Abstract
Excess reactive oxygen species (ROS) induced by physical inactivity is associated with muscle atrophy and muscle weakness. However, the role of mitochondrial ROS on disuse-induced muscle atrophy is not fully understood. The purpose of this study was to utilize a genetic strategy to examine the effect of neutralizing mitochondrial ROS on disuse-induced skeletal muscle atrophy. This was accomplished by placing wild-type (WT) and mitochondrial-targeted catalase-expressing (MCAT) littermate mice on 7 days of hindlimb unloading. After assessment of body weight and composition, muscles were analyzed for individual muscle mass, force-generating capacity, fiber type, cross-sectional area, and mitochondrial function, including H2O2 production. Despite a successful attenuation of mitochondrial ROS, MCAT mice were not protected from muscle atrophy. No differences were observed in body composition, lean mass, individual muscle masses, force-generating capacity, or muscle fiber cross-sectional area. These data suggest that neutralizing mitochondrial ROS is insufficient to suppress disuse-induced loss of skeletal muscle mass and contractile function.
NEW & NOTEWORTHY The premise of this study was to examine the efficacy of genetic suppression of mitochondrial reactive oxygen species (ROS) to attenuate disuse-induced muscle atrophy and muscle weakness. Neutralization of mitochondrial ROS by MCAT expression was insufficient to rescue muscle atrophy and muscle weakness.
Keywords: hindlimb unloading, mitochondria, muscle atrophy, oxidative stress, reactive oxygen species
INTRODUCTION
Skeletal muscle inactivity or mechanical unloading are known to cause muscle atrophy and impair contractile function (10, 33). Hindlimb unloading in mice represents an experimental animal model that is known to reproducibly induce robust muscle atrophy (4). Muscle atrophy following hindlimb unloading leads to lower protein synthesis rates and increased protein breakdown (5, 24, 46). However, the upstream mechanism by which these changes occur is not well understood.
Oxidative stress accumulates in prolonged skeletal muscle inactivity (20, 34, 37). A primary source of oxidative stress is the generation of superoxide anions by the mitochondrial electron transport chain (ETC), xanthine oxidase, and nicotinamide adenine dinucleotide phosphate oxidase. Hindlimb unloading is known to increase oxidative stress and reduce antioxidant capacity (24). In particular, the rise in mitochondrial reactive oxygen species (ROS) production has been speculated to trigger proteolysis and reduce protein synthesis with disuse (38, 54). Min et al (31) and Talbert et al. (45) demonstrated that treatment of rats with a mitochondrial-targeted antioxidant reduced mitochondrial H2O2 production and prevented casting-induced muscle atrophy. Similar findings were seen in muscle atrophy in aging (17, 49). These findings suggest that oxidative stress arising from elevated mitochondrial ROS production and impaired antioxidant defense may be involved in muscle atrophy. However, these compounds may exhibit biological activities other than rescuing mitochondrial ROS to prevent atrophy (3, 14).
Expression of catalase targeted to the mitochondria (MCAT) promotes attenuation of mitochondrial-induced oxidative stress in conditions such as insulin resistance and muscle weakness (12). In rats, MCAT expression via in vivo electroporation increased catalase activity and attenuated cast immobilization-induced muscle atrophy compared with control limbs (8). In contrast, some data suggest that mitochondrial ROS may not be important for loss of muscle mass or function associated with aging, dystrophy, or congenital mitochondrial disorders (27, 43). Electroporation of skeletal muscle is known to promote highly unpredictable and heterogenous expression in skeletal muscle tissues. To test a model system in which MCAT is expressed in all myofibers across the animal, we placed MCAT mice (18, 42) on 7-day hindlimb unloading. We hypothesized that this genetic neutralization of mitochondrial ROS would protect mice from disuse-induced skeletal muscle atrophy.
METHODS
Animals and experimental design.
Mitochondrial-targeted catalase (MCAT) transgenic mice were purchased from The Jackson Laboratory [stock no. 016197, B6.Cg-Tg(CAG-OTC/CAT) Prab/J]. The transgenic lines used in this study were maintained as hemizygous and bred onto noncarrier (C57BL/6J background). For genotyping, DNA was extracted with NaOH from the ear and purified by 40 mM Tris·HCl. Genotype was determined using the PCR as recommended by The Jackson Laboratory. Eight-month-old female MCAT and wild-type (WT) littermates were used for all experiments. Animals were assigned to one of four experimental groups (n = 5–11/group): 1) WT mice without unloading (WT non-HU; n = 5), 2) MCAT mice without unloading (MCAT non-HU; n = 5), 3) WT mice with hindlimb unloading (WT HU; n = 10), and 4) MCAT with hindlimb unloading (MCAT HU; n = 11). Non-HU animals were able to freely ambulate in their cage (3 or 4 animals/cage) and had ad libitum access to food and water. Mice were maintained in a temperature-controlled room on a 12-h light/dark cycle. All animal procedures used in this study were approved by the Institutional Animal Care and Use Committee at the University of Utah. For an unclear reason, despite MCAT expression being driven by a β-actin promoter, these mice are known to exhibit increased MCAT protein in heart and skeletal muscles but not in other tissues (12, 42). Body mass were measured every day during HU. Body composition measurements were taken immediately before terminal experiments with a Bruker Minispec MQ20 nuclear magnetic resonance (NMR) analyzer (Bruker, Rheinstetten, Germany).
Hindlimb unloading.
WT and MCAT mice underwent 7 days of HU (2 mice/cage) using a previously described protocol (21, 30, 40) based on the traditional Morey-Holton design to study disuse atrophy in rodents. Along with daily monitoring of body mass, food intake was monitored every other day to ensure that the mice did not experience excessive weight loss due to malnutrition or dehydration. Following 7 days of HU, mice were fasted for 4 h and given an intraperitoneal injection of 80 mg/kg ketamine and 10 mg/kg xylazine, after which tissues were harvested. Tibialis anterior (TA), extensor digitorum longus (EDL), soleus (SOL), plantaris (PLA), and gastrocnemius (GAS) were carefully dissected for weight measurements.
Assessments of ex vivo skeletal muscle contractility.
Maximal force production in EDL and SOL muscles were measured using an ex vivo small animal muscle contraction apparatus (Aurora Scientific), as previously described (11, 50). Briefly, the EDL and SOL muscles were carefully excised from anesthetized mice. Muscles were then attached to the anchor and force transducer of the apparatus and submerged in oxygenated Krebs-Henseleit buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.25 mM CaCl2, 1.2 mM KH2PO4, 25 mM NaHCO3, and 11 mM glucose) at 37°C. The optimal length was determined by maximum twitch force (20 V, 0.1-ms single pulse). After the muscle was set to an optimal length for maximal contraction, the buffer solution was replaced with freshly oxygenated Krebs-Henseleit Buffer (KHB). At the end of the 5-min equilibration, muscles were stimulated with pulses at 10, 20, 30, 40, 60, 80, 100, 125, 150, and 200 Hz (20 V, 0.2-ms pulse, 350-ms train duration) at 2-min intervals.
Muscle fiber-type and cross-sectional area.
The TA and SOL muscle tissues were embedded in optimal cutting temperature (OCT) compound and sectioned (10 μm) with a cryostat (Microtome Plus). Muscle sections were used for myosin heavy chain (MHC) isoform immunofluorescence (IF). The sections were incubated with MHC I (BA-D5), MHC IIa (SC-71), and MHC IIb (BF-F3) (all 3 from Developmental Studies Hybridoma Bank, University of Iowa). Negative-stained fibers were considered to be MHC IIx. Myofiber cross-sectional area was quantified using semiautomatic muscle analysis using segmentation of histology, a MATLAB application (SMASH) alongside ImageJ software.
Mitochondrial H2O2 measurements.
Mitochondrial H2O2 production was measured using the Horiba Fluoromax-4, as previously described (15, 18). Briefly, skeletal muscle was minced in mitochondria isolation medium (300 mM sucrose, 10 mM HEPES, 1 mM EGTA) and subsequently homogenized using a Teflon glass system. Homogenates were then centrifuged at 800 g for 10 min, after which the supernatant was taken and centrifuged at 12,000 g for 10 min. The resulting pellet was carefully resuspended in mitochondria isolation medium. JH2O2 was measured in buffer Z (MES potassium salt; 105 mM, KCl 30 mM, KH2PO4 10 mM, MgCl2 5 mM, and BSA 0.5 mg/ml) supplemented with 10 mM Amplex UltraRed (Invitrogen) and 20 U/mL CuZnSOD in the presence of the following substrates: 10 mM succinate, 100 µM 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU), and 1 µM auranofin. The appearance of the fluorescent product was measured with excitation/emission at 565/600 nm.
Mitochondrial respiration measurements.
Mitochondrial O2 utilization was measured using the Oroboros O2K Oxygraphs, as previously described (18, 19). Isolated mitochondria were added to the oxygraph chambers containing buffer Z. Respiration was measured in response to the following substrate concentrations: 0.5 mM malate, 5 mM pyruvate, 2 mM ADP, 10 mM succinate, and 1.5 μM FCCP.
Western blotting.
Muscle lysates or isolated mitochondria (described above) were subjected to Western blotting analyses. For muscle lysates, frozen GAS muscles were homogenized using a glass tube and mechanically driven pestle grinder in an ice-cold buffer containing 50 mM Tris (pH 7.6), 5 mM EDTA, 150 mM NaCl, 0.1% SDS, 0.1% sodium deoxycholate, and 1% Triton X-100 with a protease inhibitor cocktail. Samples were centrifuged at 4°C for 10 min at 12,000 g. Supernatant protein concentration was determined using the BCA Protein Assay Kit (Thermo Scientific). Equal amounts of protein were then mixed with Laemmeli sample buffer and loaded onto 4–15% gradient gels (Bio-Rad). Proteins were then transferred onto nitrocellulose membranes and Ponceau S stained and imaged to ensure equal protein loading between lanes. Thereafter, membranes were blocked for 1 h at room temperature with 5% bovine serum albumin in Tris-buffered saline with 0.1% Tween 20 (TBST) and subsequently treated with primary antibodies for 4-hydroxynonenal (4-HNE; ab48506; Abcam), catalase (C0979; Sigma), citrate synthase (ab96600; Abcam), SOD2 (sc-137254; Santa Cruz Biotechnology), GPx4 (ab125066; Abcam) or total OXPHOS Rodent Cocktail (ab110413; Abcam). Following incubation, blots were washed in TBST, incubated in appropriate secondary antibodies (Anti-rabbit IgG, 7074, Cell Signaling Technology; or anti-mouse IgG, 31450, Thermo Scientific), and washed in TBST. Western Lightning Plus-ECL (PerkinElmer) were used to facilitate blot detection, and blots were scanned and quantified using FluorChem E imager (ProteinSimple).
Statistical analysis.
Values are expressed as means ± SE. Statistical comparisons were performed using an unpaired two-tailed Student’s t test for two-group analyses and two-way ANOVA with Tukey’s post hoc test for multiple comparisons (GraphPad Prism 8.1.0).
RESULTS
Body mass, food consumption, and body composition.
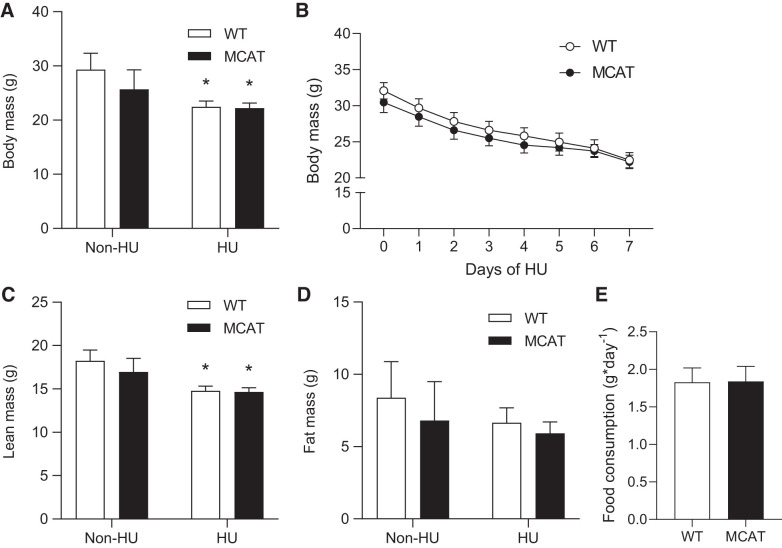
Without HU, body mass and body compositions of WT and MCAT mice were not different (Fig. 1, A–D), consistent with our previous work (18) and other published studies (12, 25). These studies also showed no differences in muscle weights and force-generating capacity between the groups. WT and MCAT mice lost weight similarly during the 7-day HU period (Fig. 1B). The reduction in body mass was largely accounted for by reduced lean mass (Fig. 1C), with no effect on fat mass (Fig. 1D). No difference was observed in food consumption during HU (Fig. 1E).
Fig. 1.
Mitochondrial-targeted catalase (MCAT) does not rescue the loss of lean mass induced by hindlimb unloading (HU). A: body mass from wild-type (WT; open bars) and MCAT (black bars) mice (WT non-HU, n = 5; MCAT non-HU, n = 5; WT HU, n = 10; MCAT HU, n = 11). B: time course changes in body mass during HU (WT HU, n = 10; MCAT HU, n = 11). C: lean mass. D: fat mass (WT non-HU, n = 5; MCAT non-HU, n = 5; WT HU, n = 10; MCAT HU, n = 11). E: daily food consumption during HU (WT HU, n = 10; MCAT HU, n = 11). *Main effect of HU (P < 0.05). Values are means ± SE.
Muscle mass.
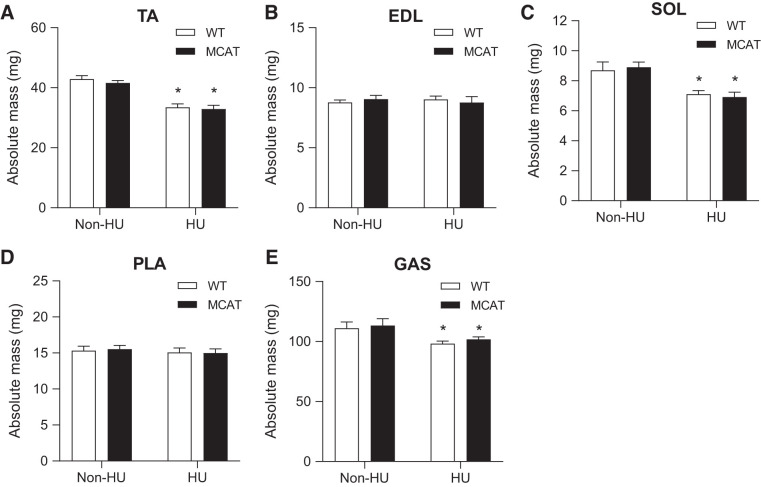
Without HU, muscle weights between WT and MCAT were not different for any of the five muscles that were studied. HU significantly reduced muscle mass in TA, SOL, and GAS muscles, but not in EDL or PLA muscles (Fig. 2, A–E). After 7 days of HU, muscle weights between WT and MCAT mice were not different for any of the five muscles.
Fig. 2.
Mitochondrial-targeted catalase (MCAT) does not rescue the loss of muscle mass induced by hindlimb unloading (HU). A–E: absolute mass of several muscle tissue from wild-type (WT; open bars) and MCAT (black bars) mice with or without HU (WT non-HU, n = 5; MCAT non-HU, n = 5; WT HU, n = 10; MCAT HU, n = 11). EDL, extensor digitorum longus; GAS, gastrocnemius; PLA, plantaris; SOL, soleus; TA, tibialis anterior. *Main effect of HU (P < 0.05). Values are means ± SE.
Contractile function.
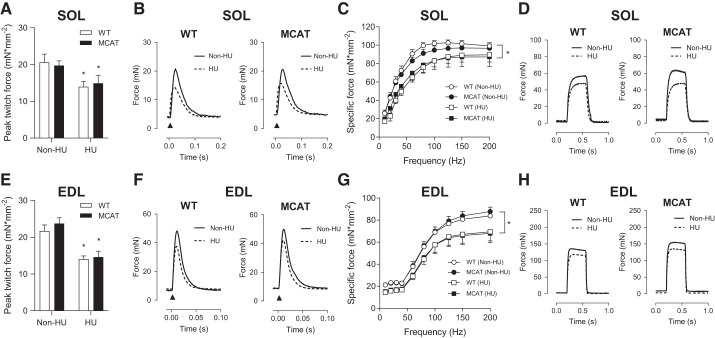
Force-generating capacity was analyzed for SOL (Fig. 3, A–D) and EDL (Fig. 3, E–H). Without HU, twitch and tetanic muscle force production were not different between WT and MCAT mice. As expected, HU significantly reduced twitch and tetanic force production in both WT and MCAT mice. After 7 days of HU, twitch and tetanic force production between WT and MCAT mice were not different. In the force-frequency curves, reduction in maximal tetanic force production induced by HU also coincided with increases in the midpoint of this relationship both in soleus (from 20 to 25 Hz to 30–35 Hz; Fig. 3C) and in EDL (from ∼60 Hz to 65–70 Hz; Fig. 3G).
Fig. 3.
Mitochondrial-targeted catalase (MCAT) does not rescue contractile dysfunction induced by hindlimb unloading (HU). A: peak twitch force for soleus (SOL) muscle in wild-type (WT; open bars) and MCAT (filled bars) mice with or without HU. B: representative twitch force tracings in SOL muscles with or without HU. C: force frequency curves for SOL muscles with or without HU. D: representative tetanic force tracings (200 Hz) for SOL muscles with or without HU (WT non-HU, n = 4; MCAT non-HU, n = 5; WT HU, n = 5; MCAT HU, n = 8). E: peak twitch force for extensor digitorum longus (EDL) muscles with or without HU. F: representative twitch force tracings in EDL muscles with or without HU. G: force-frequency curves for EDL muscles with or without HU. H: representative tetanic force tracings (200 Hz) for EDL muscles with or without HU (WT non-HU, n = 5; MCAT non-HU, n = 4; WT HU, n = 6; MCAT HU, n = 8). *Main effect of HU (P < 0.05). Values are means ± SE.
Muscle fiber-type and cross-sectional area.
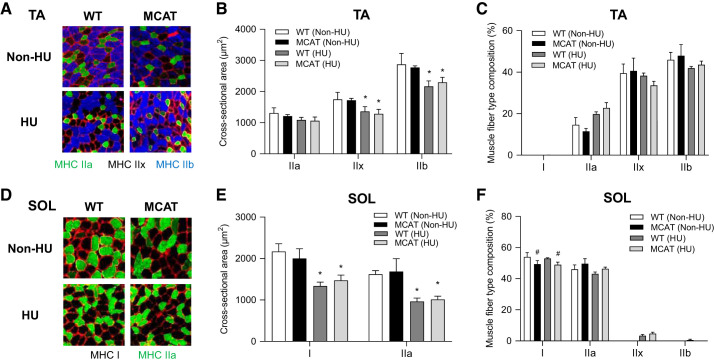
Consistent with previous studies (48), fiber cross-sectional area (CSA) was not different between WT and MCAT mice in TA or SOL muscles (Fig. 4, B and E). HU reduced fiber CSA in both TA and SOL (except for MHC IIa in TA) in both WT and MCAT mice. After 7 days of HU, fiber CSA was not different between WT and MCAT mice in TA or SOL muscles. HU did not significantly alter fiber type composition in TA or SOL muscles (Fig. 5, C and F). Independent of HU, MCAT overexpression appeared to lower the proportion of type I fibers in SOL muscle (main effect of genotype). No such difference was observed in TA muscles, where type I fibers were virtually absent.
Fig. 4.
Mitochondrial-targeted catalase (MCAT) has no effect on muscle fiber cross-sectional area. A: representative images of myosin heavy chain (MHC) immunofluorescence for tibialis anterior (TA) muscles in wild-type (WT) and MCAT mice with or without HU. B: muscle fiber cross-sectional area by fiber type for TA muscles. C: fiber-type composition for TA muscles (WT non-HU, n = 4; MCAT non-HU, n = 4; WT HU, n = 6; MCAT HU, n = 6). D: representative images of MHC immunofluorescence for soleus (SOL) muscles. E: muscle fiber cross-sectional area by fiber type for SOL muscles. F: fiber type composition for SOL muscles (WT non-HU, n = 3; MCAT non-HU, n = 3; WT HU, n = 7; MCAT HU, n = 8). *Main effect of HU (P < 0.05); #main effect of genotype (P < 0.05). Values are means ± SE.
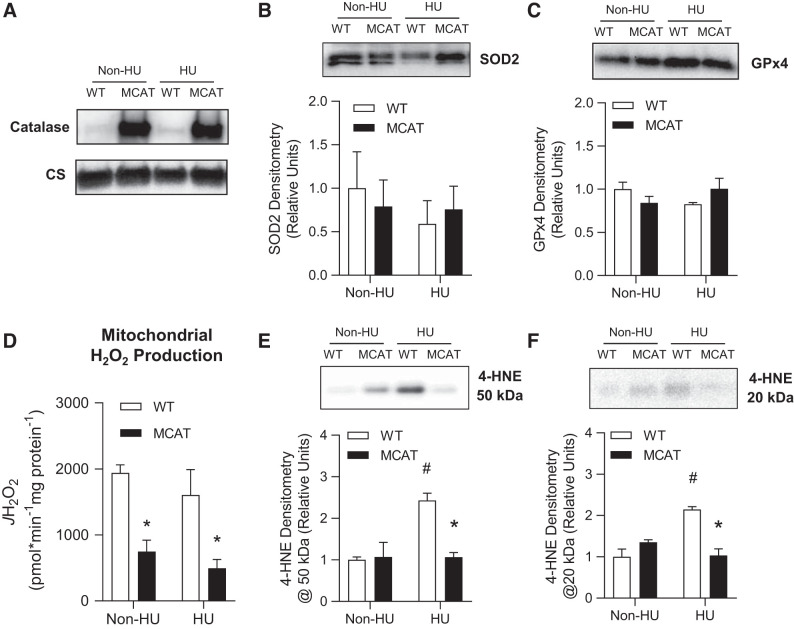
Fig. 5.
Mitochondrial-targeted catalase (MCAT) suppresses mitochondrial oxidative stress. A: representative Western blots of catalase and citrate synthase (CS; used as a loading control) in isolated skeletal muscle mitochondria from wild-type (WT) and MCAT mice with or without hindlimb unloading (HU). B: superoxide dismutase 2 (SOD2) protein abundance in skeletal muscle isolated mitochondria from WT (open bars) and MCAT (black bars) mice with or without HU (WT non-HU, n = 3; MCAT non-HU, n = 3; WT HU, n = 3; MCAT HU, n = 3). C: glutathione peroxidase 4 (GPx4) protein abundance in isolated skeletal muscle mitochondria (WT non-HU, n = 3; MCAT non-HU, n = 3; WT HU, n = 3; MCAT HU, n = 3). D: rate of mitochondrial H2O2 production in response to 10 mM succinate (WT non-HU, n = 5; MCAT non-HU, n = 5; WT HU, n = 7; MCAT HU, n = 7). E and F: representative images and quantification of muscle 4-hydroxynonenal (4-HNE) at ∼50 and ∼20 kDa in whole skeletal muscle tissues (WT non-HU, n = 3; MCAT non-HU, n = 3; WT HU, n = 3; MCAT HU, n = 3). *Significantly different from WT (P < 0.05); #significantly different from non-HU (P < 0.05). Values are means ± SE.
Mitochondrial H2O2 production and O2 consumption.
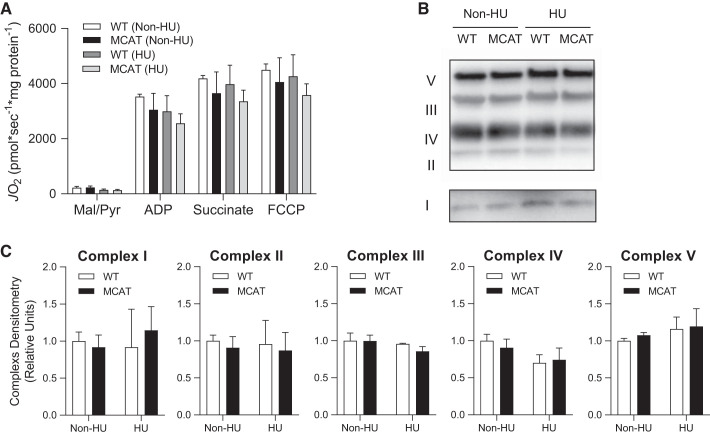
Western blotting was used to confirm MCAT expression (Fig. 5A). WT and MCAT did not differ in the amount of antioxidant enzymes SOD2 and GPx4 with or without HU (Fig. 5, B and C). As expected, H2O2 production was successfully attenuated in muscles with mitochondrial-enriched catalase compared with WT muscles (Fig. 5D). Surprisingly, rates for H2O2 production (succinate driven) were not different between non-HU and HU groups. In contrast, oxidative stress measured by 4-HNE antibody showed marked increase with HU in WT mice (Fig. 5, E and F) that was completely removed with MCAT overexpression (18). No significant differences were observed in skeletal muscle mitochondrial respiration stimulated under a variety of substrates (Fig. 6A), nor were they observed in abundance of respiratory enzymes (Fig. 6, B and C) by genotype or with/without HU.
Fig. 6.
Mitochondrial-targeted catalase (MCAT) has no effect on oxygen consumption in the mitochondria. A: rates of oxygen consumption measured in isolated skeletal muscle mitochondria with Krebs cycle substrates from wild-type (WT; open and dark gray bars) and MCAT (black and light gray bars) mice without (non-HU) or with hindlimb unloading (HU). ADP, adenosine diphosphate; FCCP, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone; Mal, malate; Pyr, pyruvate (WT non-HU, n = 3; MCAT non-HU, n = 3; WT HU, n = 3; MCAT HU, n = 3). B: representative Western blot of the Electron Transport System (ETS) protein complexes in isolated skeletal muscle mitochondria. C: quantification of ETS protein complexes (WT non-HU, n = 3; MCAT non-HU, n = 3; WT HU, n = 3; MCAT HU, n = 3). Values are means ± SE.
DISCUSSION
Oxidative stress has been implicated in disuse-induced muscle atrophy (31, 37, 45). Mitochondria in particular are responsible for generating a significant amount of ROS in the form of superoxide anions derived from electron leakage from the ETC (47). Previous studies show that antioxidants targeted to mitochondria can attenuate muscle atrophy and weakness, suggesting that mitochondrial ROS may directly induce muscle atrophy (31, 45). However, these compounds may exhibit alternative biological activity to other cellular systems to attenuate atrophy. Thus, the premise of this study was to use a genetic model to suppress mitochondrial ROS to more definitively conclude its role in mediating disuse-induced muscle atrophy. Surprisingly, neutralization of mitochondrial ROS (specifically H2O2) by MCAT expression did not alter the propensity for HU-induced loss of skeletal muscle mass or contractile function. These findings suggest that skeletal muscle mitochondrial ROS is not the mechanism by which disuse promotes skeletal muscle atrophy and weakness.
Mitochondrial ROS is thought to induce signaling events to promote muscle atrophy in various models of disuse (39). In response to denervation, whole body CuZn-superoxide dismutase (SOD1)-knockout mice show higher levels of oxidative damage and accelerated muscle atrophy and weakness (22, 32). However, a more recent study shows that muscle-specific knockout of SOD1 mice are not more prone to denervation-induced muscle atrophy (1). In another study, a mimetic of superoxide dismutase and catalase (EUK-134) attenuated HU-induced muscle atrophy (23), although this could be mediated by the action of these compounds in nonmuscle tissues. Directly relevant to the current study, in vivo electroporation of MCAT attenuated casting-induced muscle atrophy in rats (8). The differences are likely contributed by differences in the animal model, mode of disuse, and mode of overexpression. In corroboration with our previous work (18), MCAT expression in mice was sufficient to suppress H2O2 emission without affecting the capacity for mitochondrial oxidative phosphorylation (Fig. 6). In aging, some studies have also shown that mitochondrial oxidative stress may not be causal for age-related muscle atrophy (26, 28).
MCAT overexpression likely only neutralizes mitochondrial ROS (specifically H2O2), not those produced by cytosolic proteins. In this study, rates for H2O2 production were measured in isolated mitochondria. This was done so to validate that MCAT successfully attenuates H2O2 production that occurs specifically in mitochondria. Meanwhile, HU did not increase mitochondrial JH2O2, potentially suggesting that disuse may promote ROS production largely outside of mitochondria. We do not believe this is the case. HU robustly promoted an increase in whole muscle 4-HNE confirming that whole cellular ROS is increased with HU. Furthermore, MCAT overexpression completely neutralized the HU-induced increase in 4-HNE, indicating that ROS induced by HU occurs in mitochondria. Whole muscle 4-HNE likely better reflects ROS observed in vivo, whereas JH2O2 measurements were taken in isolated mitochondria that lost some of the three-dimensional structure present in vivo, studied only with succinate as the substrate.
SOD catalyzes the reaction by which superoxide anions are converted into hydrogen peroxides. Consistent with previous report (20), muscle SOD levels were not altered with MCAT overexpression or with HU. Previous studies suggest that skeletal muscle contains excess mitochondrial SOD that effectively neutralizes superoxides into hydrogen peroxide (16, 35), but we cannot completely rule out a possibility that superoxide anions, which are not scavenged by MCAT, are involved in signaling events that promote muscle atrophy and weakness.
Prolonged periods of mechanical unloading can result in muscle weakness and atrophy (2, 10, 13). Loss of contractile function in skeletal muscle has been linked to increases in oxidative stress and mitochondrial ROS production (1, 36, 44). A study using SS-31, a mitochondria-targeted antioxidant, showed no effect on fatigue-induced reduction in contractile force (7). SS-31 mostly scavenges the superoxide anion (41), suggesting that signaling from superoxide anions is likely not essential for this effect. Similar findings were observed with other antioxidants (6, 52), but it is important to recognize that each of these antioxidants may act primarily on different components of cellular ROS. Nevertheless, examination of force production in mice selectively overexpressing catalase, CuZnSOD, and MnSOD in the diaphragm also revealed no differences (29). In this study, no significant differences were observed in force-generating capacity between MCAT and WT mice following 7 days of HU. Taken together, these studies demonstrate that the involvement of oxidative stress to loss of muscle contractile function is likely intervention dependent.
After HU, muscles from WT and MCAT mice did not differ in fiber CSA or mass of individual muscles. Consistent with these findings, a previous study showed that mitochondrial antioxidant has no effect on muscle CSA (48). There was no difference in fiber type composition in fast/slow-mixed TA muscles. In contrast, the proportion of MHC I fibers from SOL muscles was slightly lower in MCAT mice compared with WT mice as a result of HU. Hindlimb unloading can induce a slow-to-fast transition of MHC isoforms in skeletal muscle, but this is largely a shift within MHC II subisoforms from IIa to IIx and IIb (9, 51, 53). Thus, the difference in fiber type composition is likely influenced by genetics during development, perhaps by the presence of MCAT in the myofibers or α-motor neurons. Regardless, these observations show that neutralization of mitochondrial ROS does not protect loss of muscle mass, even at the microscopic level.
Conclusions.
In conclusion, neutralization of mitochondrial ROS by expression of mitochondrial-targeted catalase was insufficient to rescue disuse-induced muscle atrophy and muscle weakness induced by hindlimb suspension. Consistent with our previous reports (18), the present study questions the notion that suppression of mitochondrial ROS protects muscles from loss in muscle mass or contractile function. Thus, increases in skeletal muscle oxidative stress may be coincidental, and not causal, to atrophy. It is noteworthy that MCAT only neutralizes oxidative stress derived in the mitochondria and that ROS production in other regions of cell, or ROS in other tissues for that matter, may contribute to disuse-induced muscle atrophy. Nevertheless, caution would need to be employed when utilizing mitochondrial-targeted antioxidant to treat muscle atrophy.
GRANTS
This work was supported by National Institutes of Health Grants DK-107397, DK-109888, and AG-063077 to K.F., AG-050781 to M.J.D., and HL-139451 to Z.S.M., a Larry H. & Gail Miller Foundation Grant to P.J.F., American Heart Association Grants 18PRE33960491 to A.R.P.V. and 19PRE34380991 to J.M.J, and the Uehara Memorial Foundation to H.E.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.E., P.S., M.J.D., and K.F. conceived and designed research; H.E., P.S., Z.S.M., J.M.J., P.J.F., A.R.V., and A.S. performed experiments; H.E., P.S., Z.S.M., J.M.J., P.J.F., A.R.V., A.S., M.J.D., and K.F. analyzed data; H.E., P.S., Z.S.M., J.M.J., A.R.V., A.S., M.J.D., and K.F. interpreted results of experiments; H.E., P.S., A.S., and K.F. prepared figures; H.E., P.S., and K.F. drafted manuscript; H.E., P.S., J.M.J., P.J.F., A.R.V., M.J.D., and K.F. edited and revised manuscript; K.F. approved final version of manuscript.
REFERENCES
- 1.Ahn B, Ranjit R, Premkumar P, Pharaoh G, Piekarz KM, Matsuzaki S, Claflin DR, Riddle K, Judge J, Bhaskaran S, Satara Natarajan K, Barboza E, Wronowski B, Kinter M, Humphries KM, Griffin TM, Freeman WM, Richardson A, Brooks SV, Van Remmen H. Mitochondrial oxidative stress impairs contractile function but paradoxically increases muscle mass via fibre branching. J Cachexia Sarcopenia Muscle 10: 411–428, 2019. doi: 10.1002/jcsm.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbogast S, Smith J, Matuszczak Y, Hardin BJ, Moylan JS, Smith JD, Ware J, Kennedy AR, Reid MB. Bowman-Birk inhibitor concentrate prevents atrophy, weakness, and oxidative stress in soleus muscle of hindlimb-unloaded mice. J Appl Physiol (1985) 102: 956–964, 2007. doi: 10.1152/japplphysiol.00538.2006. [DOI] [PubMed] [Google Scholar]
- 3.Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol 24: 1250–1261, 2013. doi: 10.1681/ASN.2012121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodine SC. Disuse-induced muscle wasting. Int J Biochem Cell Biol 45: 2200–2208, 2013. doi: 10.1016/j.biocel.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth FW, Criswell DS. Molecular events underlying skeletal muscle atrophy and the development of effective countermeasures. Int J Sports Med 18, Suppl 4: S265–S269, 1997. doi: 10.1055/s-2007-972723. [DOI] [PubMed] [Google Scholar]
- 6.Bruton JD, Place N, Yamada T, Silva JP, Andrade FH, Dahlstedt AJ, Zhang SJ, Katz A, Larsson NG, Westerblad H. Reactive oxygen species and fatigue-induced prolonged low-frequency force depression in skeletal muscle fibres of rats, mice and SOD2 overexpressing mice. J Physiol 586: 175–184, 2008. doi: 10.1113/jphysiol.2007.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng AJ, Bruton JD, Lanner JT, Westerblad H. Antioxidant treatments do not improve force recovery after fatiguing stimulation of mouse skeletal muscle fibres. J Physiol 593: 457–472, 2015. doi: 10.1113/jphysiol.2014.279398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodd SL, Gagnon BJ, Senf SM, Hain BA, Judge AR. Ros-mediated activation of NF-κB and Foxo during muscle disuse. Muscle Nerve 41: 110–113, 2010. doi: 10.1002/mus.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egawa T, Ohno Y, Goto A, Yokoyama S, Hayashi T, Goto K. AMPK mediates muscle mass change but not the transition of myosin heavy chain isoforms during unloading and reloading of skeletal muscles in mice. Int J Mol Sci 19: 2954, 2018. doi: 10.3390/ijms19102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng HZ, Chen X, Malek MH, Jin JP. Slow recovery of the impaired fatigue resistance in postunloading mouse soleus muscle corresponding to decreased mitochondrial function and a compensatory increase in type I slow fibers. Am J Physiol Cell Physiol 310: C27–C40, 2016. doi: 10.1152/ajpcell.00173.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrara PJ, Verkerke ARP, Brault JJ, Funai K. Hypothermia Decreases O2 Cost for Ex Vivo Contraction in Mouse Skeletal Muscle. Med Sci Sports Exerc 50: 2015–2023, 2018. doi: 10.1249/MSS.0000000000001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilliam LA, Lark DS, Reese LR, Torres MJ, Ryan TE, Lin CT, Cathey BL, Neufer PD. Targeted overexpression of mitochondrial catalase protects against cancer chemotherapy-induced skeletal muscle dysfunction. Am J Physiol Endocrinol Metab 311: E293–E301, 2016. doi: 10.1152/ajpendo.00540.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanson AM, Stodieck LS, Cannon CM, Simske SJ, Ferguson VL. Seven days of muscle re-loading and voluntary wheel running following hindlimb suspension in mice restores running performance, muscle morphology and metrics of fatigue but not muscle strength. J Muscle Res Cell Motil 31: 141–153, 2010. doi: 10.1007/s10974-010-9218-5. [DOI] [PubMed] [Google Scholar]
- 14.Hao S, Ji J, Zhao H, Shang L, Wu J, Li H, Qiao T, Li K. Mitochondrion-targeted peptide SS-31 inhibited oxidized low-density lipoproteins-induced foam cell formation through both ROS scavenging and inhibition of cholesterol influx in RAW264.7 cells. Molecules 20: 21287–21297, 2015. doi: 10.3390/molecules201219764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heden TD, Johnson JM, Ferrara PJ, Eshima H, Verkerke ARP, Wentzler EJ, Siripoksup P, Narowski TM, Coleman CB, Lin CT, Ryan TE, Reidy PT, de Castro Brás LE, Karner CM, Burant CF, Maschek JA, Cox JE, Mashek DG, Kardon G, Boudina S, Zeczycki TN, Rutter J, Shaikh SR, Vance JE, Drummond MJ, Neufer PD, Funai K. Mitochondrial PE potentiates respiratory enzymes to amplify skeletal muscle aerobic capacity. Sci Adv 5: eaax8352, 2019. doi: 10.1126/sciadv.aax8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu JL, Hsieh Y, Tu C, O’Connor D, Nick HS, Silverman DN. Catalytic properties of human manganese superoxide dismutase. J Biol Chem 271: 17687–17691, 1996. doi: 10.1074/jbc.271.30.17687. [DOI] [PubMed] [Google Scholar]
- 17.Javadov S, Jang S, Rodriguez-Reyes N, Rodriguez-Zayas AE, Soto Hernandez J, Krainz T, Wipf P, Frontera W. Mitochondria-targeted antioxidant preserves contractile properties and mitochondrial function of skeletal muscle in aged rats. Oncotarget 6: 39469–39481, 2015. doi: 10.18632/oncotarget.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson JM, Ferrara PJ, Verkerke ARP, Coleman CB, Wentzler EJ, Neufer PD, Kew KA, de Castro Brás LE, Funai K. Targeted overexpression of catalase to mitochondria does not prevent cardioskeletal myopathy in Barth syndrome. J Mol Cell Cardiol 121: 94–102, 2018. doi: 10.1016/j.yjmcc.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson JM, Verkerke ARP, Maschek JA, Ferrara PJ, Lin CT, Kew KA, Neufer PD, Lodhi IJ, Cox JE, Funai K. Alternative splicing of UCP1 by non-cell-autonomous action of PEMT. Mol Metab 31: 55–66, 2020. doi: 10.1016/j.molmet.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo H, Nakagaki I, Sasaki S, Hori S, Itokawa Y. Mechanism of oxidative stress in skeletal muscle atrophied by immobilization. Am J Physiol 265: E839–E844, 1993. doi: 10.1152/ajpendo.1993.265.6.E839. [DOI] [PubMed] [Google Scholar]
- 21.Kwon OS, Nelson DS, Barrows KM, O’Connell RM, Drummond MJ. Intramyocellular ceramides and skeletal muscle mitochondrial respiration are partially regulated by Toll-like receptor 4 during hindlimb unloading. Am J Physiol Regul Integr Comp Physiol 311: R879–R887, 2016. doi: 10.1152/ajpregu.00253.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larkin LM, Davis CS, Sims-Robinson C, Kostrominova TY, Van Remmen H, Richardson A, Feldman EL, Brooks SV. Skeletal muscle weakness due to deficiency of CuZn-superoxide dismutase is associated with loss of functional innervation. Am J Physiol Regul Integr Comp Physiol 301: R1400–R1407, 2011. doi: 10.1152/ajpregu.00093.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawler JM, Kunst M, Hord JM, Lee Y, Joshi K, Botchlett RE, Ramirez A, Martinez DA. EUK-134 ameliorates nNOSμ translocation and skeletal muscle fiber atrophy during short-term mechanical unloading. Am J Physiol Regul Integr Comp Physiol 306: R470–R482, 2014. doi: 10.1152/ajpregu.00371.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med 35: 9–16, 2003. doi: 10.1016/S0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 25.Lee HY, Lee JS, Alves T, Ladiges W, Rabinovitch PS, Jurczak MJ, Choi CS, Shulman GI, Samuel VT. Mitochondrial-targeted catalase protects against high-fat diet-induced muscle insulin resistance by decreasing intramuscular lipid accumulation. Diabetes 66: 2072–2081, 2017. doi: 10.2337/db16-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Van Remmen H, Csete M. Sod2 overexpression preserves myoblast mitochondrial mass and function, but not muscle mass with aging. Aging Cell 8: 296–310, 2009. doi: 10.1111/j.1474-9726.2009.00477.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu M, Yue Y, Li D, Duan D. Catalase overexpression does not impair extensor digitorum longus muscle function in normal mice. Muscle Nerve 36: 833–841, 2007. doi: 10.1002/mus.20874. [DOI] [PubMed] [Google Scholar]
- 28.Lustgarten MS, Jang YC, Liu Y, Qi W, Qin Y, Dahia PL, Shi Y, Bhattacharya A, Muller FL, Shimizu T, Shirasawa T, Richardson A, Van Remmen H. MnSOD deficiency results in elevated oxidative stress and decreased mitochondrial function but does not lead to muscle atrophy during aging. Aging Cell 10: 493–505, 2011. doi: 10.1111/j.1474-9726.2011.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClung JM, Deruisseau KC, Whidden MA, Van Remmen H, Richardson A, Song W, Vrabas IS, Powers SK. Overexpression of antioxidant enzymes in diaphragm muscle does not alter contraction-induced fatigue or recovery. Exp Physiol 95: 222–231, 2010. doi: 10.1113/expphysiol.2009.049650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKenzie AI, Reidy PT, Nelson DS, Mulvey JL, Yonemura NM, Petrocelli JJ, Mahmassani ZS, Tippetts TS, Summers SA, Funai K, Drummond MJ. Pharmacological inhibition of TLR4 ameliorates muscle and liver ceramide content after disuse in previously physically active mice. Am J Physiol Regul Integr Comp Physiol 318: R503–R511, 2020. doi: 10.1152/ajpregu.00330.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Min K, Smuder AJ, Kwon OS, Kavazis AN, Szeto HH, Powers SK. Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy. J Appl Physiol (1985) 111: 1459–1466, 2011. doi: 10.1152/japplphysiol.00591.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, Van Remmen H. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol 293: R1159–R1168, 2007. doi: 10.1152/ajpregu.00767.2006. [DOI] [PubMed] [Google Scholar]
- 33.Murphy KT, Cobani V, Ryall JG, Ibebunjo C, Lynch GS. Acute antibody-directed myostatin inhibition attenuates disuse muscle atrophy and weakness in mice. J Appl Physiol (1985) 110: 1065–1072, 2011. doi: 10.1152/japplphysiol.01183.2010. [DOI] [PubMed] [Google Scholar]
- 34.Nita M, Grzybowski A. The Role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev 2016: 1–23, 2016. doi: 10.1155/2016/3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereira B, Costa Rosa LF, Safi DA, Medeiros MH, Curi R, Bechara EJ. Superoxide dismutase, catalase, and glutathione peroxidase activities in muscle and lymphoid organs of sedentary and exercise-trained rats. Physiol Behav 56: 1095–1099, 1994. doi: 10.1016/0031-9384(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 36.Powers SK, Nelson WB, Hudson MB. Exercise-induced oxidative stress in humans: cause and consequences. Free Radic Biol Med 51: 942–950, 2011. doi: 10.1016/j.freeradbiomed.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Powers SK, Smuder AJ, Criswell DS. Mechanistic links between oxidative stress and disuse muscle atrophy. Antioxid Redox Signal 15: 2519–2528, 2011. doi: 10.1089/ars.2011.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powers SK, Smuder AJ, Judge AR. Oxidative stress and disuse muscle atrophy: cause or consequence? Curr Opin Clin Nutr Metab Care 15: 240–245, 2012. doi: 10.1097/MCO.0b013e328352b4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powers SK, Wiggs MP, Duarte JA, Zergeroglu AM, Demirel HA. Mitochondrial signaling contributes to disuse muscle atrophy. Am J Physiol Endocrinol Metab 303: E31–E39, 2012. doi: 10.1152/ajpendo.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reidy PT, McKenzie AI, Mahmassani ZS, Petrocelli JJ, Nelson DB, Lindsay CC, Gardner JE, Morrow VR, Keefe AC, Huffaker TB, Stoddard GJ, Kardon G, O’Connell RM, Drummond MJ. Aging impairs mouse skeletal muscle macrophage polarization and muscle-specific abundance during recovery from disuse. Am J Physiol Endocrinol Metab 317: E85–E98, 2019. doi: 10.1152/ajpendo.00422.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakellariou GK, Pearson T, Lightfoot AP, Nye GA, Wells N, Giakoumaki II, Vasilaki A, Griffiths RD, Jackson MJ, McArdle A. Mitochondrial ROS regulate oxidative damage and mitophagy but not age-related muscle fiber atrophy. Sci Rep 6: 33944, 2016. doi: 10.1038/srep33944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308: 1909–1911, 2005. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 43.Selsby JT. Increased catalase expression improves muscle function in mdx mice. Exp Physiol 96: 194–202, 2011. doi: 10.1113/expphysiol.2010.054379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith MA, Reid MB. Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol 151: 229–241, 2006. doi: 10.1016/j.resp.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Talbert EE, Smuder AJ, Min K, Kwon OS, Szeto HH, Powers SK. Immobilization-induced activation of key proteolytic systems in skeletal muscles is prevented by a mitochondria-targeted antioxidant. J Appl Physiol (1985) 115: 529–538, 2013. doi: 10.1152/japplphysiol.00471.2013. [DOI] [PubMed] [Google Scholar]
- 46.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol (1985) 68: 1–12, 1990. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 552: 335–344, 2003. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umanskaya A, Santulli G, Xie W, Andersson DC, Reiken SR, Marks AR. Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging. Proc Natl Acad Sci USA 111: 15250–15255, 2014. doi: 10.1073/pnas.1412754111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vays VB, Eldarov CM, Vangely IM, Kolosova NG, Bakeeva LE, Skulachev VP. Antioxidant SkQ1 delays sarcopenia-associated damage of mitochondrial ultrastructure. Aging (Albany NY) 6: 140–148, 2014. doi: 10.18632/aging.100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verkerke ARP, Ferrara PJ, Lin CT, Johnson JM, Ryan TE, Maschek JA, Eshima H, Paran CW, Laing BT, Siripoksup P, Tippetts TS, Wentzler EJ, Huang H, Spangenburg EE, Brault JJ, Villanueva CJ, Summers SA, Holland WL, Cox JE, Vance DE, Neufer PD, Funai K. Phospholipid methylation regulates muscle metabolic rate through Ca2+ transport efficiency. Nat Metab 1: 876–885, 2019. doi: 10.1038/s42255-019-0111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Wang F, Zhang P, Liu H, He J, Zhang C, Fan M, Chen X. PGC-1α over-expression suppresses the skeletal muscle atrophy and myofiber-type composition during hindlimb unloading. Biosci Biotechnol Biochem 81: 500–513, 2017. doi: 10.1080/09168451.2016.1254531. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe D, Aibara C, Wada M. Treatment with EUK-134 improves sarcoplasmic reticulum Ca2+ release but not myofibrillar Ca2+ sensitivity after fatiguing contraction of rat fast-twitch muscle. Am J Physiol Regul Integr Comp Physiol 316: R543–R551, 2019. doi: 10.1152/ajpregu.00387.2018. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, He Z, Gao Y, Hinghofer-Szalkay HG, Fan X. Muscle composition after 14-day hindlimb unloading in rats: effects of two herbal compounds. Aviat Space Environ Med 78: 926–931, 2007. doi: 10.3357/ASEM.2072.2007. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Trevino MB, Wang M, Gardell SJ, Ayala JE, Han X, Kelly DP, Goodpaster BH, Vega RB, Coen PM. Impaired mitochondrial energetics characterize poor early recovery of muscle mass following hind limb unloading in old mice. J Gerontol A Biol Sci Med Sci 73: 1313–1322, 2018. doi: 10.1093/gerona/gly051. [DOI] [PMC free article] [PubMed] [Google Scholar]