Abstract
Background
Chronic kidney disease (CKD) is characterized by accelerated aging, but the age-related changes in body composition and its modification by sex and race are unclear.
Methods
We assembled a cohort of 516 patients with CKD and 45 healthy controls and serially measured body composition using air-displacement plethysmography for up to 6 years. Mixed models were used to evaluate simultaneously the baseline and longitudinal changes in body composition as influenced by age, sex and race.
Results
Compared with healthy controls, patients with CKD had a greater weight, body mass index (BMI), fat mass (FM) and percent body fat (BF%), but the changes over time in body composition were similar. Older age (>60 years) was a strong determinant of loss of weight, BMI, FM and fat-free mass (FFM), but not BF%. Compared with non-blacks, blacks had a higher FFM at baseline, but they lost FFM more rapidly. Compared with women, men had an accelerated loss of FFM and accumulation of FM. Taking interactions into account, we found that young black men had no significant change in weight due to the loss of FFM and the accumulation of FM, thereby masking obesity by conventional measurements.
Conclusion
Among patients with CKD, the changes in body composition are influenced by age, sex and race. Young black men have changes in body composition that may remain undetectable by conventional methods thus masking the occurrence of obesity.
Keywords: body composition, body mass index, CKD, longitudinal study, obesity
INTRODUCTION
Chronic kidney disease (CKD) is estimated to affect 8% of the US population and is associated with increased morbidity and mortality. A large fraction of the CKD population is elderly, and it is believed that CKD itself accelerates aging [1]. Aging is associated with loss of weight, sarcopenia, and loss of muscle power and function [2]. In contrast to younger individuals, age-related changes in body composition are more pronounced in the elderly [2]. Furthermore, the patterns of age-related changes in body composition among men and women appear to follow distinct patterns [3, 4].
Because CKD is a process of accelerated aging, age-related changes in body composition may also be accelerated in those with CKD. Whether the patterns of change in body composition are influenced by age and sex in CKD is not known. CKD disproportionately affects blacks [5]. Whether race modifies the age-related changes in body composition remains unknown. This knowledge is important because it may inform us of the extent of the problem, the pattern of change and its modification with age, sex and race. It may also point to potential strategies on how to manage age-related changes in body composition.
The sparse investigation of body composition in CKD has been limited by the use of techniques with inherent limitations. For example, a longitudinal study focused on CKD patients on dialysis to evaluate body composition used bioimpedance analysis (BIA) [6]. BIA may provide crude estimates of body composition especially in dialysis patients who have large changes in body compartment volumes. A greater precision in measurement methods is needed for the evaluation of age-related changes in body composition.
We hypothesized that in comparison with healthy controls, those with CKD will have accelerated age-related changes in body composition. We also posited that as in healthy controls, age-related changes in body composition will be modified by age and sex. Age-related changes in body composition are poorly understood among blacks. Accordingly, we explored whether race modifies the age-related changes in body composition.
MATERIALS AND METHODS
This is a prospective, longitudinal study of body composition in a cohort of patients with CKD and a smaller group of healthy controls. The primary purpose of the study was to evaluate the effects of age, sex and race at baseline and its change over time.
Study sample
Participants were recruited from the renal clinics of either the Veteran’s administration hospital or Eskenazi hospital, a free-standing nonprofit community hospital, both in Indianapolis, IN, USA. To participate, these patients had to have evidence of CKD, either Stage 3 or 4; those with earlier stages of CKD (Stage 1 or 2) had to have albuminuria or some structural evidence of kidney disease to qualify. We excluded those with Stage 5 CKD, on dialysis or with kidney transplants. A group of healthy veterans (n = 45) were also recruited from the general medicine clinic. To qualify as healthy, we required these participants to be nonsmokers, without evidence of CKD, hypertension, diabetes mellitus, myocardial infarction, heart failure, stroke, malignancy, chronic liver or chronic lung disease.
Measurement of body composition
Air displacement plethysmography was used to assess body composition using the BOD POD Gold Standard Body Composition Tracking System (Life Measurement, Inc., Concord, CA, USA). This method is akin to hydrodensitometry or underwater weighing, and provides accurate estimates of body volume and body density, and therefore body composition. Percent body fat (BF%) was calculated—as is the norm—using gender and race-specific equations as follows: for nonblacks, we used the Siri equation [7], for black men the Shutte equation [8] and for black women the Ortiz equation [9]. Fat-free mass (FFM) was calculated by subtracting the fat mass (FM) from the body weight. It is acknowledged that alterations in fluid volume status particularly in the context of CKD may influence the validity of the FFM calculations. Height was measured using a wall-mounted stadiometer with an accuracy of 1 mm. The body mass index (BMI) was calculated as weight in kilograms/height in square meters.
Measurement of covariates
Race was self-described and participants were dichotomized as blacks or nonblacks. Age was dichotomized at 60 years to classify as elderly or young. After a thorough review of the medical history and electronic medical record of the participant, the diagnosis of myocardial infarction, stroke, congestive heart failure and diabetes mellitus was ascertained using conventional definitions.
Serum creatinine was measured in the hospital laboratory using isotope dilution mass spectroscopy-calibrated methods, and the CKD-EPI equation was used to calculate the estimated glomerular filtration rate (eGFR) [10]. The KDIGO classification system was used to classify the stages of CKD based on eGFR [11] and categorizing albuminuria. High albuminuria (30–300 mg/g creatinine) or very high albuminuria (>300 mg/g creatinine) was diagnosed from a spot urine specimen, or overnight urine specimen was used to measure the urine albumin to urine creatinine ratio in the hospital laboratory.
Statistical methods
The baseline characteristics and body composition were compared using a t-test or Chi-squared test for continuous and categorical variables. Longitudinal data were plotted by individuals to examine trends and visual inspection for outliers. A hierarchical approach was used for model fitting. First, a random intercept model was fitted adjusted for the indicator variable of CKD. The latter had two levels, 0 being healthy and 1 being CKD. Next, this model was extended with both random slope and random intercept. The covariance matrix was modeled as unstructured. Residual analysis was then performed at the subject level for both intercepts and slopes. Individual level data were next examined where the random slopes were >3 SDs away from the expectation of 0. These data revealed at least a few participants with biologically implausible data. Thus, all analyses excluded these nine outliers.
We next asked the question whether age, sex and race influence the rates of change in body composition among participants. Therefore, after adjusting the indicator variable of CKD, we introduced the fixed indicator intercepts for age (elderly being defined as age ≥60 years), sex or race (black or nonblack). We further introduced interaction terms for each of these three dichotomous variables with time measured in years. Three-way interaction of CKD with the explanatory variables and time were entered in the model but were not found significant (data not shown).
To further illustrate the effect of age, sex and race on body composition changes over time in those with CKD, we estimated the slopes from the models. The point estimate and their 95% confidence intervals (CIs) were used to generate a forest plot.
The study was approved by the Institutional Review Board and the Veteran’s Administration Research and Development Committee and all participants provided written informed consent.
RESULTS
Between October 2009 and October 2016, we recruited 561 participants who had 1329 measurements of body composition using BOD POD. Of the 561 participants, 45 were healthy and had 134 measurements; the remaining 516 had CKD, and had 1195 measurements. The number of measurements per participant was variable and ranged from 1 to 10 measurements over up to 6.6 years. The mean number of measurements was 2.4 per participant and distribution of the number of measurements was as follows: one measurement in 213 (38%), two in 130 (23%), three in 98 (17%), four in 78 (14%), five in 26 (5%) and more than five in 16 (3%) (Supplementary data, Figure S1).
Table 1 shows the baseline characteristics of study sample. Those with CKD were older, heavier, shorter, rounder and had greater FM and BF%. However, the FFM was similar to the healthy control group. Among those with CKD, as expected of a predominantly older male CKD population, nearly two-thirds had diabetes mellitus, a quarter had myocardial infarction, a fifth had past hospitalization for heart failure and a tenth had strokes. Two-thirds had Stage 3 CKD and a fifth had Stage 4. Approximately a third each had no albuminuria, high albuminuria and very high albuminuria.
Table 1.
Baseline characteristics of the study sample
| Clinical characteristic | Controls | CKD | Total | P-value |
|---|---|---|---|---|
| Number (%) | 45 (8) | 516 (92) | 561 (100) | |
| Age (years) | 62.3 ± 9 | 69 ± 10.1 | 68.4 ± 10.2 | <0.0001 |
| Men, n (%) | 42 (93.3) | 485 (94) | 527 (93.9) | 0.86 |
| Blacks, n (%) | 6 (13.3) | 113 (21.9) | 119 (21.2) | 0.18 |
| Age >60 years, n (%) | 29 (64.4) | 434 (84.1) | 463 (82.5) | <0.001 |
| Age categories (years) | <0.001 | |||
| <40 | 0 (0) | 4 (0.8) | 4 (0.7) | |
| 40–50 | 5 (11.1) | 18 (3.5) | 23 (4.1) | |
| 50–60 | 11 (24.4) | 60 (11.6) | 71 (12.7) | |
| 60–70 | 23 (51.1) | 204 (39.5) | 227 (40.5) | |
| 80–90 | 4 (8.9) | 153 (29.7) | 157 (28) | |
| >90 | 2 (4.4) | 77 (14.9) | 79 (14.1) | |
| Weight (kg) | 86.5 ± 15.2 | 93.2 ± 18.4 | 92.7 ± 18.2 | 0.02 |
| Height (cm) | 176.6 ± 7.6 | 173.1 ± 8 | 173.4 ± 8 | <0.01 |
| BMI (kg/m2) | 27.6 ± 3.9 | 31.1 ± 5.4 | 30.8 ± 5.4 | <0.0001 |
| FFM (kg) | 61.2 ± 8.6 | 59.5 ± 10.3 | 59.6 ± 10.2 | 0.29 |
| FM (kg) | 25.4 ± 9.6 | 33.8 ± 12.1 | 33.1 ± 12.2 | <0.0001 |
| BF% | 28.6 ± 6.9 | 35.4 ± 8 | 34.9 ± 8.2 | <0.0001 |
| Diabetes mellitus, n (%) | 0 (0) | 338 (65.5) | 338 (60.2) | <0.0001 |
| Tobacco use, n (%) | <0.01 | |||
| Never used | 13 (28.9) | 100 (19.4) | 113 (20.1) | |
| Past use | 32 (71.1) | 320 (62) | 352 (62.7) | |
| Current use | 0 (0) | 96 (18.6) | 96 (17.1) | |
| Myocardial infarction, n (%) | 0 (0) | 142 (27.5) | 142 (25.3) | <0.0001 |
| Congestive heart failure, n (%) | 0 (0) | 102 (19.8) | 102 (18.2) | <0.001 |
| Stroke, n (%) | 0 (0) | 57 (11) | 57 (10.2) | 0.02 |
| CKD stage | <0.0001 | |||
| 1 or 2 | 45 (100) | 55 (10.7) | 100 (17.8) | |
| 3 | 0 (0) | 350 (67.8) | 350 (62.4) | |
| 4 | 0 (0) | 111 (21.5) | 111 (19.8) | |
| eGFR (mL/min/1.73 m2) | 88.6 ± 11.6 | 41.5 ± 15.1 | 45.3 ± 19.6 | <0.0001 |
| Albuminuria stage | <0.0001 | |||
| No albuminuria | 44 (97.8) | 199 (38.6) | 243 (43.3) | |
| High albuminuria | 1 (2.2) | 153 (29.7) | 154 (27.5) | |
| Very high albuminuria | 0 (0) | 164 (31.8) | 164 (29.2) | |
| Albumin/creatinine ratio (mg/g) | 5.3 ± 11.8 | 602.2 ± 1316.2 | 554.3 ± 1272.6 | <0.01 |
Table 2 shows the baseline body composition of the study sample by strata of age, sex and race. Values are means and SD in each stratum.
Table 2.
Body composition by age, sex and race
| Body composition measure | Controls | CKD | Total | Control, n | CKD, n |
|---|---|---|---|---|---|
| Weight | |||||
| Age <60 years | 88.6 ± 16.4 | 100.3 ± 18.8 | 98.4 ± 18.9 | 16 | 82 |
| Age ≥60 years | 85.4 ± 14.7 | 91.9 ± 18 | 91.5 ± 17.9 | 29 | 434 |
| Women | 63 ± 4.1 | 86.6 ± 18.3 | 84.5 ± 18.8 | 3 | 31 |
| Men | 88.2 ± 14.3 | 93.7 ± 18.3 | 93.2 ± 18.1 | 42 | 485 |
| Nonblack | 86.5 ± 15.4 | 92.5 ± 17.8 | 92 ± 17.7 | 39 | 403 |
| Black | 86.7 ± 15.6 | 95.9 ± 20 | 95.4 ± 19.9 | 6 | 113 |
| BMI (kg/m2) | |||||
| Age <60 years | 28.1 ± 4.5 | 33 ± 5.6 | 32.2 ± 5.7 | 16 | 82 |
| Age ≥60 years | 27.4 ± 3.6 | 30.7 ± 5.3 | 30.5 ± 5.3 | 29 | 434 |
| Women | 23.3 ± 2.1 | 33.4 ± 5.9 | 32.5 ± 6.3 | 3 | 31 |
| Men | 27.9 ± 3.8 | 30.9 ± 5.3 | 30.7 ± 5.3 | 42 | 485 |
| Nonblack | 27.6 ± 3.9 | 30.8 ± 5.2 | 30.5 ± 5.2 | 39 | 403 |
| Black | 28 ± 4.1 | 32 ± 6 | 31.8 ± 6 | 6 | 113 |
| FFM (kg) | |||||
| Age <60 years | 61.2 ± 8.9 | 64.8 ± 11.5 | 64.2 ± 11.2 | 16 | 82 |
| Age ≥60 years | 61.1 ± 8.5 | 58.5 ± 9.8 | 58.7 ± 9.7 | 29 | 434 |
| Women | 43.4 ± 1.5 | 47 ± 6.8 | 46.7 ± 6.6 | 3 | 31 |
| Men | 62.4 ± 7.3 | 60.3 ± 10 | 60.5 ± 9.8 | 42 | 485 |
| Nonblack | 60.8 ± 8.2 | 58.9 ± 9.6 | 59.1 ± 9.5 | 39 | 403 |
| Black | 63.5 ± 11.1 | 61.7 ± 12.3 | 61.8 ± 12.2 | 6 | 113 |
| FM (kg) | |||||
| Age <60 years | 27.4 ± 10.7 | 35.5 ± 13.8 | 34.2 ± 13.7 | 16 | 82 |
| Age ≥60 years | 24.3 ± 9 | 33.4 ± 11.8 | 32.9 ± 11.8 | 29 | 434 |
| Women | 19.6 ± 2.6 | 39.7 ± 14.3 | 37.9 ± 14.8 | 3 | 31 |
| Men | 25.8 ± 9.8 | 33.4 ± 11.9 | 32.8 ± 11.9 | 42 | 485 |
| Nonblack | 25.7 ± 9.9 | 33.6 ± 12 | 32.9 ± 12 | 39 | 403 |
| Black | 23.2 ± 8.2 | 34.2 ± 12.5 | 33.7 ± 12.6 | 6 | 113 |
| BF% | |||||
| Age <60 years | 30.2 ± 7.5 | 34.5 ± 10.1 | 33.8 ± 9.8 | 16 | 82 |
| Age ≥60 years | 27.8 ± 6.5 | 35.6 ± 7.6 | 35.1 ± 7.8 | 29 | 434 |
| Women | 31.1 ± 2.2 | 44.6 ± 8 | 43.4 ± 8.6 | 3 | 31 |
| Men | 28.5 ± 7.1 | 34.8 ± 7.7 | 34.3 ± 7.8 | 42 | 485 |
| Nonblack | 29 ± 7 | 35.5 ± 7.9 | 35 ± 8.1 | 39 | 403 |
| Black | 26.5 ± 6.2 | 35 ± 8.5 | 34.6 ± 8.6 | 6 | 113 |
Values shown are mean ± SD.
Table 3 shows the estimates of body composition without considering the time variable (random intercept model) and then considering time as an explanatory variable (the combined random intercept and random slopes model). Body measurement outcomes were measured by weight, BMI, FM, FFM and BF%. In each case, the residual variance improved considerably, suggesting that compared with the random intercept model the combined random intercept and the random slopes model was better. Residual analysis revealed that the subject-specific random effect for rate of change in FFM deviated by >3 SDs in nine participants. At least some of these measurements did not appear biologically plausible, so these 9 out of 561 participants were excluded. Table 3 and all subsequent analyses exclude these nine outlier participants.
Table 3.
Hierarchical development of mixed models for each body composition measure
| Weight |
BMI |
FFM |
FM |
BF% |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Random intercept | Random Int and slope | Random intercept | Random Int and slope | Random intercept | Random Int and slope | Random intercept | Random Int and slope | Random intercept | Random Int and slope |
| Constant | 87.1 ± 2.7 | 86.6 ± 2.7 | 27.9 ± 0.8 | 27.6 ± 0.8 | 60.8 ± 1.5 | 61.3 ± 1.5 | 26.3 ± 1.7 | 25.3 ± 1.7 | 29.4 ± 1.1 | 28.5 ± 1.1 |
| CKD intercept | 5.8 ± 2.8 | 6.6 ± 2.8 | 3.2 ± 0.8 | 3.4 ± 0.8 | −1.7 ± 1.5 | −1.8 ± 1.5 | 7.5 ± 1.8 | 8.4 ± 1.8 | 6.1 ± 1.2 | 6.8 ± 1.2 |
| P-value (CKD) | 0.04 | 0.02 | <0.001 | <0.0001 | 0.26 | 0.23 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Slope | 0.361 ± 0.530 | 0.205 ± 0.177 | −0.541 ± 0.159 | 0.945 ± 0.489 | 0.898 ± 0.315 | |||||
| P-value (Slope) | 0.5 | 0.25 | <0.001 | 0.05 | <0.01 | |||||
| CKD slope | −1.062 ± 0.575 | −0.336 ± 0.192 | −0.298 ± 0.178 | −0.739 ± 0.531 | −0.547 ± 0.345 | |||||
| P-value (CKD slope) | 0.06 | 0.08 | 0.09 | 0.16 | 0.11 | |||||
| SD (intercept) | 17.9 | 17.9 | 5.2 | 5.2 | 9.6 | 9.7 | 11.3 | 11.5 | 7.1 | 7.3 |
| SD (slope) | 2.814 | 0.928 | 0.426 | 2.527 | 1.445 | |||||
| Rho | 0.119 | 0.035 | −0.015 | −0.051 | −0.159 | |||||
| Residual | 4.016 | 2.516 | 1.365 | 0.894 | 2.595 | 2.286 | 3.96 | 2.728 | 3.298 | 2.705 |
| Log likelihood | −4627.5 | −4493.2 | −3156.7 | −3044.1 | −3968.5 | −3898.7 | −4375.2 | −4279.8 | −4000 | −3953.9 |
| Chi-squared | 4.227 | 16.436 | 14.725 | 22.086 | 1.278 | 122.9 | 17.277 | 25.287 | 27.589 | 44.226 |
| AIC | 9263 | 9002.4 | 6321.5 | 6104.3 | 7945 | 7813.4 | 8758.4 | 8575.6 | 8008 | 7923.9 |
Nine outliers removed.
Table 4 shows models that take into account the CKD status, sex, age and race into account. Taking all these data shown in Table 4 into consideration, compared with healthy controls, patients with CKD had a greater weight, BMI, FM and BF%. However, the changes in body composition depicted by CKD slope in the table were similar to those without CKD.
Table 4.
Mixed model estimates of each body composition measure
| Parameter | Weight (kg) | P-value | BMI | P-value | FFM | P-value | FM | P-value | BF% | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Constant | 79.9 ± 4.1 | 29.4 ± 1.2 | 49.5 ± 2.1 | 30.3 ± 2.7 | 36.8 ± 1.7 | |||||
| CKD intercept | 8.0 ± 2.8 | <0.01 | 3.8 ± 0.8 | <0.0001 | −0.9 ± 1.4 | 0.52 | 8.9 ± 1.8 | <0.0001 | 6.7 ± 1.2 | <0.0001 |
| Elderly intercept | −9.1 ± 2.0 | <0.0001 | −2.0 ± 0.6 | <0.01 | −7.0 ± 1.0 | <0.0001 | −2.0 ± 1.4 | 0.14 | 1.4 ± 0.9 | 0.11 |
| Male intercept | 13.1 ± 3.2 | <0.0001 | −0.7 ± 1.0 | 0.49 | 17.1 ± 1.6 | <0.0001 | −4.0 ± 2.2 | 0.07 | −9.6 ± 1.4 | <0.0001 |
| Black intercept | 2.8 ± 1.9 | 0.14 | 0.7 ± 0.6 | 0.23 | 2.9 ± 1.0 | <0.01 | −0.1 ± 1.3 | 0.93 | −1.1 ± 0.8 | 0.16 |
| Slope (per year) | 0.668 ± 1.020 | 0.51 | 0.418 ± 0.343 | 0.22 | 0.401 ± 0.403 | 0.32 | 0.057 ± 0.971 | 0.95 | −0.006 ± 0.689 | 0.99 |
| CKD slope | −0.669 ± 0.567 | 0.24 | −0.207 ± 0.189 | 0.27 | −0.110 ± 0.169 | 0.51 | −0.508 ± 0.530 | 0.34 | −0.483 ± 0.351 | 0.17 |
| Elderly slope | −1.919 ± 0.512 | <0.001 | −0.642 ± 0.171 | <0.001 | −0.555 ± 0.159 | <0.001 | −1.308 ± 0.482 | <0.01 | −0.463 ± 0.326 | 0.16 |
| Male slope | 1.112 ± 0.902 | 0.22 | 0.248 ± 0.304 | 0.42 | −0.587 ± 0.391 | 0.13 | 1.866 ± 0.867 | 0.03 | 1.244 ± 0.633 | 0.05 |
| Black slope | −0.762 ± 0.499 | 0.13 | −0.230 ± 0.167 | 0.17 | −0.493 ± 0.161 | <0.01 | −0.208 ± 0.471 | 0.66 | 0.148 ± 0.322 | 0.65 |
| SD intercept | 17.4 | 5.1 | 8.7 | 11.4 | 7 | |||||
| SD slopes | 2.713 | 0.893 | 0.264 | 2.47 | 1.432 | |||||
| Rho | 0.08 | 0.01 | 0.1 | −0.04 | −0.109 | |||||
| Residual | 2.513 | 0.894 | 2.292 | 2.719 | 2.696 | |||||
| Log likelihood | −4469.9 | −3028.7 | −3826.8 | −4271 | −3928.9 | |||||
| Chi-squared | 65.205 | 54.13 | 303.69 | 43.564 | 100.37 | |||||
| AIC | 8967.7 | 6085.5 | 7681.5 | 8570.1 | 7885.8 |
Older age (>60 years) was a strong determinant of a lower weight, lower BMI and lower FFM. Furthermore, it was also a strong determinant of loss of weight, BMI and FFM over time. However, older age was not a determinant of FM. Nonetheless, it was associated with a greater loss of FM over time. Thus, the cross-sectional and longitudinal associations between older age and FM were disparate. BF% was not different between the elderly and the young at baseline. Because of the symmetric reductions in FM and FFM, the BF% did not change.
Black race was not associated with body composition when measured by body weight, BMI, FM and BF%. However, fat-free body mass among blacks and nonblacks were very different. Compared with nonblacks, blacks had a higher FFM. However, the loss of FFM was accelerated in blacks.
Unsurprisingly, men had a higher weight than women but the change in weight over time was similar. No signal was seen between sex and BMI. However, sex-specific signals emerged for body composition and its change over time. Although compared with women, men had a higher FFM, the loss of FFM was accelerated in men. Conversely, compared with women, men had a lower FM, but the accumulation of FM was accelerated in men. Thus, compared with women, the body fat percent that was lower in men at baseline accumulated over time. In other words, the effect of sex on body composition was neutralized over time.
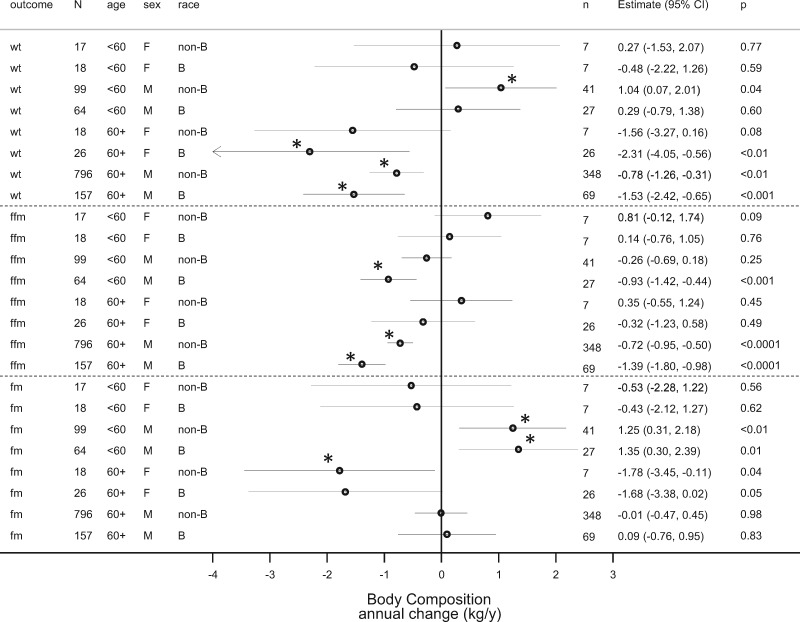
To facilitate the global comparison of age, sex and race on body composition in CKD, the estimates and their 95% CIs for the changes in weight, FM and FFM dichotomized by age, sex and race are shown in Figure 1.
FIGURE 1.
Forest plot shows the estimates of change in body composition indices over time. Weight (wt), FFM and FM are shown as point estimates (diamonds) by age, sex (M = male, F = female) and race (B = black, non-B = nonblack). N is the number of measurements and n the number of participants in the CKD stratum. The 95% CIs are shown as horizontal lines. If the line crosses the vertical line at 0, then the point estimate is not statistically significant at the 5% level. The precise value of the significance of the point estimate is shown as the P-value.
Among elderly men, there was both a decline in weight and FFM. There was no significant change in FM. In contrast, among elderly women, there was an increase in FM but no change in FFM. In younger men, there was a more complex pattern. Among blacks, there was no change in weight, but an increase in FM and a loss of FFM. Thus, over time, among those young blacks with CKD, there was an emergence of masked obesity. Among nonblacks, there was an increase in weight, no loss of FFM but an increase in FM. Thus, among young whites with CKD, there was emergence of overt obesity. In younger women with CKD, there were no changes in body composition seen, but our sample size was small to make any reliable estimates of change.
DISCUSSION
The major findings of our study are as follows. (i) Compared with healthy controls, patients with CKD have a greater weight, BMI, FM and BF%, but the changes in body composition are similar to those without CKD. (ii) Independent of CKD status, age >60 years is a strong determinant of lower weight, lower BMI and lower FFM, but not FM. However, age >60 years is a strong determinant of loss of weight, BMI, FM and FFM. Because of symmetric reductions in FM and FFM, the BF% does not change. (iii) Although compared with nonblacks, blacks have a higher FFM, the loss of FFM is accelerated in blacks. Changes in other body composition measures such as weight, BMI, FM and BF% are not modified by race. (iv) Although compared with women, men have a higher FFM, the loss of FFM is accelerated in men. Conversely, compared with women, men have a lower FM, but the accumulation of FM is accelerated in men. Thus, compared with women, the BF% that is lower in men at baseline accumulates over time.
Considerations of the above estimates allow us to propose the novel concept of masked obesity in young black men. These individuals do not lose weight because they lose FFM and gain FM. Without assessment of body composition, these changes may not be detectable. In contrast, young nonblack men have overt obesity: weight increases, FM increases, but FFM stays unchanged. We had few young women in our study but data suggest stable body composition. In contrast, fat loss is seen in older women.
Few studies evaluate body composition using direct measurements of body density. Using underwater weighing, Guo et al. reported the body composition changes in 102 men and 108 women followed between 1 and 20 years, the mean age of whom was 44.5 years (range 40–66) [3]. An increase in weight, BMI, fat weight and percent body weight was reported. The point estimates of change in weight and BMI are similar to our estimates in healthy controls. However, in contrast to their report of an increase in FM in men of 0.37 kg/year and women of 0.41 kg/year, we noted an increase of 0.95 kg/year. Possibly, our healthy subjects were less active and gained more fat weight. They reported loss of FFM of 0.07 kg/year in men and 0.11 kg/year in women, which is much smaller than the loss of 0.54 kg/year we noted in our healthy controls. Using underwater weighing, Siervogel et al. [4] in 1998 reported body composition changes in 59 men and 62 women with an age range of 45–65 years and followed on average for about 10 years [4]. They reported an increase in FM of 0.37 kg/year in men and 0.52 kg/year in women, no change in FFM and increase in BF% (0.34%/year in men and 0.47%/year in women). Perhaps, the rate of FM accretion and BF% has increased since 1998 as we observed two to three times the rate in healthy people.
There are some clinical implications of our study with respect to the care of patients with CKD. Although we did not characterize the loss of FFM, if this is because of sarcopenia, one may anticipate reduced generation of creatinine. If so, then serum creatinine as a longitudinal marker of kidney function may underestimate the decline in GFR in the elderly, in men and in blacks. Zoccali [12] points out in a review that obesity is not an innocuous bystander and may directly or indirectly damage the kidney. Since obesity is often occult in those with CKD, it calls into question even a greater contribution of this occult risk factor in causing kidney damage, particularly in young black men. In a subset of this cohort, we have measured GFR using iothalamate clearances. Accordingly, future analyses will evaluate whether greater discordance in measured and estimated GFRs are dependent on age, sex and race.
Strengths of our study are that this is the longest and largest study to our knowledge measuring body composition in CKD using one of the gold standard measurements of body composition, the air-displacement plethysmography method. The equipment was calibrated before each use and the same protocol was used to perform the longitudinal measurements of body composition. Limitations include the following: our cohort was predominantly male and did not have enough young women to provide reliable estimates of change in body composition. The measurements were not uniformly available, reflecting attrition of the cohort, which was only in part be due to death of participants.
In summary, we note accelerated loss of FFM in the elderly, men and blacks. In young black men, we describe the occurrence of masked obesity. Masked obesity is a condition where body weight stays constant, but there is a loss of FFM and a gain of FM. Detection of this condition requires the measurement of body composition as noted in this study. We note that simply measuring body weight and BMI may not be sufficient to detect changes associated specifically with CKD. Future studies should analyze the prognostic implications of these findings.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
The author thanks the participants for their time and effort. The author thanks Dr Sujuan Gao, Professor of Biostatistics, Indiana University Fairbank’s School of Public Health for grading parts of the analyses presented in this article toward a MS Biostatistics degree to R.A.
FUNDING
VA Merit review 2I01CX000829-05 and NIH R01 HL126903.
AUTHORS’ CONTRIBUTIONS
R.A. designed the study, obtained funding, supervised its approval by the IRB and the VA research and development committee, supervised the collection of data by study technicians, managed the data, analyzed the results, wrote the manuscript and submitted it for publication.
CONFLICT OF INTEREST STATEMENT
R.A. declares the following conflicts: Member data safety monitoring committees: Astra Zeneca and Ironwood Pharmaceuticals. Member steering committees of randomized trials: Akebia, Bayer, Janssen, Glaxo Smith Cline, Relypsa, and Sanofi and Genzyme US Companies. Member adjudication committees: Bayer, Boehringer Ingelheim and Janssen. Member scientific advisory board or consultant: BirdRock Bio, Celgene, Daiichi Sankyo, Inc., Eli Lilly, Relypsa, Reata, Takeda Pharmaceuticals, USA and ZS Pharma.
REFERENCES
- 1. Stenvinkel P, Larsson TE.. Chronic kidney disease: a clinical model of premature aging. Am J Kidney Dis 2013; 62: 339–351 [DOI] [PubMed] [Google Scholar]
- 2. Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol 2003; 95: 1717–1727 [DOI] [PubMed] [Google Scholar]
- 3. Guo SS, Zeller C, Chumlea WC. et al. Aging, body composition, and lifestyle: the Fels Longitudinal Study. Am J Clin Nutr 1999; 70: 405–411 [DOI] [PubMed] [Google Scholar]
- 4. Siervogel RM, Wisemandle W, Maynard LM. et al. Serial changes in body composition throughout adulthood and their relationships to changes in lipid and lipoprotein levels. The Fels Longitudinal Study. Arterioscler Thromb Vasc Biol 1998; 18: 1759–1764 [DOI] [PubMed] [Google Scholar]
- 5. Coresh J, Selvin E, Stevens LA. et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038–2047 [DOI] [PubMed] [Google Scholar]
- 6. Johansen KL, Kaysen GA, Young BS. et al. Longitudinal study of nutritional status, body composition, and physical function in hemodialysis patients. Am J Clin Nutr 2003; 77: 842–846 [DOI] [PubMed] [Google Scholar]
- 7. Siri WE. Body composition from fluid spaces and density: analysis of methods In: Brozek J, Henschel A (eds). Techniques for Measuring Body Composition. Washington, DC: National Academy of Science, 1961, 223–244 [Google Scholar]
- 8. Schutte JE, Townsend EJ, Hugg J. et al. Density of lean body mass is greater in blacks than in whites. J Appl Physiol Respir Environ Exerc Physiol 1984; 56: 1647–1649 [DOI] [PubMed] [Google Scholar]
- 9. Ortiz O, Russell M, Daley TL. et al. Differences in skeletal muscle and bone mineral mass between black and white females and their relevance to estimates of body composition. Am J Clin Nutr 1992; 55: 8–13 [DOI] [PubMed] [Google Scholar]
- 10. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levey AS, de Jong PE, Coresh J. et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011; 80: 17–28 [DOI] [PubMed] [Google Scholar]
- 12. Zoccali C. Overweight, obesity and metabolic alterations in chronic kidney disease. Prilozi 2009; 30: 17–31 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.