Abstract
Enantioselective conjunctive cross-coupling of enyne-derived boronate complexes occurs with 1,4 addition of the electrophile and migrating group across the π system. This reaction pathway furnishes α-boryl allenes as the reaction product. In the presence of a chiral catalyst, both the central and axial chirality of the product can be controlled during product formation.
Keywords: Boron, Cross-Coupling, Palladium, Catalysis
Graphical Abstract
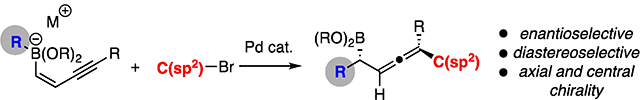
Chiral Allenes: 1,4-Addition of the migrating group and electrophile across an enyne-derived boronate complex occurs during the course of Pd-catalyzed conjunctive cross-coupling. This process generates chiral allenyl boronic esters with control of both axial and central chirality.
Allenes are important structural motifs that are found in a number of natural products1 and often find use as synthetic intermediates.2 Accordingly, a substantial body of work has been dedicated to the enantioselective synthesis of axially chiral allenes.3,4 Because of their ability to undergo cyclization to afford biologically-relevant oxygenated heterocycles5, particular attention has been paid to the synthesis of chiral substituted α-allenols. These compounds have been prepared by the stereospecific SN2’ reaction between organocuprates and propargylic alcohols, and by the transition-metal catalyzed coupling of propargylic epoxides and organic nucleophiles. In both cases, the axial chirality of the allenes arises by transfer of axial chirality from the starting materials to the products.6 More recently, Ma reported an elegant copper-catalyzed synthesis of α-allenols by coupling aldehydes and terminal alkynes wherein the axial chirality is controlled by the ligand on the copper complex.7 Despite these advances in the catalytic synthesis of α-allenols, there is still a lack of catalytic methods that allow for α-allenol construction with catalyst-based control of both axial and central chirality. Indeed, to the best of our knowledge, the Mukaiyama-type aldol reaction reported by List is the only method where a catalyst controls both axial and central chirality of α-allenols during the formation of the product.8 Herein, we report the application of catalytic conjunctive cross-coupling to the synthesis of α-allenyl boronates, which are direct precursors not only to α-allenols, but also an array of other compounds. Of note, the processes reported herein occur catalytically, and in a highly enantioselective and diastereoselective fashion.
The reaction design we undertook involves a conjunctive cross-coupling9 reaction of an enyne-derived boronate complex (A, Scheme 1) with an electrophile under the influence of a Pd catalyst to give allene-containing boronic ester B. It was expected that activation of A might occur by alkyne activation (C, inset Scheme 1), such that 1,2 boronate rearrangement would form the allenyl palladium intermediate (D). Upon reductive elimination, this sequence would generate allenyl boronic ester B. In practice, selective reaction by this pathway was found to face several challenges: first, unlike activation of simple alkenyl boronates where the chiral Pd complex directly binds to a prochiral alkene substrate, the Pd complex in C is further removed from the incipient stereogenic center such that effective stereoinduction is more challenging. Second, while activation of ate complex is proposed to occur by alkyne activation (C→D), activation of the substrate might also occur through alkene association (E→F) and this can lower the chemoselectivity of the process. Moreover, competitive if interconversion between D and F is faster than reductive elimination, then competitive activation of the alkene may also lower the stereoselectivity.
Scheme 1.
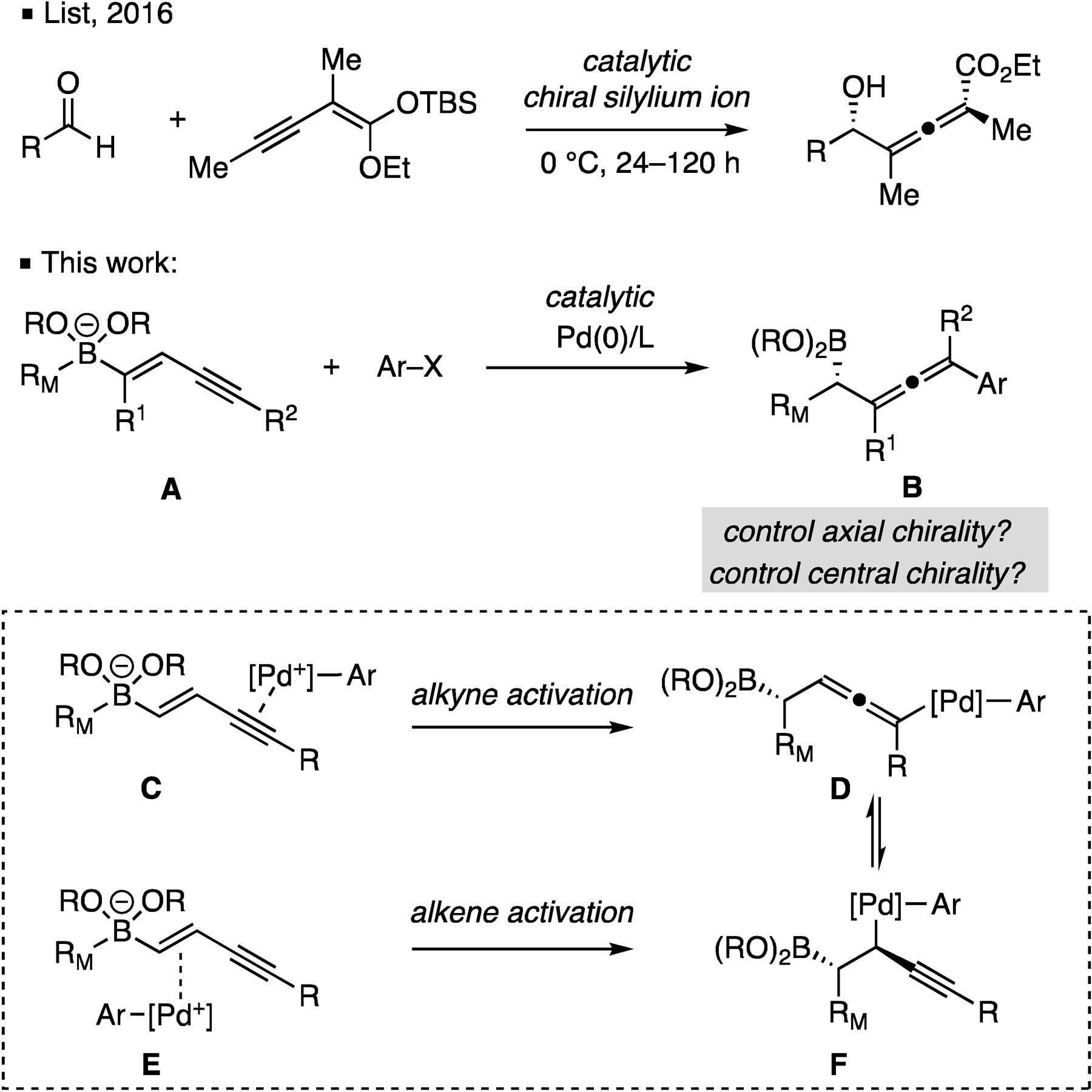
Issues pertaining to the control of both axial and central chirality in catalytic construction of chiral allenes.
To initiate our investigation of the conjunctive coupling with enynyl boronates, trans-borylenyne 1 was subjected to conjunctive coupling with 3 mol% Pd(OAc)2, 3.6 mol% MandyPhos ligand L1, and phenyl triflate. As shown in Scheme 2, this process indeed delivered the allene-containing product 2 in acceptable levels of enantioselectivity and yield; however, the reaction diastereoselectivity was poor (Scheme 2). A survey of phosphine ligands and reaction conditions (see SI) did not improve the outcome. It was reasoned that the two diastereomers in this reaction would originate from competing syn- and anti-periplanar migrations (I and II, Scheme 2) and with trans enyne-derived boronate, the Pd complex may be situated too far from the incipient stereogenic center to control the topological course of the 1,2 shift. To address this issue, we considered reaction of cis enyne-derived boronates. As depicted in III and IV (Scheme 2), this alternate olefin configuration would bring the four-coordinate boron center in closer proximity to the alkyne-bound palladium catalyst, such that anti-periplanar migration (I) would be favored in order to avoid steric repulsions between the migrating group and Pd catalyst.
Scheme 2.
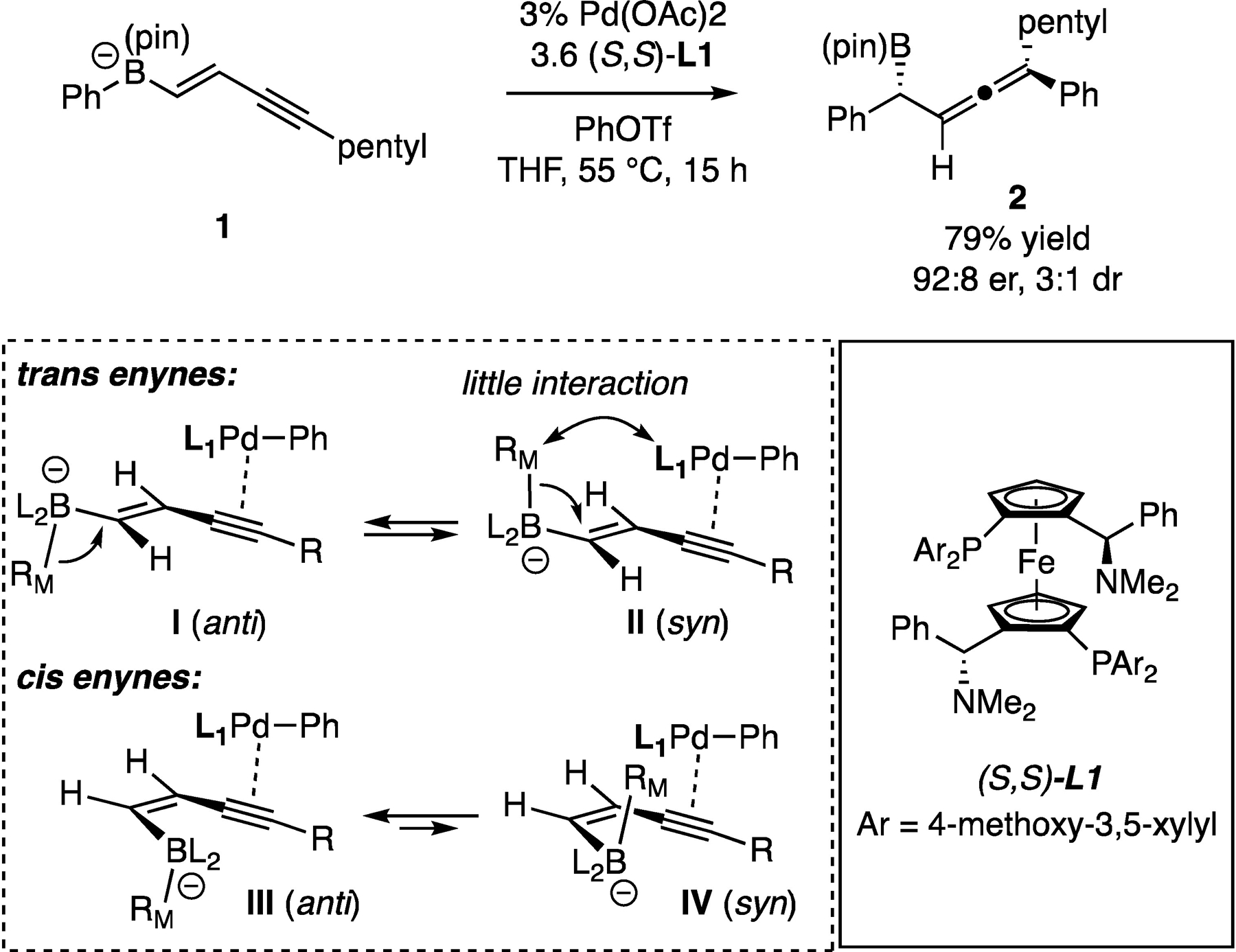
Preliminary observation in conjunctive coupling with enyne-derived boronate 1 and an analysis of the impact of alkene stereochemistry on the reaction.
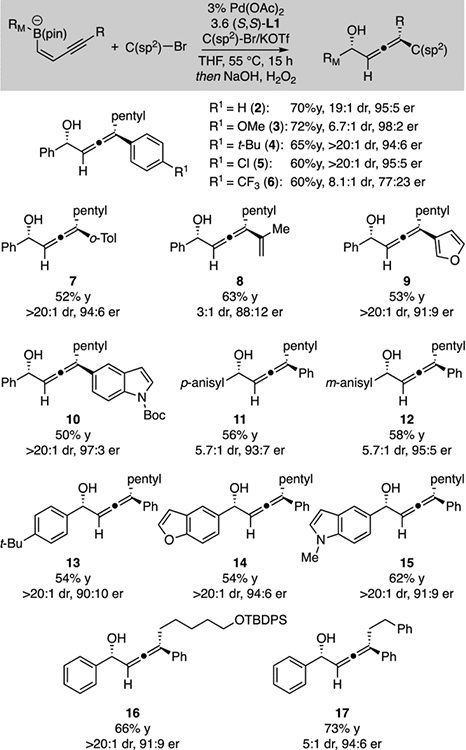
In practice, the use of cis-enyne-derived boronates10 led to significantly enhanced diastereoselectivity, while retaining high enantioselectivity and product yield in the conjunctive cross-coupling reaction. As depicted in Table 1, a number of electrophiles and migrating groups were examined with the cis-enyne substrate, and yields and selectivities are generally good. Of note, aryl bromides could be used as the electrophile and provide comparable yield and selectivity to the aryl triflates. The reaction operates with both electron-rich (3) and electron-poor (6) arene electrophiles, providing the corresponding α-allenols upon oxidative workup. An alkenyl electrophile also reacted with reasonable selectivity to furnish the enallene product (8). Also of note, heterocycles such as furan and indole derivatives can be employed as the electrophile (9, 10) or migrating group (14, 15) and alkyne substituents other than a pentyl group can be employed (16, 17).
Table 1.
Conjunctive coupling of aryl enynyl boronate complexesa
|
Yields are isolated yield of product and reflect an averaged outcome of two experiments. Diastereoselectivity (dr) determined by 1H NMR analysis of the unpurified reaction product and 13C NMR analysis of purified compounds; enantioselectivity (er) determined by chiral SFC analysis.
The conditions employed for aryl migration in Table 1 proved to be ineffective for alkyl migration, providing products with inferior enantio- and diastereoselectivity. In an attempt to improve the outcome for this important class of products, we examined other achiral boron ligands that might alter catalyst–substrate steric interactions. As depicted in Table 2, whereas the pinacol-derived boronate provided the product in 3:1 dr and in a near-racemic fashion (18), the larger “mac”-derived substrate provided the product (19) in enhanced enantioselectivity. Probing this alternate ligand framework, it was found that the secondary alcohol derived ligand, “hac”, provided the product (20) in both high enantio- and diastereoselectivity. With the “hac” ligand, the modest yield of 20 was due to competitive direct transmetallation, providing Suzuki-Miyaura coupling products. Inspired by a previous report9g, it was found that the direct transmetallation could be minimized by adding steric encumbrance near the boronate oxygen ligands that might prevent O–Pd interaction11 required for transmetallation. Thus, the 3,7-dimethyl substituted “hac*” ligand provided the desired product 21 in acceptable yield and both high enantio- and diastereoselectivity.
Table 2.
Influence of the boron ligand on conjunctive coupling with aliphatic migrating groupsa
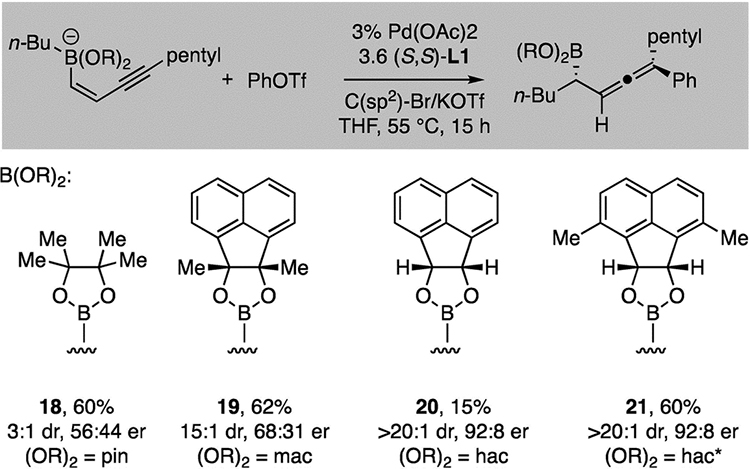
|
Yields are isolated yield of product and reflect an averaged outcome of two experiments. Diastereoselectivity (dr) determined by 1H NMR analysis of the unpurified reaction product and 13C NMR analysis of purified compounds; enantioselectivity (er) determined by chiral SFC analysis.
With an effective strategy for alkyl migration in conjunctive coupling with enyne-derived boronates, other substrates were examined in this reaction (Table 3). Because of the limited availability of functionalized alkyllithium reagents and their impractical synthesis on a laboratory scale, conditions were developed for use of more easily accessible alkyl Grignard reagents in the conjunctive coupling reaction. While a detailed optimization is described in the Supporting Information, critical features are the use of 14 equivalents of DMSO to stabilize the boronate complex, along with the use of CsF to improve reactivity in the coupling reaction, along with sodium or potassium triflate to sequester the halide which is an inhibitor of couplings.9b With these conditions, it was found that simple aliphatic groups can migrate in the coupling reaction (22, 23) and that the reaction can easily process side chains containing alkene (24), silyl ether (26), acetal (27), and alkyne (28) functional groups as well as a secondary migrating carbon atom (25), although a tert-butyl group failed to migrate under these conditions.
Table 3.
Conjunctive coupling of enynyl boronates derived from aliphatic Grignard reagentsa
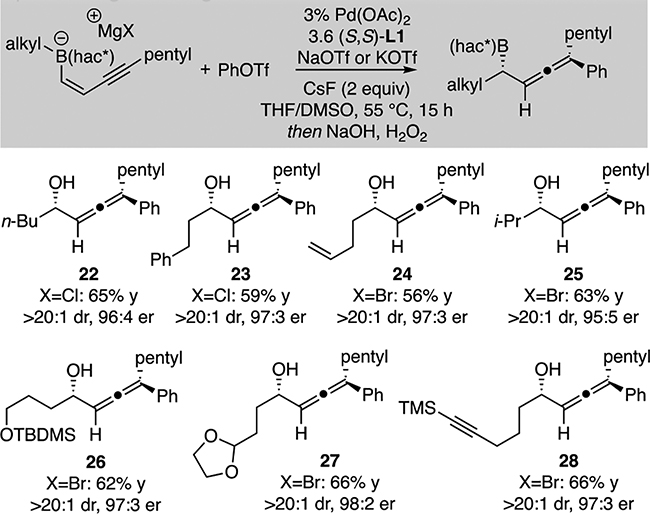
|
Yields are isolated yield of product and reflect an averaged outcome of two experiments. Diastereoselectivity (dr) determined by 1H NMR analysis of the unpurified reaction product and 13C NMR analysis of purified compounds. Enantioselectivity (er) determined by chiral SFC analysis. NaOTf used for RMgCl, KOTf added for RMgBr.
As alluded to in the introduction, activation of the enyne-derived boronate might occur through either alkene or alkyne activation, with interconversion of vinyl and propargyl palladium complexes providing a route for both activation modes to deliver the allene product. Insight into the dynamic features of this process was gained through the reaction of a more hindered boronate. As depicted in Scheme 3, when cyclohexyl-substituted boronate 29 was subjected to the reaction conditions, alkyne product 31 was produced in addition to expected allene 30. Of note, the enantioselectivity of compounds 30 and 31 is significantly different. Because the π-σ-π isomerization that would interconvert alkenyl palladium complex V and propargyl palladium VI is known to be stereospecific12, the divergent stereoselectivity observed in 30 and 31 suggests that these products are unlikely to arise from a rapidly interconverting common intermediate, but rather are formed by distinct reaction pathways, mostly likely involving alkyne versus alkene activation as depicted in Scheme 3.
Scheme 3.
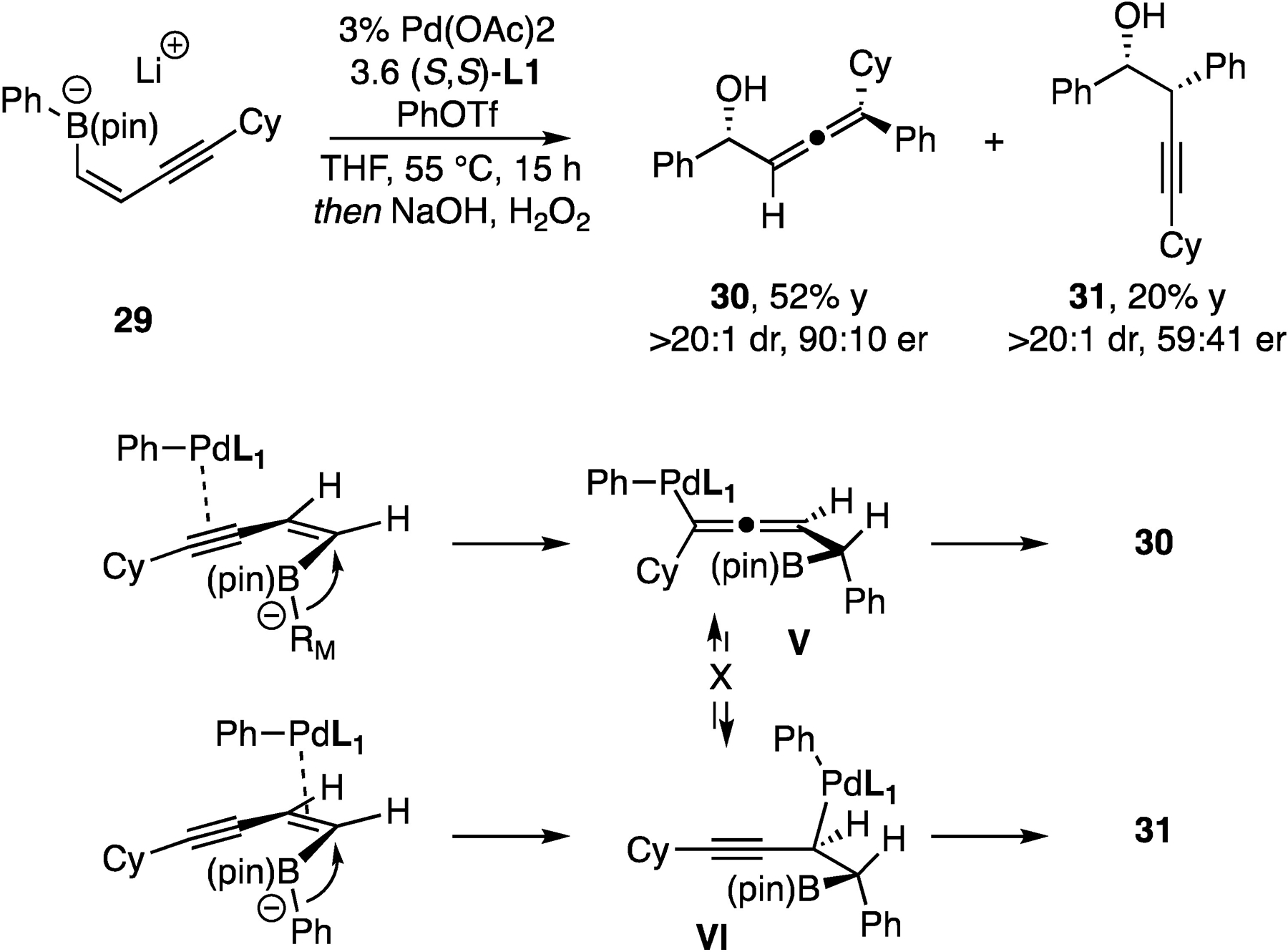
Origin of chemoselectivity in conjunctive coupling of enyne-derived boronates.
To assess the utility of the obtained α-allenols in oxygenated heterocycle synthesis, we first examined a AuCl3-catalyzed cyclization13 reaction to afford 2,5-dihydrofuran compound 32 (Scheme 4). This reaction was found to occur with excellent preservation of the stereochemical integrity of the stereogenic center in the starting material and established a second stereogenic center with a high level of selectivity (>20:1 dr). In a second reaction, it was found that the allenol substrate could undergo stereoselective epoxidation followed by intramolecular epoxide opening to afford tetrahydrofuranone 33.14 In both cases, the reactions proceeded with transfer of axial chirality to central chirality, forming a new quaternary stereogenic center with stereocontrol.
Scheme 4.
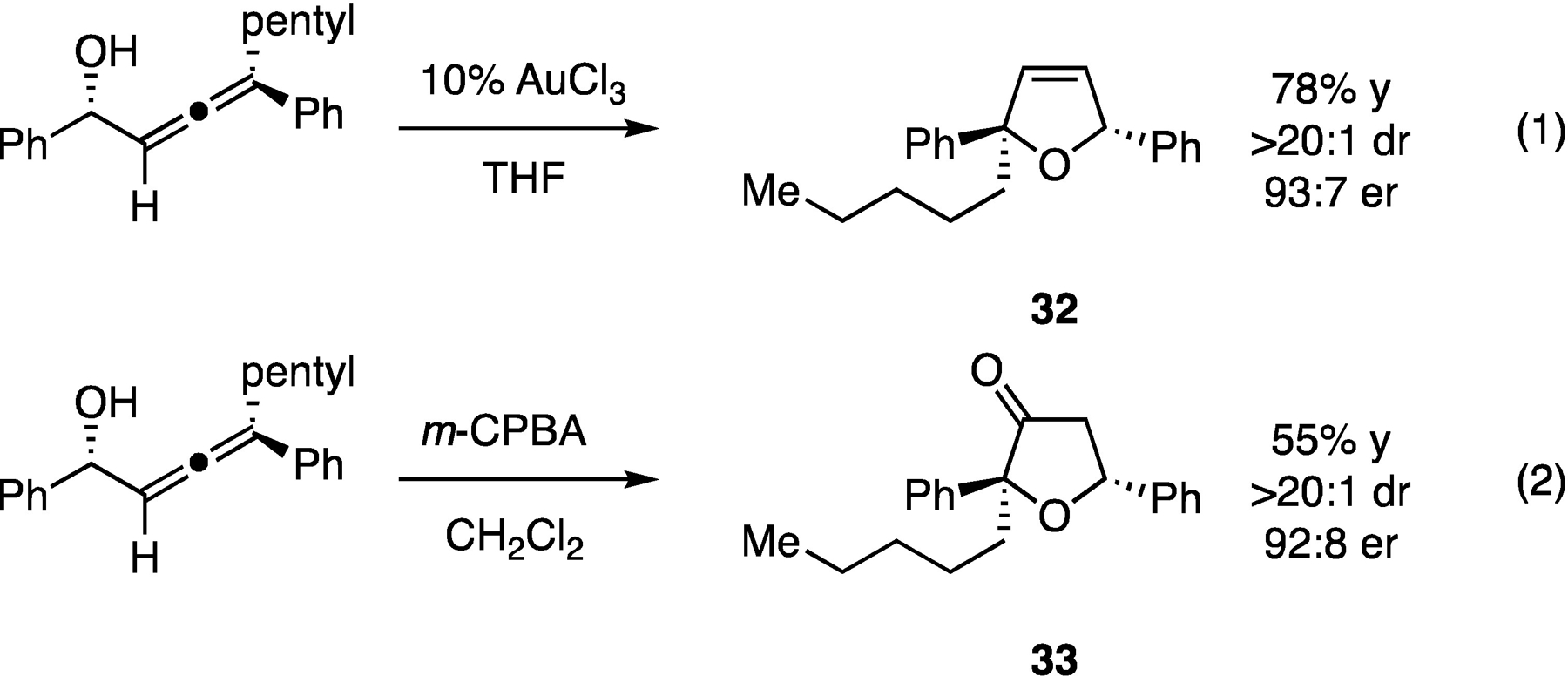
Construction of tetrahydrofuran derivatives from α-allenols.
In conclusion, we have reported a catalytic conjunctive coupling reaction that allows for the enantio- and diastereoselective synthesis of α-borylallenes from simple achiral borylenynes. Both aliphatic and aromatic groups can migrate in this process, but they require different boron ligands for the realization of both high yield and stereoselectivity. Further studies on the applications of this method in synthesis of natural products are underway.
Supplementary Material
Acknowledgements
We thank Prof. Yao Fu (USTC) for sharing procedures for the preparation of cis enyne-containing boronic esters and we thank Prof. Jay Siegel (Tianjin University) for providing substituted acenapthoquinones used in the preparation of hac*. This work was supported by a grant from the US National Institutes of Health (NIGMS GM-R35-127140).
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1]. For selected reviews on allene-containing natural products, see:; a) Krause N, Hashmi ASK, Modern Allene Chemistry; Wiley–VCH: 2004; [Google Scholar]; b) Hoffmann-Röder A, Krause N, Angew. Chem., Int. Ed 2004, 43, 1196–1216. [DOI] [PubMed] [Google Scholar]
- [2]. For selected reviews on the synthetic utility of allenes, see:; a) Brummond KM, Chen H, In Modern Allene Chemistry; Wiley-VCH: 2004; [Google Scholar]; b) Hashmi ASK, Angew. Chem. Int. Ed 2000, 39, 3590–3593; [PubMed] [Google Scholar]; e) Ma S, Chem. Rev 2005, 105, 7, 2829–2872.; [DOI] [PubMed] [Google Scholar]; d) Ma S, S. Acc. Chem. Res 2009, 42, 1679–1688; [DOI] [PubMed] [Google Scholar]; c) Yu S, Ma S, Angew. Chem. Int. Ed 2012, 51, 3074–3112; [DOI] [PubMed] [Google Scholar]; f) Neff RK, Frantz DE, Tetrahedron 2015, 71, 7–18; [Google Scholar]; e) Alonso JM, Quiros MT, Muñoz MP, Org. Chem. Front 2016, 3, 1186–1204. [Google Scholar]
- [3]. For comprehensive reviews on enantioselective synthesis of allenes, see:; a) Hoffmann-Röder A, Krause N, Angew. Chem. Int. Ed 2002, 41, 2933− 2935; [DOI] [PubMed] [Google Scholar]; b) Ogasawara M, Tetrahedron Asymmetry 2009, 20, 259–271; [Google Scholar]; c) Yu S, Ma S, Chem. Commun 2011, 47, 5384− 5418; [DOI] [PubMed] [Google Scholar]; d) Ye J, Ma S, Org. Chem. Front 2014, 1, 1210–1224; [Google Scholar]; e) Chu W, Zhang Y, Wang J, Catal. Sci. Technol 2017, 7, 4570–4579; [Google Scholar]; f) Huang X, Ma S, Acc. Chem. Rev 2019, 52, 1301–1312. [DOI] [PubMed] [Google Scholar]
- [4]. For selected recent examples of transition-metal catalyzed enantioselective synthesis of allenes, see.; a) Han JW, Tokunaga N, Hayashi T, J. Am. Chem. Soc 2001, 123, 12915–12916; [DOI] [PubMed] [Google Scholar]; b) Ogasawara M, Ito A, Yoshida K, Hayashi T, Organometallics 2006, 25, 2715–2718; [Google Scholar]; c) Li C-Y, Wang X-B, Sun X-L, Tang Y, Zheng J-C, Xu Z-H, Zhou Y-G, Dai L-X, J. Am. Chem. Soc 2007, 129, 1494–1495; [DOI] [PubMed] [Google Scholar]; d) Zhang W, Zheng S, Liu N, Werness JB, Guzei IA, Tang W, J. Am. Chem. Soc 2010, 132, 3664–3665; [DOI] [PubMed] [Google Scholar]; e) Hashimoto T, Sakata K, Tamakuni F, Dutton MJ, Maruoka K, K. Nat. Chem 2013, 5, 240–244; [DOI] [PubMed] [Google Scholar]; f) Dabrowski JA, Haeffner F, Hoveyda AH, Angew. Chem. Int. Ed 2013, 52, 7694–7699; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Qian H, Yu X, Zhang J, Sun J, J. Am. Chem. Soc 2013, 135, 18020–18023; [DOI] [PubMed] [Google Scholar]; h) Wang Y, Zhang W Ma S, J. Am. Chem. Soc 2013, 135, 11517–11520; [DOI] [PubMed] [Google Scholar]; i) Wang M, Liu Z-L, Zhang X, Tian, Xu Y-H, Loh T-P, J. Am. Chem. Soc 2015, 137, 14830–14833; [DOI] [PubMed] [Google Scholar]; j) Tang Y, Chen Q, Liu X, Wang G, Angew. Chem. Int. Ed 2015, 54, 9512–9516; [DOI] [PubMed] [Google Scholar]; k) Chu WD, Zhang L, Zhang Z, Zhou Q, Mo F, Zhang Y, Wang J, J. Am. Chem. Soc 2016, 138, 14558–14561; [DOI] [PubMed] [Google Scholar]; l) Poulsen PH, Li Y, Lauridsen VH, Jørgensen DKB, Palazzo TA, Meazza M, Jørgensen, Angew. Chem., Int. Ed 2018, 57, 10661–10665; [DOI] [PubMed] [Google Scholar]; m) Huang Y, del Pozo J, Torker S, Hoveyda AH, J. Am. Chem. Soc 2018, 140, 2643–2655; [DOI] [PMC free article] [PubMed] [Google Scholar]; n) Yu S, Sang HL, Zhang, Hong X, Ge S, Commun. Chem 2018, 1, 64; [Google Scholar]; o) Adamson N, Jeddi H, Malcolmson S, J. Am. Chem. Soc 2019, 141, 8574–8583; [DOI] [PMC free article] [PubMed] [Google Scholar]; p) Bayeh-Romero L, Buchwald SL, J. Am. Chem. Soc 2019, 141, 13788–13794; [DOI] [PMC free article] [PubMed] [Google Scholar]; q) Zhu C, Chu J, Li G, Ma S, Zhang J, J. Am. Chem. Soc 2019, 141, 19246–19251. [DOI] [PubMed] [Google Scholar]
- [5]. For reviews and references of α-allenols in nucleophilic cyclization reactions, see:; a) Satcharoen RWV, Chem. Soc. Rev 2002, 31, 12–21; [DOI] [PubMed] [Google Scholar]; b) Ma S, S. Acc. Chem. Res 2003, 36, 701–712; [DOI] [PubMed] [Google Scholar]; c) Ma S, Gu Z, Z. J. Am. Chem. Soc 2005, 127, 6182–6183; [DOI] [PubMed] [Google Scholar]; d) Alcaide B, Almendros P, Martinez del Campo T, Angew. Chem. Int. Ed 2006, 45, 4501–4504; [DOI] [PubMed] [Google Scholar]; e) Erdsack J, Krause N, N. Synthesis 2007, 3741–3750; [Google Scholar]; f) Krause N, Belting V, Deutsch C, Erdsack J, Fan H-T, Gockel B, Hoffmann-Roder, Morita N, Volz F, Pure Appl. Chem 2008, 80, 1063–1069; [Google Scholar]; g) Deng Y, Yu Y, Ma S, S., J. Org. Chem 2008, 73, 585–589. [DOI] [PubMed] [Google Scholar]; g) Deng Y, Shi Y, Ma S, Org. Lett 2009, 11, 1205–1208. [DOI] [PubMed] [Google Scholar]
- [6]. For selected examples, see:; a) Oehlschlager A, Czyzewska E, Tetrahedron Lett. 1983, 24, 5587–5590.; [Google Scholar]; b) Johnson C, Dhanoa D, J. Org. Chem 1987, 52, 1885–1888; [Google Scholar]; c) Deutsch C, Hoffmann‐Röder A, Domke A, Krause N, Synlett. 2007, 737–740; [Google Scholar]; d) e) Alexakis A, Marek I, Mangeney P, Normant J, Tetrahedron 1991, 47, 1677–1696; [Google Scholar]; f) Bertozzi C, Crotti P, Macchia F, Pineschi M, Arnold A, Feringa BA, Tetrahedron Lett. 1999, 40, 4893–4896; [Google Scholar]; g) Fürstner A, Méndez M, Angew. Chem. Int. Ed 2003, 42, 5355–5357; [DOI] [PubMed] [Google Scholar]; h) Kjellgren J, Sundén H, Szabó K, J. Am. Chem. Soc 2005, 127, 1787–1796; [DOI] [PubMed] [Google Scholar]; i) Yoshida M, Ueda H, Ihara M, Tetrahedron Lett 2005, 46, 6705–6708; [Google Scholar]; j) Deutsch C, Lipshutz BH, Krause N, Angew. Chem. Int. Ed 2007, 46, 1650–1653; [DOI] [PubMed] [Google Scholar]; k) Miura T, Shimada M, Ku S-Y, Tamai T, Murakami M, Angew. Chem. Int. Ed 2007, 46, 7101–7103. [DOI] [PubMed] [Google Scholar]
- [7].Huang X, Cao T, Han Y, Jiang X, Lin W, Zhang J, Ma S, Chem. Commun 2015, 51, 6956–6959. [DOI] [PubMed] [Google Scholar]
- [8].Tap A, Blond A, Wakchaure V, List B, Angew. Chem. Int. Ed 2016, 55, 8962–8965. [DOI] [PubMed] [Google Scholar]
- [9].a) a) Zhang L, Lovinger GJ, Edelstein EK, Szymaniak AA, Chierchia MP, Morken JP, Science 2016, 351, 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lovinger GJ, Aparece MD, Morken JP, J. Am. Chem. Soc 2017, 139, 3153–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Edelstein EK, Namirembe S, Morken JP, J. Am. Chem. Soc 2017, 139, 5027–5030; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Chierchia MP, Law C, Morken JP, Angew. Chem. Int. Ed 2017, 56, 11870–11874; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Lovinger GJ, Morken JP, J. Am. Chem. Soc 2017, 139, 17293–17296; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Myhill JA, Zhang L, Lovinger GJ, Morken JP, Angew. Chem., Int. Ed 2018, 57, 12799–12803. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Myhill JA, Wilhelmsen CA, Zhang L, Morken JP, J. Am. Chem. Soc 2018, 140, 15181–15185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. For patent on the preparation of (Z)-borylenyne, see:; Fu Y, Yu S, Zhang B, Gong T, CN108503662 (A), July 09, 2018. [Google Scholar]
- [11].a) Thomas AA, Denmark SE, Science 2016, 352, 329–332; [DOI] [PubMed] [Google Scholar]; b) Thomas AA, Wang H, Zahrt AF, Denmark SE, J. Am. Chem. Soc 2017, 139, 3805–3821; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Thomas AA, Zahrt AF, Delaney CP, Denmark SE, J. Am. Chem. Soc 2018, 140, 4401–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. For examples of isomerization of propagylic copper to allenylcopper, see:; a) Huang Y, del Pozo J, Torker S, Hoveyda AH, J. Am. Chem. Soc 2018, 140, 2643–2655; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bayeh-Romero L, Buchwald SL, J. Am. Chem. Soc 2019, 141, 35, 13788–13794. [DOI] [PMC free article] [PubMed] [Google Scholar]; For detailed investigations of interconversion of propargylic platinum and allenylplatinum species, see:; c) Ogoshi S, Nishida T, Fukunishi Y, Tsutsumi K, Kurosawa, J. Organomet. Chem 2001, 620, 190–193. [Google Scholar]; d) Ogoshi S, Fukunishi Y, Tsutsumi K, Kurosawa H, Inorganica Chim. Acta 1997, 265, 9–15, and references herein. [Google Scholar]
- [13].Hoffmann-Röder A, Krause N, Org. Lett 2001, 3, 2537–2538. [DOI] [PubMed] [Google Scholar]
- [14]. For related chemistry of allene oxide, see:; Chan T, Ong B, B. Tetrahedron 1980, 36, 2269–2289. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


