Abstract
Highly toxic DNA double-strand breaks (DSBs) readily trigger the DNA damage response (DDR) in cells, which delays cell cycle progression to ensure proper DSB repair. In Saccharomyces cerevisiae, mitotic S phase (20–30 min) is lengthened upon DNA damage. During meiosis, Spo11-induced DSB onset and repair lasts up to 5 h. We report that the NH2-terminal domain (NTD; residues 1–66) of Rad51 has dual functions for repairing DSBs during vegetative growth and meiosis. Firstly, Rad51-NTD exhibits autonomous expression-enhancing activity for high-level production of native Rad51 and when fused to exogenous β-galactosidase in vivo. Secondly, Rad51-NTD is an S/T-Q cluster domain (SCD) harboring three putative Mec1/Tel1 target sites. Mec1/Tel1-dependent phosphorylation antagonizes the proteasomal degradation pathway, increasing the half-life of Rad51 from ∼30 min to ≥180 min. Our results evidence a direct link between homologous recombination and DDR modulated by Rad51 homeostasis.
INTRODUCTION
RecA-like DNA recombinases are the central enzymes responsible for catalyzing the strand exchange reaction during homology-directed repair and recombination (HDRR). This error-free and template-dependent pathway repairs DNA double-strand breaks (DSBs) produced by collapse of replication forks, thereby upholding genome integrity (1,2). HDRR is also responsible for the repair of Spo11-induced DSBs during meiotic prophase (3,4). Most eukaryotic organisms possess two RecA-like recombinases, Rad51 and Dmc1. Rad51 is ubiquitously found in all eukaryotic cells, whereas Dmc1 is meiosis-specific (5). In Saccharomyces cerevisiae yeast, both Rad51 and Dmc1 are required for meiosis (6,7). However, the catalytic function of Rad51 is inhibited by direct interaction with a meiosis-specific protein, Hed1 (8), which renders Rad51 an accessory factor to Dmc1 during meiosis (9). It was also reported previously that Dmc1-mediated recombination is more efficient than Rad51-mediated recombination in meiosis of diploid hybrid yeasts (e.g. SK1/S288c and YJM/S288c) (10), given that the homologous chromosomes are highly polymorphic and Dmc1 is superior to Rad51 in tolerating mismatched sequences during their strand exchange reaction (11,12).
DSBs are one of the most deleterious and harmful DNA lesions. Repair of DSBs in eukaryotes is monitored by the DNA damage response (DDR), which operates similarly during mitosis and meiosis (13). ATM (ataxia-telangiectasia mutated) and ATR (RAD3-related) are the upstream sensor kinases that phosphorylate mammalian target proteins during DDR (14–16). Mec1 and Tel1 are the S. cerevisiae orthologs of mammalian ATR and ATM, respectively (17). ATRMec1 and ATMTel1 preferentially phosphorylate serines (S) and threonines (T) that are followed by glutamine (Q) (18). A large number of Mec1ATR and Tel1ATM targets contain at least one S/T-Q cluster domain (SCD), which has been defined as the presence of at least three S/T-Q sites in a stretch of 50 amino acids in S. cerevisiae or 100 amino acids in mammals (19,20). During yeast mitosis, Tel1ATM and Mec1ATR phosphorylate the Rad9 adaptor protein, which in turn activates Rad53 kinase (the yeast ortholog of CHK2) for DDR signaling (21). Rad53 amplifies the signaling cascade by phosphorylating another kinase, Dun1 (22,23).
During S. cerevisiae meiosis, Tel1ATM and Mec1ATR phosphorylate the SCD (residues 258–324) of Hop1, a Rad53-like chromosomal adaptor protein, particularly at the T318Q motif. Hop1 phosphorylation promotes its binding to the forkhead-associated (FHA) domain of Mek1, a meiosis-specific CHK2-like kinase, leading to activation of Mek1 (24,25). The Hop1–Mek1 interaction is similar to that of the Rad9–Rad53 and Rad53–Dun1 signaling pathways, which are mediated through the interaction of phosphorylated Rad9–SCD with Rad53–FHA and phosphorylated Rad53–SCD1 with Dun1–FHA, respectively (25,26). Subsequently, Mek1 promotes homolog bias via inhibition of Rad51 by: (i) phosphorylating and stabilizing Hed1, the meiosis-specific inhibitor of Rad51 and (ii) also phosphorylating the Rad51 accessory factor Rad54, leading to a reduced binding affinity of Rad54 for Rad51 (8,10,27,28).
Hop1 is not the only substrate that is phosphorylated by Tel1ATM and Mec1ATR during yeast meiosis. These two protein kinases sequentially phosphorylate several other protein targets during meiotic prophase, including γH2A (29), Zip1 (the transverse filament of the synaptonemal complex, SC) (30), Rec114 (an accessory factor of Spo11) (31), Sae2 (an endonuclease for DSB resection) (32) and RPA (a single-stranded DNA binding protein complex) (33). Also noteworthy is that Mec1ATR- and Tel1ATM-dependent γH2A phosphorylation emerges at the onset of premeiotic S phase and, therefore, it is independent of Spo11 (i.e. initiation of meiotic DSBs) and Red1, a meiosis-specific axial-element protein. Mec1ATR- and Tel1ATM-dependent Zip1 phosphorylation requires Spo11 but not Red1, whereas Mec1ATR- and Tel1ATM-dependent Hop1 phosphorylation requires both Spo11 and Red1 (34). Pachytene checkpoint protein 2 (Pch2) specifically prevents Red1-independent Hop1 phosphorylation (35). It has also been reported that Pch2 is involved in the initiation of Spo11-induced DSBs, with DSBs being reduced further when a pch2Δ mutant is combined with a hypomorphic allele of SPO11 (36). Alternatively, Pch2 might act together with Tel1ATM to control the resection and interhomolog process during early meiosis when DSB levels are low. When the levels of genome-wide DSBs reach a certain threshold, Mec1ATR takes over control and progressively strengthens the homolog bias (37).
Mec1ATR also has an essential function in regulating protein homeostasis in S. cerevisiae (38,39), but it is still unclear which Mec1ATR targets are involved in this intriguing function. In this study, we report that Tel1ATM and Mec1ATR phosphorylate the SCD (1–66 residues) of S. cerevisiae Rad51 recombinase to protect Rad51 recombinase from proteasome-mediated degradation. We also show that Rad51-NTD exhibits a novel function in promoting high steady-state levels of Rad51 during both vegetative growth and meiosis.
MATERIALS AND METHODS
Yeast strains and two-hybrid assay
All meiotic experiments were performed using diploid isogenic SK1 strains. Plasmids are listed in Supplementary Table S1, and genotypes of strains are described in Supplementary Table S2. Quantitative yeast two-hybrid assays, tetrad dissection, immunostaining of chromosome spreads, cycloheximide-shutoff experiments, and physical analyses were carried out as previously described (10,40–43).
Antisera, immunoblotting and cytology
Rabbit antisera against phosphorylated Rad51-S2Q, Rad51-S12Q, Rad51-S30Q and Rad51-S192Q were raised using the synthetic phosphopeptides M1S[P]QVQEQHISESQL14, E6QHI SES[P]QLQYGNGS20, T24VPADLS[P]QSVVDGNGN39 and E182LFGEFRTGKS[P]QLCHT197 as antigens, respectively, where S[P] is phosphorylated serine. The antisera were pre-cleaned by peptide-specific affinity chromatography using the corresponding non-phosphorylated peptides coupled to agarose beads. Phosphopeptide synthesis and animal immunization were conducted by LTK BioLaboratories, Taiwan. The rabbit antisera against Hop1, rabbit antisera against phosphorylated Hop1-T318Q and the goat antisera against Zip1 were described previously (25). The goat anti-Rad51 antibody (yN-19), goat anti-Clb1 antibody (yS-19) and rabbit anti-Sic1 antibody (FL-284) for western blot were purchased from Santa Cruz Biotechnology (CA, USA). The rabbit anti-Dmc1 antibody was a gift from Douglas Bishop (University of Chicago, IL, USA). The rat anti-HA antibody was purchased from Roche (Basel, Switzerland). The mouse anti-V5 antibody was purchased from Bio-Rad (CA, USA). The rabbit anti-Hsp104 and anti-hexokinase antisera were kindly provided by Chung Wang (Academia Sinica, Taiwan). The phospho-specific Rad51-S192Q antiserum (44) was a gift from Stephen Jackson (University of Cambridge, UK). Western blotting analyses were performed as described (41). The protein signals on Western blot membranes were exposed to X-ray films (Fujifilm Medical, Tokyo, Japan) or visualized and captured using ImageQuant LAS 4000 (GE Healthcare, IL, USA) and quantified using ImageJ (NIH, MD, USA). Cytological analyses using rabbit anti-Dmc1 and guinea pig anti-Rad51 were carried out as previously described (43). Stained samples were observed using an epi-fluorescent microscope (BX53, Olympus) with a 100X objective (NA1.4). Images were captured by a CCD camera (CoolSNAP HQ2, Teledyne Photometrics) at room temperature, and then processed using iVision software (BioVision Technologies).
Dephosphorylation assay
Bovine intestinal alkaline phosphatase (Sigma-Aldrich) was used for in vitro protein dephosphorylation reactions following the manufacturer's instructions. In brief, whole-cell extracts of ∼6 × 107 yeast cells precipitated with trichloroacetic acid (TCA, Sigma-Aldrich) were dissolved in 150 μl of phosphatase reaction buffer (5 mM Tris–HCl pH 8.0, 10 mM NaCl, 1 mM MgCl2 and 0.1 mM dithiothreitol), and then 3–4 μl of 2 M Tris–Base was added to adjust the pH to 7.9. Dephosphorylation reactions were carried out by mixing 37.5 μl of whole-cell extracts with 100 U of bovine intestinal alkaline phosphatase, followed by incubation for 4 h at 30°C. In the negative control experiments, the phosphatase inhibitor 2-glycerophosphate (Sigma-Aldrich) was added to a final concentration of 16 μM. Dephosphorylation reactions were stopped by the addition of 7% TCA for the subsequent precipitation. The pellet was resuspended in protein sample buffer and then incubated for 10 min at 65°C before analyses using SDS polyacrylamide gel electrophoresis.
RESULTS
The NH2-terminal domain (NTD) of S. cerevisiae Rad51 is critical for binding to Hed1
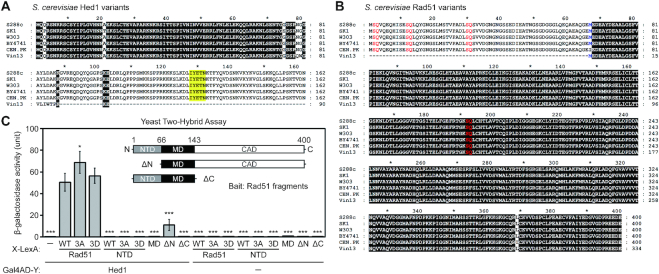
It was previously reported that the Rad51-interacting motif of Hed1 is a narrow region spanning amino acid residues 128–132, based on yeast two-hybrid (Y2H) assay (27). A homology search using NCBI BLAST revealed that Hed1 and the NTD of Rad51 (Supplementary Figure S1) are specific to the genus Saccharomyces. Multiple sequence alignments further revealed that both Rad51-NTD and the Rad51-interacting domain of Hed1 have been concomitantly lost in the wine-making strain Vin13 (Figure 1A and B). Vin13 was originally obtained by hybridizing S. bayanus and S. cerevisiae (http://www.scottlabsltd.com/product/vin-13-premium-yeast/).
Figure 1.
Interactions of Rad51 and Hed1 proteins. Sequence comparison of Hed1 and Rad51 in different yeast strains reveals loss of the COOH-terminal region of Hed1 (A), and the NH2-terminal region of Rad51 (B) in the budding yeast strain Vin13. Protein sequences were retrieved from the Saccharomyces Genome Database (SGD). Multiple sequence alignments were performed with MAFFT version 7 (63), and the results were displayed and edited with GeneDoc (version 2.7.000; https://www.softpedia.com/get/Science-CAD/GeneDoc.shtml). Identical residues are shaded in black. (A) The reported Rad51-interacting region of Hed1 is shaded in yellow. (B) The initial 66 amino acid stretch (Met1 to Glu66) of S. cerevisiae Rad51 is defined as the NH2-terminal domain (NTD). Three SQ motifs (S2Q, S12Q and S30Q) in the NTD and one S192Q motif in the ATPase domain are highlighted in red. The methionine (Met67) following the NTD is shown in blue. (C) Yeast two-hybrid assay showing interactions between Rad51 fragments and Hed1. Quantitative yeast LacZ assays were performed as described previously (40,41). Hed1 is expressed from pGADT7 vectors (Clontech, USA), whereas in X-LexA the bait protein X is NH2-terminal to LexA (64) (Supplementary Table S3). Error bars indicate standard deviation between experiments (n ≥ 3, see Supplementary Table S3 for the exact sample sizes). Asterisks indicate significant differences when compared to values of interaction between WT Rad51–LexA and Gal4AD–Hed1, with P values calculated using a two-tailed t-test (*P value < 0.05 and ***P value < 0.001).
The results of quantitative yeast two-hybrid assay (41) revealed that Rad51-ΔN, an NTD truncation mutant (residues 67–400), exhibited much weaker Y2H interactions with Hed1 than wild-type (WT), whereas the NTD alone (residues 1–66), the middle domain (MD; residues 67–143), and the CAD truncation mutant (ΔCAD; residues 1–143) were each incapable of Hed1 interaction (Figure 1C and Supplementary Table S3). The results of our immunoblotting experiments indicate that all Rad51 fragments were well expressed in the rad51Δ Y2H reporter cells (Supplementary Figure S2). We conclude that NTD is important but dispensable for the binding of Rad51 to Hed1.
Rad51-NTD itself has profound impacts for repairing DSBs during both vegetative growth and meiosis
To further examine the functional importance of Rad51-NTD in vivo, we generated both haploid and diploid rad51-ΔN mutants that express the Rad51-ΔN proteins under the control of the native RAD51 promoter. The rad51-ΔN diploid cells displayed ∼50% reduction in spore viability (Table 1). When vegetative cells were exposed to the DNA damaging agent methyl methanesulfonate (MMS), the rad51-ΔN haploid mutant, unlike rad51Δ, grew similarly to wild-type cells on YPD plates (complete media) with or without 0.01% (w/v) MMS (Figure 2A). However, the rad51-ΔN mutant displayed lower viability on YPD plates with 0.02% MMS. These results indicate that Rad51-ΔN possesses (at least in part) the capability to promote HDRR during both mitosis and meiosis, but with lower efficiency (Figure 2A and Table 1).
Table 1.
Spore viability
| Tetrad class (viable: non-viable) | ||||||
|---|---|---|---|---|---|---|
| Strain | Spore viabilitya | 4:0 | 3:1 | 2:2 | 1:3 | 0:4 |
| RAD51 | 97% (n = 152) | 92% | 5% | 3% | 0% | 0% |
| rad51Δ | 0% (n = 100) | 0% | 0% | 0% | 0% | 100% |
| rad51-ΔN | 51% (n = 216) | 7% | 30% | 28% | 28% | 7% |
| rad51-ΔN sml1Δ | 83% (n = 144) | 42% | 53% | 3% | 3% | 0% |
| rad51-S2A | 99% (n = 216) | 98% | 0% | 2% | 0% | 0% |
| rad51-S12A | 100% (n = 216) | 100% | 0% | 0% | 0% | 0% |
| rad51-S30A | 100% (n = 216) | 98% | 2% | 0% | 0% | 0% |
| rad51-S2A S12A | 100% (n = 216) | 98% | 2% | 0% | 0% | 0% |
| rad51-3A | 97% (n = 216) | 94% | 2% | 2% | 0% | 2% |
| rad51-3D | 97% (n = 288) | 96% | 2% | 0% | 0% | 2% |
| dmc1Δ hed1Δ | 74% (n = 216) | 61% | 2% | 20% | 6% | 11% |
| rad51-3A dmc1Δ | No spore | — | — | — | — | — |
| rad51-3D dmc1Δ | No spore | — | — | — | — | — |
| rad51-ΔN dmc1Δ | No spore | — | — | — | — | — |
| rad51-3A dmc1Δ hed1Δ | No spore | — | — | — | — | — |
| rad51-3D dmc1Δ hed1Δ | 70% (n = 220) | 53% | 4% | 27% | 4% | 13% |
| rad51-ΔN dmc1Δ hed1Δ | No spore | — | — | — | — | — |
| mec1-kd sml1Δ dmc1Δ hed1Δ | 7% (n = 216) | 2% | 2% | 6% | 4% | 87% |
aSpore viability was analyzed after 3 days on sporulation media at 30°C. To score spore viability, only tetrads (but not dyads or triads) were dissected on YPD.
Figure 2.
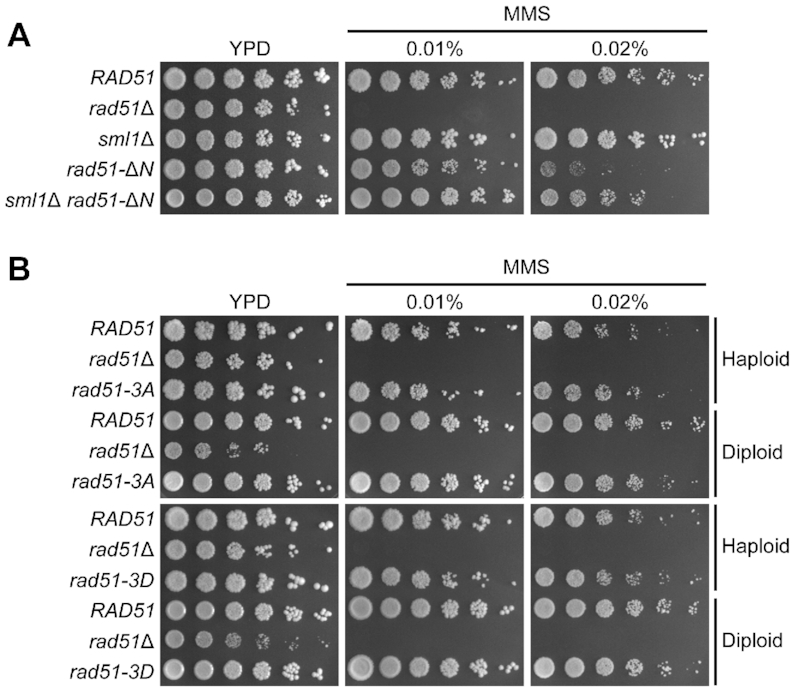
Rad51-NTD itself is more important than Rad51-NTD phosphorylation for HDRR in vegetative cells exposed to excessive DNA damaging agents. Spot assay showing five-fold serial dilutions of yeast strains grown on YPD plates with or without MMS at the indicated concentrations (w/v). (A) Analysis of haploid yeast strains, with genotypes indicated at left. (B) Analysis of both haploid and diploid yeast strains. Ploidy of the different strains is indicated at right.
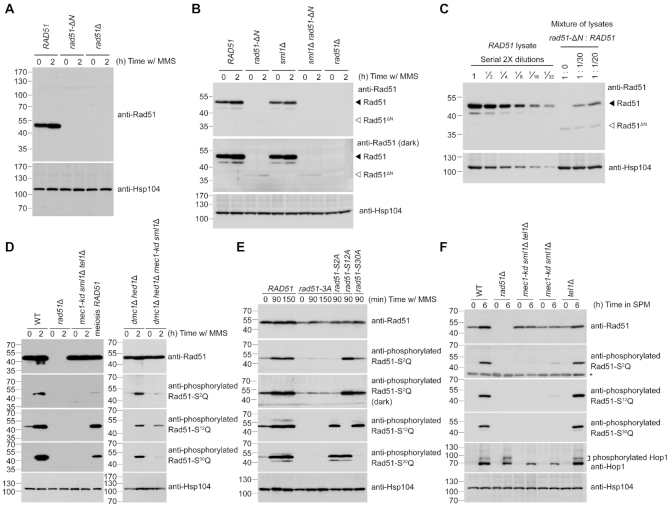
Immunoblotting time-course experiments further revealed that the steady-state Rad51-ΔN protein levels in rad51-ΔN cells were much lower than those of Rad51 protein in WT cells during vegetative growth (Figure 3A and B) or meiosis (Supplementary Figure S3A). Under vegetative conditions and upon MMS treatment to induce expression of Rad51 protein in WT, the steady-state level of Rad51-ΔN protein in rad51-ΔN cells was only ∼3% relative to that of Rad51 in WT (Figure 3C). In addition, rad51-ΔN sporulating cells exhibited a delayed meiotic progression (Figure 4A).
Figure 3.
Reduction of steady-state protein levels of Rad51-ΔN and the Mec1ATR- and Tel1ATM-dependent phosphorylation of Rad51-NTD. Total cell lysates were prepared from mitotic cells under MMS treatment (A-E), or from meiotic cells at indicated time-points after being transferred into sporulation media (SPM) (F), and then visualized by immunoblotting with the corresponding antisera. The asterisk indicates non-specific bands. Hsp104 was used as a loading control. Size in kilodaltons of standard protein markers is labeled to the left of the blots. (A) Absence of the NTD from Rad51-ΔN protein was confirmed by immunoblotting using goat anti-Rad51 antibody (yN-19) purchased from Santa Cruz Biotechnology (CA, USA). This antibody (sc-8936) was generated using a peptide mapping at the N-terminus of yeast Rad51. (B, C) Immunoblotting using guinea pig anti-Rad51 antisera that recognize the whole Rad51 protein (43), as depicted in Supplementary Figure S2A. The predicted molecular weight of Rad51-ΔN is 36,270 daltons, marked by white arrowheads to the right of the blots. Black arrowheads represent wild-type Rad51 at 42,944 daltons. (C) Total cell lysates from wild-type (WT) mitotic cells under MMS treatment were diluted with those of rad51-ΔN or empty sample buffer at indicated titers to estimate the relative steady-state protein levels of WT Rad51 and Rad51-ΔN. (E) Demonstration of the specificity of anti-phosphorylated Rad51-S2Q, Rad51-S12Q and Rad51-S30Q antisera. The darker exposure (third panel from top) illustrates that the phosphorylation-defective mutant proteins (Rad51-3A and Rad51-S2A) were slightly recognized by anti-phosphorylated Rad51-S2Q antisera.
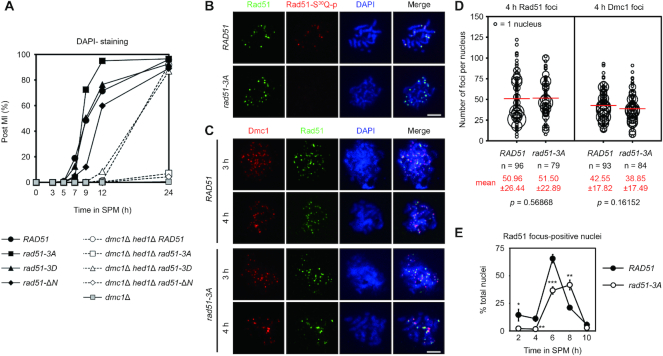
Figure 4.
Rad51-NTD and its phosphorylation are indispensable for Rad51-only meiotic recombination during dmc1Δ hed1Δ meiosis. (A) Meiotic progression was monitored by DAPI (4′,6-diamidino-2-phenylindole)-staining of nuclei. Cells harvested from SPM having two or four nuclei (as determined by fluorescence microscopy) were assessed as having completed meiosis I (MI), and the percentage of such cells over total cells counted (n = 200) per time-point was plotted. (B, C) Cytology. Representative images of meiotic nuclear surface spreading experiments using guinea pig anti-Rad51 (green) and anti-phosphorylated Rad51-S30Q (red) or anti-Dmc1 (red) antisera, respectively. Meiotic chromosomes were stained with DAPI (blue) at indicated sporulation time-points. Scale bars, 5 μm. (D) Quantification of the numbers of Rad51 and Dmc1 foci in the WT and rad51-3A strains. The numbers of foci in each foci-positive chromosome spread (with more than five foci) were counted and plotted as shown. The sizes of circles are proportional to the numbers of nuclei with a given number of foci. The mean number of foci per nucleus is shown in red (bottom), and also as a red bar in the graph. Standard deviations of numbers of foci are shown in parentheses. n represents the number of nuclei analyzed for foci-counting. The P values were calculated using a two-tailed Mann–Whitney's U-test. (E) Kinetics of Rad51 foci in meiosis of WT and rad51-3A strains. For each replicate of experiments (n = 3), Rad51-foci-positive nuclei (with more than five foci) were examined in 60 chromosome spreads. Error bars indicate standard deviation between experiments. Asterisks indicate significant difference between WT and rad51-3A strains, with P values calculated using a two-tailed t-test (*P value < 0.05; **P value < 0.01 and ***P value < 0.001).
Deficiency of the meiotic-specific recombinase Dmc1 (i.e. dmc1Δ) results in a very strong meiotic progression arrest phenotype at prophase I (MI arrest, Figure 4A) (7). However, both sporulation and spore viability are greatly improved in a dmc1Δ hed1Δ mutant, presumably due to valid interhomolog recombination being conducted by Rad51 in the absence of the inhibitory effects exerted by Hed1 (8). Like dmc1Δ, the dmc1Δ rad51-ΔN meiotic cells hardly formed any spores (Table 1). We speculate that this sever defect could be due to the extremely low protein levels of Rad51-ΔN when the other recombinase, i.e. Dmc1, is absent. It is also possible that the residual activity of Rad51-ΔN is further suppressed by Hed1 during the dmc1Δ rad51-ΔN meiosis since that our Y2H results suggest that Rad51-NTD is not the only structural region in Rad51 that mediates Rad51–Hed1 interactions (Figure 1C).
Interestingly, removal of the SML1 gene, which encodes an inhibitor of ribonucleotide reductase (45), could partially suppress the hypersensitivity of the rad51-ΔN mutant to excessive DNA damaging agents (0.02% MMS) during vegetative growth (Figure 2A) and increased its spore viability (Table 1). The sml1Δ null mutant is a suppressor of mutations of DDR checkpoint kinases, e.g. mec1-kd (kinase dead) and rad53Δ (46). Also noteworthy is that introducing the sml1Δ allele into the rad51-ΔN mutant did not significantly increase the steady-state protein levels of Rad51-ΔN (Figure 3B). These results suggest that Rad51-NTD might be functionally linked to the DNA damage checkpoint. Rad51-NTD has three SQ motifs (S2Q, S12Q and S30Q) (Figure 1B). This clustering of three SQ motifs within a stretch of 30 amino acids fulfills the criteria to define an SCD (20). One intriguing possibility is that Rad51-NTD might be phosphorylated in response to a DNA damage checkpoint.
Rad51-NTD is phosphorylated in a Mec1ATR- and Tel1ATM-dependent manner during vegetative growth and meiosis
To examine the hypothesis that the three SQ motifs in Rad51-NTD are phosphorylated in DDR, we generated antisera specific to the phosphorylated Rad51-S2Q, Rad51-S12Q and Rad51-S30Q peptides (see ‘Materials and Methods’ section). The specificity of these antisera was validated by immunoblotting experiments following protein dephosphorylation assay (Supplementary Figure S4) as well as immunoblotting experiments using yeast strains carrying different mutant alleles. The rad51-S2A, rad51-S12A and rad51-S30A variants each encodes a respective mutant protein in which the Rad51 serine residue has been mutated into alanine. In the phosphorylation-defective mutant (rad51-3A), all three of these serine residues were mutated into alanines. All three SQ motifs in Rad51-NTD were phosphorylated in a Mec1ATR- and Tel1ATM-dependent manner in vegetative cells exposed to MMS, as well as during normal meiosis (Figure 3D-F). No or negligible signals of corresponding antisera were detected in the three single-amino-acid substitution mutants (i.e. rad51-S2A, rad51-S12A or rad51-S30A), the phosphorylation-defective mutant rad51-3A (Figure 3E), or the mec1-kd sml1Δ tel1Δ triple mutants (Figure 3D and F). To elucidate which kinase is more important for phosphorylation activity, we carried out immunoblotting using cell lysates collected from meiotic cultures of the tel1Δ single mutant and the mec1-kd sml1Δ double mutant. Consistent with phosphorylation of Hop1 (detected as the slower migrating species in immunoblots) being greatly reduced in the mec1-kd sml1Δ strain during meiosis (24,25), we found that all three SQ motifs in Rad51-NTD showed dramatically reduced phosphorylation signals in the mec1-kd sml1Δ double mutant, whereas mutation of tel1Δ did not induce such an apparent effect (Figure 3F). These results suggest that Mec1ATR plays a more prominent role than Tel1ATM in Rad51-NTD phosphorylation during meiosis.
Rad51-NTD phosphorylation is not strictly dependent on SPO11, but is regulated by meiotic DSB levels
In respect of the DNA damage checkpoint-dependent phosphorylation of Rad51-NTD, we examined if Rad51-NTD phosphorylation during meiosis is Spo11-dependent using the phospho-specific Rad51-S12Q antisera. Meiosis of the mutant strains carrying spo11-da-HA, i.e. the hypomorphic allele of SPO11 resulting in ∼80% reduction of DSB formation, was used for comparison (47). Our meiotic time-course immunoblotting experiments revealed that Hop1-T318Q phosphorylation did not occur in the spo11Δ strain and it was greatly reduced in the spo11-da-HA strain relative to WT (Supplementary Figure S5A and B). In contrast, Rad51-S12Q phosphorylation remained detectable in the spo11Δ strain at a reduced level (∼29% compared to the maximum intensity in WT), but it was largely diminished in the mec1-kd sml1Δ tel1Δ triple mutants (Supplementary Figure S5A). Moreover, phosphorylation of Rad51-S12Q was reduced by ∼50% during meiosis of the spo11-da-HA strain (Supplementary Figure S5B, second panel from top), whereas protein levels of Hop1, Dmc1 or Rad51 were not obviously perturbed.
It was suggested previously that pch2Δ mutation and hypomorphic spo11 reduce DSB formation through distinct mechanisms, with DSB formation being further reduced upon combining these two mutations (36). Consistently, we found that the pch2Δ mutation did not affect Hop1-T318Q phosphorylation or Rad51-S12Q phosphorylation in the SPO11 strain (Supplementary Figure S5B). However, unlike Rad51-S12Q phosphorylation, Hop1-T318Q phosphorylation was more profoundly reduced in the pch2Δ spo11-da-HA double mutant (Supplementary Figure S5B, second panel from bottom). We conclude that phosphorylation of Hop1 and Rad51-NTD in meiosis is differentially regulated; both types of phosphorylation require Mec1ATR and Tel1ATM, but only Rad51-NTD phosphorylation can occur when cellular DSB levels are very low, even in the absence of SPO11. This outcome is not surprising given that Mec1ATR- and Tel1ATM-dependent Rad51-NTD phosphorylation also occurs during vegetative growth without genotoxin treatments (Figure 3D and E). This low level phosphorylation was not detected in G1-arrested cells (Supplementary Figure S5C.) and thus could be due to spontaneous DSBs that occur in vegetative growth, and possibly during the premeiotic S phase preceding meiotic DSBs.
Rad51-NTD phosphorylation does not significantly affect the function of Rad51 in repairing DSBs during vegetative growth or meiosis
Next, we examined if Rad51-NTD phosphorylation affects Rad51 function. For comparative purposes, we generated a phosphomimetic mutant (rad51-3D) in which all three serine residues of SQ motifs had been mutated into aspartic acids. Firstly, we found that, as for WT, not only were the phosphorylation variant mutants (rad51-3A and rad51-3D) resistant to 0.02% MMS during vegetative growth (Figure 2B), but they also generated as many viable spores as WT diploid cells did (Table 1).
Secondly, we found that, like WT Rad51, both Rad51-3A and Rad51-3D exhibited strong Y2H interactions with Hed1, with Rad51-3A presenting a slightly stronger interaction (Figure 1C). Both dmc1Δ rad51-3A and dmc1Δ rad51-3D mutants behaved like the dmc1Δ mutant in that they failed to generate spores (Table 1), likely due to the inhibitory effect conferred by Hed1 in these strains. Together, these results indicate that Mec1ATR/Tel1ATM-dependent Rad51-NTD phosphorylation does not seem to affect the Rad51–Hed1 interaction.
We show that phosphorylated Rad51 readily formed foci on chromosomes at the pachytene stage of meiosis (Figure 4B). Rad51 foci detected using antisera targeting native Rad51 and phosphorylated Rad51 did not completely co-localize, implying partial phosphorylation of Rad51 or possible competition for antigen recognition in this assay. Nevertheless, formation of Rad51 foci in the phosphorylation-defective rad51-3A mutant was indistinguishable from that of the WT control (Figure 4B–D), despite their delayed appearance on chromosomes (Figure 4E), likely due to low Rad51 protein expression levels (see below). Furthermore, formation of Dmc1 foci was not significantly perturbed in the rad51-3A mutant (Figure 4C and D), suggesting that the function of Rad51 in serving as a Dmc1 accessory factor (9) is likely preserved by phosphorylation-defective Rad51-3A.
Both NTD and Mec1ATR/Tel1ATM-dependent Rad51-NTD phosphorylation are indispensable for meiotic recombination in a dmc1Δ hed1Δ mutant
Our results reveal that the dmc1Δ hed1Δ double mutant and the dmc1Δ hed1Δ rad51-3D triple mutant exhibited similar meiotic progression and spore viability to each other (Figure 4A and Table 1), indicating that the Rad51-3D protein is functional for meiotic DSB repair. Moreover, rad51-3D behaved like WT RAD51 in the dmc1Δ hed1Δ mutant in that delayed meiotic progression and meiosis I non-dysjunction phenotypes (a preponderance of tetrads containing two or zero viable spores) were seen (Figure 4A and Table 1), as reported in a previous study using the dmc1Δ hed1Δ double mutant (48). Strikingly, both dmc1Δ hed1Δ rad51-3A and dmc1Δ hed1Δ rad51-ΔN triple mutants, like the dmc1Δ mutant, hardly sporulated (Table 1) and exhibited a strong meiotic progression arrest phenotype at prophase I (Figure 4A). Thus, both Rad51-NTD and Rad51-NTD phosphorylation are essential for Rad51-mediated meiotic recombination in the dmc1Δ hed1Δ mutant, and the phosphomimetic mutant Rad51-3D can functionally substitute for phosphorylated Rad51 in the same genetic background. This scenario is further supported by the very low spore viability following meiosis of the dmc1Δ hed1Δ mec1-kd sml1Δ strain (Table 1), in which Rad51-NTD phosphorylation is solely dependent on Tel1ATM (Figure 3D, right panel).
To directly determine the impact of Rad51-NTD phosphorylation on Rad51-mediated meiotic recombination, we performed physical analyses to examine if DSB-processing and the yield of interhomolog recombination products, crossovers (COs), at the HIS4-LEU2 hotspot differed in the rad51-3A or rad51-3D strains. This hotspot, located on chromosome III, is flanked by XhoI restriction sites (49). Both DSBs and interhomolog COs can be detected by Southern blot analysis of one-dimensional agarose gels using XhoI-digested DNA, and a probe for this region was described previously (42,50) (Supplementary Figure S6A). We found that the WT, rad51-3A, rad51-3D (Supplementary Figure S6B and C) and rad51-ΔN strains (Supplementary Figure S6D, second panel from left) all generated similar levels (15–20%) of interhomolog CO products after 12 hours in the sporulation medium (SPM) or by the 24 h time-point. In contrast, in the absence of DMC1 and HED1, these four strains only generated ∼4% interhomolog CO products at the same HIS4-LEU2 hotspot (Supplementary Figure S6D and E). Five of the mutant strains examined, namely rad51-ΔN, dmc1Δ hed1Δ, dmc1Δ hed1Δ rad51-3A, dmc1Δ hed1Δ rad51-3D, and dmc1Δ hed1Δ rad51-ΔN, exhibited less efficiency in DSB repair when compared to WT. The majority of DSBs were repaired and then disappeared in the WT, rad51-ΔN, dmc1Δ hed1Δ, and dmc1Δ hed1Δ rad51-3D strains at the 12 h time-point. However, DSBs accumulated in the dmc1Δ hed1Δ rad51-ΔN and dmc1Δ hed1Δ rad51-3A triple mutant strains (Supplementary Figure S6F). Accordingly, DDR such as recombination checkpoint was persistently activated only in the dmc1Δ hed1Δ rad51-ΔN and dmc1Δ hed1Δ rad51-3A triple mutants, leading to a strong meiotic arrest phenotype in these two mutants (Figure 4A).
Phosphorylation-defective Rad51 sustains scant protein levels during meiosis
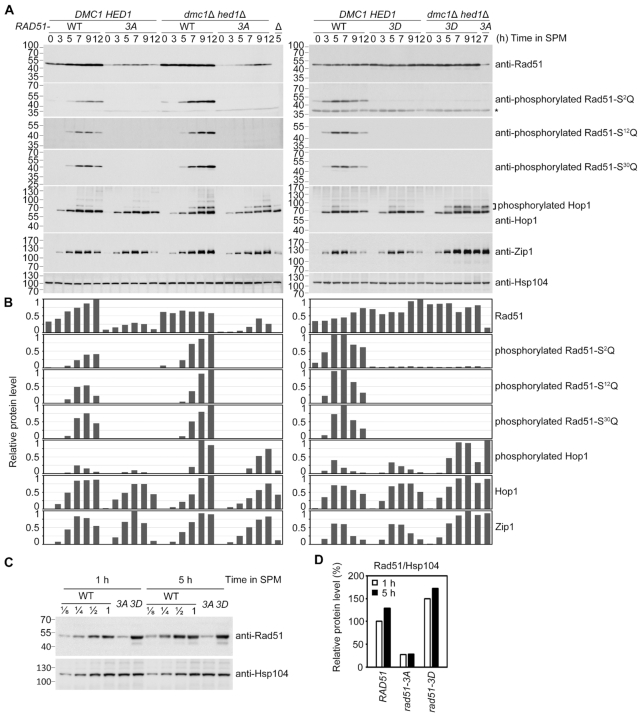
Immunoblotting experiments further revealed that the steady-state protein levels of Rad51-3A were lower than those of Rad51 and Rad51-3D in both DMC1 HED1 and dmc1Δ hed1Δ meiotic cells (Figure 5A and B). In DMC1 HED1 meiotic cells, we found the maximum steady-state levels of Rad51-3A (after 7 h in SPM) to be ∼30% those of WT Rad51 (Figure 5B). A comparison of WT Rad51, Rad51-3A and Rad51-3D protein levels in 1-h or 5-h meiotic cultures in the same immunoblot revealed that levels of Rad51-3A stalled and remained less than 30% those of WT Rad51 levels, whereas Rad51-3D was highly abundant and sustained ∼1.4-fold expression relative to WT Rad51 starting from early in meiosis (Figure 5C and D). We conducted immunoblotting analyses on Hop1 and Zip1 to rule out the possibility that the meiotic defects in the dmc1Δ hed1Δ rad51-3A strain were indirectly caused by unscheduled meiotic progression. We did not observe an apparent difference in overall protein level patterns of Hop1 or Zip1 in all six examined strains (Figure 5A and B). Given that phosphorylated Hop1 proteins migrate slower than unphosphorylated Hop1 in SDS polyacrylamide gels (24,25), it is important to note that all three strains in the dmc1Δ hed1Δ background accumulated more hyperphosphorylated Hop1 even after 12 h in SPM (Figure 5A and B). Similarly, higher levels of phosphorylated Rad51 were also seen in the dmc1Δ hed1Δ mutants in immunoblots using phospho-specific Rad51-S2Q, Rad51-S12Q, and Rad51-S30Q antisera, respectively (Figure 5A and B). These results could reflect prolonged checkpoint activation by Mec1ATR and Tel1ATM when Dmc1 is absent.
Figure 5.
Immunoblotting time-course analyses of WT and the indicated mutant strains were performed as previously described (25,34). (A) Total cell lysates were prepared from meiotic cells at indicated sporulation time-points and then visualized by immunoblotting with the corresponding antisera. Antisera against Zip1 and Hop1 were included as references for meiotic progression. Hsp104 was used as a loading control. Size in kilodaltons of standard protein markers is labeled to the left of the blots. The asterisk indicates non-specific bands. (B) Quantifications of the protein bands in (A) were normalized to those of Hsp104 at each time-point using ImageJ software and are shown as the relative steady-state levels of indicated proteins. The highest level of immunoblot signal in each blot was used as the standard for comparison. (C) Two-fold serial dilutions of WT cell lysates were used to estimate the Rad51 protein levels in rad51 mutants at 1 h and 5 h in SPM. (D) The protein levels of Rad51 variants from non-diluted lysates shown in (C) were quantified and normalized to Hsp104 levels. The Rad51 level of WT at 1 h in SPM was used as the standard.
Rad51-NTD phosphorylation results in more stable Rad51 proteins
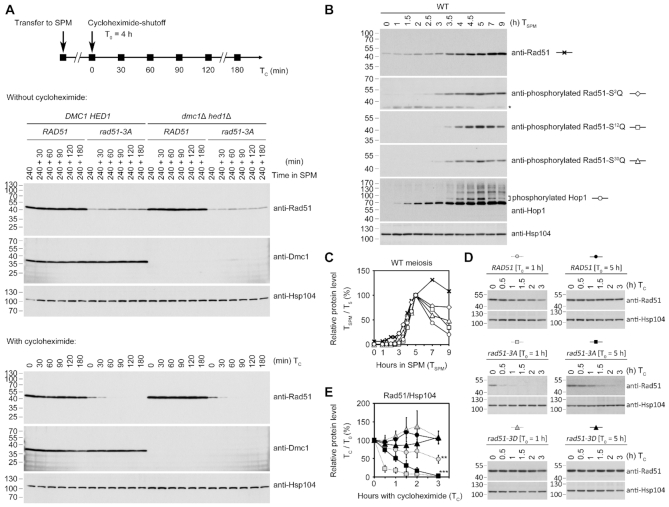
Next, we carried out cycloheximide-shutoff experiments (41) to compare the protein stability of Rad51, Rad51-3A and Rad51-3D. Protein synthesis was inhibited by adding 200 μg/ml of cycloheximide into meiotic cultures at the 4-h time-point (Figure 6A). Samples were taken at 0, 30, 60, 90, 120 and 180 min after the addition of cycloheximide. Immunoblotting analyses revealed that levels of Rad51 and Dmc1 remained steady in the presence of cycloheximide for up to 180 min. In contrast, within 30 min of adding cycloheximide, levels of Rad51-3A mutant proteins significantly decreased and were barely detectable after 60 min in both the rad51-3A and dmc1Δ hed1Δ rad51-3A strains (Figure 6A, lower panels). These results indicate that the half-life (t1/2) of Rad51-3A is ≤30 min, whereas the t1/2 of Rad51 and Dmc1 are ≥180 min.
Figure 6.
Determination of the half-lives of different Rad51 proteins. (A) Cycloheximide-shutoff experiments. Protein synthesis was inhibited by adding 200 μg/ml cycloheximide to the meiotic cultures at the indicated time-points, i.e. 4-h (A) or 1-h and 5-h (D) after the meiotic cells had been transferred into the sporulation medium. Samples untreated (upper panels) or treated (lower panels) with cycloheximide were taken at 0, 30, 60, 90, 120 and 180 min after the addition of cycloheximide for immunoblotting analysis. (B) Immunoblotting time-course analyses of WT meiosis reveal the phosphorylation status of native Rad51 and Hop1. Hsp104 was used as a loading control. (C) After normalization to Hsp104 at each time-point in SPM (TSPM), the relative steady-state protein levels in (B) were plotted as compared to those at 5-h (T5). (E) Plot showing relative protein levels in (D). Error bars indicate standard deviation between experiments (n = 3). Asterisks indicate values at TC (hours of cycloheximide treatment) = 3 h that are significantly different from that of WT [T0 = 5 h] at TC = 3 h, with P values calculated using a two-tailed t-test (**P value <0.01 and ***P value <0.001).
In our time-course experiments on WT strains with 30 min intervals between sample collection, we found that native Rad51 protein levels gradually increased along with progression of meiosis, accompanied by gradual phosphorylation of the three SQ motifs (Figure 6B and C). We speculate that during early meiosis (i.e. at the 1-h meiotic time-point), unphosphorylated native Rad51 (but not Rad51-3D) could be labile to a similar extent as Rad51-3A at the 4-h time-point (t1/2 ≤ 30 min) and would be stabilized upon phosphorylation of Rad51-NTD when entering later meiosis. We carried out cycloheximide-shutoff assays using aliquots of the same culture shown in Figure 6B at different time-points and compared the stability of WT Rad51, Rad51-3A and Rad51-3D from 1-h and 5-h meiotic cultures. Our results show that native Rad51 from the 5-h meiotic culture is indeed more stable (t1/2 ≥ 180 min) than that from the 1-h meiotic culture (t1/2 ≈ 180 min) (Figure 6D and E). We further demonstrate that the phosphomimetic mutant (Rad51-3D) in the 1-h or 5-h meiotic culture is as stable as native Rad51 from 5-h meiotic culture (t1/2 ≥ 180 min) (Figure 6D and E), suggesting that the negatively-charged NTD is sufficient to stabilize Rad51 during meiosis.
Thus, phosphorylation of Rad51-NTD plays an important role in enhancing protein stability of Rad51. When Dmc1 was present in the rad51-3A strain, low steady-state levels of Rad51-3A apparently were sufficient to facilitate Dmc1 in mediating meiotic recombination, which is consistent with our cytological observations (Figure 4C and D). In contrast, when Rad51 is the only ‘active’ recombinase in the meiotic cells (i.e. in the dmc1Δ hed1Δ strain), phosphorylation of Rad51-NTD is essential to ensure higher steady-state levels of Rad51 in order to repair the DSBs induced by Spo11. Moreover, phosphorylated Rad51 can be functionally substituted by Rad51-3D.
Rescue of the sporulation defects in dmc1Δ hed1Δ rad51-3A and dmc1Δ hed1Δ rad51-ΔN triple mutants by overexpressing WT Rad51 or Rad51-3A
Insufficient expression of Rad51 proteins may explain the sporulation defects of dmc1Δ hed1Δ rad51-3A and the lower spore viability phenotype of rad51-ΔN mutants. To test this hypothesis, we introduced high-copy number vectors expressing WT Rad51, Rad51-3A or Rad51-ΔN into four different diploid mutants that exhibited different levels of meiotic deficiencies, namely rad51Δ (no viable spores), rad51-ΔN (∼50% spore viability), dmc1Δ hed1Δ rad51-3A and dmc1Δ hed1Δ rad51-ΔN (MI arrest). Relative to yeast mutants transformed with the mock control vector, overexpression of WT Rad51 could rescue the meiotic defects (low spore viability or the sporulation deficiency phenotype) of all four of these diploid mutants (Table 2). Although overexpression of Rad51-3A failed to rescue the meiotic prophase arrest phenotype of dmc1Δ, in which Hed1 was present, overexpression of Rad51-3A not only fully restored the spore viability of rad51Δ, but also successfully rescued the sporulation defect of dmc1Δ hed1Δ rad51-3A and restored its spore viability to a level comparable to that of the dmc1Δ hed1Δ strain harboring overexpression vectors for either WT Rad51 or mock (Table 2). In addition, overexpression of Rad51-ΔN partially rescued the low spore viability of rad51Δ but not rad51-ΔN, indicating that Rad51-ΔN is capable of supporting meiotic recombination but is less efficient than WT Rad51. Overexpression of Rad51-ΔN was also unable to rescue the sporulation defects of the dmc1Δ hed1Δ rad51-ΔN strain (Table 2), suggesting that despite being expressed from a high-copy vector with its own promoter, Rad51-ΔN overexpression only exhibited mild or negligible effects when meiotic cells suffered from insufficient levels of Rad51 proteins.
Table 2.
Spore viability of yeast strains with overexpression plasmids
| Strain | Overexpression plasmida | Spore viability |
|---|---|---|
| WT | 2μ-bsd-RAD51 | 100% (n = 144) |
| rad51Δ | 2μ-bsd-RAD51 | 98% (n = 288) |
| rad51-ΔN | 2μ-bsd-RAD51 | 80% (n = 144) |
| dmc1Δ hed1Δ | 2μ-bsd-RAD51 | 83% (n = 216) |
| dmc1Δ hed1Δ rad51-3A | 2μ-bsd-RAD51 | 85% (n = 216) |
| dmc1Δ hed1Δ rad51-ΔN | 2μ-bsd-RAD51 | 81% (n = 144) |
| WT | 2μ-bsd-rad51-3A | 47% (n = 216) |
| rad51Δ | 2μ-bsd-rad51-3A | 97% (n = 216) |
| dmc1Δ | 2μ-bsd-rad51-3A | No spore formation |
| dmc1Δ hed1Δ | 2μ-bsd-rad51-3A | 86% (n = 216) |
| dmc1Δ hed1Δ rad51-3A | 2μ-bsd-rad51-3A | 79% (n = 216) |
| WT | 2μ-bsd-rad51-ΔN | 97% (n = 144) |
| rad51Δ | 2μ-bsd-rad51-ΔN | 63% (n = 144) |
| rad51-ΔN | 2μ-bsd-rad51-ΔN | 54% (n = 144) |
| dmc1Δ hed1Δ rad51-ΔN | 2μ-bsd-rad51-ΔN | No spore formation |
| rad51Δ | mock | 0% (n = 144) |
| rad51-ΔN | mock | 38% (n = 144) |
| dmc1Δ hed1Δ | mock | 88% (n = 216) |
| dmc1Δ hed1Δ rad51-3A | mock | No spore formation |
| dmc1Δ hed1Δ rad51-ΔN | mock | No spore formation |
aYeast diploid cells were respectively transformed with the indicated 2μ overexpression vector. Resistance to blasticidin is conferred by the product of the blasticidin S deaminase (bsd) gene from Aspergillus terreus. The RAD51, rad51-3A and rad51-ΔN genes were expressed under the native RAD51 promoter.
We also performed meiotic time-course immunoblotting experiments to confirm that introducing the high-copy number Rad51-3A expression vector indeed resulted in higher Rad51-3A protein levels in the dmc1Δ hed1Δ rad51-3A strain relative to introduction of mock vectors (Supplementary Figure S7). In contrast, transformation of the high-copy number Rad51-ΔN overexpression vector barely increased Rad51-ΔN protein levels in the rad51-ΔN (Supplementary Figure S3A) or dmc1Δ hed1Δ rad51-ΔN (Supplementary Figure S3B) strains compared to mock vector.
These results support our hypothesis that low steady-state levels of Rad51-3A and Rad51-ΔN proteins are the main cause of meiotic defects in the mutant strains (Tables 1 and 2).
Unphosphorylated Rad51 proteins are degraded via the proteasomal pathway
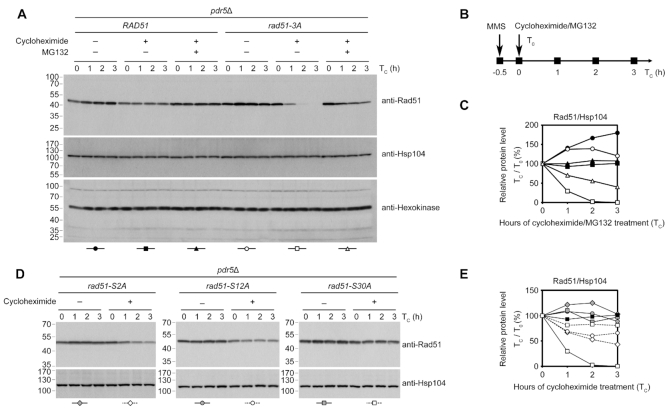
The 26S proteasome has been shown to be required for HDRR in S. cerevisiae (51). Next, we used the proteasome inhibitor MG132 to determine if WT Rad51 and Rad51-3A are degraded by proteasomes during the DNA damage response. To facilitate uptake of MG132, the strains were deleted of PDR5 to reduce efflux pump activity. After a 30-min treatment of MMS, Rad51-3A disappeared more rapidly than WT Rad51 under cycloheximide treatment (Figure 7A–C). In contrast, simultaneous addition of MG132 and cycloheximide postponed the degradation of Rad51-3A, indicating that Rad51-NTD phosphorylation stabilizes Rad51 protein by preventing proteasomal degradation during the DNA damage response.
Figure 7.
Rad51-3A is degraded by the 26S proteasome. Exponential cultures of indicated strains were treated with MMS (0.02%) 30 min prior to the addition of cycloheximide (200 μg/ml) and/or MG132 (25 μM). Protein samples were harvested at indicated time-points and immunoblotting was performed as described in Figure 6. To decrease MG132 efflux, we deleted the PDR5 gene, which encodes an ABC transporter. The protein levels of another vegetative house-keeping protein, hexokinase, were analyzed by immunoblotting using anti-hexokinase antibodies and are shown as the loading control in (A). Quantification of immunoblotting results in (A) and (D) are plotted in (C) and (E), respectively, in which total Rad51 protein levels normalized to Hsp104 levels at that time-point are plotted. Quantification results of samples of WT and rad51-3A with cycloheximide from (C) were included in (E) for comparison.
To determine phosphorylation of which SQ motif in Rad51-NTD is the most important for its stability, we carried out cycloheximide-shutoff experiments to determine the relative protein stabilities of Rad51-S2A, Rad51-S12A and Rad51-S30A (Figure 7D). Our results show that all three of these Rad51 protein variants were less stable than WT Rad51, but they were much more stable than Rad51-3A, with the least stable mutant protein Rad51-S2A exhibiting a half-life of ∼3 h (Figure 7E). Thus, phosphorylation of each of the SQ motifs in Rad51-NTD collaboratively contributes to Rad51 protein stabilization.
Rad51-NTD can act autonomously to promote expression of an exogenous protein, β-galactosidase
Our results indicate that Rad51-NTD contains an SCD and it possesses a novel function in promoting high steady-state levels of Rad51 proteins during DDR of vegetative growth as well as in meiosis. Remarkably, this function could be independent of Mec1ATR/Tel1ATM-dependent Rad51-NTD phosphorylation. To further characterize its biochemical properties, we modified an S. cerevisiae pYC2/CT/PGAL1-LacZ-V5-His6 low-copy number expression vector (Invitrogen, USA) into PRAD51-LacZ-NLS-V5-His6 by replacing the GAL1 promoter with the promoter of the wild-type RAD51 gene (PRAD51) and inserting an SV40 nuclear localization signal (NLS) peptide preceding a V5 epitope tag and a hexahistidine affinity tag. Next, we constructed three corresponding mutant vectors: PRAD51-NTDWT-LacZ-NLS-V5-His6, PRAD51-NTD3A-LacZ-NLS-V5-His6 and PRAD51-NTD3D-LacZ-NLS-V5-His6. All four of these vectors were transformed into an SK1 yeast strain. The transformants were vegetatively propagated to reach logarithmic phase and then harvested for denatured lysate preparation. Immunoblotting analyses using the antisera against phosphorylated Rad51-S12Q indicated that Tel1ATM and Mec1ATR can phosphorylate NTDWT-LacZ-NLS-V5-His6, but not NTD3A-LacZ-NLS-V5-His6 or NTD3D-LacZ-NLS-V5-His6 (Figure 8A). All three of these fusion proteins could be recognized in immunoblottings by the anti-Rad51 antiserum, and the order of steady-state protein levels was NTDWT-LacZ-NLS-V5-His6 ≈ NTD3D-LacZ-NLS-V5-His6 > NTD3A-LacZ-NLS-V5-His6 (Figure 8A), all of which showed apparently higher protein levels than for LacZ-NLS-V5-His6 (Figure 8B). A similar hierarchy of protein levels was also observed when Rad51 variants were fused to exogenous proteins such as LexA and were expressed from high-copy number vectors (Supplementary Figure S2B). Thus, NTDWT, NTD3A, and NTD3D all possess the capability to enhance expression of their fusion partner, LacZ-NLS-V5-His6. Notably, NTD3A-LacZ-NLS-V5-His6 was more labile than NTDWT-LacZ-NLS-V5-His6 and NTD3D-LacZ-NLS-V5-His6, given that there were more degraded NTD3A-LacZ-NLS-V5-His6 products in the immunoblot (Figure 8B, indicated by the black arrowhead). We also determined the β-galactosidase activities of these four fusion proteins (see ‘Materials and Methods’ section). The hierarchy (and normalized relative activity level) was NTD3D-LacZ-NLS-V5-His6 (13.2-fold) ≈ NTDWT-LacZ-NLS-V5-His6 (13.2-fold) > NTD3A-LacZ-NLS-V5-His6 (9.6-fold) >> LacZ-NLS-V5-His6 (Figure 8C). The observation that NTDWT and NTD3D exhibited almost the same ability to facilitate protein levels of their fusion partners may imply that most (if not all) of the NTDWT-LacZ-NLS-V5-His6 fusion proteins are phosphorylated dependently on Tel1ATM and Mec1ATR.
Figure 8.
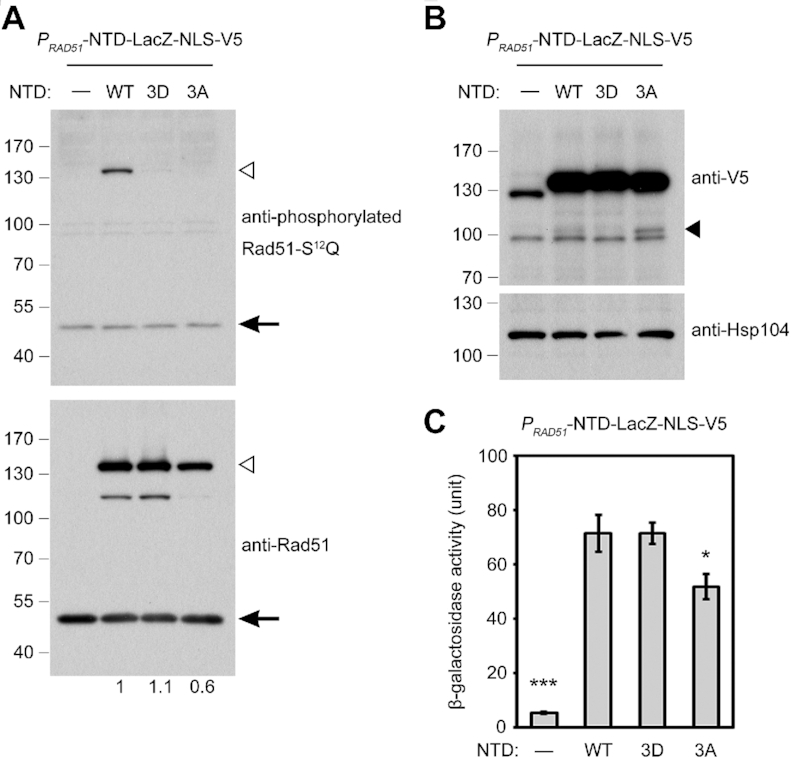
Rad51-NTD is a tunable ‘nanny’ peptide. The wild-type SK1 haploid strain was transformed with an indicated yeast expression vector, i.e. PRAD51-LacZ-NLS-V5 (mock), PRAD51-NTDWT-LacZ-NLS-V5, PRAD51-NTD3A-LacZ-NLS-V5 or PRAD51-NTD3D-LacZ-NLS-V5. (A, B) Immunoblotting. Total cell lysates were prepared from mitotic cells undergoing exponential growth for visualization by immunoblotting with the corresponding antisera and quantitative yeast LacZ assays in (C). (A) NTD-LacZ-NLS-V5 fusion proteins (∼128 kDa) are indicated by white arrowheads. Endogenous Rad51 (∼43 kDa) is indicated by black arrows. Numbers below the anti-Rad51 immunoblot indicate fold-changes of NTD-LacZ-NLS-V5 fusion protein levels normalized to endogenous Rad51 protein levels in the same blot. (B) Immunoblotting using anti-V5 antiserum. Presumptively degraded products are indicated by black arrowheads. Hsp104 was used as a loading control. (C) Quantitative yeast LacZ assays were performed as described previously (41). Error bars indicate standard deviation between experiments. (n = 3). Asterisks indicate significant differences when compared to NTDWT, with P values calculated using a two-tailed t-test (*P value < 0.05 and ***P value < 0.001).
Together with our results showing that the order of steady-state protein levels of Rad51 variants is Rad51 > Rad51-3A >> Rad51-ΔN in both vegetative and meiotic cells, we suggest that our findings reveal that Rad51-NTD has at least two independent functions for maintaining high steady-state levels of Rad51 in vivo. First, Rad51-NTD acts autonomously to promote expression (and/or folding) of its fusion partner. Second, Rad51-NTD is also an SCD that is phosphorylated by Tel1ATM and Mec1ATR to enhance Rad51 protein stability via antagonizing proteasomal degradation.
Phosphorylation on serine residues of SQ motifs but not other serines or threonines in Rad51-NTD is critical for maintaining steady-state protein levels of Rad51
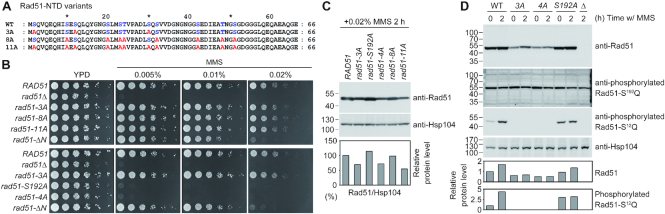
We propose that Tel1ATM- and Mec1ATR-dependent phosphorylation of Rad51-NTD at S2Q, S12Q and S30Q antagonizes the proteasomal degradation of Rad51. To decipher if other serines and threonines in the Rad51-NTD are also phosphorylated to promote Rad51 stability, we generated an additional rad51 mutant (rad51-8A) in which the three serines of the SQ motifs were retained but all the other serines and threonines in the Rad51-NTD were mutated into alanines (Figure 9A). A further rad51 mutant (rad51-11A) in which all serines and threonines of Rad51-NTD were substituted to alanines was also generated for comparison. Growth of these two new mutant strains on YPD plates containing MMS was indistinguishable from that of WT or the rad51-3A mutant strain and, unlike the rad51-ΔN strain, they did not show apparent hypersensitivity when grown on YPD plates with 0.02% MMS (Figure 9B, upper panel). Immunoblotting experiments to analyze Rad51 protein levels upon MMS treatment revealed that there was a comparable reduction of the steady-state protein levels of Rad51-11A (∼45% lower) to that of Rad51-3A (∼30% lower), whereas the rad51-8A mutant strain maintained similar levels of Rad51 protein to WT (Figure 9C). Thus, phosphorylation of the three SQ motifs in Rad51-NTD is critical for antagonizing the proteasomal degradation of Rad51, whereas phosphorylation of the other serines or threonines in Rad51-NTD is irrelevant to that function.
Figure 9.
Characterization of the functions of other probable phosphorylation targets in Rad51-NTD and the fourth SQ motif. (A) Protein sequence alignments showing Rad51-NTD variants with serines and threonines (blue) replaced by alanines (red), as indicated. (B) Spot assay showing five-fold serial dilutions of haploid yeast strains, performed as described in Figure 2. (C, D) Total cell lysates were prepared from mitotic cells under MMS (0.02%) treatment and analyzed using immunoblots, as described in Figure 3. Protein levels were quantified and normalized to Hsp104 levels. Protein levels from WT lysates of MMS-treated cultures were a standard for comparison in (C). WT lysates without MMS treatment were used as a standard for comparison in (D).
Comparative analysis of Rad51-NTD phosphorylation and Rad51-S192Q phosphorylation
It was reported previously that Rad51-S192Q, the fourth SQ motif in Rad51, might be phosphorylated by Mec1ATR (44). Those authors generated a phospho-specific Rad51-S192Q antibody to validate S192Q phosphorylation on bacterially-purified Rad51 proteins subjected to DNA-PK (DNA-dependent protein kinase)-catalyzed phosphorylation in vitro, given that DNA-PK is a phosphatidylinositol 3-kinase-related protein kinase enzyme with a similar SQ target consensus sequence to that of Mec1ATR and Tel1ATM (52). Phosphorylation of S192Q on purified yeast-expressed epitope-tagged Rad51 proteins (i.e. Rad51-TAP and Rad51-Myc) were also analyzed in that study using that antibody (44). The authors proposed that S192Q phosphorylation could influence homologous recombination by mimicking the ATP- or ADP-bound form of Rad51, which can form a catalytically inactive multimer (44). Since both the phosphorylation-defective mutation (rad51-S192A) and the phosphomimetic mutation (rad51-S192E) impaired Rad51 function in vivo (44), the exact role of Rad51-S192Q phosphorylation had remained unclear.
We were unable to generate antisera specific for phosphorylated Rad51-S192Q protein (see ‘Materials and Methods’ section) to compare the biological function(s) of Rad51-NTD phosphorylation and Rad51-S192Q phosphorylation. Instead, we generated two additional mutants, i.e. rad51-S192A and rad51-4A. In rad51-4A, all four SQ motifs (S2Q, S12Q, S30Q, and S192Q, highlighted in red in Figure 1B) of endogenous Rad51 were mutated to AQ. We first confirmed that rad51-S192A and rad51-4A, like rad51Δ (but not WT or rad51-3A), exhibited hypersensitivity to 0.005% MMS during vegetative growth (Figure 9B, lower panel). Consistent with the findings of the previous report (44), our rad51-S192A strain was indeed defective in repairing DSBs. We obtained the phospho-specific Rad51-S192Q antibody (44) as a kind gift from Stephen Jackson (University of Cambridge). However, we could not reproduce the specificity in immunoblotting experiments when examining our five yeast strains, namely RAD51, rad51-3A, rad51-4A, rad51-S192A and rad51Δ (Figure 9D), perhaps due to differences in strain background or experimental procedures. Next, we showed by immunoblotting with anti-Rad51 antisera that the state-steady protein levels of Rad51-S192A was similar to those of WT Rad51, but higher than those of Rad51-3A or Rad51-4A, during DDR in vegetative growth (Figure 9D). Using antisera specific for phosphorylated Rad51-S12Q, we also found that WT Rad51 and Rad51-S192A, but not Rad51-3A or Rad51-4A, underwent MMS-induced S12Q hyperphosphorylation (Figure 9D). Intriguingly, the rad51-S192A mutant showed a higher level of S12Q phosphorylation without MMS treatment, suggesting that DDR might be induced in this mutant even without exposure to DNA damaging agents.
We propose that phosphorylation of all three SQ motifs in Rad51-NTD plays an important role in promoting Rad51 protein stability and that this function can be recapitulated by phosphomimetic mutations. In contrast, the SQ motif (S192Q) adjacent to the ATPase domain is required for the catalytic activity of Rad51, but is not related to Rad51 protein abundance control. Since the phosphomimetic mutation rad51-S192E has been reported defective in Rad51 recombinase activity but not in protein folding and/or multimerization (44), further investigations are needed to decipher why negative charges on these four SQ motifs of Rad51 by phosphomimetic mutations render such distinct impacts.
DISCUSSION
The RAD51 gene of S. cerevisiae was first cloned in 1992 (6). Many investigations have since been conducted to establish its molecular functions in various biological processes (1,2). In this report, we show that three SQ motifs (S2Q, S12Q and S30Q) in Rad51-NTD are phosphorylated in a Tel1ATM- and Mec1ATR-dependent manner during both vegetative growth and meiosis. Thus, the function of Rad51 is directly regulated by the DNA damage checkpoint in S. cerevisiae. It was proposed previously that S. cerevisiae Rad51 was transiently phosphorylated at the S192Q motif primarily mediated by Mec1ATR (44) given that the phosphomimetic mutation of rad51-S192E could not functionally substitute this postulated phosphorylation. Thus, the biological functions involving the putative negatively charged S192Q motif remains obscure.
Here, we demonstrate that the function of Mec1ATR/Tel1ATM−dependent Rad51-NTD phosphorylation is to enhance Rad51 protein stability by antagonizing the proteasomal pathway. The half-life (t1/2) of the phosphorylation-defective Rad51-3A mutant is ≤30 min, whereas t1/2 of phosphorylated WT Rad51 or phosphomimemtic Rad51-3D is ≥180 min (Figure 6E). Based on our cycloheximide-shutoff experiments (Figure 6), which show that WT Rad51 at 5-h meiosis is as stable as Rad51-3D, we infer that most (if not all) of the Rad51 protein is likely phosphorylated in response to MMS-induced or meiotic DSBs in vivo. Basal levels of Rad51-NTD phosphorylation also occur in the absence of exogenous DNA lesions or meiotic DSBs and are likely related to spontaneous damages in S phase (Supplementary Figure S5). This unique property also readily explains why Rad51 phosphorylation has distinct impacts on Rad51-mediated DNA repair during vegetative growth and meiosis. Previous studies in S. cerevisiae have reported mitotic S phase durations of 20–30 min (53,54). During synchronous SK1 meiosis, S phase lasts 65–80 min (55,56), and Spo11-induced DSBs take place at 1.5–3.5 h (57). Subsequently, the chromosomal foci of two RecA-like recombinases were shown to appear and disappear within a single peak (2.5–5 h), with maximum abundance at 3 h (43). Accordingly, Rad51-NTD phosphorylation is dispensable for DNA damage repair during vegetative growth. In contrast, phosphorylation-defective mutant Rad51-3A proteins in the dmc1Δ hed1Δ mutant are not stable enough to support such a long period of Spo11-induced DSB repair during meiosis (Table 1). The Rad51-NTD phosphorylation pathway we describe here supports the notion that Mec1ATR alone can perform most of the functions of Tel1ATM and Mec1ATR together in S. cerevisiae (58,59), and that Mec1ATR has an essential function in regulating protein homeostasis (38,39). It will be important to further examine if genetic interaction partners of Mec1ATR that are involved in protein homeostasis (38,39) are also responsible for regulating Rad51 homeostasis, thereby affecting the outcomes of Rad51- and Dmc1-mediated meiotic recombination.
The second important finding of this study is that Rad51-NTD possesses an autonomous ‘nanny’ function and promotes expression and/or folding of its C-terminal fusion partners (e.g. Rad51-ΔN and LacZ). Although the steady-state levels of Rad51-ΔN are ≤3% those of wild-type Rad51 in both vegetative and meiotic cells, the rad51-ΔN allele only affected DSB repair when vegetative cells were exposed to high concentrations of MMS or in the dmc1Δ hed1Δ meiotic cells. Moreover, the rad51-ΔN diploid cells could still generate ∼50% viable spores (Table 1). We suggest that in order to tolerate scenarios with excessive DSBs, Rad51-NTD evolved specifically to maintain superfluous amounts of Rad51 in vivo. For example, it takes a long time (≥3 h) to repair genome-wide DSBs induced by Spo11 in S. cerevisiae meiosis, so high steady-state levels of Rad51 are required over a long time-period.
Our results have also revealed that the sml1Δ allele is a suppressor of deficiencies elicited by rad51-ΔN in terms of the less growth phenotype induced by DNA lesions during vegetative growth (Figure 2A) and the low spore viability phenotype in meiosis (Table 1). Therefore, with regard to sml1Δ repression, rad51-ΔN is phenotypically similar to mec1-kd. Further investigations will reveal whether and how the absence of Sml1 proteins in sml1Δ (or an increase of dNTP abundance) ameliorates the deleterious effects caused by rad51-ΔN. Since the steady-state levels of Rad51-ΔN in rad51-ΔN cells are not significantly different from those in rad51-ΔN sml1Δ cells during vegetative growth (Figure 3B), we reason that Rad51 might be an abundant target of Mec1ATR and Tel1ATM in S. cerevisiae in the sense that deficiency of either Rad51-NTD or Mec1 would result in a similar outcome in terms of suppression by sml1Δ. Along with this hypothesis, it was recently reported (60) (Saccharomyces Genome Database: https://www.yeastgenome.org) that the median abundance of Rad51 (6070 ± 2766 molecules/cell) in vegetative cells is higher than those of other known Mec1ATR and Tel1ATM target proteins, e.g. Rad9 (1279 ± 701 molecules/cell), Rad53 (1533 ± 520 molecules/cell) and Sae2 (1084 ± 93 molecules/cell). Whether these proteins involved in the DDR network are subjected to the same degree of protein quality control by Mec1ATR and Tel1ATM as Rad51 remains to be characterized.
In conclusion, due to the presence of the Rad51-NTD, S. cerevisiae can ensure sufficient levels of Rad51 to deal with extreme physiological conditions such as excessive DNA damage or long periods of DNA repair. This intriguing characteristic might explain two unique properties found in this important model organism: (1) HDRR is the dominant repair mode for DSBs in S. cerevisiae; and (2) inhibition of Rad51 by Hed1 ensures Dmc1-mediated HDRR outperforms Rad51-mediated HDRR during meiosis. This latter function is particularly critical for hybrid meiosis of polymorphic homologous chromosomes (10), because Dmc1 is superior to Rad51 in tolerating mismatched sequences during their strand exchange reaction (11,12). Accordingly, our results also explain why both Rad51-NTD and Hed1 (a meiosis-specific inhibitor of Rad51) are specific to the genus Saccharomyces. Since the duration of the meiotic program is, in general, longer than that of mitotic progression (61), it would be intriguing to investigate if Rad51 proteins in other sexual eukaryotes are regulated differentially during mitosis and meiosis and if novel meiosis-specific accessory factor(s) have evolved to promote or retain high steady-state Rad51 protein levels upon encountering robust DNA damage.
Inspired by the findings of this study, we recently showed that the SCDs of several other yeast DDR proteins [e.g. Rad53-SCD1 (amino acid residues 1–29), Hop1-SCD (amino acid residues 258–324) and Sml1-SCD (amino acid residues 1–50, personal communications with Rita Cha, Bangor University)] possess autonomous and exchangeable activities to enhance high-level protein expression when they are artificially designed as NH2-terminal fusion tags of LacZ, GFP, etc. We also discovered an interesting correlation between relative LacZ activities and the overall S/T/Q percentages in the total amino acid content of these intrinsically disordered regions (IDRs) given that low sequence complexity and high S/T/Q content are characteristic features of IDRs in proteins (62). To attain optimal LacZ activity of >8-fold, overall S/T/Q content of IDRs should range from 30% to 40% (unpublished results). Intriguingly, the enrichment of S/T-Q motifs in SCDs means that they exhibit low sequence complexity (19). Taken together, our results have not only revealed that SCDs can promote protein expression but also supported that Mec1ATR could regulate protein homeostasis.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Douglas Bishop (University of Chicago), Stephen Jackson (University of Cambridge) and Chung Wang (Academia Sinica) for providing antisera used in this study, Valentin Börner (Cleveland State University) for technical advice for physical analysis experiments, Rita Cha (Bangor University) for discussion of her unpublished results on Sml1 in regulating protein homeostasis, and John O′Brien for English editing.
Author contributions: T.T.W. and C.N.C. performed the experiments and analyzed the data. T.T.W. and T.F.W. conceived and designed the experiments and wrote the paper. M.H. and A.S. provided antisera and technical advice for cytological experiments. All of the authors read and approved the manuscript.
Contributor Information
Tai-Ting Woo, Institute of Molecular Biology, Academia Sinica, Taipei 115, Taiwan.
Chi-Ning Chuang, Institute of Molecular Biology, Academia Sinica, Taipei 115, Taiwan.
Mika Higashide, Laboratory of Genome-Chromosome Functions, Institute for Protein Research, Osaka University, 3-2 Yamadaoka, Suita, Japan.
Akira Shinohara, Laboratory of Genome-Chromosome Functions, Institute for Protein Research, Osaka University, 3-2 Yamadaoka, Suita, Japan.
Ting-Fang Wang, Institute of Molecular Biology, Academia Sinica, Taipei 115, Taiwan.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Academia Sinica, Taiwan [AS-105-TP-B07, AS108-TP-B07 to T.F.W.]; Ministry of Science and Technology, Taiwan, Republic of China [MOST163-2311-B-001-016-MY3 to T.F.W.]; T.T.W. was supported by postdoctoral fellowships from Academia Sinica and MOST. Funding for open access charges: Academia Sinica [AS108-TP-B07 to T.F.W.].
Conflict of interest statement. None declared.
REFERENCES
- 1. Daley J.M., Gaines W.A., Kwon Y., Sung P.. Regulation of DNA pairing in homologous recombination. Cold Spring Harb. Perspect. Biol. 2014; 6:a017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kowalczykowski S.C. An overview of the molecular mechanisms of recombinational DNA repair. Cold Spring Harb. Perspect. Biol. 2015; 7:a016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zickler D., Kleckner N.. Meiotic chromosomes: integrating structure and function. Annu. Rev. Genet. 1999; 33:603–754. [DOI] [PubMed] [Google Scholar]
- 4. Hunter N. Meiotic recombination: the essence of heredity. Cold Spring Harb. Perspect. Biol. 2015; 7:a016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown M.S., Bishop D.K.. DNA strand exchange and RecA homologs in meiosis. Cold Spring Harb. Perspect. Biol. 2014; 7:a016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shinohara A., Ogawa H., Ogawa T.. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992; 69:457–470. [DOI] [PubMed] [Google Scholar]
- 7. Bishop D.K., Park D., Xu L., Kleckner N.. DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992; 69:439–456. [DOI] [PubMed] [Google Scholar]
- 8. Tsubouchi H., Roeder G.S.. Budding yeast Hed1 down-regulates the mitotic recombination machinery when meiotic recombination is impaired. Genes Dev. 2006; 20:1766–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cloud V., Chan Y.L., Grubb J., Budke B., Bishop D.K.. Rad51 is an accessory factor for Dmc1-mediated joint molecule formation during meiosis. Science. 2012; 337:1222–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Callender T.L., Laureau R., Wan L., Chen X., Sandhu R., Laljee S., Zhou S., Suhandynata R.T., Prugar E., Gaines W.A. et al.. Mek1 down regulates Rad51 activity during yeast meiosis by phosphorylation of Hed1. PLoS Genet. 2016; 12:e1006226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee J.Y., Terakawa T., Qi Z., Steinfeld J.B., Redding S., Kwon Y., Gaines W.A., Zhao W., Sung P., Greene E.C.. DNA RECOMBINATION. Base triplet stepping by the Rad51/RecA family of recombinases. Science. 2015; 349:977–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qi Z., Redding S., Lee J.Y., Gibb B., Kwon Y., Niu H., Gaines W.A., Sung P., Greene E.C.. DNA sequence alignment by microhomology sampling during homologous recombination. Cell. 2015; 160:856–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harper J.W., Elledge S.J.. The DNA damage response: ten years after. Mol. Cell. 2007; 28:739–745. [DOI] [PubMed] [Google Scholar]
- 14. Giglia-Mari G., Zotter A., Vermeulen W.. DNA damage response. Cold Spring Harb. Perspect. Biol. 2011; 3:a000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shiloh Y. The ATM-mediated DNA-damage response: taking shape. Trends Biochem. Sci. 2006; 31:402–410. [DOI] [PubMed] [Google Scholar]
- 16. Cimprich K.A., Cortez D.. ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 2008; 9:616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Craven R.J., Greenwell P.W., Dominska M., Petes T.D.. Regulation of genome stability by TEL1 and MEC1, yeast homologs of the mammalian ATM and ATR genes. Genetics. 2002; 161:493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim S.T., Lim D.S., Canman C.E., Kastan M.B.. Substrate specificities and identification of putative substrates of ATM kinase family members. J. Biol. Chem. 1999; 274:37538–37543. [DOI] [PubMed] [Google Scholar]
- 19. Traven A., Heierhorst J.. SQ/TQ cluster domains: concentrated ATM/ATR kinase phosphorylation site regions in DNA-damage-response proteins. Bioessays. 2005; 27:397–407. [DOI] [PubMed] [Google Scholar]
- 20. Cheung H.C., San Lucas F.A., Hicks S., Chang K., Bertuch A.A., Ribes-Zamora A.. An S/T-Q cluster domain census unveils new putative targets under Tel1/Mec1 control. BMC Genomics. 2012; 13:664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schwartz M.F., Duong J.K., Sun Z., Morrow J.S., Pradhan D., Stern D.F.. Rad9 phosphorylation sites couple Rad53 to the Saccharomyces cerevisiae DNA damage checkpoint. Mol. Cell. 2002; 9:1055–1065. [DOI] [PubMed] [Google Scholar]
- 22. Lee S.J., Schwartz M.F., Duong J.K., Stern D.F.. Rad53 phosphorylation site clusters are important for Rad53 regulation and signaling. Mol. Cell. Biol. 2003; 23:6300–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen S.H., Smolka M.B., Zhou H.. Mechanism of Dun1 activation by Rad53 phosphorylation in Saccharomyces cerevisiae. J. Biol. Chem. 2007; 282:986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carballo J.A., Johnson A.L., Sedgwick S.G., Cha R.S.. Phosphorylation of the axial element protein Hop1 by Mec1/Tel1 ensures meiotic interhomolog recombination. Cell. 2008; 132:758–770. [DOI] [PubMed] [Google Scholar]
- 25. Chuang C.N., Cheng Y.H., Wang T.F.. Mek1 stabilizes Hop1-Thr318 phosphorylation to promote interhomolog recombination and checkpoint responses during yeast meiosis. Nucleic Acids Res. 2012; 40:11416–11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herruzo E., Ontoso D., Gonzalez-Arranz S., Cavero S., Lechuga A., San-Segundo P.A.. The Pch2 AAA+ ATPase promotes phosphorylation of the Hop1 meiotic checkpoint adaptor in response to synaptonemal complex defects. Nucleic Acids Res. 2016; 44:7722–7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Busygina V., Sehorn M.G., Shi I.Y., Tsubouchi H., Roeder G.S., Sung P.. Hed1 regulates Rad51-mediated recombination via a novel mechanism. Genes Dev. 2008; 22:786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Niu H., Wan L., Busygina V., Kwon Y., Allen J.A., Li X., Kunz R.C., Kubota K., Wang B., Sung P. et al.. Regulation of meiotic recombination via Mek1-mediated Rad54 phosphorylation. Mol. Cell. 2009; 36:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carballo J.A., Cha R.S.. Meiotic roles of Mec1, a budding yeast homolog of mammalian ATR/ATM. Chromosome Res. 2007; 15:539–550. [DOI] [PubMed] [Google Scholar]
- 30. Falk J.E., Chan A.C., Hoffmann E., Hochwagen A.. A Mec1- and PP4-dependent checkpoint couples centromere pairing to meiotic recombination. Dev. Cell. 2010; 19:599–611. [DOI] [PubMed] [Google Scholar]
- 31. Carballo J.A., Panizza S., Serrentino M.E., Johnson A.L., Geymonat M., Borde V., Klein F., Cha R.S.. Budding yeast ATM/ATR control meiotic double-strand break (DSB) levels by down-regulating Rec114, an essential component of the DSB-machinery. PLos Genet. 2013; 9:e1003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cartagena-Lirola H., Guerini I., Viscardi V., Lucchini G., Longhese M.P.. Budding yeast Sae2 is an in vivo target of the Mec1 and Tel1 checkpoint kinases during meiosis. Cell Cycle. 2006; 5:1549–1559. [DOI] [PubMed] [Google Scholar]
- 33. Brush G.S., Clifford D.M., Marinco S.M., Bartrand A.J.. Replication protein A is sequentially phosphorylated during meiosis. Nucleic Acids Res. 2001; 29:4808–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng Y.H., Chuang C.N., Shen H.J., Lin F.M., Wang T.F.. Three distinct modes of Mec1/ATR and Tel1/ATM activation illustrate differential checkpoint targeting during budding yeast early meiosis. Mol. Cell. Biol. 2013; 33:3365–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lo Y.H., Chuang C.N., Wang T.F.. Pch2 prevents Mec1/Tel1-mediated Hop1 phosphorylation occurring independently of Red1 in budding yeast meiosis. PLoS One. 2014; 9:e85687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Farmer S., Hong E.J., Leung W.K., Argunhan B., Terentyev Y., Humphryes N., Toyoizumi H., Tsubouchi H.. Budding yeast pch2, a widely conserved meiotic protein, is involved in the initiation of meiotic recombination. PLoS One. 2012; 7:e39724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Joshi N., Brown M.S., Bishop D.K., Borner G.V.. Gradual implementation of the meiotic recombination program via checkpoint pathways controlled by global DSB levels. Mol. Cell. 2015; 57:797–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Corcoles-Saez I., Dong K., Johnson A.L., Waskiewicz E., Costanzo M., Boone C., Cha R.S.. Essential function of Mec1, the budding yeast ATM/ATR checkpoint-response kinase, in protein homeostasis. Dev. Cell. 2018; 46:495–503. [DOI] [PubMed] [Google Scholar]
- 39. Corcoles-Saez I., Dong K., Cha R.S.. Versatility of the Mec1(ATM/ATR) signaling network in mediating resistance to replication, genotoxic, and proteotoxic stresses. Curr. Genet. 2019; 65:657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheng C.H., Lo Y.H., Liang S.S., Ti S.C., Lin F.M., Yeh C.H., Huang H.Y., Wang T.F.. SUMO modifications control assembly of synaptonemal complex and polycomplex in meiosis of Saccharomyces cerevisiae. Genes Dev. 2006; 20:2067–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin F.M., Lai Y.J., Shen H.J., Cheng Y.H., Wang T.F.. Yeast axial-element protein, Red1, binds SUMO chains to promote meiotic interhomologue recombination and chromosome synapsis. EMBO J. 2010; 29:586–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ahuja J.S., Borner G.V.. Analysis of meiotic recombination intermediates by two-dimensional gel electrophoresis. Methods Mol. Biol. 2011; 745:99–116. [DOI] [PubMed] [Google Scholar]
- 43. Shinohara M., Gasior S.L., Bishop D.K., Shinohara A.. Tid1/Rdh54 promotes colocalization of Rad51 and Dmc1 during meiotic recombination. Proc. Natl. Acad. Sci. U.S.A. 2000; 97:10814–10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Flott S., Kwon Y., Pigli Y.Z., Rice P.A., Sung P., Jackson S.P.. Regulation of Rad51 function by phosphorylation. EMBO Rep. 2011; 12:833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chabes A., Domkin V., Thelander L.. Yeast Sml1, a protein inhibitor of ribonucleotide reductase. J. Biol. Chem. 1999; 274:36679–36683. [DOI] [PubMed] [Google Scholar]
- 46. Zhao X., Muller E.G., Rothstein R.. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol. Cell. 1998; 2:329–340. [DOI] [PubMed] [Google Scholar]
- 47. Martini E., Diaz R.L., Hunter N., Keeney S.. Crossover homeostasis in yeast meiosis. Cell. 2006; 126:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lao J.P., Cloud V., Huang C.C., Grubb J., Thacker D., Lee C.Y., Dresser M.E., Hunter N., Bishop D.K.. Meiotic crossover control by concerted action of Rad51-Dmc1 in homolog template bias and robust homeostatic regulation. PLoS Genet. 2013; 9:e1003978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hunter N., Kleckner N.. The single-end invasion: an asymmetric intermediate at the double-strand break to double-Holliday junction transition of meiotic recombination. Cell. 2001; 106:59–70. [DOI] [PubMed] [Google Scholar]
- 50. Oh S.D., Jessop L., Lao J.P., Allers T., Lichten M., Hunter N.. Stabilization and electrophoretic analysis of meiotic recombination intermediates in Saccharomyces cerevisiae. Methods Mol. Biol. 2009; 557:209–234. [DOI] [PubMed] [Google Scholar]
- 51. Le Tallec B., Barrault M.B., Courbeyrette R., Guerois R., Marsolier-Kergoat M.C., Peyroche A.. 20S proteasome assembly is orchestrated by two distinct pairs of chaperones in yeast and in mammals. Mol. Cell. 2007; 27:660–674. [DOI] [PubMed] [Google Scholar]
- 52. Smith G.C., Jackson S.P.. The DNA-dependent protein kinase. Genes Dev. 1999; 13:916–934. [DOI] [PubMed] [Google Scholar]
- 53. Slater M.L., Sharrow S.O., Gart J.J.. Cell cycle of Saccharomyces cerevisiae in populations growing at different rates. Proc. Natl. Acad. Sci. U.S.A. 1977; 74:3850–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brewer B.J., Chlebowicz-Sledziewska E., Fangman W.L.. Cell cycle phases in the unequal mother/daughter cell cycles of Saccharomyces cerevisiae. Mol. Cell. Biol. 1984; 4:2529–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Padmore R., Cao L., Kleckner N.. Temporal comparison of recombination and synaptonemal complex formation during meiosis in S. cerevisiae. Cell. 1991; 66:1239–1256. [DOI] [PubMed] [Google Scholar]
- 56. Cha R.S., Weiner B.M., Keeney S., Dekker J., Kleckner N.. Progression of meiotic DNA replication is modulated by interchromosomal interaction proteins, negatively by Spo11p and positively by Rec8p. Genes Dev. 2000; 14:493–503. [PMC free article] [PubMed] [Google Scholar]
- 57. Borner G.V., Cha R.S.. Analysis of yeast sporulation efficiency, spore viability, and meiotic recombination on solid medium. Cold Spring Harb. Protoc. 2015; 2015:1003–1008. [DOI] [PubMed] [Google Scholar]
- 58. Weinert T.A., Kiser G.L., Hartwell L.H.. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev. 1994; 8:652–665. [DOI] [PubMed] [Google Scholar]
- 59. Mallory J.C., Petes T.D.. Protein kinase activity of Tel1p and Mec1p, two Saccharomyces cerevisiae proteins related to the human ATM protein kinase. Proc. Natl. Acad. Sci. USA. 2000; 97:13749–13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ho B., Baryshnikova A., Brown G.W.. Unification of protein abundance datasets yields a quantitative Saccharomyces cerevisiae proteome. Cell Syst. 2018; 6:192–205. [DOI] [PubMed] [Google Scholar]
- 61. Callan H.G. DNA replication in the chromosomes of eukaryotes. Cold Spring Harb. Symp. Quant. Biol. 1974; 38:195–203. [DOI] [PubMed] [Google Scholar]
- 62. Macossay-Castillo M., Marvelli G., Guharoy M., Jain A., Kihara D., Tompa P., Wodak S.J.. The balancing act of intrinsically disordered proteins: enabling functional diversity while minimizing promiscuity. J. Mol. Biol. 2019; 431:1650–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Katoh K., Standley D.M.. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013; 30:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Beranger F., Aresta S., de Gunzburg J., Camonis J.. Getting more from the two-hybrid system: N-terminal fusions to LexA are efficient and sensitive baits for two-hybrid studies. Nucleic Acids Res. 1997; 25:2035–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.