Watch a video presentation of this article
Abbreviations
- BIA
bioelectrical impedance analysis
- CT
computed tomography
- DEXA
dual energy X‐ray absorptiometry
- GH
growth hormone
- NAFL
nonalcoholic fatty liver without inflammation
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
Nonalcoholic fatty liver disease (NAFLD) is now the most common cause of chronic liver disease. NAFLD encompasses a group of conditions where there is an accumulation of excess fat in the liver without significant alcohol use. It includes a range of disorders from isolated nonalcoholic fatty liver without inflammation (NAFL) and nonalcoholic steatohepatitis (NASH) with inflammation, which in turn can eventually lead to fibrosis and cirrhosis. The relationship between chronic liver disease and sarcopenia has been a growing area of interest. Sarcopenia is characterized by loss of skeletal muscle mass, strength, and function. It is usually defined as a loss in appendicular muscle mass and is often assessed by either cross‐sectional or dual energy X‐ray absorptiometry. A related condition, myosteatosis, is defined as the infiltration of muscle with fat and is associated with decreased muscle function and an increased risk for mortality in cirrhosis.1 Sarcopenia is often associated with the aging process, but more recently has been recognized as also associated with metabolic syndrome, diabetes mellitus, and cardiovascular diseases. Observational studies have now identified a link between sarcopenia and chronic liver disease. Sarcopenia begins to manifest even in the early stages of liver disease and worsens with the liver disease severity, with a prevalence rate of approximately 60% in those with cirrhosis of all etiologies.2, 3
Association Between Sarcopenia and NAFLD
Within chronic liver disease, NAFLD in particular and sarcopenia share several risk factors. Multiple cross‐sectional observational studies have demonstrated that sarcopenia is associated with an increased risk for NAFLD and NAFLD‐related advanced fibrosis in NAFLD across ethnicities. This association was independent of what may be explained by obesity, metabolic syndrome, and insulin resistance. Moreover, there is growing evidence that suggests that an increase in relative skeletal muscle mass has benefits in both a lower incidence of NAFLD and the resolution of existing NAFLD.
Most of the studies that link sarcopenia and NAFLD were conducted in Asian populations, observing an association between the prevalence of sarcopenia and histological grades of steatosis. Sarcopenia was present at increasing rates in healthy control subjects without NAFLD (~8%‐22%), those with NAFL (~18%‐38%), and those with NASH (~35%‐63%).4, 5, 6, 7 Those with sarcopenia also had higher rates in NASH with fibrosis (46%) versus those without (25%), with a 2.5‐fold higher risk for NASH and significant fibrosis in patients with NAFLD, independent of obesity and insulin resistance.5 Another cohort study examined the association between sarcopenia and NAFLD, assessed by noninvasive measures.8 Among patients without baseline NAFLD, 15% developed incident NAFLD during the 7‐year study period, with a higher incidence in the lowest tertile of skeletal muscle mass as compared with the highest tertile, even after adjustment for several known risk factors. Among patients with NAFLD at baseline, the highest tertile of skeletal muscle mass was associated with resolution of existing NAFLD. Combined with multiple other studies4, 5, 6, 7, 8, 9, 10, 11 (Table 1), including in American and Italian populations, these data suggest that higher skeletal muscle mass is associated with a lower incidence of NAFLD, and perhaps even resolution of existing NAFLD. Data from varied populations and ethnicities are important, in light of observed differences in hepatic steatosis across different ethnicities, including the prevalence of lean NAFLD.12
Table 1.
Summary of Population Studies That Link Sarcopenia and NAFLD
| Study | Study Design, Population (n) | NAFLD Assessment | Sarcopenia Assessment | Key Findings: NAFLD and Complications in Patients With Sarcopenia |
|---|---|---|---|---|
| Hong et al. (2014)4 | Cross‐sectional, Korean (526) | CT imaging | DEXA | 5‐fold increase of NAFLD |
| Lee et al. (2015)6 | Cross‐sectional, Korean (15,132) | Noninvasive models | DEXA | 2.3‐ to 3.3‐fold increase of NAFLD in patients with sarcopenia |
| Lee et al. (2016)7 | Cross‐sectional, Korean (2761) | Noninvasive models | DEXA | 2‐fold increase of fibrosis in patients with sarcopenia |
| Koo et al. (2017)5 | Cross‐sectional, Korean (309) | Histology | BIA | Increasing prevalence with disease severity |
| 2.5‐fold increase of NASH and significant fibrosis in patients with sarcopenia | ||||
| Issa et al. (2014)10 | Retrospective, American (75) | Histology | CT imaging | Increasing sarcopenia in NASH and NASH cirrhosis |
| Petta et al. (2017)11 | Retrospective, Italian (225) | Histology | BIA | 2‐fold increase of fibrosis in NAFLD in patients with sarcopenia |
| Kim et al. (2018)8 | Cohort, Korean (10,534) | Noninvasive models | BIA | Increase in incident NAFLD in patients with sarcopenia |
| Increase in resolution of baseline NAFLD with higher muscle mass | ||||
| Wijarnpreecha et al. (2019)9 | Cross‐sectional, American (11,325) | Ultrasound | BIA | 2.3‐fold increase of NAFLD in patients with sarcopenia |
| 1.8‐fold increase in advanced fibrosis in patients with sarcopenia |
Data from non‐Asian populations are important, in light of the apparent greater risk for fat‐related disorders such as NAFLD in Asian populations compared with Caucasians of the same anthropomorphic characteristics.13
Mechanisms That Link Sarcopenia and NAFLD
The relationship between NAFLD and muscle metabolism is highlighted due to the essential role of skeletal muscle in energy metabolism. Indeed, NAFLD and sarcopenia share common underlying mechanisms, including systemic inflammation; insulin resistance and alterations of the growth hormone (GH)/insulin‐like growth factor 1 axis; nutritional deficiencies, such as vitamin D metabolism, myostatin, and adiponectin dysregulation; hepatic production of catabolic factors; and physical deconditioning and inactivity (reviewed by Banjhi et al.14; Fig. 1). Given the overlap in pathophysiology shared between NAFLD and sarcopenia, whether sarcopenia is a bona fide factor in the development of NAFLD or a complication thereof is difficult to determine.
Fig 1.
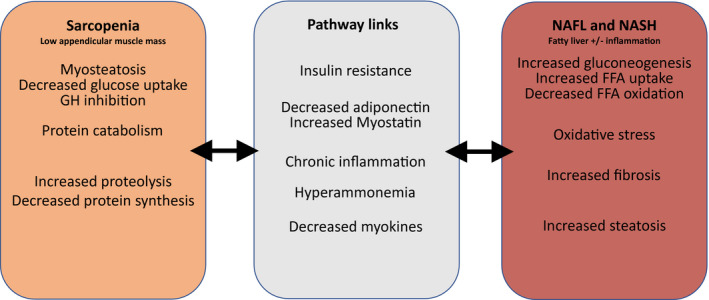
Proposed pathways linking sarcopenia and NAFLD. Sarcopenia and NAFLD share several common risk factors, and there is evidence for several pathways linking them, which are highlighted. These include insulin resistance, chronic inflammation, myostatin, adiponectin, myokines, and hyperammonia in cirrhosis.
Because skeletal muscle is a major insulin‐responsive organ, loss of skeletal muscle and myosteatosis can lead to a decrease in insulin response and energy expenditure. This, in turn, leads to increased hepatic gluconeogenesis, increased hepatic free fatty acid (FFA) uptake, and decreased FFA oxidation. Insulin resistance is therefore closely linked to the pathogenesis of NAFLD (reviewed by Banjhi et al.14). Furthermore, insulin resistance exacerbates proteolysis and contributes to skeletal muscle loss through mitochondrial dysfunction and reduction of muscle protein synthesis. Insulin resistance has also been linked to myosteatosis and GH inhibition. Myokines secreted by skeletal muscle include irisin (an exercise‐induced hormone), interleukin‐6, myostatin, and adiponectin, and are involved in the regulation of hepatic glucose and fatty acid metabolism. Altered levels of these myokines from decreases in muscle mass have been associated with hepatic fat accumulation. Chronic systemic inflammation and oxidative stress have also been linked to both catabolism (and the resulting loss in skeletal muscle mass), oxidative stress, decreased adiponectin, and the pathogenesis of NAFLD. Elevated ammonia in chronic liver disease and, more specifically, end‐stage liver disease is also associated with sarcopenia through glutamate depletion and decreased protein synthesis, the release of myostatin, and lysosomal autophagy.
Treatment Options and Lifestyle Modification
The primary intervention for both NAFLD and sarcopenia is lifestyle modification, including physical activity. In NAFLD, the degree of weight loss is proportional to the degree of improvement in liver histology. However, weight loss efforts should also be aimed toward simultaneously preserving muscle mass, for which adequate protein intake while reducing fat and fructose intake is recommended to prevent sarcopenia.15 For patients with cirrhosis, physical activity has been shown to increase both functional capacity and muscle mass.16 Assessing for diabetes, thyroid function, male testosterone status, and vitamin D, as well as suppression of ammonia excess, may also play a role in the management of sarcopenia in NAFLD. Finally, there is growing evidence for increased risk for complications in liver transplantation in patients with sarcopenia, including in NAFLD, and this should be considered accordingly (reviewed by Lai17). Providers should be cognizant of the relationship between sarcopenia and NAFLD, because sarcopenia may be a potentially intervenable, disease‐modifying state. We suggest proactively screening for sarcopenia and when advocating for lifestyle modification, to bear in mind the goal of both weight loss and muscle mass preservation.
Potential Conflict of Interest: Nothing to report.
References
- 1. Montano‐Loza AJ, Angulo P, Meza‐Junco J, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle 2016;7:126‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhanji RA, Carey EJ, Yang L, et al. The long winding road to transplant: how sarcopenia and debility impact morbidity and mortality on the waitlist. Clin Gastroenterol Hepatol 2017;15:1492‐1497. [DOI] [PubMed] [Google Scholar]
- 3. Chen HW, Dunn MA. Arresting frailty and sarcopenia in cirrhosis: future prospects. Clin Liver Dis 2018;11:52‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hong HC, Hwang SY, Choi HY, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology 2014;59:1772‐1778. [DOI] [PubMed] [Google Scholar]
- 5. Koo BK, Kim D, Joo SK, et al. Sarcopenia is an independent risk factor for non‐alcoholic steatohepatitis and significant fibrosis. J Hepatol 2017;66:123‐131. [DOI] [PubMed] [Google Scholar]
- 6. Lee YH, Jung KS, Kim SU, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: nationwide surveys (KNHANES 2008‐2011). J Hepatol 2015;63:486‐493. [DOI] [PubMed] [Google Scholar]
- 7. Lee YH, Kim SU, Song K, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: nationwide surveys (KNHANES 2008‐2011). Hepatology 2016;63:776‐786. [DOI] [PubMed] [Google Scholar]
- 8. Kim G, Lee SE, Lee YB, et al. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: a 7‐year longitudinal study. Hepatology 2018;68:1755‐1768. [DOI] [PubMed] [Google Scholar]
- 9. Wijarnpreecha K, Kim D, Raymond P, et al. Associations between sarcopenia and nonalcoholic fatty liver disease and advanced fibrosis in the USA. Eur J Gastroenterol Hepatol 2019;31:1121‐1128. [DOI] [PubMed] [Google Scholar]
- 10. Issa D, Alkhouri N, Tsien C, et al. Presence of sarcopenia (muscle wasting) in patients with nonalcoholic steatohepatitis. Hepatology 2014;60:428‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petta S, Ciminnisi S, Di Marco V, et al. Sarcopenia is associated with severe liver fibrosis in patients with non‐alcoholic fatty liver disease. Aliment Pharmacol Ther 2017;45:510‐518. [DOI] [PubMed] [Google Scholar]
- 12. Wattacheril J, Sanyal AJ. Lean NAFLD: an underrecognized outlier. Curr Hepatol Rep 2016;15:134‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Omagari K, Kadokawa Y, Masuda J, et al. Fatty liver in non‐alcoholic non‐overweight Japanese adults: Incidence and clinical characteristics. J Gastroenterol Hepatol 2002;17:1098‐1105. [DOI] [PubMed] [Google Scholar]
- 14. Bhanji RA, Narayanan P, Allen AM, et al. Sarcopenia in hiding: the risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 2017;66:2055‐2065. [DOI] [PubMed] [Google Scholar]
- 15. Yanai H. Nutrition for sarcopenia. J Clin Med Res 2015;7:926‐931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roman E, Torrades MT, Nadal MJ, et al. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci 2014;59:1966‐1975. [DOI] [PubMed] [Google Scholar]
- 17. Lai JC. A framework to determine when liver transplantation is futile. Clin Liver Dis 2016;8:137‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]


