, Muralidharan EM, Sujanapal P, Balasundaran M. Identification of market adulterants in East Indian sandalwood using DNA barcoding. Ann For Sci. 2014;71:517–522. [Google Scholar]
- Dev SA, Sijimol K, Prathibha PS, Sreekumar VB, Muralidharan EM. DNA barcoding as a valuable molecular tool for the certification of planting materials in bamboo. 3 Biotech. 2020;10:59. doi: 10.1007/s13205-019-2018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. A rapid total DNA preparation procedure for fresh plant tissue. Focus. 1990;12:13–15. [Google Scholar]
- Dransfield J. The rattans of Sarawak. Kew: Royal Botanic Gardens and Sarawak Forest Department; 1992. [Google Scholar]
- Dransfield J, Manokaran N (1994) Rattans. Plant Resources of South-East Asia No. 6. Indonesia, PROSEA Foundation, Bogor
- Duan H, Wang W, Zeng Y, Guo M, Zhou YI (2019) The screening and identification of DNA barcode sequences for Rehmannia. Sci Rep 9 (1):17295 [DOI] [PMC free article] [PubMed]
- Edwards D, Horn A, Taylor D, Savolainen V, Hawkins JA. DNA barcoding of a large genus, Aspalathus L. (Fabaceae) Taxon. 2008;57:1317–1327. [Google Scholar]
- Elansary HO, Ashfaq M, Ali HM, Yessoufou K. The first initiative of DNA barcoding of ornamental plants from Egypt and potential applications in horticulture industry. PLoS ONE. 2017;12(2):e0172170. doi: 10.1371/journal.pone.0172170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enan MR, Ahmed A. Cultivar-level phylogeny using chloroplast DNA barcode psbK-psbI spacers for identification of Emirati date palm (Phoenix dactylifera L.) varieties. Genet Mol Res. 2016;15:3. doi: 10.4238/gmr.15038470. [DOI] [PubMed] [Google Scholar]
- Erickson DL, Spouge J, Resch A, Weigt LA, Kress JW. DNA barcoding in land plants: developing standards to quantify and maximize success. Taxon. 2008;57:1304–1316. [PMC free article] [PubMed] [Google Scholar]
- Feng S, Jiang Y, Wang S, et al. Molecular identification of Dendrobium Species (Orchidaceae) based on the DNA barcode ITS2 region and its application for phylogenetic study. Int J Mol Sci. 2013;16:21975–21988. doi: 10.3390/ijms160921975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CS, Ayres KL, Toomey N, et al. Selection of candidate coding DNA barcoding regions for use on land plants. Bot J Linn Soc. 2009;159:1–11. [Google Scholar]
- Fuji T (2007) Outline of the research project “Methods to identify wood species and origin of timber of Southeast Asia”. In: Proceedings of the international symposium on development of improved methods to identify Shorea species wood and its origin, September 25–26, 2007. University of Tokyo, Tokyo and IUFRO, 19
- Gao T, Yao H, Song JY, et al. Identification of medicinal plants in the family Fabaceae using a potential DNA barcode ITS2. J Ethnopharmacol. 2010;130:116–121. doi: 10.1016/j.jep.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Gaut BS, Muse SV, Clark WD, Clegg MT. Relative rates of nucleotide substitution at the rbcL locus of the monocotyledonous plants. J Mol Evol. 1992;35:292–303. doi: 10.1007/BF00161167. [DOI] [PubMed] [Google Scholar]
- Gaut BS, Morton BR, McCaig BC, Clegg MT. Substitution rate comparisons between grasses and palms: synonymous rate differences at the nuclear gene Adh parallel rate differences at the plastid gene rbcL. Proc Natl Acad Sci USA. 1996;93:10274–10279. doi: 10.1073/pnas.93.19.10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogoi B, Bhau BS. DNA barcoding of the genus Nepenthes (Pitcher plant): a preliminary assessment towards its identification. BMC Plant Biol. 2018;18:153. doi: 10.1186/s12870-018-1375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn BF. The phylogeny of the Cocoeae (Arecaceae) with emphasis on Cocos nucifera. Ann Mo Bot Gard. 2004;91:505–522. [Google Scholar]
- Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis: Department of Microbiology, North Carolina State University
- Han YW, Duan D, Ma XF, et al. Efficient identification of the forest tree species in Aceraceae using DNA barcodes. Front Plant Sci. 2016;7:1707. doi: 10.3389/fpls.2016.01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert PDN, Cywinska A, Ball SL, deWaard JR. Biological identifications through DNA barcodes. Proc R Soc Lond B Biol Sci. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert PD, Penton EH, Burns JM, Janzen DH, Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the Neotropical skipper butterfly Astraptes fulgerator. Proc Natl Acad Sci USA. 2004;101(41):14812–14817. doi: 10.1073/pnas.0406166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth ML, Clark LA, Forrest LL, Richardson J, Pennington RT, et al. Selecting barcoding loci for plants: evaluation of seven candidate loci with species-level sampling in three divergent groups of land plants. Mol Ecol Resour. 2009;9:439–457. doi: 10.1111/j.1755-0998.2008.02439.x. [DOI] [PubMed] [Google Scholar]
- Hollingsworth PM, Graham SW, Little DP. Choosing and using a plant DNA barcode. PLoS ONE. 2011;6:e19254. doi: 10.1371/journal.pone.0019254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Mohanan N, Kariyappa K. A new species of Calamus L. (Arecaceae) from Silent Valley, the Western Ghats, India. Rheedea. 2008;18:29–31. [Google Scholar]
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. Multiple sequence alignment with Clustal X. Trends Biochem Sci. 1998;23:403–405. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Jeanson ML, Labat JN, Little DP. DNA barcoding: a new tool for palm taxonomists? Ann Bot. 2011;108:1445–1451. doi: 10.1093/aob/mcr158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly S, Davies TJ, Archambault A, et al. Ecology in the age of DNA barcoding: the resource, the promise and the challenges ahead. Mol Ecol Resour. 2014;14:221–232. doi: 10.1111/1755-0998.12173. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kress WJ, Erickson DL. A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS ONE. 2007;2:e508. doi: 10.1371/journal.pone.0000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress WJ, Wurdack KJ, Zimmer EA, Weigt LA, Janzen DH. Use of DNA barcodes to identify flowering plants. Proc Natl Acad Sci USA. 2005;102:8369–8374. doi: 10.1073/pnas.0503123102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppu R, Manoharan S, Uthandakalaipandian R. Phylogeographic study of freshwater fish Channa striata (Bloch) and Channa punctata (Bloch) construed using DNA barcodes between three rivers in southern Tamil Nadu region, India. Proc Zool Soc. 2019 doi: 10.1007/s12595-019-00299-1. [DOI] [Google Scholar]
- Kurian A (2018) Molecular systematics of rattans of south India. Doctoral thesis, Cochin University of Science and Technology, India, p 131
- Lakshmana A, Renuka C. New species of Calamus (Arecaceae) from India. JETBD. 1990;14:705–709. [Google Scholar]
- Lee HL, Yi DK, Kim JS, Kim KJ (2007) Development of plant DNA barcoding markers from the variable noncoding regions of chloroplast genome. The Second International Barcode of Life Conference, Taipei, Taiwan. pp. 18–20
- Lewis CE, Doyle JJ. Phylogenetic utility of the nuclear gene malate synthase in the palm family (Arecaceae) Mol Phylogenet Evol. 2001;19:409–420. doi: 10.1006/mpev.2001.0932. [DOI] [PubMed] [Google Scholar]
- Lewis CE, Doyle JJ. Phylogenetic analysis of tribe Areceae (Arecaceae) using two low-copy nuclear genes. Plant Syst Evol. 2002;236:1–17. [Google Scholar]
- Lewis CE, Martinez N. Identity of the Hyophorbe palms at the botanical garden of Cienfuegos, Cuba. Palms. 2000;44:93–97. [Google Scholar]
- Loo AHB, Dransfield J, Chase MW, Baker WJ. Low-copy nuclear DNA, phylogeny and the evolution of dichogamy in the betel nut palms and their relatives (Arecinae; Arecaceae) Mol Phylogenet Evol. 2006;39:598–618. doi: 10.1016/j.ympev.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Maia VH, Mata CSd, Franco LO, Cardoso MA, Cardoso SRS, et al. DNA barcoding Bromeliaceae: achievements and pitfalls. PLoS ONE. 2012;7(1):e29877. doi: 10.1371/journal.pone.0029877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier R, Shiyang K, Vaidya G, Ng PK. DNA barcoding and taxonomy in Diptera: a tale of high intraspecific variability and low identification success. Syst Biol. 2006;55:715–728. doi: 10.1080/10635150600969864. [DOI] [PubMed] [Google Scholar]
- Meyer CP, Paulay G. DNA barcoding: error rates based on comprehensive sampling. PLoS Biol. 2005;3:e422. doi: 10.1371/journal.pbio.0030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura C, Brambach F, Jair Hernandez Bado K, et al. Integrating DNA barcoding and traditional taxonomy for the identification of Dipterocarps in Remnant Lowland Forests of Sumatra. Plants (Basel, Switzerland) 2019;8(11):461. doi: 10.3390/plants8110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem A, Khan AA, Cheema HMN, Khan IA, Buerkert A. DNA barcoding for species identification in the palmae family. Genet Mol Res. 2014;13:10341–10348. doi: 10.4238/2014.December.4.29. [DOI] [PubMed] [Google Scholar]
- Newmaster SG, Fazekas A, Ragupathy S. DNA barcoding in the land plants: evaluation of rbcL in a multigene tiered approach. Can J Bot. 2006;84:335–341. [Google Scholar]
- Norup MV (2006) A molecular systematic study of Heterospathe and Rhopaloblaste (Arecaceae, Areceae). Masters Thesis, University of Aarhus
- Oxelman B, Bremer B. Discovery of paralogous nuclear gene sequences coding for the second-largest subunit of RNA polymerase II (RPB2) and their phylogenetic utility in Gentianales of the asterids. Mol Biol Evol. 2000;17:1131–1145. doi: 10.1093/oxfordjournals.molbev.a026396. [DOI] [PubMed] [Google Scholar]
- Oxelman B, Yoshikawa N, Mcconaughy BL, Luo J, Denton AL, Hall BD. RPB2 gene phylogeny in flowering plants, with particular emphasis on asterids. Mol Phylogenet Evol. 2004;32:462–479. doi: 10.1016/j.ympev.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Pang X, Song J, Zhu Y, Xu H, Huang L, et al. Applying plant DNA barcodes for Rosaceae species identification. Cladistics. 2010;27:165–170. doi: 10.1111/j.1096-0031.2010.00328.x. [DOI] [PubMed] [Google Scholar]
- Paracchini V, Petrillo M, Lievens A, et al. Novel nuclear barcode regions for the identification of flatfish species. Food Control. 2017;79:297–308. doi: 10.1016/j.foodcont.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy DM, George WA, Quentin CC, et al. Understanding the spectacular failure of DNA barcoding in willows (Salix): does this result from a trans-specific selective sweep? Mol Ecol. 2014;19:4737–4756. doi: 10.1111/mec.12837. [DOI] [PubMed] [Google Scholar]
- Pfeil BE, Brubaker CL, Craven LA, Crisp MD. Paralogy and orthology in the Malvaceae RPB2 gene family: investigation of gene duplication in hibiscus. Mol Biol Evol. 2004;21:1428–1437. doi: 10.1093/molbev/msh144. [DOI] [PubMed] [Google Scholar]
- Pillon Y, Johansen J, Sakishima T, et al. Potential use of low-copy nuclear genes in DNA barcoding: a comparison with plastid genes in two Hawaiian plant radiations. BMC Evol Biol. 2013;13:35. doi: 10.1186/1471-2148-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D, Buckley TR. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol. 2004;53:793–808. doi: 10.1080/10635150490522304. [DOI] [PubMed] [Google Scholar]
- Ran JH, Wang PP, Zhao HJ, Wang XQ. A test of seven candidate barcode regions from the plastome in Picea (Pinaceae) J Integr Plant Biol. 2010;52:1109–1126. doi: 10.1111/j.1744-7909.2010.00995.x. [DOI] [PubMed] [Google Scholar]
- SPSS Inc. Released (2007) SPSS for Windows, Version 17.0. Chicago, SPSS Inc
- Renuka C. A new species of Calamus (Palmae) from India. Kew Bull. 1986;42:433–435. [Google Scholar]
- Renuka C. Notes on the identity of Calamus delessertianus Becc. Rheedea. 1999;9:81–84. [Google Scholar]
- Renuka C. Palms of India: status, threats and conservation strategies. In: Shaanker UR, Ganeshaiah KN, Bawa KS, editors. Forest genetic resources: status, threats and conservation strategies. Calcutta: Oxford & IBH Publishing Co. Pvt. Ltd.; 2001. pp. 197–209. [Google Scholar]
- Renuka C, Sasidharan N, Anto P. A new species of Calamus (Arecaceae) from Silent Valley, Kerala, India. Rheedea. 1997;7:69–71. [Google Scholar]
- Renuka C, Bhat KV, Pandalai RC (2010) Rattans of India: taxonomy, biology and utilization. Kerala Forest Research Institute (KFRI), Thrissur, p 339
- Roncal J, Francisco-Ortega J, Asmussen CB, Lewis CE. Molecular phylogenetics of tribe Geonomeae (Arecaceae) using nuclear DNA sequences of phosphoribulokinase and RNA polymerase II. Syst Bot. 2005;30:275–283. [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Roy S, Tyagi A, Shukla V, et al. Universal plant DNA barcode loci may not work in complex groups: a case study with Indian berberis species. PLoS ONE. 2010;5:e13674. doi: 10.1371/journal.pone.0013674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees”. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sang T. Utility of low-copy nuclear gene sequences in plant phylogenetics. Crit Rev Biochem Mol Biol. 2002;37:121–147. doi: 10.1080/10409230290771474. [DOI] [PubMed] [Google Scholar]
- Sass C, Little DP, Stevenson DW, Specht CD. DNA barcoding in the Cycadales: testing the potential of proposed barcoding markers for species identification of Cycads. PLoS ONE. 2007;2(11):e1154. doi: 10.1371/journal.pone.0001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarcelli N, Barnaud A, Eiserhardt W, Treier UA, Seveno M, d’Anfray A, Vigouroux Y, Pintaud JC. A set of 100 chloroplast primer pairs to study population genetics and phylogeny in monocotyledons. PLoS ONE. 2011;6:e19954. doi: 10.1371/journal.pone.0019954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthilkumar U (2015) Phylogenetics and phylogeography of climbing palms of India (Tribe Calameae, Arecaceae). Doctoral thesis, Madras Christian College, India, p 205
- Small R, Cronn R, Wendel J. L.A.S Johnson Review No.2. Use of nuclear genes for phylogeny reconstruction in plants. Aust Syst Bot. 2004;17:145–170. [Google Scholar]
- Spooner DM. DNA barcoding will frequently fail in complicated groups: an example in wild potatoes. Am J Bot. 2009;96:1177–1189. doi: 10.3732/ajb.0800246. [DOI] [PubMed] [Google Scholar]
- Sreekumar VB (2005) Systematics and phylogeny of the genus Calamus L. (Arecaceae) in the Western Ghats. Doctoral thesis, University of Calicut, India, p 140
- Sreekumar VB, Henderson A. Nomenclatural notes on Indian Calamus (Arecaceae) Phytotaxa. 2014;166:145–149. [Google Scholar]
- Stoeckle M. Taxonomy, DNA, and the barcode of life. Bioscience. 2003;53(9):2–3. [Google Scholar]
- Tamura K, Battistuzzi FU, Billing-Ross P, Murillo O, Filipski A, Kumar S. Estimating divergence times in large molecular phylogenies. Proc Natl Acad Sci USA. 2012;109:19333–19338. doi: 10.1073/pnas.1213199109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Techen N, Parveen I, Pan Z, Khan IA. DNA barcoding of medicinal plant material for identification. Curr Opin Biotechnol. 2014;25:103–110. doi: 10.1016/j.copbio.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Thomas MM, Garwood NC, Baker WJ, et al. Molecular phylogeny of the palm genus Chamaedorea, based on the low-copy nuclear genes PRK and RPB2. Mol Phylogenet Evol. 2006;38:398–415. doi: 10.1016/j.ympev.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Uhl N, Dransfield J. Genera Palmarum. Lawrence: Allen Press; 1987. [Google Scholar]
- Uhl NW, Dransfield J, Davis JI, Luckow MA, Hansen KS, Doyle JJ. Phylogenetic relationships among palms: cladistic analyses of morphological and chloroplast DNA restriction site variation. In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ, editors. Monocotyledons: systematics and evolution. Kew: Royal Botanic Gardens; 1995. pp. 623–661. [Google Scholar]
- Umapathy S, Duvuru N, Munivenkatappa S, Uma Shaanker R, Gudasalamani R. Species delimitation in congenerics of genus Daemonorops from India using DNA barcodes. Commun Plant Sci. 2015;5(1–2):1–8. [Google Scholar]
- Valentini A, Miquel C, Nawaz MA, et al. New perspectives in diet analysis based on DNA barcoding and parallel pyrosequencing: the trnL approach. Mol Ecol Resour. 2009;9:51–60. doi: 10.1111/j.1755-0998.2008.02352.x. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego: Academic Press; 1990. pp. 315–322. [Google Scholar]
- Wilson MA, Gaut B, Clegg MT. Chloroplast DNA evolves slowly in the palm family (Arecaceae) Mol Biol Evol. 1990;7:303–314. doi: 10.1093/oxfordjournals.molbev.a040605. [DOI] [PubMed] [Google Scholar]
- Wolfe KH, Li WH, Sharp PM. Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast and nuclear DNAs. Proc Natl Acad Sci USA. 1987;84:9054–9058. doi: 10.1073/pnas.84.24.9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HQ, Dong YR, Gu ZJ, Liang N, Yang JB. A preliminary assesment of matK, rbcL and trnH-psbA as DNA barcodes for Calamus (Arecaceae) species in China with a note on ITS. Ann Bot Fenn. 2012;49:319–330. [Google Scholar]
- Yu J, Xue JH, Zhou SL. New universal matK primers for DNA barcoding angiosperms. J Syst Evol. 2011;49:176–181. [Google Scholar]
- Zona S, Ortega JF, Jestrow B, Baker WJ, Lewis CE. Molecular phylogenetics of the palm subtribe Ptychospermatinae (Arecaceae) Am J Bot. 2011;98:1716–1726. doi: 10.3732/ajb.1100218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig.1. PCR amplified products of the analysed DNA barcode loci (TIFF 202 kb)
Supplementary Fig.2. Multiple Sequence Alignment showing species specific differences in the Calamus species using RPB2 (TIFF 2057 kb)
Supplementary Fig.3. Multiple Sequence Alignment showing 300bp deletion in the C. hookerianus and C. pseudotenuis using psbA-trnH. (TIFF 580 kb)
Supplementary Fig.4. Multiple Sequence Alignment showing transversions in the Calamus gamblei species complex (TIFF 1553 kb)
Supplementary Fig.5. Neighbor-joining tree (NJ) of the genus Calamus using RPB2 based on p-distance using MEGA v.6.0 with bootstrap percentage value shown below (TIFF 53 kb)
Supplementary Fig.6. Phylogenetic tree with posterior probability using RPB2 of the genus Calamus (TIFF 553 kb)
Supplementary Fig.7. Phylogenetic tree with posterior probability using RPB2 of the family Arecaceae (TIFF 114 kb)
Supplementary Table S1. List of samples collected for DNA Barcoding, their location and GenBank Accession numbers (DOC 118 kb)
Supplementary Table S2. List of sequences downloaded from GenBank and their Accession numbers. (DOC 29 kb)
Supplementary File 1. Multiple Sequence Alignment of RPB2 (MAS 50 kb)
Data Availability Statement
All the nucleotide sequence data generated in the study was submitted to NCBI and the list of accession numbers are provided as supplementary table S1.