Abstract
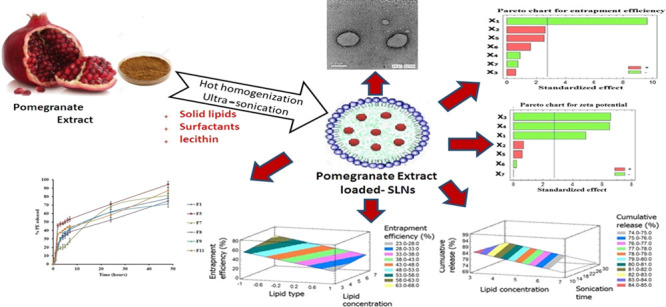
The aim of this work was to study the influence of process variables on the quality attributes of pomegranate extract loaded solid lipid nanoparticles (PE-SLNs) using Plackett–Burman design. PE-SLN formulations were prepared by hot homogenization followed by ultra-sonication technique and evaluated based on the dependent variables that were analyzed utilizing Statgraphics Centurion XV software. The lipid and surfactant (type and concentration), co-surfactant concentration, sonication time, and amplitude were selected as the independent variables (X1–X7). The dependent parameters were particle size, polydispersity index, zeta potential, entrapment efficiency, and cumulative drug release (Y1–Y5). Response surface plots, Pareto charts, and mathematical equations were generated to study the influence of independent variables on the dependent quality parameters. Out of seven variables, X1, X2, and X6 have the main significant (p value < 0.05) effect on the entrapment efficiency, the cumulative drug release, the polydispersity index, respectively, while particle size was mainly affected by X3, X6 and zeta potential by X1, X3, and X4. Consequently, this screening study revealed that stearic acid as lipid, Tween 80 as surfactant, as well as sonication with short time and high amplitude can be selected for the development of PE-SLN formulation with minimum particle size, maximum zeta potential, highest entrapment, and sustained drug release behavior. Meanwhile, concentrations of lipid, surfactant, and co-surfactant are planned to be scaled up for further optimization study. In conclusion, the Plackett–Burman design verified its influence and significance in determining and understanding both process and formulation variables affecting the quality of PE-SLNs.
Introduction
Since a long time ago, human beings have utilized natural active ingredients to treat numerous diseases. In recent eras, products of natural origin have been utilized in the prophylaxis and treatment of cancer. Although, it has been established that many of these natural products have a significant therapeutic importance, their low solubility and bioavailability have rigorously restricted their usage. In the past few periods, scientists have utilized nanoparticles, produced from natural products for the treatment and inhibition of several types of cancers.1
Pomegranate (Punica granatum) has been utilized for therapeutic purposes long periods ago, particularly, for treatment of diarrhea, parasitic, and inflammatory disorders.2 The traditional importance of pomegranate as a medicinal plant is now being reinforced by developing scientific data, which show that the fruit contains anti-oxidant and anti-inflammatory activities and may reveal anti-carcinogenic properties.3 Owing to its content from polyphenolic compounds with high antioxidant and free-radical-scavenging activity, including flavonoids, condensed tannins, and hydrolyzable tannins (ellagitannins and gallotannins), it is very useful in the protection against oxidative-stress-related diseases such as cardiovascular disorders, diabetes, and cancer.2 Antioxidant activity of pomegranate peels was found to be higher than that of pomegranate seeds, which could be a great source for the production of antioxidants with high value.4
Solid lipid nanoparticles (SLNs) are nanoparticles with submicron particle size (50–1000 nm) that were established as a substitute carrier system to conventional colloidal systems, such as emulsions, liposomes, and polymeric microparticles, and nanoparticles.5 SLNs revealed benefits over emulsions, liposomes, and polymeric nanoparticles.6 They avoid drawbacks detected with other drug carriers and protect the drug from chemical degradation compared to that observed with liposomes.7 Furthermore, SLNs may be developed because of their advantage of no or least requirement of organic solvents.8 Moreover, SLNs have drawn considerable attention as novel nanoparticulate carrier systems due to improved delivery and stability of drugs.6 SLNs consists of a solid lipid matrix at room temperature and also at body temperature, stabilized by a surfactant. SLNs have good biocompatibility and biodegradability and also have the ability of incorporating both hydrophobic and hydrophilic compounds.9 SLNs are commonly developed as suspensions or dry powders. The suspension can be transformed into a dry product by spray drying or lyophilization to avoid instability problems.10
Screening several variables that influence any process is a tedious study especially if it is carried out by a trial and error approach. This is due to the fact that it needs a huge number of experiments that require high cost and consume more time and effort. It also does not give the researchers the validated and statistically verified results.11 For these reasons, Plackett–Burman design (PBD) is considered as a highly efficient tool to screen a number of formulation and/or processing variables and explore the most significant variables that can be used in optimization of the process and fix the other insignificant at a low level.12 Several studies had been utilizing PBD in screening the formulation and/or processing variables in development of various dosage forms and drug delivery systems such as controlled release matrix tablets,13 hot melt sustained release extrudates,14 liposomes,15 niosomes,16 transferosomes,17 protein loaded PLGA nanoparticles,18 PLGA-based nanoparticles,19 alginate-reinforced chitosan nanoparticles,20 Eudragit microspheres,21 and matrix-type transdermal films.22
The emphasis of this study was to design SLNs loaded with PE and screening the effects of different formulation and processing variables, which were lipid type and concentration, surfactant type and concentration, co-surfactant concentration, sonication time, and amplitude on the characteristics of the prepared PE-SLNs. A PBD was used to determine the critical parameters that influence PE-SLN characteristics including particle size, polydispersity index, zeta potential, entrapment efficiency, and cumulative % release.
Results and Discussion
Preparation of PE-SLNs
Hot homogenization followed by the ultra-sonication method was carefully chosen for SLN preparation as it was a reliable, simple, and reproducible method. Furthermore, it produced a uniform, homogeneous SLN dispersion,23 without fearing from the incomplete evaporation of organic solvents that we faced when we tried the solvent evaporation method.24
To select the best lipids that entrap a high amount of PE, preliminary studies were done using different lipids such as stearic acid, compritol 888, geleol, and precirol ATO5 in preparation of PE-SLNs. Stearic acid and precirol ATO5 were chosen as the lipid core as it displayed the highest entrapment efficiency. In addition, PE-SLNs were stabilized using either Tween 80 or poloxamer 188 as surfactants and lecithin as a co-surfactant. Based on PBD, the composition of 12 formulations were prepared.
Evaluation of the Prepared PE-SLNs
Twelve different formulations of PE-loaded SLNs were evaluated for their particle size (Y1), PDI (Y2), zeta potential (Y3), entrapment efficiency % (Y4), and cumulative % release after 48 h (Y5), and the obtained results are shown in Table 1.
Table 1. Observed Values of Responses for 12 Formulations of Pomegranate Extract Solid Lipid Nanoparticles.
| observed
responses (Y1–Y5) |
|||||
|---|---|---|---|---|---|
| formulation code | particle sizea (nm) | PDIa | zeta potentiala (mV) | entrapment efficiencya (%) | cumulative releasea (%) |
| F1 | 529.5 ± 8.1 | 0.49 ± 0.067 | –22.6 ± 2.4 | 25.15 ± 1.2 | 78.01 ± 1.8 |
| F2 | 611.7 ± 5.6 | 0.33 ± 0.080 | –38.5 ± 2.9 | 55.60 ± 3.6 | 82.60 ± 2.1 |
| F3 | 1823.0 ± 10.2 | 0.59 ± 0.012 | –23.1 ± 1.8 | 61.11 ± 4.2 | 69.98 ± 1.5 |
| F4 | 563.3 ± 9.5 | 0.35 ± 0.050 | –21.6 ± 3.6 | 61.41 ± 3.9 | 88.30 ± 2.3 |
| F5 | 599.4 ± 8.7 | 0.11 ± 0.011 | –22.7 ± 4.7 | 15.37 ± 1.8 | 94.57 ± 3.3 |
| F6 | 938.4 ± 7.5 | 0.80 ± 0.007 | –35.7 ± 3.5 | 63.74 ± 4.4 | 87.85 ± 2.9 |
| F7 | 752.6 ± 13.4 | 1.00 ± 0.002 | –25.6 ± 2.1 | 49.50 ± 2.9 | 81.80 ± 1.6 |
| F8 | 321.9 ± 6.4 | 0.41 ± 0.020 | –30.7 ± 3.1 | 29.50 ± 1.4 | 75.10 ± 2.5 |
| F9 | 362.7 ± 5.9 | 0.42 ± 0.049 | –26.3 ± 2.3 | 31.50 ± 1.8 | 71.47 ± 1.7 |
| F10 | 965.4 ± 8.2 | 0.54 ± 0.038 | –29.2 ± 3.2 | 65.12 ± 4.8 | 75.95 ± 2.6 |
| F11 | 1670.0 ± 9.9 | 1.00 ± 0.001 | –15.6 ± 2.5 | 23.43 ± 1.7 | 87.31 ± 3.1 |
| F12 | 624.3 ± 4.9 | 0.34 ± 0.030 | –28.8 ± 2.4 | 58.71 ± 3.6 | 74.71 ± 2.9 |
Results are expressed as the mean of three replicates ± SD.
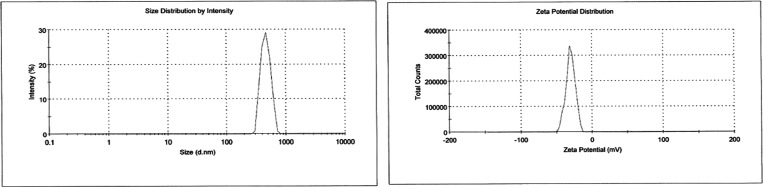
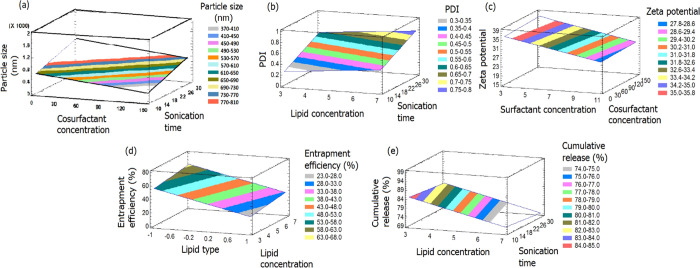
The nanoparticles showed variation in size ranging from 321.9 to 1823 nm with PDI values ranging from 0.112 to 1.0. The size distribution exhibited good unimodal behavior as deduced in Figure 1. Table 1 and Figure 1 showed the negative polarity of the prepared PE-SLNs with zeta potential value in the range of −15.6 to −38.5 mV.
Figure 1.
Particle size distribution and zeta potential for batch F4.
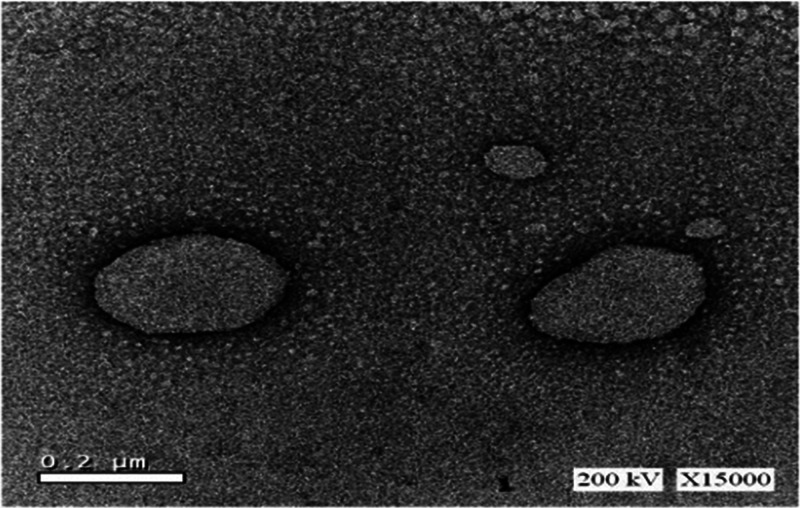
In addition, Figure 2 revealed the transmission electron microscopy (TEM) analyses of the PE-SLN samples, which showed spherical particles with no evident sign of aggregation. As all the images obtained were similar, we reported only one image as an example. Furthermore, the entrapment efficiency % was in the range of 15.37–65.12%.
Figure 2.
TEM image of pomegranate extract solid lipid nanoparticles obtained from batch F8.
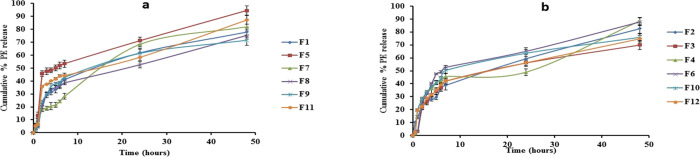
Finally, the cumulative PE release after 48 h reached 94.57% in F5 which contains the lowest value of both precirol and poloxamer. Meanwhile, the sustaining PE release was achieved in F3, which contains the highest value of both stearic acid and poloxamer. Figure 3a,b displays the release pattern of PE from all formulations.
Figure 3.
(a) In vitro release of precirol SLNs loaded PE through the cellulose membrane after 48 h. (b) In vitro release of stearic acid SLNs loaded PE the through cellulose membrane after 48 h.
Influence of Investigated Parameters on Particle Size (Y1)
One of the most essential characteristics of the nanoparticles is the particle size which affects both the release pattern of the drug and its absorption.25 The mean particle size (Y1) ranged from 321.9 nm in F8 to 1823 nm in F3 depending on the variable level selected during production (Table 1). The model that describes the influence of variables on the particle size is
| 1 |
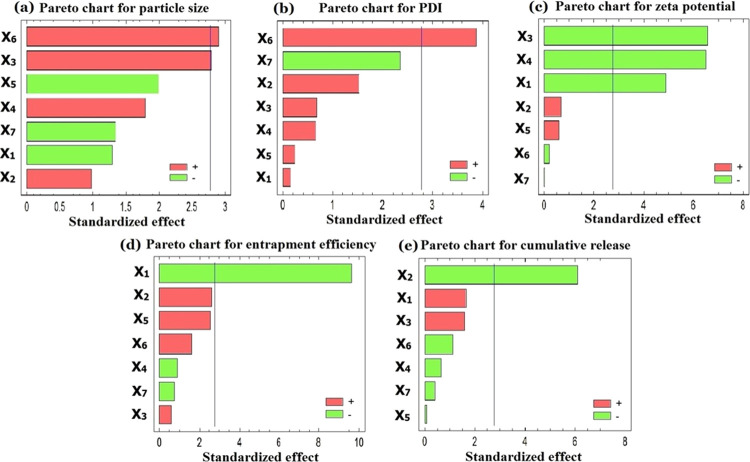
A positive value in the model for a response represents a direct relationship between the specified factor and the investigated response, and a negative value indicates an inverse relationship.26 Statistical analysis of variance of the responses (Table 2) revealed that the most significant variables affecting Y1 were the surfactant type (X3) with p value equal to 0.049 and the sonication time (X6) with p value equal to 0.044. Other investigated levels of variables did not have significant influence on the mean particle size of the prepared PE-SLNs.
Table 2. Statistical Analysis of Variance (ANOVA) of the Responses (Y1–Y5) Results.
| particle size (Y1), μm |
PDI (Y2) |
zeta potential (Y3), mV |
entrapment efficiency (Y4), % |
cumulative release (Y5), % |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| variables | estimate | p value | estimate | p value | estimate | p value | estimate | p value | estimate | p value |
| X1 | –215.0 | 0.2656 | 0.0152 | 0.8860 | –5.5667 | 0.0081a | –31.873 | 0.0007a | 3.48 | 0.1762 |
| X2 | 163.5 | 0.3811 | 0.1512 | 0.2025 | 0.7667 | 0.5369 | 8.6833 | 0.0586 | –12.92 | 0.0037a |
| X3 | 464.2 | 0.0492a | 0.0678 | 0.5319 | –7.4667 | 0.0028a | 1.9567 | 0.5863 | 3.3633 | 0.1880 |
| X4 | 297.23 | 0.1484 | 0.0662 | 0.5415 | –7.4 | 0.0029* | –2.92 | 0.4276 | –1.35 | 0.5590 |
| X5 | –330.3 | 0.1179 | 0.0242 | 0.8196 | 0.6667 | 0.5890 | 8.5567 | 0.0610 | –0.1633 | 0.9423 |
| X6 | 483.03 | 0.0439a | 0.3842 | 0.0180a | –0.2333 | 0.8474 | 5.3067 | 0.1842 | –2.3567 | 0.3288 |
| X7 | –224.13 | 0.2490 | –0.2335 | 0.0783 | –0.0333 | 0.9780 | –2.48 | 0.4955 | –0.8533 | 0.7080 |
| R2 | 87.43 | 85.62 | 96.50 | 96.51 | 91.69 | |||||
| adj. R2 | 65.44 | 60.47 | 90.38 | 90.39 | 77.14 | |||||
| SEE | 288.01 | 0.17 | 1.97 | 5.73 | 3.67 | |||||
| MAE | 162.27 | 0.08 | 0.99 | 2.59 | 1.90 | |||||
Note: Significant effect of variables on individual responses. Abbreviations: X1, lipid type; X2, lipid concentration; X3, surfactant type; X4, surfactant concentration; X5, co-surfactant concentration; X6, sonication time; X7, sonication amplitude; R2, R-squared; adj. R2, adjusted R-squared; SEE, standard error of estimate; and MAE, mean absolute error.
Figures 4a and 5a show the Pareto chart and three-dimensional response surface plot that displayed the effect of different variables on the particle size of the formulations. Particle size enlargement was observed with increasing the sonication time as reported by other investigators.27 This could be clarified by increasing the temperature of SLNs as the duration of sonication increased as stated in the study of Zhang et al.28 In addition, the sonication process simultaneously reduces the particle size of the individual particles and potentiates the agglomeration of some ultrasound-activated particles, which is in good agreement with a previous study.29 The type and concentration of surfactant had a great influence on the particle size and stability of nanoparticles. At low concentrations, the surfactant will not be enough to cover the surface of nanoparticles and the interfacial tension will be increased, resulting in particle aggregation and subsequently enlarged particle size. High concentrations of surfactant may lead to bridging between nanoparticles and may also cause toxicity.30 Two different surfactants (Tween 80 and poloxamer 188) were evaluated at two levels (3 and 10% w/v). Regarding the surfactant type (X3), it was found that Tween 80-based SLNs produce a smaller particle size than those formulations based on poloxamer. A possible clarification for this difference in particle size may be the influence of hydrocarbon chain length difference of the used surfactants.31 It may also be attributed to the higher HLB of poloxamer 188 (HLB = 29) than that of Tween 80 (HLB = 15). This led to the preferential solubility of Tween in lipid and lipophilic drug subsequently reducing the interfacial tension at the lipid–water interface. This enhancement of the emulsification process makes the lipid nanoparticles subdivided and distributed in a finely small size, which is in accordance with the previously reported finding by Das and co-workers.32
Figure 4.
Pareto charts of all independent variables on (a) particle size, (b) PDI, (c) zeta potential, (d) entrapment efficiency %, and (e) cumulative drug release %.
Figure 5.
Estimated three-dimensional response surface plot for the effect of the studied variables on (a) particle size, (b) PDI, (c) zeta potential, (d) entrapment efficiency %, and (e) cumulative drug release %.
It was also noticed that there is a direct relationship between surfactant concentration (X4) and the particle size. This phenomenon could be explained by increasing intrinsic thermodynamic instability of the nanoparticle system with increasing the concentration of surfactant, causing the adsorption of surfactant on the particle surface.33 Another explanation explains that increasing the surfactant concentration could cause the rearrangement of the surfactant molecules to form loops and tails on the particle surface, leading to the bridging among the SLNs followed by particle aggregation.34 On the contrary, lecithin concentration (X5) correlate inversely with the particle size when used as a co-surfactant. It was reported that 10% lecithin is sufficient to produce nanoparticles but, when used in combination with a surfactant, could yield a lower particle size than when used alone.35
Although the lipid type (X1) showed an insignificant effect on the particle size of the prepared PE-SLNs, stearic acid containing formulations showed relatively larger particles than those containing precirol. This difference in sizes may be due to a difference in the melting point of the utilized lipids; hence, the higher the melting point of a lipid, the larger the size.36 A high melting point of lipids results in an increase in the viscosity and leads to an insufficient homogenization, which causes an ineffective reduction of the particle size.37 The same findings were observed with lipid concentration (X2) that played a role in increasing the particle size although it was insignificant. This could be explained by the fact that, at higher lipid contents, the efficiency of sonication decreases because of a higher viscosity of the sample, resulting in larger particles.38 The results obtained are in agreement with those obtained by Mehnert and co-workers.24 In addition, a high particle concentration at high lipid contents increases the probability of particle contact and subsequent aggregation.39 Furthermore, lack of sufficient surfactant to cover the particle surface is the possible reason for increased particle size.40 On the other hand, smaller SLNs were obtained by increasing the sonication amplitude (X7) but it was an insignificant factor, which is in agreement with the previously reported results. This may be due to the fact that a higher amplitude could deliver more energy to comminute particles, leading to a smaller size.41
Influence of Investigated Parameters on Polydispersity Index (Y2)
Polydispersity index value was used to illustrate the monodispersed and polydispersed nature of nanoparticles. The higher the PDI value, the wider the droplet size distribution.42 The prepared PE-SLN formulations showed a variation in PDI values (Y2) ranging from 0.112 as in F5 to 1.0 as in F7 and F11 depending on the variables’ levels used in the formulations. The best model that describes the effects of the variables on PDI is
| 2 |
Statistical analysis of variance of the responses (Table 2) showed that the most significant independent factor affecting PDI (Y2) was sonication time (X6) with p value equal 0.018. Figures 4b and 5b show the Pareto chart and three-dimensional response surface plot that displayed the effect of different variables on PDI of the formulations. Since PDI is a dimensionless measure for the broadness of a particle size distribution, it was found that increasing X6 leads to a high PDI value.43 This could be clarified by particle aggregation that could happen during ultra-sonication technique as previously mentioned in particle size distribution.29 Quite the opposite, sonication amplitude (X7) had a negative impact on the PDI value although this factor is insignificant. This finding is in accordance with the reported results that stated that a high amplitude intensity leads to uniform distribution, which results in a low PDI value.41 On the other hand, lipid concentration (X2) had a positive effect on PDI but it was insignificant. This finding was in agreement with Tiyaboonchai et al., who reported that a high lipid concentration resulted in a larger mean particle size and broader size distribution.44 Other examined variables, which are lipid type (X1), surfactant type (X3), surfactant concentration (X4), and co-surfactant concentration (X5), did not have any significant influence on the PDI value of the prepared PE-SLNs.
Influence of Investigated Parameters on Zeta Potential (Y3)
The surface charge of the SLN dispersions is considered as a significant characteristic of the particles as this is often controlling the stability behavior when particles approach each other. The measured potential at the slipping plane of SLN dispersion is the most appropriate potential, which is known as the zeta potential.45 Zeta potential (Y3) of the prepared SLNs formulations ranged from −15.6 mV as in F11 to −38.5 mV as in F2 depending on the variable levels selected. All formulations showed a negative zeta potential since lipid nanoparticles have a negative charge on their surface due to the presence of terminal carboxylic groups in the lipids.46 This potential demonstrated that a high potential value ensures the stability of the SLN dispersions. The linear model explaining the effects of various variables on zeta potential (Y3) is
| 3 |
Statistical analysis of variance of the responses (Table 2) revealed that the most significant variables affecting Y3 were the surfactant type (X3) with p value equal to 0.0028, the surfactant concentration (X4) with p value equal to 0.0029, and the lipid type (X1) with p value equal to 0.0081. Furthermore, the lipid concentration (X2) had a positive impact on the zeta potential, however it was insignificant.47Figures 4c and 5c show the Pareto chart and three-dimensional response surface plot that displayed the effect of different variables on the zeta potential of the formulations. From the Pareto chart and the regression equation (eq 3), it was observed that there is an inverse relationship between X1 and zeta potential of the formulations. Also, it was noticed that formulations containing stearic acid showed a relatively higher zeta potential value than those containing precirol. This may be due to the highly negative charge of the residual stearic acid distributed at the surface of SLNs or due to the dissociation of stearic acid in the aqueous medium.48 The same finding was observed with the surfactant type (X3) as the zeta potential value increased by using Tween 80 rather than poloxamer 188.49 Furthermore, surfactant concentration (X4) revealed a significant impact on zeta potential as SLNs perfectly covered by a non-ionic surfactant like Tween 80 and poloxamer 188 tend to remain stable despite having a lower zeta potential. This could be attributed to greater steric stabilization and less electrostatic stabilization. In addition, surface coverage of the SLNs reduces the electrophoretic mobility of the particles and thus lowers the zeta potential.40 On the other hand, co-surfactant concentration (X5) showed a positive insignificant effect on the zeta potential. This is due to the ionic nature of lecithin that broadens the electric double layer surrounding the nanoparticles and increases the zeta potential.50 The rest of the investigated variables including the sonication time (X6) and amplitude (X7) had a non-significant effect on Y3.
Influence of Investigated Parameters on Entrapment Efficiency (Y4)
Entrapment efficiency refers to the amount, expressed as percentage, of drug entrapped by the nanoparticles compared to the amount of drug added.48 The entrapment efficiency of the prepared PE-SLN formulations varied from 15.37% in F5 to 65.12% in F10 due to the variation in variable combination. The following model can refer to the effect of the various independent variables on entrapment efficiency:
| 4 |
Statistical analysis of variance of the responses (Table 2) displayed that the most significant factor affecting the entrapment efficiency (Y4) was the lipid type (X1) with p value equal to 0.0007. Figures 4d and 5d show the Pareto chart and three-dimensional response surface plot that displayed the effect of different variables on the entrapment efficiency of the formulations. From Table 1 and the Pareto chart (Figure 4d) and the response surface plot (Figure 5d), it was noticed that the formulations prepared with stearic acid showed a higher entrapment efficiency than those prepared with precirol. This finding can be explained by the relative hydrophilicity of stearic acid (HLB = 15), which has a higher potential to solubilize PE.51 On the contrary, precirol is more hydrophobic lipid (HLB = 2) and its ability to entrap water soluble drug is low. In addition, a positive trend with increasing the lipid concentration (X2) was observed, although it was insignificant. This could be due to the fact that a higher amount of lipid provides an extra space to accommodate an excessive amount of drug during SLN preparation. This leads to reduction in the diffusion rate of the drug into the external phase as the viscosity of the lipid phase is higher and thus showed a higher entrapment efficiency.38 Similarly, there is a direct relationship between the co-surfactant concentration (X5) and the entrapment efficiency, which is in agreement with results of Rahman et al.52 On the other hand, the surfactant concentration (X4) had an inverse relationship with the entrapment efficiency, which might be due to increasing the particle size. This also explains the negative effect of co-surfactant concentration on the particle size and the positive effect on the entrapment efficiency.53 This may also be clarified by the partitioning phenomenon, which reported that the presence of a surfactant with a high concentration leads to a high solubility of the drug in the external phase, which increases the partitioning of the drug from the internal to the external phase of the medium, thus ending up with a low entrapment efficiency.38 Concerning the sonication time (X6) and amplitude (X7), the entrapment efficiency increased when X6 increased and X7 decreased. This could be explained by the fact that applying a higher kinetic energy or amplitude on previously formed lipid particles may bring out the entrapped drug into the aqueous medium, causing a low entrapment efficiency.54
Influence of Investigated Parameters on the Cumulative PE Release (Y5)
The in vitro release profiles of PE-SLN formulations prepared with precirol compared with that of stearic acid after 48 h are summarized in Figure 3a,b, respectively. In vitro release profiles of PE-SLN dispersion showed a biphasic release pattern characterized by a rapid initial burst drug release followed by sustained release, and this behavior has been reported for solid lipid nanoparticles.55 Burst drug release is likely due to the attached drug in the external shell and on the nanoparticle surface, while sustained release is commonly due to the encapsulated drug in the lipid core.56 Cumulative release after 48 h ranged from 69.98% as in F3 to 94.57% as in F5 depending on the variation in the variable’s levels. The following model (eq 5) describes the effect of the investigated variables on the cumulative % release:
| 5 |
Statistical analysis of variance of the responses (Table 2) showed that lipid concentration (X2) was the most significant variable affecting the cumulative release (Y5) with p value equal to 0.0037. Standardized Pareto chart (Figure 4e) and response surface plot (Figure 5e) showed that increasing the lipid concentration in the formulation controls the release of PE and decreases the amount released after 48 h. This could be explained by increasing the particle size and subsequently decreasing the specific surface area and therefore reducing the release rate.57 In addition, a high lipid concentration leads to a high medium viscosity and more rigid solidified nanoparticles, which would also slow down the drug diffusion to the dissolution medium.54 Despite the non-significant effect of the lipid type (X1) on the drug release, the formulations prepared with precirol showed a higher release than those prepared with stearic acid. This could be attributed to the difference in lipid melting points previously reported.58 Also, the higher amount released from precirol particles may also reflect the smaller size of these particles and hence the larger surface area as the size of precirol nanoparticles represents the smallest of the SLNs tested.59 Moreover, sonication time (X6) showed a negative effect on the cumulative release since it led to large particles and small surface area available for dissolution medium. This is in accordance with a previous study, which reported the sustained release of clozapine from tripalmitin-SLNs prepared by the hot homogenization followed by ultra-sonication method.60 Furthermore, the obtained results revealed that the surfactant type (X3) affects insignificantly the release profile. The formulations with Tween 80 showed sustaining release behavior than those with poloxamer 188. This could be attributed to the difference in HLB values of two surfactants where the higher HLB value of poloxamer facilitates the drug release from the nanoparticles. Finally, neither sonication amplitude (X7) nor co-surfactant concentration (X5) showed a significant effect on the cumulative release.
Finally, with the purpose of achieving the constraints of this study, we can select stearic acid as lipid, Tween 80 as surfactant, as well as sonication with short time and high amplitude for the development of PE-SLN formulation. On the other hand, concentrations of lipid, surfactant, and co-surfactant can be subsequently examined in a further optimization study.
Conclusions
In the present study, screening was done for seven preparation and processing variables affecting the formulation of solid lipid nanoparticles loaded with pomegranate extract. PBD was utilized efficiently to distinguish the most significant variables on the quality attributes of the prepared PE-SLNs by the hot homogenization method followed by ultra-sonication. It was revealed that lipid type and surfactant type have the main effect on the entrapment efficiency and the particle size, respectively. Furthermore, the sonication time affects significantly both particle size and polydispersity index. On the other hand, the cumulative drug release had been affected significantly with the variation in lipid concentration. Therefore, stearic acid can be used as a lipid to maximize the entrapment efficiency and Tween 80 as a surfactant to minimize the particle size and increase the zeta potential. In addition, it is preferred to use the sonication with a high amplitude and short time. To conclude, out of seven variables, only three, specifically the concentrations of lipid, surfactant, and co-surfactant, can be scaled up for subsequent optimization study. This study indicate that solid lipid nanoparticles can be indeed loaded with the pomegranate extract with minimum particle size and high entrapment efficiency and controlled release behavior. Yet, full optimization study and cytotoxicity studies for the optimized formula of PE-SLNs warrants additional investigation, which will be the forefront of our laboratory upcoming directions.
Materials and Methods
Materials
Pomegranate extract (PE) from pomegranate fruit (peel) containing ≥30% punicalagin was purchased from Shaanxi Ciyuan Biotech Co., Ltd. (Shaanxi, China). Stearic acid, disodium hydrogen phosphate, and potassium dihydrogen phosphate were purchased from El-Nasr Pharmaceutical Chemicals Co. (Cairo, Egypt). Precirol ATO5 was donated as a gift from Gattefosse (Cedex, France). Tween 80, lecithin, and poloxamer 188 were generously donated by Egyptian International Pharmaceutical Industries Co., EIPICO, (10th of Ramadan City, Egypt). Dialysis tubing cellulose membrane with a molecular weight cutoff of 12,000 Da was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were of analytical grade.
Methods
Experimental Screening Design
Development of pharmaceutical formulations conventionally is based on consuming time and energy by varying one factor at a certain time while keeping other factors constant. On the other hand, using experimental design (DOE) technique allows examining a large number of variables at the same time with a little number of experimental runs. Screening design is the greatest influential DOE methods that identify the most important factors in the pharmaceutical development. PBD is one of the commonly used screening designs, which screens a large number of factors and determines the critical one in a minimal number of runs with a high degree of precision. Generally, a number of runs required to examine the significant effects are equal to 2n or multiple of 4 in PBD instead of 2 as in the case of full factorial design.18 Seven different variables were used in SLN formulations using PBD as presented in Table 3.
Table 3. Independent Variables in Plackett–Burman Experimental Design.
| levels |
||
|---|---|---|
| independent variables | –1 | +1 |
| lipid type, (X1) | stearic acid | precirol |
| lipid concentration, % (X2) | 3 | 7 |
| surfactant type, (X3) | Tween | poloxamer |
| surfactant concentration, % (X4) | 3 | 10 |
| co-surfactant concentration, mg (X5) | 25 | 150 |
| sonication time, min (X6) | 10 | 30 |
| sonication amplitude, % (X7) | 50 | 100 |
This design was established to prepare 12 different formulations of PE-loaded SLNs as shown in Table 4. The independent variables were lipid type (X1), which was either stearic acid or precirol, while lipid concentration percent (X2) was employed either 3 or 7%. Surfactant type (X3) was either Tween 80 or poloxamer 188 with two different concentrations (X4) either 3 or 10%. In addition, co-surfactant (lecithin) concentration (X5) was 25 or 150 mg. Also, sonication time (X6) and amplitude (X7) were 10 or 30 min and 50 or 100%, respectively. The independent variables were studied for their effects on the particle size (Y1), polydispersity index (Y2), zeta potential (Y3), entrapment efficiency (Y4), and cumulative % release after 48 h (Y5).
Table 4. Formulations Composition of Solid Lipid Nanoparticles Loaded with Pomegranate Extract as Suggested by Plackett–Burman Design.
| formulation code | lipid type | lipid (%) | surfactant type | surfactant (%) | co-surfactant (mg) | sonication time (min) | sonication amplitude (%) |
|---|---|---|---|---|---|---|---|
| F1 | precirol | 7 | Tween | 10 | 25 | 10 | 50 |
| F2 | stearic acid | 3 | Tween | 3 | 25 | 10 | 50 |
| F3 | stearic acid | 7 | poloxamer | 10 | 25 | 30 | 100 |
| F4 | stearic acid | 3 | poloxamer | 10 | 150 | 10 | 100 |
| F5 | precirol | 3 | poloxamer | 3 | 25 | 10 | 100 |
| F6 | stearic acid | 7 | Tween | 3 | 25 | 30 | 100 |
| F7 | precirol | 7 | poloxamer | 3 | 150 | 30 | 50 |
| F8 | precirol | 3 | Tween | 3 | 150 | 30 | 100 |
| F9 | precirol | 7 | Tween | 10 | 150 | 10 | 100 |
| F10 | stearic acid | 7 | poloxamer | 3 | 150 | 10 | 50 |
| F11 | precirol | 3 | poloxamer | 10 | 25 | 30 | 50 |
| F12 | stearic acid | 3 | Tween | 10 | 150 | 30 | 50 |
Preparation of PE-SLNs
SLNs were prepared by the hot homogenization method followed by ultra-sonication as described by Dong et al.61 The lipid phase was prepared by melting stearic acid or precirol, lecithin, and PE at 10 °C above the lipid melting point, which is 69 °C regarding stearic acid and 56 °C regarding precirol. The aqueous phase was prepared by dissolving Tween 80 or poloxamer 188 in deionized water and heating to the same temperature of the lipid phase. After that, the hot aqueous phase was gently added into the lipid phase with constant stirring using magnetic stirrer for 30 min in a water bath at 10 °C above the melting point of the lipid. The coarse oil in water emulsion was then sonicated with a probe sonicator (Vibracell VCX130, Sonics, USA) in an ice bath at 50 or 100% amplitude for 10 or 30 min. The obtained dispersions were collected in glass containers and stored in the refrigerator for further experiments.
Characterization of the Prepared PE-SLNs
The 12 prepared formulations were investigated for particle size, polydispersity index (PDI), zeta potential, entrapment efficiency, and cumulative % release.
Measurements of Particle Size, PDI, and Zeta Potential
Particle size as z-average diameter, PDI, and zeta potential of PE-SLNs were analyzed by the dynamic light scattering (DLS) technique using a Zetasizer Nano-Zs90 (MPT-Z, Malvern Instruments Ltd., UK) after dilution with deionized water to get optimum kilo counts per second (kcps) of 50–200 for measurements.62 Results were the average of three measurements.
Morphological Analysis
The PE-SLNs were prepared, and 5 μL was placed onto the surface of a 300-mesh copper grid coated with carbon. Negative staining was performed on the samples using 2% uranyl acetate (w/v) followed by air-drying for 15 min and then the excess was removed using filter paper. The stained grids were then probed using transmission electron microscopy (TEM) (model JEM-2100, Jeol, USA) to visualize the morphology of the nanoparticles operating at an acceleration voltage of 200 kV.63
Determination of the Entrapment Efficiency (EE %)
Entrapment efficiency % was determined by measuring the amount of free drug in the aqueous phase after separation of the system using a centrifugation method. PE-SLN dispersion was centrifuged at 12,000 rpm and 4 °C for 1 h using a freeze centrifuge (2-16KL, Sigma Laborzentrifugen GmbH, Germany), and then the supernatant was collected to measure the free drug concentration after suitable dilution with a fresh phosphate-buffered saline (pH 7.4).64 The drug concentration was measured at 265 nm using an ultraviolet–visible spectrophotometer (V-630, Jasco, Japan).65 The percent of entrapped drug was calculated according to eq 6:62
| 6 |
in which Winitial drug is the amount of drug used for the assay and Wfree drug is the amount of free drug detected in the aqueous phase after centrifugation. Results were the average of three measurements.
In Vitro Drug Release Study
In vitro release study was performed by a dialysis bag method using phosphate-buffered saline pH 7.4 (PBS 7.4) as the dissolution medium and dialysis membrane with a molecular weight of 12,000 Da, which retain SLNs and allow the diffusion of free drug into the dissolution medium. The bags were soaked in PBS 7.4 for 24 h before being used, and 2 mL of PE-SLN dispersion was transferred into the bag with the two ends tied by strings.62 After that, the bags were placed in 100 mL of dissolution medium, which was maintained at 37 °C and shaken at 100 rpm in a thermostatically controlled shaking water bath (WSB-18, Dahan Scientific Co. Ltd., Korea). Cumulative % drug release was determined after 1, 2, 3, 4, 5, 6, 7, 8, 24, and 48 h time intervals. The taken samples were compensated with fresh buffer to maintain the sink condition and then analyzed spectrophotometrically at 265 nm.65 Results were the average of three measurements.
Statistical Analysis of the Data
The estimated effects of parameters on responses were represented by standardized Pareto charts and allowed us to check the statistical significance of the PBD. ANOVA was performed on experimental data to evaluate the statistical significance of the model. A p value less than 0.05 indicates the significance of model terms. The statistical software Statgraphics Centurion XV software, version 15.2.05 (StatPoint, Inc., Warrenton, VA) was used for the regression analysis and the graphical presentation. After analysis of the data, the linear correlations for each response were obtained in terms of coded variables to quantify response values.
Acknowledgments
The authors would like to thank Prof. Shaker Moussa, Professor of Pharmacology and Chairman of The Pharmaceutical Research Institute, Albany College of Pharmacy and Health Sciences for his assistance in flourishing up the idea of this work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02618.
(Figures S1–S10) The estimated three-dimensional response surface plots for the effect of the studied variables on particle size, PDI, zeta potential, entrapment efficiency %, and cumulative drug release % (PDF)
Author Contributions
All authors shared in conceiving and designing the study. N.B. performed all laboratory experiments. K.E. performed the Plackett–Burman design and prepared all figures, tables, and statistical analysis. N.B., M.T., D.A., M.E., and M.E. shared in the interpretation of the nanoparticles’ formulation and characterization. N.B. drafted the manuscript, M.T., K.E., and M.E. revised it, and then D.A. and M.E. edited the final version; all authors read and approved the final version of the manuscript.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors declare no competing financial interest.
Supplementary Material
References
- Bharali D. J.; Siddiqui I. A.; Adhami V. M.; Chamcheu J. C.; Aldahmash A. M.; Mukhtar H.; Mousa S. A. Nanoparticle delivery of natural products in the prevention and treatment of cancers: current status and future prospects. Cancers 2011, 3, 4024–4045. 10.3390/cancers3044024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirode A. B.; Bharali D. J.; Nallanthighal S.; Coon J. K.; Mousa S. A.; Reliene R. Nanoencapsulation of pomegranate bioactive compounds for breast cancer chemoprevention. Int. J. Nanomed. 2015, 10, 475. 10.2147/IJN.S65145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed D. N.; Afaq F.; Mukhtar H.. In Pomegranate derived products for cancer chemoprevention, Seminars in cancer biology; Elsevier, 2007, pp 377–385. [DOI] [PubMed] [Google Scholar]
- Topuz O. K.; Yerlikaya P.; Uçak İ.; Gümüş B.; Büyükbenli H. A.; Gökoğlu N. Influence of pomegranate peel (Punica granatum) extract on lipid oxidation in anchovy fish oil under heat accelerated conditions. J. Food Sci. Technol. 2015, 52, 625–632. 10.1007/s13197-014-1517-1. [DOI] [Google Scholar]
- Müller R.; Mehnert W.; Lucks J.-S.; Schwarz C.; Zur Mühlen A.; Meyhers H.; Freitas C.; Rühl D. Solid lipid nanoparticles (SLN): an alternative colloidal carrier system for controlled drug delivery. Eur. J. Pharm. Biopharm. 1995, 41, 62–69. [Google Scholar]
- Jain A. K.; Jain A.; Garg N. K.; Agarwal A.; Jain A.; Jain S. A.; Tyagi R. K.; Jain R. K.; Agrawal H.; Agrawal G. P. Adapalene loaded solid lipid nanoparticles gel: an effective approach for acne treatment. Colloids Surf., B 2014, 121, 222–229. 10.1016/j.colsurfb.2014.05.041. [DOI] [PubMed] [Google Scholar]
- Garg N. K.; Dwivedi P.; Campbell C.; Tyagi R. K. Site specific/targeted delivery of gemcitabine through anisamide anchored chitosan/poly ethylene glycol nanoparticles: an improved understanding of lung cancer therapeutic intervention. Eur. J. Pharm. Sci. 2012, 47, 1006–1014. 10.1016/j.ejps.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Zur Mühlen A.; Schwarz C.; Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery–drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 149–155. 10.1016/S0939-6411(97)00150-1. [DOI] [PubMed] [Google Scholar]
- Dolatabadi J. E. N.; Hamishehkar H.; Eskandani M.; Valizadeh H. Formulation, characterization and cytotoxicity studies of alendronate sodium-loaded solid lipid nanoparticles. Colloids Surf., B 2014, 117, 21–28. 10.1016/j.colsurfb.2014.01.055. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhu L.; Dong Z.; Xie S.; Chen X.; Lu M.; Wang X.; Li X.; Zhou W. Preparation and stability study of norfloxacin-loaded solid lipid nanoparticle suspensions. Colloids Surf., B 2012, 98, 105–111. 10.1016/j.colsurfb.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Lewis G.; Mathieu D.; Phan-Tan-Luu R., Mixtures in a constrained region of interest. Pharmaceutical Experimental Design .Marcel Dekker: New York: 1999, 413–54. [Google Scholar]
- Ibrahim H. M.; Ahmed T. A.; Lila A. E.; Samy A. M.; Kaseem A. A.; Nutan M. T. Mucoadhesive controlled release microcapsules of indomethacin: Optimization and stability study. J. Microencapsulation 2010, 27, 377–386. 10.3109/02652040903243445. [DOI] [PubMed] [Google Scholar]
- Jin S.-J.; Yoo Y.-H.; Kim M.-S.; Kim J.-S.; Park J.-S.; Hwang S.-J. Paroxetine hydrochloride controlled release POLYOX® matrix tablets: Screening of formulation variables using Plackett-Burman screening design. Arch. Pharmacal Res. 2008, 31, 399–405. 10.1007/s12272-001-1170-0. [DOI] [PubMed] [Google Scholar]
- Jain S. P.; Singh P. P.; Javeer S.; Amin P. D. Use of Placket–Burman statistical design to study effect of formulation variables on the release of drug from hot melt sustained release extrudates. Aaps Pharmscitech 2010, 11, 936–944. 10.1208/s12249-010-9444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbi I.; Aljaeid B.; Khalid M.; Zidan A. S. Glycosylated Sertraline-Loaded Liposomes for Brain Targeting: QbD Study of Formulation Variabilities and Brain Transport. AAPS PharmSciTech 2016, 17, 1404–1420. 10.1208/s12249-016-0481-7. [DOI] [PubMed] [Google Scholar]
- Khalid M.; Abdelaziz A. E.; Samy A. M.; Kassem A. A. Screening study for formulation variables in preparation of diclofenac sodium niosomes using plackett–burman design. Az. J. Pharm. Sci. 2008, 38, 36–53. [Google Scholar]
- Ahmed T. A. Preparation of transfersomes encapsulating sildenafil aimed for transdermal drug delivery: Plackett–Burman design and characterization. J. liposome Res. 2014, 25, 1–10. 10.3109/08982104.2014.950276. [DOI] [PubMed] [Google Scholar]
- Rahman Z.; Zidan A. S.; Habib M. J.; Khan M. A. Understanding the quality of protein loaded PLGA nanoparticles variability by Plackett–Burman design. Int. J. Pharm. 2010, 389, 186–194. 10.1016/j.ijpharm.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awotwe-Otoo D.; Zidan A. S.; Rahman Z.; Habib M. J. Evaluation of anticancer drug-loaded nanoparticle characteristics by nondestructive methodologies. AAPS PharmSciTech 2012, 13, 611–622. 10.1208/s12249-012-9782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed T. A.; El-Say K. M. Development of alginate-reinforced chitosan nanoparticles utilizing W/O nanoemulsification/internal crosslinking technique for transdermal delivery of rabeprazole. Life Sci. 2014, 110, 35–43. 10.1016/j.lfs.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Zaghloul A.; Faltinek J.; Vaithiyalingam S.; Reddy I.; Khan M. A. Naproxen-Eudragit microspheres: screening of process and formulation variables for the preparation of extended release tablets. Pharmazie 2001, 56, 321–324. [PubMed] [Google Scholar]
- El-Say K. M.; Ahmed O. A.; Aljaeid B. M.; Zidan A. S. Matrix-type transdermal films to enhance simvastatin ex vivo skin permeability. Pharm. Dev. Technol. 2017, 492–499. 10.3109/10837450.2015.1102279. [DOI] [PubMed] [Google Scholar]
- Priyanka K.; Hasan S. A. A. Preparation and evaluation of montelukast sodium loaded solid lipid nanoparticles. J. Young Pharm. 2012, 4, 129–137. 10.4103/0975-1483.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehnert W.; Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv. Drug Delivery Rev. 2001, 47, 165–196. 10.1016/S0169-409X(01)00105-3. [DOI] [PubMed] [Google Scholar]
- Gazori T.; Khoshayand M. R.; Azizi E.; Yazdizade P.; Nomani A.; Haririan I. Evaluation of Alginate/Chitosan nanoparticles as antisense delivery vector: formulation, optimization and in vitro characterization. Carbohydr. Polym. 2009, 77, 599–606. 10.1016/j.carbpol.2009.02.019. [DOI] [Google Scholar]
- Chopra S.; Patil G. V.; Motwani S. K. Release modulating hydrophilic matrix systems of losartan potassium: Optimization of formulation using statistical experimental design. European journal of pharmaceutics and Biopharmaceutics 2007, 66, 73–82. 10.1016/j.ejpb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Ariffin F. D.; Hasham R.; AbdulHamid M.; Eid A. M.; Mohamed A. T.; Keleb E. I.; Elmahgoubi A.; Issa Y. S.; Elmarzugi N. A. Effect of Techniques in Preparing VCO Nanoparticles. J. Biotechnol. Biochem. 2015, 1, 121–124. [Google Scholar]
- Zhang J.; Fan Y.; Smith E. Experimental design for the optimization of lipid nanoparticles. J. Pharm. Sci. 2009, 98, 1813–1819. 10.1002/jps.21549. [DOI] [PubMed] [Google Scholar]
- Franco F.; Pérez-Maqueda L.; Pérez-Rodríguez J. L. The effect of ultrasound on the particle size and structural disorder of a well-ordered kaolinite. J. Colloid Interface Sci. 2004, 274, 107–117. 10.1016/j.jcis.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Pooja D.; Tunki L.; Kulhari H.; Reddy B. B.; Sistla R. Optimization of solid lipid nanoparticles prepared by a single emulsification-solvent evaporation method. Data brief 2016, 6, 15–19. 10.1016/j.dib.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnarson T.; Elworthy P. H. Effects of structural variations of non-ionic surfactants on micellar properties and solubilization: surfactants containing very long hydrocarbon chains. J. Pharm. Pharmacol. 1981, 33, 141–144. 10.1111/j.2042-7158.1981.tb13736.x. [DOI] [PubMed] [Google Scholar]
- Das S.; Ng W. K.; Tan R. B. H. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs?. Eur. J. Pharm. Sci. 2012, 47, 139–151. 10.1016/j.ejps.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Göppert T. M.; Müller R. H. Protein adsorption patterns on poloxamer-and poloxamine-stabilized solid lipid nanoparticles (SLN). Eur. J. Pharm. Biopharm. 2005, 60, 361–372. 10.1016/j.ejpb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Laserra S.; Basit A.; Sozio P.; Marinelli L.; Fornasari E.; Cacciatore I.; Ciulla M.; Türkez H.; Geyikoglu F.; Di Stefano A. Solid lipid nanoparticles loaded with lipoyl–memantine codrug: Preparation and characterization. Int. J. Pharm. 2015, 485, 183–191. 10.1016/j.ijpharm.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Mulla J. A. S.; Hiremath S. P.; Sharma N. K. Repaglinide loaded solid lipid nanoparticles: design and characterization. RGUHS J. Pharm. Sci. 2012, 2, 41–49. [Google Scholar]
- Doijad R.; Manvi F.; Godhwani D.; Joseph R.; Deshmukh N. Formulation and targeting efficiency of cisplatin engineered solid lipid nanoparticles. Ind. J. Pharm. Sci. 2008, 70, 203. 10.4103/0250-474X.41456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Salam F. S.; Elkheshen S. A.; Mahmoud A. A.; Ammar H. O. Diflucortolone valerate loaded solid lipid nanoparticles as a semisolid topical delivery system. Bull. Fac. Pharm. (Cairo Univ.) 2016, 54, 1–7. 10.1016/j.bfopcu.2015.11.002. [DOI] [Google Scholar]
- Hosny K. M. Alendronate Sodium as Enteric Coated Solid Lipid Nanoparticles; Preparation, Optimization, and In Vivo Evaluation to Enhance Its Oral Bioavailability. PLoS One 2016, 11, e0154926 10.1371/journal.pone.0154926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas C.; Müller R. H. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLNTM) dispersions. Int. J. Pharm. 1998, 168, 221–229. 10.1016/S0378-5173(98)00092-1. [DOI] [Google Scholar]
- Shah R.; Eldridge D.; Palombo E.; Harding I. Optimisation and stability assessment of solid lipid nanoparticles using particle size and zeta potential. J. Phys. Sci. 2014, 25, 59. [Google Scholar]
- Jain V.; Kare P.; Jain D.; Singh R. Development and characterization of mucoadhesive nanosuspension of ciprofloxacin. Scanning Electron Microsc. 2011, 5, 9. [PubMed] [Google Scholar]
- Affandi M.; Julianto T.; Majeed A. Development and stability evaluation of astaxanthin nanoemulsion. Asian J. Pharm. Clin. Res. 2011, 4, 142–148. [Google Scholar]
- Olbrich C.; Gessner A.; Kayser O.; Müller R. H. Lipid-drug-conjugate (LDC) nanoparticles as novel carrier system for the hydrophilic antitrypanosomal drug diminazenediaceturate. J. Drug Targeting 2002, 10, 387–396. 10.1080/1061186021000001832. [DOI] [PubMed] [Google Scholar]
- Tiyaboonchai W.; Tungpradit W.; Plianbangchang P. Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int. J. Pharm. 2007, 337, 299–306. 10.1016/j.ijpharm.2006.12.043. [DOI] [PubMed] [Google Scholar]
- Shah R. M.; Rajasekaran D.; Ludford-Menting M.; Eldridge D. S.; Palombo E. A.; Harding I. H. Transport of stearic acid-based solid lipid nanoparticles (SLNs) into human epithelial cells. Colloids Surf., B 2016, 140, 204–212. 10.1016/j.colsurfb.2015.12.029. [DOI] [PubMed] [Google Scholar]
- Schwarz C. Solid lipid nanoparticles (SLN) for controlled drug delivery II. Drug incorporation and physicochemical characterization. J. Microencapsulation 1999, 16, 205–213. 10.1080/026520499289185. [DOI] [PubMed] [Google Scholar]
- Thakkar A.; Chenreddy S.; Wang J.; Prabhu S. Evaluation of ibuprofen loaded solid lipid nanoparticles and its combination regimens for pancreatic cancer chemoprevention. Int. J. Oncol. 2015, 46, 1827–1834. 10.3892/ijo.2015.2879. [DOI] [PubMed] [Google Scholar]
- Shah R. M.; Malherbe F.; Eldridge D.; Palombo E. A.; Harding I. H. Physicochemical characterization of solid lipid nanoparticles (SLNs) prepared by a novel microemulsion technique. J. Colloid Interface Sci. 2014, 428, 286–294. 10.1016/j.jcis.2014.04.057. [DOI] [PubMed] [Google Scholar]
- Han F.; Li S.; Yin R.; Liu H.; Xu L. Effect of surfactants on the formation and characterization of a new type of colloidal drug delivery system: nanostructured lipid carriers. Colloids Surf., A 2008, 315, 210–216. 10.1016/j.colsurfa.2007.08.005. [DOI] [Google Scholar]
- Kovacevic A.; Savic S.; Vuleta G.; Müller R. H.; Keck C. M. Polyhydroxy surfactants for the formulation of lipid nanoparticles (SLN and NLC): effects on size, physical stability and particle matrix structure. Int. J. Pharm. 2011, 406, 163–172. 10.1016/j.ijpharm.2010.12.036. [DOI] [PubMed] [Google Scholar]
- Ghadiri M.; Fatemi S.; Vatanara A.; Doroud D.; Najafabadi A. R.; Darabi M.; Rahimi A. A. Loading hydrophilic drug in solid lipid media as nanoparticles: Statistical modeling of entrapment efficiency and particle size. Int. J. Pharm. 2012, 424, 128–137. 10.1016/j.ijpharm.2011.12.037. [DOI] [PubMed] [Google Scholar]
- Rahman M. H.; Ramanathan M.; Sankar V. Preparation, characterization and in vitro cytotoxicity assay of curcumin loaded solid lipid nanoparticle in IMR32 neuroblastoma cell line. Pak. J. Pharm. Sci. 2014, 27, 1281–1285. [PubMed] [Google Scholar]
- Ebrahimi H. A.; Javadzadeh Y.; Hamidi M.; Jalali M. B. Repaglinide-loaded solid lipid nanoparticles: effect of using different surfactants/stabilizers on physicochemical properties of nanoparticles. DARU J. Pharm. Sci. 2015, 23, 46. 10.1186/s40199-015-0128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami J.; Mohiti H.; Hamishehkar H.; Varshosaz J. Formulation and optimization of solid lipid nanoparticle formulation for pulmonary delivery of budesonide using Taguchi and Box-Behnken design. Res. Pharm. Sci. 2015, 10, 17. [PMC free article] [PubMed] [Google Scholar]
- Nabi-Meibodi M.; Vatanara A.; Najafabadi A. R.; Rouini M. R.; Ramezani V.; Gilani K.; Etemadzadeh S. M. H.; Azadmanesh K. The effective encapsulation of a hydrophobic lipid-insoluble drug in solid lipid nanoparticles using a modified double emulsion solvent evaporation method. Colloids Surf., B 2013, 112, 408–414. 10.1016/j.colsurfb.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Tran T. H.; Choi J. Y.; Ramasamy T.; Truong D. H.; Nguyen C. N.; Choi H.-G.; Yong C. S.; Kim J. O. Hyaluronic acid-coated solid lipid nanoparticles for targeted delivery of vorinostat to CD44 overexpressing cancer cells. Carbohydr. Polym. 2014, 114, 407–415. 10.1016/j.carbpol.2014.08.026. [DOI] [PubMed] [Google Scholar]
- Mainardes R. M.; Evangelista R. C. PLGA nanoparticles containing praziquantel: effect of formulation variables on size distribution. Int. J. Pharm. 2005, 290, 137–144. 10.1016/j.ijpharm.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Paolicelli P.; Cerreto F.; Cesa S.; Feeney M.; Corrente F.; Marianecci C.; Casadei M. A. Influence of the formulation components on the properties of the system SLN-dextran hydrogel for the modified release of drugs. J. Microencapsulation 2009, 26, 355–364. 10.1080/02652040802372899. [DOI] [PubMed] [Google Scholar]
- Khalil R. M.; El-Bary A. A.; Kassem M. A.; Ghorab M. M.; Ahmed M. B. Solid lipid nanoparticles for topical delivery of meloxicam: development and in vitro characterization. Eur. Sci. J. 2013, 779. [Google Scholar]
- Venkateswarlu V.; Manjunath K. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J. Controlled Release 2004, 95, 627–638. 10.1016/j.jconrel.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Dong Z.; Xie S.; Zhu L.; Wang Y.; Wang X.; Zhou W. Preparation and in vitro, in vivo evaluations of norfloxacin-loaded solid lipid nanopartices for oral delivery. Drug Delivery 2011, 18, 441–450. 10.3109/10717544.2011.577109. [DOI] [PubMed] [Google Scholar]
- Wang S.; Chen T.; Chen R.; Hu Y.; Chen M.; Wang Y. Emodin loaded solid lipid nanoparticles: preparation, characterization and antitumor activity studies. Int. J. Pharm. 2012, 430, 238–246. 10.1016/j.ijpharm.2012.03.027. [DOI] [PubMed] [Google Scholar]
- Montenegro L.; Sinico C.; Castangia I.; Carbone C.; Puglisi G. Idebenone-loaded solid lipid nanoparticles for drug delivery to the skin: In vitroevaluation. Int. J. Pharm. 2012, 434, 169–174. 10.1016/j.ijpharm.2012.05.046. [DOI] [PubMed] [Google Scholar]
- Ekambaram P.; Sathali A. A. H. Formulation and evaluation of solid lipid nanoparticles of ramipril. J. Young Pharm. 2011, 3, 216–220. 10.4103/0975-1483.83765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil M. I.; Tomás-Barberán F. A.; Hess-Pierce B.; Holcroft D. M.; Kader A. A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.