Abstract
Background
Proof of the clinical utility of a biomarker is when its use informs a management decision and improves patient outcomes relative to when it is not.
Objective
To model the clinical benefit of the nuclear-localized AR-V7 test for men with progressing metastatic castration-resistant prostate cancer (mCRPC) at the second line of therapy or greater to inform the choice of an androgen receptor signaling inhibitor (ARSI) or a taxane.
Design, setting, and participants
The study population was a cross-sectional cohort of 193 unique patients with progressing mCRPC from whom 255 samples were drawn at the time of the second line or later treatment decision who received an ARSI or taxane, with up to 3 yr of additional follow-up. Physicians were blinded to AR-V7 status and the testing laboratory was blinded to outcomes.
Outcome measurements and statistical analyses
We measured physician propensity for choosing an ARSI or taxane based on patient prognosis, as well as overall survival (OS) adjusted for physician propensity by drug class without or with knowledge of nuclear localized AR-V7 status in circulating tumor cells (CTCs).
Results and limitations
Treating physicians had a propensity for choosing a taxane over ARSI for patients with more advanced disease or who received an ARSI as the immediate prior therapy. After adjusting for physician propensity, discernible OS differences were not observed between taxane- and ARSI-treated patients (median 15.6 vs 14.4 mo; p = 0.11). Patients with detectable nuclear-localized AR-V7 in CTCs had superior survival with taxanes over ARSIs (median 9.8 vs 5.7 mo; p = 0.041). AR-V7–negative patients had superior survival on ARSIs over taxanes (p = 0.033) but overlapping curves limit the interpretation. Mutivariable models showed a robust interaction between AR-V7 status and drug, and a lower risk of death on taxanes for AR-V7–positive men.
Conclusions
Use of the nuclear-localized AR-V7 CTC test to inform treatment choice can improve patient outcomes relative to decisions based solely on standard-of-care measures.
Patient summary
Men with metastatic prostate cancer who test positive for AR-V7 protein in circulating tumor cells are likely to live longer if taxane chemotherapy is used.
Keywords: Circulating tumor cells, AR-V7, Prostate cancer, Liquid biopsy, Treatment decisions
1. Introduction
Next-generation androgen receptor signaling inhibitors (ARSIs) such as abiraterone and enzalutamide have a proven effect on overall survival (OS) and favorable safety profiles relative to other therapies approved by the US Food and Drug Administration for metastatic castration-resistant prostate cancer (mCRPC). Most of these patients are treated in the first line with an ARSI [1] because overall response rates are high and often durable. Unfortunately, all patients eventually progress [2,3] and require additional treatment. The choice of the second or greater line of therapy is less certain, as the response to a first-line ARSI does not predict the response to a second [4–6]. Many physicians routinely prescribe a taxane in this context, while recognizing that a significant subset of patients might still benefit from a less toxic, orally administered ARSI.
There have been no prospective randomized trials comparing an ARSI to a taxane in the second-line setting. Such a trial would be challenging given the real-world bias of physicians who assume that tumors that are more aggressive according to clinically assessed parameters require a taxane over an ARSI for optimal patient benefit. Consistent with this bias is an observational study of 546 patients treated in a US community-based practice in which no significant OS difference was observed for either drug class after adjusting for known risk factors, yet the authors concluded that the data favored second-line therapy with a taxane [7] Similarly, a single-site Japanese study of 222 patients showed no discernible difference in OS after adjusting for baseline prognosis using multivariable models [8]. Reports for men treated in the post-docetaxel setting [9] or after rapid progression on an ARSI [10] suggest a modestly lower risk of death for those with high Halabi risk scores who received a taxane.
In previous work analyzing two independent cross-sectional cohorts treated primarily at a single tertiary cancer center, we showed an association between the presence of nuclear-localized AR-V7 protein in circulating tumor cells (CTCs) and a lack of response and shorter survival time following treatment with an ARSI, as well as a treatment-specific interaction showing superior survival following taxane treatment [11,12]. In both studies, men who had nuclear-localized AR-V7 protein in CTCs before therapy had more favorable OS on a taxane than on an ARSI, even though taxane-receiving patients had an inferior pretreatment prognosis on the basis of their receipt of more prior lines of systemic therapy and higher pretreatment prostate-specific antigen (PSA) and lactate dehydrogenase levels.
A limitation of these studies was that patients were not randomly assigned to treatment to specifically address the biomarker question. However, both studies incorporated the same analytically validated and locked assay, treatment decisions were made before nuclear AR-V7 status in CTCs was determined, and the laboratory personnel were blinded to patient outcomes. Combining the cohorts and using longer follow-up data, we sought to determine factors that contributed to the choice of one therapy over the other without knowledge of the biomarker result, and used them to model survival had the biomarker result been available.
To do this, we used propensity analyses of the probability of being treated with an ARSI or taxane based on known and readily available pretreatment clinical factors, without knowledge of the nuclear AR-V7 test result. Use of propensity scores provides a proxy for the degree of confidence in using either drug class per treatment decision, facilitating our estimation of the incremental survival added by AR-V7 test results to decisions made by experienced physicians in a tertiary cancer center to maximize patient survival.
2. Patients and methods
2.1. Patient population
Our analysis included a subset of mCRPC patients (255 samples for 255 treatment decisions for 193 unique patients) treated at Memorial Sloan Kettering Cancer Center (New York, NY, USA) between December 2012 and September 2016. These clinical and biomarker data were previously used in separate analyses [11,12], but up to 3 yr of additional follow-up is included in this analysis. For all patients, data on history were collected, including stage at diagnosis, initial management and all subsequent systemic therapies, physical examination, laboratory studies (complete blood count, albumin, alkaline phosphatase, lactate dehydrogenase, PSA, and hemoglobin), and serum testosterone to confirm castrate status (<50 ng/dl). Categorization of progressive disease was in accordance with Prostate Cancer Clinical Trials Working Group 2 or 3 guidelines [13,14]. All patients provided signed informed consent to participate in a protocol approved by the Memorial Sloan Kettering institutional review board. Blood samples were drawn before initiation of either an ARSI (abiraterone, enzalutamide, or apalutamide) or taxane (docetaxel, cabazitaxel, or paclitaxel) assigned at the discretion of the treating physician without knowledge of AR-V7 status (Fig. 1).
Fig. 1 –
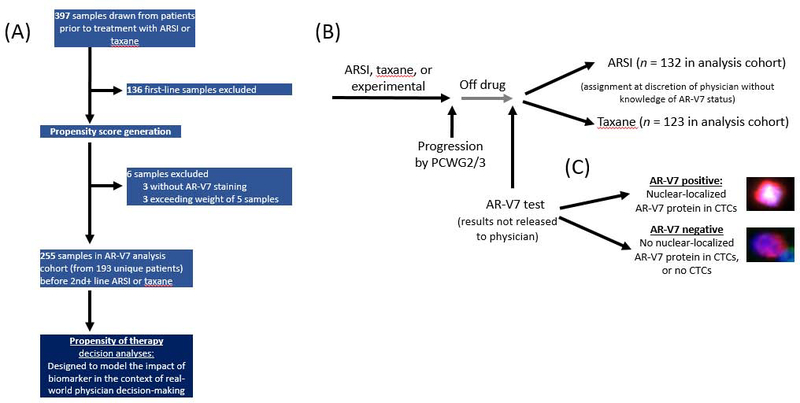
CONSORT and treatment diagrams. (A) CONSORT diagram outlining samples used for propensity score and risk score generation and subset analyzed. (B) Flow diagram illustrating cohort treatment and AR-V7 testing history. The scoring criteria for AR-V7 positivity were prespecified, and the AR-V7 results were not released to physicians. (C) Images of the presence or absence of nuclear-localized AR-V7 protein in circulating tumor cells (CTCs).
ARSI = androgen receptor signaling inhibitor; PCWG = Prostate Cancer Clinical Trials Working Group.
2.2. CTC collection
Blood (7.5 ml) from each subject was collected in Streck tubes and processed within 48 h using methods previously described [11,12,15]. In brief, red blood cells were lysed, and approximately 3 million nucleated blood cells were dispensed onto 10–16 glass microscope slides and placed at −80°C for long-term storage. Sample processing and testing were conducted in laboratories certified under US Clinical Laboratory Improvement Amendments regulations.
2.3. CTC immunofluorescent staining and analysis
CTCs were identified and characterized as previously described [11,12,15]. In brief, slides created from mCRPC patient samples underwent automated immunofluorescent staining for DNA, cytokeratins, CD45, and AR-V7. Two slides were evaluated per sample. Clinical laboratory scientists licensed in California conducted final quality control of the CTC classification and subcellular biomarker localization.
AR-V7 scoring criteria were used as previously reported [11,12,15]. In short, samples with at least one CTC with an intact nucleus and nuclear-localized AR-V7 signal-to-noise level above an established background intensity per two slides tested (~1 ml of blood) were scored as AR-V7–positive (Fig. 1C). The analytical specificity of detection in contrived CTC samples, patient CTC samples, and healthy and malignant solid tissues and blood was previously reported [11].
2.4. General statistical considerations
Patient sample demographics and clinical characteristics at the time of blood draw were evaluated in terms of descriptive statistics overall and by drug class assigned (Table 1) and AR-V7 positivity (Supplementary Table 1). Linear mixed-effects models were used to compare differences between patient sample groups, accounting for multiple records per patient (R package lme4). The unit of measure in this study was the patient sample obtained at the time of a treatment decision. Overall, 141, 43, eight, and one patient contributed one, two, three, and four samples, respectively. All treatment decisions were considered independent unless otherwise specified. OS was calculated from the time of treatment decision to death. Patients alive at last follow-up were right-censored. In multivariable Cox proportional-hazards models we used a clustered term to compute robust variances, accounting for multiple records per patient. R version 3.4.1 software was used for all statistical analyses.
Table 1 –
Patient sample demographics and clinical characteristics at the time of blood draw overall and by drug class assigned a
| Overall | Pre-ARSI | Pre-Taxane | p value | |
|---|---|---|---|---|
| Samples (n) | 255 | 132 | 123 | |
| Unique patients (n) b | 193 | 124 | 111 | |
| Sampling point, n (%) | <0.0001 | |||
| Before 2nd-line Tx | 100 (39) | 74 (56) | 26 (21) | |
| Before 3rd-line Tx | 75 (29) | 33 (25) | 42 (34) | |
| Before ≥4th-line Tx | 80 (31) | 25 (19) | 55 (45) | |
| Median age at Tx decision, yr (IQR) | 69 (62.5–75) | 70 (63–77.25) | 69 (62–73.5) | 0.13 |
| Metastatic sites, n (%) | ||||
| Lymph nodes | 169 (66) | 83 (63) | 86 (70) | 0.23 |
| Bone | 226 (89) | 111 (84) | 115(93) | 0.018 |
| Lung | 29 (11) | 12 (9) | 17 (14) | 0.23 |
| Liver | 29 (11) | 8 (6) | 21 (17) | 0.0055 |
| Blood analytes, median (IQR) | ||||
| Prostate-specific antigen (ng/ml) | 50.4 (18.2–211.2) | 30.7 (11.6–80.6) | 99.4 (28.7–517.1) | <0.0001 |
| Alkaline phosphatase (IU/l) | 111 (80–199.5) | 97.5 (76–134) | 139 (94–233.5) | 0.00012 |
| Lactate dehydrogenase (U/l) | 235 (187–294) | 211.5 (180.5–264.2) | 248 (206–347) | 0.0087 |
| Hemoglobin (g/dl) | 11.7 (10.3–12.9) | 12 (10.675–13.1) | 11.4 (10–12.35) | 0.0035 |
| Albumin (g/dl) | 4.2 (4–4.3) | 4.2 (4–4.3) | 4.2 (3.9–4.3) | 0.98 |
| Last prior Tx was ARSI, n (%) | 168 (66) | 80 (61) | 88 (72) | 0.066 |
| Pre-Tx clinical data unavailable to physician for AR-V7–positive men, n (%) | 57 (22) | 21 (16) | 36 (29) | 0.010 |
ARSI = androgen receptor signaling inhibitor; IQR = interquartile range; Tx = treatment.
All percentages are calculated using total number of samples per group as the denominator. Of the 133 samples taken before second-line or later treatment with an ARSI, 59 were taken before abiraterone, 66 before enzalutamide, and eight before apalutamide. Of the 123 samples taken before second-line or later treatment with a taxane, 85 were taken before docetaxel, 36 before cabazitaxel, and two before paclitaxel.
An individual patient could be counted more than once if, during the study, he began more than one course of therapy that was eligible for inclusion in the study.
2.5. Propensity score weighting
To gain an understanding of the factors, independent of outcome and AR-V7 status, affecting treatment decisions between ARSI and taxanes, we created a multivariable model from which propensity scores were generated. The factors evaluated were age; line of therapy to be initiated; presence of liver, bone, lung, and lymph node metastases; laboratory values for albumin, PSA, lactate dehydrogenase, alkaline phosphatase, and hemoglobin; and whether an ARSI was the immediate prior therapy (Supplementary Fig. 1). All features were included in a generalized linear model (R package glm) to develop the multivariable model before propensity score generation (R package MatchIt). Individual sample weights were created using the inverse probability of treatment weighting technique, which adjusts the weight for individual patients to the inverse of the propensity score, to estimate a randomized treatment assignment [16,17]. OS times were then estimated using the Kaplan-Meier method with and without the weights, and differences in survival between the treatment groups were assessed using the log-rank test (R package survival).
3. Results
3.1. Clinical characteristics of the analysis cohort
Overall, 397 samples from 295 unique patients were collected before starting an ARSI or taxane. Of these, 136 samples were obtained in the first line, three did not have AR-V7 staining, and three had an excessive individual sample weight after inverse probability weighting. The analysis cohort (Fig. 1A) included the remaining 255 samples from 193 unique patients obtained before starting a second-line or greater treatment, of which 132 (52%) were before starting an ARSI and 123 (48%) before starting a taxane (Fig. 1B).
Table 1 summarizes the characteristics of the patients as a group at the time of blood sample collection, and separately by the therapy selected by their treating physician without knowledge of AR-V7 status. Of the 193 unique patients, 163 were deceased. The median follow-up for surviving patients was 28.4 mo (interquartile range 24.4–33.0). Patients assigned a taxane had received more prior lines of therapy (p < 0.0001), had higher PSA, lactate dehydrogenase, and alkaline phosphatase levels, had lower hemoglobin, and were more likely to have liver (all p < 0.01) or bone metastases (p = 0.018) relative to those assigned an ARSI. They were also numerically but not significantly more likely to have had an ARSI as the immediate prior therapy (72% vs 61%; p = 0.066) and more likely to be AR-V7-positive (29% vs 16%; p = 0.010).
3.2. Propensity score
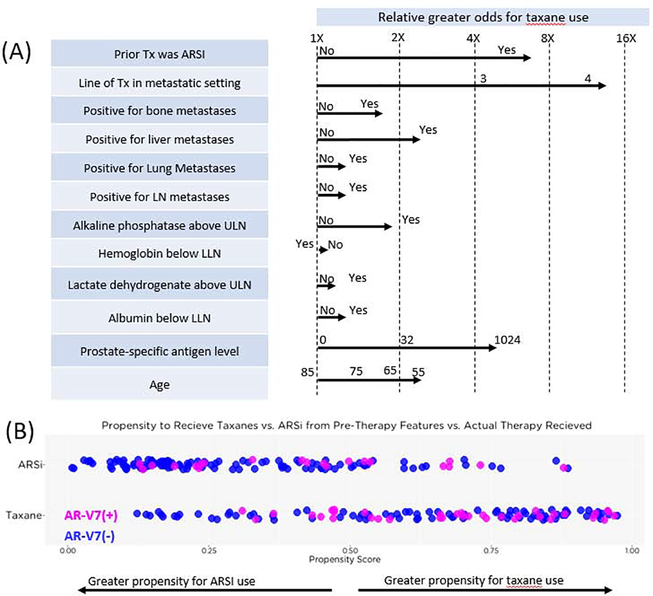
The most influential factors that led physicians to assign a taxane over an ARSI were the number of prior systemic therapies in the metastatic setting, the presence of liver metastases, PSA, and whether the immediate prior therapy was an ARSI (Fig. 2A). These factors reflect the treating physician’s propensity to use a taxane for tumors that they judge to be more aggressive and more advanced and that they suspect to be cross-resistant to a second ARSI if given sequentially (Supplementary Fig. 1).
Fig. 2 –
Factors influencing physician decisions to assign an androgen receptor signaling inhibitor (ARSI) or taxane. (A) Factors influencing physician decision to assign ARSI or taxane. Arrows indicate the relative odds for taxane use predicted by the model given the condition. (B) Distribution of patients by their propensity scores and actual therapy assigned, colored by AR-V7 status. Patients with a wide range of propensity to receive either an ARSI or a taxane could test positive for AR-V7.
LLN = lower limit of normal; Tx = treatment; ULN = upper limit of normal.
Figure 2B shows the individual propensity scores before treatment assignment. A score of 0 indicates near-complete certainty that the treatment selected would be an ARSI, and a score of 1, a taxane. With the exception of patients with a propensity score at the extremes, there was no clear tendency to give one drug class or the other (Fig. 2B). Outside of these extremes, AR-V7 positivity was independent of propensity to give one drug class or the other (Fig. 2B).
3.3. Effect of therapy choice and AR-V7 status on OS
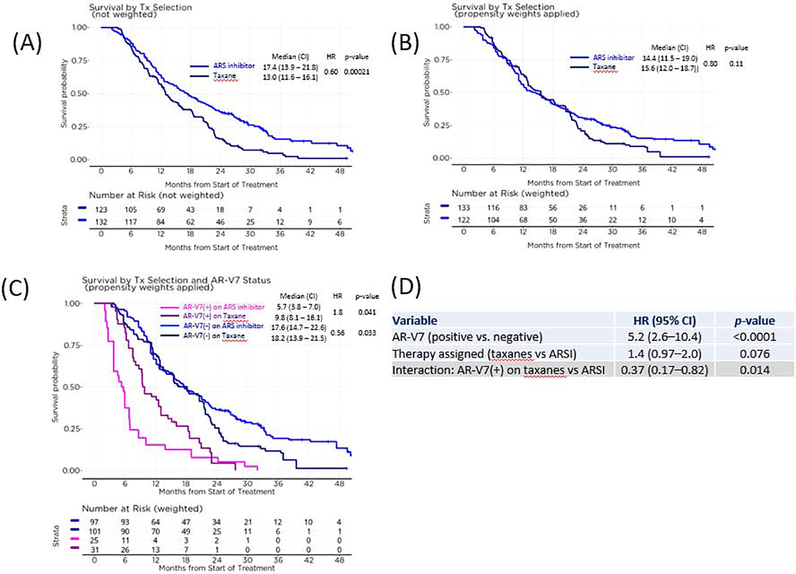
Overall, patients assigned a taxane in comparison to those assigned an ARSI had more aggressive disease, more prior lines of therapy (Table 1), and a higher risk of death (Supplementary Fig. 4). Without adjustment for physician choice propensity, those assigned an ARSI had longer OS relative to those assigned a taxane (median OS 17.4 vs 13.0 mo; hazard ratio [HR] 0.60; p = 0.00021; Fig. 3A). By contrast, after adjustment for physician propensity via inverse probability weighting, a difference in OS was not observed between the ARSI and taxane groups (14.4 vs 15.6 mo; HR 0.80; p = 0.11; Fig. 3B). However, when the patients were categorized by AR-V7 status, AR-V7–positive patients had superior OS on a taxane versus an ARSI (median 9.8 vs 5.7 mo; HR 1.8; p = 0.041; Fig. 3C). Patients testing negative for AR-V7 had a significant difference in OS favoring an ARSI over a taxane (p = 0.033), but overlapping curves limit this interpretation of the results (Fig. 3C). In practice, AR-V7 is most likely to be used to inform decisions with the greatest uncertainty. The range of propensity scores from 0.25 to 0.75 represents the area of greatest uncertainty in treatment decisions, and an analysis restricted to this range yielded similar observations (Supplementary Fig. 2).
Fig 3 –
Effect of therapy and AR-V7 status on overall survival. (A) Overall survival by drug class assigned; physicians were more likely to assign a taxane if the patient’s disease was more advanced. (B) Overall survival by drug class after weighting to adjust for physician propensity to administer either drug class influenced by pretherapy patient features, estimating drug-specific survival if cohort was randomized. (C) Overall survival by drug class and AR-V7 status in the context of (B). (D) Interaction multivariable Cox model for (C).
ARSI = androgen receptor signaling inhibitor; CI = confidence interval; HR = hazard ratio; Tx = treatment.
A Cox model evaluating this treatment interaction and incorporating treatment propensities is shown in Figure 3D. AR-V7–positive patients as a group had a higher risk of death (HR 5.2, 95% confidence interval [CI] 2.6–10.4; p < 0.0001). However, this effect was drug class–dependent, as AR-V7–positive patients receiving taxanes had a lower risk of death on taxanes than on ARSIs (HR 0.37, 95% CI 0.17–0.82; p = 0.014). Analyses with and without propensity weighting or adjustment for baseline risk factors showed similar results (Supplementary Fig. 4). We also investigated whether prior treatment alone or patient risk could be an alternative to AR-V7 status with respect to predictive associations. The interaction of AR-V7 and therapy was still additive and independent when they were considered (Supplementary Fig. 4).
4. Discussion
For a biomarker to have clinical utility, it must provide incremental information to physicians beyond what is routinely available to allow more informed therapeutic decisions that result in better patient outcomes. Here we show that for oncologists experienced in the management of prostate cancer treating men with progressing mCRPC starting the second or greater line of therapy, patient survival would have been prolonged by use of the nuclear-localized AR-V7 CTC test to guide the choice of an ARSI or a taxane relative to a decision made when AR-V7 status was unknown.
Propensity analyses adjust for the influence of known factors on treatment assignment. Randomized trials adjust for known and unknown factors and are the gold standard in determining the impact of use of a biomarker result to improve patient outcomes [18]. An alternative is a prospective-retrospective study using banked specimens that is confirmed by a second independent study [19]. This has been shown for the outcome associations of the nuclear-localized AR-V7 test investigated here [11,12] and corroborated later in a prospective trial [20].
However, the patients enrolled on the basis of defined eligibility for trials may not be fully representative of those encountered in a clinical practice setting. The propensity analysis reported here models the outcomes for unselected patients treated in the course of routine management. The results show that choice of an ARSI or taxane without knowledge of pretherapy nuclear localized AR-V7 status in CTCs was highly influenced by the line of therapy, treatment sequence, and perceived risk of death (Fig. 2). Taking these factors into account and analyzing them using multivariable treatment interaction models that included AR-V7 status (Fig. 3D, Supplementary Fig. 4), we showed that the assay result would have provided independent and additive value to both patient survival risk and prior treatment history in selecting the appropriate treatment.
Propensity weighting adjusts for the likelihood that a physician chose one drug class over the other, which is in effect a pseudorandomization [16,17]. With this adjustment, a difference in survival was not observed between ARSI and taxane use in second line or later mCRPC (Fig. 3B). When the propensity weighting was retained and AR-V7 status was added, AR-V7–positive patients had superior survival on taxanes (median 9.8 vs 5.7 mo; p = 0.041), while AR-V7–negative patients had superior survival on ARSIs (p = 0.033; Fig. 3C). The latter result, however, must be interpreted with caution given the overlap of curves observed and the difference in survival observed is primarily in the tail of the curve, beyond 20 mo.
In practice, there is physician bias against prescribing ARSIs sequentially because of presumed cross-resistance in individual patients. We observed that the interaction between prior therapy and next therapy chosen was not correlated to OS (Supplementary Fig. 4E; HR 0.61, 95% CI 0.29–1.3; p = 0.55). This, combined with the similar outcomes seen with both drug classes (Fig. 3B), with 66% having received an ARSI as their immediate prior therapy (Table 1), argues against the assumption that there can be no benefit from sequential ARSI use.
The overlap observed for propensity scores at decision points to assign an ARSI versus taxane and for types of patients chosen for each drug class shows that the second line or greater treatment decision is challenging, and supports a need for tests to better inform the decision. In this context, patients tested positive for AR-V7 across the full range of treatment propensities (Fig. 2C), showing that physician assessments of clinical and routine laboratory features alone were unable to predict resistance to ARSI, and, by extension, the value of the nuclear-localized AR-V7 test in informing this decision.
We evaluated outcomes for patients and associated decisions made at a single site by physicians experienced in the care of patients with mCRPC and assessed the additive value of a biomarker in this setting. Practice may vary significantly outside such a setting and may not use all of the factors included in our models to the same degree to guide treatment decisions. Sensitivity analyses with or without these factors to varying degrees still show an interaction between AR-V7 and therapy class–specific survival (Supplementary Fig. 4), suggesting a degree of broader applicability.
Taken together, the study results show that knowledge of AR-V7 status before making a treatment decision for mCRPC in the second line or greater might provide incremental value that could result in better survival for patients in need of a new line of therapy. Additional biomarkers are still needed to understand why some AR-V7–negative patients do not respond to an ARSI and to identify them a priori so that alternative, potentially effective treatments can be given. However, the incremental value to clinical decision-making will also need to be demonstrated for any such additional biomarkers.
5. Conclusions
These data support the notion that the nuclear-localized AR-V7 CTC test can improve clinical decision-making in choosing between ARSIs and taxanes for progressing mCRPC in a real-world setting. The results of our analysis suggest that AR-V7–positive patients would experience better survival on taxanes over ARSIs, and AR-V7–negative patients would have superior survival if given an ARSI. However, the latter finding is less clear-cut and thus individual patient assignment in the AR-V7–negative setting should still be guided by best physician judgment.
Supplementary Material
Acknowledgments
We thank the patients who took part in this study and their families, and the clinical and laboratory staff at Memorial Sloan Kettering Cancer Center and Epic Sciences. Amy Plofker of Memorial Sloan Kettering Cancer Center provided editorial assistance, without compensation for her contribution.
Funding/Support and role of the sponsor: This study was supported by NIH/NCI Prostate SPORE Grant P50-CA92629, NIH/NCI Cancer Center Support Grant P30 CA008748, the Prostate Cancer Foundation, and Department of Defense Prostate Cancer Research Program PC121111. The sponsors played no direct role in the study.
Financial disclosures: Howard I. Scher certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Ryon P. Graf and Ryan Dittamore are employees of Epic Sciences. Howard I. Scher has a leadership role with the Asterias Biotherapeutics Board of Directors and consulting roles with Janssen Biotech, Sanofi, Amgen, Janssen Research & Development, Menarini Silicon Biosystems, WIRB–Copernicus Group, ESSA Pharma, Konica Minolta, OncLive Insights, and Physician Education Resource; has received institutional research support from Janssen, Innocrin, Illumina, Epic, Menarini Silicon Biosystems, and Thermo Fisher Scientific; and has received travel and accommodation expenses from Asterias Biotherapeutics, Physician Education Resource, Sanofi, Menarini Silicon Biosystems, Amgen, WIRB–Copernicus Group, Konica Minolta, OncLive Insights, and ESSA Pharma. The remaining authors have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gillessen S, Omlin A, Attard G, et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann Oncol 2015;26:1589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015;16:152–60. [DOI] [PubMed] [Google Scholar]
- 3.Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol 2017;71:151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrader AJ, Boegemann M, Ohlmann CH, et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol 2014;65:30–6. [DOI] [PubMed] [Google Scholar]
- 5.Azad AA, Eigl BJ, Murray RN, Kollmannsberger C, Chi KN. Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur Urol 2015;67:23–9. [DOI] [PubMed] [Google Scholar]
- 6.de Bono JS, Chowdhury S, Feyerabend S, et al. Antitumour activity and safety of enzalutamide in patients with metastatic castration-resistant prostate cancer previously treated with abiraterone acetate plus prednisone for ≥24 weeks in Europe. Eur Urol 2018;74:37–45. [DOI] [PubMed] [Google Scholar]
- 7.Oh WK, Miao R, Vekeman F, et al. Real-world characteristics and outcomes of patients with metastatic castration-resistant prostate cancer receiving chemotherapy versus androgen receptor-targeted therapy after failure of first-line androgen receptor-targeted therapy in the community setting. Clin Genitourin Cancer 2018;16:50–7. [DOI] [PubMed] [Google Scholar]
- 8.Miyake H, Sugiyama T, Aki R, et al. Comparison of alternative androgen receptor-axis-targeted agent (ARATA) and docetaxel as second-line therapy for patients with metastatic castration-resistant prostate cancer with progression after initial ARATA in real-world clinical practice in Japan. Clin Genitourin Cancer 2018;16:219–25. [DOI] [PubMed] [Google Scholar]
- 9.Oh WK, Miao R, Vekeman F, et al. Patient characteristics and overall survival in patients with post-docetaxel metastatic castration-resistant prostate cancer in the community setting. Med Oncol 2017;34:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh WK, Cheng WY, Miao R, et al. Real-world outcomes in patients with metastatic castration-resistant prostate cancer receiving second-line chemotherapy versus an alternative androgen receptor-targeted agent (ARTA) following early progression on a first-line ARTA in a US community oncology setting. Urol Oncol 2018;36:500.e1–9. [DOI] [PubMed] [Google Scholar]
- 11.Scher HI, Lu D, Schreiber NA, et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol 2016;2:1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scher HI, Graf RP, Schreiber NA, et al. Assessment of the validity of nuclear-localized androgen receptor splice variant 7 in circulating tumor cells as a predictive biomarker for castration-resistant prostate cancer. JAMA Oncol 2018;4:1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 2016;34:1402–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008;26:1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scher HI, Graf RP, Schreiber NA, et al. Nuclear-specific AR-V7 protein localization is necessary to guide treatment selection in metastatic castration-resistant prostate cancer. Eur Urol 2017;71:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–81. [DOI] [PubMed] [Google Scholar]
- 17.Lanza ST, Moore JE, Butera NM. Drawing causal inferences using propensity scores: a practical guide for community psychologists. Am J Community Psychol 2013;52:380–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deverka P, Messner DA, McCormack R, et al. Generating and evaluating evidence of the clinical utility of molecular diagnostic tests in oncology. Genet Med 2015;18:780. [DOI] [PubMed] [Google Scholar]
- 19.Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst 2009;101:1446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong AJ, Halabi S, Luo J, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol 2019;37:1120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.