Abstract
As studies examining the hypertrophic effects of resistance training (RT) at the cellular level have produced inconsistent results, we performed a systematic review and meta-analysis to investigate muscle fiber size before and after a structured RT intervention in older adults. A random-effects model was used to calculate mean effect size (ES) and 95% confidence intervals (CI). Thirty-five studies were included (age range: 59.0–88.5 yr), and 44 and 30 effects were used to estimate RT impact on myosin heavy chain (MHC) I and II fiber size. RT produced moderate-to-large increases in MHC I (ES = +0.51, 95%CI +0.31 to +0.71; P < 0.001) and II (ES = +0.81, 95%CI +0.56 to +1.05; P < 0.001) fiber size, with men and women having a similar response. Age was negatively associated with change in muscle fiber size for both fiber types (MHC I: R2 = 0.11, β = −0.33, P = 0.002; MHC II: R2 = 0.10, β = −0.32, P = 0.04), indicating a less robust hypertrophic response as age increases in older adults. Unexpectedly, a higher training intensity (defined as percentage of one-repetition maximum) was associated with a smaller increase in MHC II fiber size (R2 = 15.09%, β = −0.39, P = 0.01). Notably, MHC II fiber subtypes (IIA, IIX, IIAX) were examined less frequently, but RT improved their size. Overall, our findings indicate that RT induces cellular hypertrophy in older adults, although the effect is attenuated with increasing age. In addition, hypertrophy of MHC II fibers was reduced with higher training intensity, which may suggest a failure of muscle fibers to hypertrophy in response to high loads in older adults.
Keywords: aging, cellular, exercise, hypertrophy, myosin
INTRODUCTION
Sarcopenia, or the loss of skeletal muscle mass and function with age, leads to poor physical performance in older adults (28, 53). Lifestyle and pharmacologic interventions to mitigate the effects of sarcopenia are, therefore, of great public health interest. The safest and most effective strategy for maintaining and/or increasing skeletal muscle mass is resistance training (RT), and this is broadly recognized across the scientific and clinical communities. For instance, the second edition of the Physical Activity Guidelines for Americans released in 2018 recommends muscle-strengthening activities, in addition to aerobic and balance activities, for older adults (77). Other organizations, such as the American College of Sports Medicine, also recommend the inclusion of RT in any comprehensive exercise regimen for older adults (34).
Skeletal muscle is a plastic tissue (31), and one of the most well-accepted chronic adaptations to RT exercise is an increase in muscle size, referred to as hypertrophy (2). Roughly 30 years ago, a handful of seminal studies demonstrated that RT interventions promote skeletal muscle hypertrophy in older adults (12, 27, 33). These studies used imaging techniques (e.g., computed tomography) to measure hypertrophy at the tissue level (e.g., thigh muscle cross-sectional area). Training-induced hypertrophy at the tissue level should reflect adaptations at the cellular level (e.g., single muscle fiber). Surprisingly, studies that have examined the effects of RT on single muscle fiber size in older adults have been inconsistent, with some showing hypertrophy in specific fiber types (12, 26, 47, 54, 60, 93, 95, 97, 98) or all fiber types (5, 33, 39, 42, 52, 96) while others have shown no effect in either fiber type (15, 32, 36, 38, 63, 75, 79, 84) or even a decrease in fiber size (58). Thus, while it is universally accepted that RT promotes hypertrophy based on whole muscle assessments, whether this whole muscle hypertrophy is related to increased size of individual muscle cells (e.g., fibers) in older adults is not clear.
Discrepant results could be due to a number of factors, including small sample sizes resulting in inadequate statistical power, participant characteristics (age, sex), and/or exercise program variables (training intensity, volume, etc.). Moreover, fiber type admixture of a skeletal muscle may have a role in determining hypertrophy following RT. Human skeletal muscle expresses three myosin heavy chain (MHC) isoforms (8), resulting in three pure fiber types (I, IIA, and IIX) and multiple hybrid fibers (I/IIA, IIAX, I/IIA/IIX). The predominant MHC isoforms expressed in human skeletal muscle are I and IIA (71), and these have distinctly different cellular and molecular contractile properties (64). With this in mind, RT may impact MHC I and IIA fibers differently. For instance, we recently showed that 14 wk of moderate-intensity RT in older adults with knee osteoarthritis resulted in no change in muscle fiber cross-sectional area (66). However, this was due to reciprocal changes in fiber types, with a decrease in the size of MHC I fibers and an increase in IIA fibers. A number of systematic reviews and meta-analyses have been conducted to examine the effects of RT on indexes of muscle size or mass in older adults, including quadriceps size (7), lean body mass (76), or muscle mass (19). Additionally, a meta-analytic approach has been used to investigate RT combined with concurrent creatine (14, 20) or protein (30) supplementation, or in older adults with chronic disease (13). Recently, the notion that fiber types may respond differently to RT was discussed (35), but this review did not employ a meta-analytic approach and was not focused on older adults. Thus, to our knowledge, no meta-analysis has attempted to characterize the effects of RT on single muscle fiber size in healthy older adults. This systematic review and meta-analysis seeks to provide a quantitative estimate of the effect of RT interventions on the size of skeletal muscle fibers in older adults and to examine factors that potentially moderate these effects.
METHODS
Search strategy.
This review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (68). Articles published from the origin of each electronic database until June 27, 2019, were located using searches of the PubMed (n = 235), SPORTDiscus (n = 102), and Web of Science (n = 143) online databases using combinations of the terms: resistance train*, train*, older adult, adult, fiber type, fiber, myosin. The * is a search term for electronic databases that includes the root word plus any suffix that follows (e.g., train* = train, trained, training, etc.). The search terms were intentionally broad to maximize the sensitivity of the database search. As such, specific terms related to the race/ethnicity of the sample were not included in the original search. Nonduplicate articles were independently screened by title and abstract by the authors (C.R.S. and M.V.F.) and followed by a full text-reports evaluation to determine eligibility. Additional manual searches of Google Scholar and ResearchGate were performed to identify any manuscripts not revealed as part of the electronic database search.
Study selection.
Studies included in this meta-analysis were limited to 1) peer-reviewed publications, 2) available in English, 3) involving human participants aged 55 yr and older, 4) reporting unadjusted skeletal muscle fiber size measurements as mean ± SD, or another measure of variability from which a SD could be calculated (e.g., if SE was reported, the SD was calculated by multiplying SE by the square root of the sample size), and 5) examining the change in muscle fiber size following a structured RT intervention. During the data extraction process to ensure eligibility based on the criteria provided above, disagreements were resolved by consensus. A flowchart of study selection is provided in Fig. 1.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of study selection. ES, effect size; n, number of studies; M, mean; SD, standard deviation
Statistical analysis.
The standardized mean change effect size (ES) values were calculated by subtracting the preintervention values from the postintervention values (41). A positive ES indicated an increase in muscle fiber size, with ES defined as small (0.20), medium (0.50), or large (0.80) (16). Random-effects models were used to calculate a mean ES and 95% confidence interval (CI) and potential moderator variables using macros (MeanES and MetaReg) in IBM SPSS version 25.0 (IBM SPSS Statistics; IBM Corporation, Armonk, NY). Moderator variables (Table 1) were weighted by sample size, and the inverse variance was used to weight effects for the ES. Funnel plots were created by scattering the treatment effects against their standard error (90), with the 95% CI plotted around the mean ES to indicate between-study heterogeneity (89). Potential outliers were identified by visually inspecting each funnel plot for effects outside of the 95% CI, which were subsequently removed as part of the sensitivity analysis (22–24). In addition, Egger’s test was also used to assess funnel plot asymmetry and identify potential publication bias by regressing a standardized effect size (effect size divided by the SE) against a measure of precision (inverse of the SE) (21). Data are presented as means ± SD unless otherwise indicated.
Table 1.
Definitions for levels of moderators
| Effect Moderator | Levels |
|---|---|
| Age | Continuous variable, mean age of the experimental group reported in years |
| Sex | Women: data from women only Men: data from men only Mixed: data from samples that combined women and men |
| Exercise intensity | Continuous variable, reported as a percentage of 1-repetition maximum. When the percentage was reported as a range, the average of the highest and lowest intensities was used. |
| Exercise program length | Continuous variable, reported in weeks |
| Exercise frequency | Continuous variable, reported in days/wk |
| Exercise sets | Continuous variable, reported in sets/session |
| Exercise repetitions | Continuous variable, reported in repetitions/set |
| Year published | Continuous variable, year of publication |
RESULTS
Sample characteristics.
The cumulative results are based on 97 effects gathered from 35 studies published before June 27, 2019, totaling 1,089 participants. Sample age ranged from 59.0 to 88.5 yr (68.9 ± 5.6 yr). Participant samples were mostly male (64.0 ± 48.1%), with the majority of effects gathered from sex-specific samples of men (k = 57 effects) and women (k = 40 effects). Additional study-level characteristics are presented in Table 2.
Table 2.
Characteristics of studies examining the effects of resistance training on skeletal muscle fiber size in older adults
| Study | Year | Sex (M/W) | Age, yr | Frequency, days/wk | Duration, wk | Intensity, %1-RM | Fiber Types | Classification Technique |
|---|---|---|---|---|---|---|---|---|
| Ahtiainen et al. (1) | 2016 | 10/0 | 61 | 2 | 21 | 40–90 | I, II | mATPase |
| Bamman et al. (5) | 2003 | 9/5 | 69/66 | 3 | 26 | 80 | I, IIA, IIX | mATPase |
| Bickel et al. (6)* | 2011 | 31 | 61 | 3 | 16 | 75–80 | I, II | IHC |
| Charette et al. (12) | 1991 | 0/13 | 69 | 3 | 12 | 65–75 | I, II | mATPase |
| Cooke et al. (17) | 2014 | 10/0 | 61 | 3 | 12 | 75 | I, II | mATPase |
| Ferketich et al. (26) | 1998 | 0/7 | 60–75 | 3 | 12 | 80 | I, IIA, IIX | mATPase |
| Frontera et al. (32) | 2003 | 0/7 | 74 | 3 | 12 | 80 | I | SDS-PAGE |
| Frontera et al. (33) | 1988 | 12/0 | 60–72 | 3 | 12 | 80 | I, II | mATPase |
| Grimby et al. (36) | 1992 | 9/0 | 81 | 2–3 | 12 | MVC | I, II | mATPase |
| Hagerman et al. (37) | 2000 | 9/0 | 64 | 2 | 16 | 85–90 | I, IIA, IIX | mATPase |
| Häkkinen et al. (40) | 2001 | 0/10 | 64 | 2 | 21 | 40–80 | I, IIA, IIX | mATPase |
| Häkkinen et al. (39) | 2001 | 9/8 | 72/67 | 2 | 24 | 50–80 | I, IIA, IIX | mATPase |
| Häkkinen et al. (38) | 1998 | 6/0 | 61 | 3 | 10 | 3–15-RM | I, IIA, IIX, IIAX | mATPase |
| Hikida et al. (42) | 2000 | 9/0 | 64 | 2 | 16 | 6–8-RM | I, IIA, IIX | mATPase |
| Holwerda et al. (43) | 2018 | 20/0 | 71 | 3 | 12 | 80 | I, II | IHC |
| Kosek et al. (47) | 2006 | 13/11 | 65/63 | 3 | 16 | 80 | I, II, IIA | IHC |
| Kryger and Anderson (49)* | 2007 | 9 | 89 | 3 | 12 | 80 | I, II | mATPase, IHC |
| Lange et al. (50) | 2002 | 8/0 | 75 | 3 | 12 | 8-RM | I, II | mATPase |
| Larsson (52) | 1982 | 6/0 | 59 | 2 | 15 | 20–30-RM | I, II | mATPase |
| Leenders et al. (54) | 2013 | 14/12 | 70/69 | 3 | 24 | 75–80 | I, II | IHC |
| Leenders et al. (55) | 2013 | 29/24 | 70/71 | 3 | 16 | 75–80 | I, II | IHC |
| Lexell et al. (58) | 1995 | 12/8 | 70–77 | 3 | 11 | 85 | I, II | mATPase |
| Martel et al. (60) | 2006 | 11/7 | 69/68 | 3 | 9 | 5-RM | I, IIA, IIAX | mATPase |
| Mayhew et al. (61)* | 2009 | 15 | 64 | 3 | 16 | 8–12-RM | II | IHC |
| Mero et al. (63) | 2013 | 18/0 | 61 | 2 | 21 | 40–80 | I, II | mATPase |
| Parente et al. (75) | 2008 | 0/4 | 78 | 3 | 52 | 60 | I, IIA | SDS-PAGE |
| Pyka et al. (78) | 1994 | 4/4 | 67 | 3 | 52 | 75 | I, II | mATPase |
| Raue et al. (79) | 2009 | 0/6 | 85 | 3 | 12 | 70–75 | I, IIA | SDS-PAGE |
| Slivka et al. (84) | 2008 | 6/0 | 82 | 3 | 12 | 70 | I, IIA | SDS-PAGE |
| Taaffe et al. (93) | 1996 | 0/7 | 67 | 3 | 52 | 40 or 80 | I, II | mATPase |
| Taaffe et al. (92) | 1996 | 8/0 | 65–82 | 3 | 24 | 75 | I, II | mATPase |
| Trappe et al. (97) | 2016 | 8/0 | 64 | 3 | 12 | 12-RM | I, II | mATPase |
| Trappe et al. (96) | 2000 | 7/0 | 74 | 3 | 12 | 80 | I, IIA | SDS-PAGE |
| Trappe et al. (95) | 2001 | 0/7 | 74 | 3 | 12 | 80 | I, IIA | SDS-PAGE |
| Verdijk et al. (98) | 2009 | 13/0 | 72 | 3 | 12 | 75–80 | I, II | IHC |
Age is reported as mean when available or the age range of participants included in the study. M, men; W, women; RM, repetition maximum; mATPase, myosin adenosine triphosphatase; IHC, immunohistochemistry; MVC, maximal voluntary contractions; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Study did not report breakdown of men and women in sample.
Fiber type classification.
Human skeletal muscle fiber types can be classified in a variety of ways, including for MHC isoforms and myosin adenosine triphosphatases (mATPases). Analyzing fiber types by MHC isoform in human skeletal muscle results in three pure fiber types (I, IIA, and IIX) as well as mixed fiber types (I/IIA, IIAX, and I/IIA/IIX), and is typically accomplished using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) or immunohistochemistry (IHC). Analyzing fiber types by mATPase results in three pure fiber types (I, IIA, and IIB) and mixed fiber types (IC, IIC, IIAC, and IIAB). The studies examined in this meta-analysis that used mATPase commonly report finding MHC I or II fiber types, with a few reporting IIA, IIB, and IIAB. To have consistent terminology, we will use MHC IIX and IIAX instead of MHC IIB and IIAB for the mATPase results. The classification techniques used to identify fiber types for each study are included in Table 2.
Changes in muscle fiber size.
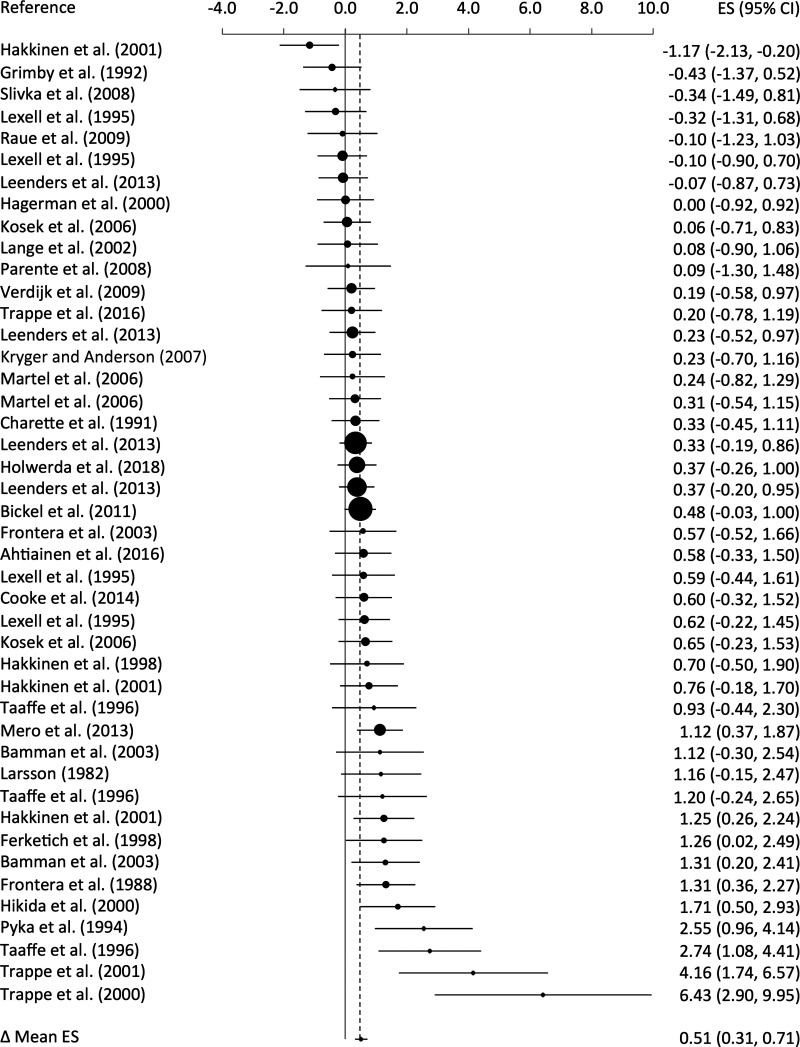
The cumulative results from 44 effects indicated that MHC I fiber size increased following a structured RT intervention (ES = +0.51, 95% CI +0.31 to +0.71; P < 0.001; Fig. 2). Significant heterogeneity was observed (Q43 = 82.74, I2 = 48.0%, P < 0.001), with sampling error accounting for 86.6% of the observed heterogeneity and ES ranging from –1.17 to 6.43. Sampling error occurs because individual studies examine participants with unique characteristics from the larger population, and these differences likely accounted for the heterogeneity in the response to RT. The increase in MHC I fiber size appeared to be consistent, since 36 effects (81.8%) were greater than zero with 11.4% small, 20.4% medium, 18.2% large, and 31.8% very large effects.
Fig. 2.
Forest plot of standardized mean change effect sizes (ES) for myosin heavy chain (MHC) I fibers. The aggregated effect size is the random-effects mean change in fiber size weighted by the inverse variance. The size of each circle reflects the respective weight of the individual effect in the overall mean effect size. CI, confidence interval.
Similar improvements were also noted for the 30 effects examining change in MHC II fiber size, with a slightly larger increase following RT (ES = +0.81, 95% CI +0.56 to +1.05; P < 0.001; Fig. 3). Significant heterogeneity was also observed for MHC II fibers (Q29 = 61.64, I2 = 53.0%, P < 0.001), with sampling error accounting for 89.8% of the observed heterogeneity and ES ranging from –0.64 to +3.87. The increase in MHC II fiber size also appeared to be consistent, since 27 effects (90.0%) were greater than zero with 3.3% small, 10.0% medium, 30.0% large, and 46.7% very large effects.
Fig. 3.
Forest plot of standardized mean change effect sizes (ES) for myosin heavy chain (MHC) II fibers. The aggregated effect size is the random-effects mean change in fiber size weighted by the inverse variance. The size of each circle reflects the respective weight of the individual effect in the overall mean effect size. CI, confidence interval.
The specific MHC II fiber subtypes were examined less frequently; however, all indicated an improvement in fiber size following RT. MHC IIA fibers (k = 18, ES = +0.88, 95% CI +0.44 to +1.32; P < 0.001), IIX fibers (k = 7, ES = +1.01, 95% CI +0.08 to +1.94; P = 0.03), and IIAX fibers (k = 5, ES = +0.98, 95% CI +0.46 to +1.51; P < 0.001) all increased in size, with significant heterogeneity observed for MHC IIA and IIX fibers (P < 0.001). No heterogeneity was observed in MHC IIAX fibers (P = 0.48), indicating all studies showed a similar hypertrophic response.
Moderator analysis.
Based on the 44 effects that evaluated the effect of RT on MHC I fibers, the number of sets (R2 = 0.04, β = −0.21, P = 0.07), number of repetitions (R2 = 0.00, β = 0.09, P = 0.46), training frequency (R2 = 0.01, β = −0.13, P = 0.28), training intensity (R2 = 0.00, β = −0.07, P = 0.57), or duration/study length (R2 = 0.01, β = 0.08, P = 0.53) were not independently associated with the observed changes in fiber size. Only age was correlated with the observed change, such that older age was associated with a smaller increase in MHC I fiber size (R2 = 0.11, β = −0.33, P = 0.002).
Based on the 30 effects that evaluated the effect of RT on MHC II fibers, the number of sets (R2 = 0.01, β = −0.09, P = 0.58), number of repetitions (R2 = 0.00, β = 0.05, P = 0.79), training frequency (R2 = 0.01, β = 0.10, P = 0.55), or duration/study length (R2 = 0.00, β = −0.06, P = 0.70) were not independently associated with the observed changes in MHC II fiber size. Age was correlated with the observed change, such that older age was associated with a smaller increase in MHC II fiber size (R2 = 0.10, β = −0.32, P = 0.04). In addition, a higher training intensity was associated with a smaller increase in MHC II fiber size (R2 = 0.15, β = −0.39, P = 0.01).
Sex was not associated with the change in size for either MHC I (R2 = 0.01, β = −0.07, P = 0.55) or II (R2 = 0.01, β = −0.08, P = 0.65) fibers, contributing <1.0% to the explained variance for either fiber type. When examining MHC I fibers, there were no differences between effects including women (k = 17, ES = 0.54, 95% CI +0.25 to +0.82; P < 0.001) or men (k = 24, ES = 0.42, 95% CI +0.20 to +0.64; P < 0.001) (between group P = 0.54). Although the overall change for MHC II fibers was larger, there were no differences between effects including women (k = 9, ES = 0.86, 95% CI +0.40 to +1.32; P < 0.001) or men (k = 17, ES = 0.73, 95% CI +0.41 to +1.05; P < 0.001) (between group P = 0.65). Based on these data, the magnitudes of improvement in MHC I and II fiber size are consistent between sexes.
To further explore the impact of age on hypertrophy, we conducted regression models and comparisons across discrete age groups. For the regression analysis, linear and quadratic models provided similar fits for MHC I (linear R2 = 0.11 vs. quadratic R2 = 0.11; P = 0.02 and 0.03, respectively) and II (linear R2 = 0.10 vs. quadratic R2 = 0.10; both P = 0.04) fiber size. For the categorical analysis, effects were divided into decades of life (i.e., 6th decade equals 50.0–59.9 yr old) and were compared within each fiber type. The mean ES for MHC I fiber size decreased from the sixth decade (k = 1, ES = +1.16, 95% CI −0.15 to +2.47; P = 0.08), seventh decade (k = 23, ES = +0.65, 95% CI +0.46 to +0.85; P < 0.001), eighth decade (k = 16, ES = +0.31, 95% CI +0.10 to +0.52; P = 0.004), and ninth decade (k = 4, ES = −0.14, 95% CI −0.66 to +0.37; P = 0.58) of life (between groups P = 0.007). Although it was not significant (P = 0.073), the mean ES for MHC II fiber size decreased from the sixth decade (k = 1, ES = +1.69, 95% CI +0.05 to +3.33; P = 0.04), seventh decade (k = 23, ES = +1.05, 95% CI +0.73 to +1.37; P < 0.001), eighth decade (k = 16, ES = +0.56, 95% CI +0.25 to +0.88; P = 0.004), and ninth decade (k = 2, ES = 0.33, 95% CI −0.51 to +1.16; P = 0.44) of life. Collectively, the results of our continuous and categorical analyses show that the effect of RT on MHC I and II fiber size is attenuated with advancing age.
Publication bias.
The number of unpublished or unretrieved null effects that would diminish the significance of observed effects to P > 0.05 was estimated as the fail-safe N+. A fail-safe N+ represents the minimal number of additional null effects from multiple studies of average sample size needed to reach a similar null conclusion (81). The fail-safe N+ for the effect of RT on MHC I fiber size using a random-effects model collapsed to a fixed-effects model, and estimated N+ = 432.09 effects. Similarly, the fail-safe N+ for the effect of RT on MHC II fiber size using a random-effects model also collapsed to a fixed-effects model, and estimated N+ = 630.75 effects. A fail-safe N+ is often considered robust when the estimated value exceeds 5 × N + 10, in which N represents the number of original effects. Given the current fail-safe N+, publication bias can be “safely ignored” (82).
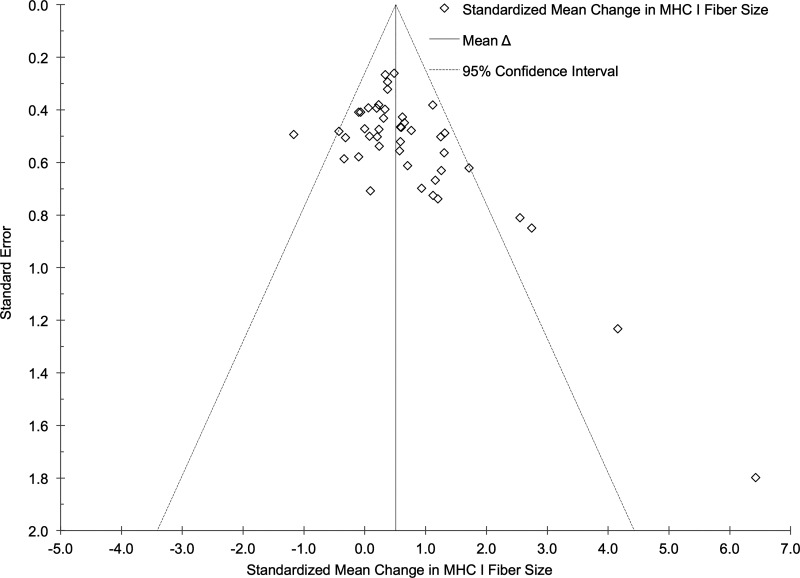
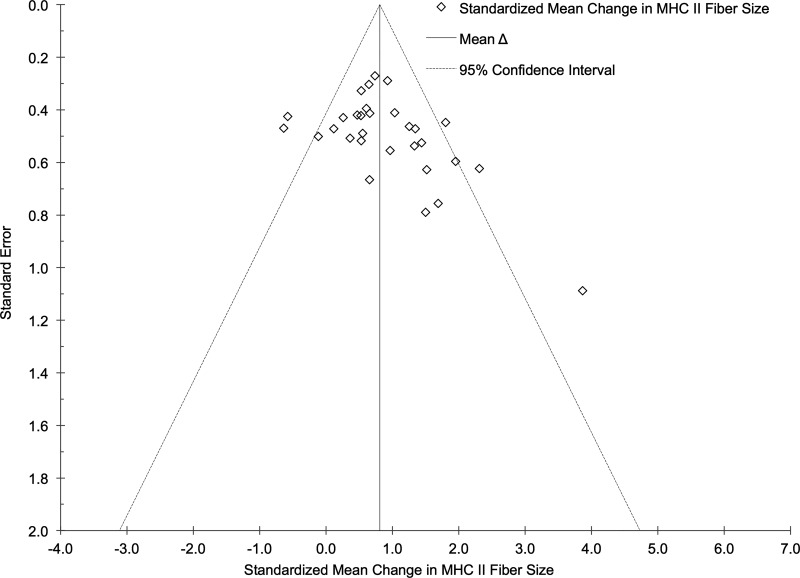
Funnel plots for the effect of RT on MHC I and II fiber size were created as exploratory assessments to address potential publication bias related to study sample size and are presented in Figs. 4 and 5. Five potential outliers were identified using the funnel plot for MHC I muscle fibers. After a sensitivity analysis excluding these effects, the cumulative results from 39 effects indicated that MHC I fiber size increased following a structured RT intervention (ES = +0.43, 95% CI +0.29 to +0.57; P < 0.001). Five potential outliers were also identified using the funnel plot for MHC II muscle fibers. Following a sensitivity analysis excluding these effects, the cumulative results from 25 effects indicated that MHC II fiber size increased following a structured RT intervention (ES = +0.77, 95% CI +0.60 to +0.94; P < 0.001). Heterogeneity was nonsignificant for both outcomes after excluding the potential outliers. The results for effect size remained similar with and without the potential outliers removed, indicating no publication bias. Potential publication bias was also addressed using Egger’s test (21). Our results indicate that the mean effect of RT on MHC I (β = −0.28, P = 0.07) and II (β = −0.05, P = 0.80) fiber size was not subject to bias.
Fig. 4.
Funnel plot of study SE vs. standardized mean change effect size in myosin heavy chain (MHC) I fibers. The aggregated effect size is the random-effects mean change in fiber size weighted by the inverse variance.
Fig. 5.
Funnel plot of study SE vs. standardized mean change effect size in myosin heavy chain (MHC) II fibers. The aggregated effect size is the random-effects mean change in fiber size weighted by the inverse variance.
DISCUSSION
Our results indicate that RT in older adults produces hypertrophy in MHC I and II muscle fibers, although the effect is larger for MHC II fibers. Despite existing research showing hypertrophy following RT at the tissue level (e.g., quadriceps cross-sectional area), studies that have examined the effects of RT on morphological adaptations at the cellular level (e.g., skeletal muscle fiber size) in older adults have been inconsistent. To our knowledge, this is the first meta-analysis to show that RT interventions increase the size of both slow-twitch, MHC I, and fast-twitch, MHC II, muscle fibers in older adults. Specific MHC II fiber types (MHC IIA, IIX, and IIAX) also show increases in size, but methods to characterize these isoforms were employed less frequently. Using metaregression, we found that age was negatively associated with the effect of RT on muscle fiber size in MHC I and II fibers, meaning the hypertrophic response in older adults was attenuated. This finding agrees with the notion that skeletal muscle becomes less sensitive to anabolic stimuli (e.g., RT or protein intake) with age, commonly referred to as anabolic resistance (10). Quite surprisingly, despite widespread recommendations advising moderate-to-high training intensities for increasing muscle size (2), we found that the magnitude of hypertrophy was reduced with higher training intensities [e.g., percentage of 1-repetition maximum (1-RM)] in MHC II fibers. Collectively, our results provide the first quantitative, meta-analytical evaluation of the effect of RT on muscle fiber size in older adults.
Whereas skeletal muscle hypertrophy is often purported as a chronic adaptation to RT, studies investigating its effects on single fiber size in older adults have yielded inconsistent results, ranging from hypertrophy in one fiber type (12, 26, 47, 54, 60, 93, 95, 97, 98), both (5, 33, 39, 42, 52, 96), neither (15, 32, 36, 38, 63, 75, 79, 84), or a decrease in size (58). Our meta-analysis shows that RT interventions resulted in hypertrophy of MHC I and II muscle fibers from older adults, and the effect was moderate-to-large for both fiber types (ES = 0.51 and 0.81 for MHC I and II fibers), with improvements being greater in MHC II fibers. The larger effect of RT on MHC II fibers may be particularly beneficial since age-related muscle atrophy is driven by these fibers (51, 57, 74). Using a meta-analysis to examine the potential change in fiber size following RT in older adults offered an opportunity to quantitatively combine all of the available and relevant research in this area to better represent changes at the population level, since we were able to combine smaller samples of participants into an aggregate analysis of >1,000 participants, with much greater statistical power than could be achieved with a single study (59). To put our findings in the context of existing literature on muscle fiber size in older adults, we extrapolated the effect sizes for MHC I and II fibers from this analysis. With the use of our effect sizes and recently published data from healthy older adults (65), a traditional RT intervention could be expected to increase size by ~20% in MHC I and ~30% in MHC II fibers. Thus, our results suggest RT is a viable countermeasure for offsetting declines in fiber size, which may ultimately lead to maintained and/or improved physical performance with age.
Our moderator analysis revealed that age (range = 59.0–88.5 yr) was negatively associated with hypertrophy in MHC I and II fibers, explaining 10–11% of the variance in fiber size and indicating an attenuated response to RT in the oldest individuals. Importantly, we also evaluated this relationship using a categorical approach, which ultimately showed that the mean ES for both MHC I and II fibers decreased from the sixth to the ninth decade of life. The observation that older age limits hypertrophy has previously been made at the tissue level (73, 101) and aligns with the concept of anabolic resistance, or the idea that skeletal muscle becomes less sensitive to anabolic stimuli (e.g., RT) with age, resulting in a diminished protein anabolic response. A number of reviews have described various studies supporting anabolic resistance with age in response to a variety of stimuli (9, 10, 70). Although a detailed discussion regarding the mechanisms of hypertrophy is beyond the scope of this review, a brief overview is useful in contextualizing the association between hypertrophy and age. Resistance training leads to greater muscle fiber cross-sectional area by increasing the size and/or number of myofibrils (the organelles containing actin and myosin) (83) within a muscle fiber. Historically, this was thought to be achieved via activation of satellite cells, which add new myonuclei to muscle fibers, although this mechanism has come under scrutiny more recently (72). Nonetheless, the population of satellite cells declines with age (45), and this may be specific to MHC II muscle fibers (99). The inverse association between age and muscle fiber hypertrophy may also be explained by factors such as a reduced ability to upregulate translational machinery (29, 88), chronic inflammation (4, 80, 94), altered hormonal responses to RT (18, 48, 73), and/or decreased muscle fiber capillarization (69, 86). These factors have not typically been assessed alongside changes in fiber size, but may shed light on the ways in which age affects the hypertrophic response to RT.
We also found that training intensity was inversely associated with the magnitude of hypertrophy in MHC II fibers, explaining roughly 15% of the variance in change in fiber size. This finding may be somewhat counterintuitive since RT regimens using moderate loads (>70% 1-RM) are typically recommended to induce muscle hypertrophy (2). However, there is evidence that a higher training intensity may not be necessary to maximize hypertrophy. A recent meta-analysis found that moderate-training intensities (51–69% 1-RM) elicited the greatest increases in whole muscle hypertrophy following RT in older adults (7). Likewise, studies of young (67), middle-aged (56), and older (100) adults have found no differences in quadriceps muscle hypertrophy when comparing low- and high-intensity RT protocols. At the single fiber level, research has shown that a higher training intensity (3–5-RM) is more effective for inducing hypertrophy in all three MHC isoforms than intermediate- (9–11-RM) and low-intensity (20–28-RM) training regimens, although this work was performed in young men (11). Successive high-intensity RT bouts, without an adequate rest period, may not be compatible with the time course of muscle damage and recovery in older adults, possibly having an unfavorable impact on anabolism. Indeed, animal models suggest a prolonged recovery period in aged mice compared with young following exercise-induced muscle damage (62, 102). Therefore, studies are needed that compare the effects of different RT intensities (e.g., low, moderate, and high), and, possibly, training frequency, on single fiber size to determine the optimal training intensity and dose for stimulating skeletal muscle hypertrophy in older muscle.
In addition to age, other participant characteristics may have a role in determining the effects of RT on fiber size. Based on obvious biological differences between men and women, such as hormone levels, a logical question is whether sex impacts the response to RT exercise in older adults. Although a few earlier studies (5, 95, 96) examined cellular hypertrophy following RT and reported substantial differences between men and women, our meta-regression, which has greater statistical power than a single study, did not reveal sex to be a significant moderator of the effect of RT on muscle fiber size (P = 0.54–0.65). Notably, 69% of the studies included in this analysis only examined one sex (either men or women; Table 2), highlighting the need for studies that include both sexes and permit direct comparisons to the same RT protocol. Nonetheless, skeletal muscle contractile function at the cellular and molecular levels can change in a sex-specific and fiber type-dependent manner in older adults following RT (66). Other studies have reported sex differences in both whole muscle hypertrophy (44) and protein synthesis (85) following RT in older adults. Thus, although some evidence points to a possible influence of sex on the effects of RT, more research is needed.
Limitations.
This study has several limitations. First, the number of potential publications not identified in the systematic review is unclear. Accidental omission of relevant articles could potentially bias the results of any meta-analysis (25). To reduce this potential bias, the authors searched multiple electronic databases for potentially relevant manuscripts and manually searched reference lists and electronic search engines for records not identified during the database search. Second, the moderator analysis yielded largely null findings when attempting to identify specific characteristics of an RT intervention that led to larger improvements in muscle fiber size. The narrow range of sets, repetitions, intensity, and frequency limited the conclusions that could be drawn. In general, studies employed interventions that largely aligned with well-established guidelines for RT exercise, which compressed the range of potential values included in the meta-regression analysis. As additional studies manipulate RT intervention characteristics to elucidate the effects on skeletal muscle fiber size in older adults, identification of program variables that lead to greater hypertrophy may be possible. In addition, using raw individual participant data would allow researchers to more accurately characterize the changes in specific fiber types following RT. However, aggregate-level data were used in the current analysis since collecting individual participant data was not feasible because of the large number of publications and wide range of years included in our electronic search (91). Third, we found that age was negatively associated with the degree of RT-induced hypertrophy in MHC I and II fibers, and this result was consistent between statistical approaches (e.g., continuous vs. categorical). However, although grouping participants by decade provided natural threshold values to determine group allocation, the restricted number of studies left only one effect per fiber type representing the sixth decade of life and very few effects (2–4) representing the ninth decade. The majority of participants were represented in the seventh and eighth decades of life (88.6 and 90.0% of effects for MHC I and II fibers) and additional data from young-old (e.g., 6th decade of life) and the oldest-old (e.g., 9th decade of life) adults are needed to better characterize the hypertrophic response across this age spectrum. Finally, the current study examined the mean change in MHC I, MHC II, and specific subgroups of MHC II fibers. However, we could not thoroughly examine potential moderators of the specific subgroups of MHC II fibers (MHC IIA, IIAX, and IIX) because of the small number of publications that include these fiber types (Table 2). Because MHC IIA and IIAX fibers may respond differently to RT (66) and older adults have a greater proportion of MHC IIAX fibers compared with young (3, 46), clearly understanding their response to exercise and potential moderators is important for designing effective exercise prescriptions. In addition to fewer effects for MHC II fiber subtypes, studies used different classification techniques to determine fiber type and this deserves comment. Performing SDS-PAGE on isolated single muscle fiber segments is considered the most rigorous means of determining MHC isoforms (71), but this process requires substantially more time and effort compared with mATPase and IHC. However, both mATPase and IHC are subject to misclassification of fiber types, particularly with regard to pure and hybrid fast-twitch fibers. Following RT, both techniques have been shown to underestimate the population of MHC IIAX fibers, leading to an overestimation of either IIA or IIX fibers (87). Indeed, spurious characterization of fiber type could be especially problematic when evaluating shifts in MHC isoform composition following exercise. Because the primary aim of this review was to quantify the hypertrophic response to RT within MHC I and II fibers, the different classification techniques most likely have no or minimal impact on the interpretation of our results. However, the different classification techniques may skew results when examining the MHC II fiber subtypes (IIA, IIX, and IIAX). There were 14 studies that examined MHC II fiber subtypes, with 5 using SDS-PAGE, 8 using mATPase, and 1 using IHC, so the most likely bias would have been toward an overestimation of IIX and an underestimation of IIAX fibers because of the greater number of mATPase compared with IHC studies (87), especially in light of recent analyses showing the rarity of pure MHC IIX fibers (71).
Conclusion.
Based on studies dating back nearly 40 years, our meta-analysis shows that RT results in hypertrophy of MHC I and II muscle fibers in older adults, although there was a diminished hypertrophic response to RT with advancing age. Thus, RT exercise is effective at inducing skeletal muscle hypertrophy in older adults. However, there is a need for further research to better understand the diminished hypertrophic response with age in older adults and the most effective training prescription to optimize skeletal muscle growth.
GRANTS
This work was supported by National Institute on Aging Grants AG-047245 (to M.S.M.) and AG-050305 (to M.J.T.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.R.S. and M.S.M. conceived and designed research; C.R.S. and M.V.F. analyzed data; C.R.S., M.V.F., M.J.T., and M.S.M. interpreted results of experiments; C.R.S. and M.V.F. prepared figures; C.R.S. drafted manuscript; C.R.S., M.V.F., M.J.T., and M.S.M. edited and revised manuscript; C.R.S., M.V.F., M.J.T., and M.S.M. approved final version of manuscript.
REFERENCES
- 1.Ahtiainen JP, Walker S, Peltonen H, Holviala J, Sillanpää E, Karavirta L, Sallinen J, Mikkola J, Valkeinen H, Mero A, Hulmi JJ, Häkkinen K. Heterogeneity in resistance training-induced muscle strength and mass responses in men and women of different ages. Age (Dordr) 38: 10, 2016. doi: 10.1007/s11357-015-9870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Sports Medicine American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 41: 687–708, 2009. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 3.Andersen JL, Terzis G, Kryger A. Increase in the degree of coexpression of myosin heavy chain isoforms in skeletal muscle fibers of the very old. Muscle Nerve 22: 449–454, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 4.Balage M, Averous J, Rémond D, Bos C, Pujos-Guillot E, Papet I, Mosoni L, Combaret L, Dardevet D. Presence of low-grade inflammation impaired postprandial stimulation of muscle protein synthesis in old rats. J Nutr Biochem 21: 325–331, 2010. doi: 10.1016/j.jnutbio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Bamman MM, Hill VJ, Adams GR, Haddad F, Wetzstein CJ, Gower BA, Ahmed A, Hunter GR. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci 58: 108–116, 2003. doi: 10.1093/gerona/58.2.B108. [DOI] [PubMed] [Google Scholar]
- 6.Bickel CS, Cross JM, Bamman MM. Exercise dosing to retain resistance training adaptations in young and older adults. Med Sci Sports Exerc 43: 1177–1187, 2011. doi: 10.1249/MSS.0b013e318207c15d. [DOI] [PubMed] [Google Scholar]
- 7.Borde R, Hortobágyi T, Granacher U. Dose-response relationships of resistance training in healthy old adults: A systematic review and meta-analysis. Sports Med 45: 1693–1720, 2015. doi: 10.1007/s40279-015-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottinelli R, Reggiani C. Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol 73: 195–262, 2000. doi: 10.1016/S0079-6107(00)00006-7. [DOI] [PubMed] [Google Scholar]
- 9.Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr Metab (Lond) 8: 68, 2011. doi: 10.1186/1743-7075-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev 41: 169–173, 2013. doi: 10.1097/JES.0b013e318292f3d5. [DOI] [PubMed] [Google Scholar]
- 11.Campos GE, Luecke TJ, Wendeln HK, Toma K, Hagerman FC, Murray TF, Ragg KE, Ratamess NA, Kraemer WJ, Staron RS. Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol 88: 50–60, 2002. doi: 10.1007/s00421-002-0681-6. [DOI] [PubMed] [Google Scholar]
- 12.Charette SL, McEvoy L, Pyka G, Snow-Harter C, Guido D, Wiswell RA, Marcus R. Muscle hypertrophy response to resistance training in older women. J Appl Physiol (1985) 70: 1912–1916, 1991. doi: 10.1152/jappl.1991.70.5.1912. [DOI] [PubMed] [Google Scholar]
- 13.Cheema BS, Chan D, Fahey P, Atlantis E. Effect of progressive resistance training on measures of skeletal muscle hypertrophy, muscular strength and health-related quality of life in patients with chronic kidney disease: a systematic review and meta-analysis. Sports Med 44: 1125–1138, 2014. doi: 10.1007/s40279-014-0176-8. [DOI] [PubMed] [Google Scholar]
- 14.Chilibeck PD, Kaviani M, Candow DG, Zello GA. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: a meta-analysis. Open Access J Sports Med 8: 213–226, 2017. doi: 10.2147/OAJSM.S123529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claflin DR, Larkin LM, Cederna PS, Horowitz JF, Alexander NB, Cole NM, Galecki AT, Chen S, Nyquist LV, Carlson BM, Faulkner JA, Ashton-Miller JA. Effects of high- and low-velocity resistance training on the contractile properties of skeletal muscle fibers from young and older humans. J Appl Physiol (1985) 111: 1021–1030, 2011. doi: 10.1152/japplphysiol.01119.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen J. A power primer. Psychol Bull 112: 155–159, 1992. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 17.Cooke MB, Brabham B, Buford TW, Shelmadine BD, McPheeters M, Hudson GM, Stathis C, Greenwood M, Kreider R, Willoughby DS. Creatine supplementation post-exercise does not enhance training-induced adaptations in middle to older aged males. Eur J Appl Physiol 114: 1321–1332, 2014. doi: 10.1007/s00421-014-2866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig BW, Brown R, Everhart J. Effects of progressive resistance training on growth hormone and testosterone levels in young and elderly subjects. Mech Ageing Dev 49: 159–169, 1989. doi: 10.1016/0047-6374(89)90099-7. [DOI] [PubMed] [Google Scholar]
- 19.Csapo R, Alegre LM. Effects of resistance training with moderate vs heavy loads on muscle mass and strength in the elderly: A meta-analysis. Scand J Med Sci Sports 26: 995–1006, 2016. doi: 10.1111/sms.12536. [DOI] [PubMed] [Google Scholar]
- 20.Devries MC, Phillips SM. Creatine supplementation during resistance training in older adults-a meta-analysis. Med Sci Sports Exerc 46: 1194–1203, 2014. doi: 10.1249/MSS.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634, 1997. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedewa MV, Gist NH, Evans EM, Dishman RK. Exercise and insulin resistance in youth: a meta-analysis. Pediatrics 133: e163–e174, 2014. doi: 10.1542/peds.2013-2718. [DOI] [PubMed] [Google Scholar]
- 23.Fedewa MV, Hathaway ED, Ward-Ritacco CL. Effect of exercise training on C reactive protein: a systematic review and meta-analysis of randomised and non-randomised controlled trials. Br J Sports Med 51: 670–676, 2017. doi: 10.1136/bjsports-2016-095999. [DOI] [PubMed] [Google Scholar]
- 24.Fedewa MV, Hathaway ED, Williams TD, Schmidt MD. Effect of exercise training on non-exercise physical activity: A systematic review and meta-analysis of randomized controlled trials. Sports Med 47: 1171–1182, 2017. doi: 10.1007/s40279-016-0649-z. [DOI] [PubMed] [Google Scholar]
- 25.Fedewa MV, MacDonald HV, Hathaway ED, Williams TD, Schmidt MD. Author’s reply to Paravidino et al.: Comment on: “Effect of exercise training on non-exercise physical activity: a systematic review and meta-analysis of randomized controlled trials”. Sports Med 47: 2131–2134, 2017. doi: 10.1007/s40279-017-0756-5. [DOI] [PubMed] [Google Scholar]
- 26.Ferketich AK, Kirby TE, Alway SE. Cardiovascular and muscular adaptations to combined endurance and strength training in elderly women. Acta Physiol Scand 164: 259–267, 1998. doi: 10.1046/j.1365-201X.1998.00428.x. [DOI] [PubMed] [Google Scholar]
- 27.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 263: 3029–3034, 1990. doi: 10.1001/jama.1990.03440220053029. [DOI] [PubMed] [Google Scholar]
- 28.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M; International Working Group on Sarcopenia . Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12: 249–256, 2011. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Figueiredo VC, McCarthy JJ. Regulation of ribosome biogenesis in skeletal muscle hypertrophy. Physiology (Bethesda) 34: 30–42, 2019. doi: 10.1152/physiol.00034.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finger D, Goltz FR, Umpierre D, Meyer E, Rosa LH, Schneider CD. Effects of protein supplementation in older adults undergoing resistance training: a systematic review and meta-analysis. Sports Med 45: 245–255, 2015. doi: 10.1007/s40279-014-0269-4. [DOI] [PubMed] [Google Scholar]
- 31.Flück M, Hoppeler H. Molecular basis of skeletal muscle plasticity–from gene to form and function. Rev Physiol Biochem Pharmacol 146: 159–216, 2003. doi: 10.1007/s10254-002-0004-7. [DOI] [PubMed] [Google Scholar]
- 32.Frontera WR, Hughes VA, Krivickas LS, Kim SK, Foldvari M, Roubenoff R. Strength training in older women: early and late changes in whole muscle and single cells. Muscle Nerve 28: 601–608, 2003. doi: 10.1002/mus.10480. [DOI] [PubMed] [Google Scholar]
- 33.Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol (1985) 64: 1038–1044, 1988. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- 34.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP; American College of Sports Medicine . American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43: 1334–1359, 2011. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 35.Grgic J, Schoenfeld BJ. Are the hypertrophic adaptations to high and low-load resistance training muscle fiber type specific? Front Physiol 9: 402, 2018. doi: 10.3389/fphys.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grimby G, Aniansson A, Hedberg M, Henning GB, Grangård U, Kvist H. Training can improve muscle strength and endurance in 78- to 84-yr-old men. J Appl Physiol (1985) 73: 2517–2523, 1992. doi: 10.1152/jappl.1992.73.6.2517. [DOI] [PubMed] [Google Scholar]
- 37.Hagerman FC, Walsh SJ, Staron RS, Hikida RS, Gilders RM, Murray TF, Toma K, Ragg KE. Effects of high-intensity resistance training on untrained older men. I. Strength, cardiovascular, and metabolic responses. J Gerontol A Biol Sci Med Sci 55: B336–B346, 2000. doi: 10.1093/gerona/55.7.B336. [DOI] [PubMed] [Google Scholar]
- 38.Häkkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Mälkiä E, Kraemer WJ, Newton RU, Alen M. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol (1985) 84: 1341–1349, 1998. doi: 10.1152/jappl.1998.84.4.1341. [DOI] [PubMed] [Google Scholar]
- 39.Häkkinen K, Kraemer WJ, Newton RU, Alen M. Changes in electromyographic activity, muscle fibre and force production characteristics during heavy resistance/power strength training in middle-aged and older men and women. Acta Physiol Scand 171: 51–62, 2001. doi: 10.1046/j.1365-201x.2001.171001051.x. [DOI] [PubMed] [Google Scholar]
- 40.Häkkinen K, Pakarinen A, Kraemer WJ, Häkkinen A, Valkeinen H, Alen M. Selective muscle hypertrophy, changes in EMG and force, and serum hormones during strength training in older women. J Appl Physiol (1985) 91: 569–580, 2001. doi: 10.1152/jappl.2001.91.2.569. [DOI] [PubMed] [Google Scholar]
- 41.Hedges L, Olkin I. Statistical Methods for Meta-Analysis. San Diego, CA: Academic Press, 1985. [Google Scholar]
- 42.Hikida RS, Staron RS, Hagerman FC, Walsh S, Kaiser E, Shell S, Hervey S. Effects of high-intensity resistance training on untrained older men. II. Muscle fiber characteristics and nucleo-cytoplasmic relationships. J Gerontol A Biol Sci Med Sci 55: B347–B354, 2000. doi: 10.1093/gerona/55.7.B347. [DOI] [PubMed] [Google Scholar]
- 43.Holwerda AM, Overkamp M, Paulussen KJM, Smeets JSJ, van Kranenburg J, Backx EMP, Gijsen AP, Goessens JPB, Verdijk LB, van Loon LJC. Protein supplementation after exercise and before sleep does not further augment muscle mass and strength gains during resistance exercise training in active older men. J Nutr 148: 1723–1732, 2018. doi: 10.1093/jn/nxy169. [DOI] [PubMed] [Google Scholar]
- 44.Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Seip RL, Clarkson PM. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc 37: 964–972, 2005. [PubMed] [Google Scholar]
- 45.Kadi F, Charifi N, Denis C, Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve 29: 120–127, 2004. doi: 10.1002/mus.10510. [DOI] [PubMed] [Google Scholar]
- 46.Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, Saltin B. Ageing alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand 140: 55–62, 1990. doi: 10.1111/j.1748-1716.1990.tb08975.x. [DOI] [PubMed] [Google Scholar]
- 47.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol (1985) 101: 531–544, 2006. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 48.Kraemer WJ, Häkkinen K, Newton RU, Nindl BC, Volek JS, McCormick M, Gotshalk LA, Gordon SE, Fleck SJ, Campbell WW, Putukian M, Evans WJ. Effects of heavy-resistance training on hormonal response patterns in younger vs. older men. J Appl Physiol (1985) 87: 982–992, 1999. doi: 10.1152/jappl.1999.87.3.982. [DOI] [PubMed] [Google Scholar]
- 49.Kryger AI, Andersen JL. Resistance training in the oldest old: consequences for muscle strength, fiber types, fiber size, and MHC isoforms. Scand J Med Sci Sports 17: 422–430, 2007. doi: 10.1111/j.1600-0838.2006.00575.x. [DOI] [PubMed] [Google Scholar]
- 50.Lange KH, Andersen JL, Beyer N, Isaksson F, Larsson B, Rasmussen MH, Juul A, Bülow J, Kjaer M. GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. J Clin Endocrinol Metab 87: 513–523, 2002. doi: 10.1210/jcem.87.2.8206. [DOI] [PubMed] [Google Scholar]
- 51.Larsson L. Histochemical characteristics of human skeletal muscle during aging. Acta Physiol Scand 117: 469–471, 1983. doi: 10.1111/j.1748-1716.1983.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 52.Larsson L. Physical training effects on muscle morphology in sedentary males at different ages. Med Sci Sports Exerc 14: 203–206, 1982. doi: 10.1249/00005768-198203000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev 99: 427–511, 2019. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Nilwik R, van Loon LJ. Elderly men and women benefit equally from prolonged resistance-type exercise training. J Gerontol A Biol Sci Med Sci 68: 769–779, 2013. doi: 10.1093/gerona/gls241. [DOI] [PubMed] [Google Scholar]
- 55.Leenders M, Verdijk LB, Van der Hoeven L, Van Kranenburg J, Nilwik R, Wodzig WK, Senden JM, Keizer HA, Van Loon LJ. Protein supplementation during resistance-type exercise training in the elderly. Med Sci Sports Exerc 45: 542–552, 2013. doi: 10.1249/MSS.0b013e318272fcdb. [DOI] [PubMed] [Google Scholar]
- 56.Léger B, Cartoni R, Praz M, Lamon S, Dériaz O, Crettenand A, Gobelet C, Rohmer P, Konzelmann M, Luthi F, Russell AP. Akt signalling through GSK-3beta, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol 576: 923–933, 2006. doi: 10.1113/jphysiol.2006.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50: 11–16, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Lexell J, Downham DY, Larsson Y, Bruhn E, Morsing B. Heavy-resistance training in older Scandinavian men and women: short- and long-term effects on arm and leg muscles. Scand J Med Sci Sports 5: 329–341, 1995. doi: 10.1111/j.1600-0838.1995.tb00055.x. [DOI] [PubMed] [Google Scholar]
- 59.Lipsey MW, Wilson DB. Practice Meta-Analysis. Thousand Oaks, CA: Sage Publications, 2001, vol. 49. [Google Scholar]
- 60.Martel GF, Roth SM, Ivey FM, Lemmer JT, Tracy BL, Hurlbut DE, Metter EJ, Hurley BF, Rogers MA. Age and sex affect human muscle fibre adaptations to heavy-resistance strength training. Exp Physiol 91: 457–464, 2006. doi: 10.1113/expphysiol.2005.032771. [DOI] [PubMed] [Google Scholar]
- 61.Mayhew DL, Kim JS, Cross JM, Ferrando AA, Bamman MM. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol (1985) 107: 1655–1662, 2009. doi: 10.1152/japplphysiol.91234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J 18: 355–357, 2004. doi: 10.1096/fj.03-0395fje. [DOI] [PubMed] [Google Scholar]
- 63.Mero AA, Hulmi JJ, Salmijärvi H, Katajavuori M, Haverinen M, Holviala J, Ridanpää T, Häkkinen K, Kovanen V, Ahtiainen JP, Selänne H. Resistance training induced increase in muscle fiber size in young and older men. Eur J Appl Physiol 113: 641–650, 2013. doi: 10.1007/s00421-012-2466-x. [DOI] [PubMed] [Google Scholar]
- 64.Miller MS, Bedrin NG, Ades PA, Palmer BM, Toth MJ. Molecular determinants of force production in human skeletal muscle fibers: effects of myosin isoform expression and cross-sectional area. Am J Physiol Cell Physiol 308: C473–C484, 2015. doi: 10.1152/ajpcell.00158.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller MS, Bedrin NG, Callahan DM, Previs MJ, Jennings ME II, Ades PA, Maughan DW, Palmer BM, Toth MJ. Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol (1985) 115: 1004–1014, 2013. doi: 10.1152/japplphysiol.00563.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller MS, Callahan DM, Tourville TW, Slauterbeck JR, Kaplan A, Fiske BR, Savage PD, Ades PA, Beynnon BD, Toth MJ. Moderate-intensity resistance exercise alters skeletal muscle molecular and cellular structure and function in inactive older adults with knee osteoarthritis. J Appl Physiol (1985) 122: 775–787, 2017. doi: 10.1152/japplphysiol.00830.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitchell CJ, Churchward-Venne TA, West DW, Burd NA, Breen L, Baker SK, Phillips SM. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol (1985) 113: 71–77, 2012. doi: 10.1152/japplphysiol.00307.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097, 2009. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moro T, Brightwell CR, Phalen DE, McKenna CF, Lane SJ, Porter C, Volpi E, Rasmussen BB, Fry CS. Low skeletal muscle capillarization limits muscle adaptation to resistance exercise training in older adults. Exp Gerontol 127: 110723, 2019. doi: 10.1016/j.exger.2019.110723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morton RW, Traylor DA, Weijs PJM, Phillips SM. Defining anabolic resistance: implications for delivery of clinical care nutrition. Curr Opin Crit Care 24: 124–130, 2018. doi: 10.1097/MCC.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 71.Murach KA, Dungan CM, Kosmac K, Voigt TB, Tourville TW, Miller MS, Bamman MM, Peterson CA, Toth MJ. Fiber typing human skeletal muscle with fluorescent immunohistochemistry. J Appl Physiol (1985) 127: 1632–1639, 2019. doi: 10.1152/japplphysiol.00624.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murach KA, Fry CS, Kirby TJ, Jackson JR, Lee JD, White SH, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Starring or supporting role? Satellite cells and skeletal muscle fiber size regulation. Physiology (Bethesda) 33: 26–38, 2018. doi: 10.1152/physiol.00019.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Negaresh R, Ranjbar R, Habibi A, Mokhtarzade M, Fokin A, Gharibvand MM. The effect of resistance training on quadriceps muscle volume and some growth factors in elderly and young men. Adv Gerontol 30: 880–887, 2017. [PubMed] [Google Scholar]
- 74.Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB, van Loon LJ. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 48: 492–498, 2013. doi: 10.1016/j.exger.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 75.Parente V, D’Antona G, Adami R, Miotti D, Capodaglio P, De Vito G, Bottinelli R. Long-term resistance training improves force and unloaded shortening velocity of single muscle fibres of elderly women. Eur J Appl Physiol 104: 885–893, 2008. doi: 10.1007/s00421-008-0845-0. [DOI] [PubMed] [Google Scholar]
- 76.Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc 43: 249–258, 2011. doi: 10.1249/MSS.0b013e3181eb6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA 320: 2020–2028, 2018. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pyka G, Lindenberger E, Charette S, Marcus R. Muscle strength and fiber adaptations to a year-long resistance training program in elderly men and women. J Gerontol 49: M22–M27, 1994. doi: 10.1093/geronj/49.1.M22. [DOI] [PubMed] [Google Scholar]
- 79.Raue U, Slivka D, Minchev K, Trappe S. Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol (1985) 106: 1611–1617, 2009. doi: 10.1152/japplphysiol.91587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, Combaret L, Dardevet D. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol 587: 5483–5492, 2009. doi: 10.1113/jphysiol.2009.178319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenberg MS. The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evolution 59: 464–468, 2005. doi: 10.1111/j.0014-3820.2005.tb01004.x. [DOI] [PubMed] [Google Scholar]
- 82.Rosenthal R. Meta-Analytic Procedures for Social Research. Thousand Oaks, CA: SAGE Publications, 1991. [Google Scholar]
- 83.Schoenfeld BJ. The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res 24: 2857–2872, 2010. doi: 10.1519/JSC.0b013e3181e840f3. [DOI] [PubMed] [Google Scholar]
- 84.Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol 295: R273–R280, 2008. doi: 10.1152/ajpregu.00093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith GI, Villareal DT, Sinacore DR, Shah K, Mittendorfer B. Muscle protein synthesis response to exercise training in obese, older men and women. Med Sci Sports Exerc 44: 1259–1266, 2012. doi: 10.1249/MSS.0b013e3182496a41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Snijders T, Nederveen JP, Joanisse S, Leenders M, Verdijk LB, van Loon LJ, Parise G. Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J Cachexia Sarcopenia Muscle 8: 267–276, 2017. doi: 10.1002/jcsm.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Staron RS, Herman JR, Schuenke MD. Misclassification of hybrid fast fibers in resistance-trained human skeletal muscle using histochemical and immunohistochemical methods. J Strength Cond Res 26: 2616–2622, 2012. doi: 10.1519/JSC.0b013e3182667095. [DOI] [PubMed] [Google Scholar]
- 88.Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC, Bamman MM. Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. Am J Physiol Endocrinol Metab 310: E652–E661, 2016. doi: 10.1152/ajpendo.00486.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 54: 1046–1055, 2001. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 90.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343: d4002, 2011. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 91.Stewart LA, Tierney JF. To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data. Eval Health Prof 25: 76–97, 2002. doi: 10.1177/0163278702025001006. [DOI] [PubMed] [Google Scholar]
- 92.Taaffe DR, Jin IH, Vu TH, Hoffman AR, Marcus R. Lack of effect of recombinant human growth hormone (GH) on muscle morphology and GH-insulin-like growth factor expression in resistance-trained elderly men. J Clin Endocrinol Metab 81: 421–425, 1996. [DOI] [PubMed] [Google Scholar]
- 93.Taaffe DR, Pruitt L, Pyka G, Guido D, Marcus R. Comparative effects of high- and low-intensity resistance training on thigh muscle strength, fiber area, and tissue composition in elderly women. Clin Physiol 16: 381–392, 1996. doi: 10.1111/j.1475-097X.1996.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 94.Toth MJ, Matthews DE, Tracy RP, Previs MJ. Age-related differences in skeletal muscle protein synthesis: relation to markers of immune activation. Am J Physiol Endocrinol Metab 288: E883–E891, 2005. doi: 10.1152/ajpendo.00353.2004. [DOI] [PubMed] [Google Scholar]
- 95.Trappe S, Godard M, Gallagher P, Carroll C, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am J Physiol Cell Physiol 281: C398–C406, 2001. doi: 10.1152/ajpcell.2001.281.2.C398. [DOI] [PubMed] [Google Scholar]
- 96.Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol (1985) 89: 143–152, 2000. doi: 10.1152/jappl.2000.89.1.143. [DOI] [PubMed] [Google Scholar]
- 97.Trappe TA, Ratchford SM, Brower BE, Liu SZ, Lavin KM, Carroll CC, Jemiolo B, Trappe SW. COX inhibitor influence on skeletal muscle fiber size and metabolic adaptations to resistance exercise in older adults. J Gerontol A Biol Sci Med Sci 71: 1289–1294, 2016. doi: 10.1093/gerona/glv231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, van Loon LJ. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci 64: 332–339, 2009. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr) 36: 545–547, 2014. doi: 10.1007/s11357-013-9583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wallerstein LF, Tricoli V, Barroso R, Rodacki A LF, Russo L, Aihara AY, da Rocha Correa Fernandes A, de Mello MT, Ugrinowitsch C. Effects of strength and power training on neuromuscular variables in older adults. J Aging Phys Act 20: 171–185, 2012. doi: 10.1123/japa.20.2.171. [DOI] [PubMed] [Google Scholar]
- 101.Welle S, Totterman S, Thornton C. Effect of age on muscle hypertrophy induced by resistance training. J Gerontol A Biol Sci Med Sci 51: M270–M275, 1996. doi: 10.1093/gerona/51A.6.M270. [DOI] [PubMed] [Google Scholar]
- 102.Zerba E, Komorowski TE, Faulkner JA. Free radical injury to skeletal muscles of young, adult, and old mice. Am J Physiol Cell Physiol 258: C429–C435, 1990. doi: 10.1152/ajpcell.1990.258.3.C429. [DOI] [PubMed] [Google Scholar]