Abstract
The World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) as a public health emergency of international concern as more than 15 million cases were reported by 24th July 2020. Angiotensin-converting enzyme 2 (ACE2) is a COVID-19 entry receptor regulating host cell infection. A recent study reported that ACE2 is expressed in cardiomyocytes. In this study, we aimed to explore if there are microRNA (miRNA) molecules which target ACE2 and which may be exploited to regulate the SARS-CoV-2 receptor. Our data reveal that both Ace2 mRNA and Ace2 protein levels are inhibited by miR-200c in rat primary cardiomyocytes and importantly, in human iPSC-derived cardiomyocytes. We report the first miRNA candidate that can target ACE2 in cardiomyocytes and thus may be exploited as a preventive strategy to treat cardiovascular complications of COVID-19.
Keywords: COVID-19, ACE2, miRNAs, Cardiomyocytes
Highlights
-
•
ACE2 is expressed in various cardiovascular cells including cardiomyocytes.
-
•
MicroRNA molecules can play an important role in ACE2 regulation.
-
•
MiR-200c can modulate ACE2 expression in both rat and human cardiomyocytes.
1. Introduction
The novel coronavirus disease 2019 (COVID-19) outbreak occurred in December 2019 and in the following months this epidemic was declared as a global health emergency by the World Health Organization (WHO) with more than 600 thousand deaths reported till date [1]. Similar to SARS-CoV and the Middle East respiratory syndrome (MERS), severe symptoms such as acute respiratory distress syndrome (ARDS) and multiple organ failure were observed in nearly 20% patients suffering from COVID-19 infection [2].
Angiotensin-converting enzyme 2 (ACE2) helps to maintain the balance of blood pressure and electrolyte in the human body. It also reduces the Angiotensin II levels in the circulation by suppressing the renin-angiotensin-aldosterone system conferring anti-hypertensive effects [3]. Recently, it has also been reported as a receptor for the spike protein of SARS-CoV-2 and plays pivotal roles during the COVID-19 infection [4]. Notably, COVID-19 patients with severe symptoms also seem to suffer from various other health conditions including cardiovascular disease (CVD), hypertension and diabetes [5]. Importantly, ACE2 is expressed in cardiomyocytes and elevated in patients with heart diseases [6]. Thus, it is likely that the extent of COVID-19 infection gets more pronounced by ACE2 in patients with comorbidities, which in turn may cause additional myocardial damage. MiRNAs are highly conserved small non-coding RNAs which are ~20–22 nucleotides in length and can negatively regulate gene expression. Several studies have reported that miRNAs could modulate ACE2 expression in a variety of cell types and diseases [7], but whether miRNA could be potential preventive target for SARS-CoV-2 is still unknown.
In our pervious review, we summarized the state of art of investigation and potential therapeutic strategies for COVID-19 patients with cardiac disease [8]. In this study, we investigate several in-silico identified miRNAs which could regulate ACE2 in vitro and therefore provide a potential strategy to develop novel therapeutic candidates for COVID-19.
2. Results
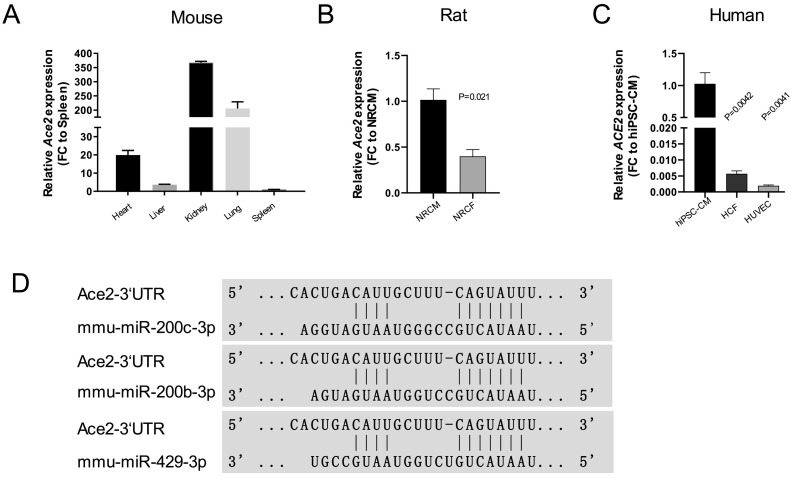
As one of the main receptors of SARS-CoV-2 [4], ACE2 is considered as a potential target for COVID-19 prevention. We first examined the expression of Ace2 in different mouse organs. Ace2 showed higher expression in kidney, lung and heart when compared to liver or spleen (Fig. 1A). To further investigate ACE2 expression pattern in different cell types (cardiomyocyte, fibroblast and endothelial cell) from human and rat were tested. Interestingly, Ace2 was highly expressed in neonatal rat cardiomyocytes (NRCMs) compared to neonatal rat cardiac fibroblasts (NRCFs) (Fig. 1B). Similarly, ACE2 demonstrated higher expression in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) compared to human cardiac fibroblasts and endothelial cells (Fig. 1C). Our next aim was to identify miRNAs which could potentially regulate ACE2 expression. A bioinformatics search using TargetScan was applied to predict miRNAs binding to the three prime untranslated region (3’UTR) of Ace2. Amongst the in silico candidates, miR-429, −200b, −200c were selected for further validation based on the highest conservation for the 3’UTR binding site (Fig. 1D). Collectively, these data suggest that cardiomyocytes are probably the major cell type which expresses ACE2 in the heart and miR-429, −200b and -200c may present as promising targets to regulate ACE2 expression.
Fig. 1.
ACE2 expression in human and rat cardiac cell types and potential binding miRNAs (A) Relative expression of Ace2 in different mouse organ panels (N = 3 mice; FC: fold change); (B) Relative expression of ACE2 in HCF (human cardiac fibroblast), hiPSC-CM (human induced Pluripotent Stem Cell-derived Cardiomyocyte), HUVEC (Human Umbilical Vein Endothelial Cells) (N = 3, independent experiments, FC: fold change, control: hiPSC-CM); (C) Relative expression of Ace2 in NRCF (Neonatal Rat Cardiac Fibroblast) and NRCM (Neonatal Rat Cardiomyocytes) (N = 3, FC: fold change, control: NRCM); (D) Bioinformatics prediction of the potential binding sites of miR-200b, −200c and −429 in 3’UTR of Ace2.
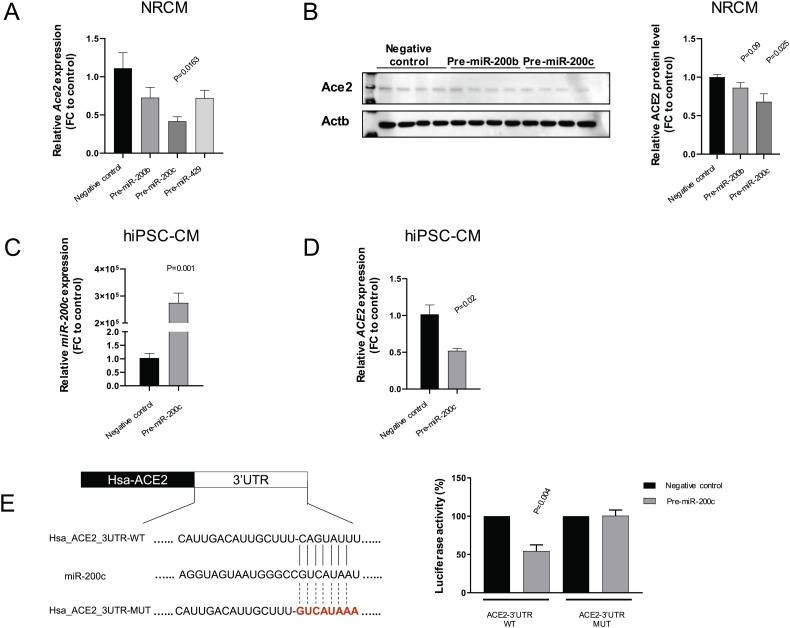
To test this hypothesis, NRCMs were first transfected with miRNA mimics pre-miR-429, pre-miR-200b and pre-miR-200c. High efficiency of miR-429, −200b and −200c overexpression was observed by miRNA specific TaqMan qPCR (Supplementary Fig. 1A). We then evaluated the Ace2 mRNA and Ace2 protein level in transfected NRCMs. Compared to the negative control, pre-miR-200c strongly diminished the expression of Ace2 as shown in qRT-PCR and western blot while the other two candidates showed a weaker downregulation that did not reach statistical significance (Fig. 2A&B, Supplementary Fig. 1B). Recently, SARS-CoV-2 infected hiPSC-CMs were reported as a promising in vitro platform to investigate the infection mechanism and screen for potential antiviral drugs [9]. In this context, hiPSC-CMs were transfected with mimics resulting in the overexpression of miR-200c and repression of ACE2 compared to the negative control (Fig. 2C&D). To further validate the interaction between miR-200c and ACE2, we constructed the luciferase reporter plasmids containing wild-type or mutated ACE2 3’UTR binding motifs (WT or MUT). Subsequently, pre-miR-200c and luciferase reporter plasmids (WT and MUT) were co-transfected in HEK-293 T cells revealing a significant reduction of luciferase activity in WT group while no change was observed when the mutated sequence was used (Fig. 2E). Collectively, these data provide direct evidence that miR-200c can regulate the expression of ACE2 in both rat and human cardiomyocytes.
Fig. 2.
MiR-200c regulates Ace2 expression in NRCM and hiPSC-CM (A) Relative expression of Ace2 in NRCMs transfected with pre-miR-200b, −200c and −429 (n = 3, technical replicates); (B) Relative protein levels of Ace2 in NRCMs transfected with pre-miR-200b and -200c (n = 4, technical replicates); (C) Relative expression of miR-200c in hiPSC-CM transfected with pre-miR-200c (n = 3, technical replicates); (D) Relative expression of ACE2 in hiPSC-CM transfected with pre-miR-200c (n = 3, technical replicates); (D) Luciferase activity in HEK293T cells transfected with pre-miR-200c (or negative control) and ACE2 3’UTR luciferase reporter plasmids (WT or MUT) (N = 3, independent experiments).
3. Discussion
In this study, high expression of Ace2 was observed in mouse kidney, lung and heart. A recent study employed single cell sequencing analysis of heart tissue showing that ACE2 expression is enriched in the cardiomyocyte fraction and is further elevated specifically in cardiomyocytes of patients with heart disease [6]. Our findings reaffirm these observations that ACE2 is abundant in cardiomyocytes compared to other cardiac cell types. Strikingly, viral particles were observed in the endo-myocardial biopsy from patients infected with SARS-CoV-2 [10]. Another multi-organ autopsy study of COVID-19 patients revealed the presence of SARS-CoV-2 viral RNA in the heart tissue [11]. Surprisingly, the SARS-Cov-2 mRNA was found in the heart tissue biopsy of patients who had already recovered from SARS-CoV-2 infection [12]. Moreover, several recent studies described that hiPSC-CMs can be efficiently infected with SARS-CoV-2 particles and such a platform can be further utilized for screening of potential anti-viral medicines [9,13]. To fight the COVID-19 pandemic in the absence of vaccines, it is necessary to identify promising drugs which act against SARS-CoV-2 infection in cardiomyocytes and such in vitro translational platforms can immensely aid in this process.
Vaccine is one of the most efficient tools to prevent COVID-19. By April 2020, 115 vaccine candidates for COVID-19 were reported, out of which 73 targets were selected for pre-clinical investigation. In addition to protein targeting vaccines, the list of most promising vaccine also includes an mRNA based vaccine from Moderna (mRNA-1273) which is currently in Phase I clinical trial [14]. However there have been no efforts, so far, to investigate the role of non-coding genome in the progression of SARS-CoV-2. It is well known that the human genome mostly comprises of non-coding transcripts which are master regulators of several physiological processes. In our study, we identified that indeed miRNAs can modulate ACE2, the key molecule for cellular SARS-CoV-2 infection. Thus, the miRNA mediated inhibition of the corona virus cell-entry receptor ACE2 could be an alternative treatment for a potential future corona virus pandemic.
The miR-200 family, comprising of miR-200b, miR-200c and miR-429, is a well-known miRNA cluster which is highly investigated in anti-cancer studies [15]. Also, it has been previously shown that miR-200c is upregulated in CVDs [16]. Here, we elucidated the role of miR-200c in SARS-CoV-2 infection where the overexpression of miR-200c represses ACE2 expression in both rat and human cardiomyocytes. Further experiments to investigate the potential of miR-200c to lower the ACE2-mediated infection of hiPSC-CMs with SARS-CoV-2 or SARS-CoV-2 spike pseudotype viruses are warranted. However, considering the correlation of miR-200c and CVDs, the activation of miR-200c should be carefully monitored.
Of note, patients with pre-existing CVDs are more likely to suffer from severe symptoms of COVID-19 [5]. Our study highlights that miR-200c can regulate the expression of ACE2 in cardiomyocytes. However, the contradicting effects of ACE2 in CVDs and COVID-19 infection should be considered carefully. On one hand, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are widely administered to patients with heart failure, which reduces stress on the heart and leads to enhanced ACE2 expression and activity. On the other hand, elevated levels of ACE2 could also render the cells more susceptible to get infected by SARS-CoV-2 [17]. Thus, it is a big challenge to ascertain the therapeutic time window for targeting ACE2 in COVID-19 patients with CVDs. Nevertheless, a miR-200c based therapy may help to bridge the time till an effective COVID-19 vaccine is available to the world. Additionally, considering that SARS-CoV-2 employs ACE2, which is also the receptor for HCoV-NL63, MERS-CoV and SARS-CoV, it does not seem too far-fetched to speculate that a novel corona virus outbreak in future will again utilize ACE2, thus making further investigations worthwhile.
4. Methods
4.1. Cell culture
Neonatal rat cardiomyocytes (NRCMs) and Neonatal rat cardiac fibroblasts (NRCFs) were isolated from one to three-day old rat pups by using the Neonatal Heart Dissociation Kit (Miltenyi). NRCMs were cultured in MEM (BioConcept) medium with 5% fetal bovine serum (FBS) (Gibco), 100 μM BrdU (Sigma) and 1% penicillin/streptomycin (Gibco). NRCFs were cultured in DMEM (Gibco) medium with 10% FBS (Gibco). Human primary cardiac fibroblasts (HCFs, Promocell) were cultivated in Fibroblast Basal medium (Promocell) supplemented with 5% FBS (Gibco). Human Umbilical Vein Endothelial Cells (HUVECs) (ATCC, UK) were cultured in EBM-2 (Lonza) supplemented with hEGF, hydrocortisone, VEGF, hFGF-B, R3-IGF-1, ascorbic acid, gentamicin/amphotericin-B and 10% FBS (Gibco). The cord blood derived human induced pluripotent stem cell line (hiPSCs) [18] was maintained on feeder-free culture conditions using Geltrex (Thermo Scientific) coated polystyrene plates (Greiner CELLSTAR) and StemMACS full medium with supplements (Miltenyi). HiPSCs were differentiated into mesodermal lineage by modulation of Wnt pathway, followed by a metabolic selection process to obtain purified population of hiPSC-CMs. Pre-miR-negative control#2 (Life Technologies), −429 (Life Technologies, PM10759), −200c (Life Technologies, PM11714) and -200b (Life Technologies, PM10492) were transfected for 48 h with Lipofectamine 2000 (Life Technologies) in OptiMEM medium.
4.2. RNA isolation and qRT-PCR
RNA isolation from in vitro and mouse organ tissue was performed by using Trifast (Peqlab) as described in manufacturer's instructions. Isolated RNA (500 ng) was reversed transcribed with random primer using iScript Select cDNA synthesis kit (Bio-Rad). Real-Time quantitative PCR was done with iQ SYBR Green mix (Bio-Rad) on C1000 Touch Thermocycler (Bio-Rad) using specific primer pairs.
The specific primers for target genes are as follows (5′–3′): rno_Ace2 forward: (ACCGGAAAGTTGTCTGCCAC) and reverse: (AGTGACATGATTTCTCCAACGGC); hsa_ACE2 forward: (GGACAAGTTTAACCACGAAGC) and reverse: (TGAGAGCACTGAAGACCCATT); rno_Hprt forward: (CAGTCAACGGGGGACATAA) and reverse: (GCTGTACTGCTTGACCAAGG); hsa_HPRT forward: (AGGACTGAACGTCTTGCTCG) and reverse: (GTCCCCTGTTGACTGGTCATT).
Taq-man assay was applied to measure miR-429 (Assay ID: 001077), −200b (Assay ID: 002251) and -200c (Assay ID: 002300) expression in NRCM and hiPSC-CM. SnoRNA (Assay ID: 001718) and RNU48 (Assay ID: 001006) were used as house keepers, respectively in rat and human.
4.3. Western blotting
Cell pellets were lysed in 1X Cell lysis buffer (Cell Signaling) and isolated protein was measured by Bradford (Bio-Rad) method. 30 μg of protein was loaded for each sample on SDS-polyacrylamide gel to resolve the proteins. Proteins were transferred to polyvinylidene fluoride membrane in Mini PROTEAN Tetra cell (Bio-Rad). Specific proteins were identified by following antibodies: ACE2 (Proteintech), and Actb (Cell Signaling). HRP conjugated secondary antibody (Cell Signaling) was used for detection of bands. Band intensity was calculated by Image J software.
4.4. Luciferase reporter assay
Luciferase reporter vector were constructed by using pMIR-Report plasmid (Ambion# Am5795) containing the binding site (or mutated) with miR-200c in 3’UTR of ACE2 (179–185). HEK 293 T cells were transfected with wild type (WT) or mutated (MUT) luciferase reporter plasmid with pre-miR-negative control#2 (Life Technologies), pre-miR-200c (Life Technologies, PM11714) and beta-Gal control plasmid (Promega). Luciferase activity was performed according to manufacturer's instructions to study the direct binding of miR-200c in 3’UTR of ACE2 normalizing with beta-Gal values via utilizing beta-Gal kit (Promega) and Luciferase Assay System (Promega).
4.5. Statistics
All data were analyzed using GraphPad Prism software. Data are presented as mean ± SEM, and an unpaired 2-tailed t-test was performed to calculate the significance between groups.
The regulation of Ace2 expression by miRNAs in NRCM (A) Relative expression of miR-200b, −200c and −429 in NRCMs transfected with pre-miR-200b, −200c and −429 (n = 4, technical replicates); (B) Relative protein levels of Ace2 in NRCMs transfected with pre-miR-429 (n = 3, technical replicates).
Author contributions
Author Contributions:Conceptualization: D.C.L., S.C., K.X., C.B., T.T.; Data curation: D.C.L., S.C., K.X., I.R.; Formal analysis: D.C.L., S.C.; Writing - original draft: D.C.L., S.C., K.X.; Writing - review & editing: D.C.L., S.C., K.X., Y.B.W, R.F., C.B., T.T.
Disclosures
T.T. is founder and shareholder of Cardior Pharmaceuticals GmbH. TT filed and licensed patents on noncoding RNAs. Y.W. is founder and shareholder of Ramino Inc. The other authors declare no competing interests.
Acknowledgements
C.B. and T.T. received funding from the German Research Foundation, DFG (SFB/Transregio TRR267).
References
- 1.Coronavirus disease (COVID-19) 2020. 2019. Situation Report – 186.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200724-covid-19-sitrep-186.pdf?sfvrsn=4da7b586_2 [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ames M.K., Atkins C.E., Pitt B. The renin-angiotensin-aldosterone system and its suppression. J. Vet. Intern. Med. 2019;33:363–382. doi: 10.1111/jvim.15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80 e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Team TNCPERE The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China. China CDC Wkly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 6.Nicin L., Abplanalp W.T., Mellentin H., Kattih B., Tombor L., John D. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur. Heart J. 2020;41:1804–1806. doi: 10.1093/eurheartj/ehaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L.J., Xu R., Yu H.M., Chang Q., Zhong J.C. The ACE2/Apelin signaling, MicroRNAs, and hypertension. Int. J. Hypertens. 2015;2015 doi: 10.1155/2015/896861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross S., Jahn C., Cushman S., Bar C., Thum T. SARS-CoV-2 receptor ACE2-dependent implications on the cardiovascular system: from basic science to clinical implications. J. Mol. Cell. Cardiol. 2020;144:47–53. doi: 10.1016/j.yjmcc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma A., Garcia G., Arumugaswami V., Svendsen C.N. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. bioRxiv. 2020 doi: 10.1016/j.xcrm.2020.100052. pre-print:2020.04.21.051912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puelles V.G., Lutgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenzel P., Kopp S., Gobel S., Jansen T., Geyer M., Hahn F. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc. Res. 2020;116:1661–1663. doi: 10.1093/cvr/cvaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bojkova D., Wagner J.U.G., Shumliakivska M., Aslan G.S., Saleem U., Hansen A. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. bioRxiv. 2020 doi: 10.1093/cvr/cvaa267. electronic pre-print: 2020.06.01.127605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thanh Le T., Andreadakis Z., Kumar A., Gomez Roman R., Tollefsen S., Saville M. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 15.Kozak J., Jonak K., Maciejewski R. The function of miR-200 family in oxidative stress response evoked in cancer chemotherapy and radiotherapy. Biomed. Pharmacother. 2020;125 doi: 10.1016/j.biopha.2020.110037. [DOI] [PubMed] [Google Scholar]
- 16.D’Agostino M., Martino F., Sileno S., Barilla F., Beji S., Marchetti L. Circulating miR-200c is up-regulated in paediatric patients with familial hypercholesterolaemia and correlates with miR-33a/b levels: implication of a ZEB1-dependent mechanism. Clin. Sci. 2017;131:2397–2408. doi: 10.1042/CS20171121. [DOI] [PubMed] [Google Scholar]
- 17.Guo J., Huang Z., Lin L., Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haase A., Gohring G., Martin U. Generation of non-transgenic iPS cells from human cord blood CD34(+) cells under animal component-free conditions. Stem Cell Res. 2017;21:71–73. doi: 10.1016/j.scr.2017.03.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The regulation of Ace2 expression by miRNAs in NRCM (A) Relative expression of miR-200b, −200c and −429 in NRCMs transfected with pre-miR-200b, −200c and −429 (n = 4, technical replicates); (B) Relative protein levels of Ace2 in NRCMs transfected with pre-miR-429 (n = 3, technical replicates).