Abstract
Background
Despite long-standing clinical use of sodium polystyrene sulphonate (SPS) for hyperkalaemia management in chronic kidney disease (CKD), its safety profile remains poorly investigated.
Methods
We undertook an observational analysis of nephrology-referred adults with incident CKD Stage 4+ in Sweden during 2006–16 and with no previous SPS use. We studied patterns of use and adverse events associated to SPS initiation during follow-up. Patterns of SPS use were defined by chronicity of treatment and by prescribed dose. We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) associated with SPS initiation (time-varying exposure) for the risk of severe (intestinal ischaemia, thrombosis or ulceration/perforation) and minor (de novo dispensation of laxatives or anti-diarrheal drugs) gastrointestinal (GI) events.
Results
Of 19 530 SPS-naïve patients with CKD, 3690 initiated SPS during follow-up. A total of 59% took SPS chronically, with an average of three dispensations/year. The majority (85%) were prescribed lower dosages than specified on the product label. During follow-up, 202 severe and 1149 minor GI events were recorded. SPS initiation was associated with a higher incidence of severe adverse events [adjusted HR 1.25 95% CI 1.05–1.49)], particularly in those receiving per label doses [1.54 (1.09–2.17)] and mainly attributed to ulcers and perforations. SPS initiation was also associated with higher incidence of minor GI events [adjusted HR 1.11 (95% CI 1.03–1.19)], regardless of dose, and mainly accounted for by de novo dispensation of laxatives.
Conclusions
Initiation of SPS in patients with advanced CKD is associated with a higher risk of severe GI complications as well as the initiation of GI-related medications, particularly when prescribed at per label doses.
Keywords: chronic haemodialysis, chronic renal failure, CKD, epidemiology, hyperkalaemia
INTRODUCTION
Hyperkalaemia is a common and potentially life-threatening complication of advanced chronic kidney disease (CKD) [1–3]. Hyperkalaemia treatment generally consists of a reduction of dietary intake, modification of contributing medications and the use of cation-exchange resins [4]. Sodium polystyrene sulphonate (SPS) is a commonly used cation-exchange resin licensed for hyperkalaemia management during the 1950s. Although maintenance treatment of hyperkalaemia with SPS is not specified in the summary of product characteristics, off-label application in everyday clinical practice is probably common [5, 6], yet uncharacterized.
Data on SPS safety are scarce and ambiguous [7]; clinical trials evaluating SPS efficacy reported no serious adverse gastrointestinal (GI) events [8–10], but they collectively included <150 patients and had a short-term duration (up to 7 days). In contrast, some case reports of serious GI injury with SPS and sorbitol [11, 12] led to the US Food and Drug Administration warning against their concurrent use [13]. GI injuries have also been noted with the use of SPS alone [11, 14–20], but these are believed to be rare [15].
To date, considerable uncertainty exists regarding practice patterns of SPS and its potential adverse event risk [5, 8]. A recent population-based Canadian study [21] of older adults initiating SPS observed a higher incidence of hospitalization for serious adverse GI events compared with non-use. We note that only 1% of the included sample in that study was undergoing dialysis. We set out to systematically examine SPS use and GI safety in a large Swedish representative cohort of nephrologist-referred persons with CKD.
MATERIALS AND METHODS
Data sources
This retrospective cohort study is based on the Swedish Renal Registry (SRR), which is a nationwide registry of patients on renal replacement therapy (RRT) or with CKD Stages 3–5 followed at any nephrology clinic in Sweden. The SRR is a platform with several interlinked registers: in the SRR-CKD register, nephrology clinics report information on outpatient visits (on average, two to three visits per year) from referred patients diagnosed with CKD from the moment they reach an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2. The SRR also encourages the inclusion of CKD referred patients earlier in the course of the disease (eGFR <45–60 mL/min/1.73 m2), given that the inclusion is performed systematically at the participating clinic. Patients are then followed prospectively until discharge from nephrology follow-up or death. Transitions to dialysis and transplantation are registered continuously into the SRR-RRT. The SRR-RRT then collect yearly information about clinical parameters, laboratory measures and treatment outcomes of all RRT patients in the country, whereas changes between treatment modalities and complications are registered consecutively. In 2017, the national coverage of kidney transplant patients was estimated as 96% and 85% of incident dialysis cases were already included in the register during their pre-dialysis phase [22]. Via each citizen’s unique personal identification number, the SRR was linked with the Swedish government 31180477 run drug dispensation and patient registers. The Swedish drug dispensation register records complete information on all prescribed drugs dispensed at a Swedish pharmacy or single dosages provided at elder care or nursing facilities [23]. The coverage of this register is considered complete. The Swedish patient register records, with no loss to follow-up, all outpatient–specialist consultations, hospitalizations and deaths occurring in Swedish health care [24].
Patient selection and study design
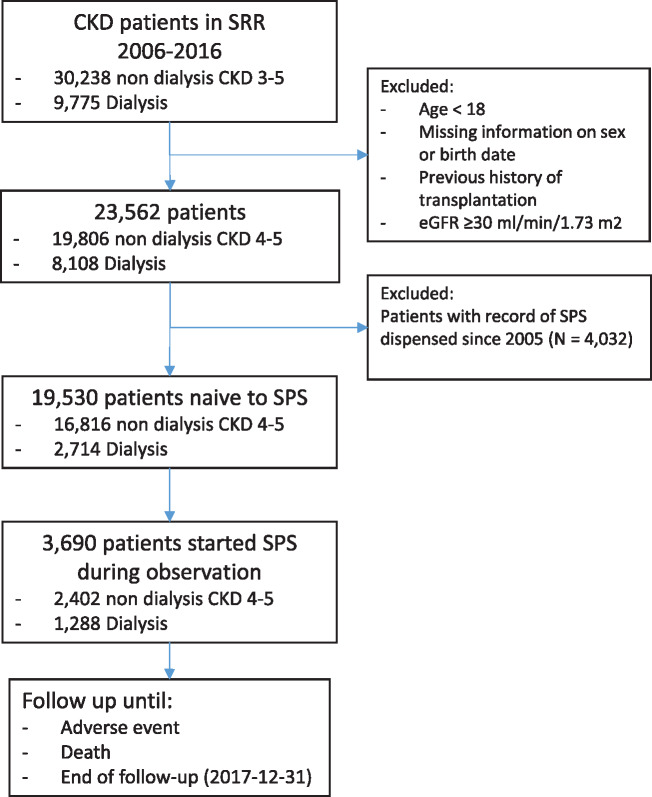
For this study we selected all adult (>18 years old) participants in the SRR with an eGFR ≤30 mL/min/1.73 m2 between 1 January 2006 and 31 December 2016. The date of the first SRR recorded visit satisfying this inclusion criterion was set as the index date. Follow-up was available until 31 December 2017, ensuring a minimum of 1 year per patient. We then excluded patients who had a history of SPS use, defined as a pharmacy dispensation of SPS recorded since the creation of the Swedish drug dispensation register in 2005.
Study exposure
The study exposure is SPS initiation. As per study design, all patients enrolled in the cohort were non-exposed and some of them initiated SPS during follow-up. In the first part of the study we describe the dosages and patterns of SPS dispensation within 1 year. We chose a 1-year period because SPS is commercialized in Sweden as a 450-g package of powder and it is difficult to quantify use as compared with other methods of administration (i.e. pills). Per label dosages, recommended for short-term hyperkalaemia treatment, are 15 g two to four times per day until hyperkalaemia is resolved (potassium <5 mmol/L). However, SPS is often prescribed at lower dosages for chronic hyperkalaemia. We consulted online regional and national expert opinion guidance documents as well as five nephrology departments at Swedish hospitals to define three commonly used patterns of SPS dosing: ‘per label dose’: this is the dosage recommended by the product label and consists of two to four SPS dosages per day, which, if consumed regularly, leads to an estimated product supply of up to 30 days; ‘moderate–low dose’: this dosing regimen ranged from three to seven SPS dosages of 15 g/week (i.e. up to one dose daily), leading to an estimated product supply of up to 3 months; and ‘low dose’: the most common dosing, consisting of one or two SPS doses of 15 g/week, leading to an estimated product supply of up to 8 months. We extracted information on all subsequent SPS dispensations within 1 year and evaluated if the treatment was isolated (a single package dispensed) or chronic (repeated dispensations) and estimated prescribed dosages by evaluating whether the time to the next dispensation fit the regimens above. For individuals dispensing SPS only once, we manually extracted the information of prescribed dosages from the text introduced by the physician in the prescription form (unstructured text). Of note, calcium polystyrene sulphonate is not available in Sweden.
Study covariates
Other covariates in the study included CKD stage or, when applicable, dialysis type, comorbidities and medications. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation from isotope dilution mass spectrometry–calibrated plasma creatinine measurements. The severity of CKD was then categorized according to Kidney Disease: Improving Global Outcomes criteria as CKD Stage 4 (eGFR <30–15 mL/min/1.73 m2), CKD Stage 5 non-dialysis (eGFR <15 mL/min/1.73 m2) and chronic dialysis (haemodialysis or peritoneal dialysis) [25, 26]. Comorbid conditions were defined using International Classification of Diseases, 10th revision (ICD-10) diagnostic codes. Comorbidities considered are defined in Supplementary data, Table S1, and included diabetes mellitus, hypertension, cardiovascular and cerebrovascular diseases, rheumatic diseases, cancer and chronic obstructive pulmonary disease, with no time limit since the implementation of ICD-10 codes in Sweden in 1997. We also derived information on the history of GI events (defined in the same way as the outcome of severe GI adverse events, see below) and hyperkalaemia in the year prior to the index date. Ongoing medications were ascertained via anatomic therapeutic classification codes as defined in Supplementary data, Table S2, and included angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEis/ARBs), β-blockers, spironolactone, non-steroidal anti-inflammatory drugs (NSAIDs), diuretics, statins, vitamin D, phosphate binders, diarrhoea medications, laxatives and sorbitol.
Study outcomes
The primary study outcome was hospitalization or a death attributed to a composite of severe adverse GI events (intestinal ischaemia or thrombosis, GI ulcers and perforation). This composite study outcome reflects the broad spectrum of reported GI injury with SPS use [17]. Definitions are further detailed in Supplementary data, Table S3. Hospitalizations, death and causes of death were ascertained via linkage with the government-run Swedish Population Registry. Patients were followed until event, death (by other causes) or end of follow-up (31 December 2017), whichever happened first. There was no loss to follow-up.
The secondary study outcome was the occurrence of minor adverse GI events, defined as the need to take a de novo dispensation of GI-related medications (laxatives or anti-diarrheal drugs). These are the most commonly reported minor adverse events with SPS use [8]. Patients were followed until event, death or end of follow-up (31 December 2012), whichever happened first. There was no loss to follow-up.
Statistical analysis
Summary statistics are presented as median and interquartile range (IQR) or number and percentage. We explored adverse events associated to SPS use by estimating cause-specific hazard ratios (HRs) using Cox proportional hazards regression with time-updated exposure. Thus a patient who started SPS during follow-up contributed to the non-exposed group during his/her time before the SPS dispensation and thereafter to the SPS-exposed group. Covariates were recalculated again at the time of incident SPS use, updating potential new comorbidities (such as hyperkalaemia and heart failure) that may have prompted the prescription. We estimated multivariable-adjusted HRs and 95% confidence intervals (CIs) in SPS-exposed versus SPS-free periods for the study outcomes. Assumptions of Cox models were assessed by looking at the Schoenfeld residuals and there were no violations in the fitted models. Additionally, we tested two-way interactions between SPS initiation and CKD status (non-dialysis CKD or undergoing dialysis) in the adjusted models.
Once SPS was initiated, we assumed the patient remained exposed for the whole period, because this drug is often prescribed at low dosages or for use when needed. For the analysis of secondary outcomes, we excluded patients with ongoing laxatives or anti-diarrheal medication (defined as a dispensation within the preceding 5 months). We purposefully decided not to study the risk of death (by any cause), because confounding by indication bias is high for SPS (i.e. patients initiating SPS do so because of hyperkalaemia, which can be life-threatening). However, we included deaths attributed to severe GI events in our primary outcome definition above. Commencement of dialysis or kidney transplantation was not considered a censoring or competing event since GI injury related to SPS use would persist regardless.
As a next step, we evaluated dose responses comparing the adverse outcome risk associated with initiating SPS with a per label dose or a lower dose. For this, individuals receiving low–moderate and low dosages (see methods) were grouped together. Some individuals (2% of the sample) did not have information recorded on their prescribed dose and these were conservatively assigned to the ‘per label dose’ group. As a sensitivity analysis, we restricted the follow-up after SPS initiation to 1 year, given that SPS intake is erratic and the time from SPS exposure to reported adverse GI events is believed to be rapid [11]. Finally, we further evaluated the risk of unmeasured confounding through the E-value methodology [27], which identifies the minimum strength of association that an unmeasured confounder would need to have with both treatment and outcome, conditional on the measured covariates, to fully explain the observed association. This estimates what the relative risk would have to be for any unmeasured confounder to overcome the observed association of SPS initiation with the risk of GI events.
RESULTS
Cohort characteristics at study entry
After applying inclusion and exclusion criteria (see methods and flow chart in Figure 1), 19 530 nephrology-referred adults with CKD Stages 4–5 and naïve to SPS were identified in Swedish nephrologist care during 2006–16 and included in the analysis. Descriptive characteristics are shown in Table 1. The median age was 73 (IQR 64–80) years and 38% were women. As per the study design, the majority of patients included had CKD Stage 4 (69%) and Stage 5 (17%), the remaining (14%) being on chronic dialysis. Briefly, hypertension was the most commonly observed comorbidity (81%), followed by diabetes (40%), coronary artery disease (31%) and congestive heart failure (27%). The concomitant use of ACEis/ARBs (62%), β-blockers (65%), vitamin D (45%) and statins (50%) was high.
FIGURE 1.
Flow chart of patient selection to the study.
Table 1.
Characteristics of the study population at cohort entry (baseline)
| Number of individuals | 19 530 |
| Age (years), median (IQR)a | 73 (64–80) |
| Women, n (%) | 7428 (38) |
| CKD stage, n (%) | |
| 15–30 mL/min/1.73 m2 | 13 494 (69) |
| <15 mL/min/1.73 m2 | 3322 (17) |
| Haemodialysis | 1932 (10) |
| Peritoneal dialysis | 782 (4) |
| Comorbid history, n (%) | |
| Hypertension | 15 873 (81) |
| Diabetes mellitus | 7717 (40) |
| Coronary artery disease | 6064 (31) |
| Heart failure | 5339 (27) |
| Angina | 4132 (21) |
| Myocardial infarction | 3906 (20) |
| Cerebrovascular disease | 3573 (18) |
| Peripheral vascular disease | 2990 (15) |
| Stroke | 2718 (14) |
| Atrial fibrillation | 3927 (20) |
| Rheumatic disease | 1181 (6) |
| Cancer | 2904 (15) |
| Chronic obstructive pulmonary disease | 2412 (12) |
| Prior history of hyperkalaemia | 253 (2) |
| Gastric ulcers | 1300 (7) |
| Prior history of severe GI complications | 367 (2) |
| Ongoing medications, n (%) | |
| ACEis/ARBs | 12 169 (62) |
| β-blockers | 12 697 (65) |
| Spironolactone | 1425 (7) |
| NSAIDs | 995 (5) |
| Non-potassium-sparing diuretics | 1248 (6) |
| Potassium-sparing diuretics | 1567 (8) |
| Statins | 9476 (50) |
| Vitamin D | 8859 (45) |
| Phosphate binders | 2701 (11) |
| Anti-diarrheal medication | 892 (5) |
| Laxatives | 3102 (16) |
| Sorbitol | 8 (<1) |
IQR is quartile 1–quartile 3.
SPS users and patterns of SPS use
Of included patients, 3690 initiated SPS during the follow-up. The characteristics of these patients at the time of SPS initiation are described in Supplementary data, Table S4. The percentage of patients with CKD Stage 5 or dialysis increased compared with cohort entry, as well as the percentage of patients with a history recent hyperkalaemia. None of the patients was consuming sorbitol at the time of SPS initiation.
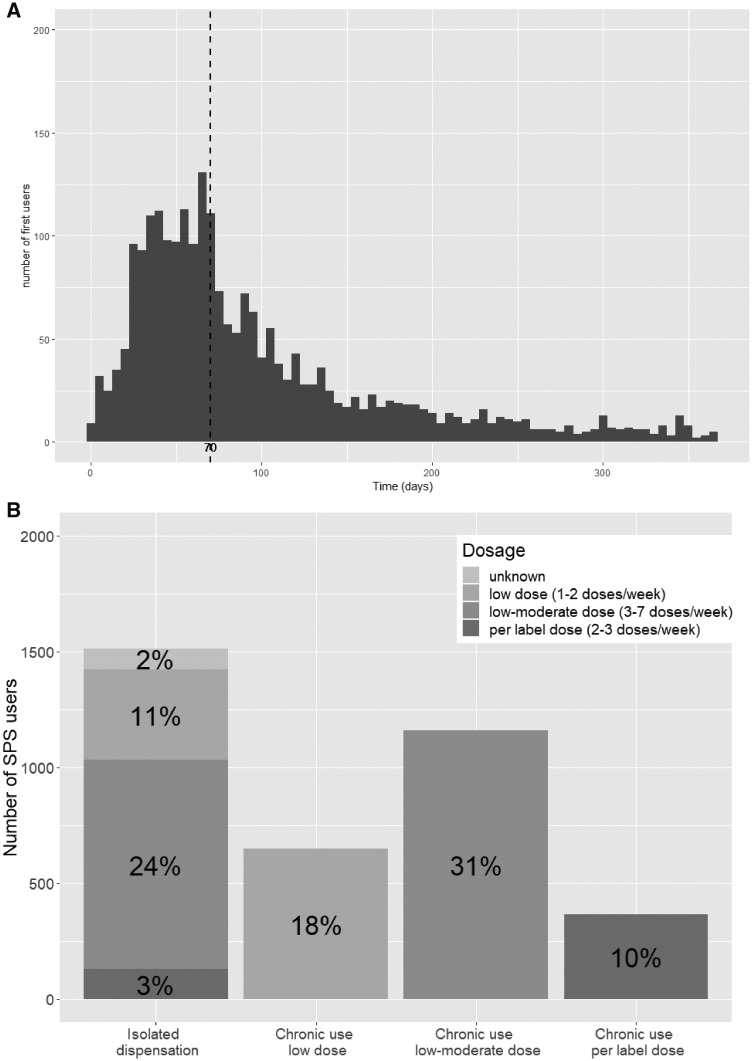
By evaluating all subsequent SPS dispensations during 1 year, we observed that 41% of individuals had a single isolated dispensation. The remaining 59% of SPS users had, on average, three dispensations per year (Supplementary data, Figure S1), with a median time between the first and second dispensation of 70 (IQR 43–125) days (Figure 2A). Only 13% of patients were estimated to consume per label dosages (10% chronically and 3% as a single dispensation—see Methods for dosing definitions). Only 2% had no information on prescribed dose and the remaining 85% used lower dosages than per label recommendations. Specifically, a moderate–low dose was used by 45% of patients (31% chronically and 24% as a single dispensation) and the remaining 29% used a low dose (18% chronically and 11% as a single dispensation; Figure 2B).
FIGURE 2.
Distribution of (A) the number of days between the first and second SPS dispensation and (B) the percentage of patients dispensing a single SPS package or receiving chronic treatment, as well as dosages used. In (A), the dashed line represents the median number of days between dispensations, which was 70 days.
Adverse events associated to SPS use
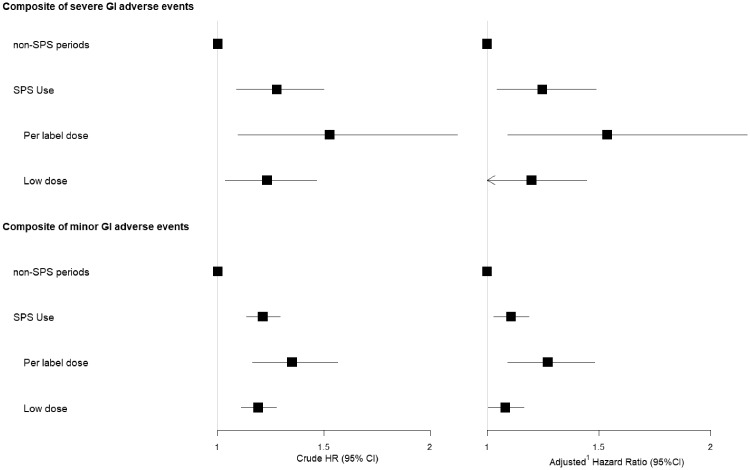
Patients were followed prospectively for the study of adverse GI events (see Methods for outcome definitions) associated with the initiation of SPS therapy, treating SPS dispensing as a time-dependent exposure (Table 2 and Figure 3). During follow-up, 1105 severe GI events (composite of hospitalization or death due to intestinal ischaemia or thrombosis, GI ulcers and perforation) were recorded. Compared with unexposed periods, SPS use was associated with the risk of severe GI adverse events [adjusted HR 1.25 (95% CI 1.05–1.49)]. This association was stronger among SPS users prescribed with a per label dose [adjusted HR 1.54 (95% CI 1.09–2.17)] than among SPS users prescribed with a lower dose [adjusted HR 1.20 (95% CI 0.99–1.45)], with the possibility of a non-association among the low-dose users. Similar results were found for the risk of the single outcome of GI ulcer and perforation. The associations for the risk of intestinal ischaemia/thrombosis were similar in magnitude but did not reach statistical significance (Table 2 and Figure 3). There were no significant two-way interactions between SPS use and CKD status for severe GI adverse events (P = 0.5), GI ulcer and perforations (P = 0.4) and intestinal ischaemia/thrombosis (P = 0.4).
Table 2.
Incidence rates (IRs) and HRs for the adverse events associated with SPS use
| Adverse events | Events, n | IR per 1000 PY (95% CI) | Crude HR (95% CI) | Adjusteda HR (95% CI) |
|---|---|---|---|---|
| Composite of severe GI adverse events | ||||
| Non-SPS periods | 903 | 15 (14–16) | REF | REF |
| SPS use | 202 | 16 (14–19) | 1.28 (1.09–1.50) | 1.25 ( 1.05–1.49) |
| Per label dose | 37 | 19 (14–27) | 1.53 (1.10–2.13) | 1.54 (1.09–2.17) |
| Low dose | 165 | 16 (13–18) | 1.23 (1.03–1.45) | 1.20 (0.99–1.45) |
| GI ulcer and perforation | ||||
| Non-SPS periods | 808 | 13 (12–14) | REF | REF |
| SPS use | 179 | 14 (12–17) | 1.27 (1.07–1.50) | 1.23 (1.02–1.48) |
| Per label dose | 33 | 17 (12–24) | 1.52 (1.07–2.16) | 1.52 (1.06–2.18) |
| Low dose | 146 | 14 (12–16) | 1.22 (1.01–1.47) | 1.18 (0.97–1.44) |
| Intestinal ischaemia or thrombosis | ||||
| Non-SPS periods | 107 | 1.7 (1.4–2.0) | REF | REF |
| SPS use | 30 | 2.3 (1.6–3.3) | 1.51 (0.98–2.32) | 1.37 (0.85–2.20) |
| Per label dose | 4 | 2.0 (0.5–5.0) | 1.28 (0.47–3.51) | –b |
| Low dose | 26 | 2.4 (1.5–3.5) | 1.55 (0.99–2.43) | 1.39 (0.84–2.28) |
| Composite of minor GI adverse events | ||||
| Non-SPS periods | 6832 | 184 (179–188) | REF | REF |
| SPS use | 1149 | 200 (189–212) | 1.21 (1.14–1.29) | 1.11 ( 1.03–1.19) |
| Per label dose | 179 | 223 (192–258) | 1.35 (1.16–1.57) | 1.27 (1.09–1.48) |
| Low dose | 970 | 196 (184–209) | 1.19 (1.11–1.28) | 1.08 (1.00–1.17) |
| De novo dispensation of anti-diarrheal medication | ||||
| Non-SPS periods | 1854 | 38 (36–40) | REF | REF |
| SPS use | 350 | 38 (34–43) | 1.08 (0.96–1.22) | 0.87 (0.76–0.99) |
| Per label dose | 65 | 49 (38–63) | 1.38 (1.08–1.77) | 1.11 (0.86–1.43) |
| Low dose | 285 | 37 (32–41) | 1.03 (0.90–1.17) | 0.83 (0.72–0.95) |
| De novo dispensation of laxatives | ||||
| Non-SPS periods | 6054 | 163 (159–167) | REF | REF |
| SPS use | 1058 | 179 (168–190) | 1.21 (1.14–1.30) | 1.16 (1.08–1.25) |
| Per label dose | 164 | 199 (170–232) | 1.31 (1.12–1.53) | 1.28 (1.09–1.51) |
| Low dose | 894 | 176 (164–187) | 1.20 (1.12–1.29) | 1.14 (1.05–1.23) |
Adjusted for age, sex, eGFR, hypertension, diabetes, coronary artery disease, heart failure, angina, cerebrovascular disease, myocardial infarction, peripheral vascular disease, stroke, atrial fibrillation, history of severe GI complications, rheumatic disease, chronic obstructive pulmonary disease, history of hyperkalaemia, current use of ACEis/ARBs, β-blockers, spironolactone, non-potassium-sparing and potassium-sparing diuretics, statins, vitamin D, phosphate binders, anti-diarrheal medication (when pertinent) or laxatives (when pertinent).
Too few events to allow multivariable adjustment. REF, reference.
FIGURE 3.
Forest plot for the severe and minor GI adverse events associated with SPS initiation in advanced CKD.
After the exclusion of patients taking laxatives or anti-diarrheal medication, a total of 15 706 individuals were followed prospectively for the de novo prescription of these drugs, taken as a surrogate of minor GI adverse events (composite). Compared with unexposed periods, SPS use was associated with a higher incidence of minor GI adverse events, with an adjusted HR of 1.11 (95% CI 1.03–1.19). Again, a dose–response relationship was observed and the association was stronger among patients using per label dosages [adjusted HR 1.27 (95% CI 1.09–1.48)] compared with lower dosages [adjusted HR 1.08 (95% CI 1.00–1.17)]. When studying single outcomes, the association was mainly observed for the incidence of new laxative use, and no association was observed for the incidence of new anti-diarrheal medication use. There were significant two-way interactions between SPS use and CKD status for the composite of minor GI adverse events (P < 0.01), as well as for the single outcomes of new anti-diarrheal medication (P < 0.01) and new laxative (P < 0.01) use. This means that the risk of initiating GI-related medications after SPS initiation is higher among patients with non-dialysis CKD compared with patients undergoing dialysis.
When restricting the follow-up after SPS initiation to 1 year, the short-term risk association between SPS use and serious GI events became less precise (CI crossing zero) but was still 29% higher overall compared with non-SPS (Supplementary data, Table S5). In the short-term, the risk of minor GI events remained, this time attributed to a higher rate of both laxatives and anti-diarrheal medication dispensations. Once more, the associations were stronger in magnitude in those patients following a per label dose. E-values for study outcomes ranged between 1.4 and 2.4, which we interpret as moderately robust to potential unmeasured confounders (Supplementary data, Table S6). For instance, E-values for the primary composite outcome indicated that the observed HR of 1.25 (95% CI 1.05–1.49) could only be explained by an unmeasured confounder that was associated with both initiation of SPS and severe GI risk by an HR >1.80 above and beyond that of the confounders that were measured in this study. We note from the multivariable-adjusted model that most confounders were generally below this value and therefore the probability that an unknown comorbidity or medication may abrogate this association may be judged moderately low, particularly for the per label dose association (E-value 2.44). For instance, the multivariable-adjusted HR in our primary Cox model was 1.25 (95% CI 1.04–1.49) for hypertension, 1.37 (1.18–1.59) for heart failure and was 1.30 (0.95–1.99) for recent hyperkalaemia diagnosis.
DISCUSSION
This is the largest evaluation to date of SPS use and safety in persons with CKD. The selection of nephrologist-referred patients in our study is important, not only for their heightened hyperkalaemia risk, but also because the vast majority of SPS case reports [11] had a history of acute kidney injury, CKD or end-stage kidney disease (ESKD). In this nationwide analysis, we found that SPS is more often prescribed chronically and at lower dosages than specified in the summary of product characteristics and SPS initiation was associated with an increased relative risk of both serious and minor GI events compared with periods of non-use. These associations were more apparent in individuals following per label dosages.
Although often speculated in narrative reviews [6], this is the first study to quantify with precision the use of SPS for hyperkalaemia prevention in the outpatient setting. We found that nephrologists in our country prescribed SPS chronically in about two-thirds of users and at low dosages in 85%, illustrating its common off-label use as a chronic hyperkalaemia prevention strategy. A strength of our study is that our exposure is based on SPS dispensations rather than prescriptions, offering a better ascertainment than previous studies. A limitation is, however, the way SPS is dispensed (450 g package), which makes it difficult to ascertain the exposure with precision despite our efforts. Another limitation is that the Swedish prescribed drug register does not record medications given in hospital, and acute hyperkalaemia patients treated in hospital may have received SPS, which is not accounted for here. This being said, such a limitation would only underestimate our risk estimates, and we hope to have minimized it to some extent by adjusting for the presence of recent hyperkalaemia diagnoses at the time of SPS initiation. Lastly, although our methods for evaluating dosages are standard in pharmacoepidemiology research, we cannot ascertain if the dose was altered later on by the physician or if the patient complied with the treatment.
The main finding in our study is the estimation of adverse risks associated with SPS use, which, to our knowledge, has not been shown before, except in case–control studies, single-centre retrospective analyses and case reports [11, 14–20]. We observe a dose-dependent increased incidence of serious GI adverse events for CKD patients initiating SPS therapy. SPS was prescribed as a solo agent, and no SPS user took concomitant sorbitol, in accordance with recommendations from the medical agencies [13]. Previous reports have focused on the risk of colonic injury/necrosis, often requiring pathological confirmation for identification [15]. This outcome is certainly a rare occurrence. In a case–control analysis of adult inpatients at a tertiary medical centre, 0.14% of individuals receiving SPS had colonic necrosis, compared with 0.07% of individuals who were not given SPS [15]. In another study of haemodialysis patients, colonic surgery (taken here as a marker of colonic necrosis) was no more common in patients who received SPS (0.6%) than in those not given SPS (1.0%) [28]. We focused instead on a composite of serious GI complications that reflects the broad spectrum of reported GI injury with SPS use [17]. In this context, we observed an increased relative risk of ulcers and perforations after SPS initiation, particularly at per label dosages. The association with the risk of intestinal ischaemia/thrombosis was less evident, possibly impacted by the limited number of these events (<3 per 1000 patient-years). Overall, although relative risks are higher, absolute risks for our composite outcome remain low. Furthermore, this information needs to be contextualized with the varying SPS prescription rates across countries. As shown by Jadoul et al. [28] in the Dialysis Outcomes and Practice Patterns Study, SPS was used by an average of 40–45% of French dialysis patients, followed by Sweden with a 20–25% SPS prescription rate. Other countries such as Italy, Belgium and Canada prescribed it to 5–15% of their patients, and countries such as the UK, USA, Australia/New Zealand and Germany have SPS prescription rates <5%.
Our results are in line and expand a recent population-based study [21] from Canada of older adults initiating SPS. The authors observed a higher incidence of hospitalization for serious adverse GI events among SPS users compared with non-users. While SPS is approved in Canada for the management of hyperkalaemia in general, in Sweden its use is specific to persons with CKD. In the Canadian study, only 1% of the included sample was undergoing dialysis. Potential limitations are that they did not have information on sorbitol use, subsequent SPS dispensations or prescribed dosages. This may be more relevant in the case of chronic hyperkalaemia of CKD, given that previous case reports suggested the existence of a dose-dependent response between SPS and intestinal injury and that low-dose SPS is less likely to cause GI injury [11, 29]. This hypothesis is supported by our findings, whereby individuals following per label SPS doses (15% of all SPS users) had a higher outcome risk than those receiving low SPS doses. Adverse events are believed to appear rapidly; in a systematic review [11] of adverse GI events across 30 case reports with SPS use (about half of them without sorbitol), symptoms were observed a median of 2 days after SPS initiation. When we attempted to address this by evaluating 1-year risk as short term, we experienced a lack of power due to the small number of events, but the overall risk for serious GI events remained 30% higher for SPS use compared with no use.
Finally, our analysis also quantifies the risk of minor adverse events associated with SPS use. We chose to model the de novo prescription of anti-diarrheal and laxative medications, for these were the most commonly reported minor adverse events in the trial of Lepage et al. [8]. In line with the clinical experience of this drug [6], we observed that laxatives are commonly initiated after SPS use in all our analyses and anti-diarrheal medication use was also increased in our short-term risk analysis, particularly among patients following a per label dose. Unfortunately we were unable to study other reported adverse effects of SPS such as hypernatraemia, hypophosphataemia or hypomagnesaemia, as the capture of these events by ICD codes has poor reliability.
Additional strengths of our study include the large sample size and nationwide inclusion of cases. The linkage via each citizen’s unique personal identification number allows a complete recollection of clinical adverse events without loss to follow-up. However, our study also has limitations. We lack information on the serum potassium level that prompted SPS initiation, which would have better allowed us to separate indications of acute versus chronic hyperkalaemia management. Although the validity of ICD diagnoses in Swedish health care is believed very high [24], we depend on the event being noticed and reported, a hindrance intrinsic to any register-based research. However, the lack of outcome reporting could be considered a non-differential misclassification and would likely affect both SPS users and non-users similarly. We lacked pathology data that could suggest a causal relationship between SPS use and adverse GI events. Finally, despite our large dataset, there remained a limited number of severe events, particularly those of intestinal ischaemia and thrombosis. Given the scarcity of these outcomes, the unspecificity of some diagnoses and the plausible risk of misclassification between them, we were unable to evaluate differential risk profiles at the stomach versus the intestine.
Our findings add to previous evidence [11, 14–20] and provoke concern, suggesting caution in the use of this medication. Observational evidence is also supported by some, but not all, animal studies, which demonstrate that the administration of SPS enemas at high doses with or without sorbitol results in colonic necrosis [30, 31]. However, neither our observational study nor previous evidence can yet prove causality in the associations reported. In our study, we cannot ascertain whether the risk observed is explained by the use of SPS or by other conditions that make the patient prone to both hyperkalaemia requiring SPS and GI complications. This reverse causality is plausible in patients with CKD, who are predisposed to non-occlusive mesenteric ischaemia by often advanced arteriosclerosis through angiotensin II–mediated vasoconstriction [32] and often subjected to concomitant hypotension, ileus-induced colonic distension (resulting in reduced colonic blood flow) and decreased gut motility as a result of opioids, uraemia and constipation [33, 34]. Constipation and reduced GI motility can theoretically increase potassium bioavailability in the intestine leading to hyperkalaemia [35].
To conclude, this real-world evidence analysis shows that SPS is frequently used by persons with advanced CKD/ESKD chronically and at lower dosages than specified on the product label. Compared with non-use, we observed an increased relative risk of the combined endpoint of intestinal ischaemia, thrombosis or ulceration/perforation for SPS use. In addition, SPS use was associated with a higher rate of laxative dispensations. These associations were more evident in patients prescribed per label SPS dosages.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
FUNDING
This study was supported by a grant from Vifor Pharma Nordiska to the Karolinska Institutet. P.L.’s stay at the Karolinska Institutet was supported by the Erasmus European programme of student exchange. M.E. has received financial support from the Stockholm County Council (ALF medicin) and a strategic research grant from CIMED and Karolinska University Hospital. She is also a board member of the Swedish Renal Register.
CONFLICT OF INTEREST STATEMENT
Results presented in this article have not been published previously in whole or part except in abstract format. J.J.C. reports funding from the Stockholm County Council and the Swedish Heart and Lung Foundation; has received institutional grants from AstraZeneca, Vifor Pharma, Astellas, Merck and Novartis and has been a speaker or consultant for Abbott, Baxter Healthcare, Astellas and Vifor Pharma. L.S. is an employee of Vifor Pharma Nordiska. M.E. reports being a speaker and advisory board member for Astellas. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
(See related article by Labriola and Jadoul. Sodium polystyrene sulfonate: still news after 60 years on the market. Nephrol Dial Transplant 2020; 35: 1455--1458)
REFERENCES
- 1. Gasparini A, Evans M, Barany P. et al. Plasma potassium ranges associated with mortality across stages of chronic kidney disease: the Stockholm CREAtinine Measurements (SCREAM) project. Nephrol Dial Transplant 2019; 34: 1534–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kovesdy CP, Matsushita K, Sang Y. et al. Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta-analysis. Eur Heart J 2018; 39: 1535–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nilsson E, Gasparini A, Arnlov J. et al. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol 2017; 245: 277–284 [DOI] [PubMed] [Google Scholar]
- 4. Kovesdy CP, Appel LJ, Grams ME. et al. Potassium homeostasis in health and disease: a scientific workshop cosponsored by the National Kidney Foundation and the American Society of Hypertension. Am J Kidney Dis 2017; 70: 844–858 [DOI] [PubMed] [Google Scholar]
- 5. Sterns RH, Rojas M, Bernstein P. et al. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol 2010; 21: 733–735 [DOI] [PubMed] [Google Scholar]
- 6. Kovesdy CP. Management of hyperkalemia: an update for the internist. Am J Med 2015; 128: 1281–1287 [DOI] [PubMed] [Google Scholar]
- 7. Watson M, Abbott KC, Yuan CM.. Damned if you do, damned if you don’t: potassium binding resins in hyperkalemia. Clin J Am Soc Nephrol 2010; 5: 1723–1726 [DOI] [PubMed] [Google Scholar]
- 8. Lepage L, Dufour AC, Doiron J. et al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol 2015; 10: 2136–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scherr L, Ogden DA, Mead AW. et al. Management of hyperkalemia with a cation-exchange resin. N Engl J Med 1961; 264: 115–119 [DOI] [PubMed] [Google Scholar]
- 10. Gruy-Kapral C, Emmett M, Santa Ana CA. et al. Effect of single dose resin-cathartic therapy on serum potassium concentration in patients with end-stage renal disease. J Am Soc Nephrol 1998; 9: 1924–1930 [DOI] [PubMed] [Google Scholar]
- 11. Harel Z, Harel S, Shah PS. et al. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013; 126: 264.e9–264.e24 [DOI] [PubMed] [Google Scholar]
- 12. McGowan CE, Saha S, Chu G. et al. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J 2009; 102: 493–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration. Kayexelate: Sodium Polystyrene Sulfonate, USP, Cation-Exchange Resin, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/011287s022lbl.pdf
- 14. Albeldawi M, Gaur V, Weber L.. Kayexalate-induced colonic ulcer. Gastroenterol Rep (Oxf) 2014; 2: 235–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Watson MA, Baker TP, Nguyen A. et al. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis 2012; 60: 409–416 [DOI] [PubMed] [Google Scholar]
- 16. Hajjar R, Sebajang H, Schwenter F. et al. Sodium polystyrene sulfonate crystals in the gastric wall of a patient with upper gastrointestinal bleeding and gastric perforation: an incidental finding or a pathogenic factor? J Surg Case Rep 2018; 6: rjy138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abraham SC, Bhagavan BS, Lee LA. et al. Upper gastrointestinal tract injury in patients receiving kayexalate (sodium polystyrene sulfonate) in sorbitol: clinical, endoscopic, and histopathologic findings. Am J Surg Pathol 2001; 25: 637–644 [DOI] [PubMed] [Google Scholar]
- 18. Edhi AI, Cappell MS, Sharma N. et al. One oral dose of sodium polystyrene sulfonate associated with ischemic colitis and crystal deposition in colonic mucosa. ACG Case Rep J 2018; 5: e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nuzzo A, Shaar-Chneker C, Maillet M. et al. Gastroduodenal injury induced by orally administered sodium polystyrene sulfonate. Clin Toxicol (Phila) 2019; 57: 75–76 [DOI] [PubMed] [Google Scholar]
- 20. Almulhim AS, Hall E, Mershid Al Rehaili B. et al. Sodium polystyrene sulfonate induced intestinal necrosis; a case report. Saudi Pharm J 2018; 26: 771–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noel JA, Bota SE, Petrcich W. et al. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med 2019; doi: 10.1001/jamainternmed.2019.0631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. https://www.medscinet.net/snr/ (January 2019, date last accessed)
- 23. Wettermark B, Hammar N, MichaelFored C. et al. The new Swedish Prescribed Drug Register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidem Drug Safe 2007; 16: 726–735 [DOI] [PubMed] [Google Scholar]
- 24. Ludvigsson JF, Andersson E, Ekbom A. et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 26. Inker LA, Astor BC, Fox CH. et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014; 63: 713–735 [DOI] [PubMed] [Google Scholar]
- 27. VanderWeele TJ, Ding P.. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017; 167: 268–274 [DOI] [PubMed] [Google Scholar]
- 28. Jadoul M, Karaboyas A, Goodkin DA. et al. Potassium-binding resins: associations with serum chemistries and interdialytic weight gain in hemodialysis patients. Am J Nephrol 2014; 39: 252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Georgianos PI, Liampas I, Kyriakou A. et al. Evaluation of the tolerability and efficacy of sodium polystyrene sulfonate for long-term management of hyperkalemia in patients with chronic kidney disease. Int Urol Nephrol 2017; 49: 2217–2221 [DOI] [PubMed] [Google Scholar]
- 30. Lillemoe KD, Romolo JL, Hamilton SR. et al. Intestinal necrosis due to sodium polystyrene (Kayexalate) in sorbitol enemas: clinical and experimental support for the hypothesis. Surgery 1987; 101: 267–272 [PubMed] [Google Scholar]
- 31. Ayoub I, Oh MS, Gupta R. et al. Colon necrosis due to sodium polystyrene sulfonate with and without sorbitol: an experimental study in rats. PLoS One 2015; 10: e0137636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Douglas JG. Mechanism of adrenal angiotensin II receptor changes after nephrectomy in rats. J Clin Invest 1981; 67: 1171–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dardik A, Moesinger RC, Efron G. et al. Acute abdomen with colonic necrosis induced by Kayexalate-sorbitol. South Med J 2000; 93: 511–513 [PubMed] [Google Scholar]
- 34. Rashid A, Hamilton SR.. Necrosis of the gastrointestinal tract in uremic patients as a result of sodium polystyrene sulfonate (Kayexalate) in sorbitol: an underrecognized condition. Am J Surg Pathol 1997; 21: 60–69 [DOI] [PubMed] [Google Scholar]
- 35. Cupisti A, Kovesdy C, D’Alessandro C. et al. Dietary approach to recurrent or chronic hyperkalaemia in patients with decreased kidney function. Nutrients 2018; 10: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.