Abstract
There has been increasing interest in the role of hypoxia in the microenvironment of organs, because of the discovery of hypoxia-inducible factor-1 (HIF1), which acts as a transcription factor for many genes activated specifically under hypoxic conditions. The ovary changes day by day during the estrous cycle as it goes through phases of follicular growth, ovulation, and formation and regression of the corpus luteum (CL). These phenomena are regulated by hypothalamic and pituitary hormones, sex steroids, peptides and cytokines, as well as oxygen conditions. Hypoxia strongly induces angiogenesis via transcription of a potent angiogenic factor, vascular endothelial growth factor (VEGF), that is regulated by HIF1. A CL forms with a rapid increase of angiogenesis that is mainly induced by HIF1-VEGF signaling. Hypoxia also contributes to luteolysis by down-regulating progesterone synthesis and by up-regulating apoptosis of luteal cells. This review focuses on recent studies on the roles of hypoxia- and HIF1-regulated genes in the regulation of bovine CL function.
Keywords: Corpus luteum, Hypoxia, Hypoxia-inducible factor-1 (HIF1), Luteal formation, Luteolysis
Introduction
The corpus luteum (CL) develops after ovulation and is accompanied by active angiogenesis. Its primary role is the establishment and maintenance of pregnancy in mammals. When conception does not occur, the CL regresses with a decrease in progesterone (P4) synthesis and increased apoptosis of luteal cells. Variable blood flow to the ovary during the ovarian cycle [1] causes changes in the transport of nutrients, hormones, and gases, including O2, to the ovary. Ovarian blood flow in cows decreases during luteal regression and is kept at low levels during luteal formation after ovulation [1]. Thus, during luteal regression and formation, the intra-luteal environment is characterized by low oxygen (hypoxic) conditions due to decreased blood supply.
The discovery that cellular responses to hypoxic conditions are mainly regulated by hypoxia-inducible factors (HIFs) was the basis of the study that won the 2019 Nobel Prize in Physiology or Medicine [2]. HIFs are hypoxia-specific transcription factors. HIF1 was first identified as an inducer of erythropoiesis in the kidney [2, 3] and was later found to be involved in inducing many physiological processes including angiogenesis, glycolysis, apoptosis and autophagy [4]. Recently, it was discovered to be a factor in the regulation of ovarian functions [5, 6].
This review focuses on the roles of hypoxia and HIF signaling in the development and death of the CL in cattle.
Luteinization
Luteinization is the process by which the follicular granulosa and theca cells are transformed into luteal cells. It begins in the developing follicle, whose interior is under hypoxic conditions [7,8,9,10]. Moderate hypoxia, such as the hypoxia in the peri-ovulatory follicles, has been shown to stimulate P4 production in granulosa cells (GCs) [11,12,13]. Culturing murine GCs [12] and bovine luteinized GCs [11, 13] under 10% O2 conditions increased the transcription of steroidogenic acute regulatory protein (STAR) as well as production of P4. In mice, HIF1-induced autophagy was found to be vital for GC proliferation through the selective degradation of damaged mitochondria during follicle-stimulating hormone (FSH) -mediated follicular development [14, 15]. This autophagy-related action has been observed in luteinization as well as luteal formation after ovulation [16]. BCL2/adenovirus E1B 19 kilodalton protein-interacting protein 3 (BNIP3) is a cell death factor that induces autophagy of the mitochondria (mitophagy) [17]. The finding that BNIP3 is abundantly expressed in the early CL supports the idea that hypoxia-induced autophagy is necessary for luteinization and luteal formation [16]. These studies suggest that the hypoxia generated in the developing follicle induces luteinization by up-regulating P4 production in GCs and this luteinization continues during early luteal formation after ovulation.
Luteal Formation
VEGF
Vascular endothelial growth factor (VEGF) – a strong angiogenic factor [18] – is the most essential factor for angiogenesis during luteal formation [5, 8, 19,20,21,22]. Soon after HIF1 was discovered [23], it was found that it profoundly induced the transcription of VEGF [24]. The early luteal tissue just after ovulation is presumed to be under hypoxic conditions, since the vasculature and the structure of follicular wall are destroyed by ovulation [25] and the blood flow to the ovary is low around the time of ovulation [1]. In bovine luteal endothelial cells, the mRNA expressions of the α subunit of HIF1 (HIF1A) and VEGF were not significantly different between normoxic (20% O2) and hypoxic (1% O2) cultures [26]. In the porcine CL, HIF1A mRNA expression was high at the early luteal stage suggesting that HIF1 assists in luteal formation [27]. The amount of HIF1 inside the cells is tightly regulated at the protein level [28]. Under normoxic conditions, HIF1A protein is rapidly degraded by the ubiquitin-proteasome pathway. In contrast, hypoxic conditions slow the degradation of HIF1A, leading to its accumulation. Consequently, HIF1 forms a dimer with the β subunit (HIF1B, also called aryl hydrocarbon receptor nuclear translocator; ARNT) to become a functional transcription factor [28]. Immunostaining of the primate ovary showed that HIF1A protein was highly expressed in the early CL in the primate ovary [29]. HIF1A protein expression was also high in the early and developing bovine CL, and along with VEGF protein, was significantly up-regulated under hypoxic conditions (3% O2) [30]. These studies suggest that the vascular and structural changes caused by ovulation lead to an acute oxygen shortage locally in follicular walls, which, in turn, strongly activates HIF1. This induces the transcription of VEGF, which is necessary for angiogenesis that occurs during luteal formation.
BNIP3
BCL-2 family proteins are well known regulators of apoptosis. BNIP3 is a member of this family that was first identified as an apoptosis promoter [31]. Subsequently, BNIP3 was found to regulate the induction of autophagy, especially mitophagy [17]. Recently, BNIP3-related autophagy was discovered in the ovarian functions. In the murine ovarian follicles, FSH induced the autophagy of murine GCs via HIF1, the latter being necessary for follicular development and atresia [15]. BNIP3 expression was also detected in bovine follicles and CLs and was found to be up-regulated by hypoxic conditions in GCs and luteal cells [16]. BNIP3 has also been suggested to have roles before ovulation, because of its increased expression in the GCs of bovine large follicles [16]. Furthermore, in the bovine CL, BNIP3 expression at the early luteal stage is much higher than that at other luteal stages. These results suggest that BNIP3 regulates mitophagy and autophagy in the early CL in order to form and establish the CL. They also suggest that the activation of BNIP3 is induced by HIF1 and hypoxia that occur during ovulation.
GLUT1
HIF1 is also known to induce genes related to glycolysis, one of which is the facilitative glucose transporter 1 (GLUT1) [4]. The expression of GLUT1 in CL has been reported in canine [32] and bovine [33, 34] ovaries. In the canine CL, GLUT1 expression was positively correlated with the plasma P4 concentration and expression of HIF1A [32]. In the bovine CL, GLUT1 is expressed throughout the estrous cycle [33] and is most highly expressed at the early luteal stage [34], when the expression of HIF1A is also high [30]. Culturing bovine luteal cells under hypoxic conditions induced GLUT1 expression, while inhibiting GLUT1 decreased P4 production [16]. These results support the idea that GLUT1 is needed for luteal P4 production, which luteal cells need to take up glucose. They also support the idea that GLUT1 expression at the early luteal stage is induced by HIF1, after HIF1 is activated by the hypoxic conditions during ovulation.
Luteal Regression
Functional luteolysis
Luteal regression is characterized by a decrease in P4 production (functional luteolysis), followed by a decrease in luteal size (structural luteolysis), which is largely achieved by apoptosis [35,36,37,38,39]. In cows, ovarian blood flow (as measured by electromagnetic probes) is low just after ovulation, increases gradually toward the luteal stage, and then decreases during luteal regression [1, 40]. Measurements with color-doppler ultrasound also show that intra-luteal blood flow decreases simultaneously with the decrease in plasma P4 concentrations during luteolysis, suggesting that the decreased blood supply is related to functional luteolysis in cows [41, 42]. Vascular occlusion was found to occur following the sloughing of endothelial cells into the lumina of small blood vessels during luteolysis, suggesting that vascular occlusion is the cause of the decreased blood supply and hypoxic conditions in the CL [43]. We found that hypoxic conditions decreased P4 production in mid luteal cells by inhibiting the expression and activity of the enzyme P450scc (cytochrome P450 side-chain cleavage enzyme), which converts pregnenolone into P4 by cleaving the side-chain [44]. This inhibitory effect was evident in the mid CL, while it was not detected in the early CL [45]. Hypoxia has been suggested to inhibit the process of side-chain cleavage of cholesterol, since molecular oxygen is required for this process [46], and the importance of oxygen has been reported in different types of cells [47, 48]. Hypoxia is also known to generate reactive oxygen species in mitochondria, thereby damaging the mitochondria [49]. The damage to mitochondria could be one of the reasons for the hypoxia-induced inhibition of P450scc activity by hypoxia. Because the expression of HIF1A in bovine CL is low during the regressed stage [30], hypoxia-induced functional luteolysis seems to occur without the activation of HIF1. However, the relationship between P450scc activity and HIFs is not known, and needs to be explored in future studies.
Structural luteolysis
Apoptosis, which is essential for structural luteolysis [36], was also induced in cultured luteal cells under hypoxic conditions. In cultured luteal cells, hypoxic conditions induced caspase-3 – an effector caspase in the apoptotic cascade [50]. BNIP3, which facilitates apoptosis [31] and mitophagy [17] under hypoxic conditions, was also induced in bovine luteal cells by hypoxia. These findings suggest that the oxygen deficiency in the CL is one of the factors that accelerate luteolysis principally induced by uterine prostaglandin F2α and other luteolytic factors, such as cytokines, peptides and gases [37].
Conclusion
The findings cumulatively suggest that hypoxia plays multiple roles in both the formation and regression of the bovine CL. During CL formation, hypoxia promotes luteinization and induces angiogenesis, glucose uptake, and mitophagy, while during CL regression, it decreases P4 synthesis and promotes apoptosis (summarized in Fig. 1). Further studies on how the length and degree of hypoxia determine the fate of cells in each luteal stage and what other factors, such as hormones, regulate HIF1 signals will contribute to a better understanding of the roles of hypoxia in ovarian physiology.
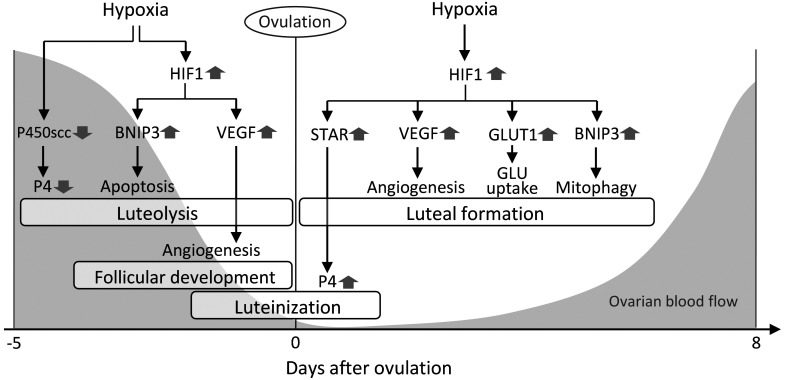
Fig. 1.
Possible roles of hypoxia in the ovary. Hypoxia generates multiple signals related to several ovarian processes. <Before ovulation> 1) In luteolysis, hypoxia down-regulates progesterone (P4) production (functional luteolysis) via cytochrome P450 side-chain cleavage enzyme (P450scc) inhibition and induces luteal cell apoptosis (structural luteolysis) via hypoxia-inducible factor-1 (HIF1)-BCL2/adenovirus E1B 19 kilodalton protein-interacting protein 3 (BNIP3) signaling. 2) In follicular development, hypoxia induces angiogenesis via HIF1-vascular endothelial growth factor (VEGF) signaling. <During the peri-ovulatory period> 3) In luteinization, moderate hypoxia stimulates P4 production via HIF1-steroidogenic acute regulatory protein (STAR) signaling. <After ovulation> 4) In luteal formation, acute hypoxia activates HIF1, leading to angiogenesis by VEGF, glucose (GLU) uptake by glucose transporter 1 (GLUT1) and mitochondrial autophagy (mitophagy) by BNIP3. Gray-colored parts show the dynamics of bovine ovarian arterial blood flow based on a previous report [1].
Acknowledgments
We thank the Society for Reproduction and Development for granting us with the Young Investigator Award and giving us the opportunity to write this review. This research was supported by the Japan Society for the Promotion of Science (JSPS) Research Fellowship for Young Scientists (Grant Number 1703589), and by the Grant-in-Aid for Young Scientists (B) (Grant Number JP16K18803) of JSPS.
References
- 1.Wise TH, Caton D, Thatcher WW, Barron DH, Fields MJ. Ovarian function during the estrous cycle of the cow: ovarian blood flow and progesterone release rate. J Anim Sci 1982; 55: 627–637. [DOI] [PubMed] [Google Scholar]
- 2.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 1992; 12: 5447–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck I, Weinmann R, Caro J. Characterization of hypoxia-responsive enhancer in the human erythropoietin gene shows presence of hypoxia-inducible 120-Kd nuclear DNA-binding protein in erythropoietin-producing and nonproducing cells. Blood 1993; 82: 704–711. [PubMed] [Google Scholar]
- 4.Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J 2002; 16: 1151–1162. [DOI] [PubMed] [Google Scholar]
- 5.Meidan R, Klipper E, Zalman Y, Yalu R. The role of hypoxia-induced genes in ovarian angiogenesis. Reprod Fertil Dev 2013; 25: 343–350. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura R, Okuda K. Multiple roles of hypoxia in ovarian function: roles of hypoxia-inducible factor-related and -unrelated signals during the luteal phase. Reprod Fertil Dev 2015; 28: 1479–1486. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Liu Z, Zhou J, Meng X, Liu S, Li W, Zhang X, Zhou J, Yao W, Dong C, Cao Y, Li R, Chen B, Jiang A, Jiang Y, Ning C, Zhao F, Wei Y, Sun SC, Tao J, Wu W, Shen M, Liu H. Insulin-like growth factor-I prevents hypoxia-inducible factor-1 alpha-dependent G1/S arrest by activating cyclin E/cyclin-dependent kinase2 via the phoshatidylinositol-3 kinase/AKT/forkhead box O1/Cdkn1b pathway in porcine granulosa cells. Biol Reprod 2019; 102: 116–132. [DOI] [PubMed] [Google Scholar]
- 8.Redmer DA, Reynolds LP. Angiogenesis in the ovary. Rev Reprod 1996; 1: 182–192. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki T, Sasano H, Takaya R, Fukaya T, Yajima A, Nagura H. Cyclic changes of vasculature and vascular phenotypes in normal human ovaries. Hum Reprod 1998; 13: 953–959. [DOI] [PubMed] [Google Scholar]
- 10.Bianco F, Basini G, Santini S, Grasselli F. Angiogenic activity of swine granulosa cells: effects of hypoxia and the role of VEGF. Vet Res Commun 2005; 29(Suppl 2): 157–159. [DOI] [PubMed] [Google Scholar]
- 11.Fadhillah, Yoshioka S, Nishimura R, Okuda K. Hypoxia promotes progesterone synthesis during luteinization in bovine granulosa cells. J Reprod Dev 2014; 60: 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowalewski MP, Gram A, Boos A. The role of hypoxia and HIF1α in the regulation of STAR-mediated steroidogenesis in granulosa cells. Mol Cell Endocrinol 2015; 401: 35–44. [DOI] [PubMed] [Google Scholar]
- 13.Fadhillah, Yoshioka S, Nishimura R, Yamamoto Y, Kimura K, Okuda K. Hypoxia-inducible factor 1 mediates hypoxia-enhanced synthesis of progesterone during luteinization of granulosa cells. J Reprod Dev 2017; 63: 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Li C, Yao W, Alsiddig MC, Huo L, Liu H, Miao YL. Hypoxia-inducible factor-1α-dependent autophagy plays a role in glycolysis switch in mouse granulosa cells. Biol Reprod 2018; 99: 308–318. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Yao W, Li C, Wu W, Li Q, Liu H. Administration of follicle-stimulating hormone induces autophagy via upregulation of HIF-1α in mouse granulosa cells. Cell Death Dis 2017; 8: e3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura R, Okuda K, Gunji Y, Khalid AM, Yamano Y, Yamashita Y, Hishinuma M. BNIP3 expression in bovine follicle and corpus luteum. J Vet Med Sci 2018; 80: 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ 2009; 16: 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 1989; 161: 851–858. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds LP, Killilea SD, Redmer DA. Angiogenesis in the female reproductive system. FASEB J 1992; 6: 886–892. [PubMed] [Google Scholar]
- 20.Grazul-Bilska AT, Redmer DA, Killilea SD, Zheng J, Reynolds LP. Initial characterization of endothelial mitogens produced by bovine corpora lutea from the estrous cycle. Biochem Cell Biol 1993; 71: 270–277. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds LP, Grazul-Bilska AT, Killilea SD, Redmer DA. Mitogenic factors of corpora lutea. Prog Growth Factor Res 1994; 5: 159–175. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds LP, Grazul-Bilska AT, Redmer DA. Angiogenesis in the corpus luteum. Endocrine 2000; 12: 1–9. [DOI] [PubMed] [Google Scholar]
- 23.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem 1995; 270: 1230–1237. [DOI] [PubMed] [Google Scholar]
- 24.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 1996; 16: 4604–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amselgruber WM, Schäfer M, Sinowatz F. Angiogenesis in the bovine corpus luteum: an immunocytochemical and ultrastructural study. Anat Histol Embryol 1999; 28: 157–166. [DOI] [PubMed] [Google Scholar]
- 26.Tscheudschilsuren G, Aust G, Nieber K, Schilling N, Spanel-Borowski K. Microvascular endothelial cells differ in basal and hypoxia-regulated expression of angiogenic factors and their receptors. Microvasc Res 2002; 63: 243–251. [DOI] [PubMed] [Google Scholar]
- 27.Boonyaprakob U, Gadsby JE, Hedgpeth V, Routh PA, Almond GW. Expression and localization of hypoxia inducible factor-1alpha mRNA in the porcine ovary. Can J Vet Res 2005; 69: 215–222. [PMC free article] [PubMed] [Google Scholar]
- 28.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem 1997; 272: 22642–22647. [DOI] [PubMed] [Google Scholar]
- 29.Duncan WC, van den Driesche S, Fraser HM. Inhibition of vascular endothelial growth factor in the primate ovary up-regulates hypoxia-inducible factor-1alpha in the follicle and corpus luteum. Endocrinology 2008; 149: 3313–3320. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura R, Okuda K. Hypoxia is important for establishing vascularization during corpus luteum formation in cattle. J Reprod Dev 2010; 56: 110–116. [DOI] [PubMed] [Google Scholar]
- 31.Bruick RK. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci USA 2000; 97: 9082–9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papa PC, Sousa LM, Silva RS, de Fátima LA, da Fonseca VU, do Amaral VC, Hoffmann B, Alves-Wagner AB, Machado UF, Kowalewski MP. Glucose transporter 1 expression accompanies hypoxia sensing in the cyclic canine corpus luteum. Reproduction 2013; 147: 81–89. [DOI] [PubMed] [Google Scholar]
- 33.Nishimoto H, Matsutani R, Yamamoto S, Takahashi T, Hayashi KG, Miyamoto A, Hamano S, Tetsuka M. Gene expression of glucose transporter (GLUT) 1, 3 and 4 in bovine follicle and corpus luteum. J Endocrinol 2006; 188: 111–119. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura R, Hasegawa H, Yamashita M, Ito N, Okamoto Y, Takeuchi T, Kubo T, Iga K, Kimura K, Hishinuma M, Okuda K. Hypoxia increases glucose transporter 1 expression in bovine corpus luteum at the early luteal stage. J Vet Med Sci 2017; 79: 1878–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bacci ML, Barazzoni AM, Forni M, Costerbosa GL. In situ detection of apoptosis in regressing corpus luteum of pregnant sow: evidence of an early presence of DNA fragmentation. Domest Anim Endocrinol 1996; 13: 361–372. [DOI] [PubMed] [Google Scholar]
- 36.Juengel JL, Garverick HA, Johnson AL, Youngquist RS, Smith MF. Apoptosis during luteal regression in cattle. Endocrinology 1993; 132: 249–254. [DOI] [PubMed] [Google Scholar]
- 37.McCracken JA, Custer EE, Lamsa JC. Luteolysis: a neuroendocrine-mediated event. Physiol Rev 1999; 79: 263–323. [DOI] [PubMed] [Google Scholar]
- 38.Rueda BR, Tilly KI, Botros IW, Jolly PD, Hansen TR, Hoyer PB, Tilly JL. Increased bax and interleukin-1beta-converting enzyme messenger ribonucleic acid levels coincide with apoptosis in the bovine corpus luteum during structural regression. Biol Reprod 1997; 56: 186–193. [DOI] [PubMed] [Google Scholar]
- 39.Rueda BR, Wegner JA, Marion SL, Wahlen DD, Hoyer PB. Internucleosomal DNA fragmentation in ovine luteal tissue associated with luteolysis: in vivo and in vitro analyses. Biol Reprod 1995; 52: 305–312. [DOI] [PubMed] [Google Scholar]
- 40.Ford SP, Chenault JR. Blood flow to the corpus luteum-bearing ovary and ipsilateral uterine horn of cows during the oestrous cycle and early pregnancy. J Reprod Fertil 1981; 62: 555–562. [DOI] [PubMed] [Google Scholar]
- 41.Acosta TJ, Yoshizawa N, Ohtani M, Miyamoto A. Local changes in blood flow within the early and midcycle corpus luteum after prostaglandin F(2 alpha) injection in the cow. Biol Reprod 2002; 66: 651–658. [DOI] [PubMed] [Google Scholar]
- 42.Miyamoto A, Shirasuna K, Hayashi KG, Kamada D, Awashima C, Kaneko E, Acosta TJ, Matsui M. A potential use of color ultrasound as a tool for reproductive management: New observations using color ultrasound scanning that were not possible with imaging only in black and white. J Reprod Dev 2006; 52: 153–160. [DOI] [PubMed] [Google Scholar]
- 43.Sawyer HR, Niswender KD, Braden TD, Niswender GD. Nuclear changes in ovine luteal cells in response to PGF2 alpha. Domest Anim Endocrinol 1990; 7: 229–237. [DOI] [PubMed] [Google Scholar]
- 44.Nishimura R, Sakumoto R, Tatsukawa Y, Acosta TJ, Okuda K. Oxygen concentration is an important factor for modulating progesterone synthesis in bovine corpus luteum. Endocrinology 2006; 147: 4273–4280. [DOI] [PubMed] [Google Scholar]
- 45.Hasegawa H, Nishimura R, Yamashita M, Yamaguchi T, Hishinuma M, Okuda K. Effect of hypoxia on progesterone production by cultured bovine early and mid luteal cells. J Reprod Dev 2019; 65: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lieberman S, Lin YY. Reflections on sterol sidechain cleavage process catalyzed by cytochrome P450(scc). J Steroid Biochem Mol Biol 2001; 78: 1–14. [DOI] [PubMed] [Google Scholar]
- 47.Raff H, Jankowski B. O2 dependence of pregnenolone and aldosterone synthesis in mitochondria from bovine zona glomerulosa cells. J Appl Physiol (1985) 1995; 78: 1625–1628. [DOI] [PubMed] [Google Scholar]
- 48.Bruder ED, Nagler AK, Raff H. Oxygen-dependence of ACTH-stimulated aldosterone and corticosterone synthesis in the rat adrenal cortex: developmental aspects. J Endocrinol 2002; 172: 595–604. [DOI] [PubMed] [Google Scholar]
- 49.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol 2006; 91: 807–819. [DOI] [PubMed] [Google Scholar]
- 50.Nishimura R, Komiyama J, Tasaki Y, Acosta TJ, Okuda K. Hypoxia promotes luteal cell death in bovine corpus luteum. Biol Reprod 2008; 78: 529–536. [DOI] [PubMed] [Google Scholar]