Abstract
Background
Propofol has been reported to be related to the migration, invasion, and epithelial‐mesenchymal transition (EMT) of esophageal cancer (EC) cells. However, the detailed mechanism has not yet been fully reported. The purpose of this research was to elucidate the function of long non‐coding RNA TMPO antisense RNA 1 (lncRNA TMPO‐AS1) and microRNA‐498 (miR‐498) in propofol‐regulated EC.
Methods
Transwell assay was performed to assess cell migratory and invasive abilities. Western blot assay was employed to determine the levels of EMT markers and hypoxia inducible factor‐1 (HIF‐1α). Quantitative real‐time polymerase chain reaction (qRT‐PCR) was carried out to detect the levels of TMPO‐AS1 and miR‐498. Moreover, the interaction between TMPO‐AS1 and miR‐498 was predicted by starBase, and then confirmed by the dual‐luciferase reporter assay and RNA immunoprecipitation (RIP) assay.
Results
Propofol suppressed hypoxia‐induced EC cell migration, invasion, and EMT. Both TMPO‐AS1 overexpression and miR‐498 knockdown weakened the effect of propofol on hypoxia‐induced EC cell progression. Interestingly, TMPO‐AS1 targeted miR‐498 and suppressed miR‐498 expression. TMPO‐AS1 regulated EC cell progression via downregulating miR‐498 expression.
Conclusions
Collectively, our findings demonstrated that propofol inhibited hypoxia‐induced EC cell mobility through modulation of the TMPO‐AS1/miR‐498 axis, providing a theoretical basis for the treatment of EC.
Keywords: Esophageal cancer, hypoxia, miR‐498, propofol, TMPO‐AS1
Introduction
Esophageal cancer (EC), a common cancer, is the leading cause of tumor‐related death with a high incidence worldwide. 1 , 2 According to the statistics in 2016, there were approximately 16 910 newly diagnosed cases and 15 910 deaths of EC patients in the USA. Moreover, the survival rate of EC patients is low, and the recurrence rate has been reported to be high after neoadjuvant chemo‐radiotherapy. 3 Therefore, it is essential to explore new methods for the treatment of EC patients.
Hypoxia is ordinary in the microenvironment of cancers and is related to the mobility of cancer cells. 4 , 5 Hypoxia‐inducible factor‐1α (HIF‐1α), a subunit of HIF, is identified as a transcription factor that is activated by hypoxia and reported to be involved in the migration and epithelial‐mesenchymal transition (EMT) of tumors. 4 , 6 Propofol, a common sedation and hypnotic agent, is universally applied in anesthesia of patients. 7 Previous evidence has suggested that propofol represses the effect of hypoxia on cell growth through suppressing HIF‐1α in prostate cancer. 8 However, the function of propofol in hypoxia‐regulated EC cell progression is unclear.
Long non‐coding RNAs (lncRNAs), more than 200 nucleotides in length, are a new group of transcripts that have been shown to exert function in a variety of aspects, such as transcription, post‐transcription, and epigenetic regulation. 9 , 10 TMPO antisense RNA 1 (TMPO‐AS1), identified as an lncRNA, regulates the development of many human cancers. For example, Yang et al. suggested that TMPO‐AS1 accelerated the proliferation and mobility, and promoted apoptosis of cervical cancer cells via regulating miR‐577. 11 Similarly, TMPO‐AS1 modulated this cell progression in prostate cancer. 12 However, the role and functional mechanism of TMPO‐AS1 in EC have not been studied.
MicroRNAs (miRNAs) are a family of small non‐coding RNAs, on average 22 nucleotides in length, that regulate the levels of downstream genes through binding to 3′‐untranslated region (UTR) of mRNA. 13 , 14 Present studies have demonstrated that miRNA acts as an oncogene or tumor‐suppressor to modulate cancer cell proliferation, mobility, apoptosis, or autophagy. 13 , 15 , 16 MiR‐498, identified as an miRNA, has been shown to be lowly expressed in a lot of cancers, including ovarian, 17 colorectal, 18 and gastric 19 cancers. Moreover, miR‐498 level has been shown to be decreased and miR‐498 upregulation suppressed cell development in esophageal squamous cell carcinoma (ESCC). 20 Therefore, it is essential to further analyze the role of miR‐498 in EC.
Here, we explored the effect of propofol on hypoxia‐induced cell mobility. Furthermore, the roles of TMPO‐AS1 and miR‐498 in propofol‐regulated EC cell progression were investigated. In addition, we analyzed the relationship between TMPO‐AS1 and miR‐498.
Methods
Cell culture and treatment
Human Het‐1A cell line and two esophageal cancer lines (EC109 and KYSE70) were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). These cells were kept in Roswell Park Memorial Institute 1640 medium (RPMI; Gibco, Grand Island, NY,USA) containing 10% fetal bovine serum (FBS; Gibco), 1 U/mL penicillin (Sigma‐Aldrich, St. Louis, MO, USA) and 1 mg/mL streptomycin sulfate (Sigma‐Aldrich) at 37°C with 5% CO2. For hypoxia incubation, the cells were incubated in a hypoxic incubator (1% O2 and 5% CO2). In addition, 5 μg/L propofol (Sigma‐Aldrich) was used to treat the cells.
Cell migration and invasion assay
A transwell chamber (BD Biosciences, Bedford, MA, USA) was used to analyze cell mobility based on the recommended manual. Briefly, treated or transfected cells were mixed with medium without FBS, and then transferred into the upper chamber. Medium was introduced into the lower chamber with 10% FBS. After 24 hours of incubation, migratory cells on the lower chamber were analyzed using a microscope. Five fields were randomly selected in every sample. Matrigel was coated on the insert of the chamber for invasion assay, and the steps were in agreement with that in migratory assay.
Western blot assay
Total proteins were isolated from EC109 and KYSE70 cells using lysis buffer (Beyotime Biotechnology, Shanghai, China). Western blot assay was carried out as described elsewhere. 21 The primary antibodies (1:1000) against E‐cadherin (E‐cad), Vimentin (Vim), hypoxia inducible factor‐1 (HIF‐1α), and β‐actin, as well as relative secondary antibodies (1:2000) were provided by Abcam (Cambridge, MA, USA).
Cell transfection
Small interfering RNA against TMPO‐AS1 (si‐TMPO‐AS1), miR‐498 mimic (miR‐498), miR‐498 inhibitor (anti‐miR‐498), and their negative controls (si‐NC, miR‐NC, and anti‐NC) were provided by GenePharma (Shanghai, China). For the overexpression of TMPO‐AS1, its sequence was cloned into the pcDNA3.1 vector (GenePharma). Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) was used to perform the cell transfection assay.
RNA extraction and quantitative real‐time polymerase chain reaction (qRT‐PCR)
Total RNA was extracted from EC109 and KYSE70 cells using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Subsequently, Prime Script RT reagent kit (TaKaRa, Otsu, Japan) was applied to synthesize complementary DNA (cDNA), and SYBR Premix ExTaq kit (TaKaRa) was employed to conduct qRT‐PCR. The quantity of gene was normalized to U6 level or glyceraldehyde 3‐phosphate dehydrogenase GAPDH level with the use of the 2‐ΔΔCt method. The primers used in this research were as follows: TMPO‐AS1 (forward (F), 5'‐CTTTTGTGCGCCGTTTCCT‐3′; Reverse (R), 5'‐CCCAGAGACGAAAGCTGCTT‐3′), miR‐498 (F, 5'‐AAGCCAGGGGGCGTTT‐3′; R, 5'‐GAACATGTCTGCGTATCTC‐3′), U6 (F, 5'‐TGCGGGTGCTCGCTTCGGCAGC‐3′; R, 5'‐CCAGTGCAGGGTCCGAGGT‐3′), and (GAPDH) (F, 5'‐ATCA CTGCCACCCAGAAGAC‐3′; R, 5'‐TTTCTAGACGGCAGGTCAGG‐3′).
Dual‐luciferase reporter assay
The interaction between TMPO‐AS1 and miR‐498 was predicted by starBase. Wild TMPO‐AS1 (TMPO‐AS1 WT) or mutant TMPO‐AS1 (TMPO‐AS1 MUT) was inserted into the pGL3 vector (Promega, Madison, WI, USA). Then, EC109 and KYSE70 cells were cotransfected with TMPO‐AS1 WT or TMPO‐AS1 MUT and miR‐338‐3p or miR‐NC. Finally, the dual‐luciferase system (Promega) was used to examine the luciferase activity.
RNA immunoprecipitation (RIP) assay
The Magna RNA immunoprecipitation kit (Millipore, Bedford, MA, USA) was employed for RIP assay in line with the user's protocol. Briefly, EC109 and KYSE70 cells were lysed using RIP buffer, and then incubated with magnetic beads coated with anti‐Argonaute2 (AGO2) or anti‐immunoglobulin G (IgG) antibodies. Then, RNA enrichment was assessed by qRT‐PCR assay.
Statistical analysis
The data are expressed as the mean ± standard deviation (SD). Statistical analysis was carried out using the Student's t‐test. P‐values < 0.05 were considered statistically significant. Each data group represented three biological replicates × 3 technical replicates, unless otherwise indicated.
Results
Propofol repressed hypoxia‐induced migration, invasion, and EMT of EC cells
First, to investigate whether propofol affected hypoxia‐induced cell mobility, a transwell assay was performed in EC cells (EC109 and KYSE70). The results suggested that cell migration and invasion were significantly promoted by hypoxia, and was then partly rescued due to propofol treatment (Fig 1a and b). Subsequently, we analyzed the levels of two EMT markers (E‐cad and Vim) and HIF‐1α, and found that E‐cad level was downregulated and the levels of Vim and HIF‐1α were upregulated by hypoxia, whereas these actions were reversed by propofol treatment (Fig 1c). Therefore, propofol suppressed hypoxia‐induced EC cell mobility via inhibiting HIF‐1α expression.
Figure 1.
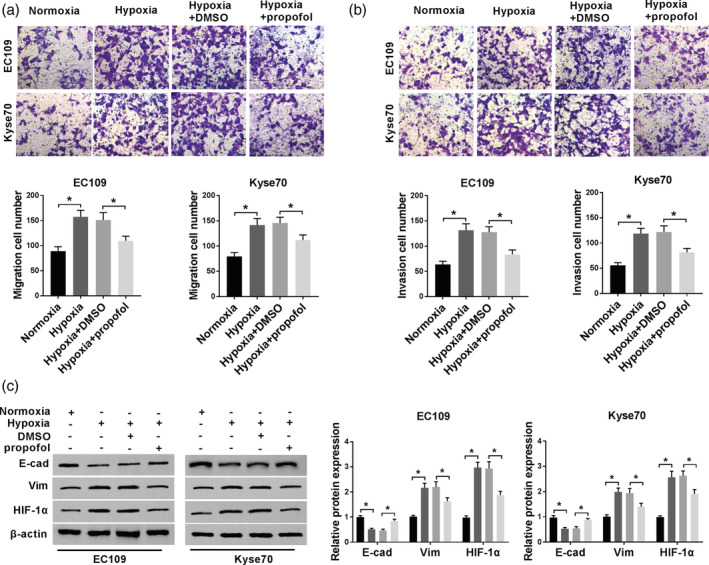
The effect of propofol on hypoxia‐induced EC cells. (a and b) Transwell assay was performed to assess cell migratory and invasive abilities. (c) Western blot assay was carried out to detect the levels of EMT‐markers and HIF‐1α. *P < 0.05. EC109:  , Normoxia;
, Normoxia;  , Hypoxia;
, Hypoxia;  , Hypoxia+DMSO;
, Hypoxia+DMSO;  , Hypoxia+propofol; and Kyse70:
, Hypoxia+propofol; and Kyse70:  , Normoxia;
, Normoxia;  , Hypoxia;
, Hypoxia;  , Hypoxia+DMSO;
, Hypoxia+DMSO;  , Hypoxia+propofol.
, Hypoxia+propofol.
TMPO‐AS1 overexpression weakened the inhibitory effect of propofol on EC cell progression
In this study, we analyzed the level of TMPO‐AS1 in normal cells (Het‐1A) and EC cells (EC109 and KYSE70), and found that TMPO‐AS1 level was increased in EC cells (Fig 2a). This result indicated that TMPO‐AS1 might be involved in EC development. To explore whether TMPO‐AS1 was related to propofol‐regulated EC cell progression, TMPO‐AS1 expression was determined in EC cells treated with hypoxia or propofol. The results demonstrated that TMPO‐AS1 expression was dramatically upregulated by hypoxia, and then partly rescued due to propofol treatment (Fig 2b and c). TMPO‐AS1 was then transfected into EC cells to increase the level of TMPO‐AS1. The efficiency was confirmed by qRT‐PCR assay (Fig 2d). Next, transwell assay was employed to assess cell mobility. As shown in Fig 2e–h, propofol treatment inhibited the migration and invasion of hypoxia‐treated EC cells, whereas this action was impaired by TMPO‐AS1 overexpression. In addition, we found that TMPO‐AS1 overexpression weakened the effect of propofol on the levels of EMT markers and HIF‐1α (Fig 2i and j). These data revealed that propofol exerted function including migration, invasion, and EMT by downregulating TMPO‐AS1 expression.
Figure 2.
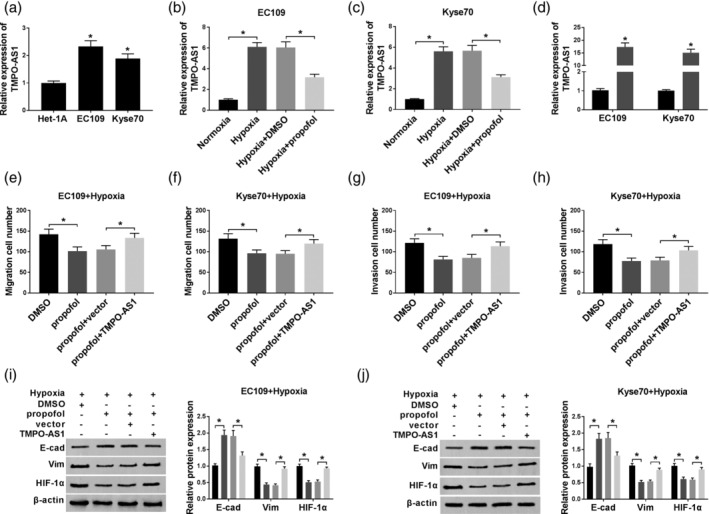
The function of TMPO‐AS1 in propofol‐regulated EC cell progression. (a) The expression of TMPO‐AS1 was determined by qRT‐PCR assay in normal cells (Het‐1A) and EC cells (EC109 and KYSE70). (b and c) TMPO‐AS1 expression was measured in EC109 and KYSE70 cells treated with normoxia, hypoxia, hypoxia + DMSO, or hypoxia + propofol, respectively. (d) TMPO‐AS1 expression was examined in EC109 and KYSE70 cells transfected with vector or TMPO‐AS1.  , vector ;
, vector ;  , TMPO‐AS1. (e–h) Cell migratory and invasive abilities were assessed in hypoxia‐administered EC109 and KYSE70 cells treated with DMSO or propofol and transfected with vector or TMPO‐AS1. (i and j) The levels of EMT markers and HIF‐1α were investigated by western blot assay. *P < 0.05. EC109+Hypoxia:
, TMPO‐AS1. (e–h) Cell migratory and invasive abilities were assessed in hypoxia‐administered EC109 and KYSE70 cells treated with DMSO or propofol and transfected with vector or TMPO‐AS1. (i and j) The levels of EMT markers and HIF‐1α were investigated by western blot assay. *P < 0.05. EC109+Hypoxia:  , DMSO;
, DMSO;  , propofol;
, propofol;  , propofol+vector;
, propofol+vector;  , propofol+TMPO‐AS1; and Kyse70+Hypoxia:
, propofol+TMPO‐AS1; and Kyse70+Hypoxia:  , DMSO;
, DMSO;  , propofol;
, propofol;  , propofol+vector;
, propofol+vector;  , propofol+TMPO‐AS1.
, propofol+TMPO‐AS1.
MiR‐498 knockdown reversed the effect of propofol on EC cell progression
In this study, we found that miR‐498 level was remarkably lower in EC cells compared with normal cells (Fig 3a). Moreover, our results revealed that the miR‐498 level was dramatically decreased in hypoxic conditions, and then increased due to propofol treatment (Fig 3b and c). Subsequently, whether miR‐498 was involved in propofol‐regulated EC cell progression was investigated via transfection of anti‐miR‐498 into hypoxia‐induced EC cells. QRT‐PCR assay confirmed that the transfection with anti‐miR‐498 significantly downregulated miR‐498 level (Fig 3d). Next, transwell assay was employed to measure cell mobility. As demonstrated in Fig 3e–h, cell migration and invasion were suppressed by propofol treatment, and then promoted by miR‐498 knockdown. Furthermore, miR‐498 knockdown reversed the effect of propofol treatment on the levels of EMT markers and HIF‐1α (Fig 3i and j). These results indicated that propofol regulated the growth of EC cells by upregulating miR‐498 expression.
Figure 3.
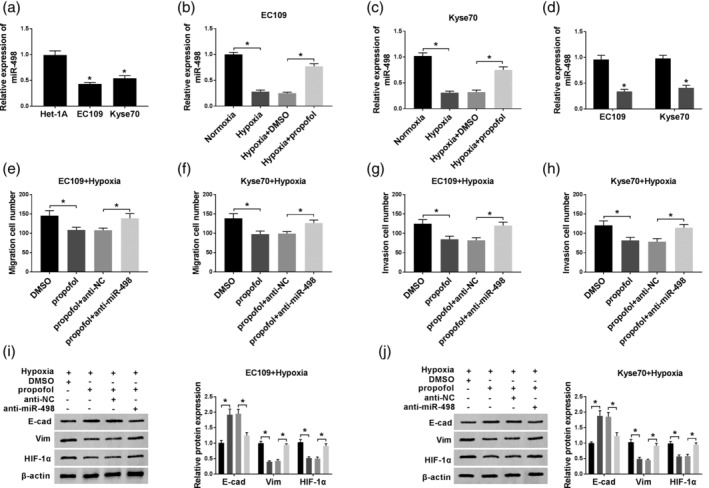
The function of miR‐498 in propofol‐regulated EC cell progression. (a) The expression of miR‐498 was detected by qRT‐PCR assay in normal and EC cells. (b and c) MiR‐498 expression was determined in EC109 and KYSE70 cells treated with normoxia, hypoxia, hypoxia + DMSO, or hypoxia + propofol, respectively. (d) MiR‐498 expression was measured in EC109 and KYSE70 cells transfected with anti‐NC or anti‐miR‐498.  , anti‐NC;
, anti‐NC;  , anti‐miR‐498. (e–h) Cell migratory and invasive abilities were explored in hypoxia‐administered EC109 and KYSE70 cells treated with DMSO or propofol and transfected with anti‐NC or anti‐miR‐498. (i and j) Western blot assay was employed to examine the levels of EMT‐markers and HIF‐1α. *P < 0.05. EC109+Hypoxia:
, anti‐miR‐498. (e–h) Cell migratory and invasive abilities were explored in hypoxia‐administered EC109 and KYSE70 cells treated with DMSO or propofol and transfected with anti‐NC or anti‐miR‐498. (i and j) Western blot assay was employed to examine the levels of EMT‐markers and HIF‐1α. *P < 0.05. EC109+Hypoxia:  , DMSO;
, DMSO;  , propofol;
, propofol;  , propofol+anti‐NC;
, propofol+anti‐NC;  , propofol+anti‐miR‐498; and Kyse70+Hypoxia:
, propofol+anti‐miR‐498; and Kyse70+Hypoxia:  , DMSO;
, DMSO;  , propofol;
, propofol;  , propofol+anti‐NC;
, propofol+anti‐NC;  , propofol+anti‐miR‐498.
, propofol+anti‐miR‐498.
TMPO‐AS1 acted as a sponge for miR‐498
Bioinformatics analysis tool starBase predicted that miR‐498 was a potential target of TMPO‐AS1 (Fig 4a). A dual‐luciferase reporter assay was carried out to verify this interaction. As shown in Fig 4b and c, miR‐498 overexpression remarkably reduced the luciferase activity of TMPO‐AS1 WT, and did not affect the luciferase activity of TMPO‐AS1 MUT, meaning that TMPO‐AS1 interacted with miR‐498. Moreover, the interaction between TMPO‐AS1 and miR‐498 was confirmed by RIP assay (Fig 4d and e). Next, TMPO‐AS1 regulated miR‐498 expression was analyzed through transfection of TMPO‐AS1 or si‐TMPO‐AS1 into EC cells. First, qRT‐PCR assay confirmed that the transfection with si‐TMPO‐AS1 significantly reduced the level of TMPO‐AS1 (Fig 4f). Subsequently, our data suggested that miR‐498 expression was remarkably upregulated by TMPO‐AS1 knockdown and downregulated by TMPO‐AS1 overexpression (Fig 4g). Thus, TMPO‐AS1 targeted miR‐498 and negatively regulated miR‐498 expression.
Figure 4.
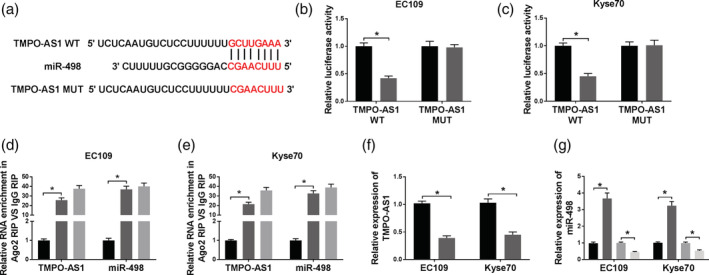
The interaction between TMPO‐AS1 and miR‐498. (a) The interaction between TMPO‐AS1 and miR‐498 was predicted by starBase. The red color represents the mutated sites. (b and c) The luciferase activity was analyzed in EC109 and KYSE70 cells transfected with TMPO‐AS1 WT or TMPO‐AS1 MUT and miR‐498 or miR‐NC. EC109:  , miR‐NC;
, miR‐NC;  , miR‐498; and Kyse70:
, miR‐498; and Kyse70:  , miR‐NC;
, miR‐NC;  , miR‐498. (d and e) RIP assay was used to verify the interaction between TMPO‐AS1 and miR‐498. EC109:
, miR‐498. (d and e) RIP assay was used to verify the interaction between TMPO‐AS1 and miR‐498. EC109:  , anti‐lgG;
, anti‐lgG;  , anti‐ago2;
, anti‐ago2;  , Input; and Kyse70:
, Input; and Kyse70:  , anti‐lgG;
, anti‐lgG;  , anti‐ago2;
, anti‐ago2;  , Input. (f) TMPO‐AS1 expression was detected in EC109 and KYSE70 cells transfected with si‐NC or si‐TMPO‐AS1.
, Input. (f) TMPO‐AS1 expression was detected in EC109 and KYSE70 cells transfected with si‐NC or si‐TMPO‐AS1.  , si‐NC;
, si‐NC;  , si‐TMPO‐AS1. (g) MiR‐498 level was investigated in EC109 and KYSE70 cells transfected with si‐NC, si‐TMPO‐AS1, vector, or TMPO‐AS1, respectively. *P < 0.05.
, si‐TMPO‐AS1. (g) MiR‐498 level was investigated in EC109 and KYSE70 cells transfected with si‐NC, si‐TMPO‐AS1, vector, or TMPO‐AS1, respectively. *P < 0.05.  , si‐NC;
, si‐NC;  , si‐TMPO‐AS1;
, si‐TMPO‐AS1;  , vector;
, vector;  , TMPO‐AS1.
, TMPO‐AS1.
MiR‐498 knockdown attenuated effect of TMPO‐AS1 depletion on EC cell progression
To explore whether TMPO‐AS1 exerted its function via downregulating miR‐498 expression, hypoxia‐administered EC cells were transfected with si‐NC, si‐TMPO‐AS1, si‐TMPO‐AS1 + anti‐NC, si‐TMPO‐AS1 + anti‐miR‐498, respectively. Then, transwell assay was applied to measure cell mobility. As demonstrated in Fig 5a–d, TMPO‐AS1 knockdown suppressed cell migration and invasion, whereas this action was weakened by miR‐498 depletion. Moreover, we found that miR‐498 depletion reversed the effect of TMPO‐AS1 knockdown on the levels of EMT markers and HIF‐1α (Fig 5e and f). Therefore, TMPO‐AS1 suppressed the expression of miR‐498 to modulate the growth of EC cells.
Figure 5.
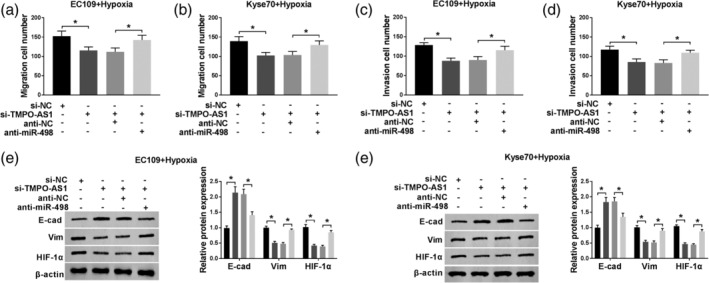
The function of miR‐498 in TMPO‐AS1‐regulated EC cell progression. (a–d) Transwell assay was used to analyze cell migratory and invasive abilities in hypoxia‐administered EC109 and KYSE70 cells transfected with si‐NC, si‐TMPO‐AS1, si‐TMPO‐AS1 + anti‐NC, si‐TMPO‐AS1 + anti‐miR‐498, respectively. (e and f) The levels of EMT markers and HIF‐1α were detected by western blot assay. *P < 0.05. EC109+Hypoxia:  , si‐NC;
, si‐NC;  , si‐TMPO‐AS1;
, si‐TMPO‐AS1;  , si‐TMPO‐AS1+anti‐NC;
, si‐TMPO‐AS1+anti‐NC;  , si‐TMPO‐AS1+anti‐miR‐498; and Kyse70+Hypoxia:
, si‐TMPO‐AS1+anti‐miR‐498; and Kyse70+Hypoxia:  , si‐NC;
, si‐NC;  , si‐TMPO‐AS1;
, si‐TMPO‐AS1;  , si‐TMPO‐AS1+anti‐NC;
, si‐TMPO‐AS1+anti‐NC;  , si‐TMPO‐AS1+anti‐miR‐498.
, si‐TMPO‐AS1+anti‐miR‐498.
Discussion
Esophageal cancer, with a high invasion and metastasis rate, is a common tumor worldwide. 22 Hypoxia exists in many solid tumor microenvironments. 23 In these tumor cells, hypoxia condition induced the increase of HIF‐1α level, and thus regulated the expression of downstream genes, which led to the change of cell progression. 24 , 25 Previous evidence has revealed that propofol is capable of reversing the effect of hypoxia on cell growth. For example, Qian et al. suggested that hypoxia upregulated HIF‐1α level to promote the EMT of prostate cancer cells, whereas propofol treatment impaired this effect via suppression of HIF‐1α. 8 Gao et al. demonstrated that propofol suppressed hypoxia‐induced cell progression in pancreatic cancer. 26 Consistent with these data, our results indicated that propofol weakened the inhibitory effect of hypoxia on EC cell migration, invasion, and EMT through downregulating HIF‐1α level. Therefore, propofol acted as a negative regulator in hypoxia‐induced EC cell progression.
In recent years, many lncRNAs have been reported to play pivotal roles in propofol‐regulated cancer cell progression. For example, Sun et al. confirmed that propofol inhibited the development of hepatocellular carcinoma via increasing the level of lncRNA DGCR5. 27 Chen et al. revealed that propofol reduced the level of lncRNA ANRIL to repress the proliferation and mobility of thyroid cancer cells. 28 In this study, we found that TMPO‐AS1 was highly expressed in EC cells, and TMPO‐AS1 expression was downregulated due to the treatment of propofol. Furthermore, TMPO‐AS1 overexpression promoted EC cell development inhibited by propofol. Nowadays, the function of TMPO‐AS1 in EC is unknown, but TMPO‐AS1 has been reported to promote cell development in many other cancers, such as breast cancer, 29 cervical cancer, 11 prostate cancer, 12 and non‐small cell lung cancer. 30 Therefore, TMPO‐AS1 acts as a positive regulator in EC cells. These data indicated that propofol inhibited hypoxia‐induced EC cell progression through downregulating TMPO‐AS1 level.
Mounting evidence demonstrates that lncRNA acts as a sponge for miRNA to regulate the level of target miRNA in human cancers. 31 For example, LncRNA PTCSC3 targeted miR‐574‐5p to decrease the level of miR‐574‐5p in cervical cancer. 32 We next used the bioinformatics analysis tool starBase to predict the potential targets of TMPO‐AS1, and found that TMPO‐AS1 was able to bind to miR‐498. The interaction between TMPO‐AS1 and miR‐498 was then verified by the dual‐luciferase reporter assay and RIP assay. Moreover, we confirmed that TMPO‐AS1 negatively regulated miR‐498 level. These results suggested that TMPO‐AS1 downregulated miR‐498 expression through interaction.
Previous studies have suggested that miR‐498 level was lower in EC tissues/cells than in normal tissues/cells. 33 Here, we detected the level of miR‐498 in EC cells. In agreement with the previous data, our results confirmed that miR‐498 expression was downregulated in EC cells. Previously, the role of miR‐498 in propofol‐regulated cell progression was unknown. In the present study, we demonstrated that propofol inhibited the mobility of EC cells through upregulating the level of miR‐498. Furthermore, we confirmed that TMPO‐AS1 exerted function via regulating miR‐498 expression in hypoxia‐induced EC cell progression. Generally, one lncRNA can modulate multiple miRNAs, 34 so it is speculated that TMPO‐AS1 regulated the growth of EC cells via mediating the levels of various miRNAs. Therefore, more experiments are needed to investigate the functional mechanism of TMPO‐AS1 in EC.
In conclusion, in this study we demonstrate that propofol suppressed hypoxia‐induced EC cell migration, invasion, and EMT through modulating TMPO‐AS1/miR‐498 axis. Our findings provide a potential target for the therapy of EC patients.
Disclosure
The authors declare that they have no competing interests.
References
- 1. Short MW, Burgers KG, Fry VT. Esophageal cancer. Am Fam Physician 2017; 95: 22–8. [PubMed] [Google Scholar]
- 2. Huang FL, Yu SJ. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J Surg 2018; 41: 210–5. [DOI] [PubMed] [Google Scholar]
- 3. Alnaji RM, Du W, Gabriel E et al Pathologic complete response is an independent predictor of improved survival following neoadjuvant Chemoradiation for Esophageal adenocarcinoma. J Gastrointest Surg 2016; 20: 1541–6. [DOI] [PubMed] [Google Scholar]
- 4. Liu Y, Liu Y, Yan X et al HIFs enhance the migratory and neoplastic capacities of hepatocellular carcinoma cells by promoting EMT. Tumour Biol 2014; 35: 8103–14. [DOI] [PubMed] [Google Scholar]
- 5. Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science 2016; 352: 175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vaupel P. The role of hypoxia‐induced factors in tumor progression. Oncologist 2004; 9 ((Suppl 5)): 10–7. [DOI] [PubMed] [Google Scholar]
- 7. Yip GMS, Chen Z‐W, Edge CJ et al A propofol binding site on mammalian GABAA receptors identified by photolabeling. Nat Chem Biol 2013; 9: 715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qian J, Shen S, Chen W et al Propofol reversed hypoxia‐induced docetaxel resistance in prostate cancer cells by preventing epithelial‐mesenchymal transition by inhibiting hypoxia‐inducible factor 1alpha. BioMed Res Int 2018; 2018: 4174232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell 2009; 136: 629–41. [DOI] [PubMed] [Google Scholar]
- 10. Salehi S, Taheri MN, Azarpira N et al State of the art technologies to explore long non‐coding RNAs in cancer. J Cell Mol Med 2017; 21: 3120–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang J, Liang B, Hou S. TMPO‐AS1 promotes cervical cancer progression by upregulating RAB14 via sponging miR‐577. J Gene Med 2019; 21: e3125. [DOI] [PubMed] [Google Scholar]
- 12. Huang W, Su X, Yan W et al Overexpression of AR‐regulated lncRNA TMPO‐AS1 correlates with tumor progression and poor prognosis in prostate cancer. Prostate 2018; 78: 1248–61. [DOI] [PubMed] [Google Scholar]
- 13. Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol Med 2014; 20: 460–9. [DOI] [PubMed] [Google Scholar]
- 14. Pang KC, Stephen S, Dinger ME, Engstrom PG, Lenhard B, Mattick JS. RNAdb 2.0—an expanded database of mammalian non‐coding RNAs. Nucleic Acids Res 2007; 35: D178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trionfini P, Benigni A. MicroRNAs as master regulators of glomerular function in health and disease. J Am Soc Nephrol 2017; 28: 1686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morishita A, Masaki T. miRNA in hepatocellular carcinoma. Hepatol Res 2015; 45: 128–41. [DOI] [PubMed] [Google Scholar]
- 17. Cong J, Liu R, Wang X, Wang J, Wang H, Hou J. Low miR‐498 expression levels are associated with poor prognosis in ovarian cancer. Eur Rev Med Pharmacol Sci 2015; 19: 4762–5. [PubMed] [Google Scholar]
- 18. Gopalan V, Smith RA, Lam AK. Downregulation of microRNA‐498 in colorectal cancers and its cellular effects. Exp Cell Res 2015; 330: 423–8. [DOI] [PubMed] [Google Scholar]
- 19. Zhao T, Chen Y, Sheng S et al Upregulating microRNA‐498 inhibits gastric cancer proliferation invasion and chemoresistance through inverse interaction of Bmi1. Cancer Gene Ther 2018; 26: 366–73. [DOI] [PubMed] [Google Scholar]
- 20. Islam F, Gopalan V, Law S, Tang JCO, Chan KW, Lam AKY. MiR‐498 in esophageal squamous cell carcinoma: Clinicopathological impacts and functional interactions. Hum Pathol 2017; 62: 141–51. [DOI] [PubMed] [Google Scholar]
- 21. Chen L, Qiu J, Yang C et al Identification of a novel estrogen receptor beta1 binding partner, inhibitor of differentiation‐1, and role of ERbeta1 in human breast cancer cells. Cancer Lett 2009; 278: 210–9. [DOI] [PubMed] [Google Scholar]
- 22. Domper Arnal MJ, Ferrandez Arenas A, Lanas AA. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol 2015; 21: 7933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kujan O, Shearston K, Farah CS. The role of hypoxia in oral cancer and potentially malignant disorders: A review. J Oral Pathol Med 2017; 46: 246–52. [DOI] [PubMed] [Google Scholar]
- 24. Wu W, Hu Q, Nie E et al Hypoxia induces H19 expression through direct and indirect Hif‐1alpha activity, promoting oncogenic effects in glioblastoma. Sci Rep 2017; 7: 45029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE 2005; 2005: re12. [DOI] [PubMed] [Google Scholar]
- 26. Gao Y, Yu X, Zhang F, Dai J. Propofol inhibits pancreatic cancer progress under hypoxia via ADAM8. J Hepatobiliary Pancreat Sci 2019; 26: 219–26. [DOI] [PubMed] [Google Scholar]
- 27. Sun Y, Sun H. Propofol exerts anticancer activity on hepatocellular carcinoma cells by raising lncRNA DGCR5. J Cell Physiol 2020; 235 (3): 2963–2972. [DOI] [PubMed] [Google Scholar]
- 28. Chen F, Li M, Zhu X. Propofol suppresses proliferation and migration of papillary thyroid cancer cells by down‐regulation of lncRNA ANRIL. Exp Mol Pathol 2019; 107: 68–76. [DOI] [PubMed] [Google Scholar]
- 29. Mitobe Y, Ikeda K, Suzuki T et al ESR1‐stabilizing lncRNA TMPO‐AS1 promotes hormone‐refractory breast cancer progression. Mol Cell Biol 2019; 39: e00261–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qin Z, Zheng X, Fang Y. Long noncoding RNA TMPO‐AS1 promotes progression of non‐small cell lung cancer through regulating its natural antisense transcript TMPO. Biochem Biophys Res Commun 2019; 516: 486–93. [DOI] [PubMed] [Google Scholar]
- 31. Wang J, Ding Y, Wu Y, Wang X. Identification of the complex regulatory relationships related to gastric cancer from lncRNA‐miRNA‐mRNA network. J Cell Biochem 2020; 121 (1): 876–887. [DOI] [PubMed] [Google Scholar]
- 32. Tong R, Zhang J, Wang C et al LncRNA PTCSC3 inhibits the proliferation, invasion and migration of cervical cancer cells via sponging miR‐574‐5p. Clin Exp Pharmacol Physiol 2020; 47: 439–448. [DOI] [PubMed] [Google Scholar]
- 33. Wang P, Liu X, Han G et al Downregulated lncRNA UCA1 acts as ceRNA to adsorb microRNA‐498 to repress proliferation, invasion and epithelial mesenchymal transition of esophageal cancer cells by decreasing ZEB2 expression. Cell Cycle 2019; 18: 2359–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liao J, Wang J, Liu Y, Li J, Duan L. Transcriptome sequencing of lncRNA, miRNA, mRNA and interaction network constructing in coronary heart disease. BMC Med Genomics 2019; 12: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]


