Abstract
Pentosan polysulfate (PPS) is a semi-synthetic sulfated polysaccharide compound which has been shown the benefits on therapeutic treatment for osteoarthritis (OA) and has been proposed as a disease modifying osteoarthritis drugs (DMOADs). This study investigated the effects of PPS on cell proliferation, particularly in cell cycle modulation and phenotype promotion of canine articular chondrocytes (AC). Canine AC were treated with PPS (0–80 µg/ml) for 24, 48 and 72 hr. The effect of PPS on cell viability, cell proliferation and cell cycle distribution were analyzed by MTT assay, DNA quantification and flow cytometry. Chondrocyte phenotype was analyzed by quantitative real-time PCR (qPCR) and glycosaminoglycan (GAG) quantification. PPS significantly reduced AC proliferation through cell cycle modulation particularly by maintaining a significantly higher proportion of chondrocytes in the G1 phase and a significantly lower proportion in the S phase of the cell cycle in a concentration- and time-dependent manner. While the proportion of chondrocytes in G1 phase corresponded with the significant downregulation of cyclin-dependent kinase (CDK) 1 and 4. Furthermore, the study confirms that PPS promotes a chondrogenic phenotype of AC through significant upregulation of collagen type II (Col2A1) mRNA and GAG synthesis. The effect of PPS on the inhibition of chondrocyte proliferation while promoting a chondrocyte phenotype could be beneficial in the early stages of OA treatment, which transient increase in proliferative activity of chondrocytes with subsequent phenotypic shift and less productive in an essential component of extracellular matrix (ECM) is observed.
Keywords: articular chondrocyte, cell cycle, cyclin-dependent kinases, osteoarthritis, pentosan polysulfate
Articular cartilage is a thin layer of hyaline cartilage covering on the ends of bones, which provides a smooth, lubricated surface of synovial joints and takes an important role to distribute mechanical loads on the joint. The structure of articular cartilage is mainly composed of a dense extracellular matrix (ECM), in which nerves, lymphatics and blood vessels are not contained but consist of only one type of cells called chondrocyte [2, 31]. Although articular cartilage is avascular, chondrocytes still can function in a low oxygen environment and only relies on nutrients from synovial fluid by diffusion through the ECM [1, 2, 31]. Chondrocytes in cartilage are specifically responsible for maintaining homeostasis and turnover rate of ECM structure [1, 31]. In adult normal articular cartilage, chondrocytes are in resting due to the synthetic activity being generally low with almost no proliferative ability [9, 12, 17, 29, 30], which is related to the molecular component of ECM that has an extensively prolonged turnover rate. Among ECM components, collagens half-life could be greater than 100 years, while the half-life of aggrecans could be up to 25 years [27, 31, 35].
Osteoarthritis (OA) is the most common type of cartilage degenerative disease that involves the entire joint structure including articular cartilage, synovial membrane, ligaments and subchondral bones [11, 16, 26]. Metabolic of osteoarthritic cartilage is characterized by an imbalance in both cartilage homeostasis and alteration in the metabolic state of chondrocytes with a shift towards a catabolic state [12, 17]. Even though several studies have improved our understanding on pathogenesis and progression of OA, the precise mechanism of this disease is still not fully identified. During OA progression, chondrocytes undergo a phenotypic shift to be hypertrophic and less productive in essential components of ECM [11, 30]. Genes associated with the chondrogenic phenotype are downregulated, while genes that indicate the hypertrophic change of chondrocytes are upregulated [23, 38]. The transient increase in proliferative activity of chondrocytes has been observed in the initial stage of OA, followed by cell accumulation and cluster formation which is a characteristic feature of OA cartilage [9, 16, 17, 26]. This alteration of cellular arrangement has been shown to affect the quantity and composition of the ECM secreted by the chondrocytes [17, 26]. Changes of the activity in chondrocytes might be due to better access of various factors in synovial fluid through the fissuring, loosening or damaged collagen network [30]. This phenotypic shift has also been observed in-vitro in monolayer chondrocyte cultures in which resting cells isolated from cartilage are cultured under two-dimensional conditions. This sudden change in microenvironment allows chondrocytes to be active and rapidly proliferate [29]. These proliferated chondrocytes gradually lose their chondrogenic phenotype and capability to proliferate, similar to the chondrocytes in OA cartilage [12, 24, 34]. Although the mechanism of phenotypic shift, metabolic activity and cell proliferation between OA cartilage and monolayer cultured chondrocytes are different, intervening in these events might provide us more understanding of chondrocytes physiology and therapeutic targets for OA treatment [8].
The pharmaceutical therapies available for OA are mostly palliative and are unable to reduce disease progression [28]. In recent years, the development of pharmacological therapies has focused not only at relieving the symptoms but on modifying the structural progression of OA. These drugs, which promote cartilage repair concurrent with halting further damage of joints are classified as disease modifying osteoarthritis drugs (DMOADs) [18, 28].
Pentosan polysulfate (PPS) is a low molecular weight heparin-like compound. It is a semi-synthetic drug manufactured from beech-wood hemicellulose that contains anticoagulant and fibrinolytic effects [14, 22]. PPS has been shown to reduce cartilage degradation, improve synovial and subchondral blood flow and to stimulate hyaluronan and proteoglycan synthesis [5, 14, 18, 22]. In fact, several studies have proved the benefits of PPS on OA treatment and recommended it as a prospective DMOADs [6, 14, 22]. However, the mechanism of action of PPS on articular cartilage remains to be fully explained [5, 6, 14, 33]. Furthermore, the effects of PPS on chondrocyte proliferation and cell cycle remain unknown. Therefore, the purpose of this study was to investigate the effects of PPS on cell proliferation, particularly in cell cycle modulation and phenotype promotion of canine AC under monolayer culture conditions.
MATERIALS AND METHODS
Chondrocytes isolation, culture and treatment
Canine articular cartilage was harvested with owners’ formal consent from femoral head cartilages of four different dogs; 4 years old Beagle, 6 years old Toy poodle, 10 years old Shetland sheepdog and 11 years old Pomeranian that underwent femoral head and neck ostectomy due to traumatic coxofemoral luxation. The use of animal samples was in accordance with Hokkaido University Institutional Animal Care and Use Committee guidelines (approval #: 12-0059). Chondrocytes were collected from cartilage by dissection into small pieces and digestion was performed at 37°C overnight using 0.3% collagenase Type I (Wako Pure Chemicals Industries, Osaka, Japan) in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Grand Island, NY, USA). Cell suspension was passed through a 40 µm filter into a sterile 50 ml conical polypropylene tube (Corning, Lowell, MA, USA). Total cell count and viability were assessed by trypan blue (Wako) exclusion test. Primary chondrocytes (P0) were plated in 100 mm diameter polystyrene culture dishes (Corning) and cultured in DMEM containing 10% Fetal bovine serum (FBS; Nichirei Biosciences Inc., Tokyo, Japan), 10 mM HEPES (Dojindo, Kumamoto, Japan), 25 mM NaHCO3 (Wako), 100 U/ml Penicillin G potassium (Wako) and 73 U/ml Streptomycin sulphate (Wako). At 80–90% confluence, P0 were washed twice with Phosphate buffered saline (PBS) and detached using 0.05% Trypsin (Wako) with 0.02% Ethylenediaminetetraacetic acid (EDTA; Dojindo) in PBS and subsequently passaged. For all the experiments, second passage (P2) chondrocytes were seeded in polystyrene culture plates at an initial cell density of 1.2 × 104 cells/cm2 and cultured in DMEM with 10% FBS for 24 hr.
Chondrocytes morphology analysis
After 24 hr culture in 6-well plates (Corning), the culture medium was changed and cells were incubated in the presence (5, 10, 20, 40 and 80 µg/ml) or absence (Control) of PPS (Cartrophen Vet injection, Biopharm Australia, NSW, Australia) for a further 72 hr. Cell morphology, confluency and attachment on culture surfaces were observed under a light microscope.
Analysis of cell viability and cytotoxicity of PPS
After 24 hr culture in 96-well plates (Corning), medium was changed and cells were incubated in the presence or absence of PPS as described above for 24, 48 and 72 hr. Cell viability was evaluated by 3-(4,5-dimehylthiazolyl-2) 2,5-diphenyltetrazolium bromide (MTT; Dojindo) colorimetric assay. After washing cells with PBS, MTT solution (0.5 mg/ml in DMEM) was added into each well and incubated for 4 hr. The solution was then removed and MTT formazan crystals that formed were dissolved by dimethyl sulfoxide (DMSO; Wako). The absorbance was quantified by a microplate reader (Multiskan FC, Thermo Scientific, Vantaa, Finland) at 570 nm. In addition, the cytotoxic effect of PPS was evaluated with Annexin V and Propidium iodide (PI) double stained using FITC Annexin V Apoptosis Detection Kit I (BD Bioscience, Heidelberg, Germany) according to manufacturer’s protocol and analyzed on a flow cytometer (FACS Verse, BD Biosciences).
Cell cycle analysis
The effect of PPS on cell cycle was assessed through PI staining and flow cytometry. After culture for 24 hr in 60 mm culture dishes (Corning), medium was changed and chondrocytes were treated with or without PPS as described above for 24, 48 and 72 hr. Chondrocytes were harvested, washed with PBS and fixed with cold 70% ethanol in distilled water overnight at −20°C. Fixed cells were treated with 100 µg/ml RNase A (Wako) for 30 min at 37°C prior to incubating with 50 µg/ml PI (Sigma-Aldrich, St. Louis, MO, USA) for 10 min at room temperature with light protection. Cell cycle analysis was performed by flow cytometry (FACS Verse) and the results were analyzed by the FlowJo software program (Treestar, Ashland, OR, USA) using Watson Pragmatic model.
DNA and GAG content analysis
After culture for 24 hr in 12-well plates (Corning), medium was changed and chondrocytes were treated with or without PPS as described above for 72 hr. Cell lysates were prepared by digesting in papain solution containing 300 µg/ml papain (Sigma-Aldrich) in 20 mM Na2HPO4 (Wako), 1 mM EDTA and 2 mM Dithiothreitol (Wako) at pH 6.8 for 18 hr at 60°C. The DNA content was determined by Hoechst 33258 assay (Wako) with a calf thymus DNA standard (Sigma-Aldrich), using 350 nm excitation and 460 nm emission filter set. The dimethylmethylene blue (DMMB) assay (Sigma-Aldrich) was used to quantify glycosaminoglycan (GAG) contents with a chondroitin sulphate standard (Wako) at 525 nm. Both assays were measured using a microplate reader (Infinite M200 Pro, Tecan, Männedorf, Switzerland).
RNA isolation and quantitative real-time PCR (qPCR)
To evaluate the effect of PPS on gene expression, P2 chondrocytes were cultured for 24 hr in 60 mm culture dishes (Corning) as described above. The medium was changed, and chondrocytes were treated with 0 (control), 5, 20 and 80 µg/ml of PPS for 24 and 72 hr. Total RNA was extracted using TRIZol reagent (Invitrogen, Carlsbad, CA, USA) and purified with NucleoSpin RNA purification kit (Macherey-Nagel, Dürren, Germany) according to the manufacturer’s instruction. Quantification of RNA was performed by spectrophotometry at 260 nm, while 260/280 nm and 260/230 nm absorbance ratio were used to evaluated RNA quality. Total of one microgram RNA was reverse transcribed into cDNA with M-MLV RT kit (Invitrogen) according to manufacturer’s recommended protocol. qPCR reaction was performed with KAPA SYBR FAST qPCR kit (KAPA Biosystems, Woburn, MA, USA) to determine changes in expression of mRNA. Each gene was validated by presence of a single peak in melt curve analysis, and standard curve was used to identify the primer efficiency. The PCR products of each gene were sequenced to confirm the specificity of primers. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization. Collagen type II (Col2A1) and matrix metalloproteinase 13 (MMP13) were used to evaluated chondrogenic phenotype. Cyclin-dependent kinases (CDK) including CDK1, 2, 4 and 6 were used to evaluate the cell cycle regulation. The level of expression of each target gene was calculated using delta delta CT (ddCT) method. Sequence of primers using in the experiment were designed according to the data published on the National Center for Biotechnology Information (NCBI) website using BLAST programs. The sequence, amplicon length and accession number for each of primers are indicated in (Table 1).
Table 1. Sequence of primers used to evaluate gene expression in the experiment.
| Target gene | Primer sequence | Amplicon length (bp) | Accession number |
|---|---|---|---|
| GAPDH | Forward: 5′-CTGAACGGGAAGCTCACTGG-3′ | 129 | NM_001003142.1 |
| Reverse: 5′-CGATGCCTGCTTCACTACCT-3′ | |||
| CDK1 | Forward: 5′-TGTATGTGCTGTGCCATCGG-3′ | 150 | XM_003639013.4 |
| Reverse: 5′-GCCTCCAGGTCTTTGAAGCA-3′ | |||
| CDK2 | Forward: 5′-CTCTAGCGCTTGCTTCATGG-3′ | 72 | XM_005625479.3 |
| Reverse: 5′-TACACAACTCCGTACGTGCC-3′ | |||
| CDK4 | Forward: 5′-TAGCTTGCGGCCTGTCTATG-3′ | 145 | XM_844780.5 |
| Reverse: 5′-CAGAGAAGACCCTCACTCGG-3′ | |||
| CDK6 | Forward: 5′-AGCCAAACGTCCCTAGAAGC-3′ | 121 | XM_022427346.1 |
| Reverse: 5′-GAGAGATGCCTGGTAGACGC-3′ | |||
| Collagen Type II | Forward: 5′-CACTGCCAACGTCCAGATGA-3′ | 215 | NM_001006951.1 |
| Reverse: 5′-GTTTCGTGCAGCCATCCTTC-3′ | |||
| MMP13 | Forward: 5′-GGCTTAGAGGTCACTGGCAAAC-3′ | 118 | XM_022418390.1 |
| Reverse: 5′-TGGACCACTTGAGAGTTCGGG-3′ |
Statistical analysis
Statistical analysis was performed using GraphPad Prism software version 8.2.0 (GraphPad Software Inc., La Jolla, CA, USA). All quantitative results are presented as mean ± standard error of mean (SEM). Statistical comparisons were performed using analysis of variance (ANOVA), with an appropriate post hoc test to compare between groups. Correlation between PPS concentration, cell viability and cell cycle distribution were calculated using Pearson correlation coefficient. P-value <0.05 was considered statistically significantly different.
RESULTS
Effect of PPS on the morphology and proliferative activity of chondrocytes
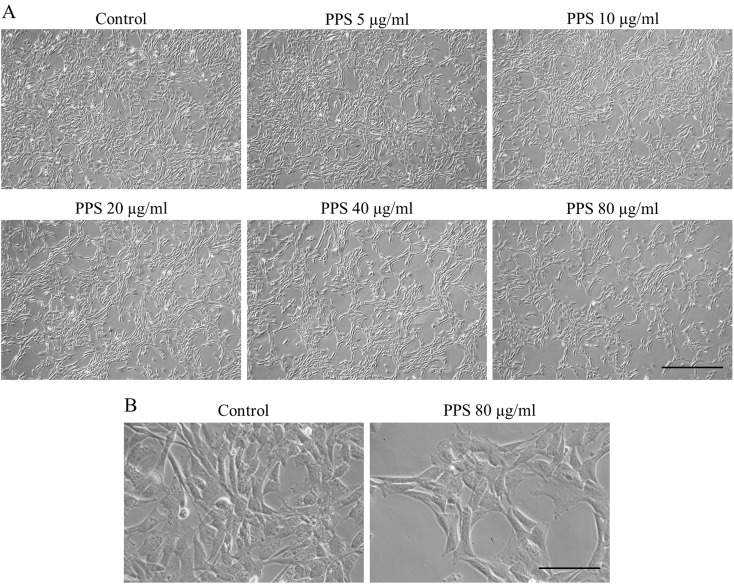
After culture with PPS for 72 hr, a decrease in chondrocyte number was observed at PPS concentration of 20 µg/ml and was evident at higher concentrations of 40 and 80 µg/ml compared to control (Fig. 1A). However, there was no morphological difference observed between the groups (Fig. 1B). As expected, chondrocytes in all treatment groups exhibited the typical fibroblast-like shape observed in monolayer culture.
Fig. 1.
Morphological appearance and confluency condition of pentosan polysulfate (PPS) treated chondrocytes observed under a light microscope. Chondrocytes were cultured as a monolayer for 24 hr prior to the treatment with various concentrations of PPS (0, 5, 10, 20, 40 and 80 µg/ml) for 72 hr. (A) Magnification: ×40, Scale bar: 500 µm. (B) Magnification: ×200, Scale bar: 100 µm.
PPS affects chondrocyte viability
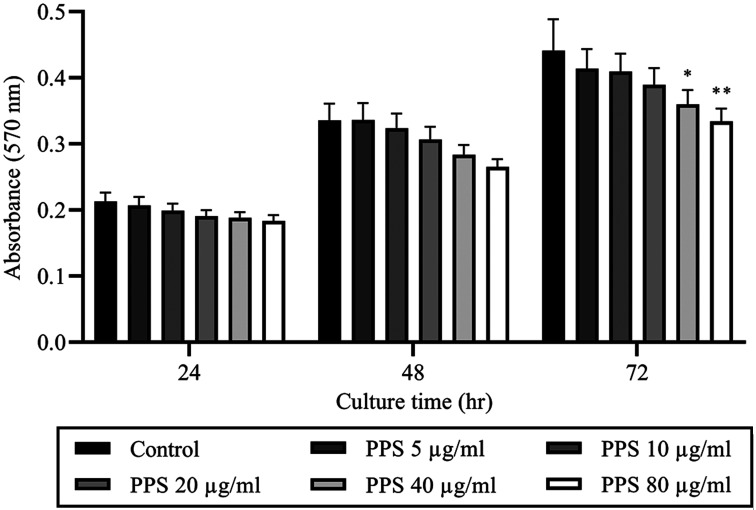
The concentration effect of PPS on chondrocyte viability was shown in (Fig. 2). The results showed that chondrocyte viability was reduced by PPS in concentration-dependent pattern while significant reduction was only observed at 40 (P=0.045) and 80 µg/ml (P=0.007) at time point of 72 hr of culture. Treatment with PPS for 72 hr at 5, 10, 20, 40 and 80 µg/ml reduced relative chondrocyte viability to 96.56 ± 4.6, 94.11 ± 4.23, 87.41 ± 3.36, 79.27 ± 2.91 and 71.42 ± 2.38%, respectively compared to control (100%) (Fig. 2). There was a significant negative correlation between PPS concentration and chondrocyte viability at 24 (r= −0.839; P=0.037), 48 (r= −0.959; P=0.003) and 72 hr (r= −0.945; P=0.005).
Fig. 2.
Treatment with pentosan polysulfate (PPS) resulted in reduced chondrocyte viability. Chondrocytes were cultured as a monolayer for 24 hr prior to the treatment with various concentrations of PPS (0, 5, 10, 20, 40 and 80 µg/ml) for 72 hr. The cell viability of cultured chondrocytes was analyzed by MTT assay at 24, 48 and 72 hr during PPS treatment. The data are expressed as the mean ± SEM (*P<0.05 and **P<0.01).
PPS (5–80 µg/ml) has no cytotoxic effect on chondrocytes
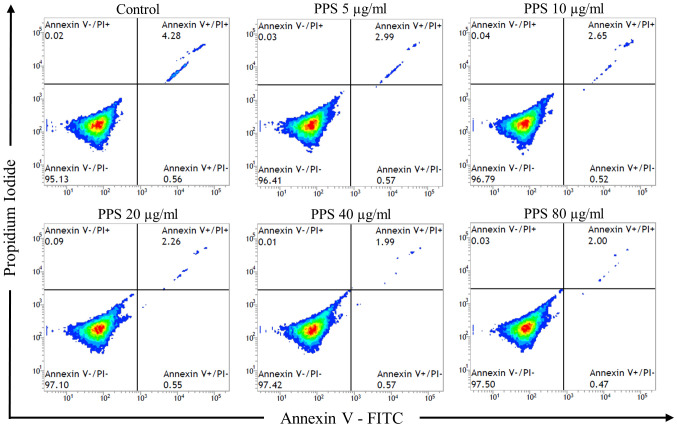
Cytotoxic effect of PPS on chondrocytes as evaluated with Annexin V and PI staining with flow cytometry revealed a similar pattern of cell distribution among chondrocytes exposed to various concentrations of PPS and the control at 72 hr (Fig. 3). The classification of viable cells and non-viable cells (including early apoptotic, late apoptotic and necrotic cells) showed no significant difference (P>0.05) between the groups. The percentage of viable cells in PPS treated chondrocytes at 72 hr with 5, 10, 20, 40 and 80 µg/ml were 95.9 ± 0.6, 96.3 ± 0.4, 95.9 ± 0.8, 96.1 ± 0.8 and 96.1 ± 0.8%, respectively compared to control (94.2 ± 0.7%).
Fig. 3.
Treatment with pentosan polysulfate (PPS) showed no cytotoxic effect on cultured chondrocytes. Chondrocytes were cultured as a monolayer for 24 hr prior to the treatment with various concentrations of PPS (0, 5, 10, 20, 40 and 80 µg/ml) for 72 hr. Cell apoptosis was evaluated by flow cytometry analysis with annexin V and propidium iodide (PI) staining at 72 hr after exposure to PPS. Flow cytometry results showed the percentage of cells binding to annexin V and PI.
PPS increases the proportion of chondrocytes in G1 phase while reducing the proportion of chondrocytes in S phase of the cell cycle
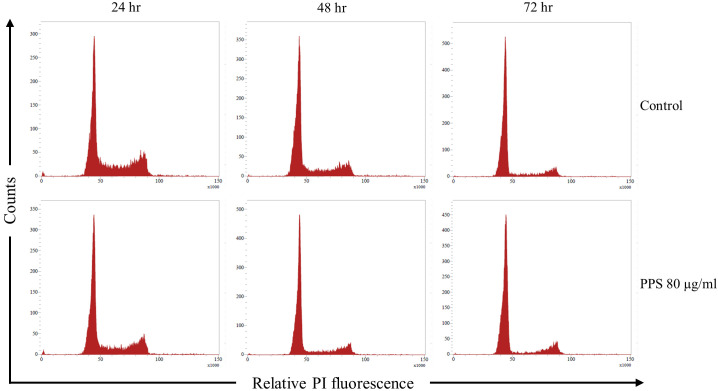
Effects of PPS on cell cycle distribution were evaluated using PI staining and flow cytometry (Fig. 4). Compared to the control, after 24 hr of treatment with PPS; chondrocytes in G1 phase were significantly increased (P=0.001) only at 80 µg/ml of PPS. Chondrocytes in S phase were significantly reduced at 20 (P=0.007), 40 (P=0.005) and 80 µg/ml (P<0.001) of PPS after 24 hr treatment (Table 2). Similarly, after 48 hr of PPS treatment; chondrocytes in S phase were significantly reduced at 40 (P=0.002) and 80 µg/ml (P<0.001) of PPS (Table 2). There was a strong positive correlation observed between the PPS concentration and number of chondrocytes distributed in G1 phase at 24 (r=0.986, P<0.001) and 48 hr (r=0.923, P<0.001) of treatment. Conversely, chondrocytes distribution in S phase demonstrated a strong negative correlation between PPS concentration and number of chondrocytes in S phase at 24 (r= −0.981, P<0.001) and 48 hr (r= −0.973, P=0.001) of treatment. However, there was no significant correlation (P>0.05) on both G1 and S phase with PPS treatment at 72 hr. There was a significant negative correlation between PPS concentration and chondrocytes distribution in G2 phase at 48 hr (r= −0.858, P=0.029) and a significant positive correlation at 72 hr (r=0.815, P=0.048) of treatment.
Fig. 4.
Pentosan polysulfate (PPS) increases the proportion of chondrocytes distributed in the G1 phase while reducing the proportion of chondrocytes distributed in the S phase of the cell cycle. Chondrocytes were cultured as a monolayer for 24 hr prior to the treatment with various concentrations of PPS (0, 5, 10, 20, 40 and 80 µg/ml) for 72 hr. Cell cycle was analyzed by flow cytometry and propidium iodide (PI) staining at 24, 48 and 72 hr during PPS treatment. A represents histogram showing the cell distribution pattern between control and treatment with PPS at 80 µg/ml. The results were analyzed by the FlowJo software program using Watson Pragmatic model.
Table 2. Percentage of cells in each phase of cell cycle analyzed by the FlowJo software program using Watson Pragmatic model.
| Cell cycle phase | Pentosan polysulfate concentration (µg/ml) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 20 | 40 | 80 | ||
| 24 hr | |||||||
| G1 (%) | 56.33 ± 2.37 | 56.65 ± 2.34 | 57.26 ± 1.95 | 59.14 ± 2.06 | 60.03 ± 2.23 | 63.41 ± 2.39a) | |
| S (%) | 30.36 ± 1.67 | 30.31 ± 1.66 | 29.11 ± 1.45 | 27.75 ± 1.48a) | 26.23 ± 1.41a) | 23.54 ± 1.44b) | |
| G2 (%) | 10.41 ± 0.67 | 10.43 ± 0.60 | 10.79 ± 0.63 | 10.25 ± 0.51 | 10.14 ± 0.74 | 9.93 ± 0.50 | |
| 48 hr | |||||||
| G1 (%) | 67.29 ± 2.21 | 66.30 ± 2.85 | 67.25 ± 3.18 | 68.49 ± ±2.67 | 70.81 ± 3.20 | 71.54 ± 3.06 | |
| S (%) | 21.86 ± 1.93 | 21.98 ± 2.17 | 21.79 ± 2.35 | 20.53 ± 2.14 | 18.30 ± 2.27b) | 16.79 ± 2.18b) | |
| G2 (%) | 7.95 ± 0.57 | 8.57 ± 0.60 | 7.99 ± 0.43 | 8.08 ± 0.50 | 7.85 ± 0.60 | 7.22 ± 0.65 | |
| 72 hr | |||||||
| G1 (%) | 77.91 ± 1.85 | 80.76 ± 0.90 | 81.49 ± 1.30 | 80.13 ± 1.45 | 80.44 ± 1.60 | 78.14 ± 1.51 | |
| S (%) | 11.95 ± 1.28 | 9.66 ± 0.86 | 10.45 ± 0.96 | 10.95 ± 0.92 | 10.82 ± 0.96 | 11.16 ± 0.90 | |
| G2 (%) | 6.15 ± 0.44 | 6.20 ± 0.43 | 6.53 ± 0.36 | 6.90 ± 0.44 | 7.39 ± 0.53 | 7.23 ± 0.57 | |
The data are expressed as the mean ± SEM (a) P<0.01 and b) P<0.001).
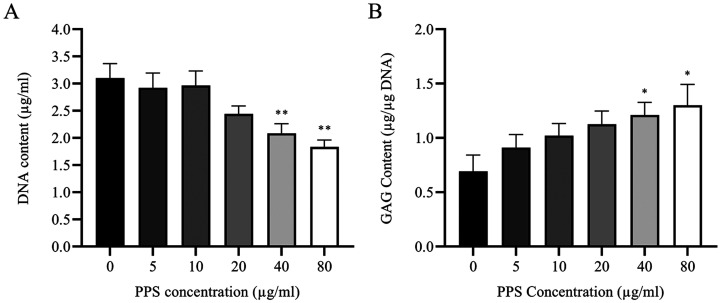
Effect of PPS on chondrocytes DNA and GAG Content
After treatment of chondrocytes with PPS for 72 hr, there was a decrease in the content of DNA in cell lysates as quantified by Hoechst 33258 assay with significant difference observed at 40 and 80 µg/ml relative to the control (Fig. 5A). DNA content of chondrocytes treated with PPS at 40 and 80 µg/ml was significantly decreased to 2.086 ± 0.175 (P=0.01) and 1.834 ± 0.128 µg/ml (P=0.001), respectively compared to control (3.101 ± 0.268 µg/ml) (Fig. 5A).
Fig. 5.
Treatment with pentosan polysulfate (PPS) promotes glycosaminoglycan (GAG) synthesis but reduces DNA content of chondrocytes in a concentration-dependent manner. Chondrocytes were cultured as a monolayer for 24 hr prior to the treatment with various concentrations of PPS (0, 5, 10, 20, 40 and 80 µg/ml) for 72 hr. Biochemical analysis was performed at 72 hr after exposure to PPS. (A) Quantification of DNA content in cell lysates by Hoechst assay. (B) Quantification of GAG content (normalized with DNA content) in cell lysates by dimethylmethylene blue (DMMB) assay. The data are expressed as the mean ± SEM (*P<0.05 and **P<0.01).
DMMB assay revealed that greater amount of GAG was synthesized in PPS treatment in a concentration-dependent pattern, although significant increase was only observed at higher PPS concentrations of 40 (P=0.049) and 80 µg/ml (P=0.016) compared to control (Fig. 5B). Total amount of GAG normalized with DNA content in PPS treated chondrocytes at 72 hr with 5, 10, 20, 40 and 80 µg/ml were 0.91 ± 0.12, 1.02 ± 0.11, 1.12 ± 0.12, 1.21 ± 0.12 and 1.30 ± 0.19 µg/µg DNA, respectively compared to control (0.69 ± 0.15 µg/µg DNA) (Fig. 5B).
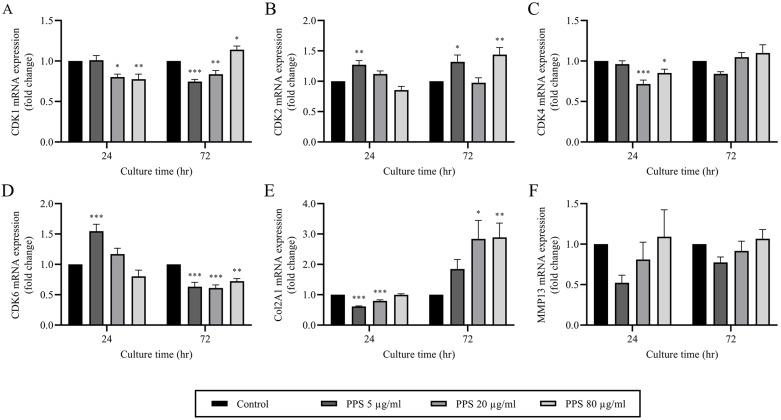
PPS downregulates cell cycle regulator genes and promotes a chondrogenic phenotype
The results from qPCR analysis revealed a marked decrease in CDK1 and 4 mRNA expression in chondrocytes treated with 40 (P=0.013 and P <0.001, respectively) and 80 µg/ml of PPS (P=0.005 and P=0.033, respectively) at 24 hr compared to control (Fig. 6A and 6C). However, CDK2 and 6 expressions remained unchanged between the treatments except in chondrocytes treated with PPS at 5 µg/ml in which both genes were significantly upregulated (P<0.05) (Fig. 6B and 6D). Notably, after 72 hr of treatment, CDK2 expression remained significantly upregulated (P<0.05) at 5 µg/ml of PPS and was also significantly upregulated at 80 µg/ml of PPS. Conversely, at 72 hr of treatment CDK6 was significantly downregulated (P<0.01) at all PPS concentrations (Fig. 6D). The expression of CDK1 was decreased in the presences of PPS at 5 and 20 µg/ml (P<0.001 and P=0.008, respectively), whereas at 80 µg/ml (P=0.024) of PPS it was significantly increased relative to the control at 72 hr of treatment. On the other hand, there was no significant difference (P>0.05) in CDK4 expression between the groups at 72 hr of treatment.
Fig. 6.
Pentosan polysulfate (PPS) downregulates cell cycle regulator genes, while promoting a chondrocyte phenotype. Chondrocytes were cultured as a monolayer for 24 hr prior to the treatment with various concentrations of PPS (0, 5, 20 and 80 µg/ml) for 72 hr. Relative mRNA expression of chondrocytes was evaluated by quantitative real-time PCR (qPCR) analysis at 24 and 72 hr during PPS treatment. The relative mRNA expression of (A) cyclin-dependent kinase (CDK) 1, (B) CDK2, (C) CDK4, (D) CDK6, (E) collagen type II (Col2A1) and (F) matrix metalloproteinase 13 (MMP13) were normalized to the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The data are expressed as the mean ± SEM (*P<0.05, **P<0.01 and ***P<0.001).
The specific gene for cartilage, Col2A1 was expressed by chondrocytes in all culture groups. Interestingly, treatment with PPS for 24 hr, demonstrated a significant decrease (P<0.001) in Col2A1 expression at 5 and 20 µg/ml relative to the control. However, at 72 hr of treatment with PPS, there was a concentration-dependent upregulation of Col2A1 with significant difference (P<0.05) being observed at 20 (P=0.011) and 80 µg/ml (P=0.009) compared to the control (Fig. 6E). There was no significant difference in the gene expression for MMP13 between PPS treated chondrocytes and the control at all treatment times (Fig. 6F).
DISCUSSION
The transient increase in proliferative activity of chondrocytes followed by cell accumulation and cluster formation are a hallmark of early stages of OA cartilage [9, 16, 17, 26]. Although the mechanism of phenotypic shift, metabolic activity and cell proliferation between OA cartilage and monolayer culture chondrocytes are different, intervening in these events might provide us more understanding about chondrocyte physiology and potential targets for OA treatment. While there are many studies that have demonstrated the anabolic and anti-inflammatory effects of PPS, to the best of our knowledge, this is the first study to demonstrate its effects on cell proliferation and cell cycle regulators. The present study demonstrates that PPS reduces chondrocyte viability and proliferation not through PPS-induced cell death but by maintaining a high proportion of cells in the G1 phase and a low proportion in the S phase of cell cycle in the early stages of monolayer culture (24 hr). The modulation of cell cycle by PPS appears to be through the short-term inhibition of cell cycle genes, particularly CDK1 and 4. Consistent with previous findings [6, 14], our results validate the use of PPS as a chondrogenic phenotype promoter as evidenced by the significant upregulation of cartilage-specific markers, Col2A1 and GAG. Therefore, the use of PPS may be beneficial in inhibiting chondrocyte proliferation while promoting a chondrocyte phenotype in early stages of OA treatment in which chondrocytes are observed to undergo transient proliferation followed by a reduction in ECM synthesis.
According to MTT colorimetric and DNA quantitative assays, PPS reduced canine AC viability in a concentration- and time-dependent pattern with a higher concentration of 40 and 80 µg/ml demonstrating a significantly lower cell viability relative to the control at 72 hr of culture but with no significant difference observed between the treatments at 24 and 48 hr of culture (Fig. 2). Contrary to the findings of this study, a previous study demonstrated that PPS possessed the ability to promote cell proliferation in mesenchymal precursor cells (MPC) [14, 32]. The disparity in cell viability between chondrocytes and MPC could reflect reduced metabolic activity in chondrocytes cultured in PPS compared to MPC in which MTT is highly reduced due to their rapid proliferation activity promoted by PPS. In fact, by Annexin V and PI staining with flow cytometry the present study demonstrated that the significant reduction in cell viability and DNA content was not due to PPS-induced cell death as confirmed by the same pattern of cell distribution among chondrocytes exposed to various concentrations of PPS and the control. The classification of viable and non-viable cells (including early apoptotic, late apoptotic and necrotic cells) also showed no significant difference (P>0.05) between the groups (Fig. 3). These findings clearly indicate the safety of PPS on chondrocytes even at high concentration and this is consistent with other related studies on cytotoxic effects of PPS on chondrocytes [5,6,7, 14, 15, 33]. The difference in response between chondrocytes and MPC has been attributed to their diversity in cell properties and expression patterns [3, 21]. Dedifferentiation of chondrocytes is an adverse phenomenon that commonly occurs in the process of expanding chondrocytes. Once chondrocytes start to proliferate, their characteristics rapidly change, together with their morphology and finally, these chondrocytes completely lose their own phenotype [36]. Moreover, synthetic activities of chondrocytes are found to be inversely related to proliferation activities [29]. Therefore, the inhibition of chondrocyte proliferation by PPS demonstrated in this study could be of benefit to maintain appropriate chondrogenic phenotype and thus could be beneficial in the early stages of OA cartilage where a transient increase in proliferative activity of chondrocytes has been observed [9, 16, 17, 26].
Cell cycle analysis showed that the treatment of chondrocytes with PPS at different time points significantly reduced the proportion of cells in the S phase while increasing the proportion of cells in the G1 phase. However, this effect was only observed during the high proliferative stage of 24 and 48 hr with no significant difference observed at 72 hr of PPS treatment. In fact, we observed a strong positive correlation between the PPS concentration and chondrocytes distributed in the G1 phase at 24 and 48 hr of treatment and not at 72 hr time point. Conversely, there was a strong negative correlation between PPS concentration and chondrocytes in the S phase at 24 and 48 hr of treatment and not 72 hr time point. There was a significant negative correlation between PPS concentration and chondrocytes in the G2 phase at 48 hr but at 72 hr time point PPS concentration demonstrated a significant positive correlation with chondrocytes in the G2 phase of the cell cycle. Taken together, this finding suggests that PPS may increase the proportion of chondrocytes in the G1 phase while reducing the number of chondrocytes in the S and G2 phases of the cell cycle. However, this effect is transient (24–48 hr), concentration-dependent and is evidently lost by 72 hr of culture. We speculate that an increase in cell density might trigger contact inhibition process, causing mediation from various pathways that are involved in the cell cycle [13].
To understand how PPS modulates the cell cycle, we assessed the expression of genes involved in the cell cycle. During cell proliferation, in G1 to S phase, CDK4 and 6 forms a complex with Cyclin D to phosphorylate the retinoblastoma protein and allow cell cycle progression through G1, while CDK2/Cyclin A and CDK2/Cyclin E complex activate DNA synthesis [4, 13, 25]. The activation of CDK1/Cyclin B control cell mitosis, and together, CDK1 is a major kinase that could form a complex with Cyclin D, E and A, instead of CDK2, 4 and 6 for correct progression in the cell cycle [4]. The increase in the proportion of cells in G1 phase and subsequent decrease in S phase under treatment PPS was observed only at a high proliferative stage (24 hr) of chondrocytes, which corresponded to the downregulation of CDK1 and 4 expressions but not CDK2 and 6. In fact, CDK2 and 6 were significantly upregulated at a low concentration of PPS. Judging from these results, we could speculate that PPS modulates the cell cycle mainly through major kinase CDK1, together with CDK4. The inhibition of cell-cycle progression has been previously shown to occur via the downregulation of nuclear factor-kappa B (NF-κB) [20] and previously our laboratory demonstrated that PPS inhibits nuclear translocation of NF-κB [5, 33]. Therefore, the inhibition of CDK1 and 4 mRNA expression in this study could be consistent with the inhibitory effects of PPS on nuclear translocation of NF-κB. Interestingly, the effect of PPS on CDK1, 2 and 4 expressions at a low proliferative stage (72 hr) were directly proportional to PPS concentration. However, cell cycle analysis revealed an unchanged cell proportion on any phase of the cell cycle.
Incubation of chondrocytes with PPS resulted in significantly higher expression of cartilage specific markers Col2A1 and GAG compared to control. These results are in agreement with previous studies [6, 14]. Furthermore, the upregulation of Col2A1 corresponded with the significant downregulation of CDK6 at 72 hr whereas the significant upregulation of CDK6 at 24 hr corresponded to a significant downregulation of Col2A1. CDK6 has been shown to induce c-Jun phosphorylation and lead to suppression of Col2A1 and Sox-9 [19]. Moreover, previous studies have demonstrated that an increase in the proliferation rate of OA chondrocytes compared to healthy chondrocytes is directly related to higher gene expression of cell cycle regulators, Cyclin D and CDK6 [10]. PPS showed no significant effect on the expression of MMP13. Previous studies have shown that PPS can downregulate Interleukin-1 (IL-1)-induced MMP13 upregulation in canine AC in-vitro [5]. This finding demonstrates the unique selective inhibition of only inflammatory cytokine-induced MMP13 upregulation by PPS but not the constitutively expressed MMP13 which is required to maintain homeostasis of ECM turnover in normal articular cartilage [37].
There were several limitations in this study, including a small sample size of only four cartilage samples. Furthermore, the exact mechanism and pathway by which PPS modulates CDK expression and the relation between cell proliferation and chondrogenic phenotypes of canine AC were not fully elucidated. Although our results suggest that PPS could preserve chondrogenic phenotypes by reducing chondrocyte proliferation via downregulating the expression of CDK, other factors related to CDK pathway and phenotypic regulators were not included in this study. In addition, CDK expression in this study was shown at mRNA level only which is considered inferior to the analysis of the active protein. While further investigation should examine these findings in-vivo, the present study brings to light for the first time the in-vitro effects of PPS on chondrocyte proliferation and cell cycle which could be beneficial to OA cartilage treatment.
In conclusion, this study demonstrates that PPS reduces canine AC proliferation in monolayer culture through cell cycle modulation particularly by maintaining a significantly higher proportion of chondrocytes in the G1 phase and a significantly lower proportion in the S phase of the cell cycle in a concentration and time-dependent manner. The inhibition of chondrocyte proliferation appears to be through the short-term inhibition of cell cycle regulator genes, particularly CDK1 and 4. The study further confirms that PPS promotes a chondrogenic phenotype of canine AC through significant upregulation of Col2A1 mRNA and GAG synthesis. The inhibition of chondrocyte proliferation while promoting a chondrocyte phenotype by PPS could be beneficial in the early stages of OA treatment in which transient increase in proliferative activity of chondrocytes with subsequent phenotypic shift and less productive in an essential component of ECM is observed.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Akkiraju H., Nohe A.2015. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J. Dev. Biol. 3: 177–192. doi: 10.3390/jdb3040177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer C. W., Francis-West P.2003. The chondrocyte. Int. J. Biochem. Cell Biol. 35: 401–404. doi: 10.1016/S1357-2725(02)00301-1 [DOI] [PubMed] [Google Scholar]
- 3.Bernstein P., Sticht C., Jacobi A., Liebers C., Manthey S., Stiehler M.2010. Expression pattern differences between osteoarthritic chondrocytes and mesenchymal stem cells during chondrogenic differentiation. Osteoarthritis Cartilage 18: 1596–1607. doi: 10.1016/j.joca.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 4.Berridge M. J.2014. Cell cycle and proliferation. Cell Signal. Biol. 6: csb0001009. doi: 10.1042/csb0001009 [DOI] [Google Scholar]
- 5.Bwalya E. C., Kim S., Fang J., Wijekoon H. M. S., Hosoya K., Okumura M.2017. Pentosan polysulfate inhibits IL-1β-induced iNOS, c-Jun and HIF-1α upregulation in canine articular chondrocytes. PLoS One 12: e0177144. doi: 10.1371/journal.pone.0177144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bwalya E. C., Kim S., Fang J., Wijekoon H. M. S., Hosoya K., Okumura M.2018. Pentosan polysulfate sodium restores the phenotype of dedifferentiated monolayer canine articular chondrocytes cultured in alginate beads. J. Tissue Sci. Eng. 09: 1–10. doi: 10.4172/2157-7552.1000218 [DOI] [Google Scholar]
- 7.Bwalya E. C., Kim S., Fang J., Wijekoon H. M. S., Hosoya K., Okumura M.2017. Effects of pentosan polysulfate and polysulfated glycosaminoglycan on chondrogenesis of canine bone marrow-derived mesenchymal stem cells in alginate and micromass culture. J. Vet. Med. Sci. 79: 1182–1190. doi: 10.1292/jvms.17-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charlier E., Deroyer C., Ciregia F., Malaise O., Neuville S., Plener Z., Malaise M., de Seny D.2019. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 165: 49–65. doi: 10.1016/j.bcp.2019.02.036 [DOI] [PubMed] [Google Scholar]
- 9.Charlier E., Relic B., Deroyer C., Malaise O., Neuville S., Collée J., Malaise M. G., De Seny D.2016. Insights on molecular mechanisms of chondrocytes death in osteoarthritis. Int. J. Mol. Sci. 17: 2146. doi: 10.3390/ijms17122146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Andrés M. C., Takahashi A., Oreffo R. O. C.2016. Demethylation of an NF-κB enhancer element orchestrates iNOS induction in osteoarthritis and is associated with altered chondrocyte cell cycle. Osteoarthritis Cartilage 24: 1951–1960. doi: 10.1016/j.joca.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 11.Dreier R.2010. Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Res. Ther. 12: 216. doi: 10.1186/ar3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García-Carvajal Z. Y.,, Garciadiego-Czares D.,, Parra- Cid C., Aguilar-Gaytn R., Velasquillo C., Ibarra C., Castro Carmo Cartilage Tissue Engineering: The Role of Extracellular Matrix (ECM) and Novel Strategies. pp. 365–397. In: Regenerative Medicine and Tissue Engineering, InTech, Rijeka. [Google Scholar]
- 13.Gérard C., Goldbeter A.2014. The balance between cell cycle arrest and cell proliferation: control by the extracellular matrix and by contact inhibition. Interface Focus 4: 20130075. doi: 10.1098/rsfs.2013.0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh P., Wu A., Shimmon S., Gronthos S., Zannettino A., Itescu S.2009. Pentosan polysulfate promotes proliferation and chondrogenic differentiation of adult human bone marrow-derived mesenchymal precursor cells. Osteoarthritis Cartilage 17: S101. doi: 10.1016/S1063-4584(09)60193-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh P.1999. The pathobiology of osteoarthritis and the rationale for the use of pentosan polysulfate for its treatment. Semin. Arthritis Rheum. 28: 211–267. doi: 10.1016/S0049-0172(99)80021-3 [DOI] [PubMed] [Google Scholar]
- 16.Goldring M. B.2012. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther. Adv. Musculoskelet. Dis. 4: 269–285. doi: 10.1177/1759720X12448454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldring M. B., Marcu K. B.2009. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 11: 224. doi: 10.1186/ar2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henrotin Y., Sanchez C., Balligand M.2005. Pharmaceutical and nutraceutical management of canine osteoarthritis: present and future perspectives. Vet. J. 170: 113–123. doi: 10.1016/j.tvjl.2004.08.014 [DOI] [PubMed] [Google Scholar]
- 19.Hwang S. G., Song S. M., Kim J. R., Park C. S., Song W. K., Chun J. S.2007. Regulation of type II collagen expression by cyclin-dependent kinase 6, cyclin D1, and p21 in articular chondrocytes. IUBMB Life 59: 90–98. doi: 10.1080/15216540701245022 [DOI] [PubMed] [Google Scholar]
- 20.Jhou R. S., Sun K. H., Sun G. H., Wang H. H., Chang C. I., Huang H. C., Lu S. Y., Tang S. J.2009. Inhibition of cyclin-dependent kinases by olomoucine and roscovitine reduces lipopolysaccharide-induced inflammatory responses via down-regulation of nuclear factor kappaB. Cell Prolif. 42: 141–149. doi: 10.1111/j.1365-2184.2009.00584.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson C., Brantsing C., Svensson T., Brisby H., Asp J., Tallheden T., Lindahl A.2007. Differentiation of human mesenchymal stem cells and articular chondrocytes: analysis of chondrogenic potential and expression pattern of differentiation-related transcription factors. J. Orthop. Res. 25: 152–163. doi: 10.1002/jor.20287 [DOI] [PubMed] [Google Scholar]
- 22.Kumagai K., Shirabe S., Miyata N., Murata M., Yamauchi A., Kataoka Y., Niwa M.2010. Sodium pentosan polysulfate resulted in cartilage improvement in knee osteoarthritis--an open clinical trial. BMC Clin. Pharmacol. 10: 7. doi: 10.1186/1472-6904-10-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H., Wang D., Yuan Y., Min J.2017. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res. Ther. 19: 248. doi: 10.1186/s13075-017-1454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Z., Fitzgerald J. B., Xu J., Willers C., Wood D., Grodzinsky A. J., Zheng M. H.2008. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J. Orthop. Res. 26: 1230–1237. doi: 10.1002/jor.20523 [DOI] [PubMed] [Google Scholar]
- 25.LuValle P., Beier F.2000. Cell cycle control in growth plate chondrocytes. Front. Biosci. 5: D493–D503. doi: 10.2741/A529 [DOI] [PubMed] [Google Scholar]
- 26.Maldonado M., Nam J.2013. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. BioMed Res. Int. 2013: 284873. doi: 10.1155/2013/284873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maroudas A., Bayliss M. T., Uchitel-Kaushansky N., Schneiderman R., Gilav E.1998. Aggrecan turnover in human articular cartilage: use of aspartic acid racemization as a marker of molecular age. Arch. Biochem. Biophys. 350: 61–71. doi: 10.1006/abbi.1997.0492 [DOI] [PubMed] [Google Scholar]
- 28.Mobasheri A.2013. The future of osteoarthritis therapeutics: targeted pharmacological therapy. Curr. Rheumatol. Rep. 15: 364. doi: 10.1007/s11926-013-0364-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otero M., Favero M., Dragomir C., Hachem K. E., Hashimoto K., Plumb D. A., Goldring M. B.2012. Human chondrocyte cultures as models of cartilage-specific gene regulation. Methods Mol. Biol. 806: 301–336. doi: 10.1007/978-1-61779-367-7_21 [DOI] [PubMed] [Google Scholar]
- 30.Sandell L. J., Aigner T.2001. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 3: 107–113. doi: 10.1186/ar148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sophia Fox A. J., Bedi A., Rodeo S. A.2009. The basic science of articular cartilage: structure, composition, and function. Sports Health 1: 461–468. doi: 10.1177/1941738109350438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sørensen H. P., Vivès R. R., Manetopoulos C., Albrechtsen R., Lydolph M. C., Jacobsen J., Couchman J. R., Wewer U. M.2008. Heparan sulfate regulates ADAM12 through a molecular switch mechanism. J. Biol. Chem. 283: 31920–31932. doi: 10.1074/jbc.M804113200 [DOI] [PubMed] [Google Scholar]
- 33.Sunaga T., Oh N., Hosoya K., Takagi S., Okumura M.2012. Inhibitory effects of pentosan polysulfate sodium on MAP-kinase pathway and NF-κB nuclear translocation in canine chondrocytes in vitro. J. Vet. Med. Sci. 74: 707–711. doi: 10.1292/jvms.11-0511 [DOI] [PubMed] [Google Scholar]
- 34.van der Kraan P. M., van den Berg W. B.2012. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage 20: 223–232. doi: 10.1016/j.joca.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 35.Verzijl N., DeGroot J., Thorpe S. R., Bank R. A., Shaw J. N., Lyons T. J., Bijlsma J. W. J., Lafeber F. P. J. G., Baynes J. W., TeKoppele J. M.2000. Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 275: 39027–39031. doi: 10.1074/jbc.M006700200 [DOI] [PubMed] [Google Scholar]
- 36.Villar-Suárez V., Calles-Venal I., Bravo I. G., Fernández-Alvarez J. G., Fernández-Caso M., Villar-Lacilla J. M.2004. Differential behavior between isolated and aggregated rabbit auricular chondrocytes on plastic surfaces. J. Biomed. Biotechnol. 2004: 86–92. doi: 10.1155/S1110724304312039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto K., Okano H., Miyagawa W., Visse R., Shitomi Y., Santamaria S., Dudhia J., Troeberg L., Strickland D. K., Hirohata S., Nagase H.2016. MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biol. 56: 57–73. doi: 10.1016/j.matbio.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong L., Huang X., Karperien M., Post J. N.2016. Correlation between gene expression and osteoarthritis progression in human. Int. J. Mol. Sci. 17: 1126. doi: 10.3390/ijms17071126 [DOI] [PMC free article] [PubMed] [Google Scholar]