Abstract
Purpose of Review:
We discuss how potentially modifiable factors including obesity, the microbiome, diet, and exercise may impact melanoma development, progression, and therapeutic response.
Recent Findings:
Obesity is unexpectedly associated with improved outcomes with immune and targeted therapy in melanoma, with early mechanistic data suggesting leptin as one mediator. The gut microbiome is both a biomarker of response to immunotherapy and a potential target. As diet is a major determinant of the gut microbiome, ongoing studies are examining the interaction between diet, the gut microbiome, and immunity. Data are emerging for a potential role of exercise in reducing hypoxia and enhancing anti-tumor immunity, though this has not yet been well-studied in the context of contemporary therapies.
Summary:
Recent data suggests energy balance may play a role in the outcomes of metastatic melanoma. Further studies are need to demonstrate mechanism and causality as well as the feasibility of targeting these factors.
Keywords: melanoma, immunotherapy, obesity, diet, microbiome, exercise
Introduction:
Outcomes for patients with metastatic melanoma have dramatically improved with the development of checkpoint inhibitor immunotherapies and MAPK-directed targeted therapies.[1–6] However, a large proportion of patients will not respond or will develop resistance. Thus, identifying predictors of response and mechanisms of resistance is of great interest to assist clinicians in personalizing treatment regimens and developing new strategies to improve patient outcomes. There is strong evidence from other diseases that host (i.e. patient level) factors can impact tumor initiation, progression, and therapeutic response. However, until recently these factors had not been well-studied in melanoma, or in the context of immune or targeted therapy in any disease. Recent data has suggested that non-modifiable host factors such as age and biological sex impact melanoma biology and therapeutic response, sometimes in surprising ways.[7–10] In this review, we discuss emerging evidence of how potentially modifiable factors related to energy balance including obesity, the microbiome, diet, and exercise, may shape melanoma biology, immunity, and outcomes (Figure 1).
Figure 1.
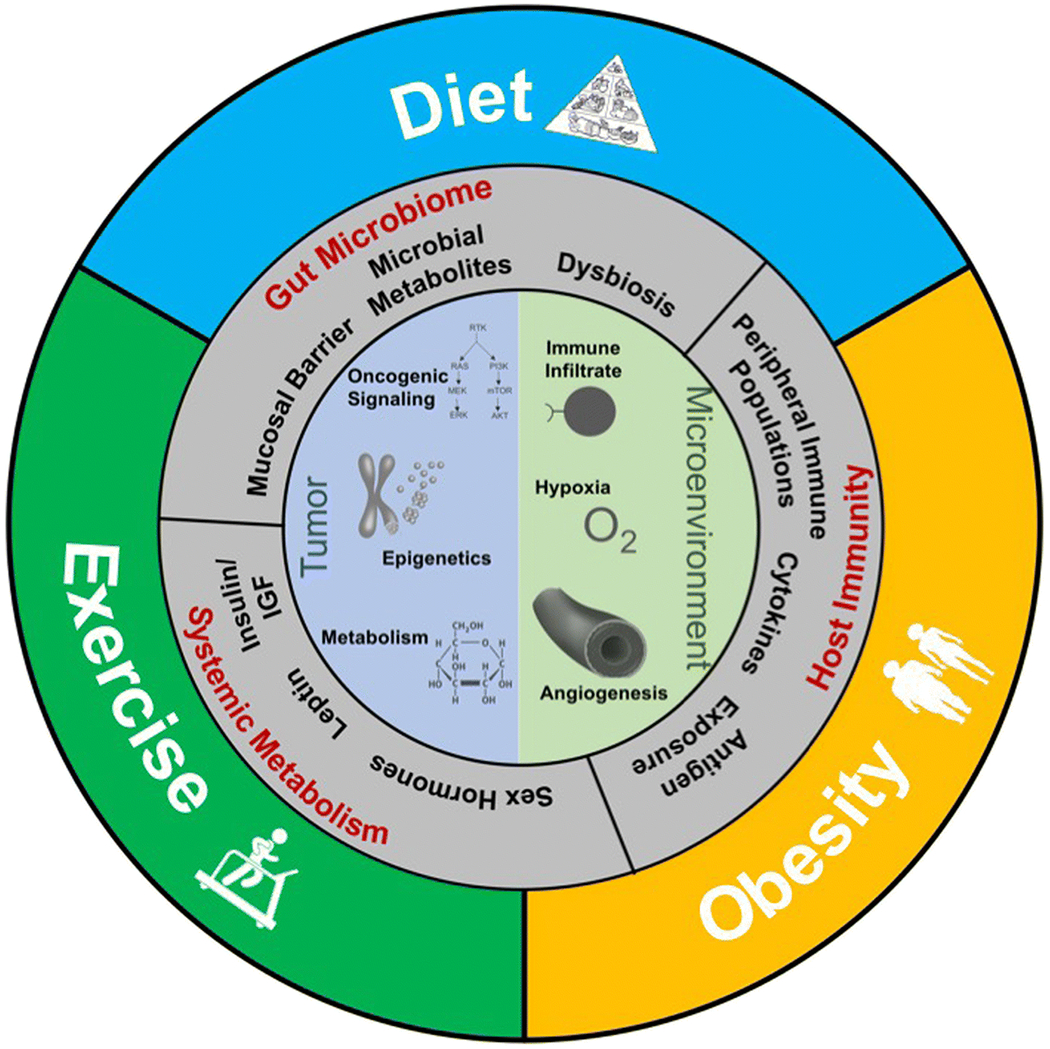
Modifiable patient-level factors such as obesity, diet, and exercise have the potential to influence systemic and tumor-level determinants of melanoma development, progression, and response to therapy. Key mechanisms are thought to be overlapping including changes in systemic and tumor level metabolism, oncogenic signaling, gut microbiome, angiogenesis and oxygenation, and immune activation and infiltration.
Obesity
Obesity is considered an established risk factor for 13 different malignancies.[11] There are multiple mechanisms linking obesity to cancer development and progression.[12–14] Adipocytes are biologically active and produce adipokines, estrogens, and cytokines. Imbalances of the adipokines leptin and adiponection can accelerate or inhibit the growth of many cancers.[15] Excess estrogen produced by adipocyte aromatase activity can fuel the growth of estrogen-responsive tumors such as endometrial and breast cancer.[16, 17] Obesity also leads to systemic metabolic derangements including metabolic syndrome and increased blood levels of insulin and IGF-1, which can feed into oncogenic signaling pathways such as the PI3K pathway.[18] Finally, obesity is considered a meta-inflammatory state characterized by chronic low-level elevation of IL-1, IL-6, and TNF-α which can both drive carcinogenesis locally and also impair systemic immunity.[19, 20]
Limited evidence [11] suggests that obesity is associated with a slightly increased risk of melanoma in men [21] and increased primary tumor Breslow thickness.[22] Previous work had implicated the adipokine leptin in directly promoting melanoma growth via leptin receptors on tumor cells [23, 24], and leptin levels in humans have been linked to both melanoma risk [25] and risk of sentinel lymph node metastases.[26] More recently, direct cross-talk between adipocytes and melanoma cells has been described with transfer of lipids from adipocytes fueling melanoma tumor growth in preclinical models.[27]
Despite these data linking obesity and melanoma development, the effects of obesity on melanoma outcomes and therapeutic response were largely unknown. Intriguingly, recent data from our group and others have demonstrated that the relationship between obesity and outcomes in melanoma may differ by stage, treatment, and even biological sex. In a study of >1100 patients with surgically resected melanoma, elevated body mass index (BMI) was associated with poorer melanoma specific and overall survival.[28] These associations remained significant after adjusting for age, sex, and stage. However, when C-reactive protein, a marker of systemic inflammation, was added to the multivariable model, the BMI association was no longer significant, supporting chronic inflammation as a potential mediator.[28]
More recently, we found that obesity was associated with significantly improved outcomes in metastatic melanoma patients treated with BRAF-targeted or immune checkpoint inhibitor therapy. This was contrary to our initial hypothesis, as it was previously shown that the IGF-1/PI3K pathway can mediate resistance to both BRAF-targeted[29] and immune therapy[30] in melanoma and insulin/IGF1 activation of the PI3K pathway is a key mediator of the obesity/cancer connection.[18] However, in this study of >1900 patients in 6 independent cohorts treated with either BRAF/MEK-directed targeted therapy, checkpoint inhibitors, or dacarbazine chemotherapy, we instead observed remarkably strong and consistent associations between obesity (BMI>30) and improved outcomes with immune and targeted therapies.[31] For example, in 599 patients treated on randomized phase III trials of dabrafenib + trametinib, the median progression-free survival (PFS) for obese (BMI>30) individuals was 15.7 months vs. 9.6 months for normal BMI (BMI 18.5-24.9). Overall survival (OS) was 33.0 vs. 19.8 months with very similar survival differences observed in an independent trial cohort of vemurafenib + cobimetinib. With immunotherapy (anti-PD-1/anti-PDL-1 or anti-CTLA4), obesity was associated with a ~40% lower risk of death after adjusting for other prognostic factors.
Interestingly, though obesity is associated with an increased risk of many malignancies, this is not the first time that paradoxical associations between obesity and improved outcomes have been observed.[32–34] Mechanisms underlying these associations remain controversial. Often, the survival advantage is limited to the overweight range (BMI 25-29.9) where adiposity may be misclassified (i.e. excess weight may be due to increased muscle mass rather than adipose tissue).[35] However, in melanoma a dose-response was observed with the risk of progression or death decreasing with increasing BMI through morbid obesity.[31] Other potential explanations for an obesity survival advantage are reverse causality wherein patients with more aggressive disease have antecedent weight loss and BMI category downward migration and enhanced “metabolic reserve” to withstand the wasting effects of cancer or its treatment in the obese.[36, 37] However, underweight BMI in metastatic melanoma is quite rare (<2%), and the BMI distribution in these cohorts mirrored that of the US population with ~65% of patients overweight or obese.[31] Most pointedly, the survival advantage of obesity in melanoma was specific to immune and targeted therapy which typically do not cause weight loss, and a BMI association was not found in chemotherapy cohorts.[38]
Prospective clinical trial data further allowed for adjustment for multiple potential confounders, including conventional prognostic factors, concomitant medications more commonly used by the obese that may have anti-cancer activity (metformin, aspirin, statins, and beta-blockers), and examination of rates of adverse events and pharmacokinetics, none of which appeared to underlie the observed associations.[31]
Interestingly, there was an interaction observed between biological sex and BMI wherein the obesity advantage was specific to males. Female sex is an established favorable prognostic factor in melanoma.[39, 40] In this study, obesity seemed to overcome the survival disadvantage associated with the male sex, with obese males and females of any BMI almost twice as likely to be alive at 2 years as normal BMI males (60-65% vs 35%).[31] This sex-BMI interaction was validated in a retrospective analysis of 139 metastatic melanoma patients treated with checkpoint inhibitors, where strong associations were again found between elevated BMI and improved survival in males (adjusted HR for OS 0.11 95% CI :0.03–0.4 for males BMI 25-35 vs. <25).[41]
This observation points to a potential hormonal mediator as the levels of both androgens and estrogens are altered in obesity due to adipocyte aromatase activity.[42] Melanoma does not express classical estrogen receptors. However, the recent discovery of a G-protein coupled estrogen receptor (GPER) in melanoma whose activation leads to increased differentiation [43, 44] may be the missing piece of the puzzle in explaining both sexual dimorphism in melanoma and the obesity paradox.[45] Alternatively, instead of effects on the tumor cells, hormones or other sex-specific factors could directly impact immune response, a reasonable alternative explanation given the sexual dimorphism in immunity in other contexts such as autoimmune disease.[46] However, why the sex differences would be consistent across stage and therapy and the obesity advantage specific to targeted and immune therapy is unclear.
Sex differences in BMI associations could also be mediated by differences in body composition (i.e. the relative amount of muscle vs adipose tissue at a given BMI), as was suggested by an interaction between serum creatinine (a surrogate of muscle mass), sex, and BMI.[41] Future investigations should assess the association of direct body composition measures of adipose and muscle tissue mass with outcomes, with preliminary data supporting a link between sarcopenia and toxicity with immune checkpoint blockade.[47]
Recent investigations suggest that the effects of obesity on response to immunotherapy may not be disease-specific. In two retrospective analyses of cohorts composed of multiple solid tumor types treated with anti-PD-1/PDL-1 immunotherapy in either Italy (n=976)[48] or the US (n=250),[49] associations between higher BMI and improved outcomes were again observed, with very similar 40-60% lower risk of death or progression in those with higher BMI Notably, the Italian cohort was predominantly composed of patients with non-small cell lung carcinoma (65%), a disease in which cachexia is prevalent and obesity has previously been associated with improved outcomes.[50] Importantly, however, elevated BMI was not just associated with survival but also a near doubling in response rate (BMI ≥25: ORR 41.2% vs. BMI<25: 20.9%, p<0.0001), supporting a true biological effect of obesity on treatment outcome.
This putative beneficial effect of obesity on response to checkpoint inhibitors is counterintuitive given the literature linking the chronic inflammatory state of obesity to an impaired adaptive immune response.[20] Indeed, in a recent study in multiple species, obese subjects demonstrated systemic T cell dysfunction characterized by higher checkpoint expression (e.g. PD-1), reduced proliferation, and diminished cytokine production, indicating the T cells were functionally “exhausted.”[49] Mechanistic studies implicated the adipokine leptin, which is known to signal through the JAK-STAT pathway, in upregulating T cell PD-1 expression. However, while tumor growth was increased in untreated obese mice with B16 melanoma, tumors in obese mice were paradoxically more responsive to anti-PD-1 than in their lean counterparts with a higher relative increase in immune infiltrate and a re-invigoration of T cells.[49] This suggests that checkpoint inhibition can overcome the obesity-induced exhausted T cell phenotype and that obesity-induced immunosuppression presents a key target that anti-PD-1 immunotherapy may overcome. However, this mechanism does not explain the survival advantage associated with obesity with targeted therapy or the sex-BMI interaction observed in melanoma.
Ultimately, there is probably not a singular mechanism, as human obesity is a complex metabolic phenotype that likely has pleotropic and potentially contradictory effects on tumor cell growth and immune surveillance. These effects may vary based on tumor stage, histology, molecular drivers, and treatment. Preclinical models are unlikely to recapitulate this heterogeneous biology; the interplay between obesity, diet, the microbiome, comorbidities, and host variables such as genetics, age, and sex in patients will require integrative modeling of human tissue and clinical variables.
Diet, the microbiome, and metabolism
The potential of using diet to influence outcomes in cancer is of great interest to patients and their caregivers. They frequently inquire about dietary guidance and are often frustrated by a perceived lack of interest in the subject by their oncologists (who are in turn frustrated by a lack of robust interventional data to guide recommendations). The role of diet on cancer risk has been well-studied, with cohesive recommendations from the American Cancer Society (ACS) and American Institute of Cancer Research (AICR) supporting a diet rich in vegetables and whole grains and low in red and processed meat and refined carbohydrates.[51] However, in melanoma specifically, there are very limited epidemiological data linking diet to risk.[52]
Preclinical mouse studies suggest that diet can impact tumor growth and treatment response as well as immune function; [53] however, though useful for isolating mechanism, mouse chow manipulation cannot reflect the complexity of human diet. Observational data in several malignancies supports that the ACS/AICR recommended diet for cancer prevention may be associated with lower risk of cancer progression or recurrence and improved survival.[54–57] However, it is difficult to fully disentangle the effects of diet from other lifestyle (obesity, physical activity) and demographic (socioeconomic status) factors in an observational study. Short-term interventional studies in humans have demonstrated that diet can impact key cancer-related biomarkers (e.g. high-fiber diet decreases colonocyte Ki67), providing an important proof-of-principal to this approach.[58, 59] However, to date, we lack conclusive interventional data that a change in diet after a diagnosis of cancer can significantly impact cancer recurrence and survival.[54, 60, 61] This is the goal of several ongoing studies.
Mounting evidence for a key role of the gut microbiome in shaping response to immunotherapy has also directed new research questions in nutrition, as diet is a key determinant of gut flora. The microbiome refers to the trillions of bacteria, viruses, and other organisms that live on and in the body. The most well-studied niche in relation to human health and disease is that of the gastrointestinal tract, i.e. the gut microbiome. The bi-directional interaction between the microbiome and the immune system has been an area of active investigation.[62] The initial work demonstrating that the microbiome may play a role in response to immune checkpoint blockade came from preclinical studies demonstrating differential response to therapy based on native gut microbiome and proof-of-principal studies that microbiome modulation can modify response to immunotherapy.[63, 64] Subsequent studies demonstrated the relevance to humans, with pro-immunotherapy-response gut microbiome profiles being described in multiple independent clinical cohorts.[65–69] Importantly, several of these studies further used germ-free mouse models to establish that responsiveness to immunotherapy was transferrable by fecal microbiota transplant (FMT) from human patients who responded to checkpoint inhibitors.[66, 67]
This work has prompted several planned or ongoing clinical trials of microbiome modulation to enhance response to immunotherapy using FMT or bacterial consortia.[70] These studies have also invigorated interest in how modifiable lifestyle factors that shape the microbiome might influence biology and outcomes in cancer, as the composition and diversity of our microbial ecosystem is mostly shaped by environmental factors and exposures.[71] Diet is a major determinant of the gut microbiome, and of particular interest is the observation that multiple cohorts have identified bacteria involved in dietary fiber digestion as being associated with response to immunotherapy.[68, 67, 66] These fiber-responsive bacteria produce short-chain fatty acids (SCFAs) which are the main nutrient source for intestinal epithelial cells, help maintain the gut mucosal barrier, and influence both mucosal and systemic immunity.[72, 73] Preliminary work by our group in an observational cohort of metastatic melanoma patients initiating therapy has further suggested that high-baseline dietary fiber intake correlates with a higher abundance of these favorable bacteria and improved response to anti-PD-1 based immunotherapy.[74, 75] However, causality and mechanism need to be established. Importantly, interventional studies in other populations have demonstrated that dietary change can rapidly alter the gut microbiome, though this implies also rapidly reversible if dietary changes not sustained [58, 76]
Beyond the microbiome, emerging data also suggests that diet may impact therapeutic response by altering the systemic metabolic phenotype. For example, the PI3K pathway is a commonly altered oncogenic signaling pathway, but its normal physiologic function is nutrient sensing and activated by binding of insulin and IGF to their cell-surface receptors. PI3K inhibitors, which are being tested as a treatment in multiple cancers, lead to disrupted glucose homeostasis and reflexive hyperinsulinemia, which can then reactivate the pathway and limit the efficacy of the compounds.[77] In preclinical models, a ketogenic diet effectively suppressed insulin feedback and potently synergized with PI3K inhibitors, decreasing PI3K signaling and tumor growth.[77] Though this approach was tested in multiple tumor models, data in melanoma are not available. However, the PI3K pathway is a key mediator of resistance to both targeted and immune therapy in melanoma [78, 30] and thus this would be a rational combinatorial strategy to test. A previous preclinical study suggested that the ketone body acetoacetate increased tumor growth in BRAF-mutant (but not NRAS-mutant) melanoma.[79] However, the diet used in the murine studies was not a true ketogenic diet and did not cause elevations in beta-hydroxybutyrate, the ketone body most commonly monitored in ketogenic diet studies. While the effects of a true ketogenic diet remain to be tested in melanoma, this study highlights potential interactions between systemic metabolism, driver mutations, and metabolic dependencies.
Ultimately, just as with drugs, there are multiple rational targets for dietary interventions, and personalized strategies will be biomarker dependent, with the added complexity that host factors (microbiome, metabolic phenotype) will likely be as important as the tumor targets (metabolic dependencies and oncogenic drivers).
Exercise
Another key player in the host energy balance equation is energy expenditure through physical activity and structured exercise training. Though exercise has been a longstanding component of therapy for many chronic conditions, only recently has it gained traction as an adjunct therapy for cancer. Exercise has been shown to be a safe intervention to improve physical functioning, fatigue, and quality of life in patients with cancer[80] and a growing body of evidence suggests that exercise may also modify the tumor microenvironment and cancer-related outcomes, though this has not been well-studied in the context of targeted or immune therapy.
Observational studies suggest correlations between self-reported physical activity and reduced risk and recurrence across many cancer types;[81–84] however, within the context of melanoma, data are mixed. A population-based study of 1.44 million Americans and Europeans suggested that leisure time physical activity is associated with a slightly higher risk of melanoma [84] though other studies have found an inverse relationship.[25, 85] These studies are likely confounded by sun exposure, complicating interpretation of these data. To our knowledge, no clinical data are available on the influence of post-diagnosis exercise on melanoma-related outcomes.
In addition to modifying energy balance and associated changes in body composition and BMI, exercise may directly influence the tumor microenvironment. Exercise is routinely incorporated into the care of patients with vascular disease because it can improve the structure and function of blood vessels. Tumor blood vessels are quite abnormal. Imbalances in pro- and anti-angiogenic signaling in a growing tumor lead to dysfunctional vessels characterized by dilatation, excessive branching, and leakiness.[86] Poorly functional vasculature causes tumor hypoxia which in turn enhances tumor invasiveness, promotes metastasis, and diminishes response to chemotherapy, targeted therapy, and radiotherapy.[86–91] Furthermore, hypoxia plays a key role in malignant transformation of melanocytes.[92] Once transformation occurs, rapid cell growth in a developing melanoma increases oxygen demand, further promoting hypoxia, leading to HIF-1α-driven angiogenesis and the development of poorly functioning tumor vasculature. Additionally, hypoxia signaling via HIF-1α likely plays a causal role in melanoma metastasis.[93]
To date, there are no approved interventions to decrease tumor hypoxia. Preclinical experiments suggest that exercise may do just that. In tumor-bearing mice, exercise enhances blood vessel structure and function, improving tumor perfusion and reducing hypoxia by as much as 50% in a process known as vascular normalization[94–96] and slows tumor growth in a variety of preclinical cancer models, including B16F10 melanoma.[97, 98] Vessel normalization also has crucial implications for therapeutic efficacy, with preclinical data from melanoma as well as other malignancies demonstrating that improvements in vascular structure, tumor oxygenation, and perfusion can improve intra-tumoral drug concentration and responsiveness to therapy.[86, 94, 96]Improvements in vessel structure and function may also facilitate recruitment of immune effector cells, because dysfunctional tumor vessels downregulate endothelial adhesion molecules critical for leukocyte infiltration of the tumor.[99]
Beyond vascular normalization, exercise may also directly influence effectors of both the innate and adaptive immune systems. In a murine models, exercise has been shown to increase NK cell infiltration [97], induce macrophage differentiation to a M1 (anti-tumor) phenotype, [100], and increase macrophage cytolytic activity.[101] With respect to the effects of exercise on adaptive anti-tumor immunity, data are scarce. Exercise intensity influences the magnitude of lymphocytosis and the distribution of T-cell subtypes[102], which in turn influences the expression of IL-2 and interferon-γ response to viral stimulation in healthy subjects.[103] Exercise studies in healthy subjects demonstrate that lymphocyte concentration in the blood peaks during or immediately after exercise, and rapidly returns to baseline or even below, likely reflecting redistribution into peripheral tissues.[102] Given the pivotal roles of immune surveillance in melanoma control and immunotherapy in the care of patients with melanoma, understanding how these pathways converge to influence anti-tumor immunity is increasingly important.
Conclusion
Modifiable patient-level factors may provide a new avenue for intervention for patients with advanced melanoma. Here we have reviewed the growing body of literature regarding the influence of obesity, the microbiome, diet, and exercise on pathways of tumor development, growth, progression, and response to targeted and immune therapies. Obesity is associated with worse prognosis in early stage melanoma but significantly improved outcomes in advanced melanoma patients treated with BRAF-targeted or immune checkpoint inhibitor therapy. Men may benefit from obesity more than women. Potential mechanisms include sex hormone effects on tumor and/or immune cells as well as leptin immunosuppressive effects on T cells which may paradoxically enhance responsiveness to anti-PD1 therapy. However, at this time it remains unclear how the “obesity paradox” could be exploited to improve outcomes. The gut microbiome has recently been shown in several seminal studies to play a role in response to immune checkpoint blockade, and modulation of the gut microbiome to enhance therapeutic response is being tested in multiple ongoing clinical studies. As diet is a key determinant of the gut microbiome, there is new interest in examining the diet/microbiome/immunity axis in the context of immunotherapy. Diet may also modulate systemic metabolism with downstream consequences on tumor growth, metabolism, and immunity. Observational data link exercise with reduced development, progression, and recurrence of many cancers, though data in melanoma are mixed. In preclinical models, exercise has been shown to slow melanoma tumor growth and improve the structure and function of tumor blood vessels, thereby decreasing tumor hypoxia and increasing delivery of anti-tumor agents. Each of these modifiable factors shows promise as a target for intervention to improve melanoma response to therapy, but interventional studies are needed to prove causality. Further, to turn these observations into safe and effective interventions for patients, we must understand mechanisms by which they influence host and tumor biology and then systematically study rational combinations.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
Allison Betof Warner declares that she has no conflict of interest.
Jennifer L. McQuade has received compensation from Merck for service as a consultant.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23.doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–9. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 3.Long GV, Weber JS, Infante JR, Kim KB, Daud A, Gonzalez R et al. Overall Survival and Durable Responses in Patients With BRAF V600–Mutant Metastatic Melanoma Receiving Dabrafenib Combined With Trametinib. Journal of Clinical Oncology. 2016;34(8):871–8. doi: 10.1200/jco.2015.62.9345. [DOI] [PubMed] [Google Scholar]
- 4.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. New England Journal of Medicine. 2015;372(26):2521–32. doi:doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 6.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM et al. Nivolumab plus Ipilimumab in Advanced Melanoma. New England Journal of Medicine. 2013;369(2):122–33. doi:doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kugel CH, Douglass SM, Webster MR, Kaur A, Liu Q, Yin X et al. Age Correlates with Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations. Clinical Cancer Research. 2018. doi: 10.1158/1078-0432.ccr-18-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ecker BL, Kaur A, Douglass SM, Webster MR, Almeida FV, Marino GE et al. Age-Related Changes in HAPLN1 Increase Lymphatic Permeability and Affect Routes of Melanoma Metastasis. Cancer Discov. 2019;9(1):82–95. doi: 10.1158/2159-8290.cd-18-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur A, Webster MR, Marchbank K, Behera R, Ndoye A, Kugel CH 3rd, et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature. 2016;532(7598):250–4. doi: 10.1038/nature17392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McQuade JL, Daniel CR, Hess KR, Davies MA. Sex as a predictor of response to cancer immunotherapy. Lancet Oncol. 2018;19(8):e376. doi: 10.1016/s1470-2045(18)30483-2. [DOI] [PubMed] [Google Scholar]
- *11.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body Fatness and Cancer — Viewpoint of the IARC Working Group. New England Journal of Medicine. 2016;375(8):794–8. doi:doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review of the evidence for obesity and cancer risk
- 12.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15(8):484–98. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 13.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11(12):886–95. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin PJ, Stambolic V. Impact of the obesity epidemic on cancer. Annu Rev Med. 2015;66:281–96. doi: 10.1146/annurev-med-051613-012328. [DOI] [PubMed] [Google Scholar]
- 15.Lee CH, Woo YC, Wang Y, Yeung CY, Xu A, Lam KS. Obesity, adipokines and cancer: an update. Clinical endocrinology. 2015;83(2):147–56. doi: 10.1111/cen.12667. [DOI] [PubMed] [Google Scholar]
- 16.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer epidemiology, biomarkers & prevention. 2002;11(12):1531–43. [PubMed] [Google Scholar]
- 17.Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis. Int J Cancer. 2009;124(3):698–712. doi: 10.1002/ijc.23943. [DOI] [PubMed] [Google Scholar]
- 18.Pollak M Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer.2008;8(12):915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 19.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J Clin Oncol. 2016;34(35):4270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen CJ, Murphy KE, Fernandez ML. Impact of Obesity and Metabolic Syndrome on Immunity. Adv Nutr. 2016;7(1):66–75. doi: 10.3945/an.115.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sergentanis TN, Antoniadis AG, Gogas HJ, Antonopoulos CN, Adami HO, Ekbom A et al. Obesity and risk of malignant melanoma: a meta-analysis of cohort and case-control studies. Eur J Cancer. 2013;49(3):642–57. doi: 10.1016/j.ejca.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 22.Skowron F, Berard F, Balme B, Maucort-Boulch D. Role of obesity on the thickness of primary cutaneous melanoma. J Eur Acad Dermatol Venereol. 2014. doi: 10.1111/jdv.12515. [DOI] [PubMed] [Google Scholar]
- 23.Brandon EL, Gu JW, Cantwell L, He Z, Wallace G, Hall JE. Obesity promotes melanoma tumor growth: role of leptin. Cancer Biol Ther. 2009;8(19):1871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amjadi F, Javanmard SH, Zarkesh-Esfahani H, Khazaei M, Narimani M. Leptin promotes melanoma tumor growth in mice related to increasing circulating endothelial progenitor cells numbers and plasma NO production. Journal of experimental & clinical cancer research : CR. 2011;30:21. doi: 10.1186/1756-9966-30-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gogas H, Trakatelli M, Dessypris N, Terzidis A, Katsambas A, Chrousos GP et al. Melanoma risk in association with serum leptin levels and lifestyle parameters: a case-control study. Ann Oncol. 2008;19(2):384–9. doi: 10.1093/annonc/mdm464. [DOI] [PubMed] [Google Scholar]
- 26.Oba J, Wei W, Gershenwald JE, Johnson MM, Wyatt CM, Ellerhorst JA et al. Elevated Serum Leptin Levels are Associated With an Increased Risk of Sentinel Lymph Node Metastasis in Cutaneous Melanoma. Medicine (Baltimore). 2016;95(11):e3073. doi: 10.1097/MD.0000000000003073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Di Martino JS, Bowman RL, Campbell NR, Baksh SC, Simon-Vermot T et al. Adipocyte-Derived Lipids Mediate Melanoma Progression via FATP Proteins. Cancer Discov. 2018;8(8):1006–25. doi: 10.1158/2159-8290.cd-17-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang S, Wang Y, Dang Y, Gagel A, Ross MI, Gershenwald JE et al. Association between Body Mass Index, C-Reactive Protein Levels, and Melanoma Patient Outcomes. J Invest Dermatol. 2017;137(8):1792–5. doi: 10.1016/j.jid.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Gopal YNV, Rizos H, Chen G, Deng W, Frederick DT, Cooper ZA et al. Inhibition of mTORC1/2 Overcomes Resistance to MAPK Pathway Inhibitors Mediated by PGC1α and Oxidative Phosphorylation in Melanoma. Cancer Research. 2014;74(23):7037–47. doi: 10.1158/0008-5472.can-14-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016;6(2):202–16. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **31.McQuade J, Daniel C, Hess K, Mak C, Wang D, Rai R et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncology. 2018;19(3):310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]; First demonstration that obesity associated with improved outcomes with immune and targeted therapy in melanoma in >1900 patients in 6 independent cohorts
- 32.Greenlee H, Unger JM, LeBlanc M, Ramsey S, Hershman DL. Association between Body Mass Index and Cancer Survival in a Pooled Analysis of 22 Clinical Trials. Cancer epidemiology, biomarkers & prevention. 2017;26(1):21–9. doi: 10.1158/1055-9965.EPI-15-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroenke CH, Neugebauer R, Meyerhardt J, Prado CM, Weltzien E, Kwan ML et al. Analysis of Body Mass Index and Mortality in Patients With Colorectal Cancer Using Causal Diagrams. JAMA Oncol. 2016;2(9):1137–45. doi: 10.1001/jamaoncol.2016.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albiges L, Hakimi AA, Xie W, McKay RR, Simantov R, Lin X et al. Body Mass Index and Metastatic Renal Cell Carcinoma: Clinical and Biological Correlations. J Clin Oncol. 2016. doi: 10.1200/JCO.2016.66.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E et al. Explaining the Obesity Paradox: The Association between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer epidemiology, biomarkers & prevention 2017;26(7):1008–15. doi: 10.1158/1055-9965.EPI-17-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lennon H, Sperrin M, Badrick E, Renehan AG. The Obesity Paradox in Cancer: a Review. Curr Oncol Rep. 2016;18(9):56. doi: 10.1007/s11912-016-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renehan AG, Sperrin M. The Obesity Paradox and Mortality After Colorectal Cancer: A Causal Conundrum. JAMA Oncol. 2016;2(9):1127–9. doi: 10.1001/jamaoncol.2016.0868. [DOI] [PubMed] [Google Scholar]
- 38.McQuade JL, Daniel CR, Davies MA. Body-mass index and metastatic melanoma outcomes - Authors’ reply. Lancet Oncol. 2018;19(5):e227–e8. doi: 10.1016/s1470-2045(18)30266-3. [DOI] [PubMed] [Google Scholar]
- 39.Joosse A, Collette S, Suciu S, Nijsten T, Lejeune F, Kleeberg UR et al. Superior outcome of women with stage I/II cutaneous melanoma: pooled analysis of four European Organisation for Research and Treatment of Cancer phase III trials. J Clin Oncol. 2012;30(18):2240–7. doi: 10.1200/JCO.2011.38.0584. [DOI] [PubMed] [Google Scholar]
- 40.Joosse A, Collette S, Suciu S, Nijsten T, Patel PM, Keilholz U et al. Sex Is an Independent Prognostic Indicator for Survival and Relapse/Progression-Free Survival in Metastasized Stage III to IV Melanoma: A Pooled Analysis of Five European Organisation for Research and Treatment of Cancer Randomized Controlled Trials. Journal of Clinical Oncology. 2013;31(18):2337–46. doi: 10.1200/jco.2012.44.5031. [DOI] [PubMed] [Google Scholar]
- 41.Naik GS, Waikar SS, Johnson AEW, Buchbinder EI, Haq R, Hodi FS et al. Complex inter-relationship of body mass index, gender and serum creatinine on survival: exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. Journal for ImmunoTherapy of Cancer. 2019;7(1):89. doi: 10.1186/s40425-019-0512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48(4):633–8. doi: 10.1210/jcem-48-4-633. [DOI] [PubMed] [Google Scholar]
- 43.Natale CA, Duperret EK, Zhang J, Sadeghi R, Dahal A, O’Brien KT et al. Sex steroids regulate skin pigmentation through nonclassical membrane-bound receptors. Elife. 2016;5. doi: 10.7554/eLife.15104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Natale CA, Li J, Zhang J, Dahal A, Dentchev T, Stanger BZ et al. Activation of G protein-coupled estrogen receptor signaling inhibits melanoma and improves response to immune checkpoint blockade. Elife. 2018;7. doi: 10.7554/eLife.31770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McQuade JL, Davies MA. Estrogen returns to the stage in melanoma. Pigment Cell Melanoma Res. 2018. doi: 10.1111/pcmr.12706. [DOI] [PubMed] [Google Scholar]
- 46.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–38. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 47.Daly LE, Power DG, O’Reilly A, Donnellan P, Cushen SJ, O’Sullivan K et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br J Cancer. 2017;116(3):310–7. doi: 10.1038/bjc.2016.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7(1):57. doi: 10.1186/s40425-019-0527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **49.Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25(1):141–51. doi: 10.1038/s41591-018-0221-5 [DOI] [PMC free article] [PubMed] [Google Scholar]; First mechanistic study of “obesity paradox” in immunotherapy demonstrating that obesity induces T cell exhaustion which can be overcome with PD1 immunotherapy
- 50.Li S, Wang Z, Huang J, Fan J, Du H, Liu L et al. Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: does the ‘obesity paradox’ really exist? European journal of cardiothoracic surgery : official journal of the European Association for Cardio-thoracic Surgery 2017;51(5):817–28. doi: 10.1093/ejcts/ezw386. [DOI] [PubMed] [Google Scholar]
- 51.Research WCRFAIfC. Diet, Nutrition, Physical Activity and Cancer: a Global Perspective. 2018. [Google Scholar]
- 52.Yang K, Fung TT, Nan H. An Epidemiological Review of Diet and Cutaneous Malignant Melanoma. Cancer epidemiology, biomarkers & prevention 2018;27(10):1115–22. doi: 10.1158/1055-9965.epi-18-0243. [DOI] [PubMed] [Google Scholar]
- 53.Soldati L, Di Renzo L, Jirillo E, Ascierto PA, Marincola FM, De Lorenzo A. The influence of diet on anti-cancer immune responsiveness. J Transl Med. 2018;16(1):75. doi: 10.1186/s12967-018-1448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rock CL, Doyle C, Demark‐Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL et al. Nutrition and physical activity guidelines for cancer survivors. CA: A Cancer Journal for Clinicians. 2012;62(4):242–74. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 55.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, Mayer RJ et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298(7):754–64. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 56.Kwan ML, Weltzien E, Kushi LH, Castillo A, Slattery ML, Caan BJ. Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer. J Clin Oncol. 2009;27(6):919–26. doi: 10.1200/jco.2008.19.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gregg JR, Zheng J, Lopez DS, Reichard C, Browman G, Chapin B et al. Diet quality and Gleason grade progression among localised prostate cancer patients on active surveillance. Br J Cancer. 2019;120(4):466–71. doi: 10.1038/s41416-019-0380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *58.O’Keefe SJ, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6:6342. doi: 10.1038/ncomms7342. [DOI] [PMC free article] [PubMed] [Google Scholar]; Controlled feeding study demonstrating that diet change can rapidly and reproducibly change gut microbiota
- 59.Demark-Wahnefried W, Polascik TJ, George SL, Switzer BR, Madden JF, Ruffin MTt et al. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer epidemiology, biomarkers & prevention 2008;17(12):3577–87. doi: 10.1158/1055-9965.epi-08-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. Jama. 2007;298(3):289–98. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006;98(24):1767–76. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 62.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature.2016;535(7610):75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 63.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–84. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–9. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frankel AE, Coughlin LA, Kim J, Froehlich TW, Xie Y, Frenkel EP et al. Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia. 2017;19(10):848–55. doi: 10.1016/j.neo.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–8. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–79. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 69.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- *70.McQuade JL, Daniel CR, Helmink BA, Wargo JA. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019;20(2):e77–e91. doi: 10.1016/s1470-2045(18)30952-5. [DOI] [PubMed] [Google Scholar]; Review of the evidence on role for microbiome in cancer as well as potential for modulation to change outcomes
- 71.Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–5. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 72.Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell. 2016;167(5):1339–53 e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luu M, Weigand K, Wedi F, Breidenbend C, Leister H, Pautz S et al. Regulation of the effector function of CD8(+) T cells by gut microbiota-derived metabolite butyrate. Sci Rep. 2018;8(1):14430. doi: 10.1038/s41598-018-32860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McQuade JL, Gopalakrishnan V, Spencer C, Andrews MA, Helmink BA, Cogdill AP et al. SMR Congress 2018 Abstracts: The gut microbiome of melanoma patients is distinct from that of healthy individuals and is impacted by probiotic and antibiotic use Pigment Cell & Melanoma Research. 2018;31(1):138–9. [Google Scholar]
- *75.Spencer C, Gopalakrishnan V, McQuade J, Andrews M, Helmink B, Khan M et al. , editors. The gut microbiome (GM) and immunotherapy response are influenced by host lifestyle factors American Association for Cancer Research; 2019. 4/2/19; Atlanta, GA. [Google Scholar]; Abstract of cohort of melanoma patients treated with anti-PD1 showing that high dietary fiber intake associated with increased abundance of pro-response bacteria and improved response to immunotherapy.
- 76.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hopkins BD, Pauli C, Du X, Wang DG, Li X, Wu D et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018. doi: 10.1038/s41586-018-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yennu Nanda VG, Hu Z, Thiele VM, Heffernan TP, DiFrancesco M, Masrszalek J et al. , editors. Targeting mitochondrial oxidative phosphorylation in de novo and acquired MAPK inhibitor-resistant melanomas Metabolism and Cancer; 2015. June 7-10; Bellevue, WA: AACR. [Google Scholar]
- 79.Xia S, Lin R, Jin L, Zhao L, Kang HB, Pan Y et al. Prevention of Dietary-Fat-Fueled Ketogenesis Attenuates BRAF V600E Tumor Growth. Cell Metab. 2017;25(2):358–73. doi: 10.1016/j.cmet.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buffart LM, Kalter J, Sweegers MG, Courneya KS, Newton RU, Aaronson NK et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91–104. doi: 10.1016/j.ctrv.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 81.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM.Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(11):815–40. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26(24):3958–64. doi:26/24/3958 [pii] 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kruk J, Aboul-Enein HY. Physical activity in the prevention of cancer. Asian Pac J Cancer Prev. 2006;7(1):11–21. [PubMed] [Google Scholar]
- 84.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern Med. 2016;176(6):816–25. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shors AR, Solomon C, McTiernan A, White E. Melanoma risk in relation to height, weight, and exercise (United States). Cancer causes & control: CCC. 2001;12(7):599–606. [DOI] [PubMed] [Google Scholar]
- 86.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071–121. doi:91/3/1071 [pii] 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giatromanolaki A, Sivridis E, Kouskoukis C, Gatter KC, Harris AL, Koukourakis MI.Hypoxia-inducible factors 1alpha and 2alpha are related to vascular endothelial growth factor expression and a poorer prognosis in nodular malignant melanomas of the skin. Melanoma Res. 2003;13(5):493–501. doi: 10.1097/01.cmr.0000056268.56735.4c. [DOI] [PubMed] [Google Scholar]
- 88.Loftus SK, Baxter LL, Cronin JC, Fufa TD, Program NCS, Pavan WJ. Hypoxia-induced HIF1alpha targets in melanocytes reveal a molecular profile associated with poor melanoma prognosis. Pigment Cell Melanoma Res. 2017;30(3):339–52. doi: 10.1111/pcmr.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee JT, Herlyn M. Microenvironmental influences in melanoma progression. Journal of cellular biochemistry. 2007;101(4):862–72. doi: 10.1002/jcb.21204. [DOI] [PubMed] [Google Scholar]
- 90.Li H, Chen J, Wang X, He M, Zhang Z, Cen Y. Nodal induced by hypoxia exposure contributes to dacarbazine resistance and the maintenance of stemness in melanoma cancer stemlike cells. Oncol Rep. 2018;39(6):2855–64. doi: 10.3892/or.2018.6387. [DOI] [PubMed] [Google Scholar]
- 91.Qin Y, Roszik J, Chattopadhyay C, Hashimoto Y, Liu C, Cooper ZA et al. Hypoxia-Driven Mechanism of Vemurafenib Resistance in Melanoma. Mol Cancer Ther. 2016;15(10):2442–54. doi: 10.1158/1535-7163.MCT-15-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bedogni B, Powell MB. Hypoxia, melanocytes and melanoma - survival and tumor development in the permissive microenvironment of the skin. Pigment Cell Melanoma Res. 2009;22(2):166–74. doi: 10.1111/j.1755-148X.2009.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hanna SC, Krishnan B, Bailey ST, Moschos SJ, Kuan PF, Shimamura T et al. HIF1alpha and HIF2alpha independently activate SRC to promote melanoma metastases. J Clin Invest. 2013;123(5):2078–93. doi: 10.1172/JCI66715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *94.Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR et al. Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. J Natl Cancer Inst. 2015;107(5). doi: 10.1093/jnci/djv040. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper was the first to demonstrate that exercise causes vascular normalization and augments chemotherapy-induced tumor growth delay in a preclinical model.
- 95.Jones LW, Antonelli J, Masko EM, Broadwater G, Lascola CD, Fels D et al. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J Appl Physiol (1985). 2012;113(2):263–72. doi: 10.1152/japplphysiol.01575.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schadler KL, Thomas NJ, Galie PA, Bhang DH, Roby KC, Addai P et al. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget. 2016;7(40):65429–40. doi: 10.18632/oncotarget.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **97.Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016;23(3):554–62. doi: 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]; This paper was the first to demonstrate exercise-induced growth delay in murine models of melanoma, with increased melanoma tumor NK cell infiltration, mediated by epinephrine and IL-6.
- *98.Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, Jones LW. Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer Res. 2016;76(14):4032–50. doi: 10.1158/0008-5472.CAN-16-0887 [DOI] [PMC free article] [PubMed] [Google Scholar]; Systematic review of the literature of 53 preclinical studies on the effects of exercise on cancer prevention and progression.
- 99.Ashcraft KA, Warner AB, Jones LW, Dewhirst MW. Exercise as Adjunct Therapy in Cancer.Semin Radiat Oncol. 2019;29(1):16–24. doi: 10.1016/j.semradonc.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abdalla DR, Aleixo AA, Murta EF, Michelin MA. Innate immune response adaptation in mice subjected to administration of DMBA and physical activity. Oncol Lett. 2014;7(3):886–90. doi: 10.3892/ol.2013.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lu Q, Ceddia MA, Price EA, Ye SM, Woods JA. Chronic exercise increases macrophage-mediated tumor cytolysis in young and old mice. Am J Physiol. 1999;276(2):R482–9. doi: 10.1152/ajpregu.1999.276.2.R482. [DOI] [PubMed] [Google Scholar]
- 102.Rooney BV, Bigley AB, LaVoy EC, Laughlin M, Pedlar C, Simpson RJ. Lymphocytes and monocytes egress peripheral blood within minutes after cessation of steady state exercise: A detailed temporal analysis of leukocyte extravasation. Physiol Behav. 2018;194:260–7. doi: 10.1016/j.physbeh.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 103.LaVoy EC, Hussain M, Reed J, Kunz H, Pistillo M, Bigley AB et al. T-cell redeployment and intracellular cytokine expression following exercise: effects of exercise intensity and cytomegalovirus infection. Physiol Rep. 2017;5(1). doi: 10.14814/phy2.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]


