Abstract
Biological phase separation is known to be important for cellular organization, recently extending to a new class of biomolecules that form liquid-like droplets coexisting with the surrounding cellular, or extracellular environment. These droplets are termed membraneless organeles (MLOs) as they lack a dividing lipid membrane, and are formed through liquid-liquid phase separation (LLPS). Elucidating the molecular determinants of phase separation is a critical challenge for the field as we are still at the early stages of understanding how cells may promote and regulate functions that are driven by LLPS. In this review, we discuss the role of disorder, perturbations to molecular interactions resulting from sequence, post-translational modifications (PTMs) and various regulatory stimuli on protein LLPS, with a particular focus on insights which may be obtained from simulation and theory. We finally discuss how these molecular driving forces alter multicomponent phase separation, and selectivity.
Keywords: phase separation, intrinsically disordered proteins, biomolecular condensates, multicomponent phase separation
1. INTRODUCTION
Membraneless organelles (MLOs) have recently been shown to occur in a variety of biological contexts, facilitating a wide array of functions requiring compartmentalization(1). These organelles, which lack a surrounding lipid membrane, have demonstrated liquid-like properties(2) and are characterized by a region of highly concentrated proteins and frequently also nucleic acids, coexisting with the surroundings through the process of liquid-liquid phase separation (LLPS)(3). MLOs differ from membrane-bound organelles in their ability to spontaneously form and dissipate(2, 4), and their permeability(5, 6). MLOs have also been linked to formation of pathological aggregates associated with neurodgenerative diseases such as Amyotrophic Lateral Sclerosis (ALS), Frontotemporal Dementia (FTD) and Alzheimer’s disease(1, 7–9). To gain a greater understanding of the normal and pathological functions of MLOs requires a clear view of the molecular interactions underlying LLPS, and how different biomolecules may contribute to the process of phase separation.
In this review, we discuss the different types of biomolecules which participate in LLPS and formation of MLOs, and provide some perspective on what interactions contribute to phase separation, how these interactions may be altered by environmental conditions, and how overall interactions between components promotes phase separation of multiple components into two or more phases. Elucidating such interactions by experiment is challenging owing to the heterogeneous structure of MLOs such that any observables are always averaged over a broad distribution of structures. Molecular simulations can play an extremely valuable role in this situation, providing detailed information on the driving forces behind phase separation (and of course subsequently be tested against experiment); they can also be used for developing and testing new analytical theories. In addition, simulations allow rapid screening of sequence changes or other modifications, which may be more costly to do experimentally. In our review, we therefore place particular emphasis on the role which simulation can play in exploring the space of sequence, structure and phase properties of intrinsically disordered proteins.
2. STRUCTURE VS. DISORDER
2.1. Role of Intrinsic Disorder in Phase Separation
Recent studies have linked protein intrinsic disorder to membraneless organelles (MLOs), showing that the proteome for MLOs has a significantly greater fraction of proteins containing intrinsically disordered regions (IDRs) than the overall proteome(10). Intrinsically disordered proteins (IDPs) are proteins which do not adopt a stable folded structure, yet are able to carry out biological functions(11). They are also highly abundant, composing a large fraction of the eukaryotic proteome(11). Different classes of proteins generally tend toward being disordered, such as typical IDPs which are rich in charged amino acids(12), elastin-like polypeptides (ELPs), which are more enriched in hydrophobic amino acids(13) and prion-like domains(14, 15) which generally have a simple repetitive sequence, and are composed largely of only a few different amino acid types. Each of these are generally enriched in glycine or proline residues which disfavor formation of normal secondary structures.
2.1.1. Why Are IDPs Important to LLPS?.
IDPs have previously been suggested as being important to LLPS because of their ability to form many contacts with one another simultaneously, having high multivalency(16). Indeed, the length of an IDP has been shown to correlate with its ability to phase separate(6, 17, 18), having phase diagrams in agreement with polymer theories such as Flory-Huggins(19). In vitro studies have shown that a fully disordered protein may undergo LLPS, and that it remains disordered while in the phase-separated state(19, 20), forming weak interactions promiscuously between all types of amino acids(21). Another advantage of IDPs is that the amino acids are more exposed, and therefore, more accessible to post-translational modification(22) which is a major regulator of biomolecular phase separation(23–27). Simulations have shown that inclusion of folded domains may drastically slow down dynamics within phase-separated compartments, indicating that intrinsic disorder may also be important for the liquid-like properties of MLOs(18).
2.1.2. Disordered Chain Dimensions as an Indicator of Phase Separation Propensity.
When studying IDPs, researchers may infer characteristics of phase behavior from simple theory, simulation or experiment from the properties of single chains(4, 28, 29). This is because the same interactions driving compaction (or otherwise) of a single chain tend to also stabilize the protein-rich phase in LLPS, thus the degree of collapse is expected to be correlated with the propensity to phase separate. Single molecule experiments, scattering experiments or simulations of IDPs can be used to identify the average size of an IDP in solution(30–32). This is related to the backbone flexibility of the protein(33), as well as the overall strength of interactions between its amino acids(18), and of course the overall chain length. Lin et al. used random phase approximation theory to study the relationship between the critical temperature of phase separation (Tc) and the radius of gyration (Rg) of a series of synthetic polyampholytic protein sequences of the same length, and found that they are highly correlated(28).
To approximately remove the effects of chain length, the size of an IDP in solution can be quantified by its Flory scaling exponent (ν)(34), for example the end-end distance R of a disordered polymer should approximately scale with the number of residues as R = bNν (for proteins b ≈ 5 Å is frequently a good approximation). In this description, the degree of collapse of the protein is entirely captured by the scaling exponent ν, which takes on distinct values in a poor solvent when the protein is collapsed, (ν ≈ 1/3), in good solvent where the protein interactions are essentially repulsive (self-avoiding random walk, ν ≈ 3/5) or if attractive and repulsive interactions are exactly balanced (ν1/2), also known as Θ-solvent conditions(34). In simulations, ν is often estimated by fitting the scaling of internal distances in the protein (35). In FRET experiments ν can be estimated from studying different labellings as pioneered by Hofmann et al (30), or from fitting polymer model distributions (32). In scattering experiments ν can in principle be obtained directly from the mass fractal dimension from the raw scattering data, although in practice this is very challenging (36). More practical alternatives are the use of ensemble fitting (36, 37), from a simple “extended Guinier” analysis (38), or from the “molecular form factor” (MFF) fitting procedure (4). This scaling exponent may be used to find the Θ-solvent temperature (TΘ) (or Θ conditions for control variables other than temperature). This has been shown to be nearly equivalent to Tc(29), in agreement with previous studies showing the same relationship for homopolymers in the limit of infinite chain length(39). Simulation studies benefit greatly from this approach as systems containing a large number of polymeric chains may easily become computationally intractable(40, 41). Simulation studies have made use of this relationship, and have been able to demonstrate the collapse(42), and phase separation of IDPs with increasing temperature(43) due to temperature-dependent solvent mediated interactions.
2.2. Role of Folded Domains in Phase Separation
In addition to IDPs and IDRs, folded proteins and domains contribute to many functions of MLOs. Folded domains involved in LLPS include RNA-recognition motifs (RRMs) which bind to specific sequences of RNA(4, 44), oligomerization domains(6, 45, 46), and other domains which carry out the intended function of the MLO such as metabolic catalysis(47), promoting gene expression(48), and recruiting specific cargo molecules(6, 25). There are some cases where IDRs have even been shown to inhibit LLPS while the folded domains are the major driving force of phase separation(4).
2.2.1. Phase Separation of Folded Proteins.
Going back many decades, X-ray crystallography studies have observed liquid-liquid phase separation at some conditions during screening for protein crystallization(49). This, however, generally requires very high protein concentration, and rather extreme conditions, unlike many IDRs which may phase separate at much lower concentrations(17, 26). Folded proteins may aggregate or crystallize, leaving only a small window of conditions where LLPS may occur(50). However, IDRs, including those involved in LLPS, have also been known to be prone to aggregation and formation of disease-causing inclusions(7, 11, 51).
2.2.2. Folded Domains Facilitate Phase Separation With IDRs.
Many proteins involved in LLPS, such as ribonucleoproteins (RNPs) include multiple folded domains tethered together with disordered linkers(15). These folded domains may contribute significantly to phase separation by oligomerizing multiple protein molecules together and effectively increasing the multivalency and number of interactions a single “particle” is able to form(45). Taking advantage of this, researchers have engineered proteins including a light-activated oligomerization domain(52), thus enabling induction of phase separation in a controlled manner inside living cells(48, 53). RNPs even more commonly include RRMs which selectively bind to particular regions of RNA and can promote LLPS in the presence of these particular RNA sequences(4, 44, 54). Partially folded structures may also contribute to the phase separation through folding upon binding to specific binding partners(55, 56), or promotion of secondary structure upon self-association(8, 57). Inclusion of short helical motifs within an ELP also contributes to phase separation with significant hysteresis(58), having a considerably higher saturation temperature (Tsat) upon heating compared to cooling.
2.2.3. Folded Domains Carry Out Orthogonal Functions.
Some folded domains in phase separating proteins are relatively passive and do not contribute appreciably to the ability of the protein to phase separate. Such domains may have an orthogonal function, such as enzymes(47, 59) and RNA-remodelling helicase domains(60, 61). Many studies use protein constructs containing green fluorescent protein (GFP) or other similar fluorescent protein domains in order to visualize LLPS within cells(23, 44). Importantly, different fluorescent tags have been shown to incorporate into droplets of the LAF-1 RGG with different preferences(6) indicating that in some contexts, the inclusion of fluorescent tags may alter LLPS.
2.3. Interactions Between Folded and Disordered Domains
Another advantage of IDPs that makes them preferable for driving LLPS is that they can interact promiscuously with a large number of binding partners (this may be why they occur at protein interaction network “hubs” with a significantly higher frequency than folded proteins (62, 63)). IDPs may interact with other IDPs, sometimes with very high affinity, while remaining fully disordered and having no specific bound complex(36). Many IDPs also interact with folded domains in a specific manner, by adopting a folded structure, usually via an induced fit mechanism(11, 55, 56), though they may simply interact with folded domains and remain disordered(64, 65). Self-complementary RNA structures also play a role in LLPS by imparting an identity to the MLO it is incorporated in, and preventing merges with other MLOs containing different folded RNAs(66, 67).
3. SEQUENCE-LEVEL DRIVING FORCES
3.1. Amino Acid Interaction Modes
Since weak multivalent interactions between disordered and folded protein domains are the major driving forces of phase separation, it is important to understand exactly what are the different modes of interaction which cause proteins to assemble, demixing from their normal solvated state. Biology has provided proteins with an incredible arsenal of amino acids with differing side-chain chemistries, and an even more extensive library of PTMs(68). The result is hundreds of different types of amino acid derivatives in addition to the 20 canonical amino acids, and many different possible interaction modes arising from these(15, 69, 70). A full understanding of each of the interaction modes, and how they contribute to or detract from a system’s ability to phase separate needs to be well understood in order to appreciate the implications of protein composition and sequence.
3.1.1. Charge-Charge Interactions.
IDPs are commonly enriched in charged amino acids(12), and thus LLPS usually involves interactions between charged amino acid side chains and termini (Fig. 1). Charge-driven LLPS can take place as a single sequence containing both cationic and anionic amino acids such as the disordered domains of LAF-1(5, 71) and Ddx4(19, 72, 73), or as co-phase separation of two oppositely charged biomolecules through a complex coacervation(74–76). A high net charge may allow a single protein to exist at high concentrations without undergoing LLPS(24). Depending on the overall charge composition, IDPs display salt-out or salt-in behavior due to screening of electrostatic interactions as well as Hofmeister effects (19, 71, 77).
Figure 1.
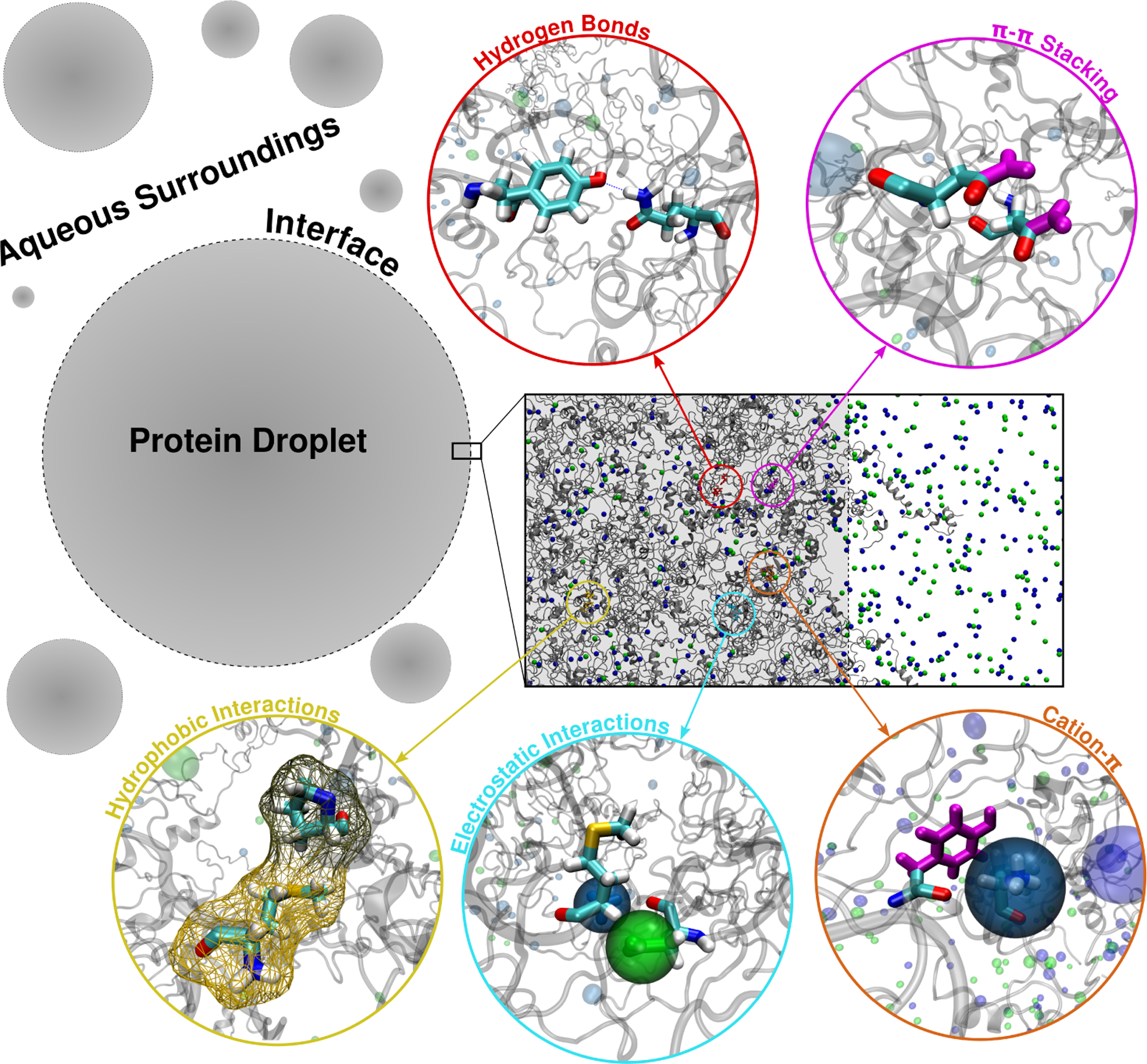
Schematic of a single-component droplet in phase coexistence with the surrounding aqueous environment. Box shows a molecular configuration of proteins stabilizing the condensed phase at the interface. Colored side chains and zoomed insets highlight the different interaction modes occurring between protein molecules.
Several factors may alter the charge state of amino acids, such as pH, which has a significant effect on histidine (side-chain pKa ~ 6), but must usually be very high or low to have significant effect on other amino acid side chains. Charge state may also be modified by various post-translational modifications, such as phosphorylation(9, 24, 78) or acetylation(27). Interestingly, the polarity of the environment may also have an impact on the formal charge of certain amino acids, such as lysine, which can shift its effective pKa when buried within a hydrophobic core of a protein(79).
3.1.2. π-Interactions.
Another mode of interaction that has been suggested as an important driver of biomolecular phase separation is planar interactions between sp2-hybridized atoms, commonly referred to as π-π interactions (Fig. 1). π-bonds occur between sp2-hybridized groups, which are most predominant in aromatic amino acids(80, 81). Since the aromatic groups of phenylalanine, tyrosine and tryptophan are electron-rich, they would most likely interact in off-center parallel, or edge-to-face perpendicular configurations(82), though aromatic rings may also become polarized and prefer face-centered stacking(83, 84). Vernon et al. suggest that interactions between all sp2-hybridized groups contribute to phase separation of proteins, and not just interactions between aromatic side chains(85). Since all amino acids contain a backbone peptide bond with a partial π bond, each may contribute to the planar sp2 interactions driving phase separation. This would result in weak multivalent interactions throughout the full protein sequence, and with the nucleotide bases in single stranded nucleic acids.
In addition to interaction between two sp2-hybridized groups, there are also interactions formed between aromatic rings and charged amino acids, particularly cationic residues(84, 86). Cation-π interactions are demonstrably important to phase separation in many commonly studied proteins(87), particularly between arginine and tyrosine residues. Dimethylation of arginine residues significantly reduces phase separation propensity in the hnRNPA2 protein(26), even though this PTM does not alter the net charge of the arginine residue. It is likely that the methylation of arginine disrupts multiple modes of arginine interactions with all other amino acids, including tyrosine, which may suggest that Arg-Tyr interactions are stabilized by multiple interaction modes in addition to cation-π. Cation-π interactions also may result in apparent non-Fickian diffusion within condensates where groups get trapped and move slowly on short length scales, while diffusing more quickly at longer length scales, suggesting that cation-π interactions are also important for tuning the material and transport properties of condensates(88).
Importantly, these interactions are all prominent within both proteins and nucleic acids, particularly single-stranded, unfolded nucleic acids which have their aromatic nucleotide bases exposed, which is consistent with studies showing that ssDNA, but not dsDNA is incorporated into IDP-rich droplets(72, 73, 88). Thus, proteins and nucleic acids may co-localize through many interactions with sp2-hybridized groups, and that hybridization or folding of proteins or nucleic acids may function as a regulator of such interactions and phase separation. Residues most prone to sp2-hybridized interactions include the aromatic residues Tyr, Phe, Trp and His, carboxyl and carboxamide groups of Asn, Asp, Gln and Glu, the guanidine group in Arg, and the exposed backbone peptide bond of Gly and other amino acids with small side chains(85).
3.1.3. Hydrophobic Contacts.
In the context of LLPS, hydrophobic interactions may be less predominant than in folded proteins(89), but are likely still important in most cases due to there still being appreciable content of hydrophobic amino acids (Fig. 1). The lower fraction of hydrophobic residues likely allows for the chains to remain disordered, and the assemblies to be liquid-like rather than solid. Rauscher and Pomès performed simulations of a large liquid-like assembly of elastin-like polypeptides (ELPs), and were also able to observe hydrophobic contacts between valine residues which largely stabilize the condensed phase(40). NMR studies on a minimal model of the processing body MLO highlights the role of hydrophobic leucine-rich helices in its formation and stabilization as a condensate(90). Importantly, hydrophobic amino acids may also interact strongly with aromatic amino acids which tend to be quite prevalent in phase-separating proteins. Even amino acids that are generally considered polar may contain nonpolar groups which participate in hydrophobic interactions, such as glutamine which contains two methylene groups and associates with other hydrophobic atoms in glutamine and tyrosine residues within FUS(21). Hydrophobic interactions are also a major driving force of protein folding and specific binding sites, promoting the formation and stability of various folded domains and oligomers that may additionally promote phase separation(45, 91), or specific binding of ligands to incorporate them preferentially into the condensed phase(56, 92).
3.1.4. Hydrogen Bonds.
While hydrogen bonds are largely considered as being responsible for solvation of polar amino acids, they also contribute much to the self-association of biomolecules, and driving forces of biomolecular phase separation. Most amino acids contain both hydrogen bond donors and acceptors, suggesting that hydrogen bonding could be very common within the densely-packed proteinaceous condensates. A combination of NMR and simulation techniques has shown that glutamine residues in the low-complexity region of FUS are important drivers of phase separation, and that hydrogen bonding is highly prevalent(21). Other studies have used atomistic molecular dynamics simulations to highlight this contribution of hydrogen bonds to phase separation(40, 42, 93). Hydrogen bonding is also a major factor in recognition of nucleotide bases(94), and is likely important for incorporation of RNA and DNA into condensates(95).
Hydrogen bonds may also contribute to biomolecular phase separation more indirectly through the formation of secondary structure in proteins(8), the hybridization and secondary structure of nucleic acids(67, 73, 88), and incorporation of water into the condensate(21, 93, 96). Hydrogen bonds also contribute to the formation of fibrillar structures in FUS, similar to amyloids, but stabilized by hydrogen bonds rather than a hydrophobic core(97), and transient formation of small fibrillar structures such as LARKS(98) or other transient interacting structures(99). All amino acid types may participate in hydrogen bonding, however, the most prone are generally those with polar or charged side chains.
3.1.5. Summary of interactions.
Proteins and nucleic acids make use of diverse chemistries in order to drive self-association and incorporation or exclusion of other molecules, and to control dynamical and transport properties of phase-separated assemblies(15, 21, 68, 81, 85, 87, 88, 100). Some amino acids may interact through several different interaction modes, which may work cooperatively to provide even stronger binding(21). Since the components of MLOs are highly dynamic, and usually disordered, it is a major challenge to directly determine which interaction modes are contributing, and the relative importance of each to LLPS(20, 24). Atomic-resolution simulations provide a promising path forward for this as they can be used to observe all of the different interaction modes directly (21, 26, 40, 42, 78). The current challenge in using simulations is the cost of running atomic-resolution simulation on a large assembly of many proteins, and so far this has only been achieved in one study(40). Studies identifying interactions within proteins may be useful in identifying which interactions contribute most significantly, and how small perturbations can be made to the sequences to greatly alter the macroscopic phase behavior, and how naturally occurring mutations may have significant physiological and biophysical repercussions(7, 14, 57).
3.2. Sequence and Arrangement of Interactions
In addition to amino acid composition, the arrangement and sequence of amino acids also has an important role in IDP properties, as well as the phase separation of biomolecules. Many decades of research have been devoted to relating protein sequence to structure, but for disordered proteins, the role of sequence is not as well understood(12, 101). This is largely due to a lack of structural information from experiment, thus necessitating alternative measurements to be used for IDPs such as size measurements (Rg, Rh and ν), and their propensity to self-associate, aggregate, or phase separate. Indeed, phase separation may serve as an excellent descriptor for studying the sequence determinants of disordered proteins, nucleic acids, and their interactions(18, 74, 95).
3.2.1. Charge Patterning.
To explore the effects of the arrangement of charged amino acids, Das et al. used all-atom implicit solvent simulations to demonstrate the wide range of single molecule behaviors for a set of proteins having identical composition(101). The specifically designed sequences are composed of 25 positively charged lysine residues, and 25 negatively charged glutamate residues arranged differently throughout the sequence, with extremes being a strictly alternating dipeptide repeat ([KE]25) and a block copolyampholyte ([K]25[E]25)(101). To quantify the degree of charge segregation, they devised a parameter, κ, defined as
| (1) |
| (2) |
| (3) |
where Nblob is the number of “blobs” or segments of 5 or 6 residues of the seqence in a moving window, f+/− is the fraction of positive or negative residues within the blob, σ is calculated over the full sequence, and δmax is the maximum possible δ value for all sequences of the given composition. A κ value near 0 indicates the strictly alternating sequence, and near 1 indicates the block copolyampholyte. They find that sequences with low κ values are more extended, while sequences which are more blocky (charge-segregated) are more collapsed(101). Sawle and Ghosh then used mean field theory to support this assessment, and proposed an alternative charge patterning metric, termed sequence charge decoration (SCD)
| (4) |
where N is the number of amino acids in the sequence, and q is the formal charge on each residue(102). A large negative value of SCD indicates a block copolyampholyte, while a value near zero would indicate an alternating polyampholyte. SCD may also be positive for sequences having a non-zero net charge. Since the single-chain size of the protein is generally correlated with its phase separation propensity, it also follows that the charge patterning values of these polyampholytic sequences are highly correlated with their critical temperatures as calculated by theory(80) and simulation(103). Importantly, charge patterning has been demonstrated to have a significant impact on the phase separation of biological IDPs such as Ddx4, which contains 25% charged amino acids(19, 72). While these two charge patterning metrics are highly correlated with each other, some sequences may be designed where one metric predicts collapse, while the other predicts extended configurations. For such sequence, neither metric is effective at predicting its single-chain or LLPS behavior(103), demonstrating that both may be limited in their predictive capabilities in special circumstances. Other metrics have also been used to quantify degree of charge patterning such as charge fluctuations(77), and average “run” length(76).
3.2.2. Hydrophobic/Aromatic Patterning.
There is also evidence that the effects of patterning are non-negligible for other types of interactions, particularly involving hydrophobic and aromatic amino acids. Previous studies have looked at the arrangement of hydrophobic and hydrophilic amino acids to find that folded proteins need to have particular patterning in order to collapse properly(104). Computational work has also shown how sequence correlation of hydrophobic residues can be used to design disordered proteins of identical hydrophobic and hydrophilic composition with very different single chain behavior(105), which should also result in differences in phase separation(29). A hydrophobic correlation parameter may be used similarly to the SCD and κ metrics in order to make predictions about IDP single chain behavior and phase separation, though when comparing, it will also be important to take into account the distance dependence of such interactions(106). Some disordered proteins with a very blocky nature may even function as biological surfactants, promoting mixing of polar and nonpolar molecules(107).
It is curious how many LLPS-enabled IDPs, particularly prion-like domains, have widely distributed aromatic residues, rather than them being clustered together(80, 108). It is possible that the dispersion of these amino acids prevents hydrophobic collapse or folding. Since cation-π interactions(87) and π-π interactions(85) are also known to be particularly competent at promoting phase separation, it is likely a patterning metric that considers cations and aromatic residues could also prove useful to predicting phase behavior directly from sequences.
3.2.3. Patterning as Mode of Recognition.
Patterning of amino acids is very important to a protein’s ability to phase separate, and also toward biological functions through molecular recognition and condensate selectivity. Lin et al. have shown that differences in charge patterning between polyampholytic sequences results in drastically different partitioning of the two components into two or three distinct phases where sequences which are similar cooperatively phase separate, while drastically different patterning results in separation of the two into separate phases(109). In addition, sequence patterning may also serve as a mode of recognition between disordered proteins and folded proteins with patchy surfaces(64) which could result in additional selectivity of condensates for both disordered and folded domains.
3.3. Stimulus Responses of Different Interaction Modes
Many perturbations have been shown to alter the phase separation of diverse proteins(21, 44, 48, 110, 111). Depending on the amino acid sequence, different stimuli may either promote or disrupt phase separation, and may change the way in which multiple components mutually or exclusively phase separate. In Fig. 2A we show an example of a 2-component system at different values of a control variable which could be temperature, salt concentration, pH, etc. The two components have different propensity to phase separate as a function of the control variable, where component A may phase separate at a wider range of conditions than B. Fig. 2A shows the single component phase diagrams of each of the two components, having a region where LLPS is permitted at low values of the control variable, and a region above the critical point where the system is in a single continuous phase. At conditions where both may phase separate, it is likely that droplets may form that contain both components, however, in the region between the two critical points, it is unclear whether component B will be able to incorporate into droplets of A. To visualize this further, Fig. 2B; shows a 2-component phase diagram at three conditions highlighted in Fig.2A. We observe a cooperative condensation of both components into a single condensed phase for the two lowest values of the control variable (green & orange), and at conditions where only A may phase separate, we observe a scaffold-client phase diagram (yellow). This example shows how altering conditions in solution may lead to different phase behaviors, and that some components may still be incorporated into condensates even if they would not undergo LLPS in isolation.
Figure 2.
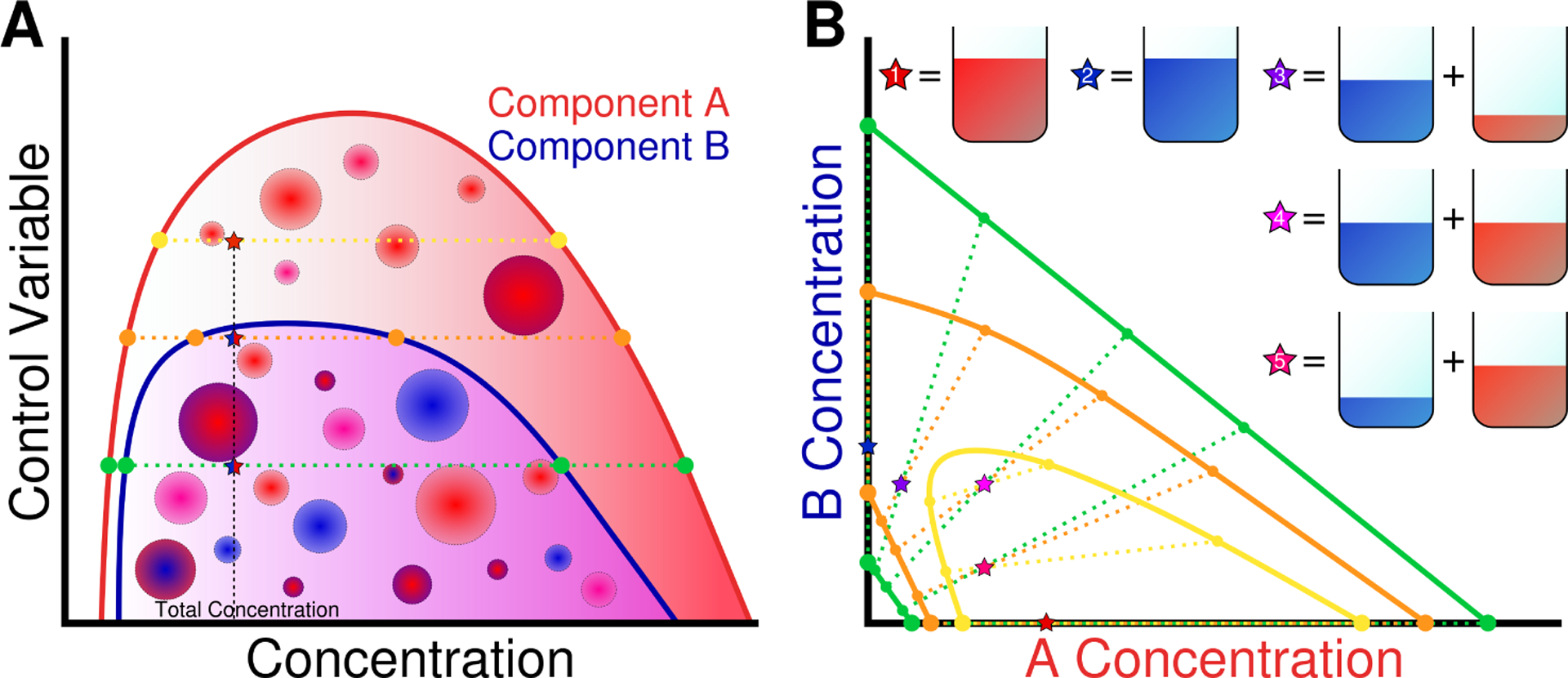
Co-phase separation of two species with similar self- and cross-interactions. A) Single component phase diagrams for Component 1 and Component 2 with tie lines at three values of the control variable: both components may phase separate (green); both 1 and 2 may phase separate, but 2 is nearing its critical point (orange); and the region where only 1 is able to form a condensed phase. B) Multicomponent phase diagram of mixtures of Components 1 and 2, with control variable indicated by color. Stars indicate different experiments conducted at different relative total compositions of the two components where 1 and 2 only contain a single component, and 3–5 contain a mixture of the two. Tie lines show the resulting concentrations within the two phases.
3.3.1. Temperature.
While cells generally exist in a narrow range of temperature, changes to interactions in response to temperature is still very important to understanding membraneless organelles, particularly the role of different interaction modes due to each one’s distinct temperature response. An increase in temperature may induce phase separation of proteins through active processes which accelerate at higher temperatures, or through thermodynamically-driven, and reversible LLPS(110). The thermodynamically-driven phase separation which is promoted at higher temperatures results in a lower critical solution temperature(LCST)-type phase transition. Garcia-Quiroz and Chilkoti have provided a comprehensive characterization of composition-dependent phase behavior of IDPs, demonstrating that sequences containing many polar and aromatic amino acids generally are follow an upper critical solution temperature (UCST) phase transition, while those containing more hydrophobic amino acids follow LCST transitions(17). Some protein sequences such as An16-resilin even show a reentrant phase behavior, where LLPS occurs at both low and high temperatures with a region of miscibility in between(112), typically referred to as having an hourglass phase diagram(43, 113).
With increasing temperature, the loss of chain entropy accompanying IDP collapse or phase separation will increase(29), which itself may fully explain the UCST phase transitions. However, to observe an LCST phase transition, it is important to consider the temperature dependence of solvent-mediated interactions, particularly with the different types of amino acids(114). Privalov and Makhatadze conducted solvent transfer experiments on small molecule analogues of the 20 different amino acid side chains and backbone to show that the free energy of solvation is temperature-dependent, and increases with temperature(115). Amino acids becoming more insoluble with increasing temperature could, in principle, overcome the effect of chain entropy and allow for LCST phase transitions. The difference in temperature-dependence between different types of amino acids based on how different interaction modes are strengthened or weakened by temperature would explain the ability to switch between UCST and LCST phase transitions based on overall composition.
Indeed, temperature-dependent solvation free energy of hydrophobic molecules is also non-monotonic, having an initial increase of “hydrophobicity” up to a turnover point, and subsequent decrease(116), which may be explained by the dominance of enthalpy at low temperatures, and entropy at high temperatures(117, 118). By fitting this temperature dependence to a thermodynamic equation, Dill et al. developed a theoretical model to explain protein folding and thermal stability, showing that temperature-dependent interactions can be used to explain increasing stability with increasing temperature, and cold denaturation(119). By using a temperature-dependent interaction potential calculated from solved solution NMR structures(120), Dignon et al. developed a coarse-grained simulation model(43), which is successful in predicting the composition-dependence of UCST and LCST phase transitions from Garcia-Quiroz and Chilkoti(17). Models such as this one can be quite helpful in elucidating the sequence determinants of temperature-controlled phase behavior.
3.3.2. Salt.
Salt is an important solution additive that can be used to tune phase separation as it is easily controlled in vitro, and is perhaps more physiologically relevant than large changes in temperature. Early studies have shown that for some proteins, increasing salt concentration may induce (salt-out) phase separation(8, 20) or prevent (salt-in) phase separation(19, 71). An obvious effect of increasing ionic strength is the screening of electrostatic interactions(121). However, for a sequence such as the low complexity (LC) domain of FUS which is nearly devoid of charged amino acids, it is unlikely that charge screening is the only factor contributing to the large effect salt concentration has on its phase separation. In our previous work, we suggest the possibility of salting-in and salting-out effects on the solubility of all amino acids by shifting effective pairwise interactions to be more or less attractive(18). Brangwynne et al. also discuss such a possibility that several other interaction modes are affected differently by salt concentration(106).
The identity of the salt ions also plays an important role in the effects on phase separation. The Hofmeister series ranks different anions and cations by their ability to solubilize or precipitate proteins(122), and have been shown to have the same effect on the phase separation of FUS(21). The kosmotropic salts were shown to promote phase separation of FUS at considerably lower saturation concentrations than the standard NaCl, and the chaotropic salts did the opposite, by greatly increasing the required FUS concentration to observe LLPS(21). Ions with higher valency such as transition metals also will also facilitate phase separation for biomolecules having an abundance of the opposite charge(50, 123). With all of these considerations, salt may present one of the most tunable handles to perturb phase separation.
3.3.3. pH.
Another stimulus likely to induce changes to phase behavior is the solution pH. Kroschwald et al. showed that reduced pH as a result of cellular starvation causes the formation of stress granules (SGs) in vivo and in vitro(44). Interestingly, they found that pH-induced phase separation results in SGs which are more dynamic than those induced by heat-shock, implying that different assembly mechanisms may be occurring(44). Changes to a system’s pH will undoubtedly alter the charges within a protein, and depending on the types and arrangement of charged residues within the sequence, this may also have strong a impact on the charge patterning of the sequence. Thus pH might also be used as a powerful tool for tuning LLPS and selectivity through altered net charge and charge patterning(113).
3.3.4. Other Stimuli.
Other factors that the cell uses to control LLPS are ATP(111, 124) and poly ADP-ribose (PAR)(125). One process by which ATP may modulate phase separation is by acting as a hydrotrope, thus solubilizing nonpolar groups of the proteins and preventing, or reducing phase separation when driven by hydrophobic interactions(126). Another consideration is that ATP-driven reactions, particularly phosphorylation of amino acid side chains may also drive or prevent phase separation(127). PAR may seed phase separation, particularly in cases where phase separation is required to aid in repair of damaged DNA(125).
LLPS may also be mediated by various small molecules such as 1,6-hexanediol(9, 48, 81, 108, 128), chemical chaperones(129), and large molecular crowders including polyethylene glycol (PEG)(130–132). Braun et al. look at effects of solvent isotope content, and find that D2O promotes hydrophobicity-driven phase separation of BSA more strongly than H2O, having important implications for NMR experiments which commonly use D2O as a solvent(50). Oxidation also plays an important role in LLPS in various ways. Reed et al. show that oxidative croslinking of a cysteine residues within a designed oleosin protein can facilitate LLPS by promoting dimerization and increasing the multivalency of the protein(133). They also find that the position of the cysteine residue within the sequence can control the degree to which LLPS is promoted(133). In contrast, oxidation of methionine side chains has been shown to prevent LLPS of the yeast ataxin-2 protein(134). Thus, oxidizing and reducing environments are able to promote, or abrogate LLPS depending on protein compositions.
3.3.5. Summary.
The stimulus-response of biomolecular condensates may currently be somewhat unpredictable, but an understanding of the molecular interactions underlying LLPS, and how such interactions are perturbed by various stimuli will go a long way in making it more predictable. This, however, is very challenging, and it is not clear what is the best way to quantify interactions. Solvation free energy is a useful metric, but only considers the interactions of the amino acid with solvent, and not with other amino acids(119), and use of small molecule analogs may also neglect the polymeric effect(115). Alternative strategies which may provide the field with much-needed insights include bioinformatics(120, 135), or experimental(136) or computational(137, 138) characterization of binding energies of all amino acid pairs, and their dependences on relevant stimuli.
4. MULTICOMPONENT PHASE SEPARATION
4.1. Modes of Two-phase Systems With Two Components
Most MLOs exist not as a single-component condensate, but as a mixture of dozens to hundreds of different components including proteins, nucleic acids, solvent, ions and other cargo molecules(110, 124, 128, 139, 140). Many of these organelles have redundancy built in, such that no single component is solely responsible for the formation of the MLO(140). To characterize the relationship between different components, and their relative abilities to phase separate, as well as their mode of phase separation (i.e. co-phase separation, scaffold/client, exclusivity, core-shell etc.) one may make use of relative interaction strengths between different components. The interaction strength between two molecules may be quantified using metrics such as the osmotic second virial coefficient (B22)(5, 29). Interaction strengths can be altered with changes in temperature, and the temperature at which B22 disappears is known as the Boyle temperature (TB), which has been shown to correlate well with the critical temperature of phase separation for a single-component system(29). For a two component system, one may consider four possible cases involving phase separation into two distinct phases.
4.1.1. Cooperative Co-Phase Separation.
The first case we consider is where both components have strong self-interaction affinity, as well as strong cross-interactions (Fig. 3A). For such a system, one may expect formation of a heterotypic droplet enriched in both components, and a surrounding aqueous phase with both components at low concentration, also known as a condensation phase transition(141). This would occur with droplets in which the constituent proteins are very similar to one another, such as co-phase separation of a wild-type protein with a fraction of the same protein with a fluorescent tag or NMR-active label(26). Depending on the relative total concentrations of the components, the droplet may be more enriched in one component than the other (Fig. 3A). The slopes of the tie lines can also be used to determine which component is contributing more to the phase separation of the system.
Figure 3.
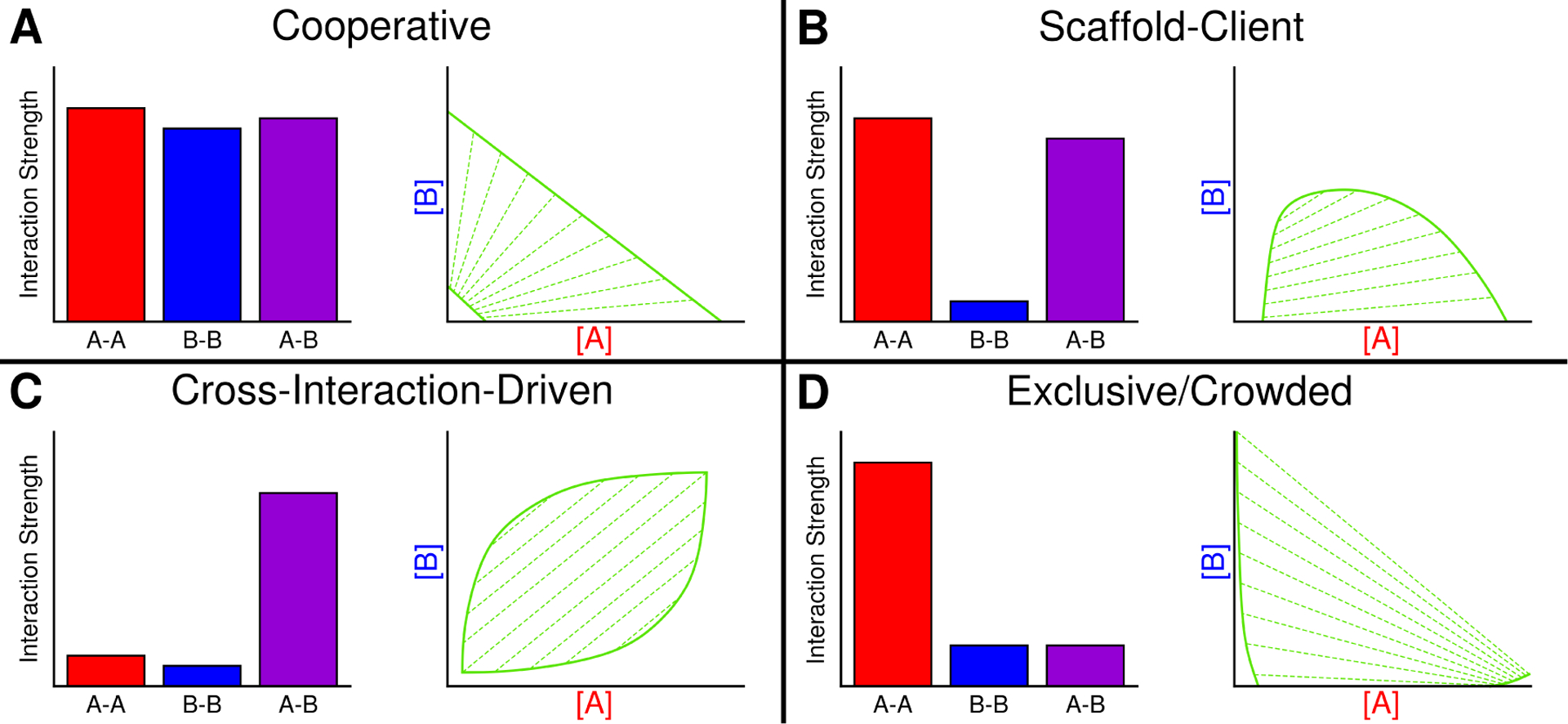
Possible shapes of multicomponent phase diagrams and the relative self- and cross-interaction strengths of components the two components. A. A cooperative condensation phase transition occurs when both components have strong self-attraction as well as a mutual attraction for one another. This is characterized by phase lines running from one axis to the other at low and high concentrations, and tie lines having a positive slope in between the two phase lines. B. Scaffold-client relationship occurs when one component has strong self-interaction and can undergo LLPS while the second cannot, but has some attraction for the first component. This results in a single phase line that intercepts the scaffold concentration axis twice. Tie lines may be positively or negatively sloped indicating recruitment and exclusion respectively. C. Cross-interaction-driven phase separation occurs when neither component has strong self-interaction, butb they have a strong mutual interaction strength. The phase lines do not intercept either axis since neither pure component is able to undergo LLPS. Tie lines are always positive because both components must be present in sufficient quantities for the condensed phase to be stabilized. D. Exclusive/Crowded phase separation occurs when one component has strong self-interaction strength, while the other has no strong interaction with itself or the first component. In this case, the first component forms a condensed phase while the second component preferentially occupies the other phase, indicated by negatively-sloped tie lines.
4.1.2. Scaffold-Client Co-Phase Separation.
The second case we consider deals with systems where one component is able to phase separate as a single component, and the second is not, but has a strong attraction for the first (Fig. 3B). The shape of the scaffold-client phase diagram may change rather significantly but should always have a continuous phase along one of the axes, and thus, the phase lines should never intercept one of the two component axes. An example of such a system would be an RNA-binding protein which, itself, is prone to phase separation, and an RNA molecule which generally would be incapable of phase separation. Here, the protein functions as a scaffold molecule, promoting phase separation, and the RNA functions as a client, being incorporated into the droplet(8, 71).
One way in which the shape of the phase diagram may vary is if the cross-interaction is considerably weaker than the self-interaction of the scaffold and the client is more excluded from the condensate, rather than recruited, which would be indicated by negatively-sloped tie-lines in the phase diagram. Another possible difference is whether the saturation concentration (csat) increases or decreases with the initial addition of client concentration. If the client molecule saturates the interaction sites of the scaffold, it may reduce the propensity of the scaffold to phase separate(142, 143) thus increasing csat as in Fig. 3B. In contrast, if the client molecule additionally promotes phase separation, the saturation concentration of scaffold will decrease initially as in Fig. 2B. In this second case, there will eventually be a turnover, and csat will begin to increase again, which has been observed as a reentrant phase separation upon increasing RNA concentration(144). In this case, the turnover occurs roughly at the charge-inversion point, when the phase-separating components balance each other out in net charge(144).
4.1.3. Cross-Interaction-Driven Co-Phase Separation.
Cross-interaction-driven phase separation is the phase separation of two mutually attractive species, which are each incapable of phase separating in the absence of the other. The phase diagram of such a system will not intersect either component axis since neither pure component may phase separate. The coexistence region occurs in the middle, always having positively-sloped tie lines to indicate cooperativity between the components (Fig. 3C). Complex coacervation is a common example of this where the cross-attraction and self-repulsion are driven by electrostatics(76) and may occur between oppositely-charged proteins(74), or positively charged proteins with RNA(145).
Similar phase diagrams may also be achieved through the use of chains of heterospecific binding partners such as the SRC homology 3 (SH3) domain with proline-rich motifs (PRMs)(3) or SUMO and SUMO Interaction Motif (SIM)(139) attached by linkers. For systems such as these with specific binding partners, the phase diagram can be tuned though modifying the number of repeats of each of the interaction sites(3, 139, 146). It should be noted that due to specificity of these interactions, the recruitment of molecules into condensates can be done with a particular stoichiometric ratio(146). Modelling studies on multivalent proteins with specific binding sites also predict a “magic number” relationship where saturation of the binding sites may actually reduce the phase-separation propensity by forming tightly-bound oligomers that do not interact strongly with each other(70, 147).
4.1.4. Exclusive Phase Separation.
The final two-phase system is the case where a single component is prone to phase separate, and a second component does not, nor does it interact strongly with the first component (Fig. 3D). Model systems may include a phase-separation-prone protein and an inert crowding agent such as ribosomes(148) or polyethylene glycol (PEG)(131, 132). In many of these cases, increasing concentration of the inert crowding agent allows the protein to phase separate at a lower csat, likely due to excluded volume(132). The phase diagram of such systems shows negatively-sloped tie lines, indicating that one component is excluded from the other. In the case shown, component A may phase separate in absence of the second, but will form condensates at lower concentration as the concentration of the second component is increased (Fig. 3D). However, one must also consider the possibility that even “inert” crowders may have some level of interaction with proteins, and may be incorporated into the condensates at low concentrations. This may have effects on the condensate itself such as altering its material properties(132).
4.2. Multiple Condensed Phases
In addition to systems which segregate into two coexisting phases, there are also cases involving more complex phase behaviors which have been observed in biology. Interestingly, some membraneless organelles may be characterized as multi-layered “core-shell” assemblies containing condensates within larger condensates such as the P granule which is characterized by a gel-like phase rich in the disordered MEG-3 protein, surrounded by a liquid-like phase rich in the PGL-3 protein(128), the related Z granule(149), and the nucleolus, which consists of three distinct layered compartments(150). Biophysical studies of the nucleolus have identified the cause for the core-shell structure as differences in surface tension between the droplets of different components with the component having the highest surface tension at the center(150). This type of architecture would be most likely to occur when both components have strong self-interaction strength, and significant, yet weaker, cross-interaction strength. Strong self-interactions and weaker cross-interactions could also result in formation of two distinct types of droplets which do not mix or fuse, which can also be represented using two-component phase diagrams with regions of two-phase and three-phase coexistence(109). Recent studies have also suggested the possibility that some MLOs may form concentration gradients between components(151).
4.3. Phase separation with many components
Studying two components forming distinct phases can be useful for characterizing the different relationships between phase-separating proteins and nucleic acids. However, most MLOs contain dozens or hundreds of different components, making in vivo condensates drastically different from in vitro reconstituted droplets, or simplified theoretical and computational models. However, useful information may arise from considering the relative interaction strengths of each component with itself and all other components, such as an interaction matrix(141, 150, 152). Jacobs and Frenkel undertook the challenge of reducing systems with many different components to a couple parameters, looking at the effects of the number of components, and the interactions between the components(141). They find that in general, systems with many components having a large distribution of interaction strengths tends toward demixing of fewer components, while a system with more homogeneous interaction strengths results in a condensation phase transition, with most of the components being incorporated into the condensed phase. As the number of components increases, the system tends toward forming a single condensed phase with the many components.
SUMMARY POINTS.
Both folded and unfolded domains are important to phase separation and function.
LLPS is driven by weak, multivalent interactions of different types.
The roles of disordered regions, folded regions and different interaction modes are highly context-specific.
Different interaction modes respond to stimuli in different ways, making biomolecular phase separation highly tunable.
Multicomponent systems can display multiple co-phase separation scenarios depending on protein/RNA composition and sequence, and can be tuned by external stimuli.
Molecular simulations are a powerful tool for relating the molecular properties of the components undergoing LLPS to the phase separation.
FUTURE ISSUES.
How applicable is the study of equilibrium phase behavior to the highly non-equilibrium processes occurring in biology?
Can the responses to different stimuli be tuned independently within a sequence?
Can robust selectivity be achieved by designing proteins with different interaction modes driving phase separation?
Can two-body interaction strengths be used to infer entire two-component phase diagrams?
Can the interaction matrix between a large library of components predict the concentration-dependent phase separation, or are higher order terms necessary for this analysis to be useful?
ACKNOWLEDGMENTS
GLD and JM acknowledge Prof. Nicolas Fawzi (Brown University) and his lab members for helpful conversations relating to many of the topics discussed in this article. GLD and JM acknowledge the US Department of Energy, Office of Science, Basic Energy Sciences Award DE-SC00013979, and the National Institutes of Health grants R01GM118530 and R01GM120537 for funding our work on this topic. This research used resources of the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility supported under Contract No. DE-AC02-05CH11231. Use of the high-performance computing capabilities of the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the National Science Foundation, project no. TG-MCB120014 is also gratefully acknowledged. RB is supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Glossary
- IDP
intrinsically disordered protein
- IDR
intrinsically disordered region
- MLO
membrane-less organelle
- LLPS
liquid-liquid phase separation
- Rg
radius of gyration
- ν
Flory scaling exponent
- Tc
critical temperature of phase separation
- θ-solvent temperature
the temperature at which a polymer chain behaves as in an ideal solvent
- RRM
RNA-recognition motif
- aggregate
Solid inclusions composed largely of misfolded protein, commonly associated with cell death and disease
- Oligomerization
assembly of a finite number of components into a single “oligomer”
- RNP
ribonucleoprotein
- Helicase
a specific class of enzyme whose function is to unwind structured or double-stranded nucleic acids
- GFP
green uorescent protein
- Tsat
saturation temperature, or cloud-point temperature
- Hysteresis
a history-dependent phase behavior, such as displaying different saturation temperatures when heating vs. cooling
- Interaction Network
A map that shows connections between each pair of proteins that are known to interact with each other
- Induced Fit
when an IDP binds to a partner which promotes folding into specific folded structure
- PTM
post-translational modification
- sp2-hybridization
arrangement of electron orbitals into a planar triangular shape, present in carbon atoms having three covalent bonds
- π-bond
occurs when two atoms form covalent bonds with more than one electron orbital each as in a double, triple, or partial double bond
- Saturation Concentration
the concentration above which a solution becomes turbid and begins to phase separate
- Block Copolymer
A polymer containing two or more types of monomer units which are arranged in blocks, having many of the same type of monomer adjacent to one another
- SCD
sequence charge decoration
- UCST
upper critical solution temperature
- LCST
lower critical solution temperature
- cold denaturation
the unfolding of proteins with decreasing temperatures
- Hofmeister series
ranking of anions and cations by their ability to precipitate proteins
- kosmotrope
Hofmeister ion which is more likely to precipitate protein from solution by increasing its hydrophobicity
- chaotrope
Hofmeister ion which is more likely to solubilize protein and decrease hydrophobicity
- B22
osmotic second virial coefficient of self interaction
- TB
Boyle temperature, where B22 disappears
Contributor Information
Gregory L. Dignon, Department of Chemical and Biomolecular Engineering, Lehigh University, Bethlehem, Pennsylvania, 18015, United States;
Robert B. Best, Laboratory of Chemical Physics, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland, 20892-0520, United States;
Jeetain Mittal, Department of Chemical and Biomolecular Engineering, Lehigh University, Bethlehem, Pennsylvania, 18015, United States;.
LITERATURE CITED
- 1.Shin Y and Brangwynne CP, “Liquid phase condensation in cell physiology and disease,” Science, vol. 357, no. 6357, p. eaaf4382, 2017. [DOI] [PubMed] [Google Scholar]
- 2.Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Jülicher F, and Hyman AA, “Germline p granules are liquid droplets that localize by controlled dissolution/condensation,” Science, vol. 324, no. 5935, pp. 1729–1732, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Li P, Banjade S, Cheng H-C, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, King DS, Banani SF, Russo PS, Jiang Q-X, Nixon BT, and Rosen MK, “Phase transitions in the assembly of multi-valent signaling proteins,” Nature, vol. 483, no. 7389, p. 336, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riback JA, Katanski CD, Kear-Scott JL, Pilipenko EV, Rojek AE, Sosnick TR, and Drummond DA, “Stress-triggered phase separation is an adaptive, evolutionarily tuned response,” Cell, vol. 168, no. 6, pp. 1028–1040, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei M-T, Elbaum-Garfinkle S, Holehouse AS, Chen CC-H, Feric M, Arnold CB, Priestley RD, Pappu RV, and Brangwynne CP, “Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles,” Nat. Chem, vol. 9, no. 11, p. 1118, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuster BS, Reed EH, Parthasarathy R, Jahnke CN, Caldwell RM, Bermudez JG, Ramage H, Good MC, and Hammer DA, “Controllable protein phase separation and modular recruitment to form responsive membraneless organelles,” Nat. Commun, vol. 9, no. 1, p. 2985, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, Pozniakovski A, Poser I, Maghelli N, Royer LA, Weigert M, Myers EW, Grill S, Dreschel D, Hyman AA, and Alberti S, “A liquid-to-solid phase transition of the als protein fus accelerated by disease mutation,” Cell, vol. 162, no. 5, pp. 1066–1077, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Conicella AE, Zerze GH, Mittal J, and Fawzi NL, “Als mutations disrupt phase separation mediated by α-helical structure in the tdp-43 low-complexity c-terminal domain,” Structure, vol. 24, no. 9, pp. 1537–1549, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wegmann S, Eftekharzadeh B, Tepper K, Zoltowska KM, Bennett RE, Dujardin S, Laskowski PR, MacKenzie D, Kamath T, Commins C, Vanderburg C, Roe AD, Fan Z, Molliex AM, Hernandez-Vega A, Muller D, Hyman AA, Mandelkow E, Taylor JP, and Hyman BT, “Tau protein liquid–liquid phase separation can initiate tau aggregation,” EMBO J., p. e98049, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darling AL, Liu Y, Oldfield CJ, and Uversky VN, “Intrinsically disordered proteome of human membrane-less organelles,” Proteomics, vol. 18, no. 5–6, p. 1700193, 2018. [DOI] [PubMed] [Google Scholar]
- 11.Van Der Lee R, Buljan M, Lang B, Weatheritt RJ, Daughdrill GW, Dunker AK, Fuxreiter M, Gough J, Gsponer J, Jones DT, Kim PM, Kriwacki RW, Oldfield CJ, Pappu RV, Tompa P, Uversky VN, Wright PE, and Babu MM, “Classification of intrinsically disordered regions and proteins,” Chemical reviews, vol. 114, no. 13, pp. 6589–6631, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uversky VN, Gillespie JR, and Fink AL, “Why are “natively unfolded” proteins unstructured under physiologic conditions?,” Proteins, vol. 41, pp. 415–427, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Muiznieks LD, Sharpe S, Pomès R, and Keeley FW, “Role of liquid–liquid phase separation in assembly of elastin and other extracellular matrix proteins,” Journal of molecular biology, vol. 430, no. 23, pp. 4741–4753, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Kim NC, Wang Y-D, Scarborough EA, Moore J, Diaz Z, MacLea KS, Freibaum B, Li S, Molliex A, Kanagaraj AP, Carter R, Boylan KB, Wojtas AM, Rademakers R, Pinkus JL, Greenberg SA, Trojanowski JQ, Traynor BJ, Smith BN, Topp S, Gkazi A-S, Miller J, Shaw CE, Kottlors M, Kirschner J, Pestronk A, Li YR, Ford AF, Gitler AD, Benatar M, King OD, Kimonis VE, Ross ED, Weihl CC, Shorter J, and Taylor JP, “Mutations in prion-like domains in hnrnpa2b1 and hnrnpa1 cause multisystem proteinopathy and als,” Nature, vol. 495, no. 7442, p. 467, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittag T and Parker R, “Multiple modes of proteinprotein interactions promote rnp granule assembly,” Journal of Molecular Biology, vol. 430, no. 23, pp. 4636–4649, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uversky VN, Kuznetsova IM, Turoverov KK, and Zaslavsky B, “Intrinsically disordered proteins as crucial constituents of cellular aqueous two phase systems and coacervates,” FEBS Lett, vol. 589, no. 1, pp. 15–22, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Quiroz FG and Chilkoti A, “Sequence heuristics to encode phase behaviour in intrinsically disordered protein polymers,” Nat. Mater, vol. 14, no. 11, p. 1164, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dignon GL, Zheng W, Kim YC, Best RB, and Mittal J, “Sequence determinants of protein phase behavior from a coarse-grained model,” PLoS Comput. Biol, vol. 14, no. 1, p. e1005941, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brady JP, Farber PJ, Sekhar A, Lin Y-H, Huang R, Bah A, Nott TJ, Chan HS, Baldwin AJ, Forman-Kay JD, and Kay LE, “Structural and hydrodynamic properties of an intrinsically disordered region of a germ cell-specific protein on phase separation,” Proc. Natl. Acad. Sci. U.S.A, vol. 114, no. 39, pp. E8194–E8203, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke KA, Janke AM, Rhine CL, and Fawzi NL, “Residue-by-residue view of in vitro fus granules that bind the c-terminal domain of rna polymerase ii,” Mol. Cell, vol. 60, no. 2, pp. 231–241, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murthy AC, Dignon GL, Kan Y, Zerze GH, Parekh SH, Mittal J, and Fawzi NL, “Molecular interactions underlying liquid-liquid phase separation of the fus low-complexity domain,” Nature Structural and Molecular Biology, vol. 26, pp. 637–648, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galea CA, Wang Y, Sivakolundu SG, and Kriwacki RW, “Regulation of cell division by intrinsically unstructured proteins: intrinsic flexibility, modularity, and signaling conduits,” Biochemistry, vol. 47, no. 29, pp. 7598–7609, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JT, Smith J, Chen B-C, Schmidt H, Rasoloson D, Paix A, Lambrus BG, Calidas D, Betzig E, and Seydoux G, “Regulation of rna granule dynamics by phosphorylation of serine-rich, intrinsically disordered proteins in c. elegans,” eLife, vol. 3, p. e04591, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monahan Z, Ryan VH, Janke AM, Burke KA, Zerze GH, O’Meally R, Dignon GL, Conicella AE, Zheng W, Best RB, Cole RN, Mittal J, and Shewmaker F, “Phosphorylation of fus low-complexity domain disrupts phase separation, aggregation, and toxicity,” EMBO J., p. e201696394, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larson AG, Elnatan D, Keenen MM, Trnka MJ, Johnston JB, Burlingame AL, Agard DA, Redding S, and Narlikar GJ, “Liquid droplet formation by hp1α suggests a role for phase separation in heterochromatin,” Nature, vol. 547, no. 7662, p. 236, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan VH, Dignon GL, Zerze GH, Chabata CV, Silva R, Conicella AE, Amaya J, Burke KA, Mittal J, and Fawzi NL, “Mechanistic view of hnrnpa2 low-complexity domain structure, interactions, and phase separation altered by mutation and arginine methylation,” Mol. Cell, vol. 39, no. 3, pp. 465–479, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito M, Hess D, Eglinger J, Fritsch AW, Kreysing M, Weinert BT, Choudhary C, and Matthias P, “Acetylation of intrinsically disordered regions regulates phase separation,” Nature chemical biology, vol. 15, no. 1, p. 51, 2019. [DOI] [PubMed] [Google Scholar]
- 28.Lin Y-H and Chan HS, “Phase separation and single-chain compactness of charged disordered proteins are strongly correlated,” Biophys. J, vol. 112, no. 10, pp. 2043–2046, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dignon GL, Zheng W, Best RB, Kim YC, and Mittal J, “Relation between single-molecule properties and phase behavior of intrinsically disordered proteins,” Proc. Natl. Acad. Sci. U.S.A, vol. 115, no. 40, pp. 9929–9934, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofmann H, Soranno A, Borgia A, Gast K, Nettels D, and Schuler B, “Polymer scaling laws of unfolded and intrinsically disordered proteins quantified with single-molecule spectroscopy,” Proc. Natl. Acad. Sci. U.S.A, vol. 109, pp. 16155–16160, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riback JA, Bowman MA, Zmyslowski AM, Knoverek CR, Jumper JM, Hinshaw JR, Kaye EB, Freed KF, Clark PL, and Sosnick TR, “Innovative scattering analysis shows that hydrophobic proteins are expanded in water,” Science, vol. 358, pp. 238–241, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng W, Zerze GH, Borgia A, Mittal J, Schuler B, and Best RB, “Inferring properties of disordered chains from fret transfer efficiencies,” J. Chem. Phys, vol. 148, no. 12, p. 123329, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsh JA and Forman-Kay JD, “Sequence determinants of compaction in intrinsically disordered proteins,” Biophysical journal, vol. 98, no. 10, pp. 2383–2390, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flory PJ, “The configuration of real polymer chains,” J. Chem. Phys, vol. 17, pp. 303–310, 1949. [Google Scholar]
- 35.Mao AH, Crick SL, Vitalis A, Chicoine C, and Pappu RV, “Net charge per residue modulates conformational ensembles of intriniscally disordered proteins,” Proc. Natl. Acad. Sci. U.S.A, vol. 107, pp. 8183–8188, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borgia A, Borgia MB, Bugge K, Kissling VM, Heidarsson PO, Fernandes CB, Sottini A, Soranno A, Buholzer KJ, Nettels D, et al. , “Extreme disorder in an ultrahigh-affinity protein complex,” Nature, vol. 555, no. 7694, p. 61, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuertes G, Banterle N, Ruff KM, Chowdhury A, Mercadante D, Koehler C, Kachala M, Girona GE, Milles S, Mishra A, Onck PR, Gräter F, Esteban-Martn S, Papu RV, Svergun DI, and Lemke EA, “Decoupling of size and shape fluctuations in heteropolymeric sequences reconciles discrepancies in saxs vs. fret measurements,” Proc. Natl. Acad. Sci. U.S.A, vol. 114, pp. E6342–E6351, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng W, Best RB. 2018. An extended Guinier analysis for intrinsically disordered proteins. J. Mol. Biol 430:2540–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panagiotopoulos AZ, Wong V, and Floriano MA, “Phase equilibria of lattice polymers from histogram reweighting monte carlo simulations,” Macromolecules, vol. 31, no. 3, pp. 912–918, 1998. [Google Scholar]
- 40.Rauscher S and Pomès R, “The liquid structure of elastin,” eLife, vol. 6, p. e26526, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dignon GL, Zheng W, and Mittal J, “Simulation methods for liquid-liquid phase separation of disordered proteins,” Curr. Opin. Chem. Eng, vol. 24, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao B, Li NK, Yingling YG, and Hall CK, “Lcst behavior is manifested in a single molecule: elastin-like polypeptide (vpgvg) n,” Biomacromolecules, vol. 17, no. 1, pp. 111–118, 2015. [DOI] [PubMed] [Google Scholar]
- 43.Dignon GL, Zheng W, Kim YC, and Mittal J, “Temperature-controlled liquid–liquid phase separation of disordered proteins,” ACS Central Science, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kroschwald S, Munder MC, Maharana S, Franzmann TM, Richter D, Ruer M, Hyman AA, and Alberti S, “Different material states of pub1 condensates define distinct modes of stress adaptation and recovery,” Cell Rep, vol. 23, no. 11, pp. 3327–3339, 2018. [DOI] [PubMed] [Google Scholar]
- 45.Wang A, Conicella AE, Schmidt HB, Martin EW, Rhoads SN, Reeb AN, Nourse A, Montero DR, Ryan VH, Rohatgi R, Shewmaker F, Naik MT, Mittag T, Ayala YM, and Fawzi NL, “A single n-terminal phosphomimic disrupts tdp-43 polymerization, phase separation, and rna splicing,” The EMBO journal, p. e97452, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitrea DM, Cika JA, Stanley CB, Nourse A, Onuchic PL, Banerjee PR, Phillips AH, Park C-G, Deniz AA, and Kriwacki RW, “Self-interaction of npm1 modulates multiple mechanisms of liquid–liquid phase separation,” Nature communications, vol. 9, no. 1, p. 842, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.An S, Kumar R, Sheets ED, and Benkovic SJ, “Reversible compartmentalization of de novo purine biosynthetic complexes in living cells,” Science, vol. 320, no. 5872, pp. 103–106, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Sabari BR, Dall’Agnese A, Boija A, Klein IA, Coffey EL, Shrinivas K, Abraham BJ, Hannett NM, Zamudio AV, Manteiga JC, Li CH, Guo YE, Day DS, Schuijers J, Vasile E, Malik S, Hnisz D, Lee TI, Cisse II, Roeder RG, Sharp PA, Chakraborty AK, and Young RA, “Coactivator condensation at super-enhancers links phase separation and gene control,” Science, vol. 361, p. eaar3958, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asherie N, “Protein crystallization and phase diagrams,” Methods, vol. 34, no. 3, pp. 266–272, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Braun MK, Wolf M, Matsarskaia O, Da Vela S, Roosen-Runge F, Sztucki M, Roth R, Zhang F, and Schreiber F, “Strong isotope effects on effective interactions and phase behavior in protein solutions in the presence of multivalent ions,” J. Phys. Chem. B, vol. 121, no. 7, pp. 1731–1739, 2017. [DOI] [PubMed] [Google Scholar]
- 51.Jiang L-L, Che M-X, Zhao J, Zhou C-J, Xie M-Y, Li H-Y, He J-H, and Hu H-Y, “Structural transformation of the amyloidogenic core region of tdp-43 protein initiates its aggregation and cytoplasmic inclusion,” Journal of Biological Chemistry, vol. 288, no. 27, pp. 19614–19624, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shin Y, Berry J, Pannucci N, Haataja MP, Toettcher JE, and Brangwynne CP, “Spatiotemporal control of intracellular phase transitions using light-activated optodroplets,” Cell, vol. 168, no. 1, pp. 159–171, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bracha D, Walls MT, Wei M-T, Zhu L, Kurian M, Avalos JL, Toettcher JE, and Brangwynne CP, “Mapping local and global liquid phase behavior in living cells using photo-oligomerizable seeds,” Cell, vol. 175, no. 6, pp. 1467–1480, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loughlin FE, Lukavsky PJ, Kazeeva T, Reber S, Hock E-M, Colombo M, Von Schroetter C, Pauli P, Cléry A, Mühlemann O, et al. , “The solution structure of fus bound to rna reveals a bipartite mode of rna recognition with both sequence and shape specificity,” Molecular cell, vol. 73, no. 3, pp. 490–504, 2019. [DOI] [PubMed] [Google Scholar]
- 55.Knott M and Best RB, “Discriminating binding mechanisms of an intrinsically disordered protein via a multistate coarse-grained model,” J. Chem. Phys, vol. 140, p. 175102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bah A, Vernon RM, Siddiqui Z, Krzeminski M, Muhandiram R, Zhao C, Sonenberg N, Kay LE, and Forman-Kay JD, “Folding of an intrinsically disordered protein by phosphorylation as a regulatory switch,” Nature, vol. 519, no. 7541, p. 106, 2015. [DOI] [PubMed] [Google Scholar]
- 57.Conicella AE, Dignon GL, Zerze GH, Schmidt HB, Alexandra M, Kim YC, Rohatgi R, Ayala YM, Mittal J, and Fawzi NL, “Tdp-43 α-helical structure tunes liquid-liquid phase separation and function,” BioRxiv, p. 640615, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts S, Harmon TS, Schaal J, Miao V, Li KJ, Hunt A, Wen Y, Oas TG, Collier JH, Pappu RV, and Chilkoti A, “Injectable tissue integrating networks from recombinant polypeptides with tunable order,” Nat. Mater, vol. 17, no. 12, pp. 1154–1163, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prouteau M and Loewith R, “Regulation of cellular metabolism through phase separation of enzymes,” Biomolecules, vol. 8, no. 4, p. 160, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jankowsky E, “Rna helicases at work: binding and rearranging,” Trends in biochemical sciences, vol. 36, no. 1, pp. 19–29, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rai AK, Chen J-X, Selbach M, and Pelkmans L, “Kinase-controlled phase transition of membraneless organelles in mitosis,” Nature, vol. 559, no. 7713, p. 211, 2018. [DOI] [PubMed] [Google Scholar]
- 62.Dunker AK, Cortese MS, Romero P, Iakoucheva LM, and Uversky VN, “Flexible nets: the roles of intrinsic disorder in protein interaction networks,” The FEBS journal, vol. 272, no. 20, pp. 5129–5148, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Markmiller S, Soltanieh S, Server KL, Mak R, Jin W, Fang MY, Luo E-C, Krach F, Yang D, Sen A, Fulzele A, Wozniak JM, Gonzalez DJ, Kankel MW, Gao F-B, Bennett EJ, Lécuyer E, and Yeo GW, “Context-dependent and disease-specific diversity in protein interactions within stress granules,” Cell, vol. 172, no. 3, pp. 590–604, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chakraborty AK, “Disordered heteropolymers: models for biomimetic polymers and polymers with frustrating quenched disorder,” Physics Reports, vol. 342, no. 1, pp. 1–61, 2001. [Google Scholar]
- 65.Amaya J, Ryan VH, and Fawzi NL, “The sh3 domain of fyn kinase interacts with and induces liquid–liquid phase separation of the low-complexity domain of hnrnpa2,” Journal of Biological Chemistry, vol. 293, no. 51, pp. 19522–19531, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang H, Elbaum-Garfinkle S, Langdon EM, Taylor N, Occhipinti P, Bridges AA, Brangwynne CP, and Gladfelter AS, “Rna controls polyq protein phase transitions,” Mol. Cell, vol. 60, no. 2, pp. 220–230, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Langdon EM, Qiu Y, Niaki AG, McLaughlin GA, Weidmann CA, Gerbich TM, Smith JA, Crutchley JM, Termini CM, Weeks KM, Myong S, and Gladfelter AS, “mrna structure determines specificity of a polyq-driven phase separation,” Science, vol. 360, no. 6391, pp. 922–927, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khoury GA, Baliban RC, and Floudas CA, “Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database,” Scientific reports, vol. 1, p. 90, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gomes E and Shorter J, “Molecular language of membraneless organelles,” Journal of Biological Chemistry, vol. 294, no. 18, pp. 7115–7127, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bentley EP, Frey BB, and Deniz AA, “Physical chemistry of cellular liquid-phase separation,” Chemistry–A European Journal, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elbaum-Garfinkle S, Kim Y, Szczepaniak K, Chen CC-H, Eckmann CR, Myong S, and Brangwynne CP, “The disordered p granule protein laf-1 drives phase separation into droplets with tunable viscosity and dynamics,” Proc. Natl. Acad. Sci. U.S.A, vol. 112, no. 23, pp. 7189–7194, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nott TJ, Petsalaki E, Farber P, Jervis D, Fussner E, Plochowietz A, Craggs TD, Bazett-Jones DP, Pawson T, Forman-Kay JD, and Baldwin AJ, “Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles,” Mol. Cell, vol. 57, no. 5, pp. 936–947, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nott TJ, Craggs TD, and Baldwin AJ, “Membraneless organelles can melt nucleic acid duplexes and act as biomolecular filters.,” Nat. Chem, vol. 8, no. 6, pp. 569–575, 2016. [DOI] [PubMed] [Google Scholar]
- 74.Pak CW, Kosno M, Holehouse AS, Padrick SB, Mittal A, Ali R, Yunus AA, Liu DR, Pappu RV, and Rosen MK, “Sequence determinants of intracellular phase separation by complex coacervation of a disordered protein,” Mol. Cell, vol. 63, no. 1, pp. 72–85, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin Y, McCarty J, Rauch JN, Delaney KT, Kosik KS, Fredrickson GH, Shea J-E, and Han S, “Narrow equilibrium window for complex coacervation of tau and rna under cellular conditions,” Elife, vol. 8, p. e42571, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lytle TK, Chang L-W, Markiewicz N, Perry SL, and Sing CE, “Designing electrostatic interactions via polyelectrolyte monomer sequence,” ACS Central Science, vol. 5, no. 4, pp. 709–718, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Samanta HS, Chakraborty D, and Thirumalai D, “Charge fluctuation effects on the shape of flexible polyampholytes with applications to intrinsically disordered proteins,” The Journal of chemical physics, vol. 149, no. 16, p. 163323, 2018. [DOI] [PubMed] [Google Scholar]
- 78.Martin EW, Holehouse AS, Grace CR, Hughes A, Pappu RV, and Mittag T, “Sequence determinants of the conformational properties of an intrinsically disordered protein prior to and upon multisite phosphorylation,” Journal of the American Chemical Society, vol. 138, no. 47, pp. 15323–15335, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Isom DG, Castañeda CA, Cannon BR, and Garcia-Moreno EB, “Large shifts in pka values of lysine residues buried inside a protein,” Proceedings of the National Academy of Sciences, vol. 108, no. 13, pp. 5260–5265, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin Y, Currie SL, and Rosen MK, “Intrinsically disordered sequences enable modulation of protein phase separation through distributed tyrosine motifs,” J. Biol. Chem, vol. 292, no. 46, pp. 19110–19120, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li H-R, Chiang W-C, Chou P-C, Wang W-J, and Huang J.-r., “Tar dna-binding protein 43 (tdp-43) liquid–liquid phase separation is mediated by just a few aromatic residues,” JBC, vol. 293, no. 16, pp. 6090–6098, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martinez CR and Iverson BL, “Rethinking the term pi-stacking,” Chemical Science, vol. 3, no. 7, pp. 2191–2201, 2012. [Google Scholar]
- 83.Schottel BL, Chifotides HT, and Dunbar KR, “Anion-π interactions,” Chemical Society Reviews, vol. 37, no. 1, pp. 68–83, 2008. [DOI] [PubMed] [Google Scholar]
- 84.Dougherty DA, “The cation-π interaction,” Accounts of chemical research, vol. 46, no. 4, pp. 885–893, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vernon RM, Chong PA, Tsang B, Kim TH, Bah A, Farber P, Lin H, and Forman-Kay JD, “Pi-pi contacts are an overlooked protein feature relevant to phase separation,” eLife, vol. 7, p. e31486, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gallivan JP and Dougherty DA, “Cation-π interactions in structural biology,” Proceedings of the National Academy of Sciences, vol. 96, no. 17, pp. 9459–9464, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang J, Choi J-M, Holehouse AS, Lee HO, Zhang X, Jahnel M, Maharana S, Lemaitre R, Pozniakovsky A, Drechsel D, Poser I, Pappu RV, Alberti S, and Hyman AA, “A molecular grammar governing the driving forces for phase separation of prion-like rna binding proteins,” Cell, vol. 174, no. 3, pp. 688–699, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shakya A and King JT, “Non-fickian molecular transport in protein–dna droplets,” ACS Macro Letters, vol. 7, no. 10, pp. 1220–1225, 2018. [DOI] [PubMed] [Google Scholar]
- 89.Dill KA, “Theory for the folding and stability of globular proteins,” Biochemistry, vol. 24, pp. 1501–1509, 1997. [DOI] [PubMed] [Google Scholar]
- 90.Fromm SA, Kamenz J, Nöldeke ER, Neu A, Zocher G, and Sprangers R, “In vitro reconstitution of a cellular phase-transition process that involves the mrna decapping machinery,” Angew. Chem. Int. Edit, vol. 53, no. 28, pp. 7354–7359, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mitrea DM, Cika JA, Guy CS, Ban D, Banerjee PR, Stanley CB, Nourse A, Deniz AA, and Kriwacki RW, “Nucleophosmin integrates within the nucleolus via multi-modal interactions with proteins displaying r-rich linear motifs and rrna,” Elife, vol. 5, p. e13571, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shih J-W, Wang W-T, Tsai T-Y, Kuo C-Y, Li H-K, and Lee Y-HW, “Critical roles of rna helicase ddx3 and its interactions with eif4e/pabp1 in stress granule assembly and stress response,” Biochemical Journal, vol. 441, no. 1, pp. 119–129, 2012. [DOI] [PubMed] [Google Scholar]
- 93.Tarakanova A, Huang W, Weiss AS, Kaplan DL, and Buehler MJ, “Computational smart polymer design based on elastin protein mutability,” Biomaterials, vol. 127, pp. 49–60, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheng AC, Chen WW, Fuhrmann CN, and Frankel AD, “Recognition of nucleic acid bases and base-pairs by hydrogen bonding to amino acid side-chains,” Journal of molecular biology, vol. 327, no. 4, pp. 781–796, 2003. [DOI] [PubMed] [Google Scholar]
- 95.Zagrovic B, Bartonek L, and Polyansky AA, “Rna-protein interactions in an unstructured context,” FEBS letters, vol. 592, no. 17, pp. 2901–2916, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zaslavsky BY, Ferreira LA, Darling AL, and Uversky VN, “The solvent side of proteinaceous membrane-less organelles in light of aqueous two-phase systems,” International Journal of Biological Macromolecules, vol. 117, pp. 1224–1251, 2018. [DOI] [PubMed] [Google Scholar]
- 97.Murray DT, Kato M, Lin Y, Thurber KR, Hung I, McKnight SL, and Tycko R, “Structure of fus protein fibrils and its relevance to self-assembly and phase separation of low-complexity domains,” Cell, vol. 171, no. 3, pp. 615–627, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hughes MP, Sawaya MR, Boyer DR, Goldschmidt L, Rodriguez JA, Cascio D, Chong L, Gonen T, and Eisenberg DS, “Atomic structures of low-complexity protein segments reveal kinked β sheets that assemble networks,” Science, vol. 359, no. 6376, pp. 698–701, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guenther EL, Cao Q, Trinh H, Lu J, Sawaya MR, Cascio D, Boyer DR, Rodriguez JA, Hughes MP, and Eisenberg DS, “Atomic structures of tdp-43 lcd segments and insights into reversible or pathogenic aggregation,” Nature structural & molecular biology, vol. 25, no. 6, p. 463, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ackermann BE and Debelouchina GT, “Heterochromatin protein hp1α gelation dynamics revealed by solid-state nmr spectroscopy,” Angewandte Chemie, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Das RK and Pappu RV, “Conformations of intrinsically disordered proteins are influenced by linear sequence distributions of oppositely charged residues,” Proc. Natl. Acad. Sci. U.S.A, vol. 110, pp. 13392–13397, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sawle L and Ghosh K, “A theoretical method to compute sequence dependent configurational properties in charged polymers and proteins,” J. Chem. Phys, vol. 143, no. 8, p. 085101, 2015. [DOI] [PubMed] [Google Scholar]
- 103.Das S, Amin AN, Lin Y-H, and Chan HS, “Coarse-grained residue-based models of disordered protein condensates: utility and limitations of simple charge pattern parameters,” Phys. Chem. Chem. Phys, vol. 20, no. 45, pp. 28558–28574, 2018. [DOI] [PubMed] [Google Scholar]
- 104.Khokhlov AR and Khalatur PG, “Conformation-dependent sequence design (engineering) of ab copolymers,” Physical review letters, vol. 82, no. 17, p. 3456, 1999. [Google Scholar]
- 105.Ashbaugh HS, “Tuning the globular assembly of hydrophobic/hydrophilic heteropolymer sequences,” The Journal of Physical Chemistry B, vol. 113, no. 43, pp. 14043–14046, 2009. [DOI] [PubMed] [Google Scholar]
- 106.Brangwynne CP, Tompa P, and Pappu RV, “Polymer physics of intracellular phase transitions,” Nat. Phys, vol. 11, no. 11, p. 899, 2015. [Google Scholar]
- 107.Cuylen S, Blaukopf C, Politi AZ, Müller-Reichert T, Neumann B, Poser I, Ellenberg J, Hyman AA, and Gerlich DW, “Ki-67 acts as a biological surfactant to disperse mitotic chromosomes,” Nature, vol. 535, no. 7611, p. 308, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T, and Taylor JP, “Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization,” Cell, vol. 163, no. 1, pp. 123–133, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin Y-H, Brady JP, Forman-Kay JD, and Chan HS, “Charge pattern matching as a fuzzymode of molecular recognition for the functional phase separations of intrinsically disordered proteins,” New J. Phys, vol. 19, no. 11, p. 115003, 2017. [Google Scholar]
- 110.Falahati H and Wieschaus E, “Independent active and thermodynamic processes govern the nucleolus assembly in vivo,” Proc. Natl. Acad. Sci. U.S.A, vol. 114, no. 6, pp. 1335–1340, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nakashima KK, Baaij JF, and Spruijt E, “Reversible generation of coacervate droplets in an enzymatic network,” Soft Matter, vol. 14, no. 3, pp. 361–367, 2018. [DOI] [PubMed] [Google Scholar]
- 112.Balu R, Dutta NK, Choudhury NR, Elvin CM, Lyons RE, Knott R, and Hill AJ, “An16-resilin: An advanced multi-stimuli-responsive resilin-mimetic protein polymer,” Acta Biomater, vol. 10, no. 11, pp. 4768–4777, 2014. [DOI] [PubMed] [Google Scholar]
- 113.Ruff KM, Roberts S, Chilkoti A, and Pappu RV, “Advances in understanding stimulus responsive phase behavior of intrinsically disordered protein polymers,” J. Mol. Biol, vol. 430, no. 23, pp. 4619–4635, 2018. [DOI] [PubMed] [Google Scholar]
- 114.Zerze GH, Best RB, and Mittal J, “Sequence-and temperature-dependent properties of unfolded and disordered proteins from atomistic simulations,” J. Phys. Chem. B, vol. 119, no. 46, pp. 14622–14630, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Privalov PL and Makhatadze GI, “Contribution of hydration to protein folding thermodynamics. II. The entropy and gibbs energy of hydration,” J. Mol. Biol, vol. 232, pp. 660–679, 1993. [DOI] [PubMed] [Google Scholar]
- 116.Garde S, Garcıa AE, Pratt LR, and Hummer G, “Temperature dependence of the solubility of non-polar gases in water,” Biophys. Chem, vol. 78, no. 1–2, pp. 21–32, 1999. [DOI] [PubMed] [Google Scholar]
- 117.Huang DM and Chandler D, “Temperature and length scale dependence of hydrophobic effects and their possible implications for protein folding,” Proc. Natl. Acad. Sci. U.S.A, vol. 97, no. 15, pp. 8324–8327, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chandler D, “Interfaces and the driving force of hydrophobic assembly,” Nature, vol. 437, pp. 640–647, 2005. [DOI] [PubMed] [Google Scholar]
- 119.Dill KA, Alonso DO, and Hutchinson K, “Thermal stabilities of globular proteins,” Biochemistry, vol. 28, no. 13, pp. 5439–5449, 1989. [DOI] [PubMed] [Google Scholar]
- 120.van Dijk E, Varilly P, Knowles TP, Frenkel D, and Abeln S, “Consistent treatment of hydrophobicity in protein lattice models accounts for cold denaturation,” Phys. Rev. Lett, vol. 116, no. 7, p. 078101, 2016. [DOI] [PubMed] [Google Scholar]
- 121.Debye P and Hückel E, “De la theorie des electrolytes. i. abaissement du point de congelation et phenomenes associes,” Physikalische Zeitschrift, vol. 24, no. 9, pp. 185–206, 1923. [Google Scholar]
- 122.Zhang Y and Cremer PS, “Chemistry of hofmeister anions and osmolytes,” Annual review of physical chemistry, vol. 61, pp. 63–83, 2010. [DOI] [PubMed] [Google Scholar]
- 123.Onuchic PL, Milin AN, Alshareedah I, Deniz AA, and Banerjee PR, “Divalent cations can control a switch-like behavior in heterotypic and homotypic rna coacervates,” bioRxiv, p. 453019, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brangwynne CP, Mitchison TJ, and Hyman AA, “Active liquid-like behavior of nucleoli determines their size and shape in xenopus laevis oocytes,” Proc. Natl. Acad. Sci. U.S.A, vol. 108, no. 11, pp. 4334–4339, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Altmeyer M, Neelsen KJ, Teloni F, Pozdnyakova I, Pellegrino S, Grøfte M, Rask M-BD, Streicher W, Jungmichel S, Nielsen ML, and Lukas J, “Liquid demixing of intrinsically disordered proteins is seeded by poly (adp-ribose),” Nat. Commun, vol. 6, p. 8088, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Patel A, Malinovska L, Saha S, Wang J, Alberti S, Krishnan Y, and Hyman AA, “Atp as a biological hydrotrope,” Science, vol. 356, no. 6339, pp. 753–756, 2017. [DOI] [PubMed] [Google Scholar]
- 127.Wurtz JD and Lee CF, “Stress granule formation via atp depletion-triggered phase separation,” New Journal of Physics, vol. 20, no. 4, p. 045008, 2018. [Google Scholar]
- 128.Putnam A, Cassani M, Smith J, and Seydoux G, “A gel phase promotes condensation of liquid p granules in caenorhabditis elegans embryos,” Nature structural & molecular biology, p. 1, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Choi K-J, Tsoi PS, Moosa MM, Paulucci-Holthauzen A, Liao S-CJ, Ferreon JC, and Ferreon ACM, “A chemical chaperone decouples tdp-43 disordered domain phase separation from fibrillation,” Biochemistry, vol. 57, no. 50, pp. 6822–6826, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin Y, Protter DS, Rosen MK, and Parker R, “Formation and maturation of phase-separated liquid droplets by rna-binding proteins,” Mol. Cell, vol. 60, no. 2, pp. 208–219, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lau HK, Paul A, Sidhu I, Li L, Sabanayagam CR, Parekh SH, and Kiick KL, “Microstructured elastomer-peg hydrogels via kinetic capture of aqueous liquid–liquid phase separation,” Advanced Science, vol. 5, no. 6, p. 1701010, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kaur T, Alshareedah I, Wang W, Ngo J, Moosa MM, and Banerjee PR, “Molecular crowding tunes material states of ribonucleoprotein condensates,” Biomolecules, vol. 9, no. 2, p. 71, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reed EH and Hammer DA, “Redox sensitive protein droplets from recombinant oleosin,” Soft matter, vol. 14, no. 31, pp. 6506–6513, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kato M, Yang Y-S, Sutter BM, Wang Y, McKnight SL, and Tu BP, “Redox state controls phase separation of the yeast ataxin-2 protein via reversible oxidation of its methioninerich low-complexity domain,” Cell, vol. 177, no. 3, pp. 711–721, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Miyazawa S and Jernigan RL, “Residue-residue potentials with a favourable contact pair term and an unfavourable high packing density term, for simulation and threading,” J. Mol. Biol, vol. 256, pp. 623–644, 1996. [DOI] [PubMed] [Google Scholar]
- 136.Du H, Hu X, Duan H, Yu L, Qu F, Huang Q, Zheng W, Xie H, Peng J, Tuo R, Yu D, Lin Y, Li W, Zheng Y, Fang X, Zou Y, Wang H, Wang M, Weiss PS, Yang Y, and Wang C, “Principles of inter-amino-acid recognition revealed by binding energies between homogeneous oligopeptides,” ACS Central Science, vol. 5, no. 1, pp. 97–108, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dias CL and Chan HS, “Pressure-dependent properties of elementary hydrophobic interactions: ramifications for activation properties of protein folding,” The Journal of Physical Chemistry B, vol. 118, no. 27, pp. 7488–7509, 2014. [DOI] [PubMed] [Google Scholar]
- 138.Winter RHA, Cinar H, Fetahaj Z, Cinar S, Vernon RM, and Chan HS, “Temperature, hydrostatic pressure, and osmolyte effects on liquid-liquid phase separation in protein condensates: Physical chemistry and biological implications,” Chemistry–A European Journal, 2019. [DOI] [PubMed] [Google Scholar]
- 139.Banani SF, Rice AM, Peeples WB, Lin Y, Jain S, Parker R, and Rosen MK, “Compositional control of phase-separated cellular bodies,” Cell, vol. 166, no. 3, pp. 651–663, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rao BS and Parker R, “Numerous interactions act redundantly to assemble a tunable size of p bodies in saccharomyces cerevisiae,” Proceedings of the National Academy of Sciences, vol. 114, no. 45, pp. E9569–E9578, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jacobs WM and Frenkel D, “Phase transitions in biological systems with many components,” Biophys. J, vol. 112, no. 4, pp. 683–691, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yoshizawa T, Ali R, Jiou J, Fung HYJ, Burke KA, Kim SJ, Lin Y, Peeples WB, Saltzberg D, Soniat M, Baumhardt JM, Oldenbourg R, Sali A, Fawzi NL, Rosen MK, and Chook YM, “Nuclear import receptor inhibits phase separation of fus through binding to multiple sites,” Cell, vol. 173, no. 3, pp. 693–705, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maharana S, Wang J, Papadopoulos DK, Richter D, Pozniakovsky A, Poser I, Bickle M, Rizk S, Guillén-Boixet J, Franzmann TM, Jahnel M, Marrone L, Chang Y-T, Sterneckert J, Tomancak P, Hyman AA, and Alberti S, “Rna buffers the phase separation behavior of prion-like rna binding proteins,” Science, vol. 360, no. 6391, pp. 918–921, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Banerjee PR, Milin AN, Moosa MM, Onuchic PL, and Deniz AA, “Reentrant phase transition drives dynamic substructure formation in ribonucleoprotein droplets,” Angewandte Chemie International Edition, vol. 56, no. 38, pp. 11354–11359, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boeynaems S, Holehouse AS, Weinhardt V, Kovacs D, Van Lindt J, Larabell C, Van Den Bosch L, Das R, Tompa PS, Pappu RV, and Gitler AD, “Spontaneous driving forces give rise to protein- rna condensates with coexisting phases and complex material properties,” Proceedings of the National Academy of Sciences, p. 201821038, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Harmon TS, Holehouse AS, and Pappu RV, “Differential solvation of intrinsically disordered linkers drives the formation of spatially organized droplets in ternary systems of linear multivalent proteins,” New Journal of Physics, vol. 20, no. 4, p. 045002, 2018. [Google Scholar]
- 147.Rosenzweig ESF, Xu B, Cuellar LK, Martinez-Sanchez A, Schaffer M, Strauss M, Cartwright HN, Ronceray P, Plitzko JM, Förster F, et al. , “The eukaryotic co2-concentrating organelle is liquid-like and exhibits dynamic reorganization,” Cell, vol. 171, no. 1, pp. 148–162, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Delarue M, Brittingham GP, Pfeffer S, Surovtsev I, Pinglay S, Kennedy K, Schaffer M, Gutierrez J, Sang D, Poterewicz G, Chung J, Plitzko J, Groves J, Jacobs-Wagner C, Engel B, and Hold L, “mtorc1 controls phase separation and the biophysical properties of the cytoplasm by tuning crowding,” Cell, vol. 174, no. 2, pp. 338–349, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wan G, Fields BD, Spracklin G, Shukla A, Phillips CM, and Kennedy S, “Spatiotemporal regulation of liquid-like condensates in epigenetic inheritance,” Nature, vol. 557, no. 7707, p. 679, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, Kriwacki RW, Pappu RV, and Brangwynne CP, “Coexisting liquid phases underlie nucleolar subcompartments,” Cell, vol. 165, no. 7, pp. 1686–1697, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gasior K, Zhao J, McLaughlin G, Forest MG, Gladfelter AS, and Newby J, “Partial demixing of rna-protein complexes leads to intradroplet patterning in phase-separated biological condensates,” Physical Review E, vol. 99, no. 1, p. 012411, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fei J, Jadaliha M, Harmon TS, Li IT, Hua B, Hao Q, Holehouse AS, Reyer M, Sun Q, Freier SM, Pappu RV, Prasanth KV, and Ha T, “Quantitative analysis of multilayer organization of proteins and rna in nuclear speckles at super resolution,” J Cell Sci, vol. 130, no. 24, pp. 4180–4192, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


