Abstract
The non-viral delivery of genes into macrophages, known as hard-to-transfect cells, is a challenge. In this study, the microporation of a CpG-free and small plasmid (pCGfd-GFP) showed high transfection efficiency, sustainable transgene expression, and good cell viability in the transfections of Raw 264.7 and primary bone marrow-derived macrophages. The non-viral method using the pCGfd vector encoding anti-EGFR single-chain Fv fused with Fc (scFv-Fc) generated the macrophages secreting anti-EGFR scFv-Fc. These macrophages effectively phagocytized tumor cells expressing EGFR through the antibody-dependent mechanism, as was proved by experiments using EGFR-knockout tumor cells. Finally, peri-tumoral injections of anti-EGFR scFv-Fc-secreting macrophages were shown to inhibit tumor growth in the xeno-graft mouse model.
Keywords: Antibody-dependent cellular phagocytosis, Antibody-secreting macrophage, CpG-free plasmid, Macrophages, Nonviral gene delivery
INTRODUCTION
Immune cell therapy has been highlighted owing to the success of the engineered T cells expressing chimeric antigen receptors (CARs) (1). Although CAR T cell (CAR-T) therapy has been used to treat B-cell-derived lymphoma, the application of CAR-T against solid tumors has not been successful. To solve this problem, alternative immune cells, such as natural killer cells and macrophages, have attracted attention (1). Macrophages are professional phagocytes that engulf and digest pathogenic microbes, dead cells, and cellular debris. It has been reported that the phago-cytic ability of macrophages plays an important role in anti-cancer therapy, using monoclonal antibodies, such as rituximab, trastuzumab, daratumumab, and elotuzumab (2-5). The antibody binding to tumor-specific antigens results in an antibody coating of the tumor cell surfaces, which is known as opsonization. Then, macrophages efficiently engulf antibody-opsonized tumor cells via antibody-dependent cellular phagocytosis (ADCP) (6).
Initial clinical trials using autologous macrophages did not result in any meaningful therapeutic effects on cancer treatment, because the tumor microenvironment can polarize macrophages into the pro-tumorigenic phenotype (7). Therefore, pre-treatments, such as by ex vivo genetic engineering, are required for the macrophages to eliminate tumor cells (8). For the genetic engi-neering of macrophages, viral gene-delivery methods have been widely used because of their high transfection efficiency and long-term expression. However, their clinical applications are highly limited because of various potential concerns, including oncogenic transformation, pathogenic risks, and immune responses (9, 10). Many efforts have been made to develop clinically safe non-viral gene-delivery systems using plasmid vectors, which have the advantage of easy production as well as a lower possibility of chromosome integration. However, these strategies suffer from low transfection efficiency and short sustainability; moreover, macrophages are known as “hard-to-transfect” cells (11). Many strategies for the engineering of plasmids have been developed to increase transfection efficiency (12). A decreased plasmid size is one of the factors known to improve transfection efficiency, indicating that shortness is a crucial factor for vector design; this has led to the development of minimized vectors, such as minicircles and MIDGE (12, 13). Manipulating vector components have also been shown to increase transgene expression (12). The plasmids produced from Escherichia coli generally contain unmethylated cytosine-phosphate-guanine (CpG) dinucleotide sequences, which are recognized by the mammalian immune system through Toll-like receptor 9 (TLR9) and are known to induce both inflammatory responses and transgene silencing (14). Plasmids devoid of CpG sequences have been previously developed and used to improve transgene expression in the various tissues (15-21).
In this study, we employed a non-viral gene-delivery method using a CpG-free plasmid for the generation of macrophages that secrete anti-EGFR antibody. They efficiently eliminated tumor cells expressing EGFR through ADCP. The peri-tumoral injection of antibody-secreting macrophages suppressed tumor growth in a xenograft mouse model, indicating their potential use in the development of immune cell therapies.
RESULTS
Plasmids lacking CpG sequences greatly improved the transfection efficiency of macrophages
Although plasmids devoid of CpG sequences have been demonstrated to have high rates of transfection in several types of tissues and cell lines (15-21), there are currently no reports on their effect on macrophage transfection. To determine whether the removal of CpG sequences from plasmids improves trans-fection efficiency in macrophages, we used a commercially available CpG-free plasmid, pCpGfree-Lucia (3.6 kb), as a plasmid backbone. To facilitate this measurement, the reporter gene expressing Lucia luciferase was replaced with the GFP gene, resulting in the pCGf-GFP plasmid (3.7 kb) (Supplementary Fig. 1). Because smaller plasmids are associated with a better transfection efficiency (12, 13), we removed two MARs (IFN-β S/MAR and β-globin MAR) from the pCGf-GFP plasmid, creating pCGfd-GFP (2.5 kb) (Supplementary Fig. 1). As a control plasmid, pcDNA3.1 expressing GFP (pcDNA3.1-GFP) was also constructed.
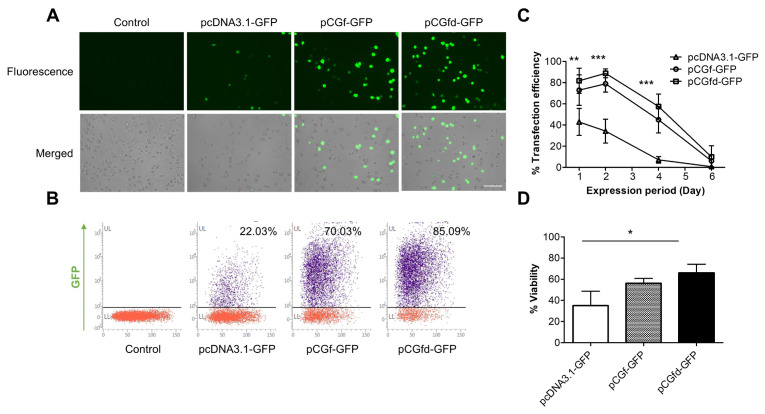
Microporation was used to deliver the plasmids into the RAW 264.7 macrophages. The microporation of the pCGf-GFP and pCGfd-GFP plasmids generated a much larger population of GFP-positive (GFP+) cells than that of pcDNA3.1-GFP (Fig. 1A). We analyzed the percentages of the GFP+ cells for pCGf-GFP and pCGfd-GFP by flow cytometry and found them to be 70% and 85%, respectively, whereas pcDNA3.1-GFP displayed a percentage of only 22% (Fig. 1B). Notably, the smallest plasmid, pCGfd-GFP, provided the highest efficiency. When the sustainability of GFP expression was analyzed for six days, the pCGf-GFP and pCGfd-GFP microporations exhibited a longer GFP expression than did pcDNA3.1-GFP (Fig. 1C). The fact that the pCGfd-GFP plasmid displayed sustainability like that of pCGf-GFP indicated that the beneficial effect of plasmid size reduction was greater than the negative effect induced by the removal of two MARs.
Fig. 1.
Improved transfection efficiency, prolonged sustainability, and increased cell viability by plasmids lacking CpG sequences. (A) GFP+ RAW 264.7 macrophages were analyzed by fluorescence microscopy two days after microporation. Scale bar = 100 µm. (B) GFP+ macro-phages were analyzed by flow cytometry. (C) The percentages of GFP+ macrophages were quantified for six days by flow cytometry. (D) Cell viability was measured using a Luna Dual fluorescence cell counter. Data represent the means ± standard deviation (SD), n = 3. Statistical significances were determined by two-way (C) or one-way (D) ANOVA, *P < 0.05, **P < 0.01, ***P < 0.001.
Because unmethylated CpG sequences have been known to induce inflammatory responses and cell cytotoxicity (15), next, we determined whether a reduction in CpG sequences increases cell viability. When the viabilities were analyzed one day after the microporation (Fig. 1D), pCGf-GFP and pCGfd-GFP showed significantly higher viabilities (56% and 66%) than did pcDNA3.1-GFP (35%).
Based on the results of the transfection efficiency, sustainability, and viability experiments, we selected pCGfd-GFP as the best plasmid for RAW 264.7 macrophage transfection.
pCGfd-GFP showed the best performance in primary macrophage transfection
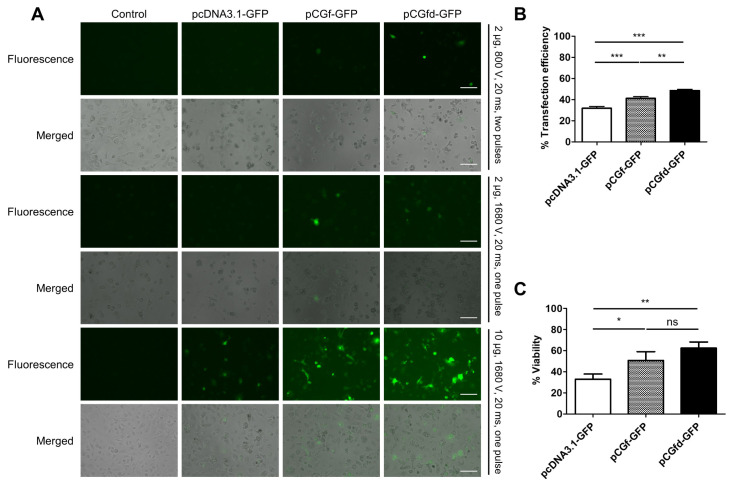
Next, we investigated the transfection of primary BMDMs because it is known to be extremely challenging (11). After testing a series of microporation conditions, we found an optimal condition (10 µg of plasmid, 1680 V, 20 ms, and one pulse) (Fig. 2A). More plasmid was required for efficient BMDM transfection than for RAW 264.7 macrophage transfection. Similar to the results for RAW 264.7 macrophage transfection, pCGfd-GFP showed the highest percentage (∼50%) of GFP+ cells and the maximum cell viability upon BMDM transfection (Fig. 2B and 2C).
Fig. 2.
Efficient transfection and good cell viability in BMDMs by pCGfd-GFP microporation. (A) GFP+ BMDMs were analyzed by fluo-rescence microscopy two days after microporation. Scale bar = 100 µm. (B) The percentages of GFP+ BMDMs were analyzed by flow cytometry. (C) Cell viability was measured using a Luna Dual fluorescence cell counter. Data represent the means ± SD, n = 3. Statisti-cal significances were determined by one-way ANOVA, *P < 0.05, **P < 0.01, ***P < 0.001.
Antibody-secreting macrophages can efficiently remove tumor cells through ADCP
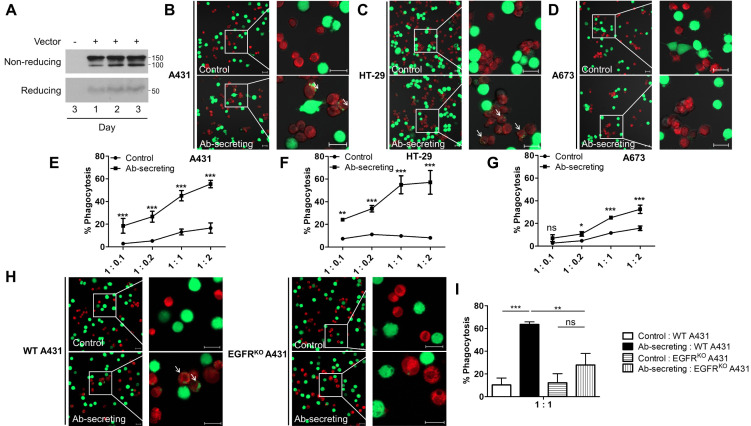
We designed the pCGfd vector for the secretion of anti-EGFR scFv fused with Fc (anti-EGFR scFv-Fc), because macrophages can recognize the Fc region of antibody-opsonized tumor cells for ADCP. To this end, the pCGfd-aEGFR-scFv-Fc encoding the signal peptide, anti-EGFR scFv, and Fc of IgG were constructed (Supplementary Fig. 2). Here, the anti-EGFR scFv sequences were derived from the light- and heavy-chain variable regions of cetuximab, which is currently used for anti-cancer therapy. After RAW 264.7 macrophage transfection, the amounts of anti-EGFR scFv-Fc gradually increased in the culture media for three days (Fig. 3A).
Fig. 3.
Anti-EGFR scFv-Fc-secreting macrophages efficiently phagocytized tumor cells expressing EGFR. (A) After pCGfd-aEGFR-scFv-Fc microporation, the culture media of the RAW 264.7 macrophages were collected for three days and analyzed in non-reducing and reducing conditions by immunoblotting using anti-human Fc antibody. (B-D) Deep red dye-stained macrophages (control and Ab-secreting) were co-incubated for 2 h with Calcein-stained (green fluorescent) A431 (B), HT-29 (C), or A673 (D) cells. The arrows indicate the macrophages phagocytizing the tumor cells. Scale bar = 20 µm. (E-G) After 2 h of co-incubating the macrophages and A431 (E), HT-29 (F), or A673 (G), the cells were analyzed by flow cytometry. The ratios of macrophage versus tumor cells were 1:0.1, 1:0.2, 1:1, and 1:2. (H, I) After co-incubating the macrophages with the WT or EGFRKO A431 cells, phagocytosis was analyzed by confocal microscopy (H) and flow cytometry (I). Scale bar = 20 µm. Data represent the means ± SD, n = 3. Statistical significances were determined by using two-way (E-G) or one-way (I) ANOVA, ns = non-significance, *P < 0.05, **P < 0.01, ***P < 0.001.
The analysis of phagocytosis was performed using three tumor cell lines, A431, HT-29, and A673, representing high-, medium-, and low-EGFR-expressing cells, respectively (Supplementary Fig. 3). The anti-EGFR scFv-Fc-secreting RAW 264.7 macrophages were found to efficiently phagocytize A431 and HT-29 cells (Fig. 3B and 3C), but the control macrophages did not. We used flow cytometry for the quantitative analysis of phagocytosis after co-incubating the macrophages and tumor cells for 2 h at various ratios (1:0.1, 1:0.2, 1:1, and 1:2) (Fig. 3E-3G). The anti-EGFR scFv-Fc-secreting macrophages showed a 3- to 7-fold higher percentage of phagocytosis (%) of A431 and HT-29 cells than did the control macrophages (Fig. 3E and 3F). The A673 cells expressing low levels of EGFR were also engulfed by the anti-EGFR scFv-Fc-secreting macrophages, although the phagocytosis was much less efficient (Fig. 3G).
To confirm that phagocytosis occurred by binding anti-EGFR scFv-Fc to EGFR on the surface of the tumor cells, we con-structed EGFRKO A431 cells using the CRISPR-Cas9 system based on the pCGfd vector (pCGfd-gR-EGFR-Cas9-2A-GFP) for efficient transfection (Supplementary Fig. 4). As expected, anti-EGFR scFv-Fc-secreting macrophages phagocytized very few EGFRKO A431 cells, which was in contrast with the efficient phagocytosis of wild-type A431 cells (Fig. 3H and 3I). These results clearly indicate that anti-EGFR scFv-Fc macrophages phagocytize EGFR-expressing tumor cells via ADCP.
Anti-EGFR scFv-Fc-secreting macrophages inhibit tumor growth in vivo
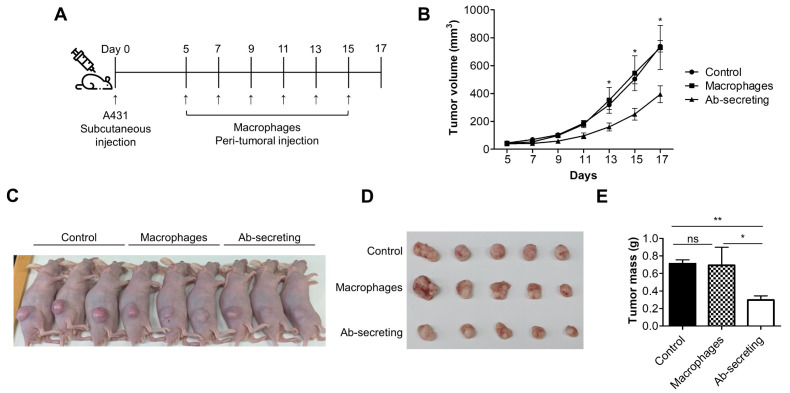
Last, we investigated whether anti-EGFR scFv-Fc-secreting macrophages were able to remove EGFR-expressing tumor cells in vivo using a xenograft mouse experiment (Fig. 4A). The peri-tumoral injection of anti-EGFR scFv-Fc-secreting RAW 264.7 macrophages clearly slowed A431 tumor growth more than did the injection of the control or the macrophages (Fig. 4B and 4C). Furthermore, the tumor volumes and masses were significantly smaller only in the mice injected with anti-EGFR scFv-Fc-secreting macrophages (Fig. 4D and 4E). No differences were found between the PBS and macrophage injections, which strongly suggests that the genetic engineering of macro-phages for the secretion of antibodies is essential for anti-tumor therapy.
Fig. 4.
Anti-EGFR scFv-Fc-secreting macrophages effectively inhibited tumor growth in vivo. (A) Schematic representation of the xenograft experiment. (B) The tumor volumes were measured on the indicated days. (C-E) Photos of the mice (C) and the incised tumors (D) were obtained after sacrifice. The tumor masses were measured (E). Data represent the means ± standard error mean (SEM), n = 5. Statistical significance was determined by using the one-tailed Mann Whitney U-test, ns = non-significance, *P < 0.05, **P < 0.01.
DISCUSSION
The potential of genetically engineering immune cells has been apparent, because CAR-T was used to achieve a successful cure for B-cell-derived lymphoma. To overcome the limitations of CAR-T, alternative immune cell therapies, including CAR-NK and CAR-macrophages, were developed (1, 22). Instead of CAR-macrophages, we developed genetically engineered macrophages that secrete anti-EGFR scFv-Fc. Compared to CAR, which is fixed on the cell surface, the secreting of scFv-Fc provides a more potent therapeutic advantage in attacking tumors, because of an increased capability for movement and penetration. Be-cause scFv-Fc can efficiently coat the tumor cells, the opsonized tumor cells should be effectively eliminated through ADCP by macrophages.
Many previous attempts to replace viral vectors for the genetic engineering of immune cells have not been successful (23). In this study, we successfully used CpG-free plasmid microporation for the efficient delivery of genes into primary BMDMs as well as RAW 264.7 macrophages. The commercially available CpG-free plasmid was engineered for size reduction by removing the S/MAR and β-globin MAR sequences. Although MARs have often been used in various vector systems to prolong transgene expression (24, 25), they have been reported to work in a context-dependent manner (26, 27). In our study, the removal of the two MARs increased the transfection efficiency, as well as the cell viability, without provoking any harmful effects on the sustainability of transgene expression. Our results indicate that the strategy used to reduce the plasmid size was better than the one used to insert beneficial cis-regulatory elements.
The highest transfection efficiency (∼50%) was obtained in the primary BMDMs, with reasonably good cell viability (∼60%), using pCGfd-GFP under the optimal microporation conditions. This result is a great improvement compared to previous reports (28). Plasmid transfection in BMDMs is a great challenge, because BMDMs can degrade nucleic acids, including DNA, into lysosomes via phagocytosis (29). The conventional methods (liposome, DEAD-dextran, and calcium phosphate co-precipitation) have previously failed to accomplish the efficient delivery of plasmids into BMDMs (30). Electroporation has been suggested as an alternative solution for BMDM transfection, because it can directly deliver plasmids; however, high levels of cell death (∼90%) have been associated with this method (28). Zhang et al. reported that nucleofection achieved high trans-fection efficiency in BMDMs. They did not, however, analyze its cell viability (11). Our strategy improved both the transfec-tion efficiency and cell viability, suggesting that unmethylated CpG sequences are the major cause of low transfection effici-ency and high levels of cell death in BMDM transfections. As such, pCGfd vector microporation could be a promising tool for the genetic engineering of macrophages.
A non-viral method using the pCGfd vector was successfully applied for the generation of antibody-secreting macrophages. We selected the antibody form of scFv-Fc to minimize the transgene size and use the opsonization function of Fc. RAW 264.7 macrophages transfected by pCGfd-aEGFR-scFv-Fc were able to secrete anti-EGFR scFv-Fc, thereby efficiently phagocytizing tumor cells expressing EGFR. The experiment using EGFRKO A431 cells proved that the process of phagocytosis occurred via ADCP. Since anti-EGFR scFv-Fc-secreting macrophages were shown to be effective, it could be extended by replacing the anti-EGFR antibody with other monoclonal antibodies already approved for use in cancer therapies. In our proof-of-concept experiments, we used only RAW 264.7 macrophages, because the use of BMDMs, which require the sacrifice of live mice, was limited. However, similar results could be obtained, considering the reasonably good transfection efficiency of the BMDMs, which was achieved by using pCGfd vector-based microporation.
We confirmed that anti-EGFR scFv-Fc-secreting macrophages retarded the growth of EGFR+ tumors in mice. Although the inhibition of tumor growth was visible in this in vivo experi-ment, it was somewhat disappointing that the anti-EGFR scFv-Fc-secreting macrophages could not fully eradicate the tumors. It was speculated that the antigen-presenting function of macro-phages did not work in the nude mice of the xenograft experi-ment, which was unable to generate mature T cells. Macrophages can present phagocytosed peptide fragments to T cells, sub-sequently inducing an adaptive immune response against the tumors. However, anti-EGFR scFv-Fc-secreting macrophages can eliminate only EGFR+ tumor cells via ADCP in nude mice, without the help of the adaptive immune response. If antibody-sec-reting macrophages were to be injected into immunocompe-tent mice, they might generate a much greater therapeutic effect via their antigen-presenting function, eliciting an adap-tive immune response in addition to ADCP.
In summary, in this study, we developed a CpG-free plasmid microporation method for the efficient transfection of macro-phages and proved that these engineered macrophages were able to secrete antibodies and eliminate tumors via ADCP. As such, the findings presented here will contribute to the development of novel immune cell therapies for the treatment of cancer.
MATERIALS AND METHODS
The detailed methods are described in the “Supplementary Materials and Methods”
Supplemental Materials
ACKNOWLEDGEMENTS
This work was supported by the grants of the National Re-search Foundation of Korea [NRF-2018R1A2B6003237], Next-Generation BioGreen 21 Program of the Rural Development Administration [PJ013320], and Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
References
- 1.Leslie M. New cancer-fighting cells enter trials. Science. 2018;361:1056–1057. doi: 10.1126/science.361.6407.1056. [DOI] [PubMed] [Google Scholar]
- 2.Overdijk MB, Verploegen S, Bogels M, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7:311–321. doi: 10.1080/19420862.2015.1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao MP, Alizadeh AA, Tang C, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y, Fan X, Deng H, et al. Trastuzumab triggers phagocytic killing of high HER2 cancer cells in vitro and in vivo by interaction with Fcgamma receptors on macro-phages. J Immunol. 2015;194:4379–4386. doi: 10.4049/jimmunol.1402891. [DOI] [PubMed] [Google Scholar]
- 5.Kurdi AT, Glavey SV, Bezman NA, et al. Antibody-Dependent Cellular Phagocytosis by Macrophages is a Novel Mechanism of Action of Elotuzumab. Mol Cancer Ther. 2018;17:1454–1463. doi: 10.1158/1535-7163.MCT-17-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michaud HA, Eliaou JF, Lafont V, Bonnefoy N, Gros L. Tumor antigen-targeting monoclonal antibody-based immunotherapy: Orchestrating combined strategies for the development of long-term antitumor immunity. Oncoimmunology. 2014;3:e955684. doi: 10.4161/21624011.2014.955684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S, Kivimae S, Dolor A, Szoka FC. Macrophage-based cell therapies: The long and winding road. J Control Release. 2016;240:527–540. doi: 10.1016/j.jconrel.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genard G, Lucas S, Michiels C. Reprogramming of Tumor-Associated Macrophages with Anticancer Therapies: Radiotherapy versus Chemo- and Immunotherapies. Front Immunol. 2017;8:828. doi: 10.3389/fimmu.2017.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelman A. The ups and downs of gene expression and retroviral DNA integration. Proc Natl Acad Sci U S A. 2005;102:1275–1276. doi: 10.1073/pnas.0409587101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Edwards JP, Mosser DM. The expression of exogenous genes in macrophages: obstacles and opportunities. Methods Mol Biol. 2009;531:123–143. doi: 10.1007/978-1-59745-396-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardee CL, Arevalo-Soliz LM, Hornstein BD, Zechiedrich L. Advances in Non-Viral DNA Vectors for Gene Therapy. Genes (Basel) 2017;(Basel):65. doi: 10.3390/genes8020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mun JY, Shin KK, Kwon O, Lim YT, Oh DB. Minicircle microporation-based non-viral gene delivery improved the targeting of mesenchymal stem cells to an injury site. Biomaterials. 2016;101:310–320. doi: 10.1016/j.biomaterials.2016.05.057. [DOI] [PubMed] [Google Scholar]
- 14.Latz E, Schoenemeyer A, Visintin A, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 15.Hyde SC, Pringle IA, Abdullah S, et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat Biotechnol. 2008;26:549–551. doi: 10.1038/nbt1399. [DOI] [PubMed] [Google Scholar]
- 16.Lesina E, Dames P, Flemmer A, et al. CpG-free plasmid DNA prevents deterioration of pulmonary function in mice. Eur J Pharm Biopharm. 2010;74:427–434. doi: 10.1016/j.ejpb.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Lesina E, Dames P, Rudolph C. The effect of CpG motifs on gene expression and clearance kinetics of aerosol administered polyethylenimine (PEI)-plasmid DNA complexes in the lung. J Control Release. 2010;143:243–250. doi: 10.1016/j.jconrel.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Pringle IA, Hyde SC, Connolly MM, et al. CpG-free plasmid expression cassettes for cystic fibrosis gene therapy. Biomaterials. 2012;33:6833–6842. doi: 10.1016/j.biomaterials.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Mann CJ, Anguela XM, Montane J, et al. Molecular signature of the immune and tissue response to non-coding plasmid DNA in skeletal muscle after electrotransfer. Gene Ther. 2012;19:1177–1186. doi: 10.1038/gt.2011.198. [DOI] [PubMed] [Google Scholar]
- 20.Yew NS, Zhao H, Przybylska M, et al. CpG-depleted plasmid DNA vectors with enhanced safety and long-term gene expression in vivo. Mol Ther. 2002;5:731–738. doi: 10.1006/mthe.2002.0598. [DOI] [PubMed] [Google Scholar]
- 21.Hodges BL, Taylor KM, Joseph MF, Bourgeois SA, Scheule RK. Long-term transgene expression from plasmid DNA gene therapy vectors is negatively affected by CpG dinucleotides. Mol Ther. 2004;10:269–278. doi: 10.1016/j.ymthe.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Morrissey MA, Williamson AP, Steinbach AM, et al. Chimeric antigen receptors that trigger phagocytosis. eLife. 2018;7:e36688. doi: 10.7554/eLife.36688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosaad YM. Hematopoietic stem cells: an overview. Transfus Apher Sci. 2014;51:68–82. doi: 10.1016/j.transci.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Piechaczek C, Fetzer C, Baiker A, Bode J, Lipps HJ. A vector based on the SV40 origin of replication and chromosomal S/MARs replicates episomally in CHO cells. Nucleic Acids Res. 1999;27:426–428. doi: 10.1093/nar/27.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramezani A, Hawley TS, Hawley RG. Perfor-mance- and safety-enhanced lentiviral vectors containing the human interferon-beta scaffold attachment region and the chicken beta-globin insulator. Blood. 2003;101:4717–4724. doi: 10.1182/blood-2002-09-2991. [DOI] [PubMed] [Google Scholar]
- 26.Schubeler D, Mielke C, Maass K, Bode J. Scaffold/matrix-attached regions act upon transcription in a context-dependent manner. Biochemistry. 1996;35:11160–11169. doi: 10.1021/bi960930o. [DOI] [PubMed] [Google Scholar]
- 27.Giannakopoulos A, Stavrou EF, Zarkadis I, Zoumbos N, Thrasher AJ, Athanassiadou A. The functional role of S/MARs in episomal vectors as defined by the stress-induced destabilization profile of the vector sequences. J Mol Biol. 2009;387:1239–1249. doi: 10.1016/j.jmb.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 28.Stacey KJ, Ross IL, Hume DA. Electroporation and DNA-dependent cell death in murine macrophages. Immunol Cell Biol 71 ( Pt. 1993;71:75–85. doi: 10.1038/icb.1993.8. [DOI] [PubMed] [Google Scholar]
- 29.Rupprecht AP, Coleman DL. Transfection of adherent murine peritoneal macrophages with a reporter gene using DEAE-dextran. J Immunol Methods. 1991;144:157–163. doi: 10.1016/0022-1759(91)90082-Q. [DOI] [PubMed] [Google Scholar]
- 30.Thompson CD, Frazier-Jessen MR, Rawat R, Nordan RP, Brown RT. Evaluation of methods for transient transfection of a murine macrophage cell line, RAW 264.7. Biotechniques. 1999;27:824–826. 828–830, 832. doi: 10.2144/99274rr05. [DOI] [PubMed] [Google Scholar]
- 31.Shin DM, Yang CS, Yuk JM, et al. Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol. 2008;10:1608–1621. doi: 10.1111/j.1462-5822.2008.01151.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.