Abstract
Complex coacervates are water droplets dispersed in water, which are formed by spontaneous liquid–liquid phase separation of an aqueous solution of two oppositely charged polyelectrolytes. Similar to the membraneless organelles that exist in biological cells, complex coacervate droplets are membraneless and have a myriad of features including easy formation, high viscosity, selective encapsulation of biomolecules, and dynamic behaviors in response to environmental stimuli, which make coacervates an excellent option for constructing artificial membraneless organelles. In this article, I first summarize recent advances in artificial compartments that are built from coacervates and their response to changes in the surrounding environment and then show the advantages of microfluidic techniques in the preparation of monodisperse coacervates and encapsulation of coacervates in droplets and liposomes to construct complex cell-like compartments, and finally discuss the future challenges of such membraneless aqueous compartments in cell mimics and origin of life.
I. INTRODUCTION
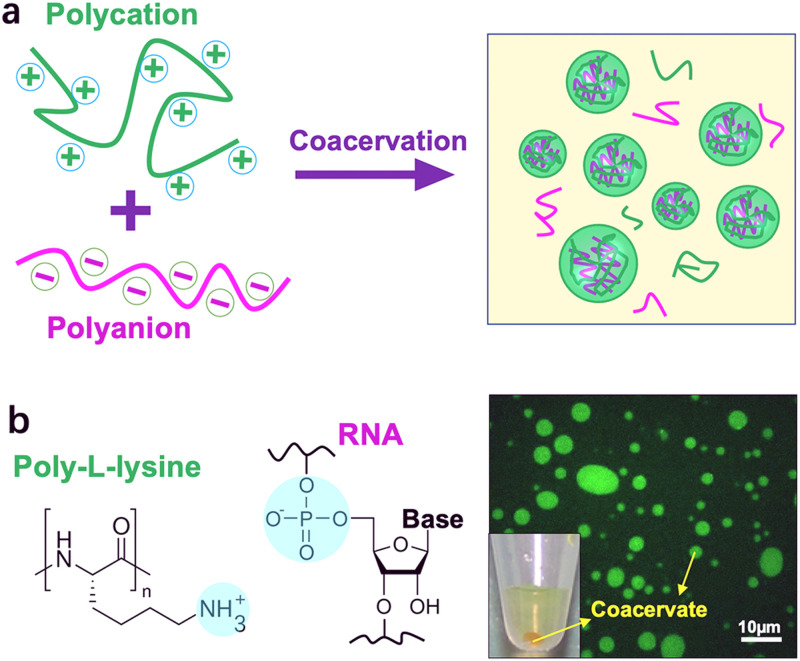
Cells compartmentalize their intracellular environment into various sub-compartments to carry out diverse biochemical processes simultaneously. Except the well-known membrane-bounded organelles, such as endoplasmic reticulum and Golgi apparatus, some of the interior subunits of cells are membraneless, such as P granules1 and stress granules.2 These membraneless architectures originate via liquid–liquid phase separation (LLPS) of intrinsically disordered proteins and RNA and act as a critical part in cellular structures and functions.3,4 Analogous to membraneless organelles, complex coacervates are water droplets dispersed in water and formed by spontaneous LLPS of an aqueous solution of two oppositely charged polyelectrolytes to form a dense polyelectrolyte-rich phase (coacervate) and a more dilute solution (Fig. 1).5–7 This associative LLPS is so-called complex coacervation and was first reported by Tiebackx.8 Bungenberg De Jong and Kruyt subsequently investigated the phenomenon systematically in the 1920s by mixing gelatin (polycation) and gum arabic (polyanion).9 Then, Oparin et al. employed complex coacervate droplets as primitive protocell models for the study of the origin of life due to the cell-like micro-compartmentalization.10 After that, a myriad of coacervate systems have been reported. Particularly, in 2011, Koga et al.11 found that coacervate droplets could be formed from components of small molecules, for example, mixing of simple peptides and nucleobases forms complex nucleotide coacervates. Such membraneless structures have good biocompatibility and striking similarity to cellular structures.
FIG. 1.
(a) Schematic illustration of the formation of coacervates via mixing polycations and polyanions. (b) An example of coacervates formed from poly-l-lysine and RNA. The right panel shows the as-formed coacervates labeled by fluorescein isothiocyanate.
Complex coacervation, together with LLPS of neutral polymer mixtures such as polyethylene glycol (PEG)/dextran system, which forms an aqueous two-phase system (ATPS) with a PEG-rich phase and a dextran-rich phase,12,13 represents a powerful means of compartmentalization at micro-scales and has been extensively studied as protocells, artificial cells, and organelles.14–16 Except for the compartmentalization, coacervate droplets also exhibit excellent functions, such as selective partitioning of biomolecules,17 enhancement of enzyme catalysis,10,18–20 and construction of cell-like crowded environments,20 which will facilitate the research of construction of synthetic cells.
In this paper, I first summarize recent advances in artificial compartments that are built from coacervates and their response to changes in the surrounding environment, and then show the advantages of microfluidic techniques in preparation of monodisperse coacervates and encapsulation of coacervates in droplets and liposomes to construct multiple compartments, and finally focus on some future challenges of such novel aqueous compartments.
II. DYNAMIC COMPARTMENTALIZATION OF COACERVATES
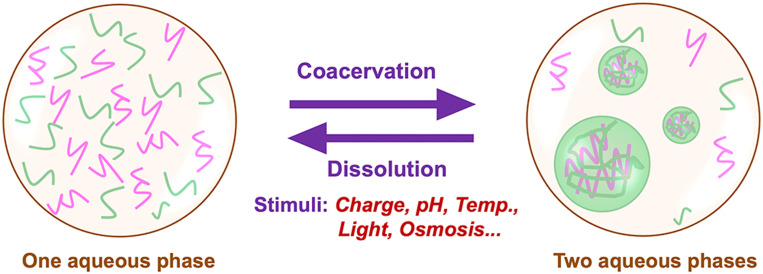
Complex coacervates, especially the bio-relevant complex nucleotide coacervates, exhibit a myriad of features including easy formation, high viscosity, selective encapsulation of biomolecules, and dynamic behaviors such as coalescence and exchange of solutes as well as adaptive response to environmental stimuli. The formation and disassembly of complex coacervates can be impacted by the mixing ratios and concentrations of the polyelectrolytes, charge densities of the polymers, and some environmental stimuli, such as pH, light, ionic strength, temperature, etc.,11,24–27 which provide possible control over the morphologies and dynamics of the compartments formed by coacervates (Fig. 2).
FIG. 2.
Schematic illustration of dynamic compartmentalization of coacervates in response to environmental stimuli.
The LLPS of polycations and polyanions to form droplets is mainly dominated by the electrostatic interactions between the oppositely charged polyelectrolytes; thus, either charge density of the polyelectrolytes or the ionic strength in the substrate could be regulated to control the dynamic assembly of the complex coacervates.26 Semenov et al.21 have demonstrated that the oscillation of trypsin concentration in an out-of-equilibrium enzymatic network enables us to control the assembly and disassembly of complex coacervates formed from polyglutamic acid and a lysine–serine polycation. Aumiller and Keating22 have mediated complex coacervation of RNAs with cationic peptides through phosphorylation in peptides. The charges in peptide molecules were tuned by using a kinase/phosphatase enzyme pair to get different phosphorylation states that induce the formation and dissolution of the complex coacervate droplets, resulting in model liquid organelles capable of reversible compartmentalization. Nakashima et al.23 have presented a method of reversible generation of adenosine triphosphate (ATP)–peptide coacervate droplets. They used two enzymatic reactions: one consumes ATP to form adenosine diphosphate (ADP) with less charges making the coacervates dissolved; the other converts ADP back to ATP to regenerate the coacervates. Recently, Donau et al.24 have reported a type of active coacervate droplets made of RNAs and peptides. The dynamics of the membraneless droplets were regulated by tuning the charges in the peptide molecules via a fuel-driven chemical reaction cycle, thereby leading to different affinities to RNAs to control the formation and dissolution of the coacervate droplets. Intriguingly, the authors also found spontaneous asymmetric division toward the end of a coacervate droplet cycle.
Similarly, other environmental stimuli including pH, temperature, light, and so forth also can be utilized to alter the dynamics of the coacervate compartments (Table I).11,25–28 Koga et al.11 have reported a coacervate system made of peptides and mononucleotides of lower molecular weight. The bio-relevant complex nucleotide coacervates are stable between pH 2 and pH 10 up to 85 °C in temperature, and able to undergo pH-induced cycles of growth and decay as well as to enrich enzymes and nanoparticles in the crowded interiors without loss of function. The authors showed the reversible growth and disassembly of coacervates with pH cycles. They prepared a clear peptide–nucleotide solution at pH 12.5 and then bubbled CO2 into the solution to produce a turbid suspension of coacervate droplets, which will be back to the clear solution again through the bubbling of NH3. The stability of the coacervate droplets is principally determined by the pKa values of the nucleotide and peptide side chains. Recently, thermo/light-stimuli-responsible complex coacervates have been reported as well, which were realized by tuning the morphologies of the applied polymers to shield or release the charges. For example, mixing of low complexity RNA, polyuridylic acid (polyU), and short polyamines, spermine can form thermo-responsible complex coacervates because polyU RNA has a melting transition temperature (Tm).27,28 Above the Tm, polyU RNA shows a random coil structure and could form coacervates with spermine at certain concentrations. As the temperature is lower than Tm, polyU RNA will form some intramolecular secondary structures due to the base stacking, which decreases the electrostatic interactions between polyU RNA and spermine, thereby making the complex coacervate droplets soluble again. The process is reversible for many times with changes in temperature of the solution.27
TABLE I.
Examples of the commonly used complex coacervate systems responsible to environmental stimuli.
| No. | Polycations | Polyanions | Stimuli | Reference |
|---|---|---|---|---|
| 1 | Poly-l-lysine | ATP | pH | 11 and 25 |
| 2 | Azobenzene cationa | Double-stranded DNA | Light | 26 |
| 3 | Spermine/spermidine | Polyuridylic acid RNA | Temp. | 27 and 28 |
Trans-azobenzenetrimethylammonium bromide.
The dynamic compartmentalization of complex coacervates offers a plausible model of membrane-free compartments for organization of prebiotic building blocks to primitive cell structures and the construction of artificial organelles. Particularly, the assembly and disassembly of coacervate droplets controlled by enzymatic processes are very analogous to the membraneless organelles in biological cells.
III. FUNCTIONAL ORGANELLE-LIKE COACERVATES
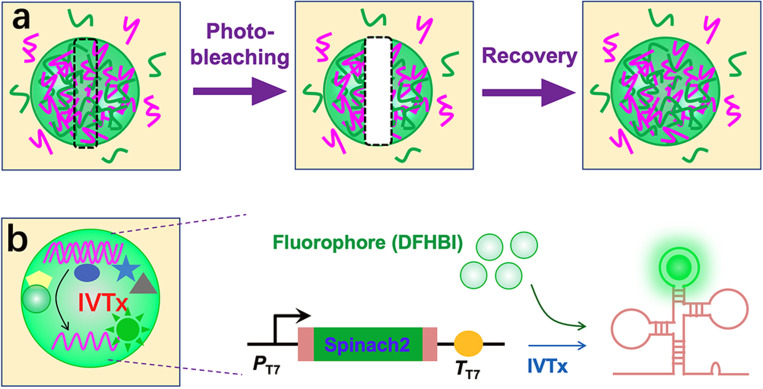
Except the dynamic compartmentalization, complex coacervates also exhibit other exceptional properties, including rapid internal mixing, fast exchange with the external environment, selective accumulation of biomolecules, and enhancement of enzymatic reactions because of their crowded interiors and different partition coefficients of biomolecules (Fig. 3). Coacervate droplets are highly adaptable and able to form well-defined structural entities through rearrangements and biopolymer reorganization. Therefore, coacervate droplets have been extensively employed as membrane-less bioreactors for constructing artificial cells and organelles.
FIG. 3.
(a) Schematic illustration of fluorescence recovery after photobleaching (FRAP) of a labeled complex coacervate. (b) Schematics showing the in vitro transcription of Spinach 2 inside coacervates.
Jia et al.29 have shown that rapid RNA exchange between ATP/poly-l-lysine coacervate droplets through the fluorescence recovery after photobleaching (FRAP) technique. Deng and Huck27 have demonstrated that DNA molecules partition efficiently into coacervates composed of polyU RNA and spermine because of electrostatic interactions and low dielectric constant. The DNA localization follows the dynamics of reversible coacervation, displaying a storage and release function of the system. Nott et al.17 have produced a range of proteinaceous bodies that resemble membraneless organelles. The liquid droplets act as biomolecular filters that are able to concentrate ssRNA and ssDNA and melt dsDNA. Sokolova et al.20 have found that membrane-free compartments formed by coacervation of cell lysates enhance the transcription rate in vitro. As-formed coacervate droplets are dense liquid and contain all macromolecular components that support mRNA synthesis. The crowded interior significantly enhances the binding constant of T7 RNA polymerase to DNA and the transcription rate constant. Moreover, Tang et al.30 have realized cell-free gene expression of a fluorescent protein (mCherry) in the molecularly crowded coacervates of polysaccharide/polypeptide.
Consequently, complex coacervate droplets are powerful, functional, organelle-like micro-compartments that will have a key role in cell mimics and construction of artificial cells or protocells.
IV. MICROFLUIDICS FOR MONODISPERSE COACERVATES
The conventional method of preparation of coacervates is simply mixing of polycations and polyanions in bulk, which, however, results in polydisperse and unstable structures. Furthermore, to mimic the sub-compartmentalization of biological organelles, complex coacervates are often used as sub-units in vesicle-based systems, but there is no proper way to achieve it in bulk. Novel techniques able to form monodisperse coacervate drops as well as combine coacervates with vesicle systems are essentially required.
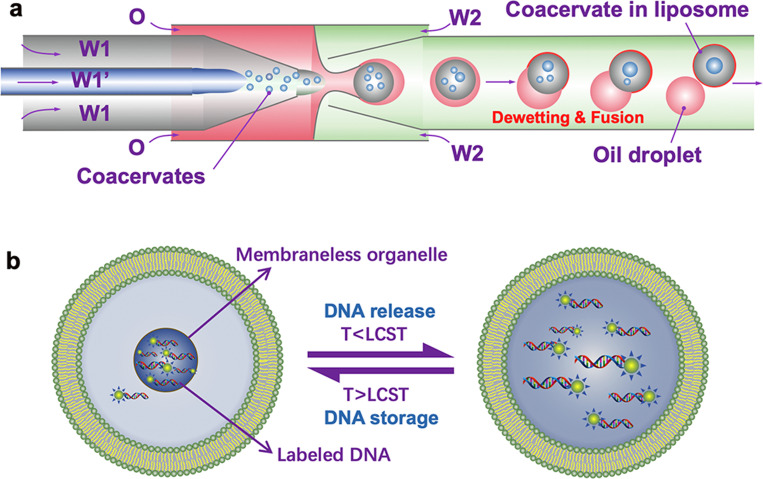
Droplet microfluidics has been emerging as an attractive approach to create monodisperse droplets, control over dimensions, and show high encapsulation efficiencies.31,32 In 2015, van Swaay et al.33 have employed a flow-focusing microfluidic device to form membrane-free aqueous coacervate droplets from poly(diallyldimethylammonium chloride) with either adenosine triphosphate or carboxymethyl-dextran. The author claimed the method improves the stability of coacervate droplets and their size distribution. In 2017, Deng and Huck27 have reported a microfluidic strategy for constructing monodisperse coacervate droplets within uniform unilamellar liposomes. The hierarchically cytomimetic compartments represent a key step toward artificial cells, which make it possible to mimic diverse intracellular activities, such as thermal-responsive reversible compartmentalization, controlled storage and release of genetic molecules, and spatial organization of bioreactions (Fig. 4). Later, other microfluidic techniques and cytomimetic structures (droplets or vesicles) containing coacervate droplets as sub-compartments have been reported extensively, enriching the research of subcellular membraneless organization, especially the use of coacervates as artificial organelles.25,34,35
FIG. 4.
(a) Schematics showing encapsulation of coacervates into liposomes by using a microcapillary-based microfluidic device. (b) Schematic illustration of thermal-triggered release and storage of labeled DNA molecules in the coacervates within liposomes.
V. OUTLOOK
Complex coacervate droplets have shown various organelle-like features and have been used in bottom–up constructions of high-order cell-like compartments, in which coupled micro-reactions can be controlled in space and time. Complex coacervate systems, however, also need to improve in some aspects as follows:
-
(1)
Stability: because of the membrane-less feature, coacervate droplets are easy to fuse with each other. Either exploring surfactants or novel preparation method may be used to enhance the stability. Attempts to form stable coacervates by using surfactants have been reported recently.36
-
(2)
Biocompatibility: the intrinsic charge interior of reported systems might be not compatible with many bio-processes, for example, gene translation. New biocompatible coacervate systems are still essentially required and need to be excavated.
-
(3)
Multiple complex coacervate droplets and their dynamics: when more than two types of polyelectrolytes are applied into the coacervate droplets, core–shell, dumbbell-shaped, or single separated coacervates may form. Although there have been some reports on this topic,37,38 detailed mechanisms and the applications will be the next step.
-
(4)
Multi-stimuli-responsible coacervate systems: exploring multi-stimuli-responsible systems will benefit for food and pharmaceutical applications as well as new functional cell-like compartments.
-
(5)
Interaction with biological cells: expression of elastic-like protein39 and spider silk protein (spidroin 1)40 in Escherichia coli can generate membraneless compartments via LLPS in living cells. Analogously, the interaction of coacervates and cells might be useful for drug delivery systems and construction of synthetic cells.
-
(6)
Prebiotic organization of lipid bilayers onto the surface of the coacervate droplets: in many cases, coacervate droplets are considered protocell models. The evolution of modern membrane on them is still unclear.
-
(7)
A big challenge: controlled growth and division of the coacervate droplets. Self-growth and self-division of the coacervate droplets are key steps to drive the evolution of coacervate-based protocells or protolives.
-
(8)
Except complex coacervates, simple coacervates formed by single component, such as elastin-like protein,41 fused in sarcoma (FUS) protein,3 and DNA,42 can also be utilized as artificial organelles toward the construction of synthetic organelle-like structures with higher complexity. Furthermore, coacervates also can be created by two like-charge polyelectrolytes, for example, 12-repetition of the decapeptide of fp-1 (Rmfp-1) and poly(2-(trimethylamino) ethyl methacrylate), the two positive charged polymers.43 There might be some interesting findings in the directions as well.
In conclusion, complex coacervation is a powerful approach to membraneless compartmentalization and will definitely be a key role in cell mimics and construction of artificial organelles or cells.
ACKNOWLEDGMENTS
This work was supported by a start-up funding from the Shanghai Jiao Tong University (WF220411020).
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Brangwynne C. P., Eckmann C. R., Courson D. S., Rybarska A., Hoege C., Gharakhani J., Jülicher F., and Hyman A. A., Science 324(5935), 1729–1732 (2009). 10.1126/science.1172046 [DOI] [PubMed] [Google Scholar]
- 2.Jain S., Wheeler J. R., Walters R. W., Agrawal A., Barsic A., and Parker R., Cell 164(3), 487–498 (2016). 10.1016/j.cell.2015.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray D. T., Kato M., Lin Y., Thurber K. R., Hung I., McKnight S. L., and Tycko R., Cell 171(3), 615–627 (2017). 10.1016/j.cell.2017.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feric M., Vaidya N., Harmon T. S., Mitrea D. M., Zhu L., Richardson T. M., Kriwacki R. W., Pappu R. V., and Brangwynne C. P., Cell 165(7), 1686–1697 (2016). 10.1016/j.cell.2016.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Gucht J., Spruijt E., Lemmers M., and Cohen Stuart M. A., J. Colloid Interface Sci. 361(2), 407–422 (2011). 10.1016/j.jcis.2011.05.080 [DOI] [PubMed] [Google Scholar]
- 6.Priftis D. and Tirrell M., Soft Matter 8(36), 9396–9405 (2012). 10.1039/C2SM25604E [DOI] [Google Scholar]
- 7.Sing C. E. and Perry S. L., Soft Matter 16(12), 2885–2914 (2020). 10.1039/D0SM00001A [DOI] [PubMed] [Google Scholar]
- 8.Tiebackx F. W., Z. Chem. Ind. Kolloide 8, 198–201 (1911). 10.1007/BF01503532 [DOI] [Google Scholar]
- 9.Bungenberg De Jong H. G. and Kruyt H. R., Proc. K. Ned. Akad. Wet. 32, 849–856 (1929). [Google Scholar]
- 10.Oparin A., Yevreinova T., Larionova T., and Davydova I., Dokl. Akad. Nauk SSSR 143, 980 (1962). [Google Scholar]
- 11.Koga S., Williams D. S., Perriman A. W., and Mann S., Nat. Chem. 3(9), 720–724 (2011). 10.1038/nchem.1110 [DOI] [PubMed] [Google Scholar]
- 12.Shum H. C., Varnell J., and Weitz D. A., Biomicrofluidics 6(1), 012808 (2012). 10.1063/1.3670365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keating C. D., Acc. Chem. Res. 45(12), 2114–2124 (2012). 10.1021/ar200294y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drino A. and Schaefer M. R., BioEssays 40(12), 1800085 (2018). 10.1002/bies.201800085 [DOI] [PubMed] [Google Scholar]
- 15.Vieregg J. R. and Tang T. Y. D., Curr. Opin. Colloid Interface Sci. 26, 50–57 (2016). 10.1016/j.cocis.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aumiller W. M. and Keating C. D., Adv. Colloid Interface Sci. 239, 75–87 (2017). 10.1016/j.cis.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 17.Nott T. J., Craggs T. D., and Baldwin A. J., Nat. Chem. 8(6), 569–575 (2016). 10.1038/nchem.2519 [DOI] [PubMed] [Google Scholar]
- 18.Crosby J., Treadwell T., Hammerton M., Vasilakis K., Crump M. P., Williams D. S., and Mann S., Chem. Comm. 48(97), 11832–11834 (2012). 10.1039/c2cc36533b [DOI] [PubMed] [Google Scholar]
- 19.Strulson C. A., Molden R. C., Keating C. D., and Bevilacqua P. C., Nat. Chem. 4(11), 941–946 (2012). 10.1038/nchem.1466 [DOI] [PubMed] [Google Scholar]
- 20.Sokolova E., Spruijt E., Hansen M. M. K., Dubuc E., Groen J., Chokkalingam V., Piruska A., Heus H. A., and Huck W. T. S., Proc. Natl. Acad. Sci. U.S.A. 110(29), 11692–11697 (2013). 10.1073/pnas.1222321110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semenov S. N., Wong A. S. Y., van der Made R. M., Postma S. G. J., Groen J., van Roekel H. W. H., de Greef T. F. A., and Huck W. T. S., Nat. Chem. 7(2), 160–165 (2015). 10.1038/nchem.2142 [DOI] [PubMed] [Google Scholar]
- 22.Aumiller W. M. and Keating C. D., Nat. Chem. 8(2), 129–137 (2016). 10.1038/nchem.2414 [DOI] [PubMed] [Google Scholar]
- 23.Nakashima K. K., Baaij J. F., and Spruijt E., Soft Matter 14(3), 361–367 (2018). 10.1039/C7SM01897E [DOI] [PubMed] [Google Scholar]
- 24.Donau C., Späth F., Sosson M., Kriebisch B., Schnitter F., Tena-Solsona M., Kang H.-S., Salibi E., Sattler M., Mutschler H., and Boekhoven J., “Active coacervate droplets as a model for membraneless organelles and a platform towards synthetic life,” chemRxiv (2020). 10.26434/chemrxiv.11648598.v1 [DOI] [PMC free article] [PubMed]
- 25.Love C., Steinkühler J., Gonzales D. T., Yandrapalli N., Robinson T., Dimova R., and Tang T.-Y. D., Angew. Chem. Int. Ed. 59(15), 5950–5957 (2020). 10.1002/anie.201914893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin N., Tian L., Spencer D., Coutable-Pennarun A., Anderson J. L. R., and Mann S., Angew. Chem. Int. Ed. 58(41), 14594–14598 (2019). 10.1002/anie.201909228 [DOI] [PubMed] [Google Scholar]
- 27.Deng N.-N. and Huck W. T. S., Angew. Chem. Int. Ed. 56(33), 9736–9740 (2017). 10.1002/anie.201703145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aumiller W. M., Pir Cakmak F., Davis B. W., and Keating C. D., Langmuir 32(39), 10042–10053 (2016). 10.1021/acs.langmuir.6b02499 [DOI] [PubMed] [Google Scholar]
- 29.Jia T. Z., Hentrich C., and Szostak J. W., Origins Life Evol. Biospheres 44(1), 1–12 (2014). 10.1007/s11084-014-9355-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang T.-Y., van Swaay D., deMello A., Anderson J. L. R., and Mann S., Chem. Comm. 51(57), 11429–11432 (2015). 10.1039/C5CC04220H [DOI] [PubMed] [Google Scholar]
- 31.Deng N.-N., Yelleswarapu M., and Huck W. T. S., J. Am. Chem. Soc. 138(24), 7584–7591 (2016). 10.1021/jacs.6b02107 [DOI] [PubMed] [Google Scholar]
- 32.Chu L.-Y., Utada A. S., Shah R. K., Kim J.-W., and Weitz D. A., Angew. Chem. Int. Ed. 46(47), 8970–8974 (2007). 10.1002/anie.200701358 [DOI] [PubMed] [Google Scholar]
- 33.van Swaay D., Tang T.-Y. D., Mann S., and de Mello A., Angew. Chem. Int. Ed. 54(29), 8398–8401 (2015). 10.1002/anie.201502886 [DOI] [PubMed] [Google Scholar]
- 34.Deshpande S., Brandenburg F., Lau A., Last M. G. F., Spoelstra W. K., Reese L., Wunnava S., Dogterom M., and Dekker C., Nat. Commun. 10(1), 1800 (2019). 10.1038/s41467-019-09855-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linsenmeier M., Kopp M. R. G., Grigolato F., Emmanoulidis L., Liu D., Zürcher D., Hondele M., Weis K., Capasso Palmiero U., and Arosio P., Angew. Chem. Int. Ed. 58(41), 14489–14494 (2019). 10.1002/anie.201907278 [DOI] [PubMed] [Google Scholar]
- 36.Mason A. F., Buddingh B. C., Williams D. S., and van Hest J. C. M., J. Am. Chem. Soc. 139(48), 17309–17312 (2017). 10.1021/jacs.7b10846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu T. and Spruijt E., J. Am. Chem. Soc. 142(6), 2905–2914 (2020). 10.1021/jacs.9b11468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mountain G. A. and Keating C. D., Biomacromolecules 21(2), 630–640 (2020). 10.1021/acs.biomac.9b01354 [DOI] [PubMed] [Google Scholar]
- 39.Ge X., Conley A. J., Brandle J. E., Truant R., and Filipe C. D. M., J. Am. Chem. Soc. 131(25), 9094–9099 (2009). 10.1021/ja902890r [DOI] [PubMed] [Google Scholar]
- 40.Wei S.-P., Qian Z.-G., Hu C.-F., Pan F., Chen M.-T., Lee S. Y., and Xia X.-X., Nat. Chem. Biol. (to be published). 10.1038/s41589-020-0579-9 [DOI] [Google Scholar]
- 41.Simon J. R., Carroll N. J., Rubinstein M., Chilkoti A., and López G. P., Nat. Chem. 9(6), 509–515 (2017). 10.1038/nchem.2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato Y., Sakamoto T., and Takinoue M., Sci. Adv. 6(23), eaba3471 (2020). 10.1126/sciadv.aba3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim S., Huang J., Lee Y., Dutta S., Yoo H. Y., Jung Y. M., Jho Y., Zeng H., and Hwang D. S., Proc. Natl. Acad. Sci. U.S.A. 113(7), E847–E853 (2016). 10.1073/pnas.1521521113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.