Abstract
Modifiable cardiometabolic risk factors induce the release of proinflammatory cytokines and reactive oxygen species from circulating peripheral blood mononuclear cells (PBMCs), resulting in increased cardiovascular disease risk and compromised immune health. These changes may be driven by metabolic reprogramming of PBMCs, resulting in reduced mitochondrial respiration; however, this has not been fully tested. We aimed to determine the independent associations between cardiometabolic risk factors including BMI, blood pressure, fasting glucose, and plasma lipids with mitochondrial respiration in PBMCs isolated from generally healthy individuals (n = 21) across the adult lifespan (12 men/9 women; age, 56 ± 21 yr; age range, 22–78 yr; body mass index, 27.9 ± 5.7 kg/m2; blood pressure, 123 ± 16/72 ± 10 mmHg; glucose, 90 ± 14 mg/dL; low-density lipoprotein cholesterol (LDL-C), 111 ± 22 mg/dL; and high-density lipoprotein cholesterol (HDL-C), 62 ± 16 mg/dL). PBMCs were isolated from whole blood by density-dependent centrifugation and used to assess mitochondrial function by respirometry. Primary outcomes included basal and maximal oxygen consumption rate (OCR), which were subsequently used to determine spare respiratory capacity and OCR metabolic potential. After we corrected for systolic blood pressure (SBP), diastolic blood pressure (DBP), and blood glucose, LDL-C was negatively associated with maximal respiration (r = −0.56, P = 0.016), spare respiratory capacity (r = −0.58, P = 0.012), and OCR metabolic potential (r = −0.71, P = 0.0011). In addition, SBP was negatively associated with OCR metabolic potential (r = −0.62, P = 0.0056) after we corrected for DBP, blood glucose, and LDL-C. These data suggest a link between blood cholesterol, SBP, and mitochondrial health that may provide insight into how cardiometabolic risk factors contribute to impaired immune cell function.
NEW & NOTEWORTHY Independent of other cardiometabolic risk factors, low-density lipoprotein cholesterol, and systolic blood pressure were found to be negatively associated with several parameters of mitochondrial respiration in peripheral blood mononuclear cells of healthy adults. These data suggest that low-density lipoprotein cholesterol and systolic blood pressure may induce metabolic reprogramming of immune cells, contributing to increased cardiovascular disease risk and impaired immune health.
Keywords: cardiovascular disease, cholesterol, immune cells, mitochondrial dysfunction, peripheral blood mononuclear cells
INTRODUCTION
Cardiometabolic risk factors including advancing age, hypertension, elevated body mass index (BMI) and fasting glucose, and dyslipidemia are associated with chronic inflammation (5, 10, 21, 32, 45, 48); however, the underlying mechanisms are incompletely understood. These risk factors not only impact cardiovascular function via an increased release of proinflammatory cytokines and reactive oxygen species (ROS) from circulating immune cells (i.e., peripheral blood mononuclear cells, PBMCs) (4, 8, 9, 15, 30, 33, 39, 42) but may also compromise immune cell metabolism (12, 29, 37, 54), making the immune system less capable of mounting an appropriate response to acute infection (37, 40). Accordingly, there is presently an urgent need to understand the relationship between cardiometabolic risk factors and immune cell function to develop more targeted therapies for improving both cardiovascular and immune health in humans.
The interaction between cardiometabolic risk factors and impaired immune cell function may be partially mediated by reduced mitochondrial respiration (11, 13, 44). In this regard, mitochondrial respiration is impaired in PBMCs of patients with early stage heart failure and is associated with elevated blood pressure and increased low-density lipoprotein cholesterol (LDL-C) concentration (29). In addition, mitochondrial dysfunction is associated with increased production of mitochondrial ROS and a greater release of proinflammatory cytokines (7, 54), contributing to oxidative stress and chronic, low-grade inflammation (11, 44). Whether these risk factors are similarly associated with reduced mitochondrial function in healthier adults, before the development of overt cardiometabolic disease, is an important question that remains to be determined. Therefore, the purpose of this study was to determine the associations between cardiometabolic risk factors and mitochondrial respiration in circulating immune cells from healthy individuals across the adult lifespan. We hypothesized that cardiometabolic factors including age, blood pressure, BMI, fasting glucose, and circulating lipids would be independently and negatively correlated with mitochondrial respiration and that these associations would occur independent of age.
METHODS
Subjects.
Twenty-one healthy adults between the ages of 18 and 79 yr were included in this study. Subjects were excluded if they were diagnosed with any chronic clinical disease or exhibited clinically abnormal blood chemistries indicative of abnormal renal, liver, thyroid, and adrenal function; past or present alcohol dependence or abuse; currently smoking; or pregnant or breastfeeding. All procedures were approved by the Institutional Review Board at the University of Delaware. The study rationale, procedures, risks, and benefits were explained to the subjects, and their written, informed consent was obtained before enrollment. Subjects were instructed to fast for ≥12 h and to refrain from prescription medications for 24 h before each study visit.
Cardiometabolic risk factors.
Seated brachial artery systolic (SBP) and diastolic (DBP) blood pressure were measured following 1 min of quiet rest in the nondominant arm using a validated semiautomated oscillometric sphygmomanometer (SunTech ADView 2, SunTech Medical) (35). Measurements were made in triplicate with 1 min of recovery in-between each measurement until three measurements were obtained that were within 5 mmHg of one another. These values were then averaged to determine resting SBP and DBP and were used to calculate brachial artery pulse pressure (SBP – DBP) and mean arterial pressure (MAP). BMI was calculated as body mass (kg)/height (m)2. Fasting blood glucose and serum lipids were measured from a comprehensive metabolic panel and standard lipid panel, respectively, by a CLIA-certified clinical laboratory (Quest Diagnostics or LabCorp). The lipid panel included total blood cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and plasma triglycerides (TGs). LDL-C concentrations were calculated from the measured variables.
Peripheral blood mononuclear cell isolation.
PBMCs were isolated from whole blood collected in EDTA-coated Vacutainer tubes, as previously described (31). Briefly, whole blood was centrifuged for 20 min at 500 g and 23°C with the acceleration and brake set to low. The majority (~2/3) of plasma was replaced with room-temperature 1× phosphate-buffered saline (PBS) to increase extraction efficiency, and the sample containing red blood cells was carefully pipetted on top of 10 mL of room-temperature Histopaque-1077 (Sigma-Aldrich) and centrifuged at 400 g for 20 min at 23°C with the acceleration and brake set to low. PBMCs were extracted and transferred to a separate tube and washed twice in PBS for 10 min each at 400 g and 23°C. The PBMCs were then counted, and viability was measured with trypan blue using the Countess II-automated cell counter (Invitrogen, No. AMQAX1000).
Mitochondrial respiration.
Isolated PBMCs were seeded in a Seahorse XFp miniplate (Agilent) for assessment of whole cell respirometry. We combined the results from two separate studies that used slightly different protocols. Cells were either incubated overnight in 120 µL of RPMI-1640 medium (ATCC 30C2001) in XFp miniplate at a density of 400,000 or 200,000 live cells/well and then washed the following day with XF DMEM at pH 7.4 (Agilent, 103575-100) or seeded the same day in XF DMEM at a density of 200,000 live cells/well. Cell-seeding density and incubation time were assessed for their potential associations with mitochondrial respiration using Pearson correlations. Neither parameter explained the variance in respiration, therefore these factors were not included as covariates in subsequent statistical models; however, all mitochondrial respiration parameters were normalized to seeding density before statistical analysis and are presented as pmol O2·min−1·10−5 cells−1.
Mitochondrial respiration was assessed using the Agilent Cell Mito Stress Test Kit (No. 103015-100) on a Seahorse XFp Analyzer (Agilent Technologies, Santa Clara, CA), which measures oxygen consumption rate (OCR) at basal and following three serial injections of oligomycin (1 µM), carbonyl cyanide-4(trifluoromethoxy)phenylhydrazone (FCCP, 2 µM), and rotenone and antimycin A (0.5 µM) to determine maximal respiration, spare respiratory capacity, coupling efficiency, and ATP-linked O2 consumption (ATPO2). Spare respiratory capacity is a measure of the cell’s capacity to increase O2 consumption above basal respiration (2). Coupling efficiency is how well ATPO2 is matched to basal respiration, and ATPO2 is calculated by measuring the decrease in OCR following the inhibition of ATP synthase (2). We also calculated the OCR metabolic potential, which reflects the percent increase (from baseline) of OCR following the acute injection of FCCP into the respirometer and represents the ability of cells to increase energy production specifically through oxidative phosphorylation (1).
Statistical analyses.
Significant cardiometabolic correlates of mitochondrial respiration were identified using bivariate correlations and subsequently assessed for their independent associations with mitochondrial respiration using partial correlations while covariates constant. Bivariate and partial correlations were performed using GraphPad Prism (version 8.2.1) and R (version 3.6.1), respectively. Data are presented as Pearson’s correlation coefficients (R) with α set at P < 0.05 for all analyses.
RESULTS
Subject characteristics.
Subject characteristics are summarized in Table 1. Subjects were recruited across the adult lifespan (22–78 yr) and were generally healthy and free of chronic diseases. While as a group, all subject characteristics were within normal ranges, we observed the necessary variation in BMI, SBP and LDL-C to assess the independent association of these risk factors with mitochondrial function.
Table 1.
Participant characteristics
| Mean ± SD | Minimum-Maximum | |
|---|---|---|
| Sex, men/women | 12/9 | - |
| Age, yr | 56 ± 21 | 22-78 |
| Height, cm | 168.0 ± 12.6 | 149.5-196.2 |
| Body mass, kg | 79.1 ± 23.3 | 52.7-148.3 |
| Body mass index, kg/m2 | 27.9 ± 5.7 | 21.9-40.8 |
| SBP, mmHg | 123 ± 16 | 100-152 |
| DBP, mmHg | 72 ± 10 | 58-89 |
| PP, mmHg | 51 ± 11 | 31-79 |
| MAP, mmHg | 89 ± 11 | 75-106 |
| HR, beats/min | 57 ± 8 | 40-71 |
| Glucose, mg/dL | 90 ± 14 | 61-131 |
| TC, mg/dL | 194 ± 31 | 143-256 |
| HDL-C, mg/dL | 62 ± 16 | 32-91 |
| LDL-C, mg/dL | 111 ± 22 | 73-161 |
| TG, mg/dL | 103 ± 60 | 38-315 |
| Statins, n (%) | 5 (24) | |
| ACE inhibitor, n (%) | 3 (14) | |
| Angiotensin II receptor blocker, n (%) | 1 (5) |
Values are means ± SD and minimum-maximum values. Characteristics for all subjects (n = 21). BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; MAP, mean arterial pressure; HR, heart rate; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; ACE, angiotensin-converting enzyme.
Cardiometabolic risk factors and mitochondrial respiration.
As reported in Table 2, maximal respiration (mean = 126.89 ± 82.31 pmol O2·min−1·10−5 cells−1) was negatively associated with SBP (r = −0.51, P = 0.018), DBP (r = −0.46, P = 0.037), blood glucose (r = −0.43, P = 0.049), and LDL-C (r = −0.55, P = 0.010). Similarly, spare respiratory capacity (mean = 104.38 ± 74.27 pmol O2·min−1·10−5 cells−1) was negatively associated with SBP (r = −0.52, P = 0.016), DBP (r = −0.44, P = 0.045), and LDL-C (r = −0.55, P = 0.0096). While nonsignificant, there was a strong association between spare respiratory capacity and blood glucose (r = −0.43, P = 0.054). Lastly, OCR metabolic potential (mean = 355.63 ± 141.55%) was negatively associated with LDL-C (r = −0.54, P = 0.011) and ATPO2 (mean = 18.31 ± 13.72 pmol O2·min−1·10−5 cells−1) was negatively associated with blood glucose (r = −0.45, P = 0.042). Basal respiration (mean = 22.50 ± 12.89 pmol O2·min−1·10−5 cells−1) and coupling efficiency (mean = 81.68 ± 35.81%) were not associated with any of the cardiometabolic risk factors tested; therefore, no subsequent analyses were performed with these parameters.
Table 2.
Bivariate correlations between mitochondrial respiration and cardiometabolic risk factors
| Age | SBP | DBP | BMI | Glucose | TC | HDL-C | LDL-C | |
|---|---|---|---|---|---|---|---|---|
| Basal respiration | ||||||||
| r | −0.06 | −0.25 | −0.38 | −0.13 | −0.32 | −0.12 | 0.20 | −0.33 |
| P value | 0.78 | 0.27 | 0.087 | 0.59 | 0.16 | 0.59 | 0.39 | 0.14 |
| Maximal respiration | ||||||||
| r | −0.26 | −0.51 | −0.46 | −0.16 | −0.43 | −0.23 | 0.31 | −0.55 |
| P value | 0.25 | 0.018 | 0.037 | 0.50 | 0.049 | 0.31 | 0.17 | 0.010 |
| Spare respiratory capacity | ||||||||
| r | −0.28 | −0.52 | −0.44 | −0.15 | −0.43 | −0.24 | 0.31 | −0.55 |
| P value | 0.22 | 0.016 | 0.045 | 0.52 | 0.054 | 0.30 | 0.18 | 0.0096 |
| ATPO2 | ||||||||
| r | 0.05 | −0.33 | −0.42 | −0.18 | −0.45 | −0.19 | 0.05 | −0.32 |
| P value | 0.82 | 0.14 | 0.060 | 0.43 | 0.042 | 0.40 | 0.84 | 0.16 |
| Coupling efficiency | ||||||||
| r | 0.18 | −0.11 | 0.02 | −0.08 | −0.40 | −0.24 | −0.26 | −0.18 |
| P value | 0.44 | 0.62 | 0.92 | 0.72 | 0.070 | 0.29 | 0.25 | 0.42 |
| Metabolic potential | ||||||||
| r | −0.22 | −0.39 | −0.21 | 0.02 | −0.17 | −0.28 | 0.26 | −0.54 |
| P value | 0.34 | 0.079 | 0.36 | 0.93 | 0.47 | 0.21 | 0.25 | 0.011 |
Pearson’s correlation coefficient between each mitochondrial respiratory parameter and cardiometabolic risk factor. Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ATPO2, ATP-linked oxygen consumption. α was set at P < 0.05 (significant associations indicated by boldface).
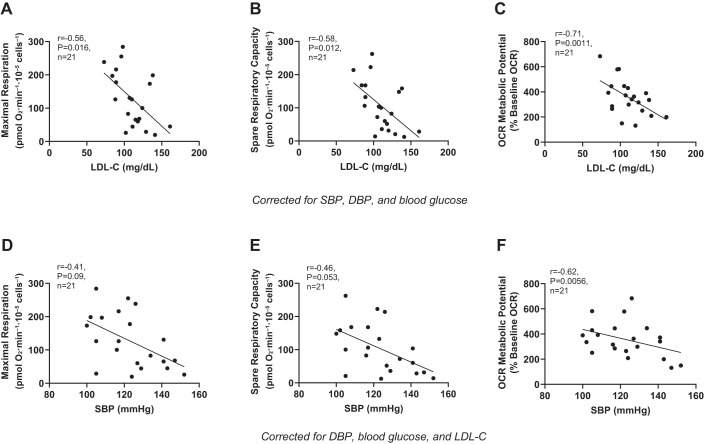
After we corrected for covariates (e.g., SBP, DBP, and blood glucose), LDL-C was negatively associated with maximal respiration (Fig. 1A), spare respiratory capacity (Fig. 1B), and OCR metabolic potential (Fig. 1C) but was not associated with ATPO2 (r = −0.13, P = 0.61). After we corrected for DBP, blood glucose, and LDL-C, SBP was also negatively associated with OCR metabolic potential (Fig. 1F), and there was a strong but nonsignificant negative association between SBP and maximal respiration (Fig. 1D) and spare respiratory capacity (Fig. 1E). There was no association between SBP and ATPO2 (r = 0.03, P = 0.90). After we corrected for SBP, blood glucose, and LDL-C, DBP was no longer associated with maximal respiration (r = 0.01, P = 0.98), spare respiratory capacity (r = 0.06, P = 0.80), OCR metabolic potential (r = 0.40, P = 0.10), or ATPO2 (r = −0.25, P = 0.32). Similarly, blood glucose was not associated with maximal respiration (r = −0.01, P = 0.97), spare respiratory capacity (r = 0.02, P = 0.93), OCR metabolic potential (r = 0.41, P = 0.09), or ATPO2 (r = 29, P = 0.25) after we corrected for covariates (e.g., SBP, DBP, and LDL-C).
Fig. 1.
Cardiometabolic risk factors and mitochondrial respiration. Independent associations between low-density lipoprotein cholesterol (LDL-C) and systolic blood pressure (SBP) with maximal respiration (A and D), spare respiratory capacity (B and E), and oxygen consumption rate (OCR) metabolic potential (C and F). Statistical analyses based on partial correlation matrices with respiratory parameters adjusted for either subject SBP or LDL-C, diastolic blood pressure (DBP), and blood glucose. α was set at P < 0.05.
DISCUSSION
In the present study, we found that LDL-C is negatively associated with maximal oxygen consumption, spare respiratory capacity, and OCR metabolic potential of PBMCs, independent of age, blood pressure, BMI, and other blood lipids. Likewise, we observed near-significant associations between SBP with maximal respiration and spare respiratory capacity and a significant association with OCR metabolic potential. Collectively, these findings suggest a potentially important link between cardiometabolic risk factors and immune cell energy metabolism in healthy humans.
Our findings are also in agreement with previous findings in a group of adults that included patients with early stage heart failure (29). The negative associations of these risk factors with both maximal respiration and spare respiratory capacity suggest that these factors may interfere with the ability of immune cells to respond to increases in energy demand, such as during acute infection. In addition, the inverse association with OCR metabolic potential suggests that elevated risk factors may also shift immune cells toward more glycolytic energy production. Immune cell metabolism is complex and cell-type dependent. As demand for ATP increases during acute infection, some lymphocytes (e.g., T effector cells) rely more on glycolysis (55) and exhibit reduced oxidative metabolism as demand for oxygen exceeds its supply (40). Importantly, the dependence on glycolysis can be reversed by improving mitochondrial function, allowing these cells to maximize energy production through oxidative phosphorylation (40). Memory T cells, while inherently more oxidative than T effector cells, demonstrate improved survival in response to reexposure to an antigen when oxidative metabolism is increased, thus highlighting the importance of mitochondrial function in the regulation of adaptive immunity (34, 49). With regard to the innate immune system, monocytes also undergo metabolic reprogramming as they differentiate into either proinflammatory M1 monocytes or anti-inflammatory M2 monocytes (27). Predifferentiated monocytes and M2 monocytes are primarily aerobic, whereas M1 monocytes primarily use anaerobic metabolism (27). Collectively, the unique metabolic requirements of lymphocytes and monocytes implies an important role of oxidative metabolism in the cellular response to acute infection and/or inflammation. This is of particular importance to the present study as lymphocytes and monocytes make up the vast majority of PBMCs (26).
The mechanisms by which blood lipids influence immune cell metabolism requires further elucidation; however, one possibility is through oxidation of LDL-C. In isolated human macrophages, oxidized LDL-C induces mitochondrial dysfunction and promotes apoptosis via the formation of peroxidases, which decreases mitochondrial membrane potential (4b). Oxidized LDL-C also induces mitochondrial membrane dysfunction in human umbilical vein endothelial cells (53) and activates calcium-dependent mitochondrial pathways that result in apoptosis (51). In addition to oxidation of LDL-C, treatment of endothelial cells with LDL-C itself reduces ATP content and decreases the expression of genes that encode for respiratory complex proteins (19). Similarly, T cells incubated with LDL-C exhibit reduced mitochondrial mass and ATP production (36), and the loading of macrophages with free cholesterol decreases mitochondrial membrane potential, triggering apoptosis (56).
In addition to attenuating immune cell function directly, LDL-C-mediated reductions in immune cell respiration may also be deleterious to the cardiovascular system. The mitochondria are major sites of ROS generation (25, 44), which can be deleterious to the vasculature by inducing endothelial dysfunction (23), a risk factor for cardiovascular disease (24, 50). Because of their location within the blood, immune cells are in constant contact with endothelial cells and may release ROS, inflammatory cytokines, and other vasoactive molecules that attenuate vascular function and increased blood pressure (4, 9, 15, 33). In this regard, ameliorating mitochondrial-derived ROS with a mitochondrial-targeted antioxidant has been shown to restore endothelial function in older or hypertensive animals and healthy middle-aged/older adults (17, 20, 38). Thus, improving mitochondrial respiration in circulating immune cells may improve endothelial function and reduce blood pressure by reducing a major source of circulating ROS. Importantly, our results suggest that the effects of LDL-C on mitochondrial function occurs even at moderate concentrations and in healthy adults without overt CVD, potentially suggesting a need for early detection and management of LDL-C for maintenance of cardiovascular and immune health.
The relations between SBP and mitochondrial respiration may be explained by elevations in oxidative stress and inflammation, which have been shown to influence respiration (29) and are characteristic of individuals with hypertension (41). Healthy mitochondria are also important for maintaining vascular function, with increased oxidative stress leading to vascular endothelial dysfunction (16, 17, 38). In addition, various animal models of induced hypertension are associated with increased mtROS production, inflammation, impaired bioenergetics, and mitochondrial damage (13). While Li et al. (29) found that DBP was correlated with mitochondrial respiration in patients with heart failure, we found no association with DBP after correcting for other risk factors. This may be due to population differences or underlying effects of SBP and LDL-C. It should be noted that blood pressure was measured in triplicate during a single visit and may not be representative of true resting blood pressure in all individuals. The interaction between mitochondrial function and more definitive measures of blood pressure (e.g., 24-h ambulatory blood pressure) should be explored in future studies.
We did not see any associations between basal respiration or coupling efficiency and any of the cardiometabolic risk factors that we studied. While ATPO2 was initially negatively correlated with blood glucose, this relationship was likely mediated by SBP, DBP, and LDL-C. Basal respiration is likely more representative of quiescent circulating immune cells, such as naïve T cells or macrophages, which are only activated when in contact with an antigen or during acute inflammation (18, 27). Thus, spare respiratory capacity and maximal respiration may be more important indexes of immune cell function, as these measures reflect the cells’ capacity to increase energy metabolism which is necessary when responding to acute infection or inflammation (18, 27). Both ATPO2 and coupling efficiency reflect how much of the basal oxygen consumption is used to facilitate ATP production. Because our results suggest that LDL-C plays a greater role in inhibiting maximal respiration, a more appropriate experiment may be to investigate the coupling of oxygen consumption to ATP production during maximal respiration.
Because of the nature of partial correlations and the limitations of retrospective analyses, we cannot determine the causal relationship between these risk factors in immune cell respiration. We believe our results may have important implications for immune cell function in adults with cardiometabolic risk factors, such as hypertension or dyslipidemia, making these groups more vulnerable to secondary infection (6) and attenuated immune system function (3, 6, 28). However, we cannot rule out the possibility that reduced mitochondrial respiration contributes to an increased serum LDL-C concentration or blood pressure; however, the effects of inflammation on LDL-C concentration are equivocal with increases in inflammation associated with both increases and decreases in hepatic LDL receptor function, resulting in hypo- and hypercholesterolemia, respectively (14, 43). Likewise, we are unable to determine whether impaired immune cell mitochondrial respiration contributes to increased SBP or reflects the mitochondrial respiration of vascular tissue. Thus, our results should be interpreted as preliminary findings to guide future exploration of these hypotheses.
A limitation of this study is that we did not directly measure physical activity and only obtained self-reported physical activity in a subset of subjects; therefore, we were unable to definitively assess how physical activity may modulate these associations. Hedges et al. (22) recently demonstrated an increase in human skeletal muscle mitochondrial respiration following a 12-wk exercise training program; however, a similar improvement was not observed in PBMCs, suggesting that physical activity primarily affects tissues that are more metabolically active during exercise. Nevertheless, increased physical activity has been shown to protect the vasculature from the damaging effects of elevated blood LDL-C concentrations and lowers systolic blood pressure (4a, 52); therefore, it is worth investigating the potential influence of physical activity on the association between of these risk factors and immune cell respiration in future studies. Additionally, we were unable to assess the role of racial and ethnic differences on the associations between cardiometabolic risk factors and mitochondrial function because of a lack of diversity in our sample. Future studies should investigate these associations in a more racially diverse population that includes underrepresented minorities, as these groups are often at elevated risk for cardiometabolic diseases. Finally, our study did not control for the use of statins or cardiovascular acting medications because of the relatively small number of subjects taking these drugs; however, the influence of these drugs on immune cell respiration is worth consideration in future studies.
Conclusions and future directions.
In summary, we have demonstrated an independent association between LDL-C, SBP, and mitochondrial respiration in circulating immune cells after controlling for cardiometabolic risk factors. Collectively, our findings suggest a possible mechanism linking aberrant blood lipids and SBP to impaired immune system health. These results may have important implications for individuals with more severe cardiometabolic disease, as these factors may compromise immune system’s ability to respond to an acute infection. Moreover, even modest increases in LDL-C as observed in the present study may contribute to cardiometabolic disease risk by promoting the production of mitochondrial-derived reactive oxygen species and/or inflammatory cytokines. Based on this exploratory analysis, future studies should aim to identify the mechanisms by which LDL-C and SBP affect mitochondrial respiration and characterize the influence of blood lipids on mitochondrial function in patients with cardiovascular disease and chronic metabolic disorders (e.g., diabetes), as well as those with and without secondary infections and following treatment.
GRANTS
This work was supported by National Institutes of Health Grants P20GM113125 and K01AG054731.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.M.D., E.R.M., and C.R.M. conceived and designed research; T.M.D., F.S., and J.C.H. performed experiments; T.M.D. analyzed data; T.M.D. and C.R.M. interpreted results of experiments; T.M.D. prepared figures; T.M.D. drafted manuscript; T.M.D., E.R.M., F.S., J.C.H., and C.R.M. edited and revised manuscript; C.R.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Wendy Nichols for assistance with phlebotomy, Benjamin Brewer for statistical consultation, and Brittany Wilson and Kyle Shuler for technical assistance with assessments of mitochondrial respiration.
REFERENCES
- 1.Agilent Technologies Agilent Seahorse XF Cell Energy Phenotype Test Kit: User Guide Kit 103325-100. Wilmington, DE: Agilent Technologies, 2017. [Online]. https://www.agilent.com/cs/library/usermanuals/public/XF_Cell_Energy_Phenotype_Test_Kit_User_Guide.pdf. [Last accessed, 7 July 2020.] [Google Scholar]
- 2.Agilent Technologies Agilent Seahorse XF Cell Mito Stress Test Kit: User Guide Kit 103015-100. Wilmington, DE: Agilent Technologies, 2019. [Online] https://www.agilent.com/cs/library/usermanuals/public/XF_Cell_Mito_Stress_Test_Kit_User_Guide.pdf. [Last accessed, 7 July 2020.] [Google Scholar]
- 3.Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One 6: e23224, 2011. doi: 10.1371/journal.pone.0023224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfatni A, Riou M, Charles AL, Meyer A, Barnig C, Andres E, Lejay A, Talha S, Geny B. Peripheral Blood Mononuclear Cells and Platelets Mitochondrial Dysfunction, Oxidative Stress, and Circulating mtDNA in Cardiovascular Diseases. J Clin Med 9: 311, 2020. doi: 10.3390/jcm9020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 140: e596–e646, 2019. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4b.Asmis R, Begley JG. Oxidized LDL promotes peroxide-mediated mitochondrial dysfunction and cell death in human macrophages: a caspase-3-independent pathway. Circ Res 92: e20–e29, 2003. doi: 10.1161/01.RES.0000051886.43510.90. [DOI] [PubMed] [Google Scholar]
- 5.Bautista LE, López-Jaramillo P, Vera LM, Casas JP, Otero AP, Guaracao AI. Is C-reactive protein an independent risk factor for essential hypertension? J Hypertens 19: 857–861, 2001. doi: 10.1097/00004872-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Case AJ, Zimmerman MC. Sympathetic-mediated activation versus suppression of the immune system: consequences for hypertension. J Physiol 594: 527–536, 2016. doi: 10.1113/JP271516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Zhou Z, Min W. Mitochondria, Oxidative Stress and Innate Immunity. Front Physiol 9: 1487, 2018. doi: 10.3389/fphys.2018.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chon H, Gaillard CA, van der Meijden BB, Dijstelbloem HM, Kraaijenhagen RJ, van Leenen D, Holstege FC, Joles JA, Bluyssen HA, Koomans HA, Braam B. Broadly altered gene expression in blood leukocytes in essential hypertension is absent during treatment. Hypertension 43: 947–951, 2004. doi: 10.1161/01.HYP.0000123071.35142.72. [DOI] [PubMed] [Google Scholar]
- 9.Chon H, Verhaar MC, Koomans HA, Joles JA, Braam B. Role of circulating karyocytes in the initiation and progression of atherosclerosis. Hypertension 47: 803–810, 2006. doi: 10.1161/01.HYP.0000210554.61293.90. [DOI] [PubMed] [Google Scholar]
- 10.Sung KC, Suh JY, Kim BS, Kang JH, Kim H, Lee MH, Park JR, Kim SW. High sensitivity C-reactive protein as an independent risk factor for essential hypertension. Am J Hypertens 16: 429–433, 2003. doi: 10.1016/S0895-7061(03)00566-1. [DOI] [PubMed] [Google Scholar]
- 11.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res 110: 1109–1124, 2012. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desdín-Micó G, Soto-Heredero G, Aranda JF, Oller J, Carrasco E, Gabandé-Rodríguez E, Blanco EM, Alfranca A, Cussó L, Desco M, Ibañez B, Gortazar AR, Fernández-Marcos P, Navarro MN, Hernaez B, Alcamí A, Baixauli F, Mittelbrunn M. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 368: 1371–1376, 2020. doi: 10.1126/science.aax0860. [DOI] [PubMed] [Google Scholar]
- 13.Eirin A, Lerman A, Lerman LO. Enhancing mitochondrial health to treat hypertension. Curr Hypertens Rep 20: 89, 2018. doi: 10.1007/s11906-018-0889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feingold KR, Moser AH, Shigenaga JK, Patzek SM, Grunfeld C. Inflammation stimulates the expression of PCSK9. Biochem Biophys Res Commun 374: 341–344, 2008. doi: 10.1016/j.bbrc.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gano LB, Donato AJ, Pierce GL, Pasha HM, Magerko KA, Roeca C, Seals DR. Increased proinflammatory and oxidant gene expression in circulating mononuclear cells in older adults: amelioration by habitual exercise. Physiol Genomics 43: 895–902, 2011. doi: 10.1152/physiolgenomics.00204.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, Seals DR. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol (1985) 124: 1194–1202, 2017. doi: 10.1152/japplphysiol.00670.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol 592: 2549–2561, 2014. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golubovskaya V, Wu L. Different subsets of T cells, memory, effector functions, and CAR-T immunotherapy. Cancers (Basel) 8: 36, 2016. doi: 10.3390/cancers8030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonnissen S, Ptok J, Goy C, Jander K, Jakobs P, Eckermann O, Kaisers W, von Ameln F, Timm J, Ale-Agha N, Haendeler J, Schaal H, Altschmied J. High concentration of low-density lipoprotein results in disturbances in mitochondrial transcription and functionality in endothelial cells. Oxid Med Cell Longev 2019: 7976382, 2019. doi: 10.1155/2019/7976382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cochemé HM, Murphy MP, Dominiczak AF. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 54: 322–328, 2009. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 21.Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep 15: 313, 2013. doi: 10.1007/s11926-012-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedges CP, Woodhead JST, Wang HW, Mitchell CJ, Cameron-Smith D, Hickey AJ, Merry TL. Peripheral blood mononuclear cells do not reflect skeletal muscle mitochondrial function or adaptation to high-intensity interval training in healthy young men. J Appl Physiol (1985) 126: 454–461, 2019. doi: 10.1152/japplphysiol.00777.2018. [DOI] [PubMed] [Google Scholar]
- 23.Incalza MA, D’Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol 100: 1–19, 2018. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Widmer RJ, Lerman A. Endothelial dysfunction and cardiovascular disease. Glob Cardiol Sci Pract 2014: 291–308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kausar S, Wang F, Cui H. The role of mitochondria in reactive oxygen species generation and its implications for neurodegenerative diseases. Cells 7: 274, 2018. doi: 10.3390/cells7120274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleiveland CR. Peripheral Blood Mononuclear Cells. In: The Impact of Food Bioactives on Health. London: Springer International, 2015, p. 161–167. [Google Scholar]
- 27.Kramer PA, Ravi S, Chacko B, Johnson MS, Darley-Usmar VM. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol 2: 206–210, 2014. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: A Cohort Study of the Veterans Affairs Healthcare System. Anesthesiology 123: 288–306, 2015. doi: 10.1097/ALN.0000000000000739. [DOI] [PubMed] [Google Scholar]
- 29.Li P, Wang B, Sun F, Li Y, Li Q, Lang H, Zhao Z, Gao P, Zhao Y, Shang Q, Liu D, Zhu Z. Mitochondrial respiratory dysfunctions of blood mononuclear cells link with cardiac disturbance in patients with early-stage heart failure. Sci Rep 5: 10229, 2015. doi: 10.1038/srep10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 32: 2045–2051, 2012. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, Chonchol M, Seals DR. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun 9: 1286, 2018. doi: 10.1038/s41467-018-03421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattace-Raso FU, Verwoert GC, Hofman A, Witteman JC. Inflammation and incident-isolated systolic hypertension in older adults: the Rotterdam study. J Hypertens 28: 892–895, 2010. doi: 10.1097/HJH.0b013e328336ed26. [DOI] [PubMed] [Google Scholar]
- 33.O’Mahony L, Holland J, Jackson J, Feighery C, Hennessy TP, Mealy K. Quantitative intracellular cytokine measurement: age-related changes in proinflammatory cytokine production. Clin Exp Immunol 113: 213–219, 1998. doi: 10.1046/j.1365-2249.1998.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460: 103–107, 2009. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polo Friz H, Facchetti R, Primitz L, Beltrame L, Galbiati V, Ricioppo A, Bombelli M, Sega R. Simultaneous validation of the SunTech 247 diagnostic station blood pressure measurement device according to the British Hypertension Society protocol, the International Protocol and the Association for the Advancement of Medical Instrumentation standards. Blood Press Monit 14: 222–227, 2009. doi: 10.1097/MBP.0b013e328330c873. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues NV, Correia DV, Mensurado S, Nóbrega-Pereira S, deBarros A, Kyle-Cezar F, Tutt A, Hayday AC, Norell H, Silva-Santos B, Dias S. Low-density lipoprotein uptake inhibits the activation and antitumor functions of human Vγ9Vδ2 T cells. Cancer Immunol Res 6: 448–457, 2018. doi: 10.1158/2326-6066.CIR-17-0327. [DOI] [PubMed] [Google Scholar]
- 37.Ron-Harel N, Notarangelo G, Ghergurovich JM, Paulo JA, Sage PT, Santos D, Satterstrom FK, Gygi SP, Rabinowitz JD, Sharpe AH, Haigis MC. Defective respiration and one-carbon metabolism contribute to impaired naïve T cell activation in aged mice. Proc Natl Acad Sci USA 115: 13347–13352, 2018. doi: 10.1073/pnas.1804149115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossman MJ, Santos-Parker JR, Steward CA, Bispham NZ, Cuevas LM, Rosenberg HL, Woodward KA, Chonchol M, Gioscia-Ryan RA, Murphy MP, Seals DR. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 71: 1056–1063, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci 53: M20–M26, 1998. doi: 10.1093/gerona/53A.1.M20. [DOI] [PubMed] [Google Scholar]
- 40.Schurich A, Pallett LJ, Jajbhay D, Wijngaarden J, Otano I, Gill US, Hansi N, Kennedy PT, Nastouli E, Gilson R, Frezza C, Henson SM, Maini MK. distinct metabolic requirements of exhausted and functional virus-specific CD8 T cells in the same host. Cell Reports 16: 1243–1252, 2016. doi: 10.1016/j.celrep.2016.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solak Y, Afsar B, Vaziri ND, Aslan G, Yalcin CE, Covic A, Kanbay M. Hypertension as an autoimmune and inflammatory disease. Hypertens Res 39: 567–573, 2016. doi: 10.1038/hr.2016.35. [DOI] [PubMed] [Google Scholar]
- 42.Sorci-Thomas MG, Thomas MJ. High density lipoprotein biogenesis, cholesterol efflux, and immune cell function. Arterioscler Thromb Vasc Biol 32: 2561–2565, 2012. doi: 10.1161/ATVBAHA.112.300135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stopeck AT, Nicholson AC, Mancini FP, Hajjar DP. Cytokine regulation of low density lipoprotein receptor gene transcription in HepG2 cells [Online]. J Biol Chem 268: 17489–17494, 1993. http://www.ncbi.nlm.nih.gov/pubmed/8349628. [PubMed] [Google Scholar]
- 44.Szewczyk A, Jarmuszkiewicz W, Koziel A, Sobieraj I, Nobik W, Lukasiak A, Skup A, Bednarczyk P, Drabarek B, Dymkowska D, Wrzosek A, Zablocki K. Mitochondrial mechanisms of endothelial dysfunction. Pharmacol Rep 67: 704–710, 2015. doi: 10.1016/j.pharep.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol 15: 104–116, 2015. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomiyama H, Shiina K, Matsumoto-Nakano C, Ninomiya T, Komatsu S, Kimura K, Chikamori T, Yamashina A. The contribution of inflammation to the development of hypertension mediated by increased arterial stiffness. J Am Heart Assoc 6: 1–11, 2017. doi: 10.1161/JAHA.117.005729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36: 68–78, 2012. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care 32, Suppl 2: S314–S321, 2009. doi: 10.2337/dc09-S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vindis C, Elbaz M, Escargueil-Blanc I, Augé N, Heniquez A, Thiers JC, Nègre-Salvayre A, Salvayre R. Two distinct calcium-dependent mitochondrial pathways are involved in oxidized LDL-induced apoptosis. Arterioscler Thromb Vasc Biol 25: 639–645, 2005. doi: 10.1161/01.ATV.0000154359.60886.33. [DOI] [PubMed] [Google Scholar]
- 52.Walker AE, Eskurza I, Pierce GL, Gates PE, Seals DR. Modulation of vascular endothelial function by low-density lipoprotein cholesterol with aging: influence of habitual exercise. Am J Hypertens 22: 250–256, 2009. doi: 10.1038/ajh.2008.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walter DH, Haendeler J, Galle J, Zeiher AM, Dimmeler S. Cyclosporin A inhibits apoptosis of human endothelial cells by preventing release of cytochrome C from mitochondria. Circulation 98: 1153–1157, 1998. doi: 10.1161/01.CIR.98.12.1153. [DOI] [PubMed] [Google Scholar]
- 54.West AP. Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology 391: 54–63, 2017. doi: 10.1016/j.tox.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 55.van der Windt GJ, Pearce EL. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol Rev 249: 27–42, 2012. doi: 10.1111/j.1600-065X.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao PM, Tabas I. Free cholesterol loading of macrophages is associated with widespread mitochondrial dysfunction and activation of the mitochondrial apoptosis pathway. J Biol Chem 276: 42468–42476, 2001. doi: 10.1074/jbc.M101419200. [DOI] [PubMed] [Google Scholar]