Abstract
Nonalcoholic fatty liver disease (NAFLD) is characterized by hepatic fat accumulation and impaired insulin sensitivity. Reduced hepatic ketogenesis may promote these pathologies, but data are inconclusive in humans and the link between NAFLD and reduced insulin sensitivity remains obscure. We investigated individuals with obesity-related NAFLD and hypothesized that β-hydroxybutyrate (βOHB; the predominant ketone species) would be reduced and related to hepatic fat accumulation and insulin sensitivity. Furthermore, we hypothesized that ketones would impact skeletal muscle mitochondrial respiration in vitro. Hepatic fat was assessed by 1H-MRS in 22 participants in a parallel design, case control study [Control: n = 7, age 50 ± 6 yr, body mass index (BMI) 30 ± 1 kg/m2; NAFLD: n = 15, age 57 ± 3 yr, BMI 35 ± 1 kg/m2]. Plasma assessments were conducted in the fasted state. Whole body insulin sensitivity was determined by the gold-standard hyperinsulinemic-euglycemic clamp. The effect of ketone dose (0.5–5.0 mM) on mitochondrial respiration was conducted in human skeletal muscle cell culture. Fasting βOHB, a surrogate measure of hepatic ketogenesis, was reduced in NAFLD (−15.6%, P < 0.01) and correlated negatively with liver fat (r2 = 0.21, P = 0.03) and positively with insulin sensitivity (r2 = 0.30, P = 0.01). Skeletal muscle mitochondrial oxygen consumption increased with low-dose ketones, attributable to increases in basal respiration (135%, P < 0.05) and ATP-linked oxygen consumption (136%, P < 0.05). NAFLD pathophysiology includes impaired hepatic ketogenesis, which is associated with hepatic fat accumulation and impaired insulin sensitivity. This reduced capacity to produce ketones may be a potential link between NAFLD and NAFLD-associated reductions in whole body insulin sensitivity, whereby ketone concentrations impact skeletal muscle mitochondrial respiration.
Keywords: insulin resistance, ketogenesis, liver, nutrient metabolism
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) affects nearly one in three Americans and one in four adults worldwide (53). NAFLD is a leading indication for liver transplantation (7) and doubles 15-yr mortality compared with the general population (46). Obesity is a primary risk factor in the onset of NAFLD (36), and as such, lifestyle interventions or surgical approaches that reduce obesity and improve insulin sensitivity, concomitantly reduce NAFLD severity (33, 49, 62). Despite extensive research, medical advances, and public health efforts, no treatments or Food and Drug Administration-approved compounds exist to address NAFLD independent of resolving obesity (48). The lack of effective approaches to treat NAFLD is due, in part, to a knowledge gap in factors underlying disease onset and progression (59). Thus understanding the pathophysiology of NAFLD within the context of obesity will aid in the development of preventative and therapeutic strategies that function independently of the treatment of obesity.
A defining clinical characteristic of NAFLD is hepatic lipid accumulation, due to an imbalance of hepatic lipid input versus output (16). This imbalance is regulated through four classically defined pathways: lipid uptake, de novo lipogenesis, fatty acid oxidation, and very-low-density lipoprotein (VLDL) export (28). An effective countermeasure to defects in these pathways is not yet available, but an emerging therapeutic target is hepatic ketogenesis (10, 20). Ketogenesis is a major metabolic pathway primarily active in the liver and is the process by which acetyl-CoA from hepatic mitochondrial β-oxidation is converted into ketone bodies, an alternate energy source, not oxidized in the liver, but exported to circulation to be used by extra-hepatic tissues. In this way, hepatic ketogenesis impacts hepatic energy and lipid flux and may therefore impact NAFLD (54). In alignment, insufficient hepatic ketogenesis causes hepatic lipid accumulation in animal models (12) while inducing ketogenesis reduces hepatic lipids in humans (5). Despite these promising developments, advancement in the field has been slowed by the high variability of reports on hepatic ketogenesis in clinical NAFLD populations, where studies report a reduction (11, 20, 27, 57), no difference (20, 32), or an increase (6, 8, 51). Thus there remains an urgent need to increase the number of well-controlled studies investigating hepatic ketogenesis in NAFLD, especially in the context of obesity.
Another defining characteristic of NAFLD is impaired whole body insulin sensitivity (22), although it remains unclear how the accumulation of fat in the liver is connected to insulin sensitivity of peripheral tissues. In alignment with reduced hepatic ketogenesis in NAFLD, circulating ketones are gaining momentum as a controller of whole body and skeletal muscle metabolism with the primary ketone body β-hydroxybutyrate (βOHB) being recently identified as an important signaling molecule (60). Furthermore, βOHB is now shown to have independent signaling properties (50) that impact whole body and cellular nutrient metabolism (e.g., insulin sensitivity) (43, 60). In this way, reduced βOHB may link liver and peripheral tissue pathology in NAFLD, which has been evidenced in animal models (9). A potential mechanism by which reductions in βOHB may reduce insulin sensitivity includes skeletal muscle mitochondrial dysfunction (23, 41, 52). Skeletal muscle plays a major role in whole body insulin sensitivity, as it comprises ~40% of total body mass and is responsible for ~80% of insulin-stimulated glucose disposal (14). Yet, human trials that experimentally elevate βOHB have been unable to further increase insulin sensitivity in healthy individuals (i.e., βOHB infusion did not impact clamp-derived insulin sensitivity) (4). This disagreement within the literature suggests there may be a dose-dependent relationship between βOHB concentrations and insulin sensitivity.
In this report, we investigated fasting hepatic ketogenesis in participants with NAFLD and concomitant overweight/obesity (NAFLD group) compared with participants with isolated overweight/obesity (Control group). We also assessed whole body insulin sensitivity using the gold-standard hyperinsulinemic-euglycemic clamp. We hypothesized that individuals with NAFLD would present with reduced fasting hepatic ketogenesis and that hepatic ketogenesis would be inversely related to hepatic lipid accumulation and positively related to insulin sensitivity. Furthermore, we aimed to determine the dose response of ketones on skeletal muscle mitochondrial function in vitro.
METHODS AND MATERIALS
Subjects.
This parallel design, case control study included 22 research volunteers, recruited from the greater Cleveland area, along with NAFLD patients at the Cleveland Clinic (Cleveland Clinic Foundation, Cleveland, OH). All subjects were screened for eligibility via health history, medical exam and fasting blood chemistry in the Clinical Research Unit of the Cleveland Clinic. Participants were excluded for lean body mass index (BMI; <25 kg/m2), morbid obesity (BMI >40 kg/m2), type 1 diabetes, recent weight gain or loss greater than 2 kg within 6 mo and taking prescribed or over the counter medications that may impact steatosis, including amiodarone, methotrexate, perhexiline maleate, estrogens, tamoxifen, nifedipine, diltiazem, or chloroquine, vitamin E, betaine, fish oil, S-adenosylmethionine (SAM-e), thiazolidinediones (TZDs), and acarbose. This research was reviewed and approved by the Cleveland Clinic’s Institutional Review Board, and signed informed consent was obtained before initiating study procedures. Participants presented in this report are an overweight/obese subset of a larger cohort that has been previously reported (18).
Hepatic lipid and body composition phenotyping.
Liver fat was quantified via 1H-magnetic resonance spectroscopy (1H-MRS) as previously described (18, 24). Scans were performed in a prone position with a Siemens Verio 3-T MRI with a spine array coil and a body surface array coil. An 8-cm3 voxel was positioned within the right posterior lobe of the liver. Manual shimming was performed at ~40 Hz to delineate water and lipid subspecies. Spectra with and without water suppression were acquired using a long repetition time (TR = 5,000 ms) and a short echo time (TE = 30 ms). Scan time was ~30 min. NAFLD designation for this study followed diagnostic criteria: hepatic total fat index (TFI) greater than 5% (Controls: n = 7, NAFLD: n = 15). Body composition was determined by dual-energy X-ray absorptiometry (model iDXA; Lunar, Madison, WI).
Metabolic control procedures.
Participants reported to the Clinical Research Unit following stringent control procedures to minimize the influence of diet and physical activity on metabolic testing (26). For 3 days before metabolic testing, subjects were counseled by a registered dietitian to eat a balanced diet that contained ~250 g carbohydrate daily to stabilize muscle and liver glycogen stores. Subjects were asked to refrain from physical activity beyond typical activities of daily living for 48 h before metabolic testing. The evening preceding metabolic tests, subjects were admitted to the Clinical Research Unit and provided a balanced meal containing 55% carbohydrate, 35% fat, and 10% protein before fasting overnight for 12 h before βOHB, free fatty acid (FFA), or insulin sensitivity measurements. This approach has been used successfully to control for the influence of diet and physical activity in metabolic research (25, 26, 30).
Hepatic ketogenesis, free fatty acids, and nutrient metabolism indexes.
During the overnight-fasted state, circulating βOHB reflects hepatic ketogenesis (44). Thus, we utilized a controlled evening meal followed by an overnight fast as a standardized physiologic stimulus to assess hepatic ketogenesis in NAFLD and Controls. Plasma βOHB was quantified via enzymatic assay (no. 700190, Cayman Chemical, Ann Arbor, MI). A contributing factor to ketogenesis is the availability of circulating free fatty acids (FFA); thus we assessed total plasma FFAs by a commercially available colorimetric assay (ab65341, Abcam, Cambridge, UK) and calculated the molar ratio of FFAs to βOHB (FFA:βOHB). The glucose-ketone ratio was calculated using the molar ratio of fasting plasma glucose to βOHB (glucose:βOHB) according to calculations by Meidenbauer et al. (37).
Assessment of insulin sensitivity and substrate utilization.
Whole body insulin sensitivity was assessed using a 3-h hyperinsulinemic (40 mU·m−2·min−1)-euglycemic (90 mg/dL) clamp procedure, as previously detailed (15, 31). Briefly, blood glucose was measured every 5 min using a YSI glucose-lactate analyzer (YSI 2300; STAT Plus, Yellow Springs, OH) and was maintained at 90 mg/dL by variable rate glucose infusion (20% dextrose), calculated according to the method of DeFronzo et al. (15). Glucose infusion rates (GIR) were calculated as the glucose required to maintain euglycemia during the steady-state (150–180 min) period of the clamp. Insulin sensitivity was expressed as the amount of glucose metabolized normalized to the prevailing insulin concentration (mg·kg−1·min−1·μU−1·ml−1) during steady state. Indirect calorimetry was performed during basal and insulin-stimulated conditions as previously described (39, 64). Briefly, expired air was continuously sampled with an automated system in a semidarkened, thermoneutral (22 ± 1°C) environment under a ventilated hood with subjects laying supine. The equations of Weir (63) and Frayn (21) were used to calculate basal metabolic rate (BMR) and substrate oxidation (carbohydrate oxidation, COX; fat oxidation, FOX). In addition, timed urinary nitrogen excretion measurements (Roche Modular Diagnostics, Indianapolis, IN) were obtained to correct for protein oxidation (21).
Skeletal muscle mitochondrial function.
Mitochondrial function from intact primary skeletal muscle cells was assessed by real-time respirometry (Seahorse XFe24; Agilent, Santa Clara, CA). Skeletal muscle myoblasts (no. CC-2580, batch no: 0000583849, Lonza, Walkersville, MD) were isolated from a young healthy donor, seeded at 10,000 cells/well in an XFe 24-well plate (Agilent) and maintained in growth medium supplemented with 0.1% human epidermal growth factor, 1% fetuin, 1% bovine serum albumin, 0.1% dexamethasone, 1% insulin, and 0.1% gentamycin/amphotericin B (no. CC-3160, SkGM Skeletal Muscle BulletKit, Lonza). Cells were kept in a water-jacketed incubator at 37°C containing 5% CO2. When cells reached ~90% confluence, growth medium was replaced with differentiation medium (DMEM-F12 supplemented with 2% horse serum and 1% antibiotic). Differentiation medium was refreshed every other day for 4 days, when multinucleated myotubes were observed throughout the culture. After the 4 days of differentiation, cells were treated with exogenous ketones (1:1, βOHB:acetoacetate) at low (0.5 mM total ketones), moderate (1.5 mM total ketones), or high (5.0 mM total ketones) concentrations for 24 h. Following treatment, media were removed and cells were incubated without CO2 for 1 h in XF DMEM medium (pH 7.4) supplemented with 1 mM pyruvate, 2 mM glutamine, and 10 mM glucose. Cells were then serially injected with 1 μM oligomycin, 1 μM FCCP, and 0.5 μM of rotenone and antimycin A. Components of mitochondrial function were calculated as described previously (3). Data were normalized to nuclear content by staining live cells after assay with 20 μM Hoechst 33342 (Thermo Fisher Scientific, Waltham, MA) and florescent detection on an automated microplate reader (Biotek Instruments, Winooski, VT).
Statistical analysis.
Data were analyzed using PRISM7 (GraphPad, La Jolla, CA). Group differences between Control and NAFLD during basal and insulin-stimulated conditions were assessed by two-way repeated measures ANOVA (Group × Time). The effect of ketone dose on mitochondrial respiration in skeletal muscle cells was assessed by one-way ANOVA. ANOVA main effects were followed by Tukey’s post hoc test. Pearson’s correlation was used to assess relationships between outcome measures of interest. Normality was verified by the Shapiro-Wilk test (alpha = 0.05). Data are expressed as means ± SD. Significance was accepted a priori at P < 0.05.
RESULTS
Subject characteristics.
Subject characteristics are detailed in Table 1. Control and NAFLD participants were similar in age and had equivalent fasting glucose and insulin levels. NAFLD participants had a higher BMI (P = 0.02) but similar body fat percentage and fat-free mass (normalized to height) compared with Controls. By design, NAFLD participants had greater liver fat content (8.3-fold, P < 0.01) and displayed impaired insulin sensitivity (58%, P < 0.01) compared with Controls.
Table 1.
Subject characteristics
| Control | NAFLD | P Value | |
|---|---|---|---|
| n (sex) | 7 (5 F) | 15 (10 F) | — |
| Race H/AA/W | 1/4/2 | 3/1/11 | — |
| Age, yr | 50 ± 16 | 57 ± 12 | 0.24 |
| Weight, kg | 86 ± 17 | 98 ± 14 | 0.11 |
| BMI, kg/m2 | 30.0 ± 2.3 | 34.6 ± 4.5 | 0.02 |
| Fasting plasma glucose, mg/dL | 100 ± 12 | 116 ± 21 | 0.07 |
| Fasting insulin, µU/ml | 18 ± 6 | 26 ± 22 | 0.37 |
| BMR, kcal/day | 1,402 ± 399 | 1,643 ± 291 | 0.16 |
| RER | 0.84 ± 0.07 | 0.87 ± 0.06 | 0.06 |
| Insulin sensitivity | 0.036 ± 0.013 | 0.021 ± 0.011 | 0.02 |
| HbA1c, % | 5.9 ± 0.9 | 6.1 ± 0.6 | 0.53 |
| Liver fat, % | 2.4 ± 0.7 | 20 ± 9 | <0.01 |
| Body fat, % | 42 ± 5 | 45 ± 6 | 0.22 |
| Fat-free mass/height, kg/m | 29 ± 6 | 31 ± 6 | 0.47 |
| Cholesterol, mg/dL | 180 ± 32 | 170 ± 23 | 0.40 |
| Triglycerides, mg/dL | 115 ± 28 | 142 ± 48 | 0.19 |
| Liver panel | |||
| ALP, U/L | 79 ± 21 | 69 ± 22 | 0.34 |
| AST, U/L | 23 ± 8 | 35 ± 23 | 0.19 |
| ALT, U/L | 28 ± 18 | 40 ± 25 | 0.30 |
| Total protein, g/dL | 6.7 ± 0.5 | 6.6 ± 0.5 | 0.71 |
| Albumin, g/dL | 4.1 ± 0.3 | 4.2 ± 0.3 | 0.72 |
| Total bilirubin, mg/dL | 0.39 ± 0.13 | 0.11 ± 0.12 | 0.36 |
Data represent means ± SD. NAFLD, nonalcoholic fatty liver disease; F, female subjects; race: H, Hispanic; AA, African American; W, white; BMI, body mass index; RER, respiratory exchange ratio, V̇o2/V̇co2; ALP, alkaline phosphatase; AST, aspartate transaminase; ALT, alanine transaminase. Insulin sensitivity is reported as glucose uptake normalized to the prevailing insulin concentration during steady-state of the hyperinsulinemic-euglycemic clamp (mg·kg−1·min−1·μU−1·ml−1).
Hepatic ketogenesis.
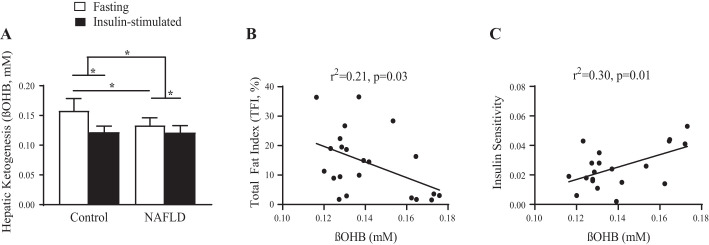
Fasting hepatic ketogenesis was reduced in NAFLD (−15.6%, P < 0.01; Fig. 1A) and was inversely related to hepatic total fat (r2 = 0.21, P = 0.03; Fig. 1B) and positively related to insulin sensitivity (r2 = 0.30, P = 0.01; Fig. 1C). Insulin-stimulated suppression of βOHB was comparable between groups. However, the change from fasting to insulin-stimulated conditions was greater in the Control group (Delta; Control: −0.036 µM; NAFLD: −0.007 µM; P < 0.01).
Fig. 1.
Hepatic ketogenesis and β-hydroxybutyrate (βOHB) during basal and insulin-stimulated conditions fasting βOHB plasma concentrations reflect hepatic ketogenesis. A: fasting ßOHB is reduced in nonalcoholic fatty liver disease (NAFLD) but is suppressed to similar concentrations as Control with insulin stimulation during the hyperinsulinemic-euglycemic clamp (Control, Fasting: 0.158 ± 0.02 mM, Insulin-stimulated: 0.122 ± 0.01 mM; NAFLD, Fasting: 0.133 ± 0.01 mM, Insulin-stimulated: 0.122 ± 0.01 mM). B and C: fasting hepatic ketogenesis correlates with hepatic fat in NAFLD (B) and whole body insulin sensitivity (C). *P < 0.05.
Free fatty acids and nutrient metabolism indexes.
Fasting free fatty acids were similar between groups (Control: 132 ± 8 µM; NAFLD: 127 ± 9 µM; Fig. 2A). With insulin stimulation, both groups reduced FFAs to minimal levels, which were below the level of detection of our quantification methods. The ratio of FFA:βOHB [Control: 0.87 ± 0.09 arbitrary units (AU); NAFLD: 0.97 ± 0.08 AU; Fig. 2B] was used as a surrogate measure of the hepatic propensity to generate βOHB while accounting for the prevailing FFA concentrations; this ratio was not different between groups. The ratio of FFA:βOHB positively correlated with body fat (r2 = 0.58, P < 0.01, Fig. 2C) and was negatively correlated with fat-free mass, adjusted for height (r2 = 0.44, P < 0.01, Fig. 2D). As expected, a higher rate of fasting fat oxidation correlated with a lower FFA:βOHB (r2 = 0.38, P < 0.01, Fig. 2E) (27).
Fig. 2.
Free fatty acid (FFA) and FFA:β-hydroxybutyrate (βOHB) ratio. A and B: plasma free fatty acid concentrations were similar between nonalcoholic fatty liver disease (NAFLD) and Controls (A) [Control, Fasting: 132 ± 22 µM, Insulin-stimulated: (not detectable); NAFLD, Fasting: 127 ± 33 µM, Insulin-stimulated: (not detectable)], along with the FFA:βOHB ratio (B) (Fasting; Control: 0.86 ± 0.22; NAFLD: 0.96 ± 0.27). C–E: the FFA:βOHB ratio was elevated with higher body fat percentage (C) but inversely associated with lean body mass (normalized to height) (D) and basal fat oxidation (E).
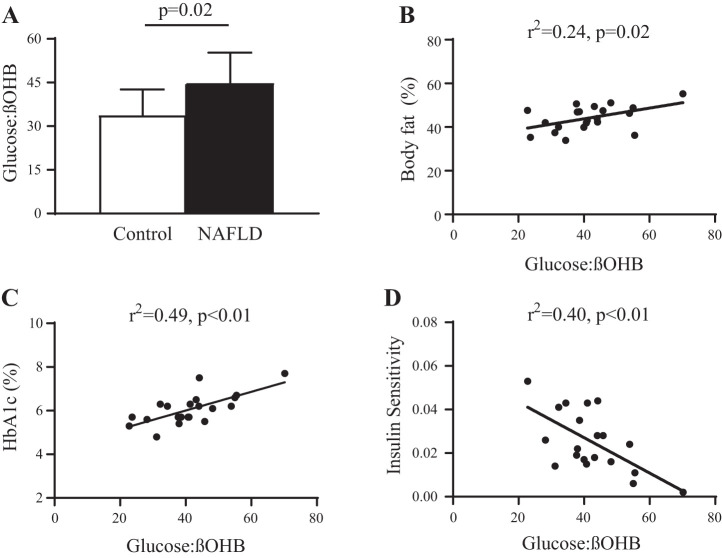
The fasting glucose:βOHB ratio was significantly higher in NAFLD compared with Controls (Fig. 3A). Elevations in the glucose:βOHB ratio were associated with greater body fat (r2 = 0.24, P = 0.02, Fig. 3B) and poorer glucose control (HbA1c, r2 = 0.49, P < 0.01, Fig. 3C). The fasting glucose:βOHB ratio also correlated with insulin sensitivity (r2 = 0.40, P < 0.01, Fig. 3D).
Fig. 3.
Glucose:β-hydroxybutyrate (βOHB) ratio. A: the glucose-ketone ratio (glucose:βOHB) was elevated in nonalcoholic fatty liver disease (NAFLD) compared with Controls (Basal; Control: 33.8 ± 8.8; NAFLD: 44.9 ± 10.4; P = 0.02). B–D: glucose:βOHB was elevated with higher body fat percentage (B) and poorer glucose control evidenced by elevated HbA1c (C) and impaired insulin sensitivity (D).
Skeletal muscle mitochondrial respiration.
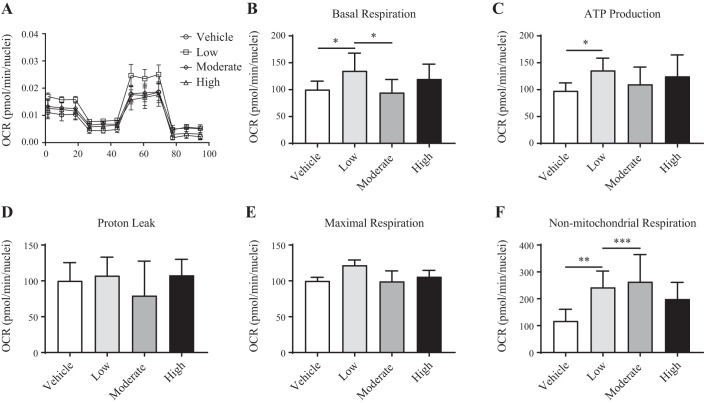
Oxygen consumption rate (OCR; Fig. 4A) was elevated only in the lowest ketone dose (Low, 0.5 mM) condition and was attributable to increases in basal (35%, P < 0.05; Fig. 4B) and ATP-linked (36%, P < 0.05; Fig. 4C) respiration. Protein leak (Fig. 4D) and maximal respiration (Fig. 4E) rates were similar between all conditions (Vehicle, Low, Moderate, and High ketones). Nonmitochondrial respiration (Fig. 4F) was elevated in Low (142%, P < 0.01) and Moderate (164%, P < 0.001) ketone conditions but did not reach significance in the High ketone condition.
Fig. 4.
Skeletal muscle mitochondrial respiration. A: representative tracing of real-time respirometry in skeletal muscle cells treated with Low, Moderate, or a High dose of ketones. B–F: oxygen consumption rates (OCR) attributable to basal (B), ATP-linked (C), proton leak (D), maximal uncoupled (E), and nonmitochondrial respiration (F). This experiment was conducted on a primary human skeletal muscle cell line (Lonza) over 3 unique culture occasions (n = 3) with 5 technical replicates per condition. Main effects of treatment were assessed by one-way ANOVA. Tukey’s multiple comparisons test was conducted on significant main effects. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
The underlying pathophysiology of human NAFLD remains incompletely understood, but recent preclinical and human studies have sparked interest in impaired hepatic ketogenesis as a causative factor (10). Our work agrees with prior research (11, 20, 57) evidencing the presence of reduced hepatic ketogenesis in a highly relevant subject population of NAFLD with concomitant obesity. Furthermore, reduced hepatic ketogenesis was associated with hepatic fat accumulation and impaired whole body insulin sensitivity. Finally, we report a dose-dependent effect of ketones on skeletal muscle mitochondrial respiration in vitro, together with the literature and our human data, suggesting circulating ketones may be a potential link between reduced hepatic ketogenesis and the NAFLD-related impairment in peripheral insulin sensitivity.
The literature on hepatic ketogenesis, whether assessed by fasting βOHB or stable isotopic tracer enrichment, is variable. We and others report reduced hepatic ketogenesis/βOHB in NAFLD (11, 20, 27, 57), while others report no differences (20, 32) or increases (6, 8, 51). Furthermore, highly technical assessments of hepatic mitochondrial metabolism or in vivo hepatic nutrient flux in NAFLD also remain inconsistent (6, 47, 58). Several explanations for this variability exist. The diversity of liver pathology ranges from simple steatosis in NAFLD to progressed stages of nonalcoholic steatohepatitis (NASH), and it is likely hepatic metabolism varies across this spectrum, as evidenced by Mannisto et al., who showed βOHB increased with NAFLD but decreased when the pathology had progressed to NASH (34). Obesity is independently associated with impairments in hepatic ketogenesis (34, 57); however, control comparison groups in these studies were sometimes (6, 27, 47, 58) but not always (11, 20, 51) matched for obesity status. Similarly, the severity of insulin sensitivity impairment of the study populations may have been variable, as the gold-standard approach to assess insulin sensitivity (the hyperinsulinemic-euglycemic clamp) was sparingly reported (6, 11, 20, 32, 51). There remains a need for well-controlled clinical trials investigating hepatic ketogenesis in NAFLD, considering these key contributing factors: progression of liver pathology, obesity status, and insulin sensitivity. Variability in hepatic ketogenesis/βOHB may also be partly explained by lack of quantification of extrahepatic ketone metabolism, including peripheral uptake, utilization, or excretion, which impact both circulating ketone concentrations and hepatocyte energy status (hepatic nutrient flux); these extrahepatic processes remain to be fully described in obesity, NAFLD, or metabolic disease. Finally, differences in the ketogenic stimulus (e.g., standardized evening meal, length of overnight fast, inpatient overnight stays on metabolic wards versus free-living conditions before assessment) may have provided variable magnitudes of ketogenic stimulation, which may lessen or exacerbate group differences in hepatic ketogenesis/βOHB measures. This particular issue was highlighted in a well-controlled study by Fletcher et al., which showed no differences in βOHB in NAFLD after a 12-h fast but a significant reduction in βOHB after a 24-h fast (20). Here, we investigated NAFLD with concomitant obesity, using a 12-h overnight fast on a metabolic ward in combination with the hyperinsulinemic-euglycemic clamp, evidencing reduced hepatic ketogenesis in NAFLD and adding that defects in hepatic ketogenesis correlate with the severity of hepatic lipid accumulation and magnitude of impaired insulin sensitivity.
Dysregulated hepatic FFA flux is integral to the development of NAFLD (2). A potential mechanism by which hepatic ketogenesis could prevent or treat NAFLD is postulated to act through diverting hepatic lipid flux towards ketone synthesis and delivery to systemic circulation (10), potentially removing lipids, lipid metabolites, and energy away from the liver microenvironment (13). In this way, animal models have evidenced impaired hepatic ketogenesis as a causative factor in the development of NAFLD (12). In the present study, we computed the FFA:βOHB ratio to assess the propensity to generate ketones in the context of prevailing FFA concentrations (38). Although βOHB was reduced in NAFLD and circulating FFAs were similar to Controls, differences in the FFA:βOHB ratio did not reach statistical significance, potentially due to our low number of participants (n = 22) or our FFA quantification methods that produced values lower than typically reported in this population. Regardless, better insight is derived from studies assessing hepatic FFA flux (16, 29, 58), evidencing increased hepatic FFA flux in NAFLD.
NAFLD occurs in concert with impaired skeletal muscle insulin sensitivity (6); however, the connection between the liver and peripheral tissue metabolism remains unclear. The hyperinsulinemic-euglycemic clamp is considered the gold-standard measure of whole body insulin sensitivity. In this study, a measure of hepatic ketogenesis (fasting βOHB) correlated with insulin sensitivity, which aligns with the concept that βOHB derived from the liver can impact whole body metabolism. Notably, skeletal muscle accounts for ~80% of insulin-stimulated glucose disposal and, as such, provides an approximation of skeletal muscle insulin sensitivity. The relationship between fasting ketogenesis and peripheral tissue insulin sensitivity is in agreement with the obesity literature, demonstrating reduced fasting ßOHB in individuals with obesity (17, 61) but not in insulin-sensitive individuals with obesity (1). This also aligns with recent research utilizing oral ketone ester administration, which experimentally elevates circulating βOHB and improves oral glucose control (42). However, these recent findings are in contrast with a long-standing concept that circulating ketone concentrations are inert to insulin sensitivity in humans. Highly-technical work, utilizing βOHB infusion in combination with the hyperinsulinemic-euglycemic clamp, has shown no relationship between experimental elevations in βOHB and insulin sensitivity (4, 40, 56). Limitations of these studies were 1) the use of exogenous/intravenous βOHB, and 2) utilizing high concentrations of βOHB, typically attainable only through dietary manipulation (with a ketogenic or low-carbohydrate diet) or prolonged fasting (>24 h). These limitations make it difficult to translate this work to NAFLD, which is characterized by reduced fasting βOHB. It is possible the variability on the impact of βOHB on insulin sensitivity is dependent on ketone dose, whereby reductions from healthy levels may impair insulin sensitivity, but increases in βOHB beyond healthy levels may not further augment insulin sensitivity, although this remains to be explicitly tested.
Emerging research suggests that defects in skeletal muscle mitochondrial function impair insulin sensitivity (23, 41, 52) while other recent research shows βOHB has independent effects on mitochondria (45). Therefore, we investigated the effect of ketone dose on mitochondrial function in primary human skeletal muscle cells. Treatment of cell culture media with 0.5 mM ketones directly impacted mitochondrial respiration, explained by differences in basal metabolism and ATP production rates. Importantly, these effects disappeared with higher ketone concentrations (1.5 mM and 5 mM) suggesting there may be a unique therapeutic window of ketone concentrations to impact metabolic signaling or skeletal muscle mitochondrial energy status. This finding of a dose-dependent relationship of ketone concentration on mitochondrial metabolism, where an effect was present at low, but not high, ketone concentrations, leads us to speculate that ketone dose is a potential explanation for the lack of consistent findings between animal and human models investigating whether ketones impact insulin sensitivity. Together with the available literature, our data support the concept that hepatic ketogenesis and βOHB play a role in regulating whole body insulin sensitivity in NAFLD, potentially by functioning as a physiological link between the liver and skeletal muscle mitochondrial metabolism. Future research may benefit from targeting populations with reduced βOHB, rather than experimentally increasing βOHB above healthy levels and by performing mechanistic investigations in skeletal muscle of individuals with NAFLD.
The ratio of glucose to ketones (glucose-ketone index) has been shown to better assess nutrient status than either glucose or ketones alone (37), while manipulation in preclinical trials has shown therapeutic potential (35, 55). The glucose:βOHB ratio was elevated in NAFLD and related to poorer long-term glucose control (elevated HbA1c). Notably, the glucose:βOHB ratio can be manipulated independently from resolving NAFLD or obesity, instead through nutritional and pharmacologic approaches, such as medium chain triglyceride provision, exogenous ketone supplementation, or sodium glucose cotransporter 2 inhibitor (SGLT2i) administration. Whether the glucose:βOHB ratio is a useful, noninvasive measure to assess nutritional and pharmacological therapies in NAFLD remains to be determined.
Together, these data support hepatic ketogenic insufficiency as part of the pathophysiology of NAFLD with concomitant obesity and that ketones have a dose-dependent effect on skeletal muscle mitochondrial respiration. It remains to be determined whether hepatic ketogenesis can be manipulated as a preventative or therapeutic measure for hepatic lipid accumulation or impaired insulin sensitivity in NAFLD.
Limitations.
We used a surrogate marker to measure hepatic ketogenesis, which could be more directly quantified by isotopic tracer enrichment. Similarly, we measured fasting FFA concentrations and the FFA:βOHB ratio to assess the propensity to produce ketones in the context of prevailing FFAs, rather than using an assessment of hepatic lipid flux, attainable through isotopic tracer approaches; NAFLD is characterized by an imbalance of lipid flux, including lipid uptake, de novo lipogenesis, export, and utilization, which has been evidenced independent of differences in circulating FFA (58). The in vitro experiments were performed on a commercially available human skeletal muscle cell line, however, the translation to NAFLD skeletal muscle pathophysiology would have been strengthened had we utilized skeletal muscle cells obtained from the participants in this study. Finally, our interpretations are limited by sample size (n = 22) and that the NAFLD participants were more overweight/obese than the overweight/obese Controls. However, both groups had similar body fat percentage, lean body mass (normalized to height), and fasting insulin, glucose, HbA1c, and substrate oxidation rates: a matched physiology that is difficult to attain in human NAFLD research. Despite these limitations, this study provides novel insight into the metabolic phenotype of NAFLD with concomitant obesity and provides proof-of-concept that ketone concentrations play a role in skeletal muscle mitochondrial respiration.
Conclusions.
NAFLD pathophysiology appears to include impaired hepatic ketogenesis, which is related hepatic fat accumulation and impaired whole body insulin sensitivity. We also evidence a dose-dependent effect of ketones on skeletal muscle mitochondrial respiration in vitro, suggesting ketones play a role in NAFLD-associated impairments in insulin sensitivity. This work adds to the emerging concept that defects in hepatic ketogenesis are intrinsic to NAFLD and agrees with the growing body of evidence that suggests future research should target hepatic ketogenesis for the treatment and prevention of NAFLD and metabolic disease, independent from resolving obesity.
GRANTS
This work was supported in part by National Institute of Health Grants UL1-RR-024989, U54-GM-104940, and DK-108089 (to J. P. Kirwan), T32-AT-004094 (to J. T. Mey, trainee), and T32-DK-064584 (to M. L. Erickson, trainee).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.A.F., A.J.M., and J.P.K. conceived and designed research; J.T.M., M.L.E., C.L.A., W.T.K., and J.P.K. performed experiments; J.T.M., M.L.E., C.L.A., W.T.K., C.A.F., A.J.M., and J.P.K. analyzed data; J.T.M., M.L.E., C.L.A., C.A.F., and J.P.K. interpreted results of experiments; J.T.M. prepared figures; J.T.M. drafted manuscript; J.T.M., M.L.E., C.L.A., W.T.K., C.A.F., A.J.M., and J.P.K. edited and revised manuscript; J.T.M., M.L.E., C.L.A., W.T.K., C.A.F., A.J.M., and J.P.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all our research participants and the Clinical Research Unit staff for assistance with subject screening, clinical visits, and hyperinsulinemic-euglycemic clamp support.
REFERENCES
- 1.Bergman BC, Cornier MA, Horton TJ, Bessesen DH. Effects of fasting on insulin action and glucose kinetics in lean and obese men and women. Am J Physiol Endocrinol Metab 293: E1103–E1111, 2007. doi: 10.1152/ajpendo.00613.2006. [DOI] [PubMed] [Google Scholar]
- 2.Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 59: 713–723, 2014. doi: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J 435: 297–312, 2011. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bratusch-Marrain PR, DeFronzo RA. Failure of hyperketonemia to alter basal and insulin-mediated glucose metabolism in man. Horm Metab Res 18: 185–189, 1986. doi: 10.1055/s-2007-1012266. [DOI] [PubMed] [Google Scholar]
- 5.Browning JD, Davis J, Saboorian MH, Burgess SC. A low-carbohydrate diet rapidly and dramatically reduces intrahepatic triglyceride content. Hepatology 44: 487–488, 2006. doi: 10.1002/hep.21264. [DOI] [PubMed] [Google Scholar]
- 6.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia 48: 634–642, 2005. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 7.Calzadilla Bertot L, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci 17: 774, 2016. doi: 10.3390/ijms17050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, Crabb DW. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology 37: 544–550, 2003. doi: 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- 9.Cotter DG, Ercal B, d’Avignon A, Dietzen DJ, Crawford PA. Impairments of hepatic gluconeogenesis and ketogenesis in PPARα-deficient neonatal mice. Am J Physiol Endocrinol Metab 307: E176–E185, 2014. doi: 10.1152/ajpendo.00087.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotter DG, Ercal B, Huang X, Leid JM, d’Avignon DA, Graham MJ, Dietzen DJ, Brunt EM, Patti GJ, Crawford PA. Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. J Clin Invest 124: 5175–5190, 2014. doi: 10.1172/JCI76388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croci I, Byrne NM, Choquette S, Hills AP, Chachay VS, Clouston AD, O’Moore-Sullivan TM, Macdonald GA, Prins JB, Hickman IJ. Whole body substrate metabolism is associated with disease severity in patients with non-alcoholic fatty liver disease. Gut 62: 1625–1633, 2013. doi: 10.1136/gutjnl-2012-302789. [DOI] [PubMed] [Google Scholar]
- 12.d’Avignon DA, Puchalska P, Ercal B, Chang Y, Martin SE, Graham MJ, Patti GJ, Han X, Crawford PA. Hepatic ketogenic insufficiency reprograms hepatic glycogen metabolism and the lipidome. JCI Insight 3: e99762, 2018. doi: 10.1172/jci.insight.99762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58: 773–795, 2009. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30: 1000–1007, 1981. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 237: E214–E223, 1979. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115: 1343–1351, 2005. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elia M, Stubbs RJ, Henry CJ. Differences in fat, carbohydrate, and protein metabolism between lean and obese subjects undergoing total starvation. Obes Res 7: 597–604, 1999. doi: 10.1002/j.1550-8528.1999.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 18.Erickson ML, Haus JM, Malin SK, Flask CA, McCullough AJ, Kirwan JP. Non-invasive assessment of hepatic lipid subspecies matched with non-alcoholic fatty liver disease phenotype. Nutr Metab Cardiovasc Dis 29: 1197–1204, 2019. doi: 10.1016/j.numecd.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher JA, Deja S, Satapati S, Fu X, Burgess SC, Browning JD. Impaired ketogenesis and increased acetyl-CoA oxidation promote hyperglycemia in human fatty liver. JCI Insight 4: e127737, 2019. doi: 10.1172/jci.insight.127737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55: 628–634, 1983. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 22.Gastaldelli A. Insulin resistance and reduced metabolic flexibility: cause or consequence of NAFLD? Clin Sci (Lond) 131: 2701–2704, 2017. doi: 10.1042/CS20170987. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Franquesa A, Patti ME. Insulin resistance and mitochondrial dysfunction. Adv Exp Med Biol 982: 465–520, 2017. doi: 10.1007/978-3-319-55330-6_25. [DOI] [PubMed] [Google Scholar]
- 24.Haus JM, Solomon TP, Kelly KR, Fealy CE, Kullman EL, Scelsi AR, Lu L, Pagadala MR, McCullough AJ, Flask CA, Kirwan JP. Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab 98: E1181–E1188, 2013. doi: 10.1210/jc.2013-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haus JM, Solomon TP, Lu L, Jesberger JA, Barkoukis H, Flask CA, Kirwan JP. Intramyocellular lipid content and insulin sensitivity are increased following a short-term low-glycemic index diet and exercise intervention. Am J Physiol Endocrinol Metab 301: E511–E516, 2011. doi: 10.1152/ajpendo.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haus JM, Solomon TP, Marchetti CM, Edmison JM, González F, Kirwan JP. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance. J Clin Endocrinol Metab 95: 323–327, 2010. doi: 10.1210/jc.2009-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inokuchi T, Orita M, Imamura K, Takao T, Isogai S. Resistance to ketosis in moderately obese patients: influence of fatty liver. Intern Med 31: 978–983, 1992. doi: 10.2169/internalmedicine.31.978. [DOI] [PubMed] [Google Scholar]
- 28.Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci 75: 3313–3327, 2018. doi: 10.1007/s00018-018-2860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacome-Sosa MM, Parks EJ. Fatty acid sources and their fluxes as they contribute to plasma triglyceride concentrations and fatty liver in humans. Curr Opin Lipidol 25: 213–220, 2014. doi: 10.1097/MOL.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 30.Kelly KR, Blaszczak A, Haus JM, Patrick-Melin A, Fealy CE, Solomon TP, Kalinski MI, Kirwan JP. A 7-d exercise program increases high-molecular weight adiponectin in obese adults. Med Sci Sports Exerc 44: 69–74, 2012. doi: 10.1249/MSS.0b013e318228bf85. [DOI] [PubMed] [Google Scholar]
- 31.Kirwan JP, del Aguila LF, Hernandez JM, Williamson DL, O’Gorman DJ, Lewis R, Krishnan RK. Regular exercise enhances insulin activation of IRS-1-associated PI3-kinase in human skeletal muscle. J Appl Physiol (1985) 88: 797–803, 2000. doi: 10.1152/jappl.2000.88.2.797. [DOI] [PubMed] [Google Scholar]
- 32.Kotronen A, Seppälä-Lindroos A, Vehkavaara S, Bergholm R, Frayn KN, Fielding BA, Yki-Järvinen H. Liver fat and lipid oxidation in humans. Liver Int 29: 1439–1446, 2009. doi: 10.1111/j.1478-3231.2009.02076.x. [DOI] [PubMed] [Google Scholar]
- 33.Ma J, Hennein R, Liu C, Long MT, Hoffmann U, Jacques PF, Lichtenstein AH, Hu FB, Levy D. Improved diet quality associates with reduction in liver fat, particularly in individuals with high genetic risk scores for nonalcoholic fatty liver disease. Gastroenterology 155: 107–117, 2018. doi: 10.1053/j.gastro.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Männistö VT, Simonen M, Hyysalo J, Soininen P, Kangas AJ, Kaminska D, Matte AK, Venesmaa S, Käkelä P, Kärjä V, Arola J, Gylling H, Cederberg H, Kuusisto J, Laakso M, Yki-Järvinen H, Ala-Korpela M, Pihlajamäki J. Ketone body production is differentially altered in steatosis and non-alcoholic steatohepatitis in obese humans. Liver Int 35: 1853–1861, 2015. doi: 10.1111/liv.12769. [DOI] [PubMed] [Google Scholar]
- 35.Mantis JG, Centeno NA, Todorova MT, McGowan R, Seyfried TN. Management of multifactorial idiopathic epilepsy in EL mice with caloric restriction and the ketogenic diet: role of glucose and ketone bodies. Nutr Metab (Lond) 1: 11, 2004. doi: 10.1186/1743-7075-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med 107: 450–455, 1999. doi: 10.1016/S0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 37.Meidenbauer JJ, Mukherjee P, Seyfried TN. The glucose ketone index calculator: a simple tool to monitor therapeutic efficacy for metabolic management of brain cancer. Nutr Metab (Lond) 12: 12, 2015. doi: 10.1186/s12986-015-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mey JT, Hari A, Axelrod CL, Fealy CE, Erickson ML, Kirwan JP, Dweik RA, Heresi GA. Lipids and ketones dominate metabolism at the expense of glucose control in pulmonary arterial hypertension: a hyperglycaemic clamp and metabolomics study. Eur Respir J 55: 1901700, 2020. doi: 10.1183/13993003.01700-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mey JT, Solomon TPJ, Kirwan JP, Haus JM. Skeletal muscle Nur77 and NOR1 insulin responsiveness is blunted in obesity and type 2 diabetes but improved after exercise training. Physiol Rep 7: e14042, 2019. doi: 10.14814/phy2.14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miles JM, Haymond MW, Gerich JE. Suppression of glucose production and stimulation of insulin secretion by physiological concentrations of ketone bodies in man. J Clin Endocrinol Metab 52: 34–37, 1981. doi: 10.1210/jcem-52-1-34. [DOI] [PubMed] [Google Scholar]
- 41.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 9: 193–205, 2008. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 42.Myette-Côté É, Neudorf H, Rafiei H, Clarke K, Little JP. Prior ingestion of exogenous ketone monoester attenuates the glycaemic response to an oral glucose tolerance test in healthy young individuals. J Physiol 596: 1385–1395, 2018. [Erratum in J Physiol 597: 5515, 2019.] doi: 10.1113/JP275709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab 25: 42–52, 2014. doi: 10.1016/j.tem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nosadini R, Avogaro A, Trevisan R, Duner E, Marescotti C, Iori E, Cobelli C, Toffolo G. Acetoacetate and 3-hydroxybutyrate kinetics in obese and insulin-dependent diabetic humans. Am J Physiol 248: R611–R620, 1985. doi: 10.1152/ajpregu.1985.248.5.R611. [DOI] [PubMed] [Google Scholar]
- 45.Parker BA, Walton CM, Carr ST, Andrus JL, Cheung EC, Duplisea MJ, Wilson EK, Draney C, Lathen DR, Kenner KB, Thomson DM, Tessem JS, Bikman BT. beta-hydroxybutyrate elicits favorable mitochondrial changes in skeletal muscle. Int J Mol Sci 19: 2247, 2018. doi: 10.3390/ijms19082247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel V, Sanyal AJ, Sterling R. Clinical presentation and patient evaluation in nonalcoholic fatty liver disease. Clin Liver Dis 20: 277–292, 2016. doi: 10.1016/j.cld.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Petersen KF, Befroy DE, Dufour S, Rothman DL, Shulman GI. Assessment of hepatic mitochondrial oxidation and pyruvate cycling in NAFLD by (13)C magnetic resonance spectroscopy. Cell Metab 24: 167–171, 2016. doi: 10.1016/j.cmet.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr 38: 485–521, 2019. doi: 10.1016/j.clnu.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 51: 121–129, 2010. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahman M, Muhammad S, Khan MA, Chen H, Ridder DA, Müller-Fielitz H, Pokorná B, Vollbrandt T, Stölting I, Nadrowitz R, Okun JG, Offermanns S, Schwaninger M. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat Commun 5: 3944, 2014. doi: 10.1038/ncomms4944. [DOI] [PubMed] [Google Scholar]
- 51.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 120: 1183–1192, 2001. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 52.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 87: 507–520, 2007. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the united states and the rest of the world. Clin Liver Dis 20: 205–214, 2016. doi: 10.1016/j.cld.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Serra D, Mera P, Malandrino MI, Mir JF, Herrero L. Mitochondrial fatty acid oxidation in obesity. Antioxid Redox Signal 19: 269–284, 2013. doi: 10.1089/ars.2012.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seyfried TN, Sanderson TM, El-Abbadi MM, McGowan R, Mukherjee P. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br J Cancer 89: 1375–1382, 2003. doi: 10.1038/sj.bjc.6601269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sherwin RS, Hendler RG, Felig P. Effect of diabetes mellitus and insulin on the turnover and metabolic response to ketones in man. Diabetes 25: 776–784, 1976. doi: 10.2337/diab.25.9.776. [DOI] [PubMed] [Google Scholar]
- 57.Soeters MR, Sauerwein HP, Faas L, Smeenge M, Duran M, Wanders RJ, Ruiter AF, Ackermans MT, Fliers E, Houten SM, Serlie MJ. Effects of insulin on ketogenesis following fasting in lean and obese men. Obesity (Silver Spring) 17: 1326–1331, 2009. doi: 10.1038/oby.2008.678. [DOI] [PubMed] [Google Scholar]
- 58.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab 14: 804–810, 2011. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thiagarajan P, Aithal GP. Drug development for nonalcoholic fatty liver disease: landscape and challenges. J Clin Exp Hepatol 9: 515–521, 2019. doi: 10.1016/j.jceh.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veech RL, Bradshaw PC, Clarke K, Curtis W, Pawlosky R, King MT. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life 69: 305–314, 2017. doi: 10.1002/iub.1627. [DOI] [PubMed] [Google Scholar]
- 61.Vice E, Privette JD, Hickner RC, Barakat HA. Ketone body metabolism in lean and obese women. Metabolism 54: 1542–1545, 2005. doi: 10.1016/j.metabol.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 62.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149: 367–78.e5, 2015. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 63.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults–a randomized clinical trial. J Gerontol A Biol Sci Med Sci 64a: 90–95, 2009. doi: 10.1093/gerona/gln032. [DOI] [PMC free article] [PubMed] [Google Scholar]